Abstract
Neuroendocrine tumors (NETs) comprise a group of rare tumors derived from the diffuse neuroendocrine system or islet endocrine cells of the pancreas. The molecular mechanisms underlying NETs are largely unknown. The tumor suppressor p53 plays a critical role in maintaining genomic stability and tumor prevention. The p53 pathway is tightly regulated by a number of proteins, among which MDM2, MDM4, and WIP1 are key negative regulators of p53 protein levels or activity. Aberrant activation of these negative regulators can attenuate the p53 function that serves as an important mechanism of tumorigenesis. In this study, several genetic alterations in pancreatic NETs were studied. These tumors exhibit various chromosomal aberrations throughout the whole genome as examined by array-based comparative genomic hybridization. Although p53 mutations are rare in NETs (<3%), this study presents evidence that the p53 pathway is altered in pancreatic NETs through aberrant activation of its negative regulators. A high percentage of pancreatic NETs contain extra gene copies of MDM2 (22%), MDM4 (30%), and WIP1 (51%), which are correlated with expression of corresponding mRNAs and proteins. In addition, there is a higher frequency (23% v. 15% in the control population) of the G/G genotype of MDM2 SNP309, a functional single-nucleotide polymorphism in the MDM2 gene that attenuates the function of the p53 protein. Overall, approximately 70% of pancreatic NETs have one or more of these genetic changes. These findings suggest that the negative regulation of p53 function could be an important mechanism for the initiation and/or progression of pancreatic NETs, and reactivation of p53 could be a potential therapeutic strategy for patients with this disease.
Keywords: p53, MDM2, MDM4, WIP1, neuroendocrine tumor
Introduction
Neuroendocrine tumors (NETs) comprise a group of tumors derived from peptide- and amine-producing cells of the neuroendocrine system, which are located largely in the gastrointestinal system but also in other tissues, including pancreas and lung.1-4 They are characterized immunohistologically by the expression of markers of endocrine tissue, such as chromogranin A, synaptophysin, and neuron-specific enolase, which can be used, in conjunction with clinical presentation, in the diagnosis of NETs. The incidence of NETs is 2 cases per 100,000 persons, and they account for 0.5% of all human malignancies.5 Despite a relatively low incidence, NETs represent a significant clinical challenge in diagnosis and clinical management. Although surgery is an effective and preferred treatment for localized tumors, there are minimal options for metastatic disease. Conventional chemotherapy lacks effectiveness and specificity and is not considered for first-line therapy in most patients.
The pathogenesis of NETs has not been studied extensively, and the molecular events underlying the initiation and progression of NETs are largely unknown. Therefore, understanding of genetic mechanisms of NETs is pressing for the development of targeted and effective treatment of the disease. The tumor suppressor p53 plays a critical role in maintaining genomic stability and tumor prevention.6,7 Disruption of p53 function commonly leads to the initiation or progression of tumors. More than 50% of all tumors harbor p53 mutations, and more than 80% of tumors have an impaired p53 signaling pathway.8 p53 is a haploinsufficient gene in both mice and humans, and a small change in levels of expression or activity of p53 protein has a significant impact on the frequency of tumorigenesis.9,10 Both p53 protein levels and activities are closely regulated in cells by a number of positive and negative regulators to maintain its proper function (Fig. 1).6,7,11 Among them, MDM2, MDM4, and WIP1 are critical negative regulators for p53.6,12 MDM2 is an E3 ubiquitin ligase that can bind to p53, resulting in its polyubiquitination and degradation.13 MDM4 can physically interact with p53 to regulate its function in a negative fashion,14 while an MDM2/4 protein complex is required for optimal MDM-2 function.6,14 WIP1 is a serine/threonine phosphatase with oncogenic activity, which can inhibit p53 activity by multiple mechanisms. WIP1 dephosphorylates the upstream activators of p53 (ATM, Chk1, and Chk2) and p53 itself to inactivate p53 function. It also dephosphorylates MDM2 at Ser395 to stabilize MDM2 and enhances MDM2-mediated p53 degradation.15,16 Amplifications of MDM2, MDM4, or WIP1 have been frequently observed in various human tumors, which are often mutually exclusive with p53 mutations.12,14 These findings strongly suggest that aberrant activation of MDM2, MDM4, or WIP1 can attenuate p53 function, which in turn serves as an important mechanism of tumorigenesis. Recently, a functional single-nucleotide polymorphism (SNP) in the promoter region of MDM2 (SNP309, a T to G change) was identified, which can increase MDM2 expression and attenuate p53 activity and thus significantly affect human tumorigenesis.17,18
Figure 1.
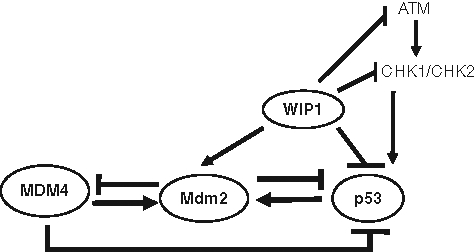
The p53 pathway detects a wide variety of stress signals. The levels and function of p53 are tightly regulated by its regulators, including MDM2, MDM4, and WIP1.
We thus hypothesized that despite previous reports that p53 mutations were rare in well-differentiated neuroendocrine tumors,19,20 the p53 pathway might be compromised in pancreatic NETs via genetic changes of key regulators of p53 protein permitting genomic rearrangements. Thus, alerted levels of MDM2, MDM4, or WIP1, which are often mutually exclusive with p53 mutations, could attenuate p53 function in tumor suppression. This potential negative regulation of p53 function could be one of the important mechanisms for the initiation and progression of NETs, and reactivation of p53 could be a potential therapeutic strategy for patients with this disease.
Results
Pancreatic NETs exhibit various chromosomal aberrations
To investigate the genetic changes in NETs, array-based comparative genomic hybridization (CGH) was employed. Genomic alterations were examined in 55 cases of pancreatic NETs collected from the tissue bank at Memorial Sloan-Kettering Cancer Center with institutional review board (IRB) and Human Tissue Utilization Committee (HTUC) approval. As shown in Table 1, pancreatic NETs exhibited extensive chromosomal alterations in most chromosomes ranging from 1 to 20 regions in each tumor examined. Nearly all tumors (98%) had chromosomal alterations, including losses and gains in multiple regions (Fig. 2). Representative cases shown in Figure 2 demonstrate that gains were observed in chromosome 4 (q13-q35), chromosome 12 (p13-p11 and q12-22), and chromosome 6 (q14). Losses were observed in chromosome 11 (telomeres, q13, q24). Furthermore, there were significantly more chromosomal alterations in larger tumors than small tumors and more in metastatic tumors compared with their primary tumors. Together, these results clearly demonstrate that although most patients with pancreatic NETs have indolent clinical behavior, these tumors exhibit significant genomic instability. These genetic alterations appear to accumulate during tumor progression. Extensive genomic instability in tumors has often been associated with p53 mutations or inactivation of the p53 pathway.
Table 1.
Various Chromosomal Aberrations in Pancreatic Neuroendocrine Tumors (n = 55) Detected by Comparative Genomic Hybridization
| Chromosome | Loss | Gain |
|---|---|---|
| 1 | p36 | q31 |
| 2 | q36 | p14, q24, q32 |
| 3 | q13 | |
| 4 | q13-35 | |
| 5 | p12, p14, q12, q13, q34 | |
| 6 | q14 | |
| 7 | q11 | p21, q21, q31 |
| 8 | p22, q21 | |
| 9 | p21, p24, q21-22, q31 | |
| 10 | q24 | q21, p15 |
| 11 | Tel, q13, q24 | |
| 12 | p13, p12, q12, q14, q21 | |
| 13 | q13, q14, q22 | |
| 14 | q21, q22, q23, q32 | |
| 15 | ||
| 16 | q13, q12 | |
| 17 | Tel, q25 | q24.3 |
| 18 | q21, q22 | |
| 19 | q13 | |
| 20 | q11 | |
| 21 | ||
| 22 | q12, q13.3 |
Note: Events of loss or gain were represented in more than 50% of cases.
Figure 2.

Representative examples of chromosomal alterations in pancreatic neuroendocrine tumors (NETs) detected by comparative genomic hybridization (CGH).
Overexpression of negative regulators in the p53 pathway in pancreatic NETs
The tumor suppressor p53 gene and its pathway play an important role in maintaining genomic stability and preventing tumor formation.6 The loss of p53 is often the prerequisite for the development and progression of many types of tumors. p53 is the most frequently mutated gene in human tumors; more than 50% of all tumors harbor p53 mutations, and more than 80% of tumors have dysfunctional p53 signaling. It has been shown that expression of SV40 T antigen, which inactivates p53 and Rb, in pancreatic endocrine β cells could lead to the development of aggressive pancreatic β-cell tumors, a type of NET, in mice.21 These observations suggest that the p53 pathway plays an important role in preventing pancreatic NETs. However, previous studies using a limited number of NETs (60 tumors) have shown that mutations in the p53 gene are rare in NETs.19,20 In this study, the immunohistochemistry (IHC) of p53 protein was used to detect p53 mutation, because mutations of the p53 gene lead to an extended half-life and increased detectable levels of p53 protein in cells. Tissue microarrays containing 101 cases of pancreatic NETs were used for immunostaining of p53. Consistent with the previous reports, no p53 mutation was detected by IHC in any pancreatic NETs examined (Table 2).
Table 2.
Protein Expression of p53, MDM2, MDM4, and WIP1 in Pancreatic Neuroendocrine Tumors by Immunohistochemistry (IHC)
| Total Case Number | IHC Positive, n (%) | IHC Negative, n (%) | |
|---|---|---|---|
| p53 | 101 | 0 | 101 (100) |
| MDM2 | 171 | 104 (61) | 67 (39) |
| MDM4 | 171 | 98 (57) | 73 (43) |
| WIP1 | 171 | 99 (58) | 72 (42) |
The expression levels of MDM2, MDM4, and WIP1 were evaluated by IHC in 171 cases of pancreatic NETs. As shown in Table 2, 61% of tumors (104/171) revealed positive MDM2 immunoreactivity, which suggests that MDM2 was highly expressed in these NETs. Similarly, 57% of tumors (98/171) showed positive MDM4 staining, and 58% of tumors (99/170) had positive WIP1 staining. The specificity of these antibody-based assays is demonstrated by the failure to detect these proteins in normal tissues in the same tissue sections nor in tumors without gene expression or amplification. Representative tumors with high levels of expression of these proteins are shown in Figure 3. The expression of MDM2, MDM4, and WIP1 in 74 cases with available material was also evaluated at the mRNA levels. Total RNA was extracted from formalin-fixed and paraffin-embedded tissue blocks. The expression levels of MDM2, MDM4, and WIP1 were measured by Taqman quantitative real-time polymerase chain reaction (PCR). The relative mRNA expression was quantified among all tumors examined, with the lowest expression being zero amplicon. There was a significant correlation between mRNA expression and positive immunoreactivity of MDM2, MDM4, and WIP1 (Fig. 4), with P < 0.0001, P = 0.005, and P < 0.0001, respectively. These results strongly suggest that several oncogenic regulators in the p53 pathway, including MDM2, MDM4, and WIP1, are expressed and activated in the majority of pancreatic NETs, which could attenuate p53 function and lead to tumorigenesis.
Figure 3.

Representative immunohistochemical (IHC) staining in pancreatic neuroendocrine tumor (NET) specimens with high expression levels of MDM2 (left), MDM4 (middle), or WIP1 (right). The positive result is depicted by strong nuclear staining. Original magnification, 200x.
Figure 4.

Correlation of protein expression by immunohistochemistry and relative mRNA expression by quantitative reverse transcription polymerase chain reaction of MDM2, MDM4, and WIP1.
Employing Taqman real-time PCR copy number assays, amplification of these genes in NETs was examined. As shown in Table 3, 22% of tumors (38 of 169 cases) showed MDM2 amplification with a range of copy number from 4 to 22. Among them, 89% of tumors (34/38) with MDM2 amplification revealed positive MDM2 immunoreactivity. In addition, 25% of tumors (45 of 150 cases) showed MDM4 amplification with a range of copy number from 4 to 10, and 76% of tumors with MDM4 amplification (34/45) showed positive immunoreactivity for MDM4. WIP1 amplification was observed in 51% of tumors (86 of 169 cases), with a range of copy number from 4 to 27, and 84% of tumors with WIP1 amplification (72/86) showed positive staining of WIP1 (Table 3). There was no significant difference in MDM2, MDM4, and WIP1 gene amplification and protein expression between male and female patients (P = 0.7). Overall, 65% of tumors showed amplification of at least 1 of the 3 genes (Fig. 5).
Table 3.
Amplification of MDM2, MDM4, and WIP1 in Pancreatic Neuroendocrine Tumors and Its Correlation with Protein Expression Detected by Immunohistochemistry (IHC)
| MDM2 | MDM4 | WIP1 | |
|---|---|---|---|
| Amplified/total cases | 38/169 (22%) | 45/150 (30%) | 86/169 (51%) |
| IHC+/amplified cases | 34/38 (89%) | 34/45 (76%) | 72/86 (84%) |
Figure 5.

Venn diagram illustrates the amplification of MDM2, MDM4, WIP1, or G/G genotype of SNP309 in pancreatic neuroendocrine tumors (NETs).
The significant enrichment of MDM2 SNP309 G allele in pancreatic NETs, especially in tumors with positive MDM2 immunoreactivity
SNP309 (a T to G change) has been found in the promoter of the MDM2 gene, whereby the G-allele resulted in higher levels of MDM2 mRNA and protein expression and is associated with the attenuation of the p53 pathway both in vitro and in vivo.17,18 In humans, SNP309 (G/G) is associated with an early age of onset of, and increased risk for, various hereditary and sporadic cancers.22 In this study, the genotype of MDM2 SNP309 in pancreatic NETs was analyzed to investigate its effect on NETs. It is worth noting that the frequency of MDM2 SNP309 differs greatly in different ethnic backgrounds; the frequency of the G allele is about 40% in Caucasian Americans and only 10% in African Americans.23 In this cohort, the great majority of patients were Caucasian, and so as to obtain statistically valid information, only Caucasian patients were analyzed for this study. Specifically, 162 Caucasian individuals with pancreatic NETs were found to have the following relative frequency of 3 different genotypes at the SNP309 locus: T/T, 29.2%; T/G, 47.8%; and G/G, 23%. Two hundred Caucasians, who had never been diagnosed with cancer, were found to have the following relative frequencies: T/T, 40.7%; T/G, 43.5%; and G/G, 15.7% (Table 4). There was a significant enrichment of SNP309 G allele in NET patients (46.9% in NET patients v. 37.5% in the normal population, P = 0.02), which strongly suggests that the SNP309 G allele is associated with an increased risk for NETs. Furthermore, tumors with positive MDM2 immunoreactivity had a much higher frequency of G/G genotype of MDM2 SNP309 (31.1%, n = 90) compared with tumors with negative MDM2 immunoreactivity (14.7%, n = 68; P = 0.02) or normal populations (15.7%, n = 200). Overall, the G allele was associated with more positive immunoreactivity of MDM2 (56.9%) than negative immunoreactivity (39%), P = 0.004 (Table 4).
Table 4.
Frequency Distribution of MDM2 SNP309 Alleles in Pancreatic Neuroendocrine Tumors
| Genotype | Net, n (%) | Con, n (%) | P Value |
|---|---|---|---|
| TT | 47 (29.2) | 81 (40.7) | 0.04 |
| TG | 77 (47.8) | 87 (43.5) | |
| GG | 37 (23) | 31 (15.7) | |
| T | 171 (53.1) | 249 (62.5) | .006 |
| G | 151 (46.9) | 149 (37.5) | |
| MDM2 IHC Staining | |||
| Genotype | Positive, n (%) | Negative, n (%) | P Value |
| TT | 20 (22.2) | 25 (36.7) | .02 |
| TG | 42 (62.2) | 33 (48.5) | |
| GG | 28 (31.1) | 10 (14.7) | |
| T | 82 (43.1) | 83 (61.0) | .004 |
| G | 98 (56.9) | 53 (39.0) | |
Note: There was a significant increase of the G allele in pancreatic neuroendocrine tumors (NETs) compared with control normal population (con), upper panel. The enrichment of the G allele was correlated with positive MDM2 immunoreactivity (lower panel). The P value was obtained using χ2 analysis comparing the differences in genotype/allele distribution.
Furthermore, the majority of genetic alterations, including DNA amplification of MDM2, MDM4, WIP, and G/G genotype of MDM2 SNP309 in these tumors, were largely mutually exclusive. Approximately 65% (112 of 171 cases) of pancreatic NETs had at least 1 of these genetic alterations (Fig. 5). These results strongly suggest that the increased levels of MDM2, MDM4, or WIP1 could significantly attenuate p53 function in tumor suppression and play an important role in the pathogenesis of neuroendocrine tumors.
Genetic alteration of the p53 pathway in cultured NET cell lines
Amplification of MDM2, MDM4, and WIP1 was also analyzed in cultured NET cell lines. NET is an understudied type of cancer, and there have been very limited NET cell lines established. In this study, pancreatic NET cell lines Qgp1 and Bon1, and lung NET cell line H727 cell line, were investigated. Significant amplification of the MDM2 gene was observed in Qgp1 cells (Table 5), with associated much higher MDM2 mRNA and protein levels measured by Taqman real-time PCR and Western blot analysis, respectively (Fig. 6). Genetic alterations in the MDM4 and WIP1 genes were not significant in these cells. These results clearly demonstrate that some of the genetic alterations in the p53 pathway, which attenuate the p53 pathway, also exist in the established NET cell lines.
Table 5.
Amplification of MDM2, MDM4, and WIP1, Expressed as Copy Numbers, in Neuroendocrine Tumor Cell Lines Qgp1, Bone 1, and H727, Respectively
| MDM2 (Copy #) | MDM4 (Copy #) | WIP1 (Copy #) | |
|---|---|---|---|
| Qgp1 | 11 | 2 | 2 |
| Bon1 | 1 | 2 | 1 |
| H727 | 4 | 2 | 2 |
Figure 6.

The mRNA and protein expression levels of MDM2 in neuroendocrine tumor (NET) cell lines were assessed by real-time polymerase chain reaction (PCR) and by Western Blot analysis, respectively. Higher MDM2 expression of both mRNA and protein levels was observed in Qgp1 cells with MDM2 amplification.
Clinical relevance of genetic alterations of MDM2, MDM4, and WIP1 in pancreatic NETs
Well-differentiated NETs have a relatively indolent clinical course when compared with other types of epithelial neoplasms. However, approximately 40% to 50% of patients with pancreatic NETs have metastatic disease either at the time of initial diagnosis or present in a metachronous fashion. The overall disease progression is slow, and metastasis may occur after 10 years of initial diagnosis or surgical resection of the primary tumor. Therefore, prediction of recurrence in association with any biomarkers is difficult with this protracted clinical course. When we evaluated cases with high levels of expression of MDM2, MDM4, and WIP1 by IHC (independent of the cause for this high IHC signal), synchronous and metachronous metastatic disease was identified in 24 of 56 (43%) patients during the clinical follow-up period of 12 months to 12 years. By contrast, only 20% of the patients (4/20) had metastatic disease without high levels of expression of any of these 3 proteins. Although the number of cases is small and some had not had extended clinical follow-up for the adequate prediction of disease outcome, the overexpression of these 3 negative regulators, MDM2, MDM4, and WIP-1, of p53 protein and activity appears to be associated with adverse clinical outcome.
Discussion
Pancreatic NETs are a group of uncommon tumors, occurring in approximately 1 individual in 100,000 of the population.24 Pancreatic NETs are categorized on the basis of their clinical manifestation into functional and nonfunctional tumors. Functional tumors are associated with a clinical syndrome caused by inappropriate secretion of hormones. Nonfunctional tumors are not associated with a distinct hormonal syndrome and often become clinically apparent only because of their large size or invasion of adjacent organs or metastases. Because more than 50% of cases of NETs have malignant clinical behavior, both the tumor burden and the excess levels of hormones released from a functional tumor require treatment. With the development of medical therapies (proton pump inhibitors, somatostatin analogues) that can control hormonal symptoms, antitumor treatment has become more important to control metastatic disease and to prolong survival. Currently, surgery is the only effective treatment for a group of selected patients, and targeted therapies have not been available for control of the tumor burden or tumor progression.25 Therefore, it is prudent to understand the genetic mechanisms underlying pancreatic NETs for the development of targeted and effective treatments.
Little is known about the involvement of particular genes and pathways in NETs. Mutations in MEN1, a putative tumor suppressor gene located on chromosome 11q13, were identified in a small fraction (15%-20%) of sporadic NETs.26-28 In this study, we have demonstrated copious chromosomal alterations in pancreatic NETs, which suggest significant genomic instability in this group of tumors.
The tumor suppressor p53 plays a critical role in maintaining genomic stability and tumor prevention.6,29 As a haplo-insufficient gene, a small change of p53 protein levels, and activity can have a significant impact on tumorigenesis in both mice and humans.30 The known genes that produce proteins that regulate p53 levels and activity are MDM2, MDM4, and WIP1. The p53 protein binds to the P2 promoter of the MDM2 gene and increases the rate of MDM2 transcription. On the other hand, MDM2 inhibits p53 by regulating its location, stability, and transcriptional activity.13 MDM4 is a p53-binding protein with structural homology to MDM2. Binding of MDM4 to p53 can inhibit transcriptional activity of p53. MDM4 can also bind to MDM2 and inhibit MDM2 degradation, thus decreasing the p53 levels.14 WIP1 is a serine/threonine phosphatase, which can be transcriptionally regulated by p53. WIP1 can inhibit p53 activity, especially in response to DNA damage, which forms another negative feedback loop to regulate p53 function.15,16 Thus, these regulators of p53 act as oncogenes when overexpressed. Amplification of MDM2, MDM4, and WIP1 has been commonly observed in various human tumors, which often correlates with their high expression levels.14,15,31 Moreover, tumors with the amplification of MDM2, MDM4, or WIP1 genes rarely contain mutations in the p53 gene. Consistent with previous investigations, we have detected no p53 mutation by overexpression of p53 in pancreatic NETs. Instead, a high percentage of pancreatic NETs overexpress the MDM2, MDM4, and WIP1 proteins in tumor cells, which may be largely contributed by DNA amplification of these genes as well as a higher frequency of the G/G genotype of MDM2 SNP309. All of these genetic changes have been known to attenuate the function of tumor suppressor p53, which in turn contributes to tumorigenesis. Results from this study strongly suggest that the dysfunction of the p53 pathway could be an important mechanism contributing to the initiation and progression of pancreatic NETs. The attenuated function of the p53 pathway may also contribute to the poor response of NETs toward chemotherapy.
The observation that amplification of these oncogenic negative regulators, MDM2, MDM4, and WIP1, is favored over p53 mutations in NETs also provides a potential therapeutic option for pancreatic NETs: reactivate p53 through inhibition of these oncogenic negative regulators. For example, Nutlin-3a is a potent and selective small-molecule antagonist of MDM2 that binds MDM2 in the p53-binding pocket and activates the p53 pathway in cells with wild-type p53.32 It is therefore possible that Nutlin-3a can overcome functional p53 inactivation associated with MDM2 overexpression in pancreatic NETs, which could be employed as a potential therapeutic strategy, especially in combination with chemotherapy.
In summary, the results from this study strongly suggest the involvement of the p53 pathway alteration in pancreatic NETs. Aberrant activation of several negative regulators of p53, including MDM2, MDM4, and WIP1, were observed in a large portion of pancreatic NETs. These findings suggest that the down-regulation of p53 function could be an important mechanism for the initiation and progression of pancreatic NETs, and reactivation of wild-type p53 could be a potential therapeutic strategy for pancreatic NET patients.
Materials and Methods
Patients
Cases of pancreatic neuroendocrine tumors (n = 171, 88 male and 83 female patients) and corresponding clinical data were collected from institution databases from 1996 to 2008 with IRB and HTUC approval.
Tissue samples
Fresh frozen tissue was available for array-based CGH analysis on 55 cases. Formalin-fixed and paraffin-embedded (FFPE) tissue blocks (n = 171) were used for DNA and RNA extraction for gene amplification and messenger RNA expression assay, respectively. In addition, tissue microarrays (TMA) were constructed from FFPE tissue for IHC staining of MDM2, MDM4, and WIP1 protein.
Cell lines
Human pancreatic NET cell line BON1 was cultured in DMEM/F12 (1:1) medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS). Human pancreatic NET cell line Qgp-1 and human bronchial NET cell line NCI-H727 purchased from the American Type Culture Collection were cultured in RPMI1640 (Invitrogen) supplemented with 10% FBS.
CGH
Chromosomal aberration analysis was carried out by CGH using a 44K oligonucleotide probe (60 mers)–based microarray with an average resolution of 35 kb (Agilent, Santa Clara, CA). A minimum of 5 µg DNA in a concentration of >0.2 µg/µL was extracted from fresh frozen tumor tissue and submitted to Sloan-Kettering Institute/Memorial Sloan-Kettering Cancer Center Genomic Core Laboratory for subsequent labeling, hybridization, and image analysis. Positive, negative, and sex-matched controls were applied. The averaged profiles were generated by CGH analysis software from at least 10 to 15 homologous chromosomes. The ratios 1.2 and 0.8 were used as diagnostic cutoff levels indicating overrepresentation (DNA amplification) and underrepresentation (DNA loss), respectively.
Determining gene copy number using Taqman real-time copy number analysis
Gene copy number was determined using a duplex Taqman copy number assay. Copy number assays were performed in triplicate with Taqman genotype Mix (Applied Biosystems, Foster City, CA) in the 7000 ABI sequence Detection System (Applied Biosystems). FAM-labeled primers for target gene and VIC-labeled primer for TERT as an endogenous control were used for duplex assay. Thermocycling conditions included a 95°C, 10-minute hot start followed by 40 cycles of 2-step PCR: 15 seconds at 95°C for denaturing and 1 minute at 60°C for annealing and extension. Two known control samples (carrying 2 alleles) were included in each reaction plate. The ΔΔCt method was used for data analysis. Primers were purchased from Applied Biosystems.
Genomic DNA extraction from FFPE tumor samples
Tumor samples were macro-dissected from FFPE tissues blocks guided with hematoxylin-and-eosin–stained sections. Following the deparaffin procedure with xylene and alcohol, genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA).
Genotyping
The status of MDM2 SNP309 (rs2279744) was determined in the study participants by using a Taqman SNP genotyping assay. Primers were purchased from Applied Biosystems.
Quantitative real-time PCR
RNA was extracted from FFPE tissue using RNeasy® FFPE Kit (Qiagen, Cat. 74404), according to the manufacturer’s protocols. The extracted RNA was reversely transcribed into cDNA and then quantified using TaqMan® real-time PCR with or without PreAmp procedure. Applied Biosystems High-Capacity cDNA Archive Kit (P/N: 4322171; Applied Biosystems) was used with the manufacturer’s protocol for reverse transcription. MDM2, MDM4, and WIP1 expression were evaluated using TaqMan® Gene Expression Assays (Applied Biosystems) with a range of amplicon sizes from 65 to 80 bp. The amplification was carried out on the Applied Biosystems 7500 Fast Real Time PCR System using TaqMan® Master Mix (Applied Biosystems).
Western blot analysis
Standard Western blot assays were used to analyze MDM2 protein expression. Whole-cell lysates were prepared using RIPA buffer (25 mM Tris, pH 7.4, 150 mM NaCl, 1% [v/v] NP-40, 1% [w/v] sodium deoxycholate, 0.1% [w/v] SDS). Protein samples were separated by 4% to 20% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Monoclonal antibody against MDM2, 2A10, was synthesized as previously described.33 The membranes were stained with Ponceau S to check for protein loading amount.
IHC staining
IHC staining for MDM2, MDM4, and WIP1 were performed on TMA from representative paraffin blocks with tumor tissues. The sections were deparaffinzed in xylene and rehydrated with ethanol. They were then steamed for 30 minutes with citrate buffer (0.01 M citric acid, pH 6.0) for antigen retrieval. Endogenous peroxidase was blocked using 95 mL of methanol plus 5 mL of 3% hydrogen peroxide solution. Preparations were washed in phosphate-buffered saline (PBS) solution. Nonspecific protein binding was blocked with 1% bovine serum albumin in PBS for 60 minutes at room temperature. Primary antibodies MDM2 (dilution 1:2000, Cat. M7146; DAKO, Glostrup, Denmark), MDM4 (dilution 1:1000, Cat. IHC-00108; Bethyl Laboratories, Montgomery, TX), and WIP1 (dilution 1:1000; Bioworld Consulting Laboratories, Mt. Airy, MD) were incubated overnight at 4°C on a leveled surface and in a dark, humid chamber. The sections were stained by the streptavidin–biotin–peroxidase method using the LSAB kit plus HRP system (DAKO). To reveal the reactive product, sections were incubated with diaminobenzidine solution and counterstained with hematoxylin. Known positive controls were included in each experiment, and negative controls were obtained by omitting the primary antibodies.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
This study is supported by the Raymond and Beverley Sackler Research Foundation (to L.H.T.), the Mushett Family Foundation (to L.H.T., D.S.K.), and the National Institutes of Health 1P30CA147892-01(to W.H., A.J.L.)
References
- 1. Yao JC. Neuroendocrine tumors: molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab. 2007;21:163-72 [DOI] [PubMed] [Google Scholar]
- 2. Duerr EM, Chung DC. Molecular genetics of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2007;21:1-14 [DOI] [PubMed] [Google Scholar]
- 3. Chan JA, Kulke MH. Progress in the treatment of neuroendocrine tumors. Curr Oncol Rep. 2009;11:193-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kang H, O’Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007;22:183-9 [DOI] [PubMed] [Google Scholar]
- 5. Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(suppl 1):3-7 [DOI] [PubMed] [Google Scholar]
- 6. Levine AJ, Hu W, Feng Z. The P53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13:1027-36 [DOI] [PubMed] [Google Scholar]
- 7. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307-10 [DOI] [PubMed] [Google Scholar]
- 8. Olivier M, Hussain SP, Caron de Fromentel C, Hainaut P, Harris CC. TP53 mutation spectra and load: a tool for generating hypotheses on the etiology of cancer. IARC Sci Publ. 2004;247-70 [PubMed] [Google Scholar]
- 9. Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356: 215-21 [DOI] [PubMed] [Google Scholar]
- 10. Malkin D, Li FP, Strong LC, Fraumeni JF, Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233-8 [DOI] [PubMed] [Google Scholar]
- 11. Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267-75 [DOI] [PubMed] [Google Scholar]
- 12. Wade M, Wahl GM. Targeting Mdm2 and Mdmx in cancer therapy: better living through medicinal chemistry? Mol Cancer Res. 2009;7:1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5:3-8 [DOI] [PubMed] [Google Scholar]
- 14. Marine JC, Dyer MA, Jochemsen AG. MDMX: from bench to bedside. J Cell Sci. 2007;120:371-8 [DOI] [PubMed] [Google Scholar]
- 15. Lu X, Nguyen TA, Moon SH, Darlington Y, Sommer M, Donehower LA. The type 2C phosphatase Wip1: an oncogenic regulator of tumor suppressor and DNA damage response pathways. Cancer Metastasis Rev. 2008;27:123-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu X, Ma O, Nguyen TA, Jones SN, Oren M, Donehower LA. The Wip1 phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell. 2007;12:342-54 [DOI] [PubMed] [Google Scholar]
- 17. Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591-602 [DOI] [PubMed] [Google Scholar]
- 18. Hu W, Feng Z, Ma L, Wagner J, Rice JJ, Stolovitzky G, et al. A single nucleotide polymorphism in the MDM2 gene disrupts the oscillation of p53 and MDM2 levels in cells. Cancer Res. 2007;67:2757-65 [DOI] [PubMed] [Google Scholar]
- 19. Lohmann DR, Funk A, Niedermeyer HP, Haupel S, Hofler H. Identification of p53 gene mutations in gastrointestinal and pancreatic carcinoids by nonradioisotopic SSCA. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:293-6 [DOI] [PubMed] [Google Scholar]
- 20. Weckstrom P, Hedrum A, Makridis C, Akerstrom G, Rastad J, Scheibenpflug L, et al. Midgut carcinoids and solid carcinomas of the intestine: differences in endocrine markers and p53 mutations. Endocr Pathol. 1996;7:273-9 [DOI] [PubMed] [Google Scholar]
- 21. Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115-22 [DOI] [PubMed] [Google Scholar]
- 22. Bond GL, Levine AJ. A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene. 2007;26:1317-23 [DOI] [PubMed] [Google Scholar]
- 23. Atwal GS, Bond GL, Metsuyanim S, Papa M, Friedman E, Distelman-Menachem T, et al. Haplotype structure and selection of the MDM2 oncogene in humans. Proc Natl Acad Sci U S A. 2007;104:4524-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753-81 [DOI] [PubMed] [Google Scholar]
- 25. Ehehalt F, Saeger HD, Schmidt CM, Grutzmann R. Neuroendocrine tumors of the pancreas. Oncologist. 2009;14:456-67 [DOI] [PubMed] [Google Scholar]
- 26. Calender A. Molecular genetics of neuroendocrine tumors. Digestion. 2000;62(suppl 1):3-18 [DOI] [PubMed] [Google Scholar]
- 27. Gortz B, Roth J, Krahenmann A, de Krijger RR, Muletta-Feurer S, Rutimann K, et al. Mutations and allelic deletions of the MEN1 gene are associated with a subset of sporadic endocrine pancreatic and neuroendocrine tumors and not restricted to foregut neoplasms. Am J Pathol. 1999;154:429-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jakobovitz O, Nass D, DeMarco L, Barbosa AJ, Simoni FB, Rechavi G, et al. Carcinoid tumors frequently display genetic abnormalities involving chromosome 11. J Clin Endocrinol Metab. 1996;81:3164-7 [DOI] [PubMed] [Google Scholar]
- 29. Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899-908 [DOI] [PubMed] [Google Scholar]
- 30. Strong LC. General keynote: hereditary cancer: lessons from Li-Fraumeni syndrome. Gynecol Oncol. 2003;88:S4-7 [DOI] [PubMed] [Google Scholar]
- 31. Momand J, Jung D, Wilczynski S, Niland J. The MDM2 gene amplification database. Nucleic Acids Res. 1998;26:3453-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844-8 [DOI] [PubMed] [Google Scholar]
- 33. Chen J, Wu X, Lin J, Levine AJ. mdm-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol Cell Biol. 1996;16:2445-52 [DOI] [PMC free article] [PubMed] [Google Scholar]


