Abstract
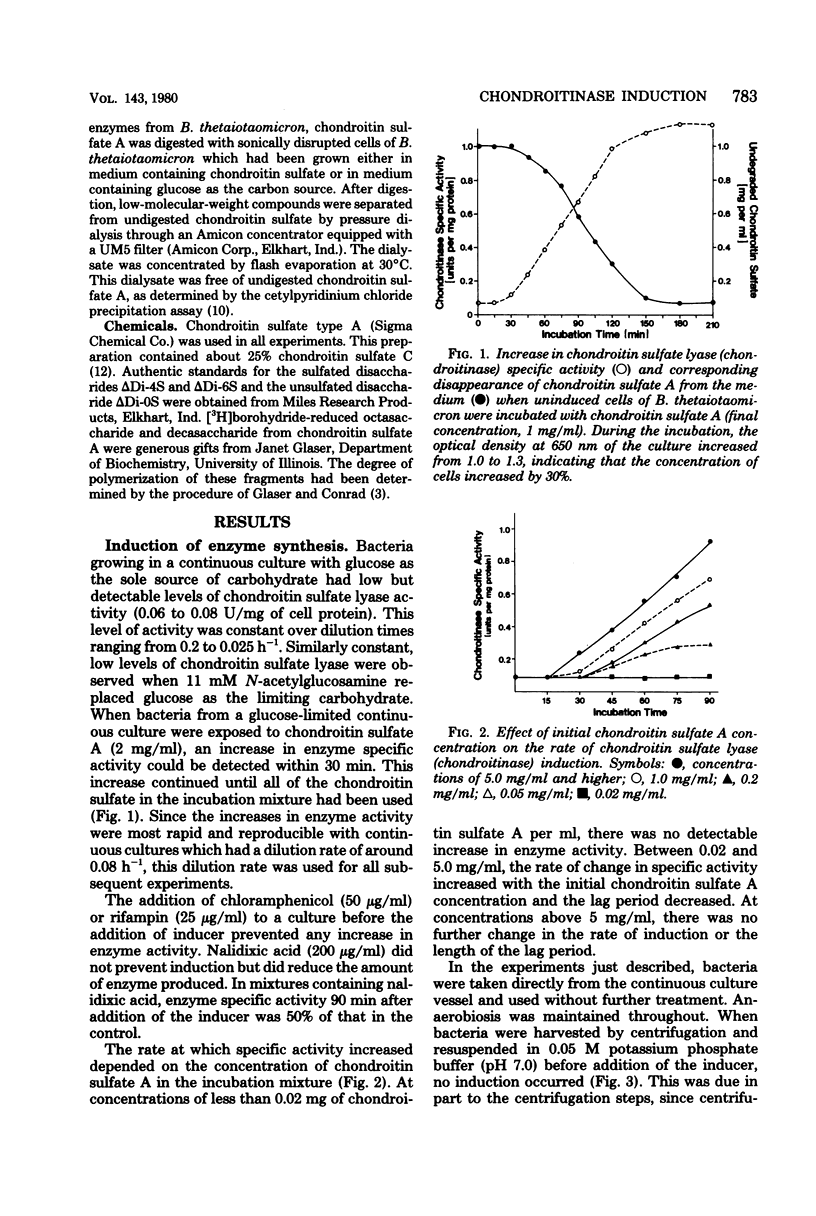
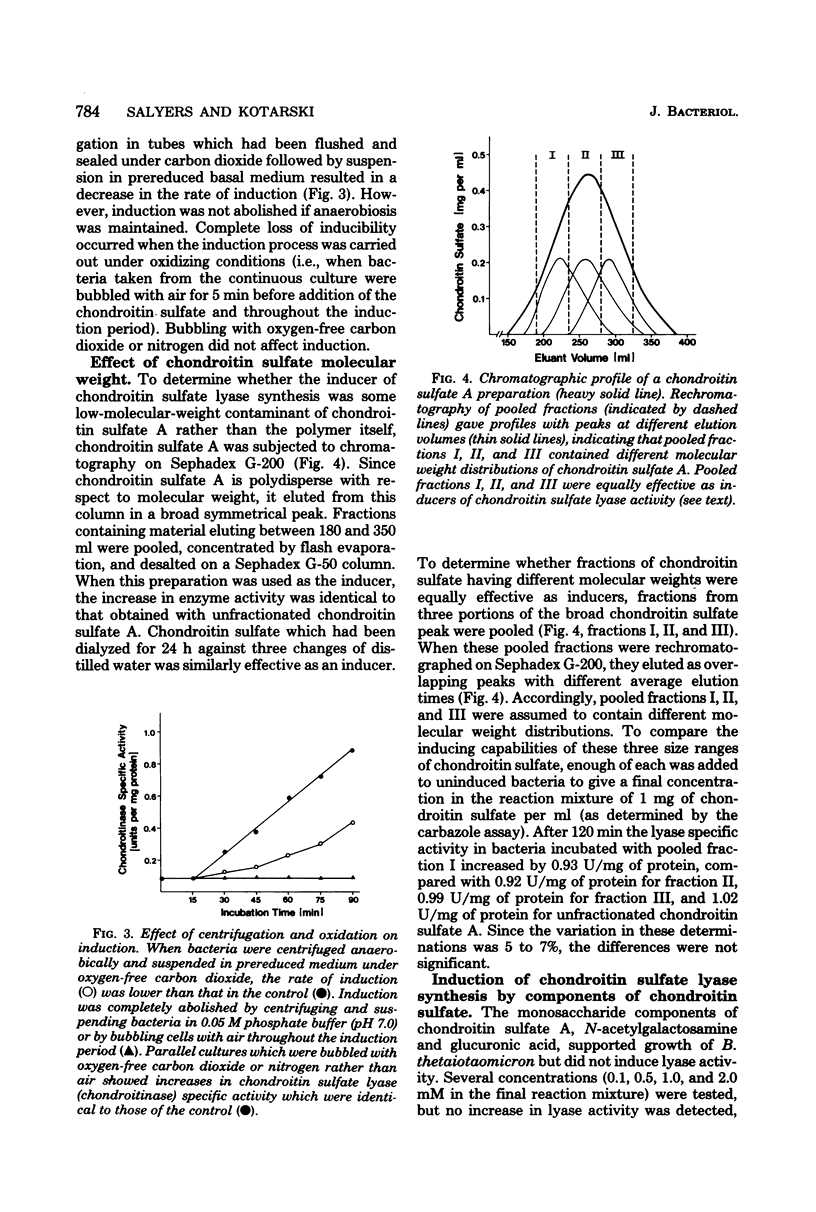
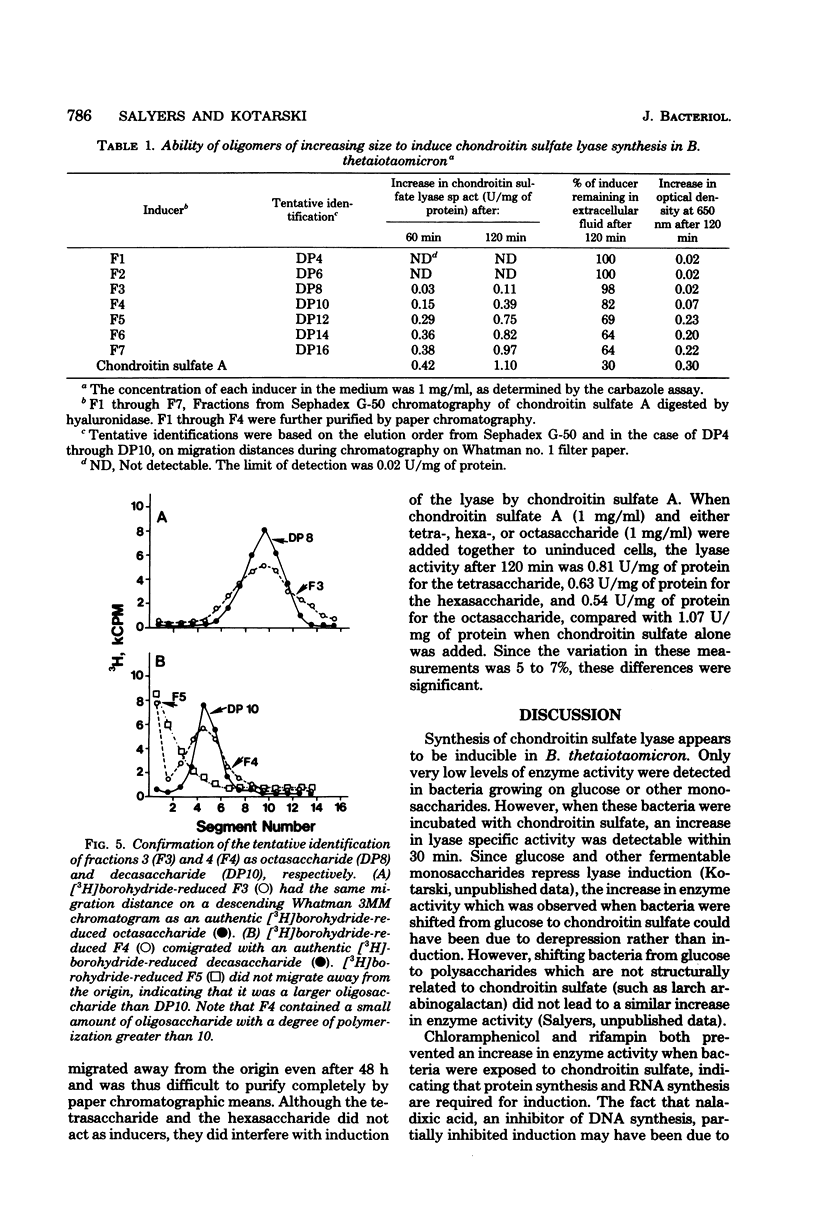
Chondroitin sulfate lyase (EC 4.2.2.4) was present constitutively at low levels (0.06 to 0.08 U/mg of protein) in cells of Bacteroides thetaiotaomicron which were growing on glucose or other monosaccharides. When these uninduced bacteria were incubated with chondroitin sulfate A (5 mg/ml), chondroitin sulfate lyase specific activity increased more than 10-fold within 90 min. Synthesis of ribonucleic acid and of protein was required for induction, and induction was sensitive to oxygen. The disaccharides which resulted from chondroitinase action did not act as inducers, nor did tetrasaccharides or hexasaccharides obtained by digestion of chondroitin sulfate with bovine testicular hyaluronidase. None of these substances was taken up by uninduced cells; they may not have been able to penetrate the outer membrane. The smallest oligomer capable of acting as an inducer was the outer membrane. The smallest oligomer capable of acting as an inducer was the octassacharide. Oligomers larger than the octassacharide induced chondroitin lyase activity nearly as well as intact chondroitin sulfate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Conrad H. E. Chondroitin SO4 catabolism in chick embryo chondrocytes. J Biol Chem. 1979 Apr 10;254(7):2316–2325. [PubMed] [Google Scholar]
- Glass T. L., Holmes W. M., Hylemon P. B., Stellwag E. J. Synthesis of guanosine tetra- and pentaphosphates by the obligately anaerobic bacterium Bacteroides thetaiotaomicron in response to molecular oxygen. J Bacteriol. 1979 Feb;137(2):956–962. doi: 10.1128/jb.137.2.956-962.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkewitz D., Iannotti E. L., Wolin M. J., Bryant M. P. An anaerobic chemostat that permits the collection and measurement of fermentation gases. Appl Microbiol. 1973 Apr;25(4):612–614. doi: 10.1128/am.25.4.612-614.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Salyers A. A. Energy sources of major intestinal fermentative anaerobes. Am J Clin Nutr. 1979 Jan;32(1):158–163. doi: 10.1093/ajcn/32.1.158. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Palmer J. K., Wilkins T. D. Laminarinase (beta-glucanase) activity in Bacteroides from the human colon. Appl Environ Microbiol. 1977 May;33(5):1118–1124. doi: 10.1128/aem.33.5.1118-1124.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]



