Abstract
Spatial variation in concentrations or flows within an organ and temporal variation in reaction rates or flows appear to broaden as one refines the scale of observation. How can we characterize heterogeneity independently of scale? Fractals come to our rescue! A system is fractal if its features adhere to the same rules through a succession of different scales. Fractals efficiently describe many types of observations, geometric and kinetic, and help to integrate physiological knowledge.
Introduction
Scatter in physiological observations is real and is more than can be accounted for by measurement error. It is a natural phenomenon; there is spatial variation in the densities of stars in various parts of the universe, in regional flows within an organ, or in regional enzyme or receptor concentrations. There is temporal variation in the intensity of the wind, the rates of opening and closing of ion channels, and of velocities of blood in capillaries.
There is a problem in knowing how to describe such variation. For example, consider the variation in local concentrations of a substance within an organ. If the organ is divided into regions, then for 16, 64, or 256 pieces we have only one estimate of the mean, but three different measures of variance. The largest estimate is that for the largest number of pieces, which is for the most refined observations on the smallest sized pieces. Likewise for channel fluctuations: when the duration of openings of an ion channel is measured, the variation is broader when the observations are made over short intervals with high-resolution instrumentation and narrower when made over long intervals with lower fidelity. While this problem seems obvious when considered directly, no standard method of handling it has evolved. In response to a question such as “What is the variance of population densities in this country?”, it is traditional for the statistician to ask, quite reasonably, “What is the size of the domain you wish to consider?” Given an arbitrary choice for size, one can calculate a variance. But our real question is, “How can we describe the system in a fashion that is independent of the magnitude of the domain or period of observation?” This is where Mandelbrot’s fractal concept comes to the rescue.
What are fractals?
Mandelbrot’s coined word “fractal,” like “fraction,” comes from the Latin adjective fractus, from the verb frangere, to break into (irregular) pieces, to fragment. Fractal systems or sets are those whose characteristic form or variation of form or degree of irregularity is the same through a succession of magnifications of scale.
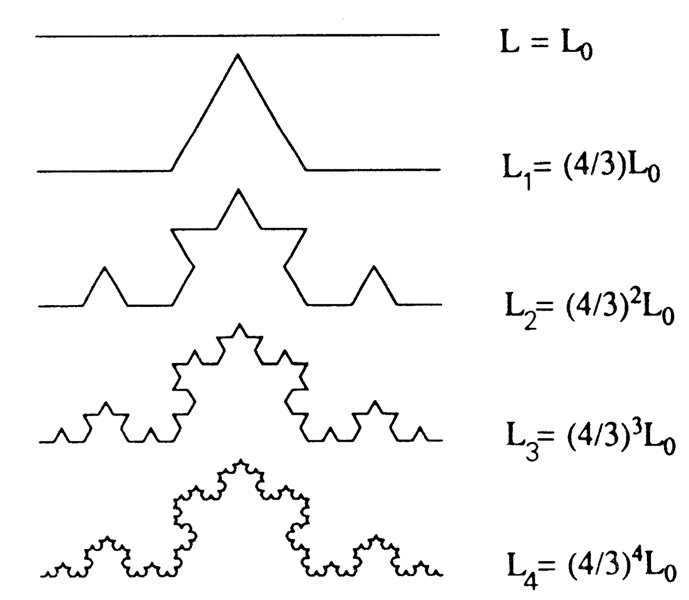
A simple example is a fractal line, shown in Fig. 1. Beginning with a straight line of length L0, the middle third of the line is replaced by two sections of length L0/3, so that the new total length is L0 · 4/3. For the next generation each straight line segment is replaced by four segments in the identical fashion so that the total length is L0 ·(4/3)2. With each succeeding generation the length increases in the same proportion so that by the Nth generation the length LN has grown to L0 · (4/3)N. The step-by-step iteration for the N + 1th step from the Nth is
and the overall expression is
As N → infinity, the length LN does also.
FIGURE 1.
Recursive extension of a line … a simple fractal system. If started from an equilateral triangle, this forms the traditional fractal snowflake. On iteration the length increases by 1/3, i.e., LN + I/LN = 4/3, going to infinity; the fractal dimension D is 1.262.
Now let us turn our viewpoint around and attempt to measure the length of the fractal profile produced as N → infinity. This is the same kind of problem as is measuring the variance in population densities or the length of a coastline: the apparent length of the contour depends on the length of the measuring stick that is used. Consider a pair of calipers set at the fixed length L0: the apparent length of each of the crooked lines in Fig. 1 (and all higher-order lines) is L0, because the calipers cannot measure the out-cropping irregularities. When the calipers are set at length L0/3, then the length for all lines except the first appears to be 4L0/3. And so on, the shorter the caliper setting the longer the measured length of the infinitely long fractal profile. If we call the length of the measuring stick ε, and ε = 1 is the initial reference length, then there is an expression that gives the length measured with each particular stick length
where D is the fractal dimension. Take a particular case, when ε is reduced from 1 to 1/3 and the measure of L is increased by 4/3. Now take logarithms of both sides
or, substituting in the actual lengths
From this, a little algebra gives the fractal dimension, D
In other words
Thus a fractal description of the length of the contour in Fig. 1 is
D is a measure of the irregularity of the system. It is always greater than one in the one-dimensional situations that we consider here. The general statement is that D must equal or exceed (the usual case) the topological dimension (0 for a point, 1 for a line, 2 for a surface, etc.). (Note that the numerator in the fraction of the “word equation” is the log of a number >1, therefore positive, whenever the ratio in the denominator is <1, having a negative log, with the result that the fraction itself is always negative.) A value of D <1 would mean that the measured length of the line gets shorter as the measuring stick gets shorter, which is absurd. A value of 1 means that the exponent 1 − D (or D − 1, see below) is 0, and the measured length is independent of the length of the measuring stick; i.e., there is no irregularity. As the irregularities increase, D also increases, so it serves as a measure of irregularity, roughness, and variation.
When this D is found to be constant over a succession of different measuring stick lengths, as it is for this example, one can say that the system is fractal over the range of the observations. (This would be true for the contour in Fig. 1 even if different ε’s and different fractional ε’s were used, but it would take a series of measurements to get D accurately.) Since real systems are probably never infinitely fractal, extrapolation beyond the observed range entails risk but may nevertheless be advantageous, as discussed below.
In general, fractal systems are those that follow simple rules of recursion, that is, undergo an iterative process by which a feature is changed generation by generation, in discrete steps. The process is “discrete” or discontinuous, as opposed to the usual continuous processes that we consider in physiology. (But the dividing line can be subtle when intervals or the degrees of change are small.) In general, the next generation, the N + 1th, is derived from the present one, the Nth, in a recursive fashion
where f(xN,) is some function of xN and c may be a constant or a random number. Mandelbrot showed that an infinite variety of patterns can be derived solely from , where c is a complex number. The phenomena are wonderfully portrayed by Peitgen and Richter (10), who also give an excellent introduction to fractals.
The structure of natural systems is often fractal
Mandelbrot’s (8) book, The Fractal Geometry of Nature, gives numerous examples of biological and other systems that appear to behave in a fractal fashion. Log-log plots have slopes of D − 1 or 1 − D depending on whether the “measure” is proportional to or reciprocally related to the “measuring stick length.” The diameters of successive branches of the bronchial tree (15) and of the arteries of an organ (13) show log-log relationships with branch length. Fractal rules have been used to construct “trees” (1).
The rules for fractal recursions can be probabilistic as well as deterministic. Random and algebraic relationships can both be encompassed. This is probably what occurs in nature. For example, the length of an unbranched arteriole may equal N diameters, with some scatter in N. If the geometry of the vascular bed is more or less fractal in nature, is it not likely that physiological functions be fractal also? Flow, being governed by the physical geometry and perfusion pressure, is a likely possibility.
Given that the vascular system has the duty to deliver nutrients to every cell in the tissue and that the bronchial tree needs to deliver gas to every alveolus, a space-filling fractal system can be expected to develop naturally. Capillaries can be expected to bud and develop as tissue grows and the vessels supplying them to enlarge as required to provide the flow, so that the end result is a network supplying the whole of the organ.
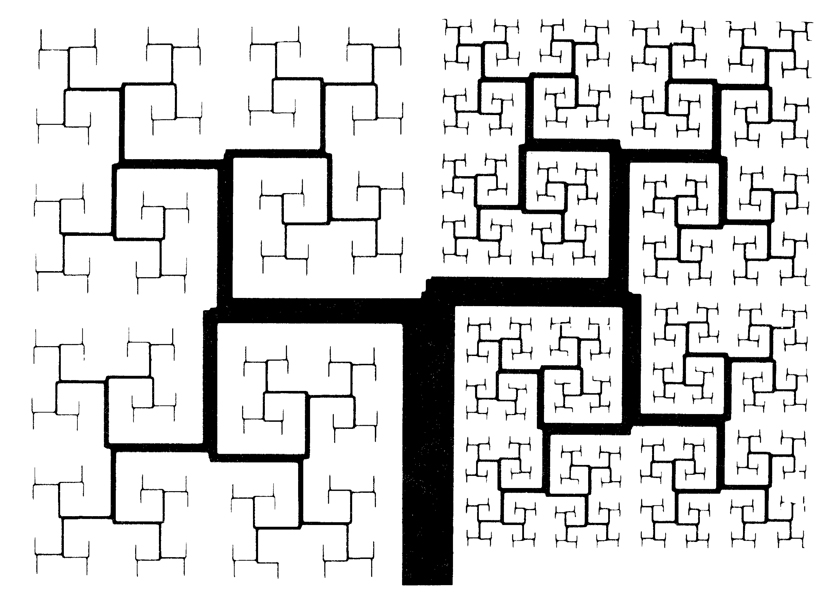
A space-filling system of branches analogous to the bronchial tree is shown in Fig. 2, a plane-filling recursion of straight line segments. The left side of the repetitive branching system has undergone one less division than the right side. The provision for a limit at a given element size, or when the residual uninvaded space reaches a minimum area or volume, renders the - system “pseudofractal,” for it does not divide indefinitely to infinitely small elemental dimension. Lefèvre (6) used a finite iterative scheme to synthesize an “optimal” form of the pulmonary arterial system. No real system is infinitely fractal, but this does not create a problem in using the fractal concept over an appropriate range.
FIGURE 2.
A pseudofractal analog to the bronchial tree, This is a plane-filling recursion in which line thicknesses (diameters) and lengths are not quite self-similar with division: the ratio of line thickness to length decreases with each iteration, going to zero. Ratio was decreased more rapidly for the left side than the right.
Fractals and heterogeneity of regional properties in an organ
Consider any intrinsic characteristic of a system, the brightness of regions in the sky, the densities of dwellings across a land, or, within an organ, the local concentrations of an enzyme or the magnitudes of regional flows. How to measure the variances of these features is the same problem as how to measure variance in population densities, namely, how to define the variance in a fashion that is independent of the size of the unit chosen for making the measurement. When the system is fractal there is a precise answer.
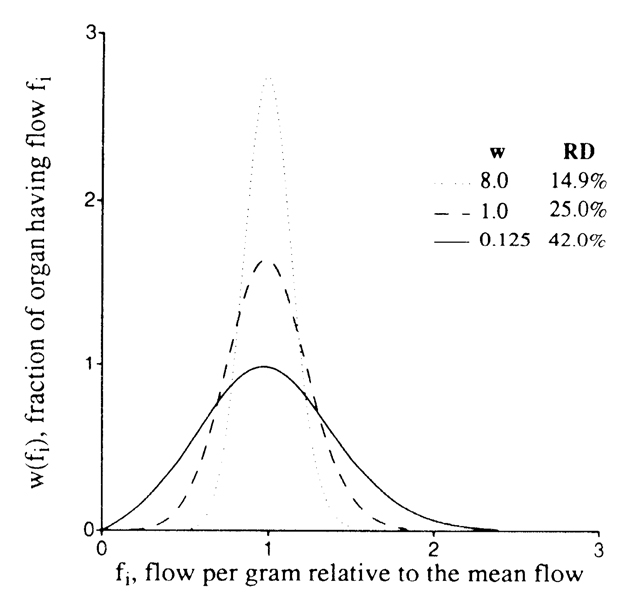
The measurement of variation in regional flows throughout an organ is our example problem. The mean flow per gram of tissue is simply the total flow divided by the mass of the organ Consider the flows every-where to be steady, setting aside consideration of fluctuations for later. The organ is divided into weighed pieces, and the flow to each piece is measured (from the deposition of indicator or microspheres, e.g., Ref. 3), giving us estimates of the flow per gram in each piece. When the organ has been divided into eight pieces, we have, of course, the same estimate of the total organ flow that we started with, but we have the additional information on the variability of regional flows. The relative dispersion (RD) of the regional flows is given by the standard deviation divided by the mean, which is merely the usual coefficient of variation. When each of the 8 pieces is further divided into 8, making 64 pieces, we find that the original 8 pieces were not internally uniform, so that the relative dispersion is larger, as depicted in Fig. 3. Dividing each of these 64 pieces into 8, to a total of 512 pieces in the hypothetical example, broadens the distribution yet further. Now we have three estimates of the variation. How do we compare our results with those found in other laboratories? Which one do we use to report the “true variation?”
FIGURE 3.
Diagram of distribution of blood flows in a 64-g sheep heart. Flows (abscissa) are relative to mean flow for the heart, and ordinate is fraction of the mass of the heart with a given local flow per gram of myocardium. Area of each curve is unity, as for any probability distribution, Fractal description is RD(w) = 25 w −0.25 with w, sample size in grams; RD, relative dispersion, equal to standard deviation divided by the mean; and fractal D = 1.25.
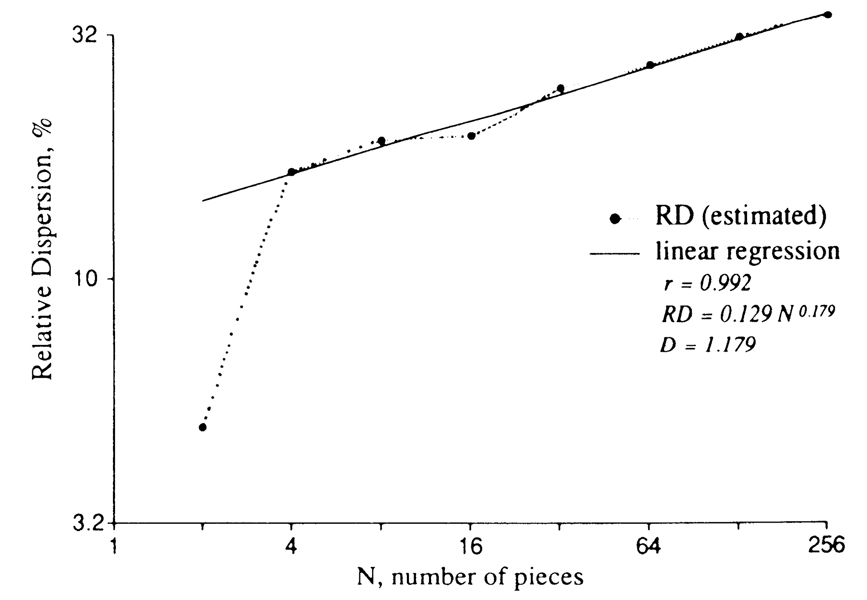
This is where the fractal approach solves our problem. In Fig. 4, the relative dispersion is plotted versus the number of pieces into which the tissue has been divided to make the measurement of variability. What is interesting, and perhaps even remarkable, is that the relative dispersion is so precisely fractal. The sizes of the pieces range from 16 g (for N = 4) to ~250 mg. Over this range the fractal expression that fits the data is
where RD is in %, the extrapolated value of RD(N = 1) = 12.9%, and the fractal dimension D is 1.18. The real value of RD(N = 1) can only be zero, and by definition it cannot be on the fractal curve; thus the use of the intercept obtained by linear extrapolation of the plot to the ordinate, where log 1 = 0, is arbitrary.
FIGURE 4.
Apparent variation in regional myocardial blood flow as a function of the number of pieces into which the heart is cut. RD, relative dispersion, is standard deviation divided by mean. Microsphere distributions in baboon hearts. Data from King et al. (6).
Translation into the size of the tissue pieces is a more general way of expressing the data
where w is the mass of the observed pieces of tissue and the fractal D is again 1.18. The exponent is 1 − D rather than D − 1, because the mass of the individual pieces is the heart mass divided by N, which is thus the reciprocal. The plot of the data expressed in this way is the mirror image of Fig. 4, and, of course, the slope is negative. The reference value of RD is arbitrarily taken to be that for 1-g pieces; this is ~17% in the normal heart.
How can we put this information to work? One application is in relating estimates from one lab to another. Each lab should calculate the fractal D and RD(w = 1 g) to describe the data. Failing that, we might assume that our D applies to their hearts and estimate a value for RD (w = 1 g) for each set of data.
What is the “true” heterogeneity?
Orbach (9) has persuasively argued that systems can be fractal over only a limited range. The concept of flow heterogeneity will not apply to single cardiac cells, because many are supplied by one capillary. At the extremes the system either appears unmeasured (whole heart) or further division is meaningless (single capillary). It is this latter issue that invites debate. When has the system been examined closely enough to reveal the ultimate heterogeneity?
The answer is not an arbitrary one but depends on the function or structure that is being studied. Obviously, the vascular tree cannot be fractal beyond the dimensions of the capillary. A more subtle question is, “What is the size of the functional unit beyond which further refinements in the measurement of local flow are useless?” The answer will depend on further questions. Flow for the delivery of substrate? For the removal of metabolites? For delivery of humoral vasodilators? For the latter, delivery to the arteriole will do, but other solutes have to go farther. Substances with higher diffusion coefficients will have more uniform distributions within the tissue, in spite of differences in flows in neighboring capillaries, than will solutes with low diffusion coefficients or with lipid solubility so low that they do not cross membranes. Presumably, also, flows in immediately adjacent regions will tend to be similar, and flows in widely separated regions less similar. Similarity with proximity, delivery from a common parent artery, diffusional spreading, etc., all have the same effect on the dispersion, namely, to tend toward uniformity within a small region. The curve of Fig. 4 must bend toward a plateau, becoming convex upward and deviating from the purely fractal relationship. Rigaut (11) has observed such curvature in fractal plots of alveolar boundary lengths. The explanation is simply that when regions are so small that they are internally uniform, further magnification or refinement of scale produces no further variance.
From the estimates of interarteriolar distances, one might estimate the size of a functional microvascular unit in the heart to be 0.2–1 mg. By extrapolating the fractal relationship down to this dimension, correcting for the increasing methodological error as the size of the pieces diminishes, we estimate the “true” heterogeneity of local flows at the functional unit level to be near 60%. This is the result we had been seeking.
This large variation has important influences on the net arteriovenous extraction of solutes. Since solute extraction across an organ is most efficient when the flow is uniform, internal nonuniformity might be inefficient unless it is primarily related to local metabolic needs. Heterogeneity partly explains the observation that coronary sinus oxygen concentrations are higher than the mean capillary concentration. Failure to account for this broad heterogeneity in calculating capillary permeability-surface area products (the conductivity of the walls of the capillary for solutes) from tracer extractions results in underestimation by >70 % (2). We take advantage of the fractal nature of flow heterogeneity to estimate the true heterogeneity approximately and to use it in the modeling analysis of tracer dilution curves to avoid the systematic errors.
Structural and dynamic fractals
It seems likely that all neural, epithelial, and endothelial branching systems are fractal in one or more features. If the fractal approach is so widely applicable, so fundamental to biological phenomena, it should be regarded as a primary and basic model for many systems. As such, it may be a replacement for more complex models that are less fundamental. At least, the fractal model should be considered and tested before being rejected in favor of more complicated models. It is very early to say how widespread the applications may be, but at least two classes of fractal phenomena in biology can be identified, structural and dynamic. The fractal structure of the vascular system seems evident from the work of Suwa and Takahashi (13). Similar work is required to describe other branching networks in blood vessels, neural networks, and excretory ducts in organs. Growth patterns are well structured (14) but nevertheless irregular and usually fill the boundaries of their space quite precisely. It is interesting to speculate on whether the basic fractal nature of growth provides some basis for the logarithmic relationship between metabolism and animal size so comprehensively portrayed by Schmidt-Nielsen (12) in his monograph, “Scaling. Why is animal size so important?”
Fractal dynamics may be seen in well-integrated systems as well as in molecular phenomena. In a case as complicated as pulmonary ventilation-perfusion ratios, the basis is in the anatomy of both the vascular and bronchial trees and in the dynamics of local flows and ventilation. Such a system will be difficult to work out in detail because of the difficulties in making refined measurements in both space and time. The fractal analysis of temporal fluctuations in local flow is accomplished by using time as the variable rather than space. Temporal heterogeneity of flow, the standard deviation of the flows over a given interval, τ, divided by the mean over a long time, is broader the shorter the interval τ over which the flow is measured. It is analogous to the spatial heterogeneity, even to the form of the equation
where the choice of the reference interval at 1 s is arbitrary. The value of Dτ, in preliminary analyses of capillary flow fluctuations appears to be ~1.3. As with spatial fluctuations, there must be a deviation from the fractal relationship to a plateau at very short intervals. For flow this must be due in part to the inertia of a moving column of fluid: it cannot be stopped or reversed at infinitely high frequencies without an infinite expenditure of energy.
At the molecular level, the simplest and most fundamental fractal phenomenon is molecular diffusion. Brownian movement by random molecular collisions was described mathematically by Einstein in 1905. For one-dimensional diffusion the fractal D is 2; the spread (standard deviation) of a group of molecules increases in proportion to time; i.e., RD(τ) is proportional to τD-−1. Solute diffusion in fractal meshes of fibers is hindered by collision with the fibers and by reduced fluid mobility in narrow passages. For transcapillary permeation through the inter-endothelial clefts we should consider the process to be hindered diffusion within a fractal fiber mesh with additional hindrance imposed by proximity to the endothelial cell surfaces on either side.
Solute interactions with proteins are probably fractal phenomena. For fatty acid binding to albumin or calcium binding to aequorin, a wide range of rate constants has been observed; the reported rate constants vary severalfold, deviating markedly from a simple first-order process. A possible cause is that multiple collision of the solvent molecules with the protein causes enough flexing of the protein to allow the solute to gain access to the binding site intermittently or with varying ease.
That ion channels are fractal has been demonstrated by Liebovitch et al. (7). Their patch-clamp data show that the durations of channel openings or closings follow a fractal model better than one with mono-or multiexponential rate constants. Their data were for a channel in lens epithelial cells. The time- and voltage-dependent potassium channel in excitable cells is probably fractal; Cole and Moore (4) observed that raising the conductance variable to a power of 25 gave a better fit to the current time course on depolarization than did the power of 4 originally assigned by Hodgkin and Huxley in 1952. Such a high exponent implies a many-level process, probably fractal.
Conclusion
The use of fractals in providing quantitative summarizing descriptions of biological systems is in its infancy. Fractal models of many forms are possible, and they can be applied widely. Their development must proceed as with any other type of model, deterministic or stochastic. This essay illustrates one of the simplest examples of application of fractals to the characterization of a system and may be useful in suggesting others.
Acknowledgments
The author has appreciated the discussions with Dr. Barry Gray (University of Oklahoma) and Dick Slaaf (Limburg University, Maastricht, the Netherlands) in the development of this approach.
This work was supported by Grants HL-19135, HL-19139, and RR-01243 from the National Institutes of Health.
References
- 1.Barnsley MF, Massopust P, Strickland H, Sloan AD. Fractal modeling of biological structures. Ann. NY Acad. Sci. 1987;504:179–194. doi: 10.1111/j.1749-6632.1987.tb48732.x. [DOI] [PubMed] [Google Scholar]
- 2.Bassingthwaighte JB, Goresky CA. Handbook of Physiology. The Cardiovascular System. Microcirculation. vol. IV. Bethesda, MD: Am. Physiol. Soc.; 1984. Modeling in the analysis of solute and water exchange in the microvasculature. chapt. 13; pp. 549–626. sect. 2. [Google Scholar]
- 3.Bassingthwaighte JB, Malone MA, Moffett TC, King RB, Little SE, Link JM, Krohn KA. Validity of microsphere depositions for regional myocardial flows. Am. J. Physiol. 1987;253(Heart Circ. Physiol. 22):H184–H193. doi: 10.1152/ajpheart.1987.253.1.H184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole KS, Moore JW. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys. J. 1960;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King RB, Bassingthwaighte JB, Hales JRS, Rowell LB. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ. Res. 1985;57:285–295. doi: 10.1161/01.res.57.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefèvre J. Teleonomical optimization of a fractal model of the pulmonary arterial bed. J. Theor. Biol. 1983;102:225–248. doi: 10.1016/0022-5193(83)90361-2. [DOI] [PubMed] [Google Scholar]
- 7.Liebovitch LS, Fischbarg J, Koniarek JP, Todorova I, Wang M. Fractal model of ion-channel kinetics. Biochim. Biophys. Acta. 1987;896:173–180. doi: 10.1016/0005-2736(87)90177-5. [DOI] [PubMed] [Google Scholar]
- 8.Mandelbrot BB. The Fractal Geometry of Nature. San Francisco, CA: Freeman; 1983. [Google Scholar]
- 9.Orbach R. Dynamics of fractal networks. Science Wash. DC. 1986;231:814–819. doi: 10.1126/science.231.4740.814. [DOI] [PubMed] [Google Scholar]
- 10.Peitgen HO, Richter PH. The Beauty of Fractals: Images of Complex Dynamical Systems. Berlin: Springer-Verlag; 1986. [Google Scholar]
- 11.Rigaut JP. An empirical formulation relating boundary lengths to resolution in specimens showing non-ideally fractal dimensions. J. Microsc. 1984;133:41–54. [Google Scholar]
- 12.Schmidt-Nielsen K. Why Is Animal Size So Important? New York: Cambridge Univ. Press; 1984. Scaling. [Google Scholar]
- 13.Suwa N, Takahashi T. Morphological and Morphometrical Analysis of Circulation in Hypertension and lschemic Kidney. Munich, FRG: Urban & Schwarzenberg; 1971. [Google Scholar]
- 14.Thompson DAW. On Growth and Form. Cambridge, UK: Cambridge Univ. Press; 1961. [Google Scholar]
- 15.Wilson TA. Design of the bronchial tree. Nature Lond. 1967;213:668–669. doi: 10.1038/213668a0. [DOI] [PubMed] [Google Scholar]