Abstract
DJ-1 and α-synuclein are leading biomarkers for Parkinson disease diagnosis and/or monitoring disease progression. A few recent investigations have determined DJ-1 and α-synuclein levels in plasma or serum, a more convenient sample source than cerebrospinal fluid; but the results were variable or even contradictory. Besides limitations in detection technology and limited number of cases in some studies, inadequate control of several important confounders likely has contributed to these inconsistent results. In this study, the relative contribution of each blood component to blood DJ-1 and α-synuclein was evaluated, followed by quantification of plasma levels of both markers in a larger cohort of patients/subjects (~300 cases) whose cerebrospinal fluid DJ-1 and α-synuclein levels have been determined recently. The results demonstrated that the DJ-1 and α-synuclein in blood resided predominantly in red blood cells (>95%), followed by platelets (1-4%), white blood cells and plasma (≤1%), indicating that variations in hemolysis and/or platelet contamination could have a significant effect on plasma/serum DJ-1 and α-synuclein levels. Nonetheless, after adjusting for the age, although there was a trend of decrease in DJ-1 and α-synuclein in patients with Parkinson or Alzheimer disease compared with healthy controls, no statistical difference was observed in this cohort between any groups, even when the extent of hemolysis and platelet contamination were controlled for. Additionally, no correlation between DJ-1 or α-synuclein and Parkinson disease severity was identified. In conclusion, unlike in cerebrospinal fluid, total DJ-1 or α-synuclein in plasma alone is not useful as biomarkers for Parkinson disease diagnosis or progression/severity.
Keywords: Biomarker, DJ-1, α-synuclein, plasma, Parkinson disease, Alzheimer disease
Introduction
Parkinson disease (PD), the second most common neurodegenerative disorder after Alzheimer disease (AD), remains difficult to diagnose clinically due to its overlapping symptoms with other diseases, such as essential tremor, multiple system atrophy and progressive supranuclear palsy, particularly during the early course of the disease [6, 10]. There is currently no definitive laboratory test for diagnostic purposes, or differentiating the level of motor impairment of PD as signified by the Hoehn and Yahr (H&Y) stage. The development of PD biomarkers is essential as management of PD increasingly focuses on early disease diagnosis and intervention as well as potential disease-altering therapies.
DJ-1 and α-synuclein (α-syn), two proteins intimately involved in familial and sporadic PD pathogenesis [16], have been tested as biomarkers in human cerebrospinal fluid (CSF) for PD diagnosis as well as for PD severity/progression [13, 14, 17, 20]. In a recently finished investigation with a large cohort, where ~300 subjects were included, we have confirmed that, as compared to healthy controls and patients with AD, a decrease in CSF DJ-1 and/or α-syn is a good index for PD diagnosis, but not for PD severity [5].
CSF, though closer to the main site of pathology in PD and related degenerative disorders in the central nervous system (CNS), is not readily accessible in most clinical settings, particularly in developing countries or in remote areas of developed countries; thus, from a clinical diagnostic point of view, one obvious question is whether DJ-1 and α-syn in plasma/serum behave similarly to those in CSF. Indeed, several groups have measured plasma/serum DJ-1 and/or α-syn in controls and PD patients. Unfortunately, the results are inconsistent, and often contradictory to each other [2, 3, 7, 9, 11, 19]. For example, the plasma DJ-1 levels measured by Western blotting and enzyme-linked immunosorbent assay (ELISA) were reported to be increased in PD patients compared with controls, and correlated with the disease severity in PD [19]. Yet none of the results could be confirmed by another study using ELISA [11]. As for α-syn, Lee et al. reported an increase in its plasma levels in PD patients with ELISA [7], while Li et al. showed a decrease in plasma α-syn levels in PD patients compared to controls by Western blotting [9]. Several factors could have potentially accounted for the conflicting results, including variations in the degree of hemolysis and/or contamination of platelets in plasma, as well as variations in assay detection, sensitivity/accuracy, and/or in antibodies used that might detect different species of the proteins.
To address some of the problems discussed above, and to further evaluate the utility of DJ-1 or α-syn in plasma as biomarkers for PD diagnosis and/or severity, we quantified DJ-1 and α-syn levels in plasma samples using the Luminex assays that have been established recently to measure DJ-1 and α-syn in CSF samples [5]. Of note, Luminex assays typically show much higher sensitivity, throughput and efficiency when compared to ELISA or Western blotting. Furthermore, several factors, i.e., hemolysis, platelet contamination, and age-dependence, were evaluated for their effects on plasma DJ-1 and α-syn levels.
Materials and methods
1. Participants
Among 299 subjects evaluated for CSF DJ-1 and α-syn in a recent investigation [5], 122 normal controls, 126 PD and 33 AD patients (a total of 281 subjects) had matching plasma samples, and therefore were included in this study. All individuals provided informed consent, and underwent evaluation that consisted of medical history, physical and neurological examinations, laboratory tests, and neuropsychological assessments. The inclusion and exclusion criteria for controls and patients with AD or PD were described previously for this cohort [5]. Demographic information is listed in Table 1 for all subjects/patients. The study was approved by the Institutional Review Boards of Baylor College of Medicine, Oregon Health and Science University, University of California at San Diego, VA Puget Sound Health Care System and University of Washington, respectively.
Table 1.
Summary of demographics and plasma DJ-1 and α-synuclein values of donors
| CTL | PD stage (H&Y Scale) |
AD | |||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | Total | |||
| Number of cases (age 50+) |
122 (95) |
22 (20) |
60 (53) |
34 (34) |
10 (10) |
126 (117) |
33 (33) |
| Gender (F/M) | 55/67 | 7/15 | 13/47 | 9/25 | 4/6 | 33/93 | 14/19 |
| Age (years) Mean±SD (Range) |
58.9±17.7 (21 - 89) |
59.9±9.0 (42-75) |
60.8±10.7 (37-83) |
69.2±7.4 (55-84) |
72.8±8.8 (58-83) |
63.7±10.6 (37 - 84) |
66.8±10.2 (51 - 86) |
| Duration of disease (years) Mean±SD (Range) |
- | 3.1±2.5 (0-8) |
8.6±5.9 (1-27) |
10.1±8.2 (1-42) |
13.5±5.6 (4-20) |
8.3±6.7 (0-42) |
- |
| Plasma HGB (× 103 ng/mL) Mean±SD (Range) |
98.9±470.8 (2.2-4540.2) |
88.8±137.0 (4.1-650.2) |
67.1±82.9 (4.2-495.5) |
69.7±131.6 (3.2-731.9) |
96.0±221.5 (5.7-724.2) |
74.1±120.8 (3.2-731.9) |
47.7±100.6 (3.4-594.9) |
| Plasma sP-Selectin (ng/mL) Mean±SD (Range) |
32.3±14.4 (9.4-93.9) |
29.7±8.7 (18.6-47.3) |
30.9±11.5 (8.1-73.2) |
29.2±8.8 (6.7-48.4) |
37.1±14.1 (19.8-68.9) |
30.7±10.7 (6.7-73.2) |
32.6±22.3 (11.2-144.8) |
| Plasma DJ-1 (ng/mL; age 50+) Mean±SD (Range) |
63.8±75.3 (2.1-539.3) |
40.0±35.2 (3.3-126.7) |
55.1±54.8 (6.4-374.7) |
45.3±28.8 (4.7-134.8) |
38.9±28.9 (4.4-73.4) |
48.3±43.5 (3.3–374.7) |
45.3±32.9 (4.1-159.5) |
| Plasma DJ-1 (ng/mL; age 50+, HGB <60800 ng/mL and sP-Selectin <34.5 ng/mL) Mean±SD Range |
40.1±30.7 (2.7-150.8) |
37.7±39.8 (5.0-126.7) |
55.0±76.3 (10.6-374.7) |
33.2±21.4 (6.8-77.2) |
28.6±28.0 (4.4-64.1) |
43.0±54.7 (4.4-374.7) |
43.5±26.3 (11.9-128.2) |
| Plasma α-syn (ng/mL; age 50+) Mean±SD (Range) |
49.2±40.0 (8.4-243.2) |
40.0±28.1 (1.7-116.7) |
47.1±25.3 (5.3-102.9) |
42.3±31.1 (5.9-140.9) |
36.3±35.7 (7.0-126.0) |
43.6±28.4 (1.7-140.9) |
36.8±32.2 (0.6-142.4) |
| Plasma α-syn (ng/mL; age 50+, HGB <63900 ng/mL and sP-Selectin <44.8 ng/mL) Mean±SD (Range) |
39.5±25.7 (8.4-113.1) |
40.3±33.3 (1.7-116.7) |
41.3±21.9 (5.3-97.8) |
33.5±22.1 (5.9-71.5) |
25.1±18.5 (7.0-63.0) |
36.8±23.9 (1.7-116.7) |
32.4±28.5 (0.6-142.4) |
PD, Parkinson disease; AD, Alzheimer disease; CTL, healthy controls; HGB, hemoglobin; sP-Selectin, soluble P-selectin.
2. Collection of plasma and quality control
Whole blood was collected from subjects in EDTA-coated tubes and centrifuged at 1500 × g for 15 minutes (4°C). Plasma was transferred to sterile polypropylene tubes on ice, and centrifuged again at 3200 × g for 15 minutes (4°C) to remove platelets. Platelet-free plasma samples were then aliquoted into 1 mL per tube, flash frozen, and stored at −80°C within 90 minutes of blood collection. Before analysis, all samples were only thawed once, with 10% protease inhibitor cocktail (Sigma, St Louis, MO, USA) added and the samples further aliquoted.
3. Blood component separation
To evaluate the contributions of different blood components to plasma DJ-1 and α-syn levels in whole blood, whole blood from seven (7) healthy controls were collected. The platelet-free plasma was collected as described above. Pelleted platelets collected during the second spin were washed once with 2% bovine serum albumin (BSA)/ phosphate buffered saline (PBS) (pH 7.4) and resuspended in 2% BSA/PBS. The white blood cell (WBC)-containing buffy coat from the first spin was poured off from the vacutainer into a 50-mL centrifuge tube. The remaining red blood cells (RBCs) from the bottom of the vacutainer were transferred into a new tube, washed twice with 2% BSA/PBS and resuspended in the same buffer. Residual RBCs in the WBC-containing buffy coat were removed by adding 10 volumes of RBC lysis buffer (131 mmol/L NH4Cl, 0.9 mmol/L NH4HCO3) and incubating for 10 minutes at room temperature. WBCs were then washed with 2% BSA/PBS and centrifuged at 1500 × g for 15 minutes (4°C) several times until the pellet was white, and the pellet was then resuspended in 2% BSA/PBS. The whole blood and separated components all underwent a complete blood count using a XE 2100 hematology analyzer (Sysmex, Mundelein, IL, USA) in the clinical laboratory at Harborview Medical Center (Seattle, WA) and was stored at −80°C before use. The purity of each separated blood component was about 90-100% except for the WBC fraction, thus a sorted WBC preparation was used for further analysis. More specifically, a purer (~100%) WBC preparation was obtained by incubating whole blood with a phycoerythrin-conjugated anti-human CD45 (LCA) antibody (1:60 final; eBiosciences, San Diego, CA, USA) in 2% fetal bovine serum (FBS)/PBS (pH 7.2) for 30 minutes followed by ~3 volumes of RBC lysis buffer for 10 minutes and then sorting WBCs using a Becton Dickinson Aria II flow cytometer (BD, Franklin Lakes, NJ, USA).
4. Bead-based Luminex assays
Plasma DJ-1 and α-syn levels were measured using established Luminex assays as described previously [5] with minor modifications. Briefly, for DJ-1 measurements, plasma samples were diluted directly with 0.1% BSA/PBS (pH 7.4) for a final dilution of 1:8 before assay; for α-syn measurements, plasma samples were treated with equal volume of 2× RIPA buffer and then diluted with 0.1% BSA/PBS (pH 7.4) for a final dilution of 1:100 before incubating with capturing antibody-coupled beads for 3 hours. The incubation time with the detection antibody was 4 hours instead of 3 hours used for CSF samples. All samples were analyzed using a LiquiChip Luminex 200™ Workstation (Qiagen, Valencia, CA, USA).
4. Hemoglobin test
The hemoglobin (HGB) levels in the samples were measured as described previously [5] to establish an index of the degree of RBC contamination or hemolysis in plasma.
5. Soluble P-Selectin test
The soluble P-Selectin (sP-Selectin) levels were chosen as an index of the degree of platelet contamination in plasma and were measured using a Human sP-Selectin/CD62P ELISA Quantitation Kit from R&D Systems (Minneapolis, MN, USA) according to the manufacturer's instructions.
6. Statistical analysis
All analyses were performed with Prism 4.0 (Graphpad, San Diego, CA, USA). To assess differences between groups, one-way ANOVA followed by the Tukey test was used, and correlations were evaluated by Spearman's correlation test and/or linear regression analysis. Values with P<0.05 were regarded as significant.
Results and Discussion
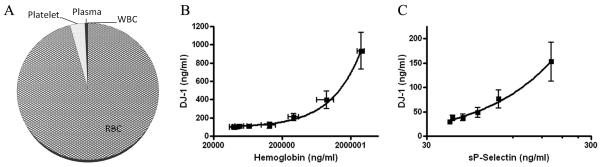
As discussed earlier, the reported results on plasma/serum DJ-1 in PD versus controls are inconsistent or contradictory thus far, and we have hypothesized that there are many variables that need to be controlled for before a meaningful conclusion can be drawn. The first question we asked is what might be the potential sources of plasma/serum DJ-1. To this end, DJ-1 has been identified in the erythrocytes (RBCs) from normal controls and PD patients [15], and it is suggested that DJ-1 may be secreted from cells through microdomains [18]. However, a complete analysis of the levels of DJ-1 in blood has not been performed. To determine the quantitative distribution of DJ-1 in the plasma and different cellular fractions of human blood and to study the potential effects of RBC contamination (or hemolysis in vivo or ex vivo) on plasma DJ-1 levels, we measured DJ-1 levels in different fractions of blood collected from seven (7) healthy subjects. As shown in Fig. 1A, more than 95% of the DJ-1 in blood resides in RBCs with 3.5% of the total detected in platelets and less than 1% in the plasma and WBCs. It is unclear, however, how much, if any, of the DJ-1 detected in plasma is derived from the CNS.
Fig. 1. Quantitative contribution of blood components to DJ-1 levels.
(A) Individual blood components were separated and purified from whole blood from seven healthy subjects, with the cell numbers counted and the DJ-1 levels determined by Luminex assays in the lysed components. The percentage contribution of each component in 1 mL of whole blood was calculated: red blood cell (RBCs) = 95.8%, platelets = 3.5%, white blood cells (WBCs) =0.1%, platelet-free plasma = 0.6%. (B) Purified RBCs from five healthy individuals were lysed and spiked in platelet-free plasma in a series of dilutions respectively, and the DJ-1 levels (shown as mean ± S.D. of 5 subjects) were measured by Luminex, while the hemoglobin levels (shown as mean ± S.D. of 5 subjects), as an index of hemolysis/RBC contamination, were measured using an ELISA kit. A positive correlation between hemoglobin and DJ-1 levels was observed (R2=0.996, P<0.0001). Hemoglobin at 60,800 ng/mL correlated with a DJ-1 level that would increase plasma DJ-1 levels by 10%. (C) Similarly, separated platelets were lysed and spiked in platelet-free plasma and the DJ-1 levels were measured by Luminex, while the soluble P-selectin (sP-Selectin) levels, as an index of residual platelets, were measured using an ELISA kit. A positive correlation was also found between sP-Selection and DJ-1 levels (R2=0.994, P<0.0001). sP-Selectin at 34.5 ng/mL corresponded to a DJ-1 level that would increase plasma DJ-1 levels by 10%.
Given that a majority of DJ-1 in whole blood came from RBCs (Fig. 1A), it is obvious that the DJ-1 levels measured in plasma will be influenced significantly if there were major difference in the scope of hemolysis (in vivo or ex vivo) or RBC contamination during sample preparation. The second major contributor to whole blood DJ-1 is the number of platelets, another variable that is often encountered even if the same protocol was followed to obtain plasma samples. Direct measurements of our samples indicated that HGB and sP-Selectin levels varied significantly among our cohort with a range of ~2,000-5,000,000 ng/mL and ~10-150 ng/mL for HGB and sP-Selectin, respectively. To find out the amounts of RBC and platelet contamination (indexed by HGB and sP-Selectin levels, respectively), which would increase the plasma DJ-1 levels significantly in plasma, we spiked different numbers of RBCs or platelets in pooled platelet-free plasma and measured alterations in DJ-1 amount as a function of HGB or sP-Selectin levels. Based on the spiking experiments, it appeared that when cut-off values of 60,800 ng/mL for HGB and 34.5 ng/mL for sP-Selectin were chosen, the contribution of RBCs and/or platelets to plasma DJ-1 values would be less than 10% (Fig. 1B, 1C). Thus, in addition to analyzing all the samples, to minimize the influence of RBC and platelet contamination in the DJ-1 results, additional analysis was also performed in the cases with HGB levels < 60,800 ng/mL and/or sP-Selectin levels <34.5 ng/mL. As to WBCs, at the proposed HGB cut-off level, <0.01% of total RBCs in blood would remain or lyse in plasma. Assuming the same percentage of total WBCs also remain in the plasma, the contribution of contaminated WBCs to the plasma DJ-1 levels should be minimal.
Similarly, α-syn has been identified in platelets [8, 12] and more recently in RBCs, which appears to be the major source of α-syn in blood [1]. In the current study, we largely confirmed these previous observations and found that more than 98% of the α-syn in blood was detected in RBCs with about 1% of the total in platelets (Supplementary Fig. 1A). The spiking-in experiments identified cut-off values of 63,900 ng/mL for HGB and 44.8 ng/mL for sP-Selectin, respectively, where contribution of RBC and platelet to plasma α-syn would be less than 10% (Supplementary Fig. 1B, 1C). Consequently, to minimize the influence of RBC and platelet contamination in the α-syn analysis, additional analysis was also performed in the cases with HGB levels < 63,900 ng/mL and/or sP-Selectin levels <44.8 ng/mL. Again, it cannot be determined currently as to how much, if any, of the α-syn detected in plasma is derived from the CNS.
The age dependence of DJ-1 and α-syn levels in plasma was also determined in this study. Linear regression and association analyses revealed that, largely consistent with previous studies [11, 19], there was no significant association between DJ-1 plasma levels and age in controls or in AD patients. Nonetheless, a significant decrease in DJ-1 values was detected with aging in PD patients (Supplementary Fig. 2A). In contrast, an age-dependant decrease in α-syn levels was observed in both PD patients and controls, although the statistical difference was achieved only for the latter. A non-statistically significant age-dependant increase in α-syn levels was also observed in AD patients (Supplementary Fig. 2B). It should be noted that a few other earlier studies did not observe a significant association between plasma α-syn levels and aging [2, 4, 7]. One of the potential explanations for this discrepancy could be that a much wider age range of controls (21-89 years; see also Table 1) was investigated in this study. Hence, to control for age-dependent changes, when comparing plasma DJ-1 and α-syn values among different groups, only those aged 50 years and older were included (number of cases for controls, PD and AD were 95, 117, and 33, respectively, in this comparison).
When total plasma DJ-1 levels were measured, the average values were 63.8, 48.3, and 45.3 ng/mL for the control, PD and AD groups, respectively (Table 1). ANOVA analysis revealed that, although there was a slight decrease in DJ-1 in PD and AD patients compared to controls, no statistical difference was observed. Similarly, the average values for plasma α-syn were 49.2, 43.6, and 36.8 ng/mL, respectively (Table 1), again without any statistical significance present by ANOVA analysis in any of the compared groups. Based on the spiking experiments, the data were further analyzed with arbitrary cut-off values for HGB and sP-Selectin for the extent of hemolysis and platelet contamination, respectively. Remarkably, even after eliminating the samples with high HGB and/or sP-Selectin levels, there were still no significant differences between the control group and PD or AD groups. Consequently, the diagnostic sensitivity and specificity of two markers were not evaluated further with Receiver Operating Characteristics (ROC) method as we did for CSF samples [5].
To determine whether there was any correlation between plasma DJ-1 and/or α-syn with PD severity, both values were also stratified against H&Y stages. As shown in Table 1 and Supplementary Fig. 3, although there was a trend for both DJ-1 and α-syn to decrease in later PD stages, no statistically significant correlations were observed for either marker, with or without eliminating cases with significant hemolysis or contamination of platelets. Notably DJ-1 tended to decrease with aging (Supplementary Fig. 2A), meaning that an age effect could have also contributed to this non-significant decrease in DJ-1 levels in PD cases at later stages since these patients were older (Table 1)
Our conclusion drawn on plasma DJ-1 as a PD biomarker is in line with what has been reported by Maita et al. [11], but disagrees with those reported by Waragai et al. who demonstrated that DJ-1 was not only different in PD patients as compared to controls but also correlated with H&Y stages, with higher values in more advanced PD patients [19]. Additionally, the absolute values reported in the current investigation are comparable with Maita's [11], but much higher than those (0.3-3.4 ng/mL) reported by Waragai, et al. [19]. It appears that the same Human DJ-1/PARK7 ELISA kit from CyLex, Japan was used in both previous studies, meaning that the disparity in the DJ-1 levels between these two experiments cannot be simply explained by the different antibodies or ELISA kits used in the studies. Controls for hemolysis and platelet contaminations in plasma/serum were not mentioned in any of these previous investigations. It should also be noted that ELISA-based assays typically demonstrate more noise in blood components (plasma or serum) than in CSF, likely due to a much higher protein content and/or related matrix effect in plasma/serum than in CSF. Matrix effects on DJ-1 and α-syn have been clearly shown in our previous investigations in human CSF samples [5]. Thus, detailed investigation on the effects of matrix should be encouraged for future biomarker discovery or validation in human plasma or serum.
Like plasma DJ-1, accumulated reports on plasma α-syn are also highly variable. While one previous study using Western blotting reported decreased plasma α-syn levels in PD patients compared to normal controls [9], two other studies reported that the plasma α-syn levels increased in PD versus controls with ELISA [2, 7]. The plasma α-syn levels reported in one of the ELISA studies were significantly lower (<100 pg/mL) [7], even below the reported detection range of the kit used in the study [4]; thus it is difficult to interpret the data generated. The discrepancy among studies could also be due to additionally quantified oligomers and other cross-reactive molecules by the ELISA technique [9]. Interestingly, preliminary findings have also shown a significant increase in α-syn oligomers in the plasma of patients with PD compared with controls [3]. Nonetheless, controls for variables such as hemolysis and platelet contaminations in plasma/serum were not mentioned in all the previous reports. Another unanswered question is what it means when extrapolating biomarker protein profiles related to short-lived cells without nuclei (RBCs and platelets) to biochemical properties and changes resulting from neurodegenerative processes in neurons.
Two major advances were made in this study. First, we determined the quantitative distribution of DJ-1 and α-syn in the plasma and different cellular fractions of human blood and confirmed the potential effects of RBC and platelet contamination on the plasma levels of these two proteins. Next, by controlling for the extent of hemolysis and platelet contamination as well as other variables, for the first time, and using a rigorous quantitative technology, we evaluated the utility of plasma DJ-1 and α-syn as biomarkers for PD diagnosis and severity correlation. We conclude that, unlike in the CSF, total α-syn and DJ-1 levels in the blood are not useful as biomarkers for PD diagnosis and/or progression/severity. However, there is a trend for plasma α-syn and DJ-1 levels to decrease in PD patients. Thus, it is possible that these markers could be helpful when combined with other potential plasma biomarkers. Another future direction might be identifying and measuring the species derived from the CNS specifically, or species/isoforms correlating with PD diagnosis/PD progression, such as α-syn oligomers [3] and oxidized DJ-1 [15] in plasma or blood, whether they are derived from the CNS or not.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health grants (ES004696, NS057567, AG025327, AG033398, NS060252, NS062684, AG005136 and AG008017) as well as grants from the Michael J. Fox Foundation. We thank Mr. Joshua Bradner and Travis Cook for their assistance on sample preparation and assays, and Mr. Thomas Quinn for manuscript preparation. We also deeply appreciate those who have donated their blood for our studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, Chilcote TJ. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener. Dis. 2008;5:55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 2.Duran R, Barrero FJ, Morales B, Luna JD, Ramirez M, Vives F. Plasma alpha-synuclein in patients with Parkinson's disease with and without treatment. Mov. Disord. 2010 doi: 10.1002/mds.22928. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 4.Fjorback AW, Varming K, Jensen PH. Determination of alpha-synuclein concentration in human plasma using ELISA. Scand. J. Clin. Lab. Invest. 2007;67:431–435. doi: 10.1080/00365510601161497. [DOI] [PubMed] [Google Scholar]
- 5.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133:713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov. Disord. 2003;18(Suppl 6):S2–12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- 7.Lee PH, Lee G, Park HJ, Bang OY, Joo IS, Huh K. The plasma alpha-synuclein levels in patients with Parkinson's disease and multiple system atrophy. J. Neural Transm. 2006;113:1435–1439. doi: 10.1007/s00702-005-0427-9. [DOI] [PubMed] [Google Scholar]
- 8.Li QX, Campbell BC, McLean CA, Thyagarajan D, Gai WP, Kapsa RM, Beyreuther K, Masters CL, Culvenor JG. Platelet alpha- and gamma-synucleins in Parkinson's disease and normal control subjects. J. Alzheimers Dis. 2002;4:309–315. doi: 10.3233/jad-2002-4406. [DOI] [PubMed] [Google Scholar]
- 9.Li QX, Mok SS, Laughton KM, McLean CA, Cappai R, Masters CL, Culvenor JG, Horne MK. Plasma alpha-synuclein is decreased in subjects with Parkinson's disease. Exp. Neurol. 2007;204:583–588. doi: 10.1016/j.expneurol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Litvan I, Halliday G, Hallett M, Goetz CG, Rocca W, Duyckaerts C, Ben-Shlomo Y, Dickson DW, Lang AE, Chesselet MF, Langston WJ, Di Monte DA, Gasser T, Hagg T, Hardy J, Jenner P, Melamed E, Myers RH, Parker D, Jr., Price DL. The etiopathogenesis of Parkinson disease and suggestions for future research. Part I. J. Neuropathol. Exp. Neurol. 2007;66:251–257. doi: 10.1097/nen.0b013e3180415e42. [DOI] [PubMed] [Google Scholar]
- 11.Maita C, Tsuji S, Yabe I, Hamada S, Ogata A, Maita H, Iguchi-Ariga SM, Sasaki H, Ariga H. Secretion of DJ-1 into the serum of patients with Parkinson's disease. Neurosci. Lett. 2008;431:86–89. doi: 10.1016/j.neulet.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Michell AW, Luheshi LM, Barker RA. Skin and platelet alpha-synuclein as peripheral biomarkers of Parkinson's disease. Neurosci. Lett. 2005;381:294–298. doi: 10.1016/j.neulet.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, Ng J, Schulz-Schaeffer W, Kretzschmar HA, McLean PJ, Trenkwalder C, Sarracino DA, Vonsattel JP, Locascio JJ, El-Agnaf OM, Schlossmacher MG. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol. 2008;213:315–325. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Ohrfelt A, Grognet P, Andreasen N, Wallin A, Vanmechelen E, Blennow K, Zetterberg H. Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neurosci. Lett. 2009;450:332–335. doi: 10.1016/j.neulet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Hamakubo T, Yoshida Y, Ogawa Y, Hara Y, Fujimura H, Imai Y, Iwanari H, Mochizuki Y, Shichiri M, Nishio K, Kinumi T, Noguchi N, Kodama T, Niki E. Preparation and application of monoclonal antibodies against oxidized DJ-1. Significant elevation of oxidized DJ-1 in erythrocytes of early-stage Parkinson disease patients. Neurosci. Lett. 2009;465:1–5. doi: 10.1016/j.neulet.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 16.Thomas B, Beal MF. Parkinson's disease. Hum. Mol. Genet. 2007;16:R183–194. doi: 10.1093/hmg/ddm159. Spec No. 2. [DOI] [PubMed] [Google Scholar]
- 17.Tokuda T, Salem SA, Allsop D, Mizuno T, Nakagawa M, Qureshi MM, Locascio JJ, Schlossmacher MG, El-Agnaf OM. Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem. Biophys. Res. Commun. 2006;349:162–166. doi: 10.1016/j.bbrc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Tsuboi Y, Munemoto H, Ishikawa S, Matsumoto K, Iguchi-Ariga SM, Ariga H. DJ-1, a causative gene product of a familial form of Parkinson's disease, is secreted through microdomains. FEBS Lett. 2008;582:2643–2649. doi: 10.1016/j.febslet.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Waragai M, Nakai M, Wei J, Fujita M, Mizuno H, Ho G, Masliah E, Akatsu H, Yokochi F, Hashimoto M. Plasma levels of DJ-1 as a possible marker for progression of sporadic Parkinson's disease. Neurosci. Lett. 2007;425:18–22. doi: 10.1016/j.neulet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Waragai M, Wei J, Fujita M, Nakai M, Ho GJ, Masliah E, Akatsu H, Yamada T, Hashimoto M. Increased level of DJ-1 in the cerebrospinal fluids of sporadic Parkinson's disease. Biochem. Biophys. Res. Commun. 2006;345:967–972. doi: 10.1016/j.bbrc.2006.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.