Abstract
The analysis of low abundance and low molecular weight biomolecules is challenging due to their labile nature and the presence of high abundance, high molecular weight species such as serum albumin, which can hinder their detection. Functionalized hydrogel particles have proven to be ideally suited for this application. We here report the synthesis of hydrogel core and core-shell particles with incorporated Cibacron Blue F3G-A, and analysis of their harvesting properties. Hydrogel particle scaffolds consisting of cross-linked N-isopropylacrylamide and allylamine copolymers were synthesized via surfactant-free precipitation polymerization, with the blue dye subsequently affixed via a nucleophilic substitution reaction. The dye-functionalized core and core-shell particles were found to efficiently harvest and sequester dilute low molecular weight peptides and proteins from solution, with the core-shell particles more effectively excluding larger proteins. Moreover, proteins bound by core and core-shell particles containing blue dye were protected from tryptic degradation. These findings suggest that core and core-shell hydrogel particles containing Cibacron Blue F3G-A constitute promising new tools for peptide/protein biomarker harvesting applications.
Keywords: hydrogels, responsive materials, biomarkers, affinity dyes, Cibacron Blue F3G-A
1. Introduction
There is a growing interest in the analysis of dilute and rare components of aqueous solutions and complex mixtures such as serum and other biological fluids. These low abundance, low-molecular weight biomolecules, broadly described as biomarkers, can provide insights into key physiological and pharmacological processes. Biomarkers have been identified in serum that are associated with diseases such as cancer [1], diabetes [2], and various cardiovascular and infectious diseases [3]. While the complexity of serum makes it a promising and rich resource of informative peptide and protein biomarkers, the presence of high molecular weight, high abundance proteins such as serum albumin and immunoglobulins, which combine to account for 55–97% of total serum protein, can hinder the detection and identification of potential low-molecular weight, low abundance proteins and peptides [4].
Because target biomarkers are present at very low concentrations in solution, current separation techniques such as two-dimensional gel electrophoresis and chromatography are not ideally suited for this application. For example, one- and two-dimensional gel electrophoresis, techniques commonly used to separate complex protein mixtures based on molecular weight, lack the sensitivity and resolution to detect trace amounts of protein. Affinity chromatography and other chromatography techniques can result in the depletion of small biomolecules that are tightly associated with other molecules present in the solution. Moreover, the discovery of new biomarkers by affinity chromatography and immunoassays is hampered due to lack of specific antibodies and affinity information for the target molecules. Currently, mass spectrometry is the foremost technique in serum proteomic diagnostics [4]. While the throughput and sensitivity of this mass spectrometry allows for the identification of proteins using peptide mass fingerprinting, this method requires purified target protein [5]. Because it is not the aim of mass spectrometry-based biomarker discovery to isolate one specific protein species [5], new tools that can efficiently separate and enrich a mixture of only the rare and low-molecular weight components from complex mixtures need to be developed.
Recently, hydrogel particles have garnered considerable attention as potential tools for the collection, concentration and preservation of low abundance, low-molecular weight peptides, proteins and other biomolecules [6]. Hydrogel particles, consisting of highly hydrated networks of cross-linked polymer chains, possess a considerable degree of flexibility and porosity, and their high water content allows small molecules access to the interior space of the particle. These qualities make hydrogel particles particularly attractive tools for collecting and concentrating dilute analytes from aqueous solutions [6,7]. Particles based on N-isopropylacrylamide (NIPAm) have been of particular interest due to their stability [6–8], versatility [8], reproducibility, and low cost of production. These acylamide-based hydrogel particles, which shrink and swell in response to changes in environmental conditions such as pH and temperature, are referred to as thermoresponsive hydrogels. Such particles have been extensively studied for drug delivery applications [8], and the knowledge obtained from these studies is applicable in engineering hydrogel particles to function as potential biomarker harvesting tools [9].
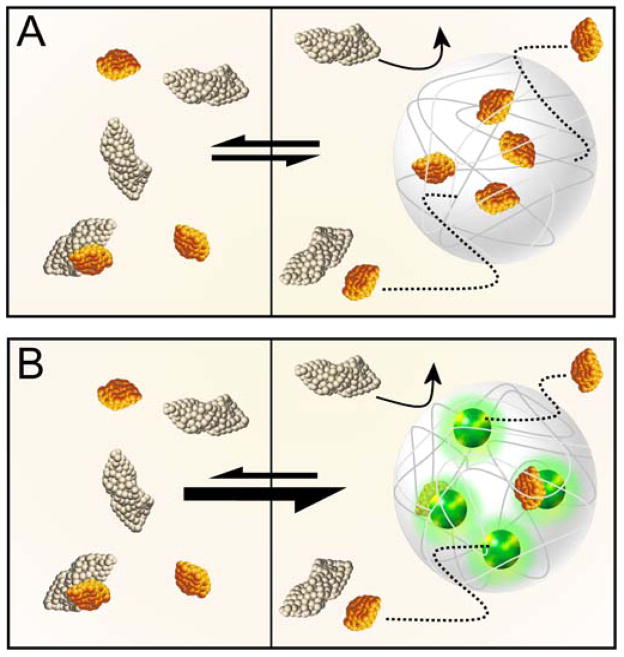
However, because the concentration of the targeted proteins and peptides within the particles is dependent on the equilibrium between the rates at which they enter and exit the particle and on their relative concentrations in solution, particles consisting of cross-linked p-NIPAm are diffusion-limited and are thus unable to effectively harvest and concentrate target proteins and peptides (Fig. 1A) [9]. To resolve this limitation, hydrogel particles have been generated that incorporate affinity baits to facilitate the capture of target proteins and to prevent them from escaping the particle (Fig. 1B) [8]. Cross-linked p-NIPAm hydrogel particles with the incorporated affinity bait acrylic acid (AAc) have been investigated for their ability to rapidly harvest blood-derived biomarkers for isolation and analysis [8]. The p-NIPAm-co-AAc particles have demonstrated the ability to rapidly, in one step, sequester, concentrate, and protect from enzymatic degradation low molecular weight proteins and peptides from serum, thereby allowing the analysis of low abundance labile biomarkers. Core-shell p-NIPAm-co-AAc particles have demonstrated the ability to sequester platelet derived growth factor (PDGF) from a solution containing the biomarker at non-detectable levels (less than 20 pg mL−1), and in turn concentrate the biomarker to levels detectable by ELISA and mass spectrometry [10]. Moreover, the p-NIPAm-co-AAc core-shell particles were also shown to protect the sequestered PDGF from tryptic degradation.
Fig. 1.
Sequestered low molecular weight species can diffuse out of underivatized hydrogel particles (A). Incorporation of an affinity bait within the particle matrix enables the particle to effectively retain the harvested low molecular weight species and prevent them from escaping the particle matrix (B).
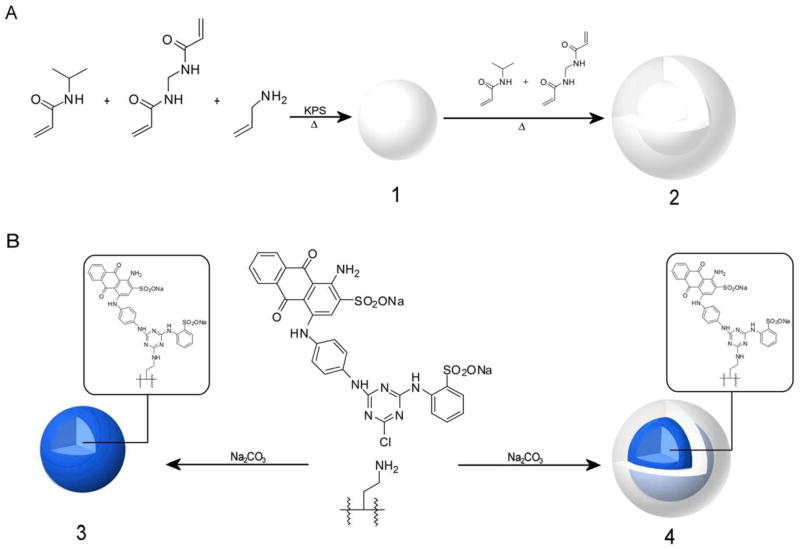
Hydrogel particles incorporating Cibacron Blue F3G-A, a triazinyl-based reactive dye, have also been investigated for their ability to harvest proteins and peptides from solution [11]. These hydrogel particles consisted of a cross-linked p-NIPAm copolymerized with allylamine (AA), which provided the scaffold onto which Cibacron Blue F3G-A was derivatized (Fig. 2A1-2). The reactive triazine dye was immobilized via direct reaction with the amine group of the allylamine units within the particles, displacing the lone chlorine on the disubstituted triazine ring of the dye (Fig. 2B3-4). These particles proved capable of capturing cadaveric human growth hormone (hGH) from synthetic and human urine. Elution of protein from the particles following extraction of hGH from a 0.05 ng mL−1 solution (in 1 mL of urine) afforded a solution with a final hGH concentration of 2 ng mL−1, which is a 40-fold increase in the hGH concentration [11].
Fig. 2.
Hydrogel particles with incorporated AA were generated to serve as the stationary scaffold onto which the reactive dye was immobilized (A1). Addition of a NIPAm and BIS mixture after the initial core polymerization yielded a core-shell particle consisting of a p-NIPAm-co-AA core encased within an inert p-NIPAm/BIS shell (A2). To immobilize the dye onto the particle matrix, a suspension of either the plain core (A1) or core-shell (A2) particles were mixed in an aqueous solution of dye and sodium carbonate and allowed to react for at least 48 h at room temperature (B). The dye bound to the particle through a direct covalent interaction between the amine group on the particle and the chlorine atom of the triazine ring (B3-4).
Reactive dyes are commonly used as ligands for affinity chromatography applications, due to their ability to reversibly bind a wide array of proteins with varied degrees of selectivity [12]. By mimicking the structures of natural substrates, these dyes demonstrate broad spectrum protein binding with particular specificities for dehydrogenases, reductases, kinases, and nucleases [12,13]. High production costs, stability concerns, and extensive purification steps make natural ligands, such as enzymes, antibodies, amino acids, and nucleic acids unappealing as affinity ligands or baits [12–15]. Affinity dyes are considered to be an appealing alternative to their natural counterparts because of their inexpensive cost, commercial availability, and ease of immobilization onto the supporting matrix [12].
In this present study, we have expanded on the functionality of dye-loaded particles through the addition of a shell to the derivatized p-NIPAm-co-AA core (Fig. 2A2). By encapsulating the dye-containing core within an inert shell, we introduce a shell-limited sieving functionality to the particle architecture. Here, we report on the synthesis of the dye-loaded core-shell particles and their physical properties and harvesting capabilities relative to dye-containing particles lacking the outer p-NIPAm shell.
2. Experimental
2.1. Materials
N-Isopropylacrylamide (NIPAm), N-N′-methylenebis(acrylamide) (BIS), potassium persulfate (KPS) and allylamine (AA) were purchased from Sigma-Aldrich, Corp., Cibacron Blue F3G-A was purchased from Polysciences, Inc. All reagents were used as received. 18% Tris-Glycine gels and Tris-Glycine SDS running buffer were purchased from Invitrogen Corporation. Water for all reactions, solution preparation, and polymer washing was distilled/purified using a Millipore Milli-Q water purification system to a resistance of 18 MΩ and passed through a 0.2 μm nylon filter.
2.2. p-NIPAm-co-AA core synthesis
p-NIPAm-co-AA particles with specified mmol percentages of AA relative to the total monomer were prepared via precipitation polymerization [11]. NIPAm (7.92 mmol, 0.89 g) and BIS (0.18 mmol, 0.03 g) were dissolved in 30 mL of dH2O, and the solution was then partially degassed by vacuum filtration through a 0.45 μm nylon filter. The filtered solution was purged with nitrogen at room temperature and a medium rate of stirring for 15 min, before AA (0.90 mmol, 0.05 g) was added to the reaction. Following the addition of AA, the solution was purged with nitrogen for another 15 min and then heated to 75°C. Once the reaction mixture had attained a stable temperature of 75°C, polymerization was initiated with the addition of KPS (4.30 × 10−2 mmol, 0.01 g) in 1.0 mL of dH2O. The reaction was maintained at a constant temperature of 75°C with stirring under nitrogen for 3 h. After this time, the reaction was allowed to cool to room temperature overnight with stirring under nitrogen. The particles were then harvested and washed via centrifugation (Eppendorf 5415R centrifuge). In this process, 1ml aliquots of particles were centrifuged for 20 minutes at 23°C and 16,100 relative centrifugal force (rcf), with the supernatant subsequently discarded. The pelleted particles were then resuspended in 1.0 mL dH2O, and the suspended particles pelleted by centrifugation. This centrifugation/redispersion process was repeated for a total of 5 times. Following the fifth centrifugation step, each aliquot of pelleted particles was resuspended in 1.0 mL of dH2O and stored as a suspension.
2.3. p-NIPAm-co-AA core-shell synthesis
The shell monomer solution was prepared by dissolving NIPAm (8.82 mmol, 0.99 g) and BIS (0.18 mmol, 0.03 g) in 30 mL of dH2O. The solution was partially degassed by vacuum filtration through a 0.45 μm nylon filter and purged with nitrogen for 2 h at room temperature with a medium stir rate.
The p-NIPAm-co-AA core solution was prepared by dissolving NIPAm (7.92 mmol, 0.89 g) and BIS (0.18 mmol, 0.03g) in 30 mL of dH2O, then partially degassed by vacuum filtration through a 0.45 μm nylon filter. The filtered solution was purged with nitrogen for 15 min at room temperature and a medium rate of stirring before adding AA (0.90 mmol, 0.05 g) to the reaction. The solution was purged with nitrogen for an additional 15 min and then heated to 75°C under nitrogen. Once the reaction mixture had attained a stable temperature of 75°C, polymerization was initiated with the addition of KPS (4.30 × 10−2 mmol, 0.01 g) in 1.0 mL of dH2O. The reaction was maintained at a constant temperature of 75°C with stirring under nitrogen for 3 h.
After the p-NIPAm-co-AA core reaction had stirred for 3 h at 75°C, the p-NIPAm shell solution was introduced into the reaction flask via addition funnel at a drop rate of 1 drop per second. After all of the p-NIPAm shell solution had been added, the reaction was maintained at 75°C under nitrogen with medium stirring for another 3 h. After this time, the reaction was allowed to cool to room temperature with stirring overnight under nitrogen. The particles were then harvested and washed via centrifugation as described above in the preparation of the p-NIPAm-co-AA core particles.
2.4. Incorporation of blue-dye in p-NIPAm-co-AA core and core-shell particles
The synthesis of dye-loaded p-NIPAm-co-AA particles has been previously described [11]. Similar conditions were used for the incorporation of blue dye in plain core and core-shell p-NIPAm-co-AA particles. The protocol described here addresses the incorporation of Cibacron Blue F3G-A in core-shell p-NIPAm-co-AA particles.
Cibacron Blue F3G-A (1.80 mmol, 1.51 g) was dissolved in 30 mL 0.1 M aqueous sodium carbonate. The p-NIPAm-co-AA core-shell suspension (30 mL volume) was purged with nitrogen for 15 min at room temperature with a medium stir rate in a 100 mL three-neck round-bottom flask, after which solid sodium carbonate (3.00 mmol, 0.32 g) was added. The alkaline particle suspension was allowed to stir at room temperature under nitrogen for 1 min, at which point, the Cibacron Blue F3G-A solution was added, and the combined reaction mixture allowed to continue stirring at room temperature under nitrogen for 48 h. After this time, 1ml aliquots of blue particles were centrifuged for 20 minutes at 23°C and 16,100 rcf. The supernatants were decanted and each tube of particles was redispersed in 1.0 mL of dH2O. This centrifugation/redispersion process was repeated until the supernatant was clear. The absorbance (610 nm) of the combined supernatants from the entire centrifugation/redispersion process was used to determine the concentration of Cibacron Blue F3G-A in the solution. Dye loading was then determined by comparing the total Cibacron Blue F3G-A in the combined supernatants to that of the parent solution, the difference corresponding to the number of moles of dye incorporated in the particles.
2.5. Light scattering
Average particle size and response to environmental conditions in aqueous solutions were determined at varied temperatures and pH values using photon correlation spectroscopy (N5 Submicron Particle Size Analyzer, Beckman Coulter). The measurement of the particles was performed using an equilibration time of 10 min and 200 s integration times. Water was used as the diluent (refractive index (RI) = 1.333, diluent viscosity = 0.890 cP), and the test angle for all light scattering experiments was 90°. Each measurement was performed in triplicate, and the average mean diameter for each was determined by converting the observed values to particle sizes via the Stokes-Einstein relationship [16].
2.6 Atomic force microscopy (AFM)
AFM was used to obtain information on the size distribution of the particles and determine homogeneity or the presence of particle subpopulations. All images and measurements of the particles were performed using an NSCRIPTOR DPN System (NanoInk) under AC mode with a silicon tip and a resonance frequency of 300 kHz. A 15 μL aliquot of a 1:10 dilution of the particles in dH2O was placed on a freshly cleaved piece of mica. The solution was incubated for 15 min in a humid atmosphere and allowed to dry at room temperature, after which the remaining residue was withdrawn from the edge of the mica using a soft tissue. One sample from each batch was randomly selected for scanning. Each sample was evaluated in triplicate.
2.7 Flow cytometry
Core and core-shell p-NIPAm-co-AA hydrogel particles with and without bound Cibacron Blue F3G-A were subjected to analysis by flow cytometry. Forward vs. side scatter histograms were obtained for particles using a FACScalibur flow cytometer (Becton Dickinson). For analysis, each particle suspension was diluted (10 mL particle suspension in 990 mL dH2O). Voltages and amplifier gains were E03 V and 1.00 for forward scatter and 667 V and 4.10 for side scatter, respectively. Approximately 50,000 events were acquired per sample.
2.8. Swelling studies
The swelling behavior of the dye-loaded core and core-shell particles was investigated as a function of dye incorporation. All swelling studies were conducted in triplicate at room temperature. The particles were first lyophilized to remove the water from the particle matrix, and then weighed (recorded as dry weight). The samples were then immersed in 1.0 mL Tris-HCl buffer (50 mM, pH 7.0) and gently agitated on a nutator for 3 days. After 3 days, the samples were centrifuged for 20 min at 16,100 rcf to separate the particles from the bulk aqueous medium. For each sample, the supernatant was removed and discarded. The resulting pellets were blotted quickly with a soft tissue to remove any remaining surface droplets, and weighed (recorded as wet weight). The degree of hydration of the particles, in terms of percentage of total mass of hydrated particles attributable to water, was calculated from the average masses of the particles dry (Wdry) and hydrated (Wwet) using Eq. (1) (below) [17]:
| (1) |
2.9. Protein binding and sieving performance
The protein binding and sieving properties of the dye-loaded particles were evaluated by incubating the particles in a protein solution consisting of a combination of molecular weight markers in order to test their protein binding and sieving performance. The protein solution consisted of 0.5 mg/mL of each of the following proteins: aprotinin (MW 6500 Da, pI 10.5, Sigma-Aldrich), lysozyme (MW 14,400 Da, pI 11.1, Sigma-Aldrich), trypsin inhibitor (MW 21,500 Da, pI 4.6, Invitrogen), carbonic anhydrase (MW 31,000 Da, pI 6.0, Sigma-Aldrich), ovalbumin (MW 45,000 Da, pI 4.6, Sigma-Aldrich), and BSA (MW 66,000 Da, pI 4.7, Fisher Scientific) dissolved in Tris-HCl buffer (pH 7, 100 mM). After the 1 h incubation, the samples were centrifuged at 16,100 rcf for 7 min to separate the particles from the aqueous medium. The pelleted particles were washed 3 times with 1.0 mL aliquots of water, and the captured proteins were electroeluted onto an SDS polyacrylamide gel (18% Tris-glycine, Invitrogen) at 200 V for 1 h. Silver staining was used to stain the gels.
Image analysis of each gel was performed by capturing the image of each gel with a PC scanner (HP ScanJet 5400c) and saving it as graphic files in JPEG format. Densitometric analysis was performed by means of a gel analysis software (ImageJ for Windows) and densitograms were then performed.
2.10. Protection from degradation
In order to test their ability to protect small protein species from enzymatic degradation, the dye-loaded core and core-shell particles were incubated for 1 h at 37°C in a NH4HCO3 (50 mM, pH 8.0) solution containing reduced and alkylated (RA) lysozyme (0.5 mg/mL, MW 14,400 Da, Sigma-Aldrich) and ovalbumin (0.5 mg/mL, MW 45,000 Da, Sigma-Aldrich) that have been digested with trypsin (0.025 mg/mL, MW 24,000 Da, pI 10.5, Promega). After 1 h, the samples were centrifuged at 16,100 rcf for 7 min to separate the particles from the aqueous medium. The pelleted particles were washed 3 times with 1.0 mL aliquots of water, and the sequestered proteins were electroeluted onto an SDS polyacrylamide gel (18%, Invitrogen) at 200 V for 1 h. Silver staining was used to develop the gels.
3. Results and discussion
3.1. p-NIPAm/Cibacron Blue F3G-A hydrogel particle synthesis and characterization
The successes and limitations of p-NIPAm-co-AAc core-shell particles at harvesting peptides and proteins from aqueous solutions suggest that particles containing affinity baits other than the carboxyl groups of AAc could also be generated for this function, and that these particles would likely demonstrate their own distinct harvesting properties. Affinity dyes, such as Cibacron Blue F3G-A, had previously been used in protein affinity chromatography, and the qualities that make Cibracron Blue F3G-A appealing for chromatographic applications also make it an attractive bait for incorporation into particles. Toward these ends, simple hydrogel particles based on cross-linked p-NIPAm with incorporated AA were generated for affinity dye immobilization (Fig. 2A1). Moreover, a second class of particles was generated that consisted of a cross-linked p-NIPAm-co-AA core encased within an inert p-NIPAm shell, so as to increase the sieving capabilities of the particles (Fig. 2A2). Both classes of particles were synthesized via precipitation polymerization in the absence of surfactant under a nitrogen atmosphere using KPS as the initiator and BIS as the cross-linker.
These two classes of AA-containing particles were used as the stationary scaffold onto which Cibacron Blue F3G-A was immobilized. The amino groups of the AA units present in the cross-linked copolymer provide a convenient avenue for chemically modifying the particles. In order to obtain the Cibacron Blue particles, the reactive dye was bound to the particles via a direct reaction between the reactive triazine rings of the dye molecules and the amino groups in the p-NIPAm-co-AA particles, displacing a chlorine group present in the reactive dye (Fig. 2B3-4). The number of moles of dye bound to the particles was ascertained by comparing the number of moles of unbound dye in the combined washes, determined spectrophotometrically, to the number present in the original reaction mixture. In order to determine the degree of dye incorporation, a small aliquot (200 μL) of the particles was lyophilized and weighed in order to obtain the dry weight of the sample. These values were in turn used, along with the total volume of particle suspension, to determine the dye density. By using this method, p-NIPAm-co-AA core particles with a nominal AA loading of 10 mmol % was estimated to have a dye loading of 1.78 mmol of dye per gram (mmol g−1) of dried particle. For the p-NIPAm-co-AA core-shell particles, which have a nominal AA loading of 10 mmol %, the dye loading was determined to be 0.74 mmol g−1.
3.1.1. Atomic force microscopy of allylamine and dye-loaded core and core-Shell particles
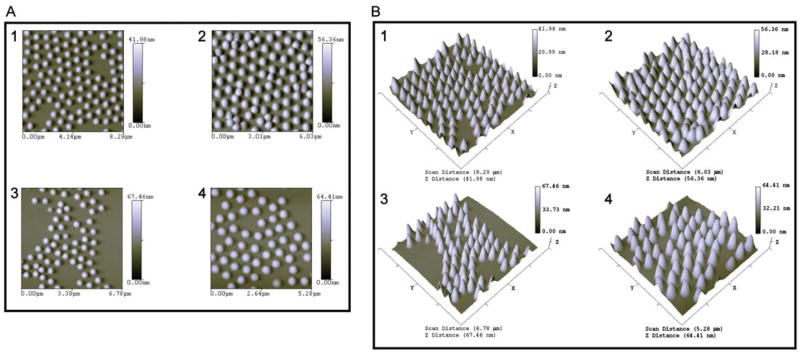
A series of images were obtained of the various preparations of hydrogel particles using atomic force microscopy (Fig. 3A-D). For both plain core and core-shell particles, atomic force microscopy shows homogenous particle populations with average diameters of approximately 300 nm for the p-NIPAm-co-AA core particles (Fig. 3A1) and 480 nm for the p-NIPAm-co-AA core-shell particles (Fig. 3A3). In comparison, average diameters for the dye-loaded core and core-shell particles are 340 nm for the core particles (Fig. 3A2) and 520 nm for the core-shell particles (Fig. 3A4). These diameters are slightly larger than the diameters exhibited by the p-NIPAm-co-AA core and core-shell parent particles, and are presumably due to the presence of the large dye molecules incorporated within the particle matrix. Based on the topography plots of the particle distribution, all of the nanoparticle samples show a relatively narrow size distribution (Fig. 3B1-4).
Fig. 3.
Atomic force microscopy shows a homogenous distribution of particle sizes for the plain AA core (A1) and core-shell (A3) particles, as well as for the dye-loaded core (A2) and core-shell (A4) particles. Topography plots of the plain AA core (B1), AA core-shell (B3), and the dye-loaded core (B2) and core-shell (B4) verify the homogenous particle size distribution.
It is important to note that the average diameters obtained by AFM are slightly smaller than those obtained through light scattering. However this discrepancy can be explained by differences in the sample preparation methodologies and experimental conditions. In the light scattering experiments, the particles samples were in aqueous suspensions. In contrast, samples were placed on a mica surface and excess moisture was removed prior to scanning with the AFM, and the overall decrease in the average measured diameters of the particles observed in AFM could be attributed to the absence of the closely associated water molecules that surround the particles in aqueous suspensions and/or the loss of water from within the particle matrix due to evaporation during the preparation of the samples.
3.1.2. Temperature dependence
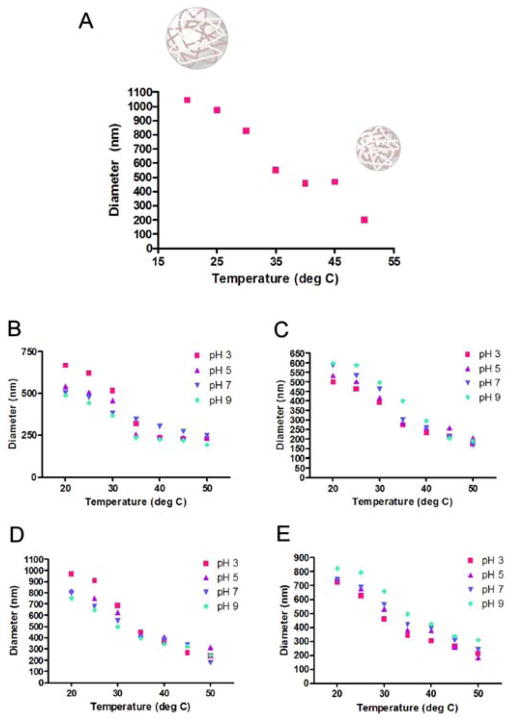
Hydrogels based on NIPAm polymers and copolymers shrink and swell in response to changes in temperature, and are therefore referred to as being thermoresponsive. Light scattering was used to record the temperature dependence of the plain and dye-incorporated p-NIPAm-co-AA core and core-shell particles in water (pH 5.0) over a temperature range of 20–50°C. The results of these studies are given in Fig. 4. While the results indicate that the sizes of the particles decrease with increasing temperature, which is characteristic of thermoresponsive hydrogels, two distinct thermoresponsive behavior profiles can be distinguished from the plots. Similar to the underivatized p-NIPAm particles (Fig. 4A), the p-NIPAm-co-AA core (Fig. 4B) and core-shell particles (Fig. 4D) show a clear size decrease at temperatures above the lower critical solution temperature (LCST). On the other hand, the p-NIPAm-co-AA core (Fig. 4C) and core-shell particles (Fig. 4E) with incorporated affinity dye exhibited a less dramatic volume phase transition than that of the plain core and core-shell particles. The behavior exhibited by the dye-loaded core and core-shell particles with respect to the p-NIPAm-co-AA particles lacking immobilized dye could be reflect the differences in their solvation properties due to the presence of the incorporated dye molecules. For the p-NIPAm-co-AA core particles, the maximum average diameter of 541.8 nm measured at 20°C decreased to a minimum average diameter of 231.1 nm measured at 50°C. For the dye-incorporated p-NIPAm-co-AA core particles, the maximum average diameter of 535.8 nm at 20°C decreased to a minimum average diameter of 205.7 nm at 50°C. The average diameter of p-NIPAm-co-AA core-shell particles is larger than that of the plain p-NIPAm-co-AA core particles due to the presence of the inert p-NIPAm shell. For the p-NIPAm-co-AA core-shell particles, the maximum average diameter of 820.8 nm measured at 20°C decreased to a minimum average diameter of 313.5 nm at 50°C. The core of the p-NIPAm-co-AA core-shell particles had a maximum average diameter of 458.9 nm at ambient temperature, which suggests that the p-NIPAm-co-AA core-shell particles have a shell with an approximate thickness of 180.9 nm. For the dye-incorporated p-NIPAm-co-AA core-shell particles, the maximum average diameter of 751.2 nm measured at 20°C decreased to a minimum average diameter of 183.7 nm at 50°C. This observed trend might reflect differences in solvent and interactions within the particle that are associated with the introduction of the dye groups.
Fig. 4.
Thermoresponsive hydrogels show a typical phase transition, beginning at approximately 32°C (A). At this LCST, the hydrogel particle begins the transition from a swollen, hydrophilic state to hydrophobic state. Diameter measurements obtained by DLS showed the characteristic thermoresponsive and pH-responsive behavior for the plain AA core (B) and core-shell (D) particles. The environmentally responsive behavior exhibited by the dye-loaded core (C) and core-shell particles (E) is different from that of the corresponding AA particles.
3.1.3. pH dependence
In prior studies, core-shell p-NIPAm-co-AAc particles have been shown to respond not only to changes in temperature, but also to increases or decreases in the pH of the solution in which they are suspended. Therefore, photon correlation spectroscopy was also used to determine the pH dependence of the plain and dye-loaded p-NIPAm-co-AA core and core-shell particle sizes. Measurements were taken for particles in water adjusted to pH values of 3.0, 5.0, 7.0, and 9.0, at temperatures ranging from 20 to 50°C.
At low pH (3.0), the majority of the AA groups of the p-NIPAm-co-AA core (Fig. 4B) and core-shell (Fig. 4D) particles are in their protonated state. Protonation of the AA groups results in two events. First, the high positive charge density results in increased charge-charge repulsion between the cross-linked polymer chains of the particle, thereby contributing to the swelling behavior exhibited in the graphs (Fig. 4B and D). Second, protonation of the AA groups leads to a phenomenon known as the Donnan equilibrium, in which solvated counterions enter the particle matrix in order to maintain the electroneutrality [17]. Both events result in an increase in the degree of hydration of the particles, causing it to swell and expand in size. These results are consistent with previous studies reporting the effects of pH and temperature on hydrogel particles containing ionizable groups [8, 17]. At pH values above 3.0 (e.g. pH 5.0 and 7.0), the p-NIPAm-co-AA core (Fig. 4B) and core-shell particles (Fig. 4D) display a decreasing trend in the degree of hydration, which is probably due to the decrease in the fraction of protonated AA groups on the particle matrix. At pH 9.0, which is close to the pKa of allylamine (the pKa for allylamine is reported to be 9.69 [18]), the nonionized amine groups are more hydrophobic and are more likely to interact favorably with each other and with other groups in the polymer scaffold, resulting in the “collapse” of the particle backbone and a decrease in the particle size.
Because it contains multiple acidic sulfonate groups and basic amino groups, the acid-base properties of Cibacron Blue F3G-A are more complex. Therefore, incorporation of the dye into the p-NIPAm-co-AA core (Fig. 4C) and core-shell particles (Fig. 4E) would be expected to alter the acid-base and solvation properties of the particles. Unlike the p-NIPAm-co-AA parent particles, the particles containing Cibacron Blue F3G-A decrease in size under acidic conditions (pH 3). The results (Fig. 4C and E) indicate that the dye-incorporated particles do not exhibit as great a pH dependence as do the p-NIPAm-co-AA parent particles. This is consistent with the hypothesis that the low pKa of the sulfonate groups (the typical pKa is reported to be less than zero [19]) will not be affected over the range of pH values selected for this study, while secondary amines will be protonated at lower pH conditions. It is likely that protonation of the amino groups will partially neutralize the negatively charged sulfonate groups, thereby decreasing their contribution to particle swelling.
3.1.4. Light scattering measurements of allylamine and dye-loaded core and core-shell particles by flow cytometry
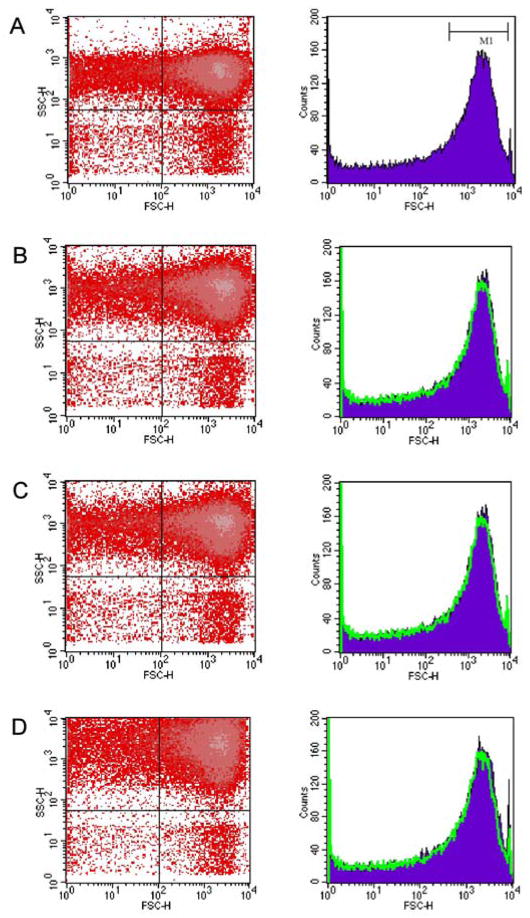
The ability of flow cytometry to rapidly make measurements and sort subpopulations based on surface properties makes it a useful method for microbiology and nanotechnology applications, and is commonly employed in the physical characterization of individual cells/particles. The technique involves a hydrodynamically focused stream of a cell/particle suspension flowing through a focused beam of light. As each individual cell/particle passes through the beam, light will be scattered in all directions. The scattered light is then collected by a forward scatter detector and a side scatter detector, with the detectors generating electronic signals that are proportional to light intensity. To evaluate the light scattering properties of core and core-shell particles, samples were introduced into the flow cytometer, which produced side scatter (SSC-H) vs. forward scatter (FSC-H) histograms for each particle species.
The differences in the scattered light intensities of the various particles are shown in Fig. 5, and these results correlate favorably with the theoretical predictions based on results obtained from photon correlation spectroscopy. The intensity of the acquired events is presented in the histogram FSC-H height curve, and the curve of the p-NIPAm-co-AA core particles, which serves as the reference, is overlaid on it (Fig. 5, right). Displayed on all of the histograms is the M1 region, which is defined as the background region, and contains all of the regular emission events [20]. Because the forward scatter is approximately proportional to the particle size, it is possible to approximate the size distribution of the particles. For the plots shown in Fig. 5, the four samples analyzed showed a narrow distribution of particle sizes, which reflect the results obtained from the photon correlation spectroscopy experiments.
Fig. 5.
Light scattering measurements (SSC-H vs. FSC-H dot plots and FSC-H histogram plots) for plain AA core (A) and core-shell (C) particles, and the dye-loaded core (B) and core-shell (D) particles.
Additional information about the surface properties of the particles can be acquired from the dot plots (SSC-H vs. FSC-H), shown in Fig. 5 (left). Each dot represents one event, with their location on the plot defined by two values, SSC-H and FSC-H. The side scatter parameter detects the light that is scattered by the particles. Because side scatter light intensity is proportional to surface complexity, this parameter can provide information about the structural and surface complexities of the particle [20]. Therefore, a dot plot that shows an increase in the side scatter intensity suggests particles with complex structures. The forward scatter parameter is roughly proportional to the size of the particle; the higher the forward scatter intensity, the greater the particle volume [20, 21]. The displayed dot plot of the plain AA core particles (Fig. 5A) showed a narrow distribution in particle morphology and surface properties, with 57.6% of the particle population found in the upper-right quadrant of the plot. However, the dye-loaded core particles (Fig. 5B, left) showed 2-fold increase in the side scatter intensity and a slight increase in the forward scatter intensity relative to the reference in the plot region occupied by the p-NIPAm-co-AA particles. The particle size population for the dye-loaded core particles (Fig. 5B) was relatively narrow, with 60.88% of the population occupying the upper-right quadrant of the plot. The p-NIPAm-co-AA core-shell particles (Fig. 5C), with 63.59% of the population occupying the upper-right quadrant of the plot, showed approximately 2–3 times greater side scatter intensity and a 1-fold increase in the forward scatter intensity relative to the reference. The dye-loaded core-shell particles (Fig. 5D) showed a uniform size distribution, with 60.72% of the population located in the upper-right-hand region of the plot. These particles displayed a slight increase in the forward scatter intensity and an increase in the side scatter intensity over 4 times greater than that of the reference.
Based on these results, all four particle subtypes exhibited a narrow size population, indicating a homogenous particle size distribution. Dot plots of the dye-loaded core and core-shell particles suggest that the presence of the dye increases the complexity of the particle structure, which may relate in some way to the distinct thermoresponsive profiles observed from the photon correlation spectroscopy experiments for the dye-loaded core and core-shell particles (Fig. 4C and E).
3.1.5. Swelling behavior
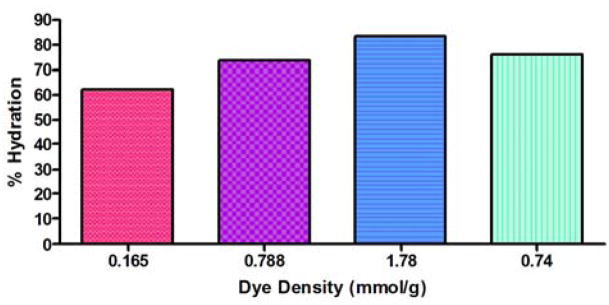
A unique property of hydrogels is their ability to swell isotropically, in which the hydrogel matrix is able to keep its original shape during and after swelling [22]. For the present study, hydrogel particles with varied loadings of incorporated Cibacron Blue F3G-A were generated (Table 1). Aliquots of each particle type were lyophilized in triplicate to obtain the dry weight. The samples were then resuspended in a 50 mM Tris-HCl buffer solution of pH 7.0 and allowed to equilibrate over a period of three days. As seen in Fig. 6, the degree of hydration is linked to dye incorporation, increasing with increased dye content. The degree of hydration increased from 62.1 % to 73.8% as the dye loading was increased from 0.165 mmol g−1 to 0.788 mmol g−1. Furthermore, the degree of hydration increased to 83.4% in the particles with a loading of 1.78 mmol g−1. The dye-incorporated core-shell particles, which have a total dye loading of 0.74 mmol g−1, were determined to have a 76.0% degree of hydration. Because the dye loading for the core-shell particles was similar to that of the dye-loaded core particles with 0.788 mmol g−1, similar degrees of hydration between the two particle types were expected. These results suggest that the presence of the sulfonate and amine groups on Cibacron Blue F3G-A increase solvation of the dye-loaded core and core-shell particles [23].
Table 1.
Comparison of the theoretical dye incorporation based on theoretical AA incorporation on the particle matrix to actual dye loading in the particles.
| Theoretical (mmol%) | Dye loading (mmol g−1) |
|---|---|
| 1% core | 0.165 |
| 5% core | 0.788 |
| 10% core | 1.780 |
| 8% core-shell | 0.740 |
Fig. 6.
Extent of hydration of the particles was dependent on the amount of dye incorporated into the particle matrix.
3.2. Molecular sieving and protection ability of the hydrogel particles
3.2.1. Sieving performance
Core-shell p-NIPAm-co-AAc particles have demonstrated the ability to bind and concentrate low molecular weight proteins from complex solutions. Similarly, p-NIPAm-co-AA based particles have been used to collect human growth hormone from urine [11]. In order to more thoroughly investigate the effects that incorporation of blue dye and encapsulation within a p-NIPAm shell have on the protein uptake properties of p-NIPAm-co-AA particles, assorted particles were incubated for 1 h in solutions containing a defined combination of reference proteins. SDS gel electrophoresis was then used to evaluate both protein uptake and the molecular sieving properties of the dye-loaded particles. Of particular interest, was the efficiency with which the particles captured and concentrated protein species with molecular weights of approximately 20,000 Da or less. This size range corresponds to that of physiologically important and informative peptides and proteins present in biological fluids [8]. Unfortunately, the presence of high abundant proteins such as albumin makes it extremely challenging to separate lower molecular weight species using traditional proteomic methods [24]. Therefore, if hydrogel particles are to be useful for this application, it is not sufficient that they simply bind these low molecular weight species. They must also exclude larger proteins that may be present.
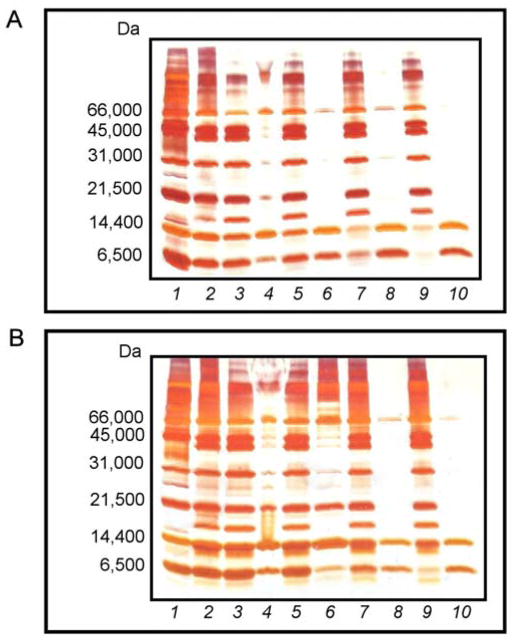
Densitometric analysis of the protein bands in the silver-stained gels indicates that the extent of dye loading plays an essential role in the harvesting performance of the particles (Fig. 7A). The peak areas, expressed in arbitrary units of optical density, were used to quantify the degree of protein harvesting by the particles. After incubation in the protein solution, core particles with a dye loading of 0.165 mmol g−1 exhibited multiple bands by SDS-PAGE (Fig. 7A, Lane 4). The pattern for the 0.165 mmol g−1 dye-loaded core particles showed two bands corresponding to lysozyme (14,400 Da) and aprotinin (6500 Da) with peak areas of 4,815.406 and 2513.456, respectively. However, the presence of intermediate bands (with peak areas of 444.263 and 1209.506) in the 21,500–45,000 Da molecular weight regions and one prominent band with a peak area of 2342.042 in the 66,000 Da molecular weight region is suggestive of considerable nonspecific binding of BSA (66,000 Da) and other proteins greater than 20,000 Da in size. An increase in the dye content of the core particles from 0.165 mmol g−1 to 0.788 mmol g−1 resulted in an 84.9% increase in aprotinin binding and a 59.6% decrease in BSA (66,000 Da) binding (Fig. 7A, Lane 6). Increasing the dye loading of the core particles from 0.165 mmol g−1 to 1.78 mmol g−1 resulted in a 147% increase in aprotinin binding and a 54.4% decrease in BSA binding, demonstrating superior harvesting and sieving performance (Fig. 7A, Lane 8). Similar analysis of harvesting by core-shell particles, with a dye loading of 0.740 mmol g−1, revealed only two prominent bands, one at 14,400 and the other at 6500 Da (Fig. 7A, Lane 10). The particles bound 95.5% more aprotinin than the core particles with 0.165 mmol g−1 dye loading, corresponding to a 20.7% decrease in the harvesting of aprotinin relative to core particles with a dye loading of 1.78 mmol g−1. More importantly, the core-shell particles showed no detectable binding of larger proteins such as BSA. All of the particles tested demonstrated comparable lysozyme binding. The ability of the dye-loaded core-shell particles to completely exclude all BSA binding demonstrates the more stringent sieving of the core-shell dye-loaded particles.
Fig. 7.
(A) SDS-PAGE analysis of dye-loaded particles incubated with MW markers: (1) MW ladder; (2) model protein solution; (3) supernatant from 0.165 mmol g−1 dye-loaded core particles; (4) bound by 0.165 mmol g−1 dye-loaded core particles; (5) supernatant from 0.788 mmol g−1 dye-loaded core particles; (6) bound by 0.788 mmol g−1 dye-loaded core particles; (7) supernatant from 1.78 mmol g−1 dye-loaded core particles; (8) bound by 1.78 mmol g−1 dye-loaded core particles; (9) supernatant from 0.74 mmol g−1 dye-loaded core-shell particles; and (10) bound by 0.74 mmol g−1 dye-loaded core-shell particles. (B) SDS-PAGE analysis comparing harvesting performance of AA and dye-loaded particles incubated with MW markers: (1) MW ladder; (2) model protein solution; (3) supernatant from plain AA core particles; (4) bound by plain AA core particles; (5) supernatant from plain AA core-shell particles; (6) bound by plain AA core-shell particles; (7) supernatant from 1.78 mmol g−1 dye-loaded core particles; (8) bound by 1.78 mmol g−1 dye-loaded core particles; (9) supernatant from 0.74 mmol g−1 dye-loaded core-shell particles; and (10) bound by 0.74 mmol g−1 dye-loaded core-shell particles.
In order to further demonstrate the exceptional harvesting ability associated with the dye-loaded core-shell particles and the sieving performance of the shell component of the particle architecture, particles were incubated for 1 h in solutions containing a defined mixture of proteins, as described earlier (Fig. 7B). Even though the resulting gel pattern of the p-NIPAm-co-AA core particles showed two bands corresponding to proteins with molecular weights of 14,400 and 6500 Da with peak areas of 5826.196 and 4123.991, the presence of a band with a peak area of 2538.577 in the 66,000 Da molecular weight region is indicative of significant nonspecific BSA binding (Fig. 7B, Lane 4). The p-NIPAm-co-AA core-shell particles exhibited similar protein binding behavior to the plain p-NIPAm-co-AA core particles (Fig. 7B, Lane 6). In comparison to the p-NIPAm-co-AA core particles, the dye-loaded core particles bound 63.8% less BSA, while capturing 23.4% less aprotinin and 41.9% less lysozyme (Fig. 7B, Lane 8). For the dye-loaded core-shell particles, the BSA binding was reduced by 90.9% with respect to the p-NIPAm-co-AA core particles, constituting a 74.7% improvement over dye-loaded particles lacking the shell (Fig. 7B, Lane 10). These results suggest that the incorporated blue dye contributes to the exclusion of high molecular weight proteins present in the test solution. Similar behavior was observed in the studies described above that were focused on the impact of dye loading on particle performance (Fig. 7A). The superior sieving capabilities of the dye-loaded core-shell particles are consistent with the results illustrated in Fig. 7A, and they indicate that the inert p-NIPAm shell encapsulating the particle used in combination with the incorporation of Cibacron Blue results in near complete exclusion of BSA and other high molecular weight species in the test solution. Furthermore, the dye-loaded core-shell particles demonstrated slightly improved harvesting of low molecular weight proteins relative to dye-loaded particles lacking the shell in this experiment, capturing 14.3% more lysozyme and 35.0% more aprotinin. These results reaffirm that the presence of the inert polymer shell did not negatively impacting the harvesting performance for proteins with MW of 14,400 Da or smaller.
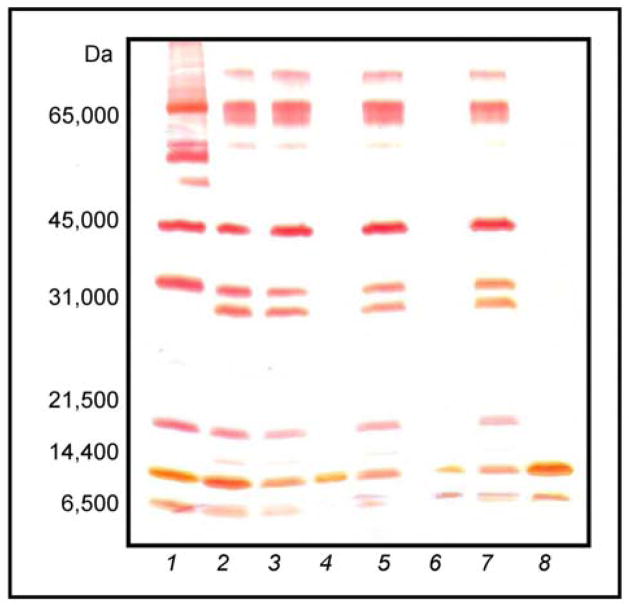
In order to assess the relationship between degree of dye loading and the sieving/harvesting performance of particles with a core-shell architecture, core-shell particles with varying degrees of dye loading (0.0821, 0.363, 0.740 mmol g−1) were incubated in the protein solution for 1 h (Fig. 8). The presence of the inert shell provided all three dye-loaded core-shell particles exceptional sieving capability. Variation in the degree of dye loading has a direct effect on the harvesting performance of the particles. When analyzed by SDS-PAGE, core-shell particles with a dye loading of 0.0821 mmol g−1 showed bands corresponding to aprotinin and lysozyme with respective peak areas of 4546.062 and 162.092 (Fig. 8, Lane 4). In contrast, core-shell particles with a dye loading of 0.363 mmol g−1 showed complete exclusion of BSA and revealed a 14-fold increase in aprotinin binding (Fig. 8, Lane 6). These particles demonstrated a decrease in the uptake of lysozyme (approximately 50.1%) relative to particles with a dye loading of 0.0821 mmol g−1. The core-shell particles with a dye loading of 0.740 mmol g−1 demonstrated better harvesting performance, sequestering 142% more lysozyme and exhibiting a 20-fold increase in aprotinin binding relative to core-shell particles with a dye loading of 0.0821 mmol g−1, which corresponds to a 385% increase in lysozyme binding and a 44.4% increase in aprotinin binding, relative to the core-shell particles with a dye loading of 0.363 mmol g−1 (Fig. 8, Lane 8). These particles also demonstrated complete exclusion of BSA.
Fig. 8.
SDS-PAGE analysis of dye-loaded core-shell particles incubated with MW markers: (1) MW ladder; (2) model protein solution; (3) supernatant from 0.0821 mmol g−1 dye-loaded core particles; (4) bound by 0.0821 mmol g−1 dye-loaded core-shell particles; (5) supernatant from 0.363 mmol g−1 dye-loaded core-shell particles; (6) bound by 0.363 mmol g−1 dye-loaded core-shell particles; (7) supernatant from 0.740 mmol g−1 dye-loaded core-shell particles; (8) bound by 0.740 mmol g−1 dye-loaded core-shell particles.
It is important to note, that while silver staining provides sensitivity that is superior to traditional staining methods using coomassie brilliant blue, it is not as amenable to consistent quantitative densitometric analysis and cannot be readily used to quantitatively compare different proteins on the same gel. That being said, the results of these studies clearly indicate that increased dye loading (within the range of loadings tested) translates to improved harvesting efficiency for low molecular weight peptides and proteins. These studies also show that the presence of the inert p-NIPAm shell, used in combination with the incorporation of Cibacron Blue dye, significantly enhanced the ability of the particles to exclude higher molecular weight proteins, such as BSA, without negatively impacting their harvesting performance for proteins with MW of 14,000 Da or smaller. Furthermore, the results illustrated in Fig. 7A and B suggest that the incorporated blue dye contributes to the ability of the particles to effectively exclude higher molecular weight proteins, although whether this is the due to electrostatics, crowding or both is not clear.
Because the particles are in a liquid suspension, the pores are not readily accessible to mercury or nitrogen, making traditional methods such as mercury porosimetry and nitrogen adsorption, impractical for this application [25]. Therefore, the size exclusion effect was used as an indirect means of estimating the minimum pore size of the particles. By assuming that the protein is a single globular domain, the radius of the protein can be calculated using Eq. (2):
| (2) |
where Rmin is the minimum radius in nm of a protein of a given mass M in Da [26]. Based on the observed molecular weight cutoff (MWCO) (Fig. 7A and B) and the molecular weights of trypsin and lysozyme, it is possible to calculate a rough approximation of pore size for the particles using Eq. (2). In this fashion, the core-shell blue hydrogel particles were determined to have pores between 3.2 and 3.9 nm in diameter. Furthermore, the relative uniformity of the molecular weight cutoff observed in the gels (Fig. 7A and B) suggests a narrow distribution in the pore sizes of the particles.
3.2.2. Protection from proteolytic degradation
In our previous work, we demonstrated that p-NIPAm-co-AAc core-shell particles were able to bind small peptides and protect them from proteolytic degradation. Therefore, the ability of simple and core-shell blue dye particles to protect sequestered proteins from proteolytic degradation was similarly evaluated. For these studies, reduced and alkylated derivatives of the model proteins, lysozyme and ovalbumin, were used because these derivatives are more susceptible to tryptic digestion than are their oxidized counterparts. Analysis of the sequestered proteins by SDS-PAGE after incubation for 1 h in the presence of trypsin indicated that the particles had shielded the small bound proteins, in this case RA lysozyme, from enzymatic degradation (Fig. 9). While RA ovalbumin, which is not bound by the particles, was completely degraded under these conditions. In the absence of the particles, both RA lysozyme and RA ovalbumin were degraded by trypsin. Furthermore, SDS-PAGE revealed that core particles with a dye loading of 1.78 mmol g−1 protected bound RA lysozyme from degradation, however some trypsin association was also noted. The core-shell particles with a dye loading of 0.74 mmol g−1 similarly protected the harvested RA lysozyme from degradation, without the trypsin binding associated with the core particles, suggesting that the presence of the inert shell increases the sieving properties of the particle. It is also interesting to note that the protection mechanism of the particles does not appear to involve the inhibition of trypsin activity. If Cibacron Blue had inhibited the activity of trypsin, then RA albumin would be expected to appear in the particle lanes (Fig. 9). However, the observed enzymatic degradation of RA albumin suggests that protection is associated with the sequestration of potential peptide substrates by the particles.
Fig. 9.
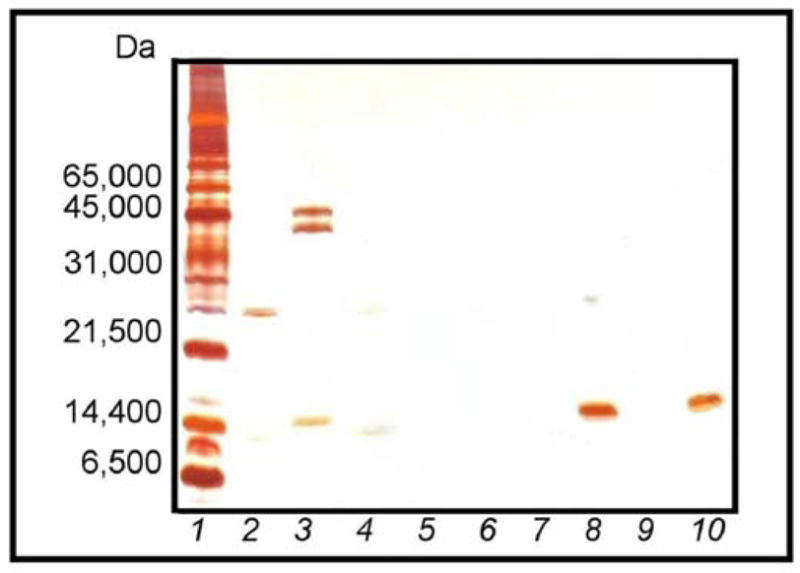
SDS-PAGE analysis of 1.78 mmol g−1 dye-loaded core and 0.74 mmol g−1 core-shell particles incubated with reduced-alkylated (RA) BSA, RA lysozyme, and trypsin: (1) MW ladder; (2) trypsin; (3) RA BSA and RA lysozyme prior to particle introduction; (4) trypsin and RA albumin; (5) trypsin and RA lysozyme; (6) trypsin, RA albumin, and RA lysozyme; (7) supernatant from 1.78 mmol g−1 dye-loaded core particles; (8) bound by 1.78 mmol g−1 dye-loaded core particles; (9) supernatant from 0.74 mmol g−1 dye-loaded core-shell particles; and (10) bound by 0.74 mmol g−1 dye-loaded core-shell particles.
4. Conclusion
Because they are secreted in response to key pharmacological processes or specific disease states, biomarkers can provide useful information regarding the physiological state of an individual. These low abundance, low molecular weight species can provide a means of diagnosing diseases such as cancer in their early stages, which can improve the overall prognosis. However, biomarker concentrations in human serum and other biological fluids are frequently well below the detection limit of mass spectrometry and immunoassays. In addition, the presence of high abundance, high molecular weight species such as serum albumin complicates the detection and identification of elusive biomarkers.
Our previous studies have established the advantages of employing temperature-sensitive hydrogel particles as biomarker harvesting platforms. These macromolecular networks can be designed to provide binding sites for target biomolecules by varying the functional groups incorporated within the polymer matrix and changing particle surface properties such as porosity. We have already shown the ability of the p-NIPAm-co-AAc hydrogel particles to rapidly sequester and concentrate small labile biomolecules such as PDGF from complex solutions in one step [8,10]. Furthermore, these particles have also demonstrated the ability to preserve the fidelity of these low molecular weight species by protecting them from proteolytic degradation [8,10]. In addition to the p-NIPAm-co-AAc core and core-shell particles, particles containing Cibacron Blue were also generated, and these particles demonstrated the ability to harvest hGH present at very low levels in urine and concentrate the hormone to levels within the detectable range of a clinical immunometric assay [11].
In the present study, the functionality of the Cibacron Blue hydrogel particles has been expanded with the fabrication of Cibacron Blue containing core-shell particles by enveloping the core particle within an inert p-NIPAm/BIS shell. A simple but effective method was developed for the synthesis of thermoresponsive dye-incorporated core-shell particles with uniform size distributions via precipitation polymerization in the absence of surfactants. While both dye-incorporated core and core-shell particle types showed the ability to capture, concentrate, and protect low molecular weight proteins from enzymatic degradation, the presence of the shell on the dye-incorporated core-shell particles was found to improve their sieving properties relative to particles lacking the p-NIPAm shell. Overall, the core-shell particles containing Cibacron Blue demonstrated efficient sieving under the conditions tested. As expected, it was found that the amount of immobilized reactive dye directly influences the ability of the hydrogel particles to sequester proteins: particles with lower dye densities captured less protein and exhibited inferior harvesting selectivity. In addition, the low pKa of the sulfonate moieties on the dye gives the dye-incorporated core and core-shell particles stability over a useful pH range. The results presented here and in previous studies provide insights into the promise that these dye-incorporated particles present for biomarker harvesting applications and other innovative diagnostic technologies.
Acknowledgments
This work was partially supported by the College of Science at George Mason University and grant number 1R21CA137706-01 from the National Cancer Institute at the National Institutes of Health. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding organizations.
References
- 1.Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, Simone C, Fishman DA, Kohn EC, Liotta LA. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–577. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- 2.Basso D, Valerio A, Seraglia R, Mazza S, Piva MG, Greco E, Fogar P, Gallo N, Pedrazzoli S, Tiengo A, Plebani M. Putative pancreatic cancer-associated diabetogenic factor: 2030 MW peptide. Pancreas. 2002;24:8–14. doi: 10.1097/00006676-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rubin RB, Merchant M. A rapid protein profiling system that speeds study of cancer and other diseases. Am Clin Lab. 2000;19:28–29. [PubMed] [Google Scholar]
- 4.Merrell K, Southwick K, Esplin MS, Lewis NE, Thulin CD. Analysis of low-abundance, low-molecular-weight serum proteins using mass spectrometry. J Biomol Technol. 2004;15:238–48. [PMC free article] [PubMed] [Google Scholar]
- 5.Kischel P, Waltregny D, Castronovo V. Identification of accessible human cancer biomarkers using ex vivo chemical proteomic strategies. Expert Rev Proteomics. 2007;4:727–739. doi: 10.1586/14789450.4.6.727. [DOI] [PubMed] [Google Scholar]
- 6.Pelton R. Temperature-sensitive aqueous microgels. Adv Colloid Interface Sci. 2000;85:1–33. doi: 10.1016/s0001-8686(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 7.Jones CD, Lyon LA. Synthesis and characterization of multiresponsive core-shell microgels. Macromolecules. 2000;33:8301–6. [Google Scholar]
- 8.Luchini A, Geho DH, Bishop B, Tran D, Xia C, Dufour R, Jones CD, Espina V, Patanarut A, Weidong S, Ross MM, Tessitore A, Petricoin EF, Liotta LA. Smart hydrogel particles: biomarker harvesting: one-step affinity purification, size exclusion, and protection against degradation. Nano Lett. 2008;8:350–61. doi: 10.1021/nl072174l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamidi M, Amir A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Longo C, Patanarut A, George T, Bishop B, Zhou W, Fredolini C, Ross MM, Espina V, Pellacani G, Petricoin EF, Liotta LA, Luchini A. Core-shell hydrogel particles harvest, concentrate and preserve labile low abundance biomarkers. PloS ONE. 2008;4:1–14. doi: 10.1371/journal.pone.0004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredolini C, Meani F, Reeder KA, Rucker S, Patanarut A, Botterell PJ, Bisho B, Longo C, Espina V, Petricoin EF, Liotta LA, Luchini A. Concentration and preservation of very low abundance biomarkers in urine, such as human growth hormone (hGH), by Cibacron Blue F3G-A loaded hydrogel particles. Nano Res. 2008;1:502–518. doi: 10.1007/s12274-008-8054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denizli A, Rad AY, Piskin E. Protein A immobilized polyhydroxyethylmethacrylate beads for affinity sorption of human immunoglobulin G. J Chromatogr B. 1995;668:13–19. doi: 10.1016/0378-4347(95)00047-m. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Lin H, Wang X, Hsiung K, Liu Y. Optimized dyeing conditions of immunoprotein with reactive dye Procion Blue MX-7RX. Anal Biochem. 2007;361:190–196. doi: 10.1016/j.ab.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Denizli A, Piskin E. Heparin-immobilized polyhydroxyethylmethacrylate microbeads for cholesterol removal: a preliminary report. J Chromatogr B. 1995;670:157–161. doi: 10.1016/0378-4347(95)00144-8. [DOI] [PubMed] [Google Scholar]
- 15.Denizli A, Piskin E. Dye-ligand affinity systems. J Biochem Biophys Methods. 2001;49:391–416. doi: 10.1016/s0165-022x(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 16.Pecora R. Dynamic Light Scattering: Applications of Photo Correlation Spectroscopy. Plenum; New York: 1985. [Google Scholar]
- 17.Brahim S, Narinesingh D, Guiseppi-Elie A. Synthesis and hydration properties of pH-sensitive p(HEMA)-based hydrogels containing 3-(trimethoxysilyl)propyl methacrylate. Biomolecules. 2003;4:497–503. doi: 10.1021/bm020080u. [DOI] [PubMed] [Google Scholar]
- 18.Braude EA, Nachod FC. Determination of Organic Structures by Physical Methods. Academic Press; New York: 1955. [Google Scholar]
- 19.Hage DS. Handbook of Affinity Chromatography. 2. CRC; New York: 2005. [Google Scholar]
- 20.Hai M, Bernath K, Tawfik D, Magdassi S. Flow cytometry: a new method to investigate the properties of water-in-oil-in-water emulsions. Langmuir. 2004;20:2081–2085. doi: 10.1021/la035402+. [DOI] [PubMed] [Google Scholar]
- 21.Siiman O, Burshteyn A. Preparation, Microscopy, and flow cytometry with excitation into surface plasmon resonance bances of gold or silver nanoparticles on aminodextran-coated polystyrene beads. J Phys Chem B. 2000;104:9795–9810. [Google Scholar]
- 22.Kwon GS. Polymeric Drug Delivery Systems. Informa Health Care; Boca Raton: 2005. [Google Scholar]
- 23.Hihara T, Okada Y, Morita Z. Azo-hydrazone tautomerism of phenylazonaphthol sulfonates and their analysis using the semiempirical molecular orbital PM5 method. Dyes Pigments. 2003;59:25–41. [Google Scholar]
- 24.Tirumalai RS, Chan KC, Prieto DRA, Issaq HJ, Conrads TP, Veenstra TD. Characterization of the low molecular weight human serum proteome. Mol Cell Proteomics. 2003;2:1096–1103. doi: 10.1074/mcp.M300031-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Dembczynski R, Jankowski T. Determination of pore diameter and molecular weight cut-off of hydrogel-membrane liquid-core capsules for immunoisolation. J Biomater Sci Polym Ed. 2001;12:1051–1058. doi: 10.1163/156856201753252552. [DOI] [PubMed] [Google Scholar]
- 26.Erickson HP. Protein structure–size and shape at the nm level [PDF document] Retrieved from http://www.cellbio.duke.edu/faculty/Erickson/pdf's/Prot-pbiophysCh1.pdf.