Abstract
The apolipoprotein ε4 allele (APOE*4) is a major genetic risk factor for Alzheimer’s disease (AD) and has been associated with altered cortical activation as assessed by functional neuroimaging in cognitively normal younger and older carriers. We chose to evaluate medial temporal lobe (MTL) activation during encoding and recognition using a perspective dependent (route or survey) visuospatial memory task by monitoring the blood-oxygen-level-dependent (BOLD) fMRI response in older, non-demented APOE*4 carriers (APOE*4+) and non-carriers (APOE*4−). During encoding, the APOE*4− group had greater average task-associated BOLD responses in ventral visual pathways, including the MTLs, as compared to the APOE*4+ group. Furthermore, MTL activation was greater during route encoding than survey encoding on average in APOE*4−, but not APOE*4+, subjects. During recognition, both groups performed similarly and no BOLD signal differences were found. Finally, within-group analysis revealed MTL activation during encoding was correlated with recognition performance in APOE*4−, but not APOE*4+ subjects. Reduced task-associated MTL activation that does not correlate with either visuospatial perspective or task performance suggests that MTL dysregulation occurs prior to clinical symptoms of dementia in APOE*4 carriers.
Keywords: Alzheimer, Apolipoprotein, Hippocampus, APOE*4, Perspective, Visuospatial learning, Encoding, Recognition, fMRI, Route, Survey
1. Introduction
Dementia occurs in 10–20% of the population over 65, with Alzheimer’s disease (AD), the most common cause of dementia, affecting about 4 million Americans (60). Although cognitive impairment precedes overt AD it can have multiple non-AD etiologies including depression, vascular disease, poor medical health and other forms of dementia (34). Differentiating the multiple etiologies of mild cognitive problems remains clinically difficult and no current test can provide accurate prognostic information regarding the extent of memory impairment an individual may suffer (21). This is especially relevant in AD given that pathological changes may begin before cognitive decline (45) and new treatments are being sought to modify this process (70). Thus identifying individuals who harbor clinically undetectable AD pathology and the ability to monitor their response to treatment will be important for drug development and clinical care.
One approach to clarifying presymptomatic neurophysiologic changes in AD is to study those who are at increased risk of developing AD prior to their manifesting cognitive problems. Carriers of the APOE*4 allele (APOE*4+) have an increased risk of developing mild cognitive impairment (MCI) and AD (11, 50, 66) and appear to have increased pathologic burden (54, 67). Imaging with PET and SPECT lend support to the hypothesis that the APOE*4 allele worsens AD, with both increased (29, 39) and decreased (17, 35, 39, 51) resting metabolism being reported in demented APOE*4 carriers as compared to demented non-carriers. In MCI, carriers of the APOE*4 allele reduced metabolism in the parietal-temporal regions and medial parietal lobe, including the posterior cingulate and retrosplenial area (40), perhaps reflecting greater pathologic burden and worse prognosis for these patients (for review see (10, 41)). PET and SPECT metabolic studies also reveal hypometabolism in younger (47, 53) and older (48, 52, 59) cognitively unimpaired APOE*4+ subjects. Studies with fMRI have been less consistent; both increased (3, 19, 26, 62, 71), decreased (36, 61) and unchanged (1) BOLD responses have been reported in APOE*4+ subjects and no clear anatomic region is reliably affected. Furthermore, recent fMRI studies suggest that a family history of dementia may modulate the effects APOE*4 on brain activation (33) or more robustly impact brain activation than APOE genotype (1).
The medial temporal lobes (MTLs), including the hippocampus, are essential for visuospatial learning (6) and visuospatial abilities are compromised in pre-clinical AD (58). Likewise, neurofibrillary tangles characteristic of AD are initially found in the MTLs and then later spread dorsolaterally throughout the cortex (4). Although an increased rate of hippocampal volume loss has been found in non-demented APOE*4+ as compared to APOE*4− subjects (9) whether the MTLs in non-demented APOE*4+ subjects are dysfunctional remains unclear. Robust metabolic changes in MTLs of healthy APOE*4 carriers have not been found (14) or not reported alongside consistent metabolic changes in the parietal and retrosplenial regions (48). Several fMRI studies have reported increased MTL activation in cognitively normal APOE*4 carriers using word pair (3, 19) or picture learning paradigms (2, 33) while reduced MTL activation has also been reported (36, 63). Thus the neurophysiologic significance of early volume loss and pathology in the MTLs of APOE*4 carriers remains to be clarified.
In this study we examined older, cognitively normal APOE*4+ and APOE*4− subjects while they engaged in a perspective dependent spatial memory task which has been shown to activate the MTLs in young subjects (55, 57). Separate encoding and recognition periods were evaluated to determine the extent that MTL activation is influenced by the APOE*4 allele and to explore if memory processing (encoding) and retrieval (recognition) are similarly affected. During encoding, subjects viewed animated movies of complex environments from either the route perspective, as if they were walking, or from a survey perspective, as if they were studying a map. During recognition still images of the previous viewed environments were judged as correct or rearranged. This task allows differences in brain activation during allocentric (survey, i.e., world centered) and egocentric (route, i.e., body-centered) encoding and recognition to be explored (42). The MTLs of young subjects during this task respond differently during route and survey encoding, with route encoding producing more robust MTL activation then survey encoding (55) and thus employing this paradigm allows us to explore the regulation of MTL activation during visuospatial memory tasks that vary only in perspective.
2. METHODS
2.1 Participants
The Human Subjects Institutional Review Board of the University of Washington approved this study and written informed consent was obtained. Subjects were recruited via posted flyers and print advertisements that sought older, healthy controls to be part of ongoing aging studies. Their initial evaluation included a thorough history and directed physical exam by a physician, screening cognitive testing, an EKG (when appropriate), and laboratory evaluation including a chemistry panel, compete blood count, thyroid stimulating hormone and B12 levels. Seven APOE*4+ (one 2/4, five 3/4 and one 4/4) and 7 APOE*4− (one 2/3 and six 3/3), with no history of altered social, occupational or cognitive function were recruited. Three APOE*4+ and two APOE*4− subjects had a positive family history for dementia as defined by a parent or sibling having been diagnosed with AD. The Mini Mental State Examination (MMSE) (20) and the logical memory subtest (or similar paragraph (12)) of Wechsler Memory Scale revised (WMS-III), were used to establish normal cognitive functioning for all subjects. In addition, 10 of the 14 subjects (5 APOE*4+ and 5 APOE*4−) completed the Mattis Dementia Rating Scale (DRS) (38); 4 subjects were not given the Mattis DRS secondary to our simplifying cognitive screening in these healthy, normally functioning adults. Exclusion criteria included a) neurologic or psychiatric disease b) alcohol or drug dependence c) unstable or clinically significant systemic medical problems d) visual or hearing deficits which would interfere with cognitive testing e) concurrent use of psychotropic medications including antidepressants, antipsychotics, benzodiazepines, narcotics, anticonvulsants, anti-Parkinsonian medications, cholinesterase inhibitors, glucocorticoids, sedating antihistamines, and CNS acting antihypertensives.
APOE genotyping was done at the Genotyping Core of the UW Alzheimer’s Disease Research Center by Hha1 restriction digests of PCR amplified APOE fragments (30). Subjects were age matched and did not differ in their baseline cognitive testing or ability to perform the task (Table 1). Each subject’s high resolution structural MRI scan was reviewed for unsuspected pathology. Two APOE*4+ subjects had mild pariventricular white matter (PWM) changes and either a right unilateral, or bilateral, subcaudal lacunae (<10 mm in diameter). One APOE*4− individual had mild PWM changes. Neither the PWM or the lacunae had obvious effects on the functional data from these subjects (data not shown).
Table 1.
Cognitive screening and recognition performance
| APOE*4− (n=7) | APOE*4+ (n=7) | |
|---|---|---|
| Age | 73.3 ± 7.4 | 72.4 ± 7.1 |
| MMSE | 29.1 ± 1.7 | 28.1 ± 1.6 |
| Paragraph memory | ||
| -immediate | 14.6 ± 4.3 | 10.4 ± 4.4 |
| -delayed | 11.9 ± 6.4 | 9.3 ± 4.5 |
| Mattis DRS* | ||
| Total Score | 137.0 ± 2.3 | 139.6 ± 3.1 |
| Subscores | ||
| attention | 35.8 ± 1.8 | 36.2 ± 0.8 |
| initiation | 35.8 ± 0.8 | 36.2 ± 1.1 |
| construction | 5.8 ± 0.4 | 5.6 ± 0.9 |
| conceptualization | 37.6 ± 2.2 | 38.6 ± 0.9 |
| memory | 22.4 ± 0.5 | 23.0 ± 1.0 |
| visual memory | 4.0 ± 0.0 | 3.8 ± 0.4 |
|
| ||
| Correct route (%) | 57.9 ± 4.3 | 59.1 ± 5.1 |
| Correct survey (%) | 62.9 ± 13.1 | 60.5 ± 10.9 |
| “Don’t know” route (%) | 10.7 ± 7.3 | 5.4 ± 4.6 |
| “Don’t know” survey (%) | 9.8 ± 9.1 | 9.8 ± 6.8 |
Performance on all cognitive screening tests were within the normal range for both groups and all differences between groups are not significant (p > 0.05). The correct route (%) and correct survey (%) during recognition are significantly different from chance (50%) p < 0.05 for both genotypes. All values are means ± standard deviation. Mini-Mental Status Exam (MMSE).
Data from 5 APOE*4− and 5 APOE*4+ subjects.
2.2 Task procedure
Subjects underwent BOLD-fMRI imaging while performing an environmental memory task designed by Shelton and colleagues (55–57) modified for older individuals by using a single environment during both encoding and recognition portions and allowing subjects six seconds (rather then three) to identify still images during recognition (details to follow). This task is composed of separate, sequentially presented encoding and recognition runs lasting 342 seconds and 472 seconds, respectively. To ensure that subjects were familiar with identifying objects from the route and survey perspective and understood the requirements of the task they completed practice session outside the MRI suite with an environment not subsequently used during the scanning session. All subjects demonstrated satisfactory understanding and performance of the task after this practice session. During the fMRI session verbal instructions were provided though headphones and visual stimuli through goggles connected via high-resolution fiber optic cables. Stimuli were presented using an Apple computer running PsyScope (psyscope.psy.cmu.edu).
2.2.1. Encoding task
During the encoding run subjects viewed animated movies of an environment (either a park or market) presented from both the route perspective, as if they were walking through it, and the survey perspective, as if flying over it. The environment was shown six times, 3x from the route and 3x from the survey perspective, in an alternating quasi-random order. The individual movies were 46 seconds long and were preceded by 2 seconds of fixation. Three, 18 second fixation blocks (crosshair) were intermixed in the sequence. The run was thus composed of 3 fixation blocks (18 seconds each) + 3 repetitions of the route perspective movie (46 + 2 seconds each) + 3 repetitions of the survey perspective movie (46 + 2 seconds each) in a quasi-random order for a total run time or 342 seconds (TR=2, 171 volumes). Immediately following encoding subjects were tested on their knowledge of the complex environment during the recognition run (see below). Subjects were informed that their memory for the environment would be subsequently tested but active responses were not required during encoding.
2.2.2 Recognition task
During the recognition run subjects viewed still images of the previously studied environment and determined if objects within the environment were; i) correctly positioned (previously seen items in appropriate environmental locations), ii) rearranged (previously seen items shuffled within the environment), or iii) they couldn’t tell (don’t know). A total of 64 images were presented; 32 from the route perspective (4 blocks of 8 images, each block preceded by 2 seconds of fixation) and 32 images from the survey perspective (4 blocks of 8 images, each block preceded by 2 seconds of fixation). Four, 18 second fixation blocks (crosshair) were intermixed in the sequence. The entire run was thus composed of 4 fixation blocks (18 seconds each) + 4 route perspective picture blocks (48 + 2 seconds each) + 4 survey perspective picture blocks (48 + 2 seconds each) in a quasi-random order for a total run time of 472 seconds (TR=2, 236 volumes). Following the fMRI session subjects were asked to draw the environment to ensure they had completed the task appropriately (data not scored).
2.3. Imaging and Analysis
2.3.1 BOLD imaging
Structural and functional MRI were performed on a 1.5 T MR imaging system (General Electric, Waukesha, WI). Coronal anatomic (TR/TE 200/2.2 msec) and functional (TR 2000/50 msec, flip angle 90°, 64 × 64 matrix, in plane resolution of 3.75 mm) images were collected using a two-dimensional gradient-echo echo-planar pulse sequence. Each volume consisted of 20 coronal slices spaced 5.5 mm apart (total anterior-posterior imaged area = 11 cm) to optimize the visualization of the MTL, thus the frontal and occipital poles were not included in the functional data set. Given this partial coverage of our functional images we refer to “whole image analysis” rather then “whole brain analysis” when performing group modeling.
2.3.2. Whole image analysis
Functional data were processed using tools from the FMRIB Software Library (www.fmrib.ox.ac.uk and (64)). Prior to automated procedures each functional series was manually reviewed and aberrant volumes or extreme motion artifacts were removed. Motion correction (31), spatial smoothing with a 7mm at half-maximum Gaussian kernel and low pass and high pass (sigma = 150 sec) filtering were applied. The first 5 volumes of each functional series were discarded to allow T1 equilibrium and statistical analysis was carried out using a general linear model (GLM). Each subject’s data was modeled two ways allowing for both task-fixation and route-survey contrasts could be explored. First, route and survey were each assigned their own explanatory variable (EVs) to compare task-associated activation with fixation. Second, the fixation volumes (27 from encoding and 36 from recognition) were removed from the data allowing a single EV to model the route-survey contrast. This approach was used given the imbalanced nature of our paradigm that included more task blocks then fixation blocks. Absolute motion varied from 0.1mm – 1.2 mm and relative motion varied between 0.04 mm – 0.22 mm.
Group comparisons were obtained by mapping each subjects EPI functional images onto their high resolution structural images using 7 DOF and into standard space (MNI152 brain) with a 12 DOF affine transformation (32). Group mixed effects analysis (72) was used and activated clusters were identified based on random field theory and cluster analysis which corrects for multiple comparisons. APOE genotype, task performance, age and the interaction of task performance x genotype were included as covariates in the general linear model (GLM). Both whole-group contrasts (n=14 subjects) and between-group contrasts (n=2 groups, seven subjects per group) were explored to determine whether age, genotype and/or performance could predict BOLD signal changes. Neither whole-group or within-group analysis revealed activation that was correlated with age at reported z-thresholds (data not shown).
2.3.3. ROI analysis
A MTL region of interest (MTLroi) was demarcated for each subject on their high-resolution structural image and used as a mask to determine average z-scores from the individual’s functional scan. The MTLroi consisted of the hippocampus and parahippocampal gyrus anteriorly from the final section containing the inferior horn of the lateral ventricle, restricted laterally by the collateral sulcus and extending posteriorly until the disappearance of the corpus callosum. The posterior MTLroi included the isthmus connecting the parahippocampal and cingulate gyrus. Average MTLroi volume was 19.3 ± 2.0 cm3 (ave. ± s.d.) in APOE*4− subjects and 18.8 ± 2.5 cm3 in APOE*4+ subjects (n.s.). MTLroi statistics were performed using STATA™.
RESULTS
3.1. Activation during encoding
3.1.1. Whole image analysis
Viewing the environmental movies from either the route or survey perspective induced BOLD signal changes in the lingual and fusiform gyri, retrosplenial regions and MTLs in APOE*4− subjects (within-group contrasts, Fig. 1a). The route perspective further activated regions of the parietal (Fig. 1a, top) and frontal lobes (not shown) that are not activated during survey encoding (Fig. 1a, bottom). Visual inspection suggests that APOE*4+ subjects displayed less encoding task-associated activation as compared to APOE*4− subjects (Fig. 1b). In APOE*4+ subjects, both route and survey encoding activated the fusiform gyrus while deactivations in lateral temporal-parietal regions were found during survey encoding (Fig. 1b, bottom). No regions of deactivation were found in APOE*4− subjects. Between-group contrasts revealed significantly greater task-associated activation in APOE*4− subjects during both route and survey encoding in multiple posterior temporal, parietal and occipital regions including the MTL bilaterally (Fig. 2a).
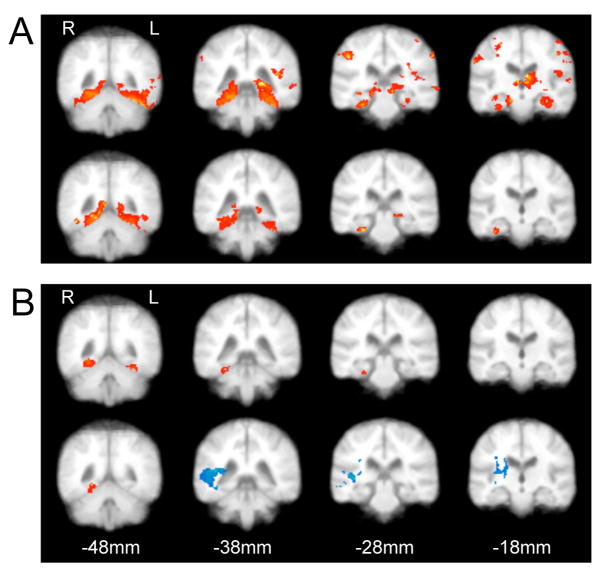
Figure 1.
Cortical activation during route and survey encoding contrasted with fixation. (a) APOE*4− subjects displayed robust activation of MTLs, ventral occipital regons areas and prefrontal cortex (not shown) during both route (upper) and survey (lower) encoding. (b) APOE*4+ subjects had less overall task associated activation during encoding which statistically was limited to the fusiform gyrus during route (upper) and survey (lower) encoding. An area of deactivation (blue) in the temporal/parietal regions was evident during survey encoding. Within-group contrasts with z-threshold = 2.7 and p < 0.01 (n=7 per group).
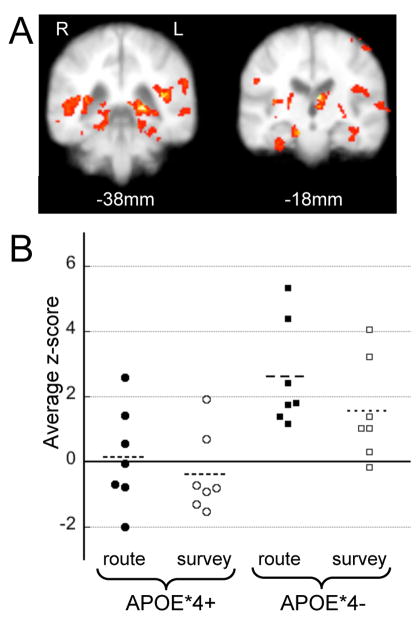
Figure 2.
Regions of greater activation in APOE*4− than APOE*4+ subjects during encoding. (a) Regions where APOE*4− subjects had greater BOLD signal during either route or survey encoding include the posterior (left) and anterior (right) MTLs and areas around the occipital-temporal-parietal junction. Between-group contrasts with z-threshold = 2.5 and p < 0.01 (n=14). (b) Average z-scores for individual subjects in the MTLroi during route (filled symbols:●,■) and survey (open symbols: ○, □) encoding. APOE*4− subjects (■, □) and APOE*4+ subjects (●, ○).
Perspective-dependent MTL activation was determined by comparing BOLD responses during route and survey encoding within individual subjects (route-survey differential). Within the APOE*4− group, route encoding activated the bilateral MTLs, left frontal (BA 4 & 6) and right temporal-parietal regions (BA 37, 39 & 40) more than survey encoding (Figure 3a), whereas survey encoding did not induce more activation than route encoding in any region. A route-survey differential was not found in the APOE*4+ group even when less stringent comparisons were performed (data not shown). Between-group contrasts of the route-survey differential in the MTL revealed a significant group difference, with APOE*4− subjects having a greater route-survey differential then APOE*4− subjects (data not shown, p = 0.009 (right MTL) and p = 0.037 (left MTL)).
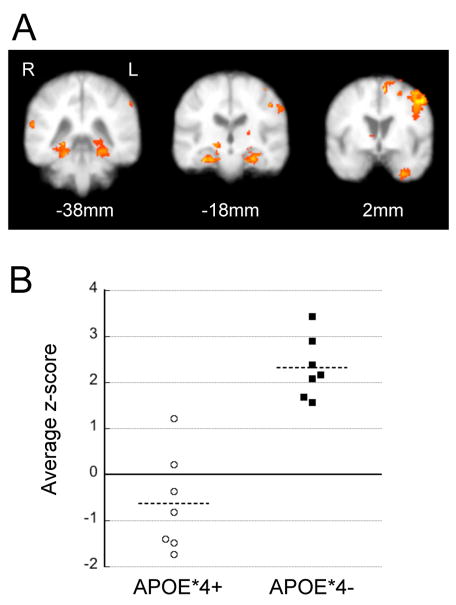
Figure 3.
Route/survey activation differential in APOE*4− subjects. (a) Regions of greater activation during route than survey encoding in APOE*4− subjects include the MTLs bilaterally, the left frontal and the right parietal cortex. Within-group contrasts with z-threshold = 2.5 and p < 0.01 (n=7). (b) Average route/survey differential z-scores in individual subjects within the MTLroi. APOE*4− subjects (■) and APOE*4+ subjects (○).
3.1.2. ROI analysis of the MTLs
Given our a priori hypothesis that MTL activation would be modulated by the APOE*4 allele and our results from the whole image analysis we chose to further explore MTL activation through ROI analysis (MTLroi which included the entire anterior-posterior extent of the hippocampus and parahippocampal gyrus). Average task-associated and route-survey differential MTLroi z-scores were determined in each subject. Within the MTLroi, on average, APOE*4− subjects had greater task-associated activation then APOE*4+ subjects during both route encoding (APOE*4− 2.6 ± 1.6 and APOE*4+ 0.1 ± 1.5, p = 0.012 unpaired t-test) and survey encoding (APOE*4− 1.6 ±1.5 and APOE*4+ −0.4 ± 1.2, p = 0.023 unpaired t-test) (Fig. 2b). Likewise, the APOE*4− group, but not the APOE*4+ group, had a significant route-survey differential in the MTLroi during encoding (APOE*4− = 1.69 ± 1.22, APOE*4+ = 0.04 ± 1.04, p = 0.02 unpaired t-test). All 7 APOE*4− subjects had an average route-survey differential z-score of greater than 1.5, whereas none of the APOE*4− subjects had an average MTLroi positive z-score of this magnitude (Figure 3b).
3.2. Activation during recognition
Immediately following the encoding scan, subjects were imaged while identifying still images from the previously seen environments as correct or rearranged. Activation of the lingual and fusiform gyri, retrosplenial areas, and medial and dorsal prefrontal regions (BA 6, 8, 24 and 32) was apparent in both groups while making recognition judgments (data not shown). However, no significant activation differences were found between groups or within groups (i.e., the route-survey differential) even when the z-threshold was made less stringent. MTLroi analysis revealed that average z-scores within the MTLroi during recognition were less than during encoding but they did not differ for APOE*4− and APOE*4+ subjects during route image (APOE*4− 0.5 ± 1.5 and APOE*4+ 0.2 ± 2.4, n.s.) or survey image (APOE*4− 0.4 ± 1.9 and APOE*4+ 0.1 ± 3.2, n.s.) viewing.
3.3. Performance correlated activation
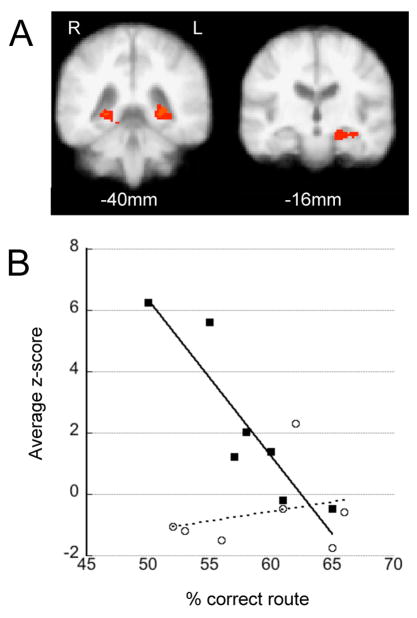
During recognition, APOE*4− and APOE*4+ subjects distinguished still images as previously seen vs. rearranged with similar efficacy (Table 1) and whole-group analysis showed that performance worsened with age (F(1,12) = 8.55, p = 0.012, r2=0.42). A full ANOVA within the FSL environment was used to assess between-group differences in how task-associated activation during encoding and recognition predicted recognition performance (RP) (dependent variable = 3D task-associated activation maps; predictor variables = genotype, RP, age, RP*genotype). During route encoding we found a significant route-RP*genotype interaction in the left MTL (p=0.01) and a potential interaction in the posterior right parahippocampal region (p=0.08) (Figure 4a). ROI analysis of these combined clusters reveals that greater route-RP was associated with less task-associated MTL activation in APOE*4−, but not APOE*4+, subjects (Figure 4b). Average MTLroi z-scores are similarly correlated with route-RP with lower MTLroi task-associated activation predicting better route-RP in APOE*4−, but not APOE*4+, subjects (data not shown; dependent variable = average MTLroi z-scores; predictor variables = genotype, route-RP, route-RP*genotype; F(3,10), p=0.01, r2=0.65)). Survey-RP was did not correlate with MTL activation in either genotype during encoding and no performance x activation interactions were identified for either genotype during the recognition period.
Figure 4.
Performance correlated activation in the MTLs during encoding. (a) Areas where there is a genotype x performance interaction in the left (p=0.01) and posterior right (p=0.08) MTLs during route encoding. Z-threshold = 2.3. (b) Average z-scores within these areas (combined) during route encoding correlate with performance in APOE*4− but not APOE*4+ subjects. APOE*4− subjects (■) and APOE*4+ subjects (○).
4. DISCUSSION
Using a visuospatial memory task we evaluated BOLD signal responses in cognitively normal APOE*4+ and APOE*4− subjects. During memory encoding there was robust task-associated activation of the MTL in APOE*4− subjects that varied with perspective and recognition performance. APOE*4+ subjects had minimal MTL task-associated activation that was not modulated by perspective or recognition performance. No significant APOE*4 effects were found during recognition. This suggests that the MTL/cortical systems are utilized differently or dysregulated in APOE*4 carriers during visuospatial memory encoding prior to their having visuospatial difficulties. Moreover, it appears the neural systems utilized during encoding are more affected by the APOE*4 allele than those used during recognition in this visuospatial memory task.
4.1 Altered brain activation in APOE*4 carriers
Prior studies have reported fMRI alterations in cognitively normal (2, 7, 19, 33, 36, 61, 71) or mildly impaired (3, 15) APOE*4+ individuals. However, there is considerable variability in both the direction and pattern of the differences. Increased activation has been reported during verbal (3, 19) and visual (2) learning paradigms while decreased activation has been found during a naming task (61) and a semantic judgment task (36). Furthermore, not all tasks result in consistently different BOLD responses in APOE*4+ and APOE*4− subjects (see (1) vs (3) (7). The extent of BOLD signal differences also varies. Both large regions of the cortex (3) and more selected regions (2, 19, 36, 61) have been found to differ between APOE*4 carriers and non-carriers. The sundry cognitive protocols used, various analysis methods and heterogeneous subject populations complicates direct comparison of these reports. Not all APOE*4 carriers will develop dementia, thus it is not possible to estimate if/when a given APOE*4+ individual will develop cognitive symptoms. In fact, two recent reports have assessed very similar contrasts (novel vs. familiar words) and reported conflicting findings regarding MTL activation (19, 36).
Our study contributes to previous work in several regards. First, it is the only study to use a complex visual environment to assess visuospatial learning. The MTLs are known to be involved in visuospatial tasks (6) and thus we chose our task to specifically evaluate this region. Second, we used whole image group analysis to establish the relative specificity of BOLD signal changes during our task to the MTLs and restricted our ROI analysis to this a priori identified ROI. Third, a single previous study (3) reported that APOE*4 mediated BOLD responses were similar during encoding and recall period, and in their analysis encoding and recall data were pooled in order to analyze overall task related activation independent of baseline. Our evaluation of encoding and recognition separately suggests that encoding may be more affected by the APOE*4 allele than recognition. Finally, the environmental memory paradigm was designed to evaluate the tight contrast between route and survey perspectives, a comparison not dependent on baseline activation. “Loose” contrasts compare dissimilar cognitive states, such as a demanding memory task vs. fixation, while “tight” contrasts compare highly similar conditions theoretically allowing for greater specificity when reporting activation differences (16). Designating loose and tight contrasts is somewhat arbitrary, but only two prior APOE*4 fMRI studies have clearly used tight contrasts (19, 36).
4.2. MTL activation in APOE*4 carriers
On average, we found less task-associated activation of the MTLs in APOE*4 carriers than non-carriers. “Task-associated” activation is defined here as the loose contrast between the BOLD signal while looking at a cross-hair (rest) vs. the BOLD signal induced when viewing the route or survey movie. Reduced task-associated activation could reflect either an increased BOLD signal at rest, a decreased BOLD signal during the task, or a combination of both. The baseline state is thought to be altered in AD (25, 37) and thus we have chosen to use the term “task associated activation” rather than “total activation” to reflect our inability to actually compare blood flow in our subjects. Our APOE*4 carriers and non-carriers performed similarly during the task and during baseline neuropsychological testing implying that reduced task-associated activation in APOE*4+ subjects does not reflect ineffectual brain use, only altered/different regulation of cortical systems. Our paradigms contained more task volumes (144 for encoding and 200 for recognition) then fixation volumes (27 for encoding and 36 for recognition). This imbalanced design reduces our power to detect task-associated differences secondary to the comparatively high variance of the short fixation blocks. Thus although task-associated activation is reduced in APOE*4− subjects, the lack of any significant activation in visuospatial regions should be interpreted cautiously.
Maintained cognitive performance in the elderly has been associated with increased BOLD responses as compared to similarly performing younger subjects suggesting that compensation occurs in older, less efficient, brains (23). Likewise, greater activation in APOE*4+ subject groups has been observed (2, 3, 19) and suggested to reflect a compensatory response which burns out if dementia ensues (2). However, we found on average less, rather than more, task-associated activation in the MTL of APOE*4+ subjects, and no regions where APOE*4+ subjects had greater activation than APOE*4− subjects. Thus our findings do not support the hypothesis that there are region-specific compensatory increases in task-associated activation in cognitively normal APOE*4 carriers.
4.3 Perspective dependent MTL activation
Route perspective encoding led to greater MTL activation than survey encoding in APOE*4−, but not APOE*4+ subjects. The APOE*4− response is consistent with the original findings with this task in younger individuals (mean age 23) (55) and with greater inferior occipital gyral activation found when a scene is viewed from the ground/immersed-observer perspective versus the aerial/external-observer perspective (68). These results appear at odds with the long standing theory that allocentric memory is dependent on the hippocampus (42). However, the human hippocampus contains place responsive cells while the parahippocampal cortex contains objects responsive cells (18). The resolution and distortion of our fMRI data does not allow us to independently visualize these regions. Thus, we do not believe that greater activation of the MTLs during route encoding should be interpreted as suggesting that the MTLs are more important during egocentric than allocentric learning. Rather, greater cognitive demands of route perspective learning which requires binding of extrapersonal and intrapersonal information may have lead to greater MTL activation during egocentric encoding. Curiously, our subjects generally reported that the route perspective was more difficult to learn then the survey perspective (unquantified). Right frontal (~BA 4, 6) and left parietal (~BA 37, 39, 40) areas also appeared more active during route than survey encoding in APOE*4− subjects. Failure of prefrontal activation to increase with increasing task demands has been observed in APOE*4+ subjects (44) and thus the lack of a prefrontal route/survey differential may illustrate impaired prefrontal recruitment during difficult tasks in APOE*4− subjects.
Lack of perspective dependent MTL activation in APOE*4+ subjects could reflect neurophysiologic changes in several regions. Damage to the parietal cortex has been associated with problems in the egocentric reference frame and hemineglect (6), while MTL dysfunction severely compromises allocentric processes (27) and survey memory (6). Dorsal and medial parietal regions are hypometabolic in APOE*4 carriers (48), while the MTLs are the initial site of AD-like pathology (4) and thus dysfunction of either anatomical site could account for the lack of differential MTL route/survey activation. Alternatively, dedifferentiation of visuospatial information processing and activation of a more generalized network may be occurring in APOE*4+ subjects. Dedifferentiation of neural systems is thought to be one cause of altered activation patterns in elderly subjects (46) and AD subjects activate cortical networks during easy tasks that are only activated by difficult tasks in control subjects (65). Furthermore, both visual processing (43) and the specificity of the dorsal-ventral visual pathways (8, 22) can become less cortically distinct with aging. Thus either dysfunction of a single region or dedifferentiation of cortical networks could account for the lack of a route/survey MTL activation differential in APOE*4+ subjects.
4.4. Performance correlated MTL activation
Numerous studies have reported associations between brain activation and memory performance in the prefrontal, medial temporal and parietal lobes (for reviews see (49, 69). Objects that are remembered are often associated with a greater BOLD signal than forgotten items (49). The block design of our experiment does not allow for the comparison of the BOLD signal during encoding (when viewing a specific object) and whether the position of that object is subsequently correctly recognized. However, we were still able to observe a relationship between MTL encoding activation and recognition performance in APOE*4− subjects, with better recognition performers having less MTL activation during encoding (Figure 4). This may reflect the greater efficiency of the MTL lobes in good route recognizers (for review, (28). Alternatively, good route-encoders could rely on other brain areas (i.e. right frontal or left parietal) when learning egocentric visuospatial material. This is in agreement with the hypothesis that parietal-based egocentric reference frame is converted into the medial temporal-based allocentric reference through a pathway that involves retrosplenial region (5), an area activated in APOE*4− than APOE*4+ subjects during both the route and survey encoding periods.
4.5 Family history and study limitations
Having a family history of dementia (FH+) has been reported to effect BOLD responses in both APOE carriers and non-carriers (1, 33). Our study was not designed to address this issue and our subjects were not initially matched for this confound. Fortunately, our groups were relatively balanced with three of our APOE*4 carriers and two of our non-carriers being FH+. Direct contrasts of subjects with (n=5) and without (n=9) a FH+ (simple t-test) or inclusion of family history as a covariate in our group comparisons (full ANOVA) did not result in significant family history associated activation differences in any brain region (data not shown, low stringency contrast with z-threshold=2.0 and p < 0.1). The small number of individuals and unbalanced comparison reduce our power to detect an influence of FH and future studies will need to explore this issue. However, it does appear that APOE genotype influences the BOLD response more robustly than family history in our subjects and thus our primary comparison remains valid.
The small sample size of this study reduces our power to detect within-group and between-group differences (type II error) while increasing the likelihood of accepting false results (type I error) especially in our whole-brain analysis using FSL. For this reason we obtained the average z-scores from MTLroi to illustrate activation in individual subjects and to confirm our whole-brain results. Although average task-associated activation overlaps between groups (Figure 2b), within the MTL there is a non-overlapping distribution of route-survey differential z-scores (Figure 3b). Uncorrected p-values are reported for these z-score comparisons because we are essentially contrasting regions that had been previously found to be significantly different by our corrected, mixed-effects GLM analysis. Thus the simple t-tests of average z-scores within the MTLroi may be unnecessary, but informative.
4.6 Summary
In summary, we found that the APOE*4 allele modulates MTL activation in cognitively normal older subjects. Task-associated, perspective dependent and performance correlated BOLD signal changes in the MTLs were all different on average in APOE*4 carriers and non-carriers. Reports of altered visuospatial abilities in cognitively normal middle-aged APOE*4 carriers (24) and brain metabolic changes in young APOE*4 carriers (47, 53) raise the possibility that neurophysiologic alterations seen in APOE*4 carriers could reflect a “trait” rather than a “state”. This raises the question of whether altered BOLD responses in these older APOE*4+ subjects represents a pre-dementia state or reflects a life-long difference in neuronal activation or neurovascular coupling. Neurovascular coupling is affected by aging and disease (13) and whether AD or the APOE*4 allele affects the process remains unknown. Future fMRI studies could address this issue by quantifying BOLD signal changes in younger APOE*4+ populations.
Acknowledgments
This research was supported by ADRC pilot award P50 AG05136. PR Borghesani is supported by the T32 AG00258 post-doctoral fellowship. We thank Darcy Vavrek, Steve Millard and Todd Richards for advice on statistics and image processing.
Footnotes
Disclosure statement
There are no actual or potential conflicts of interest for the authors that could have inappropriately influenced this work. Subjects were recruited in accordance with the University of Washington Internal Review Board (IRB) approved policies and procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL. Familial risk for Alzheimer’s disease alters fMRI activation patterns. Brain. 2006;129 (Pt 5):1229–39. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64 (3):501–8. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343 (7):450–6. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18 (4 Suppl):S85–8. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 5.Burgess N, Becker S, King JA, O’Keefe J. Memory for events and their spatial context: models and experiments. Philos Trans R Soc Lond B Biol Sci. 2001;356 (1413):1493–503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35 (4):625–41. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 7.Burggren AC, Small GW, Sabb FW, Bookheimer SY. Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am J Geriatr Psychiatry. 2002;10 (1):44–51. [PubMed] [Google Scholar]
- 8.Chen J, Myerson J, Hale S. Age-related dedifferentiation of visuospatial abilities. Neuropsychologia. 2002;40 (12):2050–6. doi: 10.1016/s0028-3932(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen RM, Small C, Lalonde F, Friz J, Sunderland T. Effect of apolipoprotein E genotype on hippocampal volume loss in aging healthy women. Neurology. 2001;57 (12):2223–8. doi: 10.1212/wnl.57.12.2223. [DOI] [PubMed] [Google Scholar]
- 10.Coleman RE. Positron emission tomography diagnosis of Alzheimer’s disease. Neuroimaging Clin N Am. 2005;15(4):837–46. x. doi: 10.1016/j.nic.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261 (5123):921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 12.Craft S, Zallen G, Baker LD. Glucose and memory in mild senile dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1992;14 (2):253–67. doi: 10.1080/01688639208402827. [DOI] [PubMed] [Google Scholar]
- 13.D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4 (11):863–72. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- 14.de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98 (19):10966–71. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65 (3):404–11. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson DI, Buckner RL. Effective paradigm design. Oxford University Press; 2001. [Google Scholar]
- 17.Drzezga A, Riemenschneider M, Strassner B, Grimmer T, Peller M, Knoll A, Wagenpfeil S, Minoshima S, Schwaiger M, Kurz A. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology. 2005;64 (1):102–7. doi: 10.1212/01.WNL.0000148478.39691.D3. [DOI] [PubMed] [Google Scholar]
- 18.Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425 (6954):184–8. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 19.Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, Bondi MW. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol. 2005;62 (12):1881–8. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12 (3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Galasko D. Biomarkers for Alzheimer’s disease - Clinical needs and application. J Alzheimers Dis. 2005;8 (4):339–46. doi: 10.3233/jad-2005-8403. [DOI] [PubMed] [Google Scholar]
- 22.Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14 (3 Pt 2):1450–62. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci. 2003;23 (3):986–93. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19 (2):199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101 (13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey-Bloom J, Salmon DP, Thal LJ, Bondi MW. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37 (5):877–88. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 28.Henson R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B. 2005;58 (3–4):340–60. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi M, Arai H, Nakagawa T, Higuchi S, Muramatsu T, Matsushita S, Kosaka Y, Itoh M, Sasaki H. Regional cerebral glucose utilization is modulated by the dosage of apolipoprotein E type 4 allele and alpha1-antichymotrypsin type A allele in Alzheimer’s disease. Neuroreport. 1997;8 (12):2639–43. doi: 10.1097/00001756-199708180-00001. [DOI] [PubMed] [Google Scholar]
- 30.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31 (3):545–8. [PubMed] [Google Scholar]
- 31.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17 (2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5 (2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 33.Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006;26 (22):6069–76. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc. 2003;78 (10):1290–308. doi: 10.4065/78.10.1290. [DOI] [PubMed] [Google Scholar]
- 35.Lehtovirta M, Soininen H, Laakso MP, Partanen K, Helisalmi S, Mannermaa A, Ryynanen M, Kuikka J, Hartikainen P, Riekkinen PJ., Sr SPECT and MRI analysis in Alzheimer’s disease: relation to apolipoprotein E epsilon 4 allele. J Neurol Neurosurg Psychiatry. 1996;60 (6):644–9. doi: 10.1136/jnnp.60.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Backman L, Nilsson LG, Petersson KM, Nyberg L. Reduced functional brain activity response in cognitively intact apolipoprotein E {varepsilon}4 carriers. Brain. 2006 doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- 37.Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100 (24):14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattis S. Dementia Rating Scale: Professional Manual. Odessa, FL: Psychological Assessments Resources; 2004. [Google Scholar]
- 39.Mielke R, Zerres K, Uhlhaas S, Kessler J, Heiss WD. Apolipoprotein E polymorphism influences the cerebral metabolic pattern in Alzheimer’s disease. Neurosci Lett. 1998;254 (1):49–52. doi: 10.1016/s0304-3940(98)00673-9. [DOI] [PubMed] [Google Scholar]
- 40.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32 (4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 41.Mosconi L, De Santi S, Rusinek H, Convit A, de Leon MJ. Magnetic resonance and PET studies in the early diagnosis of Alzheimer’s disease. Expert Rev Neurother. 2004;4 (5):831–49. doi: 10.1586/14737175.4.5.831. [DOI] [PubMed] [Google Scholar]
- 42.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Claredon Press; 1978. [Google Scholar]
- 43.Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci U S A. 2004;101 (35):13091–5. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrella JR, Lustig C, Bucher LA, Jha AP, Doraiswamy PM. Prefrontal activation patterns in subjects at risk for Alzheimer disease. Am J Geriatr Psychiatry. 2002;10 (1):112–3. [PubMed] [Google Scholar]
- 45.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45 (3):358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 46.Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128 (Pt 9):1964–83. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 47.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101 (1):284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102 (23):8299–302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357 (1424):1097–110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43 (8):1467–72. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 51.Scarmeas N, Anderson KE, Hilton J, Park A, Habeck C, Flynn J, Tycko B, Stern Y. APOE-dependent PET patterns of brain activation in Alzheimer disease. Neurology. 2004;63 (5):913–5. doi: 10.1212/01.wnl.0000137274.93125.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarmeas N, Habeck C, Anderson KE, Hilton J, Devanand DP, Pelton GH, Tabert MH, Flynn J, Park A, Ciappa A, Tycko B, Stern Y. Altered PET functional brain responses in cognitively intact elderly persons at risk for Alzheimer disease (carriers of the epsilon4 allele) Am J Geriatr Psychiatry. 2004;12 (6):596–605. doi: 10.1176/appi.ajgp.12.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, Stern Y. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005;76 (10):1440–4. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90 (20):9649–53. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shelton AL, Gabrieli JD. Neural correlates of encoding space from route and survey perspectives. J Neurosci. 2002;22 (7):2711–7. doi: 10.1523/JNEUROSCI.22-07-02711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shelton AL, McNamara TP. Orientation and perspective dependence in route and survey learning. J Exp Psychol Learn Mem Cogn. 2004;30 (1):158–70. doi: 10.1037/0278-7393.30.1.158. [DOI] [PubMed] [Google Scholar]
- 57.Shelton AL, Pippett HA. Fixed versus dynamic orientations in environmental learning from ground-level and aerial perspectives. Psychological Research. doi: 10.1007/s00426-006-0088-9. in press. [DOI] [PubMed] [Google Scholar]
- 58.Small BJ, Herlitz A, Fratiglioni L, Almkvist O, Backman L. Cognitive predictors of incident Alzheimer’s disease: a prospective longitudinal study. Neuropsychology. 1997;11 (3):413–20. doi: 10.1037//0894-4105.11.3.413. [DOI] [PubMed] [Google Scholar]
- 59.Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, Kaplan A, La Rue A, Adamson CF, Chang L, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. Jama. 1995;273 (12):942–7. [PubMed] [Google Scholar]
- 60.Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, Finkel SI, Gwyther LP, Khachaturian ZS, Lebowitz BD, McRae TD, Morris JC, Oakley F, Schneider LS, Streim JE, Sunderland T, Teri LA, Tune LE. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. Jama. 1997;278 (16):1363–71. [PubMed] [Google Scholar]
- 61.Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ. Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology. 1999;53 (7):1391–6. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- 62.Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ. Women at risk for AD show increased parietal activation during a fluency task. Neurology. 2002;58 (8):1197–202. doi: 10.1212/wnl.58.8.1197. [DOI] [PubMed] [Google Scholar]
- 63.Smith CD, Kryscio RJ, Schmitt FA, Lovell MA, Blonder LX, Rayens WS, Andersen AH. Longitudinal functional alterations in asymptomatic women at risk for Alzheimer’s disease. J Neuroimaging. 2005;15 (3):271–7. doi: 10.1177/1051228405277340. [DOI] [PubMed] [Google Scholar]
- 64.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 65.Starr JM, Loeffler B, Abousleiman Y, Simonotto E, Marshall I, Goddard N, Wardlaw JM. Episodic and semantic memory tasks activate different brain regions in Alzheimer disease. Neurology. 2005;65 (2):266–9. doi: 10.1212/01.wnl.0000168907.44632.55. [DOI] [PubMed] [Google Scholar]
- 66.Tanzi RE, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron. 2001;32 (2):181–4. doi: 10.1016/s0896-6273(01)00476-7. [DOI] [PubMed] [Google Scholar]
- 67.Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62 (11):1977–83. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- 68.Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cogn Neurosci. 2004;16 (5):817–27. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- 69.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9 (9):445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Walker LC, Ibegbu CC, Todd CW, Robinson HL, Jucker M, LeVine H, 3rd, Gandy S. Emerging prospects for the disease-modifying treatment of Alzheimer’s disease. Biochem Pharmacol. 2005;69 (7):1001–8. doi: 10.1016/j.bcp.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 71.Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, Mamourian AC, Belloni DR, Rhodes CH, McAllister TW. Increased brain activation during working memory in cognitively intact adults with the APOE epsilon4 allele. Am J Psychiatry. 2006;163 (9):1603–10. doi: 10.1176/ajp.2006.163.9.1603. [DOI] [PubMed] [Google Scholar]
- 72.Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21 (4):1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]