Abstract
Principle
The orexin system has been hypothesized to regulate drug-seeking and drug self-administration behaviors, including ethanol (EtOH) seeking and consumption. However, studies on the effects of orexin receptor antagonists have not been conducted on robust alcohol-relapse behavior.
Objectives
This study assessed the effects of the orexin-1 receptor antagonist, SB-334867, on alcohol-seeking behavior and responding for alcohol under relapse conditions.
Methods
Adult alcohol-preferring (P) rats self-trained in 2-lever operant chambers to administer 15% EtOH (vol/vol) on a fixed-ratio-5 and water on a fixed-ratio-1 schedule of reinforcement. After 10 weeks, rats underwent extinction training for 7 sessions. Animals were then maintained in their home cages for 2 weeks before being tested for Pavlovian Spontaneous Recovery (PSR; a measure of alcohol seeking) for 4 sessions. Rats were then allowed a week in their home cages before being returned to the operant chamber with access to EtOH and water (relapse). Thirty minutes before the PSR and relapse test sessions, rats received 0, 10, or 20 mg/kg SB-334867.
Results
Responses on the EtOH lever during the 1st PSR test session were ~70 presses/session (3-fold higher than baseline); SB-334867 did not alter responses on the EtOH lever. Under relapse conditions, P rats increased responding on the EtOH lever from 250 (at baseline) to 350 responses/session; both doses of SD-334867 prevented this increase.
Conclusions
The results of this study suggest that activation of orexin-1 receptors is not involved in intrinsically initiated EtOH seeking, but may regulate the consummatory behavior of EtOH consumption.
Keywords: ethanol reinforcement, alcohol seeking, Pavlovian Spontaneous Recovery, alcohol relapse
Neurons that contain orexin (hypocretin) are solely located in the hypothalamus.1 Yet, orexin-positive neurons have a dispersed and prominent innervation pattern of cortical and subcortical regions. In particular, orexin neurons innervate the mesolimbic dopamine system and other regions associated with obtainment of positive reinforcers. For example, orexin-containing neurons are activated during foraging (food seeking2) and are thought to modulate satiety and approach behaviors. The regulation of alcohol dependence and self-administration by the orexin system is thought to be mediated through a brain-arousal stress function.3
Orexin has been hypothesized to alter the HPA axis by activating CRF-expressing neurons in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala.4 Conversely, there are reciprocal connections from CRF containing neurons in the extended amygdala to orexin neurons in the lateral hypothalamus.5 Thus, orexin is thought to mediate drug reinforcement/reward through regulation of the stress pathway that may impact on some aspects of drug addiction.3
The involvement of orexin in drug reinforcement and/or the development of drug addiction has been examined recently. Stimulation of the orexin system by injections of leptin potentiated heroin seeking in rats that were food restricted.6 Orexin-A, the endogenous agonist of the orexin-1 receptor, can reinstate responding on a lever previously associated with the self-administration of cocaine (cocaine seeking) and increase the threshold for intracranial self-stimulation.7 In addition, an orexin-1 receptor antagonist eliminated footshock-induced cocaine seeking.7
The effects of manipulations of the orexin system on EtOH intake and EtOH-seeking behaviors have been examined. Administration of the orexin-1 receptor antagonist, SB-334867, reduced olfactory cue-elicited reinstatement of responding in inbred P rats.8 In Long-Evans rats, SB-334867 reduced maintenance EtOH self-administration and responding during yohimbine-induced (anxiety-induced) reinstatement of EtOH and sucrose responding.9 In addition, although basal levels of mRNA expression were similar between inbred P and inbred nonalcohol preferring rats, chronic EtOH consumption increased the number of orexin mRNA positive neurons in the hypothalamus (Lawrence et al, 2005). Cue-elicited EtOH seeking increased the number of Fos-positive CART neurons within the accurate nucleus and hypothalamic orexin neurons.10 The effects of microinjection of orexigenic peptides into discrete brain regions have also been examined. Microinjections of orexin and galanin into the paraventricular nucleus significantly increased EtOH intake in Sprague-Dawley rats.11 Orexin microinjected into the lateral hypothalamus increased EtOH consumption, whereas orexin microinjected into the nucleus accumbens failed to alter EtOH consumption.11
Animal models of drug seeking have been developed, which attempt to parallel drug craving that occurs in humans. It has been hypothesized that drug craving is a critical precipitating factor to relapse.12 The reinstatement of responding model can be considered a drug-elicited craving model, because priming injections of drugs of abuse are used to elicit drug seeking (de Wit and Stewart 1983).13,14 Exposure to a physical stressor (eg, footshock) or creating a physical state of extreme distress (yohimbine) can elicit behaviors that were previously associated with drug self-administration (stress-induced drug seeking).9,15 Cue-elicited drug seeking is readily observed after presentation of discriminative stimuli previously paired with the availability of a drug.16,17 Contextual drug-seeking models are unique in that the drug self-administration environment is used to elicit drug seeking. The 2 models that examine contextual drug seeking are the renewal and spontaneous recovery paradigms. The renewal model can be described as the recovery of an extinguished behavior that is dependent on a change in context (Bouton and Bolles, 1979). Briefly, subjects are trained to self-administer a drug in one environment (A), the behaviors are extinguished in a different context (B), and responding is returned when the animal is returned to the original context (A). The renewal model has been recently used to study drug-seeking behaviors.18,19
Spontaneous recovery is defined as a recovery of responding, in the absence of the previously trained reward, which is observed after a period of rest after extinction.20,21 In the alcohol field, the term spontaneous recovery has been used to define the phenomenon of human alcoholics terminating alcohol consumption without any outside intervention. Therefore, to avoid confusion, we have used the term Pavlovian Spontaneous Recovery (PSR). Conceptually, PSR is a unique phenomenon in which it is time dependent, and the behavior seems to be dependent on the reexposure of the organism to all the cues in the behavioral environment previously associated with the reinforcer. The expression of a PSR is directly correlated to reward saliency,21,22 contextual cues associated with first-learned signals, and the amount of first- and second-learned associations.23 In general, the PSR phenomenon has been asserted to be the result of an intrinsic shift away from the recent extinction (second) learning to the initial reinforced learning responses, which reflects an intrinsic motivation to obtain the previously administered reward.24 –26 Therefore, the PSR model may represent a unique paradigm to study craving-like behaviors.
P rats readily express a PSR for EtOH.27,28 Periadolescent EtOH drinking potentiates the expression of an EtOH PSR when tested during adulthood.27 Additionally, the expression of an EtOH PSR can be enhanced by exposure to EtOH odor or EtOH priming.27,28 Thus, responding in the PSR test has a high degree of face validity for an animal model of alcohol-seeking behavior.29
The alcohol deprivation effect (ADE) is defined as a temporary increase in the voluntary intake of EtOH when EtOH is reinstated after a period of alcohol deprivation.30,31 The ADE has been used to examine the efficacy of pharmacological agents to reduce or prevent alcohol relapse.32–34 Under operant or free-choice alcohol drinking conditions, P rats exhibit a robust ADE (McKinzie et al, 1998; Rodd-Henricks et al, 2000).35
Pharmacological studies suggest that different mechanisms may underlie relapse drinking and on-going alcohol drinking. For example, serotonin-3 receptor antagonists were less effective in reducing 24-hour EtOH intakes of P rats during relapse conditions than in reducing EtOH intakes under on-going maintenance conditions (Rodd-Henricks et al, 2000). Moreover, the operant paradigm used in this study has been used to examine the involvement of metabotropic glutamate 2/3 receptors (mGluR2/3) in EtOH-seeking and relapse behaviors.36 The results of this study indicated that the mGluR2/3 agonist LY404039 effectively reduced both EtOH-seeking and EtOH-relapse responding but had little effect on on-going EtOH responding.
The objective of this study was to examine, using P rats, the effects of the orexin-1 receptor antagonist SB-334867 on operant EtOH-responding during relapse and EtOH-seeking conditions. The overall hypothesis to be tested was that antagonism of the orexin-1 receptor would reduce EtOH-seeking behavior and EtOH self-administration under relapse conditions.
MATERIALS AND METHODS
Animals
Selectively bred adult female P rats from the 57th to 58th generations weighing 250 to 325g at the start of the experiment were used. Rats were maintained on a 12-hour reversed light-dark cycle (lights off at 0900 hours). Past research has indicated that there are sex differences in the orexin system of rats. Specifically, the expression of mRNA, which encode orexin-1 receptors in the hypothalamus, is greater in female rats than that in male rats.37 Conversely, the mRNA expression level of orexin-1 receptors in the pituitary gland and orexin-2 receptors in the adrenal gland is higher in male rats than that in female rats.37 To date, there have been no reported sex differences in the efficacy of orexin agents to alter behavioral responses.38
Food and water were available ad libitum throughout the experiment, except during operant testing. The animals used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996).
Operant Apparatus
EtOH self-administration was conducted in standard two-lever experimental chambers (Coulbourn Instruments, Whitehall, PA) contained within ventilated, sound-attenuated enclosures. Two operant levers were located on the same wall and were placed 15 cm above a grid floor and 13 cm apart. Directly beneath each lever was a trough through which a dipper cup (0.1 mL) was raised to deliver response-contingent fluid. On a reinforced response, a small light cue was illuminated in the drinking trough during the 4-second dipper cup access. A personal computer controlled all operant chamber functions and recorded lever responses and dipper presentations. Levers associated with EtOH or water were counterbalanced among rats but remained constant for each animal. Operant sessions were 60 minutes in duration and were conducted daily.
Operant Training
Without any prior training, exposure to the experimental set-up, or access to EtOH, rats were placed in the operant chambers. The EtOH (15% vol/vol) and water levers were maintained on a fixed-ratio (FR)-1 schedule of reinforcement for the first 5 weeks. Subsequently, the reinforcement schedule on the EtOH lever was increased to FR3 in weeks 6 and 7 and to FR5 in weeks 8 to 10. The reinforcement requirement was increased for 2 reasons: (1) to demonstrate that EtOH was a more potent reinforcer than water and (2) to have a high baseline of responding for EtOH. Water was always reinforced on an FR1 schedule because increasing the requirement would result in a further reduction in the low level of responding. The responses on the water lever were important during the spontaneous recovery and reinstatement test sessions to help evaluate a nonspecific general increase in motor activity from goal-directed responding on the EtOH lever. The number of responses on the active and inactive lever and the number of EtOH and water reinforcements were recorded.
Extinction
After the P rats had established stable levels of responding on the FR5 schedule for EtOH and FR1 for water, rats were placed in the operant chambers during the 60-minute time period, but neither water nor EtOH was available. Water was also not available during the extinction procedure because of the potential of unwanted idiosyncratic behaviors (ie, superstitious behaviors).21 The lever previously associated with the delivery of EtOH was maintained on an FR5 schedule, and the lever previously associated with the delivery of water was maintained on an FR1 schedule. With the exception of no fluid being presented, the delivery system operated exactly as during acquisition; rats still received the auditory stimulus of the dipper raising and the visual cue of the small light being illuminated above the dipper trough. Rats were exposed to the extinction sessions for 7 consecutive days.
PSR Testing (EtOH Seeking)
After extinction training, all rats were maintained in the home cages for 14 days. Rats were exposed to the PSR testing for 4 consecutive days. Similar to the extinction phase of the experiment, both the EtOH and water troughs were empty. The lever previously associated with the delivery of EtOH was maintained on an FR5 schedule and the lever previously associated with the delivery of water was maintained on an FR1 schedule. Except for the absence of fluids, the delivery system operated exactly as during acquisition.
Relapse
After the PSR phase of the experiment, all rats were maintained in the home cages for 14 days. Rats were then transferred to the operant chambers with both EtOH and water available for the 60-minute sessions. The EtOH lever was maintained on an FR5 schedule and the water lever on an FR1 schedule.
Drug Preparation
SB-334867 was purchased from Sigma (St. Louis). The antagonist was suspended in 3% dimethyl sulfoxide in water, and administered intraperitoneally 30 minutes before the operant test session.
Effects of SB-334867 on EtOH-Seeking and EtOH-Relapse Drinking
After acquisition and extinction training, 23 adult P female rats were randomly assigned to 1 of the 3 groups, which received 0, 10, or 20 mg/kg SB-334867 before the 1st PSR test session. These same rats were also used to test the effects of SB-334867 during relapse, using a counterbalanced design (ie, rats that were administered 10 mg/kg SB-334867 during the PSR test sessions were equally distributed to separate groups that received 1 of the 3 doses of SB-334867 during the relapse testing). For EtOH reinstatement/relapse testing, rats received 0, 10, or 20 mg/kg SB-334867 before the 1st relapse session.
Statistical Analyses
Overall, operant responding (60 min) data were analyzed with a mixed factorial analysis of variance with a between-subject factor of “dose of SB-334867” and a repeated measure of “session.” For the PSR experiments, the baseline measure for the factor of session was the average number of responses on the EtOH lever for the last 3 extinction sessions. For the deprivation studies, the baseline measure for the factor of session was the average number of responses on the EtOH lever for the 3 sessions immediately before deprivation. Post hoc comparisons (Tukey β) were performed to determine individual differences. To determine whether there were within-group effects (an increase in EtOH seeking or EtOH consumption in a particular group), t tests were used to compare baseline levels of responding with that observed during PSR and ADE testing.
RESULTS
Effects of SB-344867 on EtOH Seeking
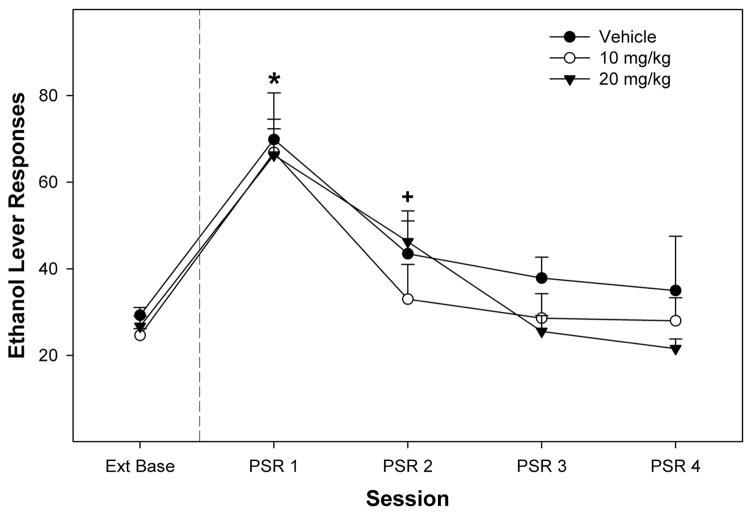
Administration of SB-344867 did not alter responding on the EtOH lever in the PSR test (Fig. 1). Statistically, there was a significant effect of the within subject factor “day” (F4,17 = 31.91; P < 0.0001), but no significant effect of “dose” (F2,20 = 0.96; P = 0.401) or a dose × day interaction term (F8,36 = 0.90; P = 0.535). The significant effect of day was based on each group responding more on the lever previously associated with the delivery of EtOH during 1st PSR test session compared with extinction baseline levels (paired t tests; P < 0.009).
FIGURE 1.
Effects of intraperitoneal injections of SB-334867 on responses on the EtOH lever during the PSR test. Data are mean (±SEM) number of responses on the lever previously associated with the delivery of EtOH (n = 7 or 8/group). The * indicates that all groups are statistically different from extinction baseline levels. The symbol “+” indicates that rats administered 20 mg/kg SB-334867 were elevated, when compared with extinction levels.
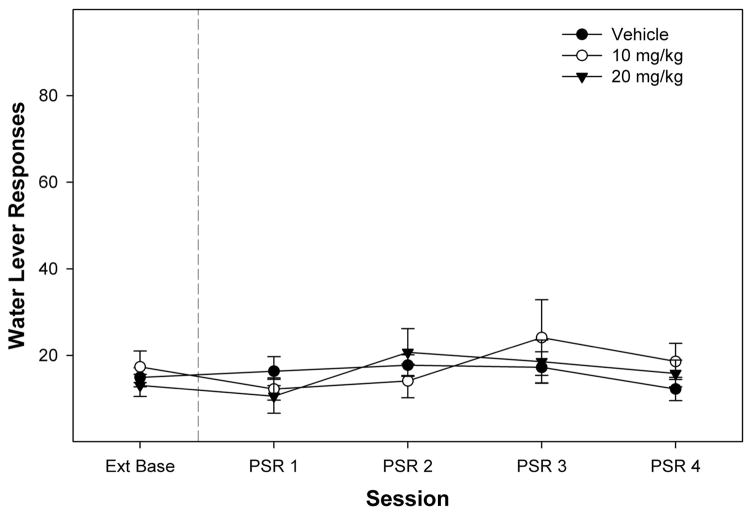
Examining the number of responses on the water lever indicate no effect of SB-334867 treatment or any difference in responding between extinction and PSR testing (Fig. 2). Statistically, there was no effect of the within-subject factor day (F4,17 = 1.1; P = 0.389), dose (F2,20 = 0.1; P = 0.905), or a dose × day interaction term (F8,36 = 1.19; P = 0.331).
FIGURE 2.
Effects of intraperitoneal injections of SB-334867 on responses on the water lever during the PSR test. Data are mean (±SEM) number of responses on the lever previously associated with the delivery of water (n = 7 or 8/group). There were no significant dose or session differences.
Effects of SB-344867 on EtOH Relapse Drinking
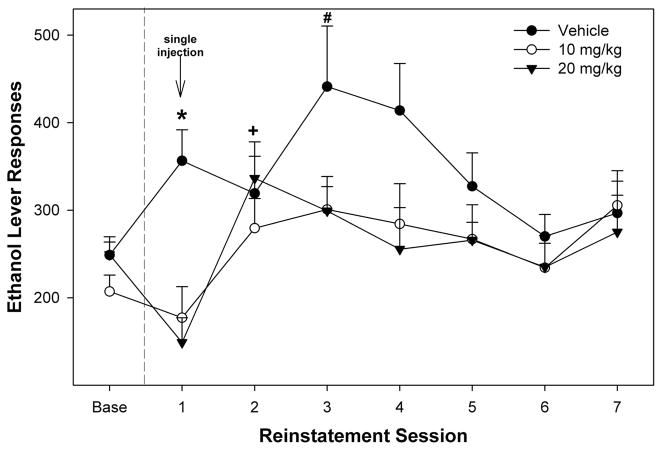
Administration of SB-334867 prevented the increase in EtOH consumption typically observed in the 1st session of relapse drinking (Fig. 3). Statistically, there was a significant effect of day (F4,16 = 5.3; P = 0.006), a significant effect of dose (F2,19 = 6.35; P = 0.008), and a significant dose × day interaction term (F8,32 = 2.86; P = 0.016). An analysis of variance performed on the 1st relapse session indicated a significant effect of dose on responding (F2,19 = 11.660; P < 0.001). Post hoc comparisons indicated that rats administered 10 or 20 mg/kg SB-334867 responded less for EtOH during the 1st relapse session compared with vehicle controls. There was no significant effect of administration of SB-334867 on any other relapse session (P > 0.75). For rats administered vehicle before the 1st relapse session, EtOH responding was increased during the 1st, 3rd, and 4th sessions (paired t tests; P < 0.044), when compared with baseline levels (preextinction responding). In P rats given 10 mg/kg SB-334867 before the 1st relapse session, there was no difference in responding compared with that in the baseline levels for the 1st relapse session (P = 0.248), but intake was increased during the 2nd and 3rd relapse sessions (P < 0.05). In P rats given 20 mg/kg SB-334867, responding for EtOH was significantly reduced compared with that in the baseline levels during the 1st relapse session (P = 0.002), but significantly increased during the 2nd relapse session (P = 0.023). There was no significant difference in baseline (predeprivation) EtOH self-administration between the groups (F2,19 = 1.6; P = 0.221).
FIGURE 3.
Effects of intraperitoneal injections of SB-334867 on EtOH self-administration under relapse conditions. Data are mean (±SEM) of responses on the EtOH lever. Baseline values are the averages of the last 3 acquisition sessions before extinction training. The symbol “*” indicates that the vehicle group was different from the other 2 groups, and responding was higher in vehicle and lower in the 20 mg/kg group, when compared with baseline. The symbol “+” indicates that the 10 and 20 mg/kg group responded more during the 2nd relapse session, when compared with baseline. The symbol “#” indicates that the vehicle and 10 mg/kg groups responded more than baseline.
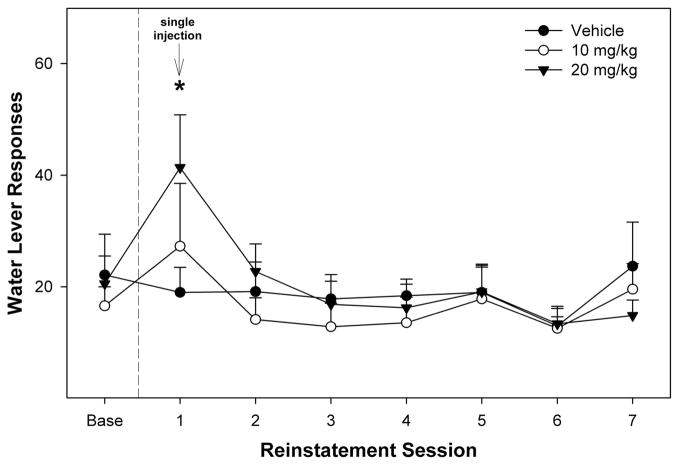
The overall analysis indicated that SB-334867 did not alter water responding during EtOH relapse drinking testing day (F4,16 = 1.3; P = 0.32), dose (F2,19 = 0.8; P = 0.47), or dose × day interaction term (F8,34 = 0.57; P = 0.80; Fig. 4). Yet, performing similar within-subject post hoc comparisons for the water data as with the EtOH data, rats administered 20 mg/kg SB-334867 before the 1st relapse session increased water self-administration (t test, P < 0.004) during this session.
FIGURE 4.
Effects of intraperitoneal injections of SB-334867 on water self-administration under relapse conditions. Data are the mean (±SEM) of responses on the water operant lever. The symbol “*” indicates that responses on water lever were higher than baseline for the 20 mg/kg group.
DISCUSSION
The current data indicate that the involvement of the orexin-1 receptor system in modulating EtOH-seeking and -relapse drinking is complex. The orexin-1 receptor antagonist SB-334867 is not effective at reducing EtOH seeking in the PSR test (Fig. 1). In contrast, SB-334867 can delay the expression of an ADE when administered before the 1st relapse session (Fig. 3), but the efficacy of SB-334867 to maintain the postrelapse reduction in EtOH drinking was not examined in this study. These results suggest that different mechanisms may be involved in EtOH-seeking behavior and alcohol relapse and that orexin-1 receptors are not involved in intrinsic EtOH seeking but may be involved in alcohol drinking.
The current findings conflict with published reports indicating that SB-334867 reduced odor cue-induced EtOH seeking in iP rats8 and yohimbine-induced EtOH or sucrose seeking in Long-Evans rats.9 The major difference between the current experiment and those reports is that EtOH-seeking in the PSR paradigm is intrinsically initiated. Systemic administration of SB-334867 produces a number of behavioral effects including reduced olfactory sensation,39 decreased locomotor activity, and a reduction in anxiety-like behaviors (anxiolytic).40 Therefore, it is impossible to divorce the physical effects of peripheral administration of SB-334867 on olfaction8 and anxiety7,9 from any possible reduction in drug seeking.
Orexin-containing neurons in the hypothalamus have been proposed to influence specific behaviors. Specifically, orexin neurons in the lateral hypothalamus have been postulated to influence various aspect of drug reward, whereas arousal and stress is regulated by orexin neurons that originate from the perifornical and dorsomedial hypothalamus.41 The activation of orexin neurons in the hypothalamus by context-induced drug-seeking behaviors has been examined.18 In the key lateral hypothalamus region, an increase in c-fos expression in orexin-negative, but not orexin-positive, neurons was observed following an EtOH ABA renewal procedure.18 The level of activation of nonorexin neurons in the lateral hypothalamus was positively correlated with EtOH-seeking.18 Context-induced EtOH-seeking (ABA renewal) did increase activity within orexin neurons in the perifornical hypothalamus, and this activation was prevented by D1 antagonists.18 Yet, the researchers concluded that the activation of orexin neurons in the perifornical hypothalamus was based primarily on the arousal of being tested.19 A similar immunohistochemical study examining activation in the hypothalamus that resulted form context-induced cocaine-seeking revealed no activation of orexin neurons in the hypothalamus.19
Overall, the results suggest that the orexin-1 receptor antagonist SB-334867 is not effective in reducing EtOH-seeking behavior when the expression is context induced. However, SB-334867 may be efficacious in reducing the effects of precipitating environmental factors (stress and conditioned discriminative stimuli) on EtOH seeking and relapse.
The involvement of the orexin-1 receptor system on relapse EtOH consumption is partially elucidated by the current findings. In this study, the 10 and 20 mg/kg doses were effective in preventing the expression of an ADE, but did not reduce EtOH self-administration below baseline levels (Fig. 3). In the absence of SB-334867, P rats expressed an ADE in the subsequent operant session (2nd reinstatement session). The efficacy of the orexin-1 receptor antagonist to chronically block the expression of an ADE was not assessed in this study.
The effects of SB-334867 were not tested in this study on maintenance of responding for EtOH. Richards et al8 reported that SB-334867 reduced on-going EtOH self-administration, but not sucrose. Orexin-A microinjected into the paraventricular nucleus or LH increased EtOH intake in Sprague-Dawley rats.11 In inbred P rats, SB-334867 reduced maintenance oral EtOH self-administration.8 The data indicate that the orexin system may regulate the consummatory behaviors associated with EtOH self-administration.
The involvement of the orexin system with consummatory behaviors has been extensively studied.1 In general, there are neuropeptide systems that stimulate (neuropeptide Y, agouti gene-related peptide, and orexin) and inhibit (melanocortins and α′-melanocortins) consummatory behaviors.42 The ability of SB-334867 to regulate EtOH consumption may be dependent on inhibiting consummatory behaviors. Experiments assessing the effects of SB-334867 on the reinforcing properties (ie, EtOH-induced CPP, progressive ratio self-administration) have not been conducted.
The current data indicate that inhibition of the orexin-1 receptor system does not alter intrinsically initiated EtOH-seeking. Previous reports have indicated that orexin-1 receptor antagonists may be efficacious at reducing environmental factors that may precipitate EtOH seeking. The current findings, and previous reports, indicate that the orexin system regulates a consummatory aspect of EtOH self-administration and may be used to alter such behaviors in humans.
Acknowledgments
Supported by AA07611, AA10717, AA10021, AA07462, and Eli Lilly & Co.
The skillful technical assistance of Tylene Pommer and Victoria McQueen are gratefully acknowledged.
References
- 1.de Lecea L, Kilduff TS, Peyron C, et al. They hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–33. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamus paraventricular nucleus and central amygdaloid nucleus of rats: Possible involvement of central orexins on stress–activated central CRF neurons. Regul Pept. 2004;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Winsky-Sommerer R, Yamanaka A, Diano S, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): A novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shalev U, Yap J, Shaham Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutrel B, Kenny J, Specio SE, et al. Role of hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence AH, Cowen MS, Yang HJ, et al. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards JK, Simms JA, Steensland P, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayas CV, McGranahan TM, Martin-Fardon R, et al. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Schneider ER, Rada P, Darby RD, et al. Orexigenic peptides and alcohol intake: Differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien CP, Childress AR, McLellan T, et al. Integrating systematic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1998;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- 13.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 14.Shaham Y, Adamson LK, Grocki S, et al. Reinstatement and spontaneous recovery of nicotine-seeking in rats. Psychopharmacology. 1997;130:396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- 15.Le AD, Quan B, Juzytch W, et al. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 16.Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 17.Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- 18.Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 19.Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Domjan M, Burkhard B. The Principles of Learning and Behavior. Monterey, CA: Brooks/Cole Publishing; 1982. [Google Scholar]
- 21.Macintosh JJ. Stimulus Control: Attentional Factors. In: Honig WK, Staddon JER, editors. Handbook on Operant Behavior. NJ: Englewood Cliffs, Prentice-Hall; 1977. pp. 162–241. [Google Scholar]
- 22.Robbins SJ. Mechanisms underlying spontaneous recovery in autoshaping. J Exper Psychol Anim Behav Processes. 1990;16:235–249. [Google Scholar]
- 23.Brooks DC. Recent and remote extinction cues reduce spontaneous recovery. Q J Exper Psycho. 2000;153:25–58. doi: 10.1080/027249900392986. [DOI] [PubMed] [Google Scholar]
- 24.Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Bio Psych. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 25.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 26.Rescorla RA. Experimental Extinction. In: Mowrer RR, Klein SB, editors. Handbook of Contemporary Learning Theories. NJ: Mahwah, Erlbaum; 2001. pp. 119–154. [Google Scholar]
- 27.Rodd-Henricks ZA, Bell RL, Kuc KA, et al. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats. I. Periadolescent exposure. Alcohol Clin Exp Res. 2002;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- 28.Rodd-Henricks ZA, Bell RL, Kuc KA, et al. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats. II. Adult exposure. Alcohol Clin Exp Res. 2002;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- 29.Rodd ZA, Bell RL, Sable HJK, et al. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;33:107–115. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Sinclair JD, Senter RJ. Increased preference for ethanol in rats following deprivation. Psychonomic Sci. 1967;8:11–12. [Google Scholar]
- 31.Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- 32.Heyser CJ, Schulteis G, Durbin P, et al. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- 33.Kornet M, Goosen C, Van Ree JM. The effect of interrupted alcohol supply on spontaneous alcohol consumption by rhesus monkeys. Alcohol Alcohol. 1990;4:407–412. [PubMed] [Google Scholar]
- 34.Spanagel R, Zieglgansberger W. Anti-craving compounds for ethanol: New pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–59. [PubMed] [Google Scholar]
- 35.Rodd ZA, Bell RL, Kuc KA, et al. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- 36.Rodd ZA, McKinzie DL, Bell RL, et al. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Jöhren O, Neidert SJ, Kummer M, et al. Prepro-Orexin and orexin receptor mRNA are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142:3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- 38.Jöhren O, Neidert SJ, Kummer M, et al. Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides. 2002;23:1177–1180. doi: 10.1016/s0196-9781(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 39.Aplebaum AF, Perrut A, Chaput M. Orexin A effects on the olfactory bulb spontaneous activity and odor responsiveness in freely breathing rats. Regul Pept. 2005;129:49–61. doi: 10.1016/j.regpep.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki M, Beuchmann CT, Shikata K, et al. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behaviors. Brain Res. 2005;1044:116–121. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Harris JA, Aston-Jones G. Arousal and reward: A dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Naslund E, Hellstrom PM. Appetite signaling: From gut peptides and enteric nerves to brain. Physiol Behav. 2007;92:256–262. doi: 10.1016/j.physbeh.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Boutrel B, de Lecea L. Addiction and arousal: The hypocretin connection. Physiol Behav. 2008;93:947–951. doi: 10.1016/j.physbeh.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardy AB, Aioun J, Baly C, et al. Orexin A modulates mitral cell activity in the rat olfactory bulb: Patch-clamp study on slices and immunocytochemical localization of orexin receptors. Endocrinology. 2005;146:4042–4053. doi: 10.1210/en.2005-0020. [DOI] [PubMed] [Google Scholar]
- 45.Heyser CJ, Schulteis G, Koob GF. Increased ethanol self-administration after a period of imposed ethanol deprivation in rats trained in a limited access paradigm. Alcohol Clin Exp Res. 1997;21:784–791. [PubMed] [Google Scholar]
- 46.Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: Implications for novel treatments. Am J Med Gen. 2008;148C:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- 47.Shibata M, Mondal MS, Date Y, et al. Distribution of orexins-containing fibers and content of orexins in the rat olfactory bulb. Neurosci Res. 2008;61:99–105. doi: 10.1016/j.neures.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Zebrowska-Lupina I, Kleinrok Z. Behavioural effects of yohimbine administered intraventricularly in the rat. Psychopharmacolgia. 1973;33:267–275. doi: 10.1007/BF00423061. [DOI] [PubMed] [Google Scholar]