Abstract
The molecular regulation of smooth muscle cell (SMC) behavior is reviewed, with particular emphasis on stimuli that promote the contractile phenotype. SMCs can shift reversibly along a continuum from a quiescent, contractile phenotype to a synthetic phenotype, which is characterized by proliferation and extracellular matrix (ECM) synthesis. This phenotypic plasticity can be harnessed for tissue engineering. Cultured synthetic SMCs have been used to engineer smooth muscle tissues with organized ECM and cell populations. However, returning SMCs to a contractile phenotype remains a key challenge. This review will integrate recent work on how soluble signaling factors, ECM, mechanical stimulation, and other cells contribute to the regulation of contractile SMC phenotype. The signal transduction pathways and mechanisms of gene expression induced by these stimuli are beginning to be elucidated and provide useful information for the quantitative analysis of SMC phenotype in engineered tissues. Progress in the development of tissue-engineered scaffold systems that implement biochemical, mechanical, or novel polymer fabrication approaches to promote contractile phenotype will also be reviewed. The application of an improved molecular understanding of SMC biology will facilitate the design of more potent cell-instructive scaffold systems to regulate SMC behavior.
Introduction
Each year, 250,000 patients undergo coronary artery bypass grafting operations.1 Unfortunately, 20%–30% of patients who require coronary artery bypass grafting do not have suitable autologous vessels for the procedure.2,3 The goal of vascular tissue engineering is to generate functional vascular replacements that provide an option for these patients. One of the limitations of current engineered vascular prostheses is stenosis caused by excessive proliferation of smooth muscle tissue, known as intimal hyperplasia (IH). During the development of IH and other vascular pathologies, such as restenosis and atherosclerosis, smooth muscle cells (SMCs) lose their contractile proteins and cellular quiescence and increase their proliferation, migration, and production of extracellular matrix (ECM) proteins. These processes define a shift from normal, “contractile” SMC phenotype along a continuum toward a phenotype described as “synthetic” or “proliferative” (Fig. 1). For this review, the term “de-differentiation” will be used to describe this shifting of contractile SMCs toward synthetic phenotype. Promoting well-differentiated contractile SMC phenotype is one strategy to minimize the development of IH. Contractile SMCs also regulate the diameter of normal blood vessels. Developing approaches to impart this function to engineered vascular conduits is also an important goal. In this review, the molecular regulation of SMC behavior will be reviewed, with particular emphasis on stimuli that promote the contractile phenotype.
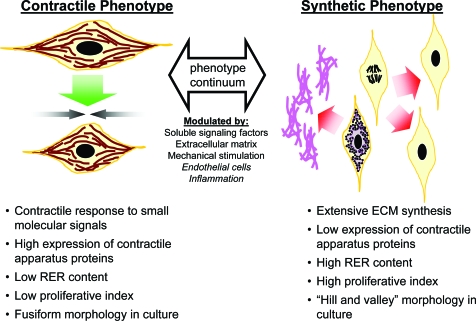
FIG. 1.
Summary of characteristics of SMC phenotypes, which vary along a continuum from synthetic and proliferative to contractile and quiescent. The position along this continuum is modulated by a variety of extracellular signals. ECM, extracellular matrix; RER, rough endoplasmic reticulum; SMC, smooth muscle cell. Color images available online at www.liebertonline.com/ten.
SMCs with a synthetic phenotype eventually can reacquire many of the characteristics of normal contractile SMCs, suggesting that phenotype switching can occur in both directions.4–10 It may be possible to harness this phenotypic plasticity to form autologous, functional arteries ex vivo11 or even in vivo from adjacent native SMCs. Proliferation of synthetic SMCs is required to populate the construct, and ECM deposition and remodeling are required to provide the appropriate mechanical strength and tissue architecture. Eventually these proliferative, synthetic SMCs must re-differentiate to a quiescent, contractile state, where they are refractory to signals that drive IH. For this review, the term “re-differentiation” will be used to describe such shifting of synthetic SMCs back toward a contractile phenotype. These processes require activation of diverse (and often opposing) cellular programs that must be appropriately controlled both spatially and temporally. Although expansion culture of synthetic SMCs has become routine, less is known about promoting contractile SMC phenotype from synthetic SMCs. Recent work on the cell and molecular biology of SMCs has elucidated many intra- and extracellular factors that affect SMC phenotype.12 Application of this information to the field of vascular tissue engineering is critical for the development of bioactive scaffold systems that can control SMC behavior.
The Continuum of SMC Phenotypes
In vivo, smooth muscle tissues play an important role in a wide range of systems from the vasculature to reproduction. To perform a diversity of functions, SMC phenotype spans a continuum from quiescent and contractile to proliferative and synthetic13,14 (Fig. 1). At the contractile extreme are SMCs with a fully functional contractile apparatus that responds to small molecule signals such as acetylcholine and norepinephrine. In early studies, these cells were characterized by an ultrastructure, observed by transmission electron microscopy, composed of tightly bundled myofilaments and minimal rough endoplasmic reticulum, Golgi, or free ribosomes.15–17 Contractile smooth muscle tissues also generally contain little connective tissue that would necessitate extensive SMC synthetic capacity.15 In culture, these cells possess a dense fusiform morphology.15,18 At the synthetic phenotype extreme of this continuum are fibroblast-like SMCs, which contain minimal contractile proteins and secrete ECM. The ultrastructure of these cells shows a cytoplasm devoid of contractile bundles with extensive rough endoplasmic reticulum, Golgi, and ribosomes.15–17 In culture, these cells initially adopt a broad, spread shape, and then begin to grow over one another in a “hill-and-valley” morphology.15,18 A synthetic phenotype is also correlated with SMC proliferation, with the number of S-phase cultured SMCs increasing from 3%–5% to 40%–60% during primary culture and pathologies such as IH.15 Most SMCs, even SMCs in contractile tissues, lie somewhere along the continuum. For example, SMCs in small muscular arteries typically have 80%–90% of their cytoplasm filled by myofilaments, whereas SMCs in the aorta typically contain only 60%–70%, indicating that aortic SMCs have both contractile and synthetic functions.15
The expression patterns of a wide range of protein markers have been characterized to describe the phenotypic state of SMCs (Table 1). Contractile SMCs, which predominate in normal vessels, exhibit a mature contractile apparatus including smooth muscle α-actin (SMαA), smooth muscle myosin heavy chains SM-1 and SM-2, calponin, SM-22α, and smoothelin (Fig. 2). Relative expression of these and other marker proteins can be used to localize SMCs on the contractile-synthetic continuum.13,19 Marker protein expression also can be correlated with an SMC's likelihood to respond to mitogens,20,21 or can predict the composition of secreted ECM proteins, which differ between contractile and synthetic SMCs.22 There also are several markers of synthetic SMC phenotype (Table 2), but these have been utilized less widely in the literature. Generally, these markers have less SMC specificity and their expression must be interpreted in the context of cells with known SMC lineage, if used to assess SMC phenotype.
Table 1.
Selected Markers of Contractile Smooth Muscle Cell Phenotype
| Name | Protein products | Protein size (kDa) | Expression in development13 | Function | Contractile SMC phenotype specificity | Additional notes |
|---|---|---|---|---|---|---|
| Smooth muscle α-actin (gene: ACTA2) | 1 | 43 | Early, increases to final level through postnatal period247 | Force generation248 | Low–intermediate: transient expression in skeletal muscle during development,249 expressed in myofibroblasts,187 endothelial cells250,251 | Most common marker of smooth muscle lineage use for research purposes |
| Smooth muscle basic (h1) calponin (gene: CNN1) | 1 | 34 | Intermediate249 | Modulation of myosin ATP-ase activity252 Signaling scaffold protein for ERK and PKC253–255 | Intermediate: transient expression in cardiomyocytes256 | Colocalizes with actin filaments in the central regions of cell cytoplasm257 Knockout mice display dysregulation of bone formation, decreased α-adrenergic vasoconstriction, impaired regulation of blood flow, and increased unloaded smooth muscle shortening velocity258–261 |
| SM-22α (transgelin) (gene: TAGLN) | 1 | 22 | Early intermediate249 | Modulation of contraction, especially that not depending on myosin light chain phosphorylation262 | Intermediate: transient expression in cardiomyocytes but restricted to smooth muscle in adults263–265 Expression in myofibroblasts266 | Knockout mice do not display any overt deficits in physiologic smooth muscle functions264 |
| Caldesmon (gene: CALD1) | 5 3 “heavy” 2 “light”267 |
h-caldesmon: 120–150 l-caldesmon: 70–80 |
Intermediate | Regulation of smooth muscle contraction255 | High: h-caldesmon is the predominant isoform expressed in smooth muscle, l-caldesmon is expressed in nonmuscle tissue24,267 | |
| Smooth muscle myosin heavy chain (gene: MYH11) | 4 SM-1A SM-1B SM-2A SM-2B268–270 |
200–204 | Late, especially SM-2 isoform which appears late in gestation and increases through the early postnatal period269 | Force generation270,271 | High: the SM-2 isoforms are lost rapidly from cultured SMCs272 and in intimal lesions273 SM-MHC, especially the SM-2 isoform, has been proposed as the most definitive marker of differentiated vascular SMCs13,274 | Differential expression of the A and B isoforms may contribute to functional (tonic vs. phasic) differences in contractility271 Transgenic mice with a deletion of the SM-2-specific exon died within 30 days after birth and had hypercontractile smooth muscle tissues275 |
| Smoothelin (gene: SMTN) | 6 A1–A3 B1–B3276 |
Smoothelin-A: 59 Smoothelin-B: 110 |
Smoothelin-B (in vasculature): intermediate Smoothelin-A: predominant expression in visceral smooth muscle of the adult277 |
Possible regulation of the contractile apparatus278 | High: smoothelin-B is predominantly expressed in the vasculature of the adult, although it is transiently expressed in visceral smooth muscle279 | Smoothelin-A and -B are expressed from the same gene by different promoters276 Smoothelin-B knockout mouse display decreased vascular contractility, hypertension, and cardiac hypertrophy280 Smoothelin binds with actin and colocalizes with actin filaments at the subcellular level64,278 |
This table covers only selected marker proteins and is not intended to be a comprehensive listing. Other markers used include the aortic-carboxypeptidase-like protein,176,281–283 smooth muscle myosin light chain kinase,179,192 telokin (a c-terminal fragment of smooth muscle myosin light chain kinase),283 cysteine- and glycine-rich protein 1,179 desmin,176 and focal adhesion kinase related nonkinase.283 A partial list of the SMC transcriptome can be found in an excellent review by Miano et al.169
ERK, extracellular signal-regulated kinase; PKC, protein kinase C; MHC, major histocompatibility complex; SMC, smooth muscle cell.
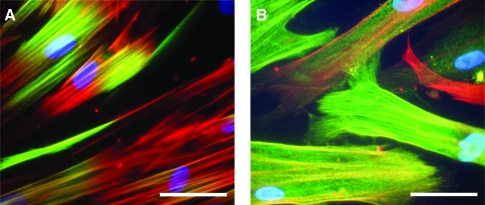
FIG. 2.
Expression of contractile apparatus proteins in human coronary artery SMCs that have been cultured to re-induce contractile phenotype, observed by immunofluorescent staining.103 (A) Calponin (green) colocalizes (yellow) with SMαA (red) fibrils in the central region of the cells. (B) SM-22α (green) colocalizes (yellow) along the length of SMαA (red) fibrils. Variable staining between cells highlights the heterogeneity of cell populations along the contractile-synthetic phenotype continuum. Nuclei are counterstained with DAPI (blue). Scale bars: 50 μm. SMαA, smooth muscle α-actin. Color images available online at www.liebertonline.com/ten.
Table 2.
Selected Markers of Synthetic Smooth Muscle Cell Phenotype
| Name | Protein products | Protein size (kDa) | Expression pattern | Function | Synthetic SMC phenotype specificity | Additional notes |
|---|---|---|---|---|---|---|
| Caldesmon light chain (l-caldesmon) (gene: CALD1) |
2 light chains | 70–80 | Expressed in synthetic SMCs267,284,285 and in nonmuscle tissue24,267,286 | Involved in calcium-dependent regulation of actin–myosin interactions,287 regulation of smooth muscle contraction255 | Expressed in synthetic SMCs267,284,285 and in nonmuscle tissue24,267,286 | |
| Vimentin (gene: VIM) | 1 | 52–58 | Widely expressed in embryos,288–290 fibroblasts, lymphocytes, endothelial cells, and other mesenchymal tissue291,292 | Type III intermediate filament; maintains cell shape and integrity of cytoplasm293,294 | One of the most common markers of synthetic SMC phenotype295,296; increased in synthetic SMCs cultured in vitro297 | Biomarker of epithelial to mesenchymal transition and tumor invasive phenotype marker298,299 |
| Nonmuscle myosin heavy chain B (SMemb) (gene: MYH10) | 1 | 200 | Expressed in embryonic SMCs, SMCs during vascular development, and SMCs in atherosclerosis273,300,301; abundantly expressed in brain302,303 | Actin–myosin force generation; involved in the growth cones of neurons304 | Expression re-induced in synthetic SMCs in atherosclerosis and after vascular injury273,300,301 | |
| Tropomyosin 4 (gene: TPM4) | 2 | 30 | Expressed in SMCs in atherosclerosis,305 striated muscle,306 and cardiac muscle307 | Actin-binding protein that regulates actin mechanics; synergistic interaction with low molecular weight topomyosin isoforms in cardiomyocytes308 | Expressed at a basal level in contractile SMCs, but strongly increased in synthetic SMCs in vitro and in atherogenesis305 | Marker of regenerative and repair processes308 |
| Cellular-retinol binding-protein-1 (gene: RBP1) | 3: a, b, c |
18.3–23.6 | Transiently expressed by SMCs during vascular repair309; mainly expressed in fibroblasts and myofibroblasts310,311 | Involved in retinoid metabolism; may play a role in the evolution of granulation tissue311 | Marker of SMC de-differentiation after endothelial injury in vivo309; may be selectively expressed by a subpopulation of SMCs prone to give rise to intimal hyperplasia309 | Associated with smooth muscle malignancy312 |
Functional contractility is the most robust indicator of contractile SMC phenotype. Contraction of individual cells can be assessed by observing the shortening of SMαA stress fiber-like structures by confocal microscopy23 or by direct observation of SMC morphology.24,25 To assess the force generation of single cells, SMCs have been cultured on microfabricated poly(dimethylsiloxane) posts coated with fibronectin (FN) where the force is proportional to the deflection of the posts.26 In native smooth muscle tissue, one of the key second messengers for contraction is cytosolic Ca2+. Calcium concentration can be modulated using Ca2+ ionophore A23187 or by depolarization with potassium chloride, and the status of the contractile apparatus can be assessed by microscopy.23,25 Upstream signaling in response to physiologically relevant small molecular signals, such as cholinergic agonists (typically simulated with carbachol), angiotensin II (Ang-II), and endothelin, can be assessed by observation of SMC contraction.11,24,25,27 Electrophysiological recording of membrane potential can also be used to assess the status of the contractile signaling apparatus.25 Ang-II, epinephrine, and carbachol inhibit the smooth-muscle-specific ATP-sensitive potassium channels (IKATP) and the large-conductance Ca2+-activated K+ channels, which contribute to SMC contraction.25,28–31 The functional contraction of whole engineered tissues also can be assessed. Tissue contraction in response to serotonin, endothelin-1, prostaglandin F2α, histamine, bradykinin, ATP, and UTP has been measured by conventional myography that has been adapted to test engineered tissues.11,32 These approaches allow for more definitive characterization of SMC contractility, but may have limited utility in SMCs that have an intermediate phenotype.
Mediators of SMC Phenotype
Because of the role SMC proliferation plays in vascular pathology, disproportionate effort has been allocated to studying the mechanisms that promote SMC proliferation, migration, and other markers of synthetic phenotype. However, the focus of this section will be weighted toward factors that promote contractile SMC phenotype, since this poses the greatest challenge to vascular tissue engineering, especially in the context of re-differentiating synthetic cells toward a contractile phenotype.
The role of soluble signaling factors
Extracellular signaling molecules play a major role in determining the phenotypic fate of vascular SMCs. A wide variety of signaling factors have been implicated in the transition of SMCs into the proliferative, synthetic phenotype, including platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), insulin-like growth factors (IGFs), epidermal growth factor, α-thrombin, factor Xa, Ang-II, endothelin-1, and unsaturated lysophosphatidic acids.24,33–37 In vitro fetal bovine serum is commonly used to stimulate SMC proliferation and de-differentiation. An overview of these signaling pathways is shown in Figure 3. The array of extracellular signaling factors that can prevent SMC de-differentiation and proliferation and/or promote contractile phenotype are fewer in number and include soluble heparin, transforming growth factor beta 1 (TGF-β1), Ang-II, and IGF-1 (limited to primary SMC isolates).38
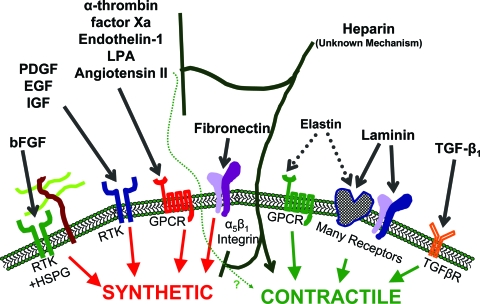
FIG. 3.
Brief overview of mechanisms involved in the modulation of SMC phenotype. The mechanism of action for heparin is unclear. Heparin may act by inhibiting binding of extracellular growth factors or secondary autocrine signaling factors, inhibiting intracellular signal transduction by these stimuli, and/or directly promoting contractile phenotype. Angiotensin II action can induce both synthetic and contractile characteristics. bFGF, basic fibroblast growth factor; PDGF, platelet-derived growth factors; EGF, epidermal growth factors; IGF, insulin-like growth factors; LPA, lysophosphatidic acid; TGF-β1, transforming growth factor beta 1; RTK, receptor tyrosine kinase; HSPG, heparan sulfate proteoglycan; GPCR, G-protein coupled receptor; TGFβR, TGF-β receptor. Color images available online at www.liebertonline.com/ten.
Heparin
The ability of heparin to inhibit SMC proliferation has been well described in vivo and in vitro.35,36,39,40 Although the effect of heparin has been known for some time, the mechanism of this effect remains incompletely understood and appears to be multifactorial in nature.
bFGF can promote proliferative, synthetic SMC phenotype directly or can stimulate growth in an autocrine fashion after release secondary to stimulation by another factor such as PDGF, thrombin, or factor Xa.37,41,42 It is well known that heparan sulfate proteoglycans, which are structurally similar to heparin, on the cell surface, act as low affinity receptors for bFGF and are necessary for full activation of the high affinity FGF receptor.43–45 The hypothesis that heparin disrupts bFGF signaling cannot explain the range of observations concerning the role of heparin in inhibiting many stimuli. When soluble bFGF is presented to SMCs in relatively high concentrations, it can both potentiate36,46,47 and inhibit40,43 its signaling. The effect of heparin on other stimulatory signals, such as PDGF, has not been consistent. In some studies, heparin inhibits PDGF-stimulated proliferation,34,41,48 whereas in others it has no effect.36,47 Differences in response may be related to species differences or specific culture conditions such as serum concentration. However, heparin consistently inhibits serum-stimulated SMC proliferation.36,46,47,49,50
The inconsistencies in these experiments have prompted the exploration of other mechanisms of heparin regulation of SMC growth. Heparin can be internalized51 via cell-surface heparin sulfate proteoglycans52 and can activate the double-stranded RNA protein kinase, PKR, which blocks the G1–S transition.49 Other possible mechanisms have been proposed, including direct signaling through an unspecified surface receptor, activation of protein phosphatases, and modulation of cell cycle progression machinery.48,50,53 It is likely that heparin utilizes more than one of these proposed pathways to modulate cell phenotype.
The precise structural determinants of antiproliferative activity have yet to be fully explained. Nonanticoagulant heparin effectively inhibits SMC proliferation in vitro54 and in vivo,55 and it has been well established that heparin's antiproliferative activity is unrelated to its anticoagulant activity. Heparin chain lengths >10–12 repeats (∼7 kDa) seem to exhibit roughly similar degrees of anti-proliferative activity (as a function of mass concentration). Sulfation patterns at the four sulfation sites in heparin also influence antiproliferative activity. In general, the overall level of sulfation (beyond some critical level, as found in unmodified heparin preparations) is the most important determinant of antiproliferative activity, but there is not one critical sulfation site or structural motif that mediates the antiproliferative effect.54,56–59
The well-established antiproliferative effect of heparin does not necessarily infer that heparin can promote a contractile SMC phenotype. A limited number of studies have shown that heparin can induce expression of SMαA60–63 and other smooth muscle contractile markers.60,63 Heparin also has been shown to delay the loss of smoothelin expression in cultured SMCs.64 However, the role that heparin's antiproliferative signal transduction pathways play in contractile gene expression remains unclear.
Transforming growth factor beta 1
TGF-β1 has a well-described ability to both inhibit proliferation and induce expression of contractile SMC marker genes, in the absence of stimuli. Active TGF-β1 is a 25 kDa homodimer of two 112 amino acid polypeptide chains, which are cleaved from longer propeptides.65 In cultured vascular SMCs, TGF-β1 inhibits growth induced by serum, PDGF, and epidermal growth factor,66–68 although the specific response to TGF-β1 may depend upon the vascular origin of the SMCs.69 TGF-β1 also has been shown to enhance expression and organization of SMαA, SM-major histocompatibility complex (MHC), and SM-22α in SMC lines as well as primary rat and human SMC cultures.66,68,70–72 Furthermore, in rodent models, the level of TGF-β1 in the neointima and damaged media of injured vessels is decreased and is correlated with a decrease in SMαA, type IV collagen, and SM-MHC.73
Classically, TGF-β1 signals via the Smad family of signaling molecules.74 Smad-2 nuclear translocation has been correlated with growth inhibition and SMαA expression in ocular microvascular pericytes,72 and Smad-3 has been associated with increased contractile marker gene expression via interaction with δEF-1.75 Other signaling pathways have been implicated in TGF-β1-mediated stimulation of contractile SMC phenotype involving the intracellular Src tyrosine kinases or RhoA tyrosine kinases and protein kinase N (PKN).68,71 It should also be noted that while TGF-β1 has been shown to reduce proliferation and induce contractile SMC marker gene expression, it is unclear whether TGF-β1 stimulation is sufficient to restore ligand induced contractility to cultured SMCs.
Other factors
Ang-II plays an important role in normal vascular physiology and cardiovascular disease. SMC response to Ang-II differs among individual cells, arteries, or arterial layers.76–81 Ang-II interacts primarily with two receptors on SMCs, the type 1 (AT1) receptor and type 2 (AT2) receptor.82 The AT1 receptor mediates changes in intracellular calcium that are a major determinant of SMC contraction.76 Ang-II may stimulate SMC proliferation83,84 or stimulate hypertrophy without proliferation.85 AT1 receptor signaling modulates SMC proliferation and hypertrophy through a complex, calcium-dependent pathway.24 Ang-II also may stimulate the selective apoptosis of synthetic SMCs through the AT1 receptor.86 Ang-II induces expression of SMαA in cultured rat aortic SMC through the AT2 receptor by increasing expression of the transcription factor myocardin.87 This result suggests that Ang-II also would upregulate expression of other myocardin-dependent marker genes such as SM-MHC, which was observed before the discovery of myocardin.88 Ang-II may also inhibit SMC migration through the AT2 receptor by increasing cellular FN synthesis and associated cell binding.89
IGF-1, a small polypeptide with structural homology to proinsulin, is produced by many cell types and acts as an autocrine/paracrine growth factor. It has been postulated to play a role in SMC growth in the bladder, uterus, and vasculature.90 In vascular SMCs, IGF-1 may stimulate proliferation, migration, and hypertrophy and its effects may interact with those of insulin and other growth factors.91 Like Ang-II, IGF-1 may have dual roles in the modulation of SMC phenotype. Hayashi et al. demonstrated that IGF-1 can maintain contractile phenotype in differentiated primary SMCs via a protein kinase B (PKB)-mediated pathway, while IGF-1 promotes proliferation of cultured de-differentiated SMCs.24,92 The phenotype-dependent response appears to be mediated, at least in part, by the interaction of the phosphatase, Src homology 2 domain-containing tyrosine phosphatase-2 (SHP-2), with the IGF receptor complex, blocking its signaling via extracellular signal-regulated kinase (ERK) and p38-mitogen-activated protein kinase (MAPK) pathways.38 However, overexpression of IGF-1 in a transgenic mouse model resulted in increased SMC proliferation and migration in both the media and neointima following mechanical injury compared with wild-type mice.93 Although the precise role of Ang-II and IGF-1 on SMC phenotype remains to be fully elucidated, these signaling molecules clearly play a role in phenotype modulation and the resulting effect may depend on the receptor profile, intracellular signaling, and overall phenotype of the target SMC.
The role of the ECM
It has been known for some time that SMCs rapidly loose their contractile apparatus and adopt a synthetic phenotype in culture.16 Early reports demonstrated FN, derived from the serum, typically used to coat the substrates for these cultures, most potently supported a loss of contractile phenotype.17 Normally, SMCs are surrounded by a basal lamina composed predominantly of type IV collagen and laminin (LN). It appears that this basal lamina is critical for the maintenance of contractile smooth muscle phenotype, perhaps in part because it forms the interface between the SMC's contractile apparatus and the ECM.94 It was later discovered that, in contrast to FN, the basement membrane proteins LN and/or type IV collagen could delay, but not eliminate, the transition to the synthetic phenotype, even when cells were cultured under serum-free conditions.24,95–98 SMCs seeded on these substrates rapidly began to produce their own provisional FN matrix, which became the dominant cell–ECM interaction and was correlated with an eventual phenotypic shift.95,99,100 RGD-peptide-dependent interactions are critical for this transition. Soluble RGD peptide can delay the transition to the synthetic phenotype on FN97,98,100 or enhance LN's mitigating effects on SMC response to mitogens like PDGF.17 Furthermore, a substrate of RGD peptide alone was sufficient to induce SMC de-differentiation.100
Some evidence exists suggesting that LN can promote expression of contractile markers in cultured vascular SMCs, as well as mitigate their response to mitogens,99,101,102 although serum-starved cultured SMCs on LN do not show increased contractile marker expression.103 LN also can attenuate the response to PDGF and thrombin in airway SMCs.104 The study of airway SMCs also has suggested that re-differentiation of cultured SMCs may involve the production of endogenous basement membrane LN. However, the predominant LN produced, α2β1γ1, is of a different form from LN-1 (α1β1γ1), which typically has been used to promote contractile phenotype.105 Interestingly, the nonbasement membrane ECM protein elastin also can promote contractile phenotype.98,106
The signaling pathways involved with ECM-dependent modulation of phenotype have been explored to a limited degree. Inhibition of tyrosine kinases by genistein resulted in decreased cell spreading and an attenuated progression to the synthetic phenotype in primary rat SMCs.107 Although decreased focal adhesion kinase activity was observed, it is unclear the extent to which these effects were regulated by focal adhesion kinase. Furthermore, the loss of α7β1 integrin, which is an LN receptor that links the contractile apparatus to the basement membrane,94 resulted in decreased expression of contractile SMC markers and increased proliferation through a Ras-MAPK-mediated signaling pathway.108 This suggests that ECM–α7β1 interactions may normally check this proliferation-inducing signaling pathway.109 It has also been suggested that autocrine/paracrine IGF-1 signaling may be involved in maintenance of contractile marker expression in primary SMCs cultured on LN.24,92 Furthermore, it appears that this signaling pathway is available only to freshly isolated cells, since subcultured rat SMCs are stimulated to proliferate by IGF-1.102
Matrix metalloproteinases (MMPs), which are critical for ECM dynamics, also play an important role in migration of synthetic SMCs and also may contribute to the de-differentiation process.110–112 SMCs produce MMP-1, -2, -3, -7, -9, and -14 (membrane type 1-MMP [MT1-MMP]).113 SMC migration, after arterial balloon injury, has been associated with MMP expression and activity, and MMP inhibition decreases SMC migration.114,115 In vitro, overexpression of MT1- and MT3-MMPs was found to result in reduced SMC adhesion and increased migration.116 Overexpression of MMP-9 also enhanced migration of rat SMCs in a collagen invasion assay.117 Furthermore, upregulation of MMPs by synthetic SMCs may contribute to aneurysm formation118 and to failure of vascular grafts.119 MMPs are clearly responsible for the breakdown of vascular matrix and especially of the internal elastic lamina, which is composed of contractile phenotype promoting type IV collagen and LN.120–122 It has been observed that the de-differentiation of isolated SMCs is preceded by a dramatic upregulation of MT1-MMP.123 However, it is not clear whether MMP expression actively drives de-differentiation per se or if increased MMP expression is simply a characteristic of the synthetic SMCs that the de-differentiation process creates.
The role of mechanical stimulation
The vascular media is subjected to continuous cyclic mechanical loading in vivo. As a result, it has been assumed that the mechanical environment plays an important role in determining SMC phenotype. Although the effects of cyclic mechanical strain have been studied extensively, the precise mechanisms that dictate the effects of cyclic strain on SMC phenotype still are understood poorly.124
Early studies with embryonic rat aortic SMCs demonstrated increased cell proliferation in response to cyclic strain that was, in part, due to release of paracrine/autocrine PDGF.125,126 Subsequent studies by other groups have confirmed this response in neonatal rat cells,127,128 but have shown increased,129 decreased,127,130 or unchanged128,131 proliferation with adult rat aortic cells or cell lines. SMCs derived from other species have a variety of responses to cyclic mechanical strain. Rabbit and bovine SMCs increased proliferation,132,133 while the growth of human and canine SMCs was unchanged.134,135
Some studies have suggested that an LN or elastin ECM attenuates the ability of SMCs to proliferate in response to cyclic strain,126,135 but this has not been a consistent finding.127,128 It has been suggested that FN–cell interactions are important for transducing strain into proliferation signals, since the RGD peptide or soluble FN can inhibit neonatal rat SMC proliferation in response to strain.126 It is interesting that the effects of the ECM on the response to cyclic strain follows a similar pattern to the effects of ECM on SMC phenotype in static culture; that is, LN and elastin prevent de-differentiation, while FN promotes the proliferative, synthetic phenotype.24,95–98
SMCs, typically of rat or rabbit origin, cultured in three-dimensional (3D) scaffold systems seem to proliferate only slightly in response to mechanical stimulation.136–138 However, the culture duration in these systems is typically longer than two-dimensional (2D) Flexcell-based experiments and unstrained samples tend to lose cell population, suggesting that the enhanced growth might not be due to strain per se but due to enhanced convective transport of nutrients into the scaffolds, which simply enhances cell viability. The effect of cyclic strain on intrascaffold transport has not been well characterized for smooth muscle tissue-engineered constructs and may be an important element underlying higher cell population in these systems, compared with static controls.
Cyclic mechanical strain also can stimulate the production of ECM components such as collagen and elastin in tissue culture models.134,139 There is some evidence to suggest that this response is mediated by paracrine release of TGF-β1, which is known to directly stimulate collagen production.140 This effect has led to the implementation of cyclic loading protocols to improve the mechanical properties of engineered vascular tissues. Cyclic mechanical strain increased the collagen and elastin content, organization, and overall strength of 3D smooth muscle tissues.136–138 Many studies have used these results as a rationale for mechanical stimulation of tissue-engineered blood vessels (TEBVs).11,137,141,142
In apparent conflict with the synthetic phenotypic response outlined above, cyclic strain can also increase expression of markers of contractile phenotype, including SMαA,128,131 calponin,131 SM-22α,131 h-caldesmon,132 and SM-MHC.131,143 This change in marker expression appeared to be related to intracellular signaling or short-lived paracrine signaling, since medium conditioned by strained SMCs did not induce contractile marker expression.131 However, these results were not consistent in all studies. Some reports indicate that strain has no effect on expression of marker proteins.136,144
Given the inconsistencies in the phenotypic response of SMCs to cyclic strain, it is not surprising that the signaling mechanisms underlying these responses are not well understood. Immediately following the initiation of cyclic strain, all three classical MAPK systems (ERK1/2, c-Jun N-terminal kinase [JNK], and p38) are activated in a transient fashion with a peak response about 10–15 min after initiation and a return to baseline after 30–60 min.131,133,144,145 In particular, inhibition of p38 activation prevents SMαA promoter activity.128 While p38 may be necessary for these responses, it is not clear whether strain directly signals through this pathway. p38 simply may be a more globally required element of the system, especially since blocking p38 tends to reduce marker gene transcript even in the absence of strain.131 Putative roles for calcium channels and tyrosine kinases also have been proposed.129,139 It is also clear that paracrine release of soluble mediators, including Ang-II, PDGF, TGF-β1, and IGF-1, play an important role and may antagonize each other's effects.125,129,130,139,146,147 It has been suggested that phenotypic outcome in response to cyclic strain may depend on the phenotype of the cells before strain148 or on the magnitude and duration of the strain.124 Future studies will provide a more complete understanding of the signaling processes involved in mechanical stimulation of vascular SMCs.
In addition to the role of cyclic, circumferential mechanical strain, there also is evidence that SMCs may respond to uniaxial strain. Increasing the axial strain of rabbit carotid arteries from 62% to 92% increased endothelial and SMC proliferation dramatically, while also causing ECM deposition to increase and remain elevated over a 12-week period.149 Ex vivo engineered vessels that were elongated by 50% over 9 days under both physiological and subphysiological perfusion conditions showed significant increases in proliferation and collagen mass, and similar viability and appearance native tissue.150 These data suggest that there are substantial interactions between cyclic strain conditions and axial strain that modulates arterial remodeling. The full extent to which these effects alter expression of contractile SMC phenotype is not known.
Clearly, the SMC response to cyclic mechanical strain depends on the state of the cells (both origin and phenotype),148 and additional work is needed to better understand the conditions that regulate this response. While it is clear that mechanical input plays an important role in the phenotypic modulation of SMCs, a lack of knowledge regarding the mechanism by which cyclic strain exerts its effects on cells limits its utility for tissue engineering. Pathways that regulate conflicting phenotypic outcomes such as proliferation and ECM production (synthetic properties) and expression of contractile markers must be more clearly defined so the tissue engineer can specifically target the appropriate cell behavior.
The role of endothelium
Endothelial cells (ECs) play an important role in guiding SMC behavior.151 Small molecules released from ECs in vivo such as nitric oxide152 and endothelin-1153 have been shown in vitro to inhibit or stimulate SMC growth, respectively. SMCs also are known to send projections toward the endothelium.154 A variety of coculture systems have been utilized to explore these interactions, including direct coculture,155 transmembrane culture,156–158 and bioreactor systems.142,159 ECs in these studies tend to increase SMC proliferation,154,158,159 suggesting that ECs' presence promotes synthetic vascular SMC phenotype. However, many of these studies are performed under static culture conditions, which likely alters the response of the ECs compared with ECs under shear. However, increased SMC proliferation has also been observed in a 3D tissue construct with monolayer ECs cultured under shear.159 It is important to note that hyperplastic smooth muscle lesions have been identified in the “floor” region of the distal anastomoses of vascular reconstructions, a region that contains native endothelial and smooth muscle tissue but where there is abnormal (zero) shear stress.160 SMCs in the presence of ECs also tend to upregulate expression of the mitogen PDGF155 and the inflammatory cytokines interleukin-8 (IL-8) and monocyte chemotactic protein-1,156 even as other proteins associated with the synthetic SMC phenotype are downregulated, such as collagen159 and bFGF.155 Furthermore, EC–SMC interactions may depend on the SMC phenotype. The synthetic SMC phenotypic state during coculture has been shown to increase expression of inflammatory signals in both SMCs and ECs.156,157 Early studies indicated that nonsheared ECs can delay the contractile-to-synthetic transition of primary SMCs.15 While ECs clearly play a critical role in modulating SMC behavior in vivo, this regulation process is complex. Neither the simple presence or absence of ECs will result in appropriate SMC behavior, but the appropriate environment must be provided to both cell types to achieve control of SMC phenotype.
The role of inflammation
Inflammation, which follows implantation of a vascular prosthesis, contributes to a loss of contractile SMC phenotype. Cytokines released from inflammatory cells can directly stimulate SMC growth and play an important role in the development of IH.161,162 Several days after endothelial denudation injury, macrophages appear in the resulting lesion of proliferating, synthetic SMCs.163,164 Disrupting the accumulation of macrophages resulted in decreased SMC hyperplasia in vivo, suggesting that these cells play an important role in the process.162 Furthermore, many of the factors generated by macrophages have been linked directly with SMC proliferation, including IL-1α,165 IL-8,166 C-reactive protein,167 and tumor necrosis factor-α.168 Various stimuli from activated endothelium and inflammatory cells can also induce the endogenous production of inflammatory cytokines and markers in SMCs, such as monocyte chemotactic protein-1,156 IL-8,156 vascular cell adhesion molecule-1,164 intercellular adhesion molecule-1,163 and class II major histocompatibility complex (MHC).163 Expression of these molecules by synthetic SMCs provides a mechanism of positive feedback, accelerating SMC proliferation. These inflammatory processes may also result in downregulation of contractile marker proteins, which generally has been correlated with SMC proliferation, but this effect has not been studied.
Molecular Regulation of SMC Gene Expression
This section will briefly review the mechanisms of phenotypic regulation in SMCs. Additional discussion can be found in reviews by Miano et al.169 and Kawai-Kowase and Owens.12
Serum response factor
Serum response factor (SRF) is a 62–67 kDa transcription factor involved in the regulation of a diverse set of cell programs including proliferation and differentiation of SMCs.170 SRF was initially identified as a transcription factor that acts as a promoter for c-fos, a gene involved in the early stages of cell proliferation.171,172 SRF is activated by transcription following serum stimulation and does not require additional protein translation to exert its effects.173 However, SRF also is active in the promoters of muscle-specific genes during differentiation.174
SRF binds as a homodimer to a consensus sequence in DNA of CC(A or T)6GG, called a CArG box.169,173 Putative CArG elements have been identified in the promoter/enhancer regions of nearly 200 genes,175 and many of these genes are involved in formation and regulation of the cytoskeleton or contractile apparatus.169 Most, but not all, markers of contractile SMC phenotype contain at least one CArG box including SMαA, calponin, SM-22α, and SM-MHC.169 The ability for SRF to activate specific transcriptional programs within the wide range of genes containing CArG boxes depends upon the presence of program-specific coactivators and repressors. Specific expression of many smooth-muscle-specific genes is substantially enhanced by the coactivator myocardin.
Myocardin
Myocardin is thought to be a central regulator of the SMC-specific expression program and can drive expression for most, but not all, contractile marker genes.176 Myocardin is a 96 kDa transcription factor that directly interacts with SRF dimers rather than binding directly to DNA.177 Myocardin appears to be regulated, at least in part, at the transcriptional level and is generally restricted to the nucleus.176,178 Myocardin also contains a leucine zipper domain, which allows myocardin dimers to bridge adjacent CArG boxes in the promoter region of many SMC marker genes, including SMαA, calponin, SM-22α, and SM-MHC.170,179 This six member myocardin2–SRF4 dimer complex seems to enhance activation of these genes.179 It has also been suggested that myocardin's interaction with SRF enhances SRF's binding to degenerate CArG boxes in the promoters of some SMC marker genes,180 such as SM-MHC, SMαA, and calponin, which typically contain one guanine substitution in the A/T rich part of the CArG sequence.170
Myocardin coactivation alone, however, does not fully explain the transcriptional control of SMC marker genes for several reasons. Overexpression of myocardin in rat aortic SMCs, mesenchymal stem cells (MSCs), and fibroblast results in inappropriate activation of skeletal and cardiac muscle genes and fails to activate non-CArG containing markers such as smoothelin-B.176,181 Dominant negative myocardin also fails to interrupt marker expression in an SMC differentiation model cell line A404.176,182 Even among genes with known CArG-containing promoters, some possess only a single CArG box such as h-caldesmon and telokin, suggesting that the myocardin dimerization hypothesis179 has limitations. Strikingly, although myocardin-null mice die in utero,183 myocardin-null mouse embryonic stem cells (ESCs) can express some SMC markers in vitro.184 The vasculature of embryos formed from a chimera of wild-type and myocardin-null stem cells contains myocardin-null SMCs expressing a normal complement of SMC markers.184
Other coactivation systems
Other coactivation schemes can function in conjunction with the myocardin–SRF system to drive marker expression. The SMαA promoter has been studied most thoroughly and, in addition to three CArG boxes, contains two E-boxes (CANNTG motifs), two muscle CAT (MCAT) elements (AGGAATG motifs), and a TGF-β control element (Fig. 4).185 The E-boxes are bound by basic helix-loop-helix transcription factor dimers that enhance SRF-dependent transcription via the protein inhibitor of activated signal transducer and activator of transcription-1 (STAT-1).186 The two MCAT boxes may be involved in parallel SMαA regulatory pathways in myofibroblasts that are not critical for SMCs.187 The homeobox-binding protein Prx-1 also has been shown to be important in SMαA transcription.87 Myocardin-related transcription factors may also play a role in modulating gene expression in a fashion similar to myocardin via sensing changes in the actin cytoskeleton.169,188
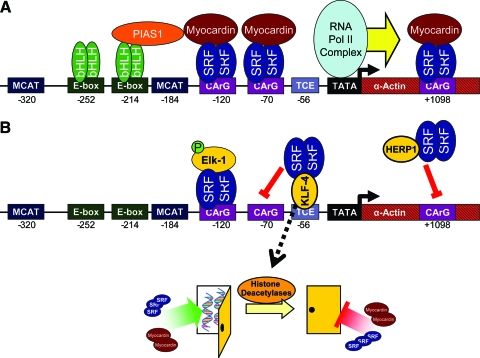
FIG. 4.
Molecular regulation of SMαA transcription, illustrating example mechanisms of transcriptional activation in differentiated SMCs (A) and mechanisms of downregulation (B). (A) Transcription is activated by SRF binding to CArG box up- and downstream of the TATA box, enhanced by the coactivator, myocardin. Additional elements further enhance transcription, such as bHLH transcription factors via PIAS-1. (B) Transcription is downregulated by phospho-Elk-1 blocking myocardin interactions with SRF. KLF-4 and HERP-1 block SRF binding to CArG boxes via sequestration. KLF-4 also activates histone deacetylases that close chromatin structure (represented as a closed door in the diagram), limiting transcription factor access to the promoter region. bHLH, basic helix-loop-helix; PIAS-1, protein inhibitor of activated STAT-1; SRF, serum response factor; KLF-4, Kruppel-like factor 4; HERP-1, Hairy- and enhancer of split-like-related repressor protein-1; TCE, TGF-β control element. Color images available online at www.liebertonline.com/ten.
Repressor systems
Several repressor pathways are known to affect the regulation of many of the CArG-box-regulated SMC marker genes. Two examples are Elk-1 and Kruppel-like factor 4 (KLF-4). Elk-1 is a transcriptional cofactor that, like myocardin, interacts with SRF to modulate transcription. Elk-1 is activated by phosphorylation by ERK1/2 and JNK MAPKs, depending on the stimulus, and increases SRF-dependent transcription of c-fos.189 Thus, Elk-1 promotes cell proliferation.189 Elk-1 can interact with SRF and DNA in the promoter region of several SMC marker genes to inhibit myocardin binding and marker gene activation,190–192 and this activity seems to vary between genes.190 KLF-4 binds in the promoter region of several SMC marker genes,193,194 which may allow it to block SRF binding to SMC marker gene promoters195 and recruit histone deacetylases that alter chromatin structure to limit transcription factor access to the promoter regions.191 KLF-4 can also suppress myocardin expression.195 In addition to enhancing SMαA transcription via interactions with E-box-binding proteins, protein inhibitor of activated STAT-1 may promote gene expression by inhibiting the action of KLF-4.196 In addition to these modifiers, additional inhibitory pathways have been identified. Hairy- and enhancer of split-like-related repressor protein-1 (HERP-1), which is upregulated in cultured SMCs, binds to SRF to inhibit its binding to CArG boxes.197 Ets-1, which is related to Elk-1, is upregulated in vascular injury and suppresses marker gene expression.198 An overview of both transcriptional activation and repression mechanisms, using SMαA transcription as an example, can be found in Figure 4.
Engineered Biomaterial Approaches to Regulate SMC Phenotype
Biomaterial and tissue engineers have begun to investigate the effects of scaffold chemistry and structure on SMC phenotype. These studies have used engineered materials as model systems to explore factors that affect SMC behavior. Novel materials and fabrication strategies also have been developed to modulate SMC phenotype.199,200
Effects of cell–scaffold interactions on phenotype
Early work in smooth muscle tissue engineering explored the effect simple scaffold materials such as poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid), and type I collagen gels had on SMC behavior.22 SMCs in polyester scaffolds showed a higher ratio of elastin:collagen production than collagen gels, suggesting a shift toward a contractile-like phenotype.22 Although the 3D structure of these matrices was dissimilar, the observed differences likely were due, at least in part, to differences in the scaffold chemistry either by affecting SMCs directly or by inducing differences in the adsorbed protein content on the scaffolds.22 Despite these promising results, SMCs in PGA vascular grafts11 showed incomplete differentiation and contractility in vivo, and a later study suggested that breakdown products from PGA may promote synthetic SMC phenotype.201
In an effort to understand the role-specific cell–ECM interactions play in SMC phenotype modulation, cell-adhesive peptides and sugars have been immobilized on various surfaces and their effects on cell behavior studied. For SMCs seeded on modified glass, collagen production decreased with ligand concentration and collagen per cell was highest on weakly adhesive substrates (such as RGE peptide-modified glass), though these results were confounded by high nonspecific cell binding to the substrates.202 However, similar results were obtained for SMCs seeded on poly(ethylene glycol) (PEG)-based hydrogels,203 which resist nonspecific attachment.103 The use of tethered TGF-β1 also increased the synthesis of ECM components on RGD-containing PEG-based gel systems.140 Short hyaluronic acid fragments tethered to glass induced increased elastin production and crosslinking.204 However, the biological characterization of SMC phenotype in these studies was limited to indirect markers. SMCs, attached to RGD-bearing PEG-based hydrogels, have been shown to express markers of contractile phenotype.205 This expression may be related to the mechanical characteristics of the underlying substrate.205 Recently, it has been shown that RGD-bearing hydrogels with highly specific cell–matrix interactions, can support a robust, quantitative re-expression of contractile marker mRNA and proteins.103
Effects of scaffold geometry on phenotype
Many groups have noted that SMC behavior depends on scaffold geometry (2D vs. 3D). Culture in 3D type I collagen gels resulted in decreased proliferation and increased collagen synthesis compared with 2D type I collagen cultures.206 Furthermore, inhibition of ERK activation using PD98059 induced cell proliferation for SMCS in 3D type I collagen matrices, whereas it inhibited growth in 2D cultures on the same substrate, suggesting that culture geometry plays an important role modulating this signal.207 Synthetic scaffolds must be biodegradable and/or highly porous to permit 3D SMC culture. To allow for degradation in synthetic gel scaffold systems, the gel network must incorporate a degradable component. SMCs cultured in 3D PEG-based gel systems with an elastase degradable sequence showed higher collagen production than SMCs in nondegradable controls.208 Culture in 3D PEG-based hydrogel scaffolds resulted in small but significant increases in the SMC markers SMαA and SM-MHC, compared with control SMCs cultured on tissue culture polystyrene, although it was difficult to discern from this study the contributions of the scaffold geometry from substrate chemistry or mechanical properties (gel vs. tissue culture polystyrene).209 Increased mechanical modulus in 3D fibrinogen/PEG-based hydrogel scaffolds was also correlated with increased expression of vinculin, a marker of focal adhesions, and SMC differentiation markers, but only for SMCs overexpressing RhoA, which is a signaling protein that plays an important role in focal adhesion formation.210
Combined biochemical and mechanical stimulation in engineered scaffolds
The interplay between the scaffold chemistry, mechanical stimulation, and external biochemical stimulation has also been explored. SMCs on FN-coated PGA-based scaffolds increased elastin production in response to mechanical stimulation more than SMCs in type I collagen gels.138 Mechanical stimulation of rat aortic SMCs, when seeded in a type I collagen–based tubular-graft-like construct, resulted in increased compaction of the collagen, which could be further enhanced by stimulation with TGF-β1.136 TGF-β1 also improved histological organization and increased SMαA expression.136 Interestingly, SMCs transfected with cyclic guanosine monophosphate (GMP)-dependent protein kinase, which promotes contractile SMC phenotype,20,211 did not affect histological organization in type I collagen gels although SMαA expression was increased dramatically, especially with exogenous TGF-β1.212 These results suggest that forced expression of contractile SMC markers per se may not substantially facilitate the organization of vascular tissues in vitro. Recently, RGD-bearing PEG diacrylate hydrogels were utilized as a 3D scaffold material to examine the effects of both SMC coculture with ECs and cyclic mechanical loading.142 When subjected to both conditions, SMCs showed a modest upregulation of elastin, calponin, and myocardin as well as a slight decrease in collagen production.142 However, this study was limited by the use of nondegradable PEG diacrylate, which does not permit normal SMC morphology postencapsulation, and the nonphysiologic 3D encapsulation of the ECs (as opposed to monolayer culture).142
Micropatterning
Rat A7r5 cells organized into aligned patterns when seeded in 160-μm-wide channels of photopolymerized poly(caprolactone-lactide-glycolide) diacrylate microchannels with some evidence of increased SMαA production in these cells.199 However, the mechanism by which the channels induced alignment was unclear. Since cultured SMCs have a propensity toward alignment, the uniform cell orientation in this system may be attributed to preclusion of alignment perpendicular to the channels (because they are narrower than the length of an SMC). It is also unclear if this alignment directly affects SMC phenotype, although some limited data suggest that SMCs in these microchannels may upregulate SMαA.199
Conducting polymers
Cyclic electrical stimulation of vascular SMCs using an ECM-coated, conducting polymeric (polypyrrole/hyaluronic acid) scaffold stimulated SMC proliferation and concomitantly stimulated upregulation of SMαA and SM-MHC.200 Blockade of L-type calcium channels abrogated this effect,200 consistent with others that have shown that calcium influx through L-type calcium channels (induced by depolarization with potassium chloride) can result in increased expression of CArG-dependent marker genes.213
SMC Phenotype in TEBVs
The smooth muscle layer of TEBVs has been generated using natural and polymeric scaffold systems as well as engineered directly from sheets of cultured cells.214–216 Primary SMCs have been used to populate these scaffolds, although recently there has been interest in alternative cell sources. Progress in the development of TEBVs has been reviewed extensively elsewhere.217,218 Here, we briefly review SMC behavior in TEBVs.
Several groups have demonstrated that decellularized scaffold systems, derived from harvested vasculature or other tubular structures, can accommodate the in-growth and remodeling of differentiated, functional smooth muscle tissue.219–221 Scaffold systems derived from a variety of natural materials have been explored to increase the engineering control of scaffold properties, while retaining the remodeling potential observed with decellularized TEBVs. In particular, type I collagen has been used widely as a scaffold material for TEBVs because it is one of the major structural ECM proteins in the vasculature.216,222,223 SMCs can proliferate in and remodel these matrices and these processes can be enhanced by mechanical and biological stimulation of the cells, as discussed elsewhere in this review.136,224 SMCs also can readily remodel fibrin scaffolds to form mechanically robust vascular structures and support differentiated, contractile SMC-like cells.225,226 Polymeric scaffold materials for TEBVs also have been explored because their properties can be controlled to a much greater extent than natural scaffold materials. Although the overall results of these synthetic TEBVs have been promising,215,227,228 these scaffolds have supported only limited SMC remodeling, penetration, and functional contractility.201,215,229 Likewise, electrospun scaffold materials made from synthetic and natural materials have demonstrated promising results as blood conduits, but SMC response has been suboptimal with poor infiltration and limited study of SMC phenotype and functional capacity.230–232
Another approach has been the use of blood vessels engineered from sheets of cultured human cells to recapitulate the layered structure of native blood vessels.214,233–235 Constructs containing both smooth muscle and adventitial (fibroblast) layers developed robust mechanical properties in vitro and were able to be implanted in vivo.233 Subsequent testing of similar constructs showed differentiated SMCs with SMαA expression and functional contraction (Fig. 5).214 However, continued study of this approach indicated that adequate mechanical properties of these constructs can be conferred by a fibroblast layer, obtained from cells that are easier to isolate and culture. Composite grafts formed from human fibroblasts have been tested in a variety of animal models (dog, athymic rat, and macaque) to demonstrate surgical feasibility and long-term patency.234 Recently, autologous graft materials formed using these techniques have been used as shunts for hemodialysis in human trials with moderate success (60% patency at 6 months).235
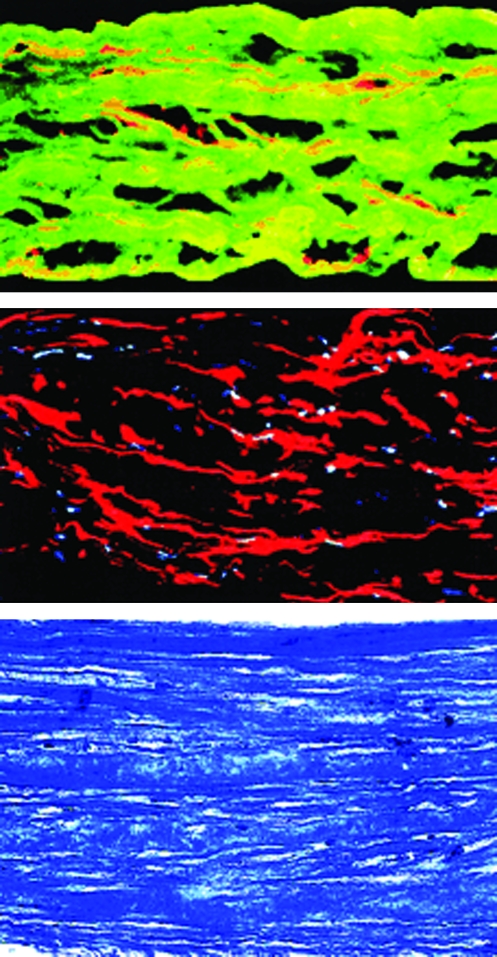
FIG. 5.
Histology of a human TEVM (from Ref.214). Top: Cross section of the TEVM immunolabeled for desmin (red) and type I collagen (green). Middle: Cross section of the TEVM immunolabeled for SMαA (red). Nuclei are stained blue. Bottom: Cross section of the TEVM stained with Masson's trichrome shows collagen in blue and cells in purple. TEVM, tissue-engineered vascular media. Color images available online at www.liebertonline.com/ten.
SMCs Derived from Stem Cells
The derivation of SMCs from progenitor cells has attracted extensive research interest. SMCs have been derived from ESCs, induced pluripotent stem cells (iPSCs), MSCs, and hair follicle stem cells.25,236–238 Contractile SMCs have been derived from ESCs using all-trans retinoic acid for over a decade.239,240 Analysis of the transcriptome of ESC-derived SMCs has suggested that these cells can express a full complement of contractile phenotype markers, SMC-specific ion channels, functional contractility, and limited proliferation.25 Recently, the use of iPSCs has attracted interest to avoid the ethical issues surrounding the use of ESCs. These cells, derived from genetically reprogrammed somatic cells, can generate SMC-like cells that have expression profiles similar to native SMCs.236,241 However, significant variability in the expression patterns of contractile marker genes have been observed among different lines of iPSCs and ESCs, suggesting that additional work is needed to better define robust protocols for these differentiation processes.236,241 Smooth muscle progenitor cells also have been isolated from bone marrow or hair follicles using a smooth-muscle-tissue-specific promoter and fluorescence-activated cell sorting.237,238 These stem-cell-derived SMCs showed high proliferation potential, exhibited similar morphology to primary SMCs, expressed several SMC markers, showed a contractile response to vasoactive agonists, and organized into a fibrillar network similar to that of native vessels.237,238
Many of the factors that affect the differentiation status of primary SMCs also contribute to stem-cell-to-SMC differentiation. For example, mechanical strain increases SMαA and SM-22α gene expression in MSCs.242 TGF-β1 and bone morphogenetic protein-4 stimulate expression of SMC-contractile markers in adipose-derived stem cells.243 Study of MSC-to-SMC differentiation also provides an opportunity to study signaling involved in SMC lineage differentiation.244,245 During this process, ECM such as LN plays an important role in upregulation of SMC-specific genes.246
Discussion and Conclusions
The phenotypic plasticity of SMCs confers these cells with great regenerative potential. However, to engineer functional smooth muscle tissues while minimizing development of hyperplastic pathologies such as IH, SMC phenotype must be well controlled. In the last several decades, a better understanding of the myriad factors that regulate SMC phenotype has emerged. The intracellular signaling machinery that relays these signals and the transcriptional machinery that ultimately results in phenotype changes are also becoming clearer. A panel of markers that can be utilized to quantitatively characterize SMC phenotype has been well established (Tables 1 and 2). However, the best approaches to modulate synthetic, cultured SMCs toward a functional contractile phenotype remain largely unknown, though the fact that SMCs seem to re-differentiate in vivo4–10 suggests that given the right conditions, this goal is attainable.
Despite these advances, the tissue engineering literature for smooth muscle tissues remains largely observational in nature. Few studies (e.g., Refs.136,142) exploit the well-established basic science literature on the topic, to generate scaffold systems specifically designed to modulate SMCs toward a contractile phenotype. Mechanical stimulation, which has yielded inconsistent results in the basic science literature, has been the most commonly used strategy, although this approach does tend to yield tissues that are mechanically robust. Critical parameters such as cell origin and cell phenotype during stimulation generally have been ignored. Studies on scaffolds that promote contractile phenotype using stimulation with exogenous signaling factors such as heparin and TGF-β1 has been surprisingly limited, given the clear evidence of the efficacy of these approaches.
Further confounding the interpretation of these studies is a lack of clear, consistent, and quantitative readouts of SMC phenotype. Most studies have employed indirect measures such as ECM synthesis and cell proliferation. Given the diversity of factors that can affect these nonspecific cell behaviors, it is challenging to compare true SMC phenotypes between studies. Studies that have examined SMC phenotype markers directly have used qualitative techniques such as immunostaining, which, without quantification, yield results that are difficult to compare between studies. Expanded use of appropriate (semi)quantitative techniques for assessing markers of contractile SMC phenotype, including quantitative polymerase chain reaction (qPCR), Western blot, enzyme-linked immunosorbent assay, and flow cytometry, will aid in the comparison of tissue engineering strategies to promote the formation of contractile smooth muscle tissue. Marker expression relative to cultured, synthetic control SMCs and, where possible, functional assessment of SMC contractility can improve the overall assessment of contractile phenotype promoting approaches. Expanded analysis of the transcriptional regulation of marker gene expression142 will lend further insight into mechanisms by which novel scaffold designs are capable of modulating SMC phenotype. Future work that utilizes quantitative methods to study scaffold systems with well-defined cell–material interactions, soluble signals, and mechanical stimulation will likely succeed in devising systems that are capable of inducing re-expression of true contractile SMC phenotype.
Acknowledgments
This work was supported by Grant Number 5R01EB002067 from the National Institute of Biomedical Imaging and Bioengineering and Grant Number 1R01HL087843 from the National Heart, Lung, and Blood Institute. J.A.B. also was supported by NIH T32GM07250 and American Heart Association Predoctoral Fellowship 0715422B.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lloyd-Jones D. Adams R. Carnethon M. De Simone G. Ferguson T.B. Flegal K. Ford E. Furie K. Go A. Greenlund K. Haase N. Hailpern S. Ho M. Howard V. Kissela B. Kittner S. Lackland D. Lisabeth L. Marelli A. McDermott M. Meigs J. Mozaffarian D. Nichol G. O'Donnell C. Roger V. Rosamond W. Sacco R. Sorlie P. Stafford R. Steinberger J. Thom T. Wasserthiel-Smoller S. Wong N. Wylie-Rosett J. Hong Y. for the American Heart Association Statistics Committee and Stroke Statistics S. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Laube H.R. Duwe J. Rutsch W. Konertz W. Clinical experience with autologous endothelial cell-seeded polytetrafluoroethylene coronary artery bypass grafts. J Thorac Cardiovasc Surg. 2000;120:134. doi: 10.1067/mtc.2000.106327. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald S.E. Berry C.L. Improving vascular grafts: the importance of mechanical and haemodynamic properties. J Pathol. 2000;190:292. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Christen T. Verin V. Bochaton-Piallat M. Popowski Y. Ramaekers F. Debruyne P. Camenzind E. van Eys G. Gabbiani G. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation. 2001;103:882. doi: 10.1161/01.cir.103.6.882. [DOI] [PubMed] [Google Scholar]
- 5.Sottiurai V.S. Yao J.S. Batson R.C. Sue S.L. Jones R. Nakamura Y.A. Distal anastomotic intimal hyperplasia: histopathologic character and biogenesis. Ann Vasc Surg. 1989;3:26. doi: 10.1016/S0890-5096(06)62381-9. [DOI] [PubMed] [Google Scholar]
- 6.Thyberg J. Blomgren K. Hedin U. Dryjski M. Phenotypic modulation of smooth muscle cells during the formation of neointimal thickenings in the rat carotid artery after balloon injury: an electron-microscopic and stereological study. Cell Tissue Res. 1995;281:421. doi: 10.1007/BF00417860. [DOI] [PubMed] [Google Scholar]
- 7.Manderson J.A. Mosse P.R.L. Safstrom J.A. Young S.B. Campbell G.R. Balloon catheter injury to rabbit carotid-artery .1. Changes in smooth-muscle phenotype. Arteriosclerosis. 1989;9:289. doi: 10.1161/01.atv.9.3.289. [DOI] [PubMed] [Google Scholar]
- 8.Aikawa M. Sakomura Y. Ueda M. Kimura K. Manabe I. Ishiwata S. Komiyama N. Yamaguchi H. Yazaki Y. Nagai R. Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation. 1997;96:82. doi: 10.1161/01.cir.96.1.82. [DOI] [PubMed] [Google Scholar]
- 9.Kocher O. Gabbiani F. Gabbiani G. Reidy M.A. Cokay M.S. Peters H. Huttner I. Phenotypic features of smooth-muscle cells during the evolution of experimental carotid-artery intimal thickening—biochemical and morphological-studies. Lab Invest. 1991;65:459. [PubMed] [Google Scholar]
- 10.Thyberg J. Blomgren K. Roy J. Tran P.K. Hedin U. Phenotypic modulation of smooth muscle cells after arterial injury is associated with changes in the distribution of laminin and fibronectin. J Histochem Cytochem. 1997;45:837. doi: 10.1177/002215549704500608. [DOI] [PubMed] [Google Scholar]
- 11.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 12.Kawai-Kowase K. Owens G.K. Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C59. doi: 10.1152/ajpcell.00394.2006. [DOI] [PubMed] [Google Scholar]
- 13.Owens G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 14.Stegemann J.P. Hong H. Nerem R.M. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98:2321. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 15.Campbell J.H. Campbell G.R. Vascular Smooth Muscle in Culture. Boca Raton, FL: CRC Press; 1987. [Google Scholar]
- 16.Chamley-Campbell J. Campbell G.R. Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Hedin U. Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation. 1987;33:239. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 18.Rzucidlo E.M. Martin K.A. Powell R.J. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007;45(Suppl A):A25. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Raines E.W. Koyama H. Carragher N.O. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann N Y Acad Sci. 2000;902:39. doi: 10.1111/j.1749-6632.2000.tb06299.x. discussion 2. [DOI] [PubMed] [Google Scholar]
- 20.Boerth N.J. Dey N.B. Cornwell T.L. Lincoln T.M. Cyclic GMP-dependent protein kinase regulates vascular smooth muscle cell phenotype. J Vasc Res. 1997;34:245. doi: 10.1159/000159231. [DOI] [PubMed] [Google Scholar]
- 21.Chamley-Campbell J.H. Campbell G.R. Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981;89:379. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim B.S. Nikolovski J. Bonadio J. Smiley E. Mooney D.J. Engineered smooth muscle tissues: regulating cell phenotype with the scaffold. Exp Cell Res. 1999;251:318. doi: 10.1006/excr.1999.4595. [DOI] [PubMed] [Google Scholar]
- 23.Moussallem M.D. Olenych S.G. Scott S.L. Keller T.C., 3rd Schlenoff J.B. Smooth muscle cell phenotype modulation and contraction on native and cross-linked polyelectrolyte multilayers. Biomacromolecules. 2009;10:3062. doi: 10.1021/bm9007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi K. Saga H. Chimori Y. Kimura K. Yamanaka Y. Sobue K. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J Biol Chem. 1998;273:28860. doi: 10.1074/jbc.273.44.28860. [DOI] [PubMed] [Google Scholar]
- 25.Potta S.P. Liang H. Pfannkuche K. Winkler J. Chen S. Doss M.X. Obernier K. Kamisetti N. Schulz H. Hubner N. Hescheler J. Sachinidis A. Functional characterization and transcriptome analysis of embryonic stem cell-derived contractile smooth muscle cells. Hypertension. 2009;53:196. doi: 10.1161/HYPERTENSIONAHA.108.121863. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y. Zheng X.R. Riddick N. Bryden M. Baur W. Zhang X. Surks H.K. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531. doi: 10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rattan S. Puri R.N. Fan Y.P. Involvement of rho and rho-associated kinase in sphincteric smooth muscle contraction by angiotensin II. Exp Biol Med (Maywood) 2003;228:972. doi: 10.1177/153537020322800814. [DOI] [PubMed] [Google Scholar]
- 28.Ledoux J. Werner M.E. Brayden J.E. Nelson M.T. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 29.Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol. 2006;572:617. doi: 10.1113/jphysiol.2006.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semenov I. Wang B. Herlihy J.T. Brenner R. BK channel beta1-subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L802. doi: 10.1152/ajplung.00104.2006. [DOI] [PubMed] [Google Scholar]
- 31.Yunoki T. Zhu H.L. Iwasa K. Tomoda T. Aishima M. Shibata A. Naito S. Teramoto N. Comparative studies of ZD0947, a novel ATP-sensitive K(+) channel opener, on guinea pig detrusor and aortic smooth muscles. Naunyn Schmiedebergs Arch Pharmacol. 2008;376:309. doi: 10.1007/s00210-007-0241-z. [DOI] [PubMed] [Google Scholar]
- 32.L'Heureux N. Stoclet J.C. Auger F.A. Lagaud G.J. Germain L. Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J. 2001;15:515. doi: 10.1096/fj.00-0283com. [DOI] [PubMed] [Google Scholar]
- 33.Hedin U. Daum G. Clowes A.W. Heparin inhibits thrombin-induced mitogen-activated protein kinase signaling in arterial smooth muscle cells. J Vasc Surg. 1998;27:512. doi: 10.1016/s0741-5214(98)70326-x. [DOI] [PubMed] [Google Scholar]
- 34.Pukac L. Huangpu J. Karnovsky M.J. Platelet-derived growth factor-BB, insulin-like growth factor-I, and phorbol ester activate different signaling pathways for stimulation of vascular smooth muscle cell migration. Exp Cell Res. 1998;242:548. doi: 10.1006/excr.1998.4138. [DOI] [PubMed] [Google Scholar]
- 35.Pukac L.A. Carter J.E. Ottlinger M.E. Karnovsky M.J. Mechanisms of inhibition by heparin of PDGF stimulated MAP kinase activation in vascular smooth muscle cells. J Cell Physiol. 1997;172:69. doi: 10.1002/(SICI)1097-4652(199707)172:1<69::AID-JCP8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Daum G. Hedin U. Wang Y. Wang T. Clowes A.W. Diverse effects of heparin on mitogen-activated protein kinase-dependent signal transduction in vascular smooth muscle cells. Circ Res. 1997;81:17. doi: 10.1161/01.res.81.1.17. [DOI] [PubMed] [Google Scholar]
- 37.Rauch B.H. Millette E. Kenagy R.D. Daum G. Clowes A.W. Thrombin- and factor Xa-induced DNA synthesis is mediated by transactivation of fibroblast growth factor receptor-1 in human vascular smooth muscle cells. Circ Res. 2004;94:340. doi: 10.1161/01.RES.0000111805.09592.D8. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi K. Shibata K. Morita T. Iwasaki K. Watanabe M. Sobue K. Insulin receptor substrate-1/SHP-2 interaction, a phenotype-dependent switching machinery of insulin-like growth factor-I signaling in vascular smooth muscle cells. J Biol Chem. 2004;279:40807. doi: 10.1074/jbc.M405100200. [DOI] [PubMed] [Google Scholar]
- 39.Bingley J.A. Hayward I.P. Campbell J.H. Campbell G.R. Arterial heparan sulfate proteoglycans inhibit vascular smooth muscle cell proliferation and phenotype change in vitro and neointimal formation in vivo. J Vasc Surg. 1998;28:308. doi: 10.1016/s0741-5214(98)70167-3. [DOI] [PubMed] [Google Scholar]
- 40.Lindner V. Olson N.E. Clowes A.W. Reidy M.A. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992;90:2044. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millette E. Rauch B.H. Defawe O. Kenagy R.D. Daum G. Clowes A.W. Platelet-derived growth factor-BB-induced human smooth muscle cell proliferation depends on basic FGF release and FGFR-1 activation. Circ Res. 2005;96:172. doi: 10.1161/01.RES.0000154595.87608.db. [DOI] [PubMed] [Google Scholar]
- 42.Rauch B.H. Millette E. Kenagy R.D. Daum G. Fischer J.W. Clowes A.W. Syndecan-4 is required for thrombin-induced migration and proliferation in human vascular smooth muscle cells. J Biol Chem. 2005;280:17507. doi: 10.1074/jbc.M410848200. [DOI] [PubMed] [Google Scholar]
- 43.Bono F. Rigon P. Lamarche I. Savi P. Salel V. Herbert J.M. Heparin inhibits the binding of basic fibroblast growth factor to cultured human aortic smooth-muscle cells. Biochem J. 1997;326(Pt 3):661. doi: 10.1042/bj3260661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rapraeger A.C. Krufka A. Olwin B.B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991;252:1705. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 45.Yayon A. Klagsbrun M. Esko J.D. Leder P. Ornitz D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 46.Kazi M. Lundmark K. Religa P. Gouda I. Larm O. Ray A. Swedenborg J. Hedin U. Inhibition of rat smooth muscle cell adhesion and proliferation by non-anticoagulant heparins. J Cell Physiol. 2002;193:365. doi: 10.1002/jcp.10184. [DOI] [PubMed] [Google Scholar]
- 47.Kenagy R.D. Clowes A.W. Regulation of baboon arterial smooth muscle cell plasminogen activators by heparin and growth factors. Thromb Res. 1995;77:55. doi: 10.1016/0049-3848(95)90864-c. [DOI] [PubMed] [Google Scholar]
- 48.Savage J.M. Gilotti A.C. Granzow C.A. Molina F. Lowe-Krentz L.J. Antibodies against a putative heparin receptor slow cell proliferation and decrease MAPK activation in vascular smooth muscle cells. J Cell Physiol. 2001;187:283. doi: 10.1002/jcp.1076. [DOI] [PubMed] [Google Scholar]
- 49.Patel R.C. Handy I. Patel C.V. Contribution of double-stranded RNA-activated protein kinase toward antiproliferative actions of heparin on vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:1439. doi: 10.1161/01.atv.0000028817.20351.fe. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y. Xiao W. Templeton D.M. Suppression of mitogen-activated protein kinase phosphatase-1 (MKP-1) by heparin in vascular smooth muscle cells. Biochem Pharmacol. 2003;66:769. doi: 10.1016/s0006-2952(03)00405-2. [DOI] [PubMed] [Google Scholar]
- 51.Castellot J.J. Wong K. Herman B. Hoover R.L. Albertini D.F. Wright T.C. Caleb B.L. Karnovsky M.J. Binding and internalization of heparin by vascular smooth-muscle cells. J Cell Physiol. 1985;124:13. doi: 10.1002/jcp.1041240104. [DOI] [PubMed] [Google Scholar]
- 52.Quarto N. Amalric F. Heparan sulfate proteoglycans as transducers of FGF-2 signalling. J Cell Sci. 1994;107(Pt 11):3201. doi: 10.1242/jcs.107.11.3201. [DOI] [PubMed] [Google Scholar]
- 53.Fasciano S. Patel R.C. Handy I. Patel C.V. Regulation of vascular smooth muscle proliferation by heparin: inhibition of cyclin-dependent kinase 2 activity by p27(kip1) J Biol Chem. 2005;280:15682. doi: 10.1074/jbc.M411458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright T.C. Castellot J.J. Petitou M. Lormeau J.C. Choay J. Karnovsky M.J. Structural determinants of heparins growth inhibitory activity—interdependence of oligosaccharide size and charge. J Biol Chem. 1989;264:1534. [PubMed] [Google Scholar]
- 55.Guyton J.R. Rosenberg R.D. Clowes A.W. Karnovsky M.J. Inhibition of rat arterial smooth-muscle cell-proliferation by heparin—in vivo studies with anti-coagulant and non-anticoagulant heparin. Circ Res. 1980;46:625. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- 56.Castellot J.J. Choay J. Lormeau J.C. Petitou M. Sache E. Karnovsky M.J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth-muscle cells .2. Evidence for a pentasaccharide sequence that contains a 3-O-sulfate group. J Cell Biol. 1986;102:1979. doi: 10.1083/jcb.102.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garg H.G. Yu L. Hales C.A. Toida T. Islam T. Linhardt R.J. Sulfation patterns in heparin and heparan sulfate: effects on the proliferation of bovine pulmonary artery smooth muscle cells. Biochim Biophys Acta. 2003;1639:225. doi: 10.1016/j.bbadis.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Garg H.G. Mrabat H. Yu L. Freeman C. Li B. Zhang F. Linhardt R.J. Hales C.A. Significance of the 2-O-sulfo group of L-iduronic acid residues in heparin on the growth inhibition of bovine pulmonary artery smooth muscle cells. Carbohydr Res. 2008;343:2406. doi: 10.1016/j.carres.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garg H.G. Joseph P.A.M. Thompson B.T. Hales C.A. Toida T. Imanari T. Capila I. Linhardt R.J. Effect of fully sulfated glycosaminoglycans on pulmonary artery smooth muscle cell proliferation. Arch Biochem Biophys. 1999;371:228. doi: 10.1006/abbi.1999.1456. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto T. Kihara M. Sato K. Imai N. Tanaka Y. Sakai M. Tamura K. Hirawa N. Toya Y. Kitamura H. Umemura S. Heparin recovers AT1 receptor and its intracellular signal transduction in cultured vascular smooth muscle cells. FEBS Lett. 2005;579:281. doi: 10.1016/j.febslet.2004.11.093. [DOI] [PubMed] [Google Scholar]
- 61.Orlandi A. Ropraz P. Gabbiani G. Proliferative activity and alpha-smooth muscle actin expression in cultured rat aortic smooth-muscle cells are differently modulated by transforming growth-factor-beta-1 and heparin. Exp Cell Res. 1994;214:528. doi: 10.1006/excr.1994.1290. [DOI] [PubMed] [Google Scholar]
- 62.Stegemann J.P. Nerem R.M. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp Cell Res. 2003;283:146. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 63.Beamish J.A. Geyer L.C. Haq-Siddiqi N.A. Kottke-Marchant K. Marchant R.E. The effects of heparin releasing hydrogels on vascular smooth muscle cell phenotype. Biomaterials. 2009;30:6286. doi: 10.1016/j.biomaterials.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christen T. Bochaton-Piallat M.L. Neuville P. Rensen S. Redard M. van Eys G. Gabbiani G. Cultured porcine coronary artery smooth muscle cells. A new model with advanced differentiation. Circ Res. 1999;85:99. doi: 10.1161/01.res.85.1.99. [DOI] [PubMed] [Google Scholar]
- 65.Hinck A.P. Archer S.J. Qian S.W. Roberts A.B. Sporn M.B. Weatherbee J.A. Tsang M.L. Lucas R. Zhang B.L. Wenker J. Torchia D.A. Transforming growth factor beta 1: three-dimensional structure in solution and comparison with the X-ray structure of transforming growth factor beta 2. Biochemistry. 1996;35:8517. doi: 10.1021/bi9604946. [DOI] [PubMed] [Google Scholar]
- 66.Bjorkerud S. Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb. 1991;11:892. [PubMed] [Google Scholar]
- 67.Owens G.K. Geisterfer A.A. Yang Y.W. Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol. 1988;107:771. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deaton R.A. Su C. Valencia T.G. Grant S.R. Transforming growth factor-beta 1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J Biol Chem. 2005;280:31172. doi: 10.1074/jbc.M504774200. [DOI] [PubMed] [Google Scholar]
- 69.Topouzis S. Majesky M.W. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev Biol. 1996;178:430. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- 70.Hautmann M.B. Madsen C.S. Owens G.K. A transforming growth factor beta (TGFbeta) control element drives TGFbeta-induced stimulation of smooth muscle alpha-actin gene expression in concert with two CArG elements. J Biol Chem. 1997;272:10948. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- 71.Kawai-Kowase K. Sato H. Oyama Y. Kanai H. Sato M. Doi H. Kurabayashi M. Basic fibroblast growth factor antagonizes transforming growth factor-beta1-induced smooth muscle gene expression through extracellular signal-regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol. 2004;24:1384. doi: 10.1161/01.ATV.0000136548.17816.07. [DOI] [PubMed] [Google Scholar]
- 72.Papetti M. Shujath J. Riley K.N. Herman I.M. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994. doi: 10.1167/iovs.03-0291. [DOI] [PubMed] [Google Scholar]
- 73.Grainger D.J. Metcalfe J.C. Grace A.A. Mosedale D.E. Transforming growth factor-beta dynamically regulates vascular smooth muscle differentiation in vivo. J Cell Sci. 1998;111(Pt 19):2977. doi: 10.1242/jcs.111.19.2977. [DOI] [PubMed] [Google Scholar]
- 74.Alberts B. Molecular Biology of the Cell. 4th. New York: Garland Science; 2002. [Google Scholar]
- 75.Nishimura G. Manabe I. Tsushima K. Fujiu K. Oishi Y. Imai Y. Maemura K. Miyagishi M. Higashi Y. Kondoh H. Nagai R. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev Cell. 2006;11:93. doi: 10.1016/j.devcel.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 76.Hughes A.D. Molecular and cellular mechanisms of action of angiotensin II (AT1) receptors in vascular smooth muscle. J Hum Hypertens. 1998;12:275. doi: 10.1038/sj.jhh.1000635. [DOI] [PubMed] [Google Scholar]
- 77.Johnson E.M. Theler J.M. Capponi A.M. Vallotton M.B. Characterization of oscillations in cytosolic free Ca2+ concentration and measurement of cytosolic Na+ concentration changes evoked by angiotensin II and vasopressin in individual rat aortic smooth muscle cells. Use of microfluorometry and digital imaging. J Biol Chem. 1991;266:12618. [PubMed] [Google Scholar]
- 78.Helou C.M. Marchetti J. Morphological heterogeneity of renal glomerular arterioles and distinct [Ca2+]i responses to ANG II. Am J Physiol. 1997;273:F84. doi: 10.1152/ajprenal.1997.273.1.F84. [DOI] [PubMed] [Google Scholar]
- 79.Geisterfer A.A. Peach M.J. Owens G.K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 80.Touyz R.M. Deng L.Y. He G. Wu X.H. Schiffrin E.L. Angiotensin II stimulates DNA and protein synthesis in vascular smooth muscle cells from human arteries: role of extracellular signal-regulated kinases. J Hypertens. 1999;17:907. doi: 10.1097/00004872-199917070-00006. [DOI] [PubMed] [Google Scholar]
- 81.deBlois D. Viswanathan M. Su J.E. Clowes A.W. Saavedra J.M. Schwartz S.M. Smooth muscle DNA replication in response to angiotensin II is regulated differently in the neointima and media at different times after balloon injury in the rat carotid artery. Role of AT1 receptor expression. Arterioscler Thromb Vasc Biol. 1996;16:1130. doi: 10.1161/01.atv.16.9.1130. [DOI] [PubMed] [Google Scholar]
- 82.Ardaillou R. Angiotensin II receptors. J Am Soc Nephrol. 1999;10(Suppl 11):S30. [PubMed] [Google Scholar]
- 83.Zhang F. Sun A.S. Yu L.M. Wu Q. Gong Q.H. Effects of isorhynchophylline on angiotensin II-induced proliferation in rat vascular smooth muscle cells. J Pharm Pharmacol. 2008;60:1673. doi: 10.1211/jpp/60.12.0014. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe T. Pakala R. Katagiri T. Benedict C.R. Serotonin potentiates angiotensin II-induced vascular smooth muscle cell proliferation. Atherosclerosis. 2001;159:269. doi: 10.1016/s0021-9150(01)00505-6. [DOI] [PubMed] [Google Scholar]
- 85.Geisterfer A.A.T. Peach M.J. Owens G.K. Angiotensin-Ii induces hypertrophy, not hyperplasia, of cultured rat aortic smooth-muscle cells. Circ Res. 1988;62:749. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 86.Bascands J.L. Girolami J.P. Troly M. Escargueil-Blanc I. Nazzal D. Salvayre R. Blaes N. Angiotensin II induces phenotype-dependent apoptosis in vascular smooth muscle cells. Hypertension. 2001;38:1294. doi: 10.1161/hy1201.096540. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida T. Hoofnagle M.H. Owens G.K. Myocardin and Prx1 contribute to angiotensin II-induced expression of smooth muscle alpha-actin. Circ Res. 2004;94:1075. doi: 10.1161/01.RES.0000125622.46280.95. [DOI] [PubMed] [Google Scholar]
- 88.Turla M.B. Thompson M.M. Corjay M.H. Owens G.K. Mechanisms of angiotensin II- and arginine vasopressin-induced increases in protein synthesis and content in cultured rat aortic smooth muscle cells. Evidence for selective increases in smooth muscle isoactin expression. Circ Res. 1991;68:288. doi: 10.1161/01.res.68.1.288. [DOI] [PubMed] [Google Scholar]
- 89.Chassagne C. Adamy C. Ratajczak P. Gingras B. Teiger E. Planus E. Oliviero P. Rappaport L. Samuel J.L. Meloche S. Angiotensin II AT(2) receptor inhibits smooth muscle cell migration via fibronectin cell production and binding. Am J Physiol Cell Physiol. 2002;282:C654. doi: 10.1152/ajpcell.00318.2001. [DOI] [PubMed] [Google Scholar]
- 90.Banskota N.K. Taub R. Zellner K. King G.L. Insulin, insulin-like growth factor I and platelet-derived growth factor interact additively in the induction of the protooncogene c-myc and cellular proliferation in cultured bovine aortic smooth muscle cells. Mol Endocrinol. 1989;3:1183. doi: 10.1210/mend-3-8-1183. [DOI] [PubMed] [Google Scholar]
- 91.Arnqvist H.J. Bornfeldt K.E. Chen Y. Lindstrom T. The insulin-like growth factor system in vascular smooth muscle: interaction with insulin and growth factors. Metabolism. 1995;44:58. doi: 10.1016/0026-0495(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 92.Hayashi K. Takahashi M. Kimura K. Nishida W. Saga H. Sobue K. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J Cell Biol. 1999;145:727. doi: 10.1083/jcb.145.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu B. Zhao G. Witte D.P. Hui D.Y. Fagin J.A. Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinology. 2001;142:3598. doi: 10.1210/endo.142.8.8331. [DOI] [PubMed] [Google Scholar]
- 94.Moiseeva E.P. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52:372. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 95.Hedin U. Bottger B.A. Forsberg E. Johansson S. Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988;107:307. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayward I.P. Bridle K.R. Campbell G.R. Underwood P.A. Campbell J.H. Effect of extracellular matrix proteins on vascular smooth muscle cell phenotype. Cell Biol Int. 1995;19:839. doi: 10.1006/cbir.1995.1019. [DOI] [PubMed] [Google Scholar]
- 97.Thyberg J. Hultgardh-Nilsson A. Fibronectin and the basement membrane components laminin and collagen type IV influence the phenotypic properties of subcultured rat aortic smooth muscle cells differently. Cell Tissue Res. 1994;276:263. doi: 10.1007/BF00306112. [DOI] [PubMed] [Google Scholar]
- 98.Yamamoto M. Yamamoto K. Noumura T. Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp Cell Res. 1993;204:121. doi: 10.1006/excr.1993.1016. [DOI] [PubMed] [Google Scholar]
- 99.Hedin U.L. Daum G. Clowes A.W. Disruption of integrin alpha 5 beta 1 signaling does not impair PDGF-BB-mediated stimulation of the extracellular signal-regulated kinase pathway in smooth muscle cells. J Cell Physiol. 1997;172:109. doi: 10.1002/(SICI)1097-4652(199707)172:1<109::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 100.Hedin U. Bottger B.A. Luthman J. Johansson S. Thyberg J. A substrate of the cell-attachment sequence of fibronectin (Arg-Gly-Asp-Ser) is sufficient to promote transition of arterial smooth muscle cells from a contractile to a synthetic phenotype. Dev Biol. 1989;133:489. doi: 10.1016/0012-1606(89)90052-3. [DOI] [PubMed] [Google Scholar]
- 101.Qin H. Ishiwata T. Wang R. Kudo M. Yokoyama M. Naito Z. Asano G. Effects of extracellular matrix on phenotype modulation and MAPK transduction of rat aortic smooth muscle cells in vitro. Exp Mol Pathol. 2000;69:79. doi: 10.1006/exmp.2000.2321. [DOI] [PubMed] [Google Scholar]
- 102.Zheng B. Duan C. Clemmons D.R. The effect of extracellular matrix proteins on porcine smooth muscle cell insulin-like growth factor (IGF) binding protein-5 synthesis and responsiveness to IGF-I. J Biol Chem. 1998;273:8994. doi: 10.1074/jbc.273.15.8994. [DOI] [PubMed] [Google Scholar]
- 103.Beamish J.A. Fu A.Y. Choi A.-J. Haq N.A. Kottke-Marchant K. Marchant R.E. The influence of RGD-bearing hydrogels on the re-expression of contractile vascular smooth muscle cell phenotype. Biomaterials. 2009;30:4127. doi: 10.1016/j.biomaterials.2009.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hirst S.J. Twort C.H. Lee T.H. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol. 2000;23:335. doi: 10.1165/ajrcmb.23.3.3990. [DOI] [PubMed] [Google Scholar]
- 105.Tran T. McNeill K.D. Gerthoffer W.T. Unruh H. Halayko A.J. Endogenous laminin is required for human airway smooth muscle cell maturation. Respir Res. 2006;7:117. doi: 10.1186/1465-9921-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karnik S.K. Brooke B.S. Bayes-Genis A. Sorensen L. Wythe J.D. Schwartz R.S. Keating M.T. Li D.Y. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- 107.Hedin U. Thyberg J. Roy J. Dumitrescu A. Tran P.K. Role of tyrosine kinases in extracellular matrix-mediated modulation of arterial smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 1997;17:1977. doi: 10.1161/01.atv.17.10.1977. [DOI] [PubMed] [Google Scholar]
- 108.Welser J.V. Lange N. Singer C.A. Elorza M. Scowen P. Keef K.D. Gerthoffer W.T. Burkin D.J. Loss of the alpha7 integrin promotes extracellular signal-regulated kinase activation and altered vascular remodeling. Circ Res. 2007;101:672. doi: 10.1161/CIRCRESAHA.107.151415. [DOI] [PubMed] [Google Scholar]
- 109.Wilson E. Alpha 7 beta 1 integrin: putting the brakes on smooth muscle cell proliferation. Circ Res. 2007;101:651. doi: 10.1161/CIRCRESAHA.107.161877. [DOI] [PubMed] [Google Scholar]
- 110.Lijnen H.R. Metalloproteinases in development and progression of vascular disease. Pathophysiol Haemost Thromb. 2003;33:275. doi: 10.1159/000083814. [DOI] [PubMed] [Google Scholar]
- 111.Filippov S. Koenig G.C. Chun T.H. Hotary K.B. Ota I. Bugge T.H. Roberts J.D. Fay W.P. Birkedal-Hansen H. Holmbeck K. Sabeh F. Allen E.D. Weiss S.J. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202:663. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dollery C.M. Libby P. Atherosclerosis and proteinase activation. Cardiovasc Res. 2006;69:625. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 113.Galis Z.S. Khatri J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251. [PubMed] [Google Scholar]
- 114.Forough R. Koyama N. Hasenstab D. Lea H. Clowes M. Nikkari S.T. Clowes A.W. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res. 1996;79:812. doi: 10.1161/01.res.79.4.812. [DOI] [PubMed] [Google Scholar]
- 115.Southgate K.M. Mehta D. Izzat M.B. Newby A.C. Angelini G.D. Increased secretion of basement membrane-degrading metalloproteinases in pig saphenous vein into carotid artery interposition grafts. Arterioscler Thromb Vasc Biol. 1999;19:1640. doi: 10.1161/01.atv.19.7.1640. [DOI] [PubMed] [Google Scholar]
- 116.Shofuda K.I. Hasenstab D. Kenagy R.D. Shofuda T. Li Z.Y. Lieber A. Clowes A.W. Membrane-type matrix metalloproteinase-1 and -3 activity in primate smooth muscle cells. FASEB J. 2001;15:2010. doi: 10.1096/fj.00-0871fje. [DOI] [PubMed] [Google Scholar]
- 117.Mason D.P. Kenagy R.D. Hasenstab D. Bowen-Pope D.F. Seifert R.A. Coats S. Hawkins S.M. Clowes A.W. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999;85:1179. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 118.Ailawadi G. Moehle C.W. Pei H. Walton S.P. Yang Z. Kron I.L. Lau C.L. Owens G.K. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009;138:1392. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnson J.L. van Eys G.J. Angelini G.D. George S.J. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol. 2001;21:1146. doi: 10.1161/hq0701.092106. [DOI] [PubMed] [Google Scholar]
- 120.Bendeck M.P. Zempo N. Clowes A.W. Galardy R.E. Reidy M.A. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 121.Zempo N. Kenagy R.D. Au Y.P. Bendeck M. Clowes M.M. Reidy M.A. Clowes A.W. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg. 1994;20:209. doi: 10.1016/0741-5214(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 122.Bendeck M.P. Irvin C. Reidy M.A. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res. 1996;78:38. doi: 10.1161/01.res.78.1.38. [DOI] [PubMed] [Google Scholar]
- 123.Lehti K. Rose N.F. Valavaara S. Weiss S.J. Keski-Oja J. MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J Cell Sci. 2009;122:126. doi: 10.1242/jcs.035279. [DOI] [PubMed] [Google Scholar]
- 124.Williams B. Mechanical influences on vascular smooth muscle cell function. J Hypertens. 1998;16:1921. doi: 10.1097/00004872-199816121-00011. [DOI] [PubMed] [Google Scholar]
- 125.Wilson E. Mai Q. Sudhir K. Weiss R.H. Ives H.E. Mechanical strain induces growth of vascular smooth muscle cells via autocrine action of PDGF. J Cell Biol. 1993;123:741. doi: 10.1083/jcb.123.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson E. Sudhir K. Ives H.E. Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J Clin Invest. 1995;96:2364. doi: 10.1172/JCI118293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hipper A. Isenberg G. Cyclic mechanical strain decreases the DNA synthesis of vascular smooth muscle cells. Pflugers Arch. 2000;440:19. doi: 10.1007/s004240000246. [DOI] [PubMed] [Google Scholar]
- 128.Tock J. Van Putten V. Stenmark K.R. Nemenoff R.A. Induction of SM-alpha-actin expression by mechanical strain in adult vascular smooth muscle cells is mediated through activation of JNK and p38 MAP kinase. Biochem Biophys Res Commun. 2003;301:1116. doi: 10.1016/s0006-291x(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 129.Standley P.R. Obards T.J. Martina C.L. Cyclic stretch regulates autocrine IGF-I in vascular smooth muscle cells: implications in vascular hyperplasia. Am J Physiol. 1999;276:E697. doi: 10.1152/ajpendo.1999.276.4.E697. [DOI] [PubMed] [Google Scholar]
- 130.Chapman G.B. Durante W. Hellums J.D. Schafer A.I. Physiological cyclic stretch causes cell cycle arrest in cultured vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;278:H748. doi: 10.1152/ajpheart.2000.278.3.H748. [DOI] [PubMed] [Google Scholar]
- 131.Qu M.J. Liu B. Wang H.Q. Yan Z.Q. Shen B.R. Jiang Z.L. Frequency-dependent phenotype modulation of vascular smooth muscle cells under cyclic mechanical strain. J Vasc Res. 2007;44:345. doi: 10.1159/000102278. [DOI] [PubMed] [Google Scholar]
- 132.Birukov K.G. Shirinsky V.P. Stepanova O.V. Tkachuk V.A. Hahn A.W. Resink T.J. Smirnov V.N. Stretch affects phenotype and proliferation of vascular smooth muscle cells. Mol Cell Biochem. 1995;144:131. doi: 10.1007/BF00944392. [DOI] [PubMed] [Google Scholar]
- 133.Li W. Chen Q. Mills I. Sumpio B.E. Involvement of S6 kinase and p38 mitogen activated protein kinase pathways in strain-induced alignment and proliferation of bovine aortic smooth muscle cells. J Cell Physiol. 2003;195:202. doi: 10.1002/jcp.10230. [DOI] [PubMed] [Google Scholar]
- 134.O'Callaghan C.J. Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1) Hypertension. 2000;36:319. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 135.Spofford C.M. Chilian W.M. The elastin-laminin receptor functions as a mechanotransducer in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2001;280:H1354. doi: 10.1152/ajpheart.2001.280.3.H1354. [DOI] [PubMed] [Google Scholar]
- 136.Stegemann J.P. Nerem R.M. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31:391. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- 137.Jeong S.I. Kwon J.H. Lim J.I. Cho S.W. Jung Y. Sung W.J. Kim S.H. Kim Y.H. Lee Y.M. Kim B.S. Choi C.Y. Kim S.J. Mechano-active tissue engineering of vascular smooth muscle using pulsatile perfusion bioreactors and elastic PLCL scaffolds. Biomaterials. 2005;26:1405. doi: 10.1016/j.biomaterials.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 138.Kim B.S. Nikolovski J. Bonadio J. Mooney D.J. Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nat Biotechnol. 1999;17:979. doi: 10.1038/13671. [DOI] [PubMed] [Google Scholar]
- 139.Joki N. Kaname S. Hirakata M. Hori Y. Yamaguchi T. Fujita T. Katoh T. Kurokawa K. Tyrosine-kinase dependent TGF-beta and extracellular matrix expression by mechanical stretch in vascular smooth muscle cells. Hypertens Res. 2000;23:91. doi: 10.1291/hypres.23.91. [DOI] [PubMed] [Google Scholar]
- 140.Mann B.K. Schmedlen R.H. West J.L. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 141.Hahn M.S. McHale M.K. Wang E. Schmedlen R.H. West J.L. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann Biomed Eng. 2007;35:190. doi: 10.1007/s10439-006-9099-3. [DOI] [PubMed] [Google Scholar]
- 142.Bulick A.S. Munoz-Pinto D.J. Qu X. Mani M. Cristancho D. Urban M. Hahn M.S. Impact of endothelial cells and mechanical conditioning on smooth muscle cell extracellular matrix production and differentiation. Tissue Eng Part A. 2009;15:815. doi: 10.1089/ten.tea.2008.0179. [DOI] [PubMed] [Google Scholar]
- 143.Reusch P. Wagdy H. Reusch R. Wilson E. Ives H.E. Mechanical strain increases smooth muscle and decreases nonmuscle myosin expression in rat vascular smooth muscle cells. Circ Res. 1996;79:1046. doi: 10.1161/01.res.79.5.1046. [DOI] [PubMed] [Google Scholar]
- 144.Reusch H.P. Chan G. Ives H.E. Nemenoff R.A. Activation of JNK/SAPK and ERK by mechanical strain in vascular smooth muscle cells depends on extracellular matrix composition. Biochem Biophys Res Commun. 1997;237:239. doi: 10.1006/bbrc.1997.7121. [DOI] [PubMed] [Google Scholar]
- 145.Chen Q. Li W. Quan Z. Sumpio B.E. Modulation of vascular smooth muscle cell alignment by cyclic strain is dependent on reactive oxygen species and P38 mitogen-activated protein kinase. J Vasc Surg. 2003;37:660. doi: 10.1067/mva.2003.95. [DOI] [PubMed] [Google Scholar]
- 146.Standley P.R. Cammarata A. Nolan B.P. Purgason C.T. Stanley M.A. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am J Physiol Heart Circ Physiol. 2002;283:H1907. doi: 10.1152/ajpheart.01043.2001. [DOI] [PubMed] [Google Scholar]
- 147.Li Q. Muragaki Y. Hatamura I. Ueno H. Ooshima A. Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-beta. J Vasc Res. 1998;35:93. doi: 10.1159/000025570. [DOI] [PubMed] [Google Scholar]
- 148.Cappadona C. Redmond E.M. Theodorakis N.G. McKillop I.H. Hendrickson R. Chhabra A. Sitzmann J.V. Cahill P.A. Phenotype dictates the growth response of vascular smooth muscle cells to pulse pressure in vitro. Exp Cell Res. 1999;250:174. doi: 10.1006/excr.1999.4502. [DOI] [PubMed] [Google Scholar]
- 149.Jackson Z.S. Gotlieb A.I. Langille B.L. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res. 2002;90:918. doi: 10.1161/01.res.0000016481.87703.cc. [DOI] [PubMed] [Google Scholar]
- 150.Nichol J.W. Petko M. Myung R.J. Gaynor J.W. Gooch K.J. Hemodynamic conditions alter axial and circumferential remodeling of arteries engineered ex vivo. Ann Biomed Eng. 2005;33:721. doi: 10.1007/s10439-005-4494-8. [DOI] [PubMed] [Google Scholar]
- 151.Davies P.F. Olesen S.P. Clapham D.E. Morrel E.M. Schoen F.J. Endothelial communication—state of the art lecture. Hypertension. 1988;11:563. doi: 10.1161/01.hyp.11.6.563.a. [DOI] [PubMed] [Google Scholar]
- 152.Thomae K.R. Nakayama D.K. Billiar T.R. Simmons R.L. Pitt B.R. Davies P. The effect of nitric oxide on fetal pulmonary artery smooth muscle growth. J Surg Res. 1995;59:337. doi: 10.1006/jsre.1995.1173. [DOI] [PubMed] [Google Scholar]
- 153.Janakidevi K. Fisher M.A. Del Vecchio P.J. Tiruppathi C. Figge J. Malik A.B. Endothelin-1 stimulates DNA synthesis and proliferation of pulmonary artery smooth muscle cells. Am J Physiol. 1992;263:C1295. doi: 10.1152/ajpcell.1992.263.6.C1295. [DOI] [PubMed] [Google Scholar]
- 154.Fillinger M.F. Sampson L.N. Cronenwett J.L. Powell R.J. Wagner R.J. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res. 1997;67:169. doi: 10.1006/jsre.1996.4978. [DOI] [PubMed] [Google Scholar]
- 155.Heydarkhan-Hagvall S. Helenius G. Johansson B.R. Li J.Y. Mattsson E. Risberg B. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J Cell Biochem. 2003;89:1250. doi: 10.1002/jcb.10583. [DOI] [PubMed] [Google Scholar]
- 156.Rose S.L. Babensee J.E. Complimentary endothelial cell/smooth muscle cell co-culture systems with alternate smooth muscle cell phenotypes. Ann Biomed Eng. 2007;35:1382. doi: 10.1007/s10439-007-9311-0. [DOI] [PubMed] [Google Scholar]
- 157.Rose S.L. Babensee J.E. Smooth muscle cell phenotype alters cocultured endothelial cell response to biomaterial-pretreated leukocytes. J Biomed Mater Res A. 2008;84:661. doi: 10.1002/jbm.a.31305. [DOI] [PubMed] [Google Scholar]
- 158.Yoshida H. Nakamura M. Makita S. Hiramori K. Paracrine effect of human vascular endothelial cells on human vascular smooth muscle cell proliferation: transmembrane co-culture method. Heart Vessels. 1996;11:229. doi: 10.1007/BF01746202. [DOI] [PubMed] [Google Scholar]
- 159.Williams C. Wick T.M. Endothelial cell-smooth muscle cell co-culture in a perfusion bioreactor system. Ann Biomed Eng. 2005;33:920. doi: 10.1007/s10439-005-3238-0. [DOI] [PubMed] [Google Scholar]
- 160.Haruguchi H. Teraoka S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J Artif Organs. 2003;6:227. doi: 10.1007/s10047-003-0232-x. [DOI] [PubMed] [Google Scholar]
- 161.Motwani J.G. Topol E.J. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 162.Hancock W.W. Adams D.H. Wyner L.R. Sayegh M.H. Karnovsky M.J. CD4+ mononuclear cells induce cytokine expression, vascular smooth muscle cell proliferation, and arterial occlusion after endothelial injury. Am J Pathol. 1994;145:1008. [PMC free article] [PubMed] [Google Scholar]
- 163.Tanaka H. Sukhova G.K. Swanson S.J. Clinton S.K. Ganz P. Cybulsky M.I. Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 164.Okamoto E. Couse T. De Leon H. Vinten-Johansen J. Goodman R.B. Scott N.A. Wilcox J.N. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 165.Schultz K. Murthy V. Tatro J.B. Beasley D. Endogenous interleukin-1 alpha promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2927. doi: 10.1152/ajpheart.00700.2006. [DOI] [PubMed] [Google Scholar]
- 166.Yue T.L. Wang X. Sung C.P. Olson B. McKenna P.J. Gu J.L. Feuerstein G.Z. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994;75:1. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]
- 167.Cirillo P. Golino P. Calabro P. Cali G. Ragni M. De Rosa S. Cimmino G. Pacileo M. De Palma R. Forte L. Gargiulo A. Corigliano F.G. Angri V. Spagnuolo R. Nitsch L. Chiariello M. C-Reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res. 2005;68:47. doi: 10.1016/j.cardiores.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 168.Heo S.K. Yun H.J. Park W.H. Park S.D. Emodin inhibits TNF-alpha-induced human aortic smooth-muscle cell proliferation via caspase- and mitochondrial-dependent apoptosis. J Cell Biochem. 2008;105:70. doi: 10.1002/jcb.21805. [DOI] [PubMed] [Google Scholar]
- 169.Miano J.M. Long X. Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 170.Miano J.M. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 171.Prywes R. Roeder R.G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987;7:3482. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986;46:567. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- 173.Norman C. Runswick M. Pollock R. Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 174.Boxer L.M. Prywes R. Roeder R.G. Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989;9:515. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun Q. Chen G. Streb J.W. Long X. Yang Y. Stoeckert C.J., Jr. Miano J.M. Defining the mammalian CArGome. Genome Res. 2006;16:197. doi: 10.1101/gr.4108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yoshida T. Kawai-Kowase K. Owens G.K. Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential embryonic cells. Arterioscler Thromb Vasc Biol. 2004;24:1596. doi: 10.1161/01.ATV.0000137190.63214.c5. [DOI] [PubMed] [Google Scholar]
- 177.Wang D. Chang P.S. Wang Z. Sutherland L. Richardson J.A. Small E. Krieg P.A. Olson E.N. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 178.Mack C.P. Hinson J.S. Regulation of smooth muscle differentiation by the myocardin family of serum response factor co-factors. J Thromb Haemost. 2005;3:1976. doi: 10.1111/j.1538-7836.2005.01316.x. [DOI] [PubMed] [Google Scholar]
- 179.Wang Z. Wang D.Z. Pipes G.C. Olson E.N. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 2003;100:7129. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hendrix J.A. Wamhoff B.R. McDonald O.G. Sinha S. Yoshida T. Owens G.K. 5' CArG degeneracy in smooth muscle alpha-actin is required for injury-induced gene suppression in vivo. J Clin Invest. 2005;115:418. doi: 10.1172/JCI22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van Tuyn J. Knaan-Shanzer S. van de Watering M.J. de Graaf M. van der Laarse A. Schalij M.J. van der Wall E.E. de Vries A.A. Atsma D.E. Activation of cardiac and smooth muscle-specific genes in primary human cells after forced expression of human myocardin. Cardiovasc Res. 2005;67:245. doi: 10.1016/j.cardiores.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 182.Manabe I. Owens G.K. Recruitment of serum response factor and hyperacetylation of histones at smooth muscle-specific regulatory regions during differentiation of a novel P19-derived in vitro smooth muscle differentiation system. Circ Res. 2001;88:1127. doi: 10.1161/hh1101.091339. [DOI] [PubMed] [Google Scholar]
- 183.Li S. Wang D.Z. Wang Z. Richardson J.A. Olson E.N. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2003;100:9366. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pipes G.C. Sinha S. Qi X. Zhu C.H. Gallardo T.D. Shelton J. Creemers E.E. Sutherland L. Richardson J.A. Garry D.J. Wright W.E. Owens G.K. Olson E.N. Stem cells and their derivatives can bypass the requirement of myocardin for smooth muscle gene expression. Dev Biol. 2005;288:502. doi: 10.1016/j.ydbio.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 185.Owens G.K. Kumar M.S. Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 186.Kawai-Kowase K. Kumar M.S. Hoofnagle M.H. Yoshida T. Owens G.K. PIAS1 activates the expression of smooth muscle cell differentiation marker genes by interacting with serum response factor and class I basic helix-loop-helix proteins. Mol Cell Biol. 2005;25:8009. doi: 10.1128/MCB.25.18.8009-8023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gan Q. Yoshida T. Li J. Owens G.K. Smooth muscle cells and myofibroblasts use distinct transcriptional mechanisms for smooth muscle alpha-actin expression. Circ Res. 2007;101:883. doi: 10.1161/CIRCRESAHA.107.154831. [DOI] [PubMed] [Google Scholar]
- 188.Miralles F. Posern G. Zaromytidou A.I. Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 189.Cavigelli M. Dolfi F. Claret F.X. Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14:5957. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou J. Hu G. Herring B.P. Smooth muscle-specific genes are differentially sensitive to inhibition by Elk-1. Mol Cell Biol. 2005;25:9874. doi: 10.1128/MCB.25.22.9874-9885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yoshida T. Gan Q. Owens G.K. Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Physiol Cell Physiol. 2008;295:C1175. doi: 10.1152/ajpcell.00288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Z. Wang D.Z. Hockemeyer D. McAnally J. Nordheim A. Olson E.N. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 193.Liu Y. Sinha S. Owens G. A transforming growth factor-beta control element required for SM alpha-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J Biol Chem. 2003;278:48004. doi: 10.1074/jbc.M301902200. [DOI] [PubMed] [Google Scholar]
- 194.Adam P.J. Regan C.P. Hautmann M.B. Owens G.K. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22alpha in vivo. J Biol Chem. 2000;275:37798. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- 195.Liu Y. Sinha S. McDonald O.G. Shang Y. Hoofnagle M.H. Owens G.K. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 196.Kawai-Kowase K. Ohshima T. Matsui H. Tanaka T. Shimizu T. Iso T. Arai M. Owens G.K. Kurabayashi M. PIAS1 mediates TGFbeta-induced SM alpha-actin gene expression through inhibition of KLF4 function-expression by protein sumoylation. Arterioscler Thromb Vasc Biol. 2009;29:99. doi: 10.1161/ATVBAHA.108.172700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Doi H. Iso T. Yamazaki M. Akiyama H. Kanai H. Sato H. Kawai-Kowase K. Tanaka T. Maeno T. Okamoto E. Arai M. Kedes L. Kurabayashi M. HERP1 inhibits myocardin-induced vascular smooth muscle cell differentiation by interfering with SRF binding to CArG box. Arterioscler Thromb Vasc Biol. 2005;25:2328. doi: 10.1161/01.ATV.0000185829.47163.32. [DOI] [PubMed] [Google Scholar]
- 198.Dandre F. Owens G.K. Platelet-derived growth factor-BB and Ets-1 transcription factor negatively regulate transcription of multiple smooth muscle cell differentiation marker genes. Am J Physiol Heart Circ Physiol. 2004;286:H2042. doi: 10.1152/ajpheart.00625.2003. [DOI] [PubMed] [Google Scholar]
- 199.Shen J.Y. Chan-Park M.B. He B. Zhu A.P. Zhu X. Beuerman R.W. Yang E.B. Chen W. Chan V. Three-dimensional microchannels in biodegradable polymeric films for control orientation and phenotype of vascular smooth muscle cells. Tissue Eng. 2006;12:2229. doi: 10.1089/ten.2006.12.2229. [DOI] [PubMed] [Google Scholar]
- 200.Rowlands A.S. Cooper-White J.J. Directing phenotype of vascular smooth muscle cells using electrically stimulated conducting polymer. Biomaterials. 2008;29:4510. doi: 10.1016/j.biomaterials.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 201.Higgins S.P. Solan A.K. Niklason L.E. Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation. J Biomed Mater Res A. 2003;67:295. doi: 10.1002/jbm.a.10599. [DOI] [PubMed] [Google Scholar]
- 202.Mann B.K. Tsai A.T. Scott-Burden T. West J.L. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20:2281. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 203.Mann B.K. West J.L. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60:86. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 204.Joddar B. Ibrahim S. Ramamurthi A. Impact of delivery mode of hyaluronan oligomers on elastogenic responses of adult vascular smooth muscle cells. Biomaterials. 2007;28:3918. doi: 10.1016/j.biomaterials.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Peyton S.R. Raub C.B. Keschrumrus V.P. Putnam A.J. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881. doi: 10.1016/j.biomaterials.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 206.Li S. Lao J. Chen B.P. Li Y.S. Zhao Y. Chu J. Chen K.D. Tsou T.C. Peck K. Chien S. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J. 2003;17:97. doi: 10.1096/fj.02-0256fje. [DOI] [PubMed] [Google Scholar]
- 207.Hong H. McCullough C.M. Stegemann J.P. The role of ERK signaling in protein hydrogel remodeling by vascular smooth muscle cells. Biomaterials. 2007;28:3824. doi: 10.1016/j.biomaterials.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mann B.K. Gobin A.S. Tsai A.T. Schmedlen R.H. West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 209.Adelow C. Segura T. Hubbell J.A. Frey P. The effect of enzymatically degradable poly(ethylene glycol) hydrogels on smooth muscle cell phenotype. Biomaterials. 2008;29:314. doi: 10.1016/j.biomaterials.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 210.Peyton S.R. Kim P.D. Ghajar C.M. Seliktar D. Putnam A.J. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29:2597. doi: 10.1016/j.biomaterials.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brophy C.M. Woodrum D.A. Pollock J. Dickinson M. Komalavilas P. Cornwell T.L. Lincoln T.M. cGMP-dependent protein kinase expression restores contractile function in cultured vascular smooth muscle cells. J Vasc Res. 2002;39:95. doi: 10.1159/000057758. [DOI] [PubMed] [Google Scholar]
- 212.Stegemann J.P. Dey N.B. Lincoln T.M. Nerem R.M. Genetic modification of smooth muscle cells to control phenotype and function in vascular tissue engineering. Tissue Eng. 2004;10:189. doi: 10.1089/107632704322791844. [DOI] [PubMed] [Google Scholar]
- 213.Wamhoff B.R. Bowles D.K. McDonald O.G. Sinha S. Somlyo A.P. Somlyo A.V. Owens G.K. L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circ Res. 2004;95:406. doi: 10.1161/01.RES.0000138582.36921.9e. [DOI] [PubMed] [Google Scholar]
- 214.L'Heureux N. Stoclet J.-C. Auger F.A. Lagaud G.J.-L. Germain L. Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J. 2001;15:515. doi: 10.1096/fj.00-0283com. [DOI] [PubMed] [Google Scholar]
- 215.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 216.Weinberg C.B. Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 217.Chan-Park M.B. Shen J.Y. Cao Y. Xiong Y. Liu Y. Rayatpisheh S. Kang G.C. Greisler H.P. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels. J Biomed Mater Res A. 2009;88:1104. doi: 10.1002/jbm.a.32318. [DOI] [PubMed] [Google Scholar]
- 218.Kakisis J.D. Liapis C.D. Breuer C. Sumpio B.E. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 219.Borschel G.H. Huang Y.C. Calve S. Arruda E.M. Lynch J.B. Dow D.E. Kuzon W.M. Dennis R.G. Brown D.L. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005;11:778. doi: 10.1089/ten.2005.11.778. [DOI] [PubMed] [Google Scholar]
- 220.Kaushal S. Amiel G.E. Guleserian K.J. Shapira O.M. Perry T. Sutherland F.W. Rabkin E. Moran A.M. Schoen F.J. Atala A. Soker S. Bischoff J. Mayer J.E. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Huynh T. Abraham G. Murray J. Brockbank K. Hagen P.O. Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat Biotechnol. 1999;17:1083. doi: 10.1038/15062. [DOI] [PubMed] [Google Scholar]
- 222.Tranquillo R.T. Girton T.S. Bromberek B.A. Triebes T.G. Mooradian D.L. Magnetically orientated tissue-equivalent tubes: application to a circumferentially orientated media-equivalent. Biomaterials. 1996;17:349. doi: 10.1016/0142-9612(96)85573-6. [DOI] [PubMed] [Google Scholar]
- 223.Seliktar D. Black R.A. Vito R.P. Nerem R.M. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28:351. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- 224.Seliktar D. Nerem R.M. Galis Z.S. Mechanical strain-stimulated remodeling of tissue-engineered blood vessel constructs. Tissue Eng. 2003;9:657. doi: 10.1089/107632703768247359. [DOI] [PubMed] [Google Scholar]
- 225.Grassl E.D. Oegema T.R. Tranquillo R.T. A fibrin-based arterial media equivalent. J Biomed Mater Res Part A. 2003;66A:550. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 226.Swartz D.D. Russell J.A. Andreadis S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- 227.Matsumura G. Hibino N. Ikada Y. Kurosawa H. Shin'oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24:2303. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 228.Shin'oka T. Matsumura G. Hibino N. Naito Y. Watanabe M. Konuma T. Sakamoto T. Nagatsu M. Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 229.Shum-Tim D. Stock U. Hrkach J. Shinoka T. Lien J. Moses M.A. Stamp A. Taylor G. Moran A.M. Landis W. Langer R. Vacanti J.P. Mayer J.E. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg. 1999;68:2298. doi: 10.1016/s0003-4975(99)01055-3. [DOI] [PubMed] [Google Scholar]
- 230.Tillman B.W. Yazdani S.K. Lee S.J. Geary R.L. Atala A. Yoo J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials. 2009;30:583. doi: 10.1016/j.biomaterials.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 231.Hashi C.K. Zhu Y.Q. Yang G.Y. Young W.L. Hsiao B.S. Wang K. Chu B. Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104:11915. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Stitzel J. Liu L. Lee S.J. Komura M. Berry J. Soker S. Lim G. Van Dyke M. Czerw R. Yoo J.J. Atala A. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 233.L'Heureux N. Paquet S. Labbe R. Germain L. Auger F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 234.L'Heureux N. Dusserre N. Konig G. Victor B. Keire P. Wight T.N. Chronos N.A. Kyles A.E. Gregory C.R. Hoyt G. Robbins R.C. McAllister T.N. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.McAllister T.N. Maruszewski M. Garrido S.A. Wystrychowski W. Dusserre N. Marini A. Zagalski K. Fiorillo A. Avila H. Manglano X. Antonelli J. Kocher A. Zembala M. Cierpka L. de la Fuente L.M. L'Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet. 2009;373:1440. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 236.Xie C.Q. Huang H. Wei S. Song L.S. Zhang J. Ritchie R.P. Chen L. Zhang M. Chen Y.E. A comparison of murine smooth muscle cells generated from embryonic versus induced pluripotent stem cells. Stem Cells Dev. 2009;18:741. doi: 10.1089/scd.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu J.Y. Swartz D.D. Peng H.F. Gugino S.F. Russell J.A. Andreadis S.T. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 238.Liu J.Y. Peng H.F. Andreadis S.T. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res. 2008;79:24. doi: 10.1093/cvr/cvn059. [DOI] [PubMed] [Google Scholar]
- 239.Huang H. Zhao X. Chen L. Xu C. Yao X. Lu Y. Dai L. Zhang M. Differentiation of human embryonic stem cells into smooth muscle cells in adherent monolayer culture. Biochem Biophys Res Commun. 2006;351:321. doi: 10.1016/j.bbrc.2006.09.171. [DOI] [PubMed] [Google Scholar]
- 240.Drab M. Haller H. Bychkov R. Erdmann B. Lindschau C. Haase H. Morano I. Luft F.C. Wobus A.M. From totipotent embryonic stem cells to spontaneously contracting smooth muscle cells: a retinoic acid and db-cAMP in vitro differentiation model. FASEB J. 1997;11:905. doi: 10.1096/fasebj.11.11.9285489. [DOI] [PubMed] [Google Scholar]
- 241.Lee T.H. Song S.H. Kim K.L. Yi J.Y. Shin G.H. Kim J.Y. Kim J. Han Y.M. Lee S.H. Lee S.H. Shim S.H. Suh W. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ Res. 2010;106:120. doi: 10.1161/CIRCRESAHA.109.207902. [DOI] [PubMed] [Google Scholar]
- 242.Liao S.W. Hida K. Park J.S. Li S. Mechanical regulation of matrix reorganization and phenotype of smooth muscle cells and mesenchymal stem cells in 3D matrix. Conf Proc IEEE Eng Med Biol Soc. 2004;7:5024. doi: 10.1109/IEMBS.2004.1404388. [DOI] [PubMed] [Google Scholar]
- 243.Wang C. Yin S. Cen L. Liu Q. Liu W. Cao Y. Cui L. Differentiation of adipose derived stem cells into contractile smooth muscle cells induced by TGF-beta1 and BMP4. Tissue Eng Part A. 2010;16:1201. doi: 10.1089/ten.TEA.2009.0303. [DOI] [PubMed] [Google Scholar]
- 244.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 245.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Suzuki S. Narita Y. Yamawaki A. Murase Y. Satake M. Mutsuga M. Okamoto H. Kagami H. Ueda M. Ueda Y. Effects of extracellular matrix on differentiation of human bone marrow-derived mesenchymal stem cells into smooth muscle cell lineage: utility for cardiovascular tissue engineering. Cells Tissues Organs. 2009;191:269. doi: 10.1159/000260061. [DOI] [PubMed] [Google Scholar]
- 247.Owens G.K. Loeb A. Gordon D. Thompson M.M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986;102:343. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kim H.R. Gallant C. Leavis P.C. Gunst S.J. Morgan K.G. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am J Physiol Cell Physiol. 2008;295:C768. doi: 10.1152/ajpcell.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Duband J.L. Gimona M. Scatena M. Sartore S. Small J.V. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55:1. doi: 10.1111/j.1432-0436.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 250.Deissler H. Deissler H. Lang G.K. Lang G.E. TGFbeta induces transdifferentiation of iBREC to alphaSMA-expressing cells. Int J Mol Med. 2006;18:577. [PubMed] [Google Scholar]
- 251.Arciniegas E. Sutton A.B. Allen T.D. Schor A.M. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103(Pt 2):521. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- 252.Winder S.J. Walsh M.P. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990;265:10148. [PubMed] [Google Scholar]
- 253.Leinweber B. Parissenti A.M. Gallant C. Gangopadhyay S.S. Kirwan-Rhude A. Leavis P.C. Morgan K.G. Regulation of protein kinase C by the cytoskeletal protein calponin. J Biol Chem. 2000;275:40329. doi: 10.1074/jbc.M008257200. [DOI] [PubMed] [Google Scholar]
- 254.Leinweber B.D. Leavis P.C. Grabarek Z. Wang C.L. Morgan K.G. Extracellular regulated kinase (ERK) interaction with actin and the calponin homology (CH) domain of actin-binding proteins. Biochem J. 1999;344(Pt 1):117. [PMC free article] [PubMed] [Google Scholar]
- 255.Morgan K.G. Gangopadhyay S.S. Invited review: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol. 2001;91:953. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- 256.Miano J.M. Olson E.N. Expression of the smooth muscle cell calponin gene marks the early cardiac and smooth muscle cell lineages during mouse embryogenesis. J Biol Chem. 1996;271:7095. doi: 10.1074/jbc.271.12.7095. [DOI] [PubMed] [Google Scholar]
- 257.Burgstaller G. Kranewitter W.J. Gimona M. The molecular basis for the autoregulation of calponin by isoform-specific C-terminal tail sequences. J Cell Sci. 2002;115:2021. doi: 10.1242/jcs.115.10.2021. [DOI] [PubMed] [Google Scholar]
- 258.Yoshikawa H. Taniguchi S.I. Yamamura H. Mori S. Sugimoto M. Miyado K. Nakamura K. Nakao K. Katsuki M. Shibata N. Takahashi K. Mice lacking smooth muscle calponin display increased bone formation that is associated with enhancement of bone morphogenetic protein responses. Genes Cells. 1998;3:685. doi: 10.1046/j.1365-2443.1998.00214.x. [DOI] [PubMed] [Google Scholar]
- 259.Masuki S. Takeoka M. Taniguchi S. Nose H. Enhanced baroreflex sensitivity in free-moving calponin knockout mice. Am J Physiol Heart Circ Physiol. 2003;284:H939. doi: 10.1152/ajpheart.00610.2002. [DOI] [PubMed] [Google Scholar]
- 260.Masuki S. Takeoka M. Taniguchi S. Yokoyama M. Nose H. Impaired arterial pressure regulation during exercise due to enhanced muscular vasodilatation in calponin knockout mice. J Physiol. 2003;553:203. doi: 10.1113/jphysiol.2003.047803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Matthew J.D. Khromov A.S. McDuffie M.J. Somlyo A.V. Somlyo A.P. Taniguchi S. Takahashi K. Contractile properties and proteins of smooth muscles of a calponin knockout mouse. J Physiol. 2000;529(Pt 3):811. doi: 10.1111/j.1469-7793.2000.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Je H.D. Sohn U.D. SM22alpha is required for agonist-induced regulation of contractility: evidence from SM22alpha knockout mice. Mol Cells. 2007;23:175. [PubMed] [Google Scholar]
- 263.Lepore J.J. Cheng L. Min Lu M. Mericko P.A. Morrisey E.E. Parmacek M.S. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis. 2005;41:179. doi: 10.1002/gene.20112. [DOI] [PubMed] [Google Scholar]
- 264.Zhang J.C. Kim S. Helmke B.P. Yu W.W. Du K.L. Lu M.M. Strobeck M. Yu Q. Parmacek M.S. Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol Cell Biol. 2001;21:1336. doi: 10.1128/MCB.2001.21.4.1336-1344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Li L. Miano J.M. Cserjesi P. Olson E.N. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78:188. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 266.Faggin E. Puato M. Zardo L. Franch R. Millino C. Sarinella F. Pauletto P. Sartore S. Chiavegato A. Smooth muscle-specific SM22 protein is expressed in the adventitial cells of balloon-injured rabbit carotid artery. Arterioscler Thromb Vasc Biol. 1999;19:1393. doi: 10.1161/01.atv.19.6.1393. [DOI] [PubMed] [Google Scholar]
- 267.Sobue K. Sellers J.R. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991;266:12115. [PubMed] [Google Scholar]
- 268.Babu G.J. Warshaw D.M. Periasamy M. Smooth muscle myosin heavy chain isoforms and their role in muscle physiology. Microsc Res Tech. 2000;50:532. doi: 10.1002/1097-0029(20000915)50:6<532::AID-JEMT10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 269.Frid M.G. Printesva O.Y. Chiavegato A. Faggin E. Scatena M. Koteliansky V.E. Pauletto P. Glukhova M.A. Sartore S. Myosin heavy-chain isoform composition and distribution in developing and adult human aortic smooth muscle. J Vasc Res. 1993;30:279. doi: 10.1159/000159007. [DOI] [PubMed] [Google Scholar]
- 270.Babu G.J. Pyne G.J. Zhou Y. Okwuchukuasanya C. Brayden J.E. Osol G. Paul R.J. Low R.B. Periasamy M. Isoform switching from SM-B to SM-A myosin results in decreased contractility and altered expression of thin filament regulatory proteins. Am J Physiol Cell Physiol. 2004;287:C723. doi: 10.1152/ajpcell.00029.2004. [DOI] [PubMed] [Google Scholar]
- 271.Lauzon A.M. Tyska M.J. Rovner A.S. Freyzon Y. Warshaw D.M. Trybus K.M. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. J Muscle Res Cell Motil. 1998;19:825. doi: 10.1023/a:1005489501357. [DOI] [PubMed] [Google Scholar]
- 272.Babij P. Kawamoto S. White S. Adelstein R.S. Periasamy M. Differential expression of SM1 and SM2 myosin isoforms in cultured vascular smooth muscle. Am J Physiol. 1992;262:C607. doi: 10.1152/ajpcell.1992.262.3.C607. [DOI] [PubMed] [Google Scholar]
- 273.Kuro-o M. Nagai R. Nakahara K. Katoh H. Tsai R.C. Tsuchimochi H. Yazaki Y. Ohkubo A. Takaku F. cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and in arteriosclerosis. J Biol Chem. 1991;266:3768. [PubMed] [Google Scholar]
- 274.Miano J.M. Cserjesi P. Ligon K.L. Periasamy M. Olson E.N. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75:803. doi: 10.1161/01.res.75.5.803. [DOI] [PubMed] [Google Scholar]
- 275.Chi M. Zhou Y. Vedamoorthyrao S. Babu G.J. Periasamy M. Ablation of smooth muscle myosin heavy chain SM2 increases smooth muscle contraction and results in postnatal death in mice. Proc Natl Acad Sci U S A. 2008;105:18614. doi: 10.1073/pnas.0808162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rensen S.S. Thijssen V.L. De Vries C.J. Doevendans P.A. Detera-Wadleigh S.D. Van Eys G.J. Expression of the smoothelin gene is mediated by alternative promoters. Cardiovasc Res. 2002;55:850. doi: 10.1016/s0008-6363(02)00491-1. [DOI] [PubMed] [Google Scholar]
- 277.van Eys G.J. Niessen P.M. Rensen S.S. Smoothelin in vascular smooth muscle cells. Trends Cardiovasc Med. 2007;17:26. doi: 10.1016/j.tcm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 278.Niessen P. Clement S. Fontao L. Chaponnier C. Teunissen B. Rensen S. van Eys G. Gabbiani G. Biochemical evidence for interaction between smoothelin and filamentous actin. Exp Cell Res. 2004;292:170. doi: 10.1016/j.yexcr.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 279.Deruiter M.C. Rensen S.S. Coolen G.P. Hierck B.P. Bergwerff M. Debie W.M. Gittenberger-De Groot A.C. Van Eys G.J. Smoothelin expression during chicken embryogenesis: detection of an embryonic isoform. Dev Dyn. 2001;221:460. doi: 10.1002/dvdy.1156. [DOI] [PubMed] [Google Scholar]
- 280.Rensen S.S. Niessen P.M. van Deursen J.M. Janssen B.J. Heijman E. Hermeling E. Meens M. Lie N. Gijbels M.J. Strijkers G.J. Doevendans P.A. Hofker M.H. De Mey J.G. van Eys G.J. Smoothelin-B deficiency results in reduced arterial contractility, hypertension, and cardiac hypertrophy in mice. Circulation. 2008;118:828. doi: 10.1161/CIRCULATIONAHA.107.743690. [DOI] [PubMed] [Google Scholar]
- 281.Layne M.D. Endege W.O. Jain M.K. Yet S.F. Hsieh C.M. Chin M.T. Perrella M.A. Blanar M.A. Haber E. Lee M.E. Aortic carboxypeptidase-like protein, a novel protein with discoidin and carboxypeptidase-like domains, is up-regulated during vascular smooth muscle cell differentiation. J Biol Chem. 1998;273:15654. doi: 10.1074/jbc.273.25.15654. [DOI] [PubMed] [Google Scholar]
- 282.Layne M.D. Yet S.F. Maemura K. Hsieh C.M. Liu X. Ith B. Lee M.E. Perrella M.A. Characterization of the mouse aortic carboxypeptidase-like protein promoter reveals activity in differentiated and dedifferentiated vascular smooth muscle cells. Circ Res. 2002;90:728. doi: 10.1161/01.res.0000013289.97650.c8. [DOI] [PubMed] [Google Scholar]
- 283.Yoshida T. Gan Q. Shang Y. Owens G.K. Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am J Physiol Cell Physiol. 2007;292:C886. doi: 10.1152/ajpcell.00449.2006. [DOI] [PubMed] [Google Scholar]
- 284.Li S. Sims S. Jiao Y. Chow L.H. Pickering J.G. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ Res. 1999;85:338. doi: 10.1161/01.res.85.4.338. [DOI] [PubMed] [Google Scholar]
- 285.Sobue K. Hayashi K. Nishida W. Expressional regulation of smooth muscle cell-specific genes in association with phenotypic modulation. Mol Cell Biochem. 1999;190:105. [PubMed] [Google Scholar]
- 286.Hayashi K. Kanda K. Kimizuka F. Kato I. Sobue K. Primary structure and functional expression of h-caldesmon complementary DNA. Biochem Biophys Res Commun. 1989;164:503. doi: 10.1016/0006-291x(89)91748-8. [DOI] [PubMed] [Google Scholar]
- 287.Hayashi K. Fujio Y. Kato I. Sobue K. Structural and functional relationships between h- and l-caldesmons. J Biol Chem. 1991;266:355. [PubMed] [Google Scholar]
- 288.Lane E.B. Hogan B.L. Kurkinen M. Garrels J.I. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature. 1983;303:701. doi: 10.1038/303701a0. [DOI] [PubMed] [Google Scholar]
- 289.Kim S. Coulombe P.A. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21:1581. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- 290.Tapscott S.J. Bennett G.S. Toyama Y. Kleinbart F. Holtzer H. Intermediate filament proteins in the developing chick spinal cord. Dev Biol. 1981;86:40. doi: 10.1016/0012-1606(81)90313-4. [DOI] [PubMed] [Google Scholar]
- 291.Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980;283:249. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- 292.Osborn M. Intermediate filaments as histologic markers: an overview. J Invest Dermatol. 1983;81:104s. doi: 10.1111/1523-1747.ep12540811. [DOI] [PubMed] [Google Scholar]
- 293.Goldman R.D. Khuon S. Chou Y.H. Opal P. Steinert P.M. The function of intermediate filaments in cell shape and cytoskeletal integrity. J Cell Biol. 1996;134:971. doi: 10.1083/jcb.134.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang N. Stamenovic D. Mechanics of vimentin intermediate filaments. J Muscle Res Cell Motil. 2002;23:535. doi: 10.1023/a:1023470709071. [DOI] [PubMed] [Google Scholar]
- 295.Asada H. Paszkowiak J. Teso D. Alvi K. Thorisson A. Frattini J.C. Kudo F.A. Sumpio B.E. Dardik A. Sustained orbital shear stress stimulates smooth muscle cell proliferation via the extracellular signal-regulated protein kinase 1/2 pathway. J Vasc Surg. 2005;42:772. doi: 10.1016/j.jvs.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 296.Zhou W. Dasgupta C. Negash S. Raj J.U. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1459. doi: 10.1152/ajplung.00143.2006. [DOI] [PubMed] [Google Scholar]
- 297.Skalli O. Bloom W.S. Ropraz P. Azzarone B. Gabbiani G. Cytoskeletal remodeling of rat aortic smooth muscle cells in vitro: relationships to culture conditions and analogies to in vivo situations. J Submicrosc Cytol. 1986;18:481. [PubMed] [Google Scholar]
- 298.Gilles C. Polette M. Piette J. Delvigne A.C. Thompson E.W. Foidart J.M. Birembaut P. Vimentin expression in cervical carcinomas: association with invasive and migratory potential. J Pathol. 1996;180:175. doi: 10.1002/(SICI)1096-9896(199610)180:2<175::AID-PATH630>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 299.Singh S. Sadacharan S. Su S. Belldegrun A. Persad S. Singh G. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003;63:2306. [PubMed] [Google Scholar]
- 300.Kuro-o M. Nagai R. Tsuchimochi H. Katoh H. Yazaki Y. Ohkubo A. Takaku F. Developmentally regulated expression of vascular smooth muscle myosin heavy chain isoforms. J Biol Chem. 1989;264:18272. [PubMed] [Google Scholar]
- 301.Aikawa M. Sivam P.N. Kuro-o M. Kimura K. Nakahara K. Takewaki S. Ueda M. Yamaguchi H. Yazaki Y. Periasamy M., et al. Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res. 1993;73:1000. doi: 10.1161/01.res.73.6.1000. [DOI] [PubMed] [Google Scholar]
- 302.Itoh K. Adelstein R.S. Neuronal cell expression of inserted isoforms of vertebrate nonmuscle myosin heavy chain II-B. J Biol Chem. 1995;270:14533. doi: 10.1074/jbc.270.24.14533. [DOI] [PubMed] [Google Scholar]
- 303.Kawamoto S. Neuron-specific alternative splicing of nonmuscle myosin II heavy chain-B pre-mRNA requires a cis-acting intron sequence. J Biol Chem. 1996;271:17613. [PubMed] [Google Scholar]
- 304.Rochlin M.W. Itoh K. Adelstein R.S. Bridgman P.C. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108(Pt 12):3661. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- 305.Abouhamed M. Reichenberg S. Robenek H. Plenz G. Tropomyosin 4 expression is enhanced in dedifferentiating smooth muscle cells in vitro and during atherogenesis. Eur J Cell Biol. 2003;82:473. doi: 10.1078/0171-9335-00333. [DOI] [PubMed] [Google Scholar]
- 306.Gunning P. Gordon M. Wade R. Gahlmann R. Lin C.S. Hardeman E. Differential control of tropomyosin mRNA levels during myogenesis suggests the existence of an isoform competition-autoregulatory compensation control mechanism. Dev Biol. 1990;138:443. doi: 10.1016/0012-1606(90)90210-a. [DOI] [PubMed] [Google Scholar]
- 307.Helfman D.M. Berthier C. Grossman J. Leu M. Ehler E. Perriard E. Perriard J.C. Nonmuscle tropomyosin-4 requires coexpression with other low molecular weight isoforms for binding to thin filaments in cardiomyocytes. J Cell Sci. 1999;112(Pt 3):371. doi: 10.1242/jcs.112.3.371. [DOI] [PubMed] [Google Scholar]
- 308.Vlahovich N. Schevzov G. Nair-Shaliker V. Ilkovski B. Artap S.T. Joya J.E. Kee A.J. North K.N. Gunning P.W. Hardeman E.C. Tropomyosin 4 defines novel filaments in skeletal muscle associated with muscle remodelling/regeneration in normal and diseased muscle. Cell Motil Cytoskeleton. 2008;65:73. doi: 10.1002/cm.20245. [DOI] [PubMed] [Google Scholar]
- 309.Neuville P. Geinoz A. Benzonana G. Redard M. Gabbiani F. Ropraz P. Gabbiani G. Cellular retinol-binding protein-1 is expressed by distinct subsets of rat arterial smooth muscle cells in vitro and in vivo. Am J Pathol. 1997;150:509. [PMC free article] [PubMed] [Google Scholar]
- 310.Uchio K. Tuchweber B. Manabe N. Gabbiani G. Rosenbaum J. Desmouliere A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest. 2002;82:619. doi: 10.1038/labinvest.3780456. [DOI] [PubMed] [Google Scholar]
- 311.Xu G. Redard M. Gabbiani G. Neuville P. Cellular retinol-binding protein-1 is transiently expressed in granulation tissue fibroblasts and differentially expressed in fibroblasts cultured from different organs. Am J Pathol. 1997;151:1741. [PMC free article] [PubMed] [Google Scholar]
- 312.Orlandi A. Francesconi A. Clement S. Ropraz P. Spagnoli L.G. Gabbiani G. High levels of cellular retinol binding protein-1 expression in leiomyosarcoma: possible implications for diagnostic evaluation. Virchows Arch. 2002;441:31. doi: 10.1007/s00428-001-0576-7. [DOI] [PubMed] [Google Scholar]