Abstract
An overview of all methodologies published during the last few years focused to the stereoselective (diastereoselective or enantioselective) synthesis of α-aminophosphonic acids and derivatives is reported. The procedures have been classified according a retrosynthetic strategy and taking into account the formation of each one of the bonds connected to the chiral centre.
Keywords: α-Aminophosphonic acids, α-Aminophosphonates, Stereoselective Synthesis, Resolution, Chiral Pool
1. Introducción
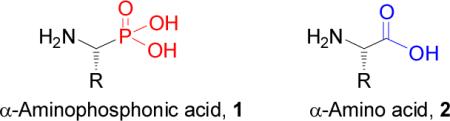
α-Aminoalkylphosphonic acids 1 are structurally analogous to α-amino acids 2, obtained by isosteric substitution of planar and less bulky carboxylic acid (CO2H) by a tetrahedral phosphonic acid functionality (PO3H2). Several aminophosphonic, aminophosphinic and aminophosphonous acids have been isolated from various natural sources either as free amino acids or as constituents of more complex molecules.1 Many natural and synthetic aminophosphonic acids, their phosphonate esters and short peptides incorporating this unit, exhibit a variety of biological properties.2 Their diverse applications include enzyme inhibitors3 such as synthase,4 HIV protease,5 rennin,6 phosphatasa activity,7 PTPases,8 and potent antibiotics,9 as antibacterial agents,10 antiviral,11 antifungal,12 herbicides,13 antitumor agents.14 Their role for antibody generation is also well documented.15 In addition, the incorporation of cyclic amino acids of medium ring size into key positions in peptide chains plays an important role, and constitutes the most prominent pathway to conformationally constrained peptidomimetics, a tool in modern drug discovery.16
In addition, α-aminophosphonic acids and their monoalkyl esters are also of interest in hydrometallurgy in order to extract metals17 and in diagnostic medicine as screening agents, once complexed with lanthanides and actinides.18,19
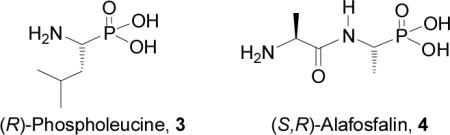
It is well known that the biological activity of α-aminophosphonic acids and derivatives depends on the absolute configuration of the stereogenic α carbon to phosphorous.20 For example, (R)-phospholeucine 3 is a more potent inhibitor of leucine aminopeptidase than the S enantiomer,21 and (S,R)-alafosfalin 4 shows higher antibacterial activity against both Gram-positive and Gram-negative microorganisms than the other three diastereoisomers.22
In view of the different biological and chemical applications of the α-aminophosphonic acids and derivatives, in the last 35 years the development of suitable synthetic methodologies for their preparation in optically pure form has been a topic of great interest in several research groups. In this context, several protocols for efficient asymmetric synthesis of α-aminophosphonic acids and derivatives have emerged in the recent years and several reviews have been published.23 Now we would like to report herein an update over stereoselective synthesis of α-aminophosphonic acids and derivatives from 1998-2007. The principal synthetic strategies of α-aminophosphonic acids and derivatives optically pure can be classified in C-P bond formation using the Strecker type process, C-C bond formation derived from diastereoselective alkylation of phosphonoglycine equivalents, C-N bond formation derived from diastereoselective electrophilic amination, catalytic hydrogenation of dehydro-aminophosphonates, resolution and chiral pool processes.
2. Stereoselective synthesis of α-aminophosphonic acids and derivatives
2.1. Stereoselective C-P bond formation
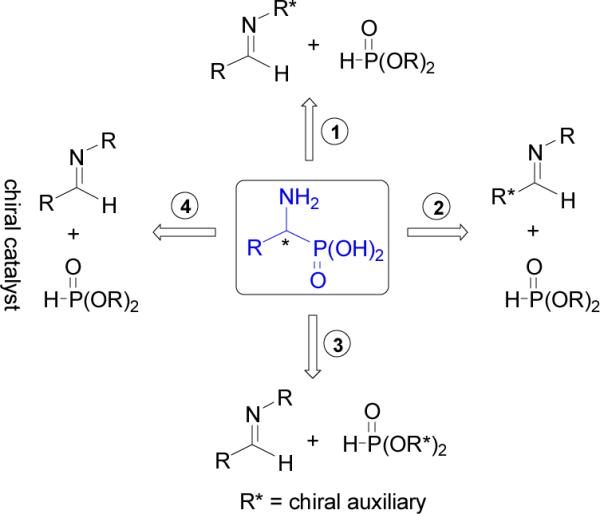
The nucleophilic addition of dialkyl- or diaryl phosphite to imines or oxoiminium derivatives, the Pudovik reaction,24 is one of the most convenient methods for the preparation of α-aminophosphonates, key intermediates in the synthesis of α-aminophosphonic acids. In this context, the stereoselective synthesis of α-aminophosphonates can be carried out by four routes: (1) addition of alkylphosphites to chiral imines readily obtained by condensation of aldehydes with chiral amines, (2) addition of alkyl phosphites to chiral imines readily obtained by condensation of chiral aldehydes with non-chiral amines, (3) addition of chiral alkyl phosphites to non-chiral imines, and (4) addition of non-chiral alkyl phosphites to non-chiral imines in the presence of a chiral catalyst (Scheme1).
Scheme 1.
2.1.1. Addition of alkyl phosphites to imines derived from chiral amines
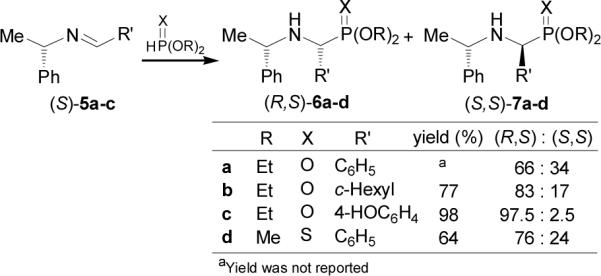
The first synthesis of enantiomerically pure α-aminophosphonic acids was described by Gilmore and McBride in 1972.25 They reported that the addition of diethyl phosphite to the imine (S)-5a readily obtained from condensation of benzaldehyde and (S)-α-methylbenzylamine [(S)-α-MBA], afforded the α-aminophosphonates (R,S)-6a and (S,S)-7a (X = O) with a 66:34 diastereoisomeric ratio.26 A better diastereoselectivity was obtained when the addition of diethyl phosphite to the imine (S)-5b derived from cyclohexanecarboxaldehyde (R' = cyclohexyl) was carried out, where the diastereoisomeric ratio was 83:17.27 Recently, Vovk et al.28 found that the reaction of imine (S)-5c (R' = 4-HOC6H4) with an excess of sodium diethyl phosphite solution gave the α-aminophosphonates (R,S)-6c and (S,S)-7c (X = O) in 98% yield and 95% diastereoisomeric excess. On the other hand, Thompson et al.29 reported that the addition of dimethyl thiophosphite (DMTP) to the imine (R)-5a (R' = C6H5), led to the α-aminophosphonothionates (R,S)-6d and (S,S)-7d (X = S) in 64% yield and 76:24 diastereoisomeric ratio (Scheme 2).
Scheme 2.
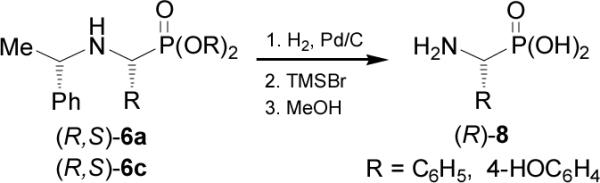
Hydrogenolysis of (R,S)-6a and (R,S)-6c (X = O) followed by hydrolysis with trimethylsilyl bromide (TMSBr) gave the enantiomerically pure (R)-α-aminophosphonic acids (Scheme 3).
Scheme 3.
This methodology has been used in the preparation of calix[4]arene α-aminophosphonic acids, which show inhibitory activity toward porcine kidney alkaline phosphatase.28 In this context, addition of sodium salt of diethyl phosphite to iminocalix[4]arenes 9 and 12 easily obtained through of the condensation of mono- or 1,3-diformilcalix[4]arenes with (S)- or (R)-α-MBA, afforded the corresponding α-aminophosphonates 10 and 13 in 60-80% yield and 75-85% diastereoisomeric excess. Hydrogenolysis of chiral auxiliary in 10 and 13, followed by the hydrolysis of phosphonic esters with TMSBr and methanol gave the mono- and di-α-aminophosphonic acids 11 and 14 in quantitative yield (Scheme 4).
Scheme 4.
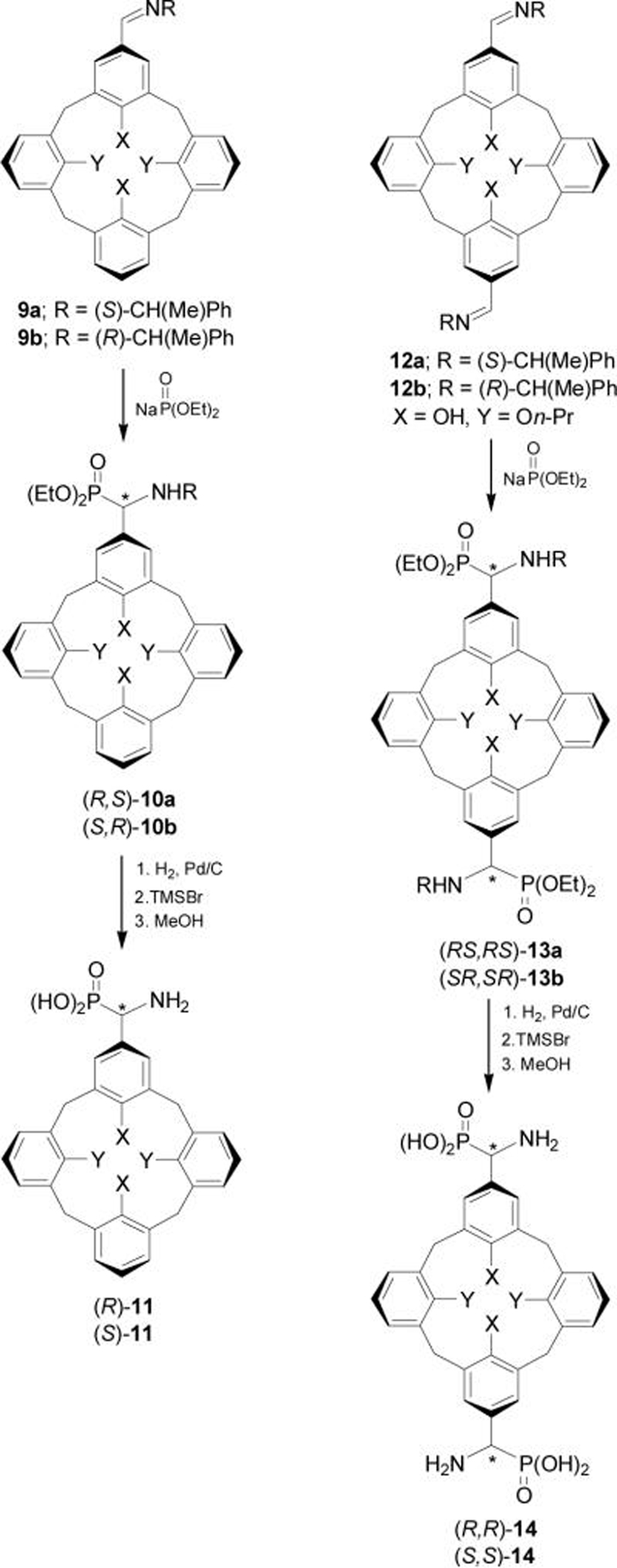
The inhibitory activity of α-aminophosphonic acids (R)-8c, 11 and 14 toward porcine kidney alkaline phosphatase (PKAP) depends considerably on the absolute configuration at the α-carbon atoms. For example, the Ki value for (S)-11 is about two times smaller than that the enantiomer (R)-11, and (R,R)-14 binds to PKAP about 50 times stronger than the (S,S)-14 enantiomer.
Molecular mechanics study on this type of reaction revealed that the diastereoisomeric excess values and the induced direction are controlled by the conformation of the imine substrate.30 In imines such as (S)-5, conformations A and C are destabilized because of allylic 1,3-strain (Figure 1).31 Thus, addition of alkyl phosphites to more stable imine B bearing (S)-α-MBA, takes place by the re face generating the (R,S)-6 diastereoisomer as major product. As consequence, the imines bearing (S)-α-MBA give rise to (R)-α-aminophosphonic acids, whereas using (R)-α-MBA affords the (S)-α-aminophosphonic acids.
Figure 1.
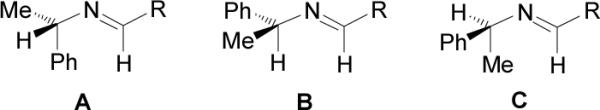
Conformers for imine (S)-5.
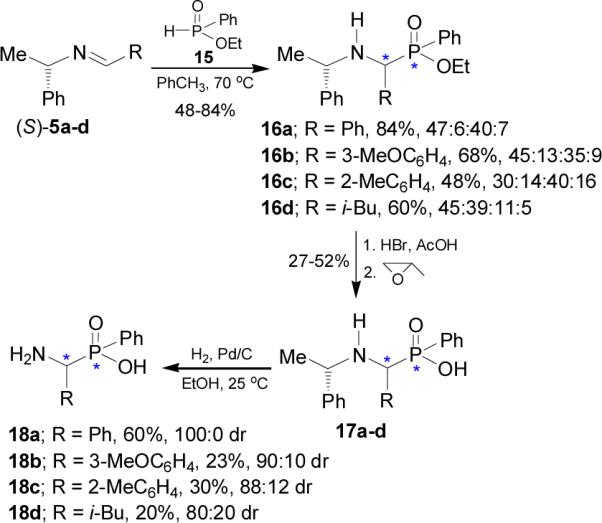
Petneházy et al.32 found that the addition of ethyl phenylphosphinate 15 to imines (S)-5a-d at 70 °C in toluene afforded the α-aminophosphinates 16a-d with a predominance of two of the four diastereoisomers. Hydrolysis of 16a-d with HCl or HBr solution in glacial acetic acid gave the corresponding derivatives 17a-d, which by hydrogenolysis led to α-aminophosphinic acids 18a-d with good to excellent diastereoisomeric ratio (80:20 to 100:0), (Scheme 5).33
Scheme 5.
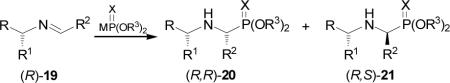
On the other hand, addition of diethyl phosphite to imine 19 obtained in excellent yield from condensation of (R)-phenylglycine t-butyl ester in the presence of Lewis acids such as ZnCl2, MgBr2 and trifluoroacetic acid (TFA), afforded the corresponding α-aminophosphonates (R,R)-20 and (R,S)-21 (Table 1, entries 1-4).34 All three catalysts led to increased rates of addition, indicative of the desired imine activation, but none afforded an increase of the diastereoselectivity. The poor diastereoselectivity obtained indicated that the chelate 22 was not formed. A smaller diastereoselectivity was obtained in the addition of diethyl phosphite or dimethyl phosphite to imine 19, derived from (R)-leucine benzyl ester (Table 1, entries 5-7).35 Similar results were obtained in the reaction of imine 19 with diethyl thiophosphite (Table 1, entries 8-11). However, the addition of lithium salt of diethyl phosphite prepared by treatment of diethyl phosphite with n-BuLi in THF at -78→25 °C, gave the α-aminophosphonates (R,R)-20 and (R,S)-21 with 80% yield and high diastereoselectivity (Table 1, entry 12).
Table 1.
Addition of the salt of alkyl phosphites to imines (S)-19.
| Entry | R | R1 | R2 | R3 | X | Conditions | Yield (%) | 20 : 21 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CO2t-Bu | Ph | c-Hexyl | Et | O | Toluene/r.t. | 87a | 69 : 31 | 34 |
| 2 | CO2t-Bu | Ph | c-Hexyl | Et | O | Toluene/r.t. | 84b | 74 : 26 | 34 |
| 3 | CO2t-Bu | Ph | c-Hexyl | Et | O | Toluene/r.t. | 40c | 69 : 31 | 34 |
| 4 | CO2t-Bu | Ph | c-Hexyl | Et | O | Toluene/r.t. | 46d | 57 : 43 | 34 |
| 5 | CO2Bn | i-Bu | Ph | Et | O | Neat/100 °C | 78 | 50 : 50 | 35 |
| 6 | CO2Bn | i-Bu | Ph | Me | O | Neat/100 °C | 73 | 50 : 50 | 35 |
| 7 | CO2Bn | i-Bu | 2-Furyl | Me | O | Neat/100 °C | 74 | 50 : 50 | 35 |
| 8 | CO2Me | Me | Ph | Me | S | Toluene/r.t. | 17e | 47 : 53 | 29 |
| 9 | CO2Me | CH2OH | Me | Me | S | Toluene/r.t. | 33e | 45 : 55 | 29 |
| 10 | CO2Me | CH2OH | i-Pr | Me | S | Toluene/r.t. | 15e | 70 : 30 | 29 |
| 11 | CO2Me | CH2OH | Ph | Me | S | Toluene/r.t. | 62e | 27 : 73 | 29 |
| 12 | CO2t-Bu | Ph | c-Hexyl | Et | O | n-BuLi/THF | 80 | 98 : 02 | 34 |
120 h
7 h in the presence of ZnCl2
48 h in the presence of MgBr2
48 h in the presence of TFA
The configuration of chiral auxiliar was (S) and the products were (S,S)-20 and (S,R)-2.
The high diastereoselectivity has been explained as result of the coordination of nucleophile with the corresponding imine 19, generating the chelate 23, with a trans relationship between the nucleophile and the stereodirecting phenyl group, and the addition of dialkyl phosphite on the re face of the imine double bound (Figure 2).
Figure 2.
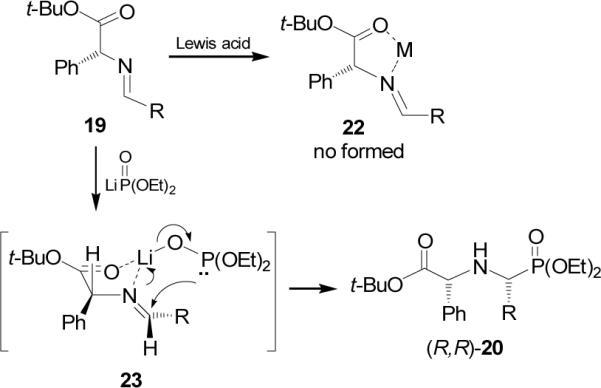
Proposed of addition of LiP(O)(OEt)2to 19.
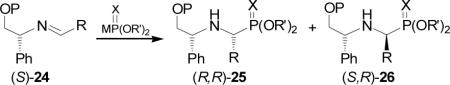
A most effective diastereoselectivity in favour of diastereoisomer (R,R)-25 was obtained in the addition of lithium salt of diethyl phosphite to aldimines 24 bearing methoxymethyl ether derived from (R)-2-phenylglycinol (Table 2, entries 1-10).36 However, the addition of dimethyl thiophosphite to imine 24 gave the α-aminophosphonates (R,R)-25 and (S,R)-26 in moderated and reverse diastereo-selectivity (Table 2, entry 11).29
Table 2.
Addition of the salt of alkyl phosphites to (S)-24.
| Entry | P | R | R' | X | Conditions | Yield (%) | 25 : 26 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Me | c-Hexyl | Et | O | n-BuLi/THF | 68 | 49 : 1 | 36 |
| 2 | Me | c-HexylCH2 | Et | O | n-BuLi/THF | 70 | 114 : 1 | 36 |
| 3 | Me | i-Pr | Et | O | n-BuLi/THF | 82 | 55 : 1 | 36 |
| 4 | Me | i-Bu | Et | O | n-BuLi/THF | 81 | 114 : 1 | 36 |
| 5 | Me | Me | Et | O | n-BuLi/THF | 77 | 41 : 1 | 36 |
| 6 | Me | n-Hexyl | Et | O | n-BuLi/THF | 78 | 49 : 1 | 36 |
| 7 | Me | Ph | Et | O | n-BuLi/THF | 90 | 7.3 : 1 | 36 |
| 8 | Me | CH2OBn | Et | O | n-BuLi/THF | 36 | 49 : 1 | 36 |
| 9 | Me | CH2CH2SMe | Et | O | n-BuLi/THF | 69 | 55 : 1 | 36 |
| 10 | Me | CH2CH2CO2t-Bu | Et | O | n-BuLi/THF | 37 | 49 : 1 | 36 |
| 11 | H | Ph | Me | S | Toluene/ r.t. | 76 | 1 : 3 | 29 |
To explain the results obtained in the Table 2, the authors suggest a chelated intermediate 27 analogous to the structure 23 (Figure 2). Thus, the enantiofacial preference of attack on the aldimine carbon by the phosphorous atom is due to the formation of highly organized cyclic transition state 27 by chelation with the lithium cation, and the anti disposition of the phenyl and phosphite groups presumably directs the addition to the re face of the imine, affording the α-aminophosphonates (R,R)-25 as principal products,37 which by hydrogenolysis over Pd(OH)2 afforded the enantiomerically pure (R)-α-aminophosphonates 28 (Scheme 6).
Scheme 6.
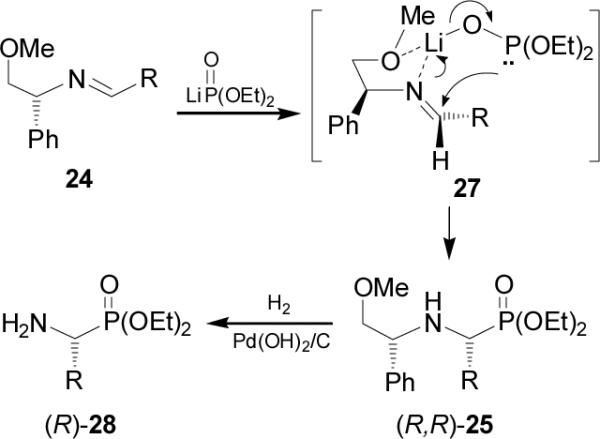
The intramolecular version of nucleophilic addition of phosphites to imines was reported by Dimukhametov et al.38 In this context, the reaction of the chlorophosphite 30 with (R)-N-(benzylidene)-2-aminobutan-1-ol 29 gave the phosphite 31, which after intramolecular cyclization followed of the Michaelis-Arbuzov reaction,39 afforded the 1,4,2-oxazaphosphinanes 32 and 33 in 88% yield and 70:30 diastereoisomeric ratio, which are precursors of chiral α-aminophosphonic acids (Scheme 7).
Scheme 7.
To explain the stereochemistry of this reaction, the authors suggest that the nucleophilic attack by phosphite group on electrophilic C=N group proceeds stereospecifically by the re-face, generating the R configuration at α-C atom to phosphorous as principal product. The attack on the si-face is hindered, in this case the ethyl group would have to adopt an unfavorable axial position in the transition state (Figure 3).
Figure 3.
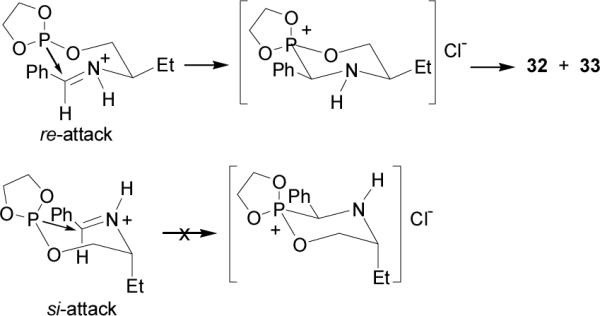
Intramolecular cyclization of 31.
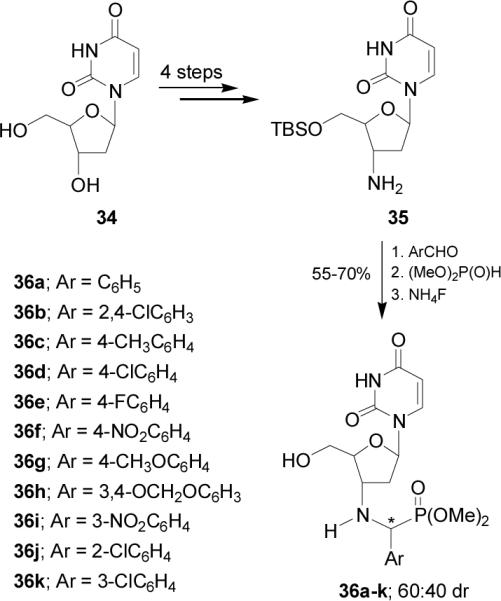
Recently Chen et al.40 reported the synthesis of the α-aminophosphonates 36a-k derivatives of 2'-deoxyuridine 34. In this context, nucleophilic addition of dimethyl phosphite to corresponding imines obtained from condensation of arylaldehydes with the amine 35 obtained in 4 steps from 34, followed by treatment with ammonium fluoride provided the α-aminophosphonates 36a-k in 55-70% yield and 60:40 diastereoisomeric ratio (Scheme 8). The configurations of the three chiral carbon atoms of 35 are known, but the newly formed chiral carbon atom resulting from the addition reaction was not established.
Scheme 8.
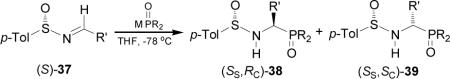
The chiral sulfinimides readily available41 containing an arylsufinyl moiety constitutes a valuable target molecules in asymmetric synthesis.42 For example,43 the addition of lithium or sodium salt of alkyl phosphites to p-toluenesulfinyl imine (S)-3744,45 gave the N-sulfinyl-α-aminophosphonates 38 and 39 in moderated yield and excellent diastereoselectivity with preference of (SS,RC)-38 (Table 3, entries 1-12). On the other hand, the reaction of the lithium salt of bis(diethylamido) phosphite with (S)-37 afforded the α-aminophosphonates (Ss,Rc)-38 and (Ss,Sc)-39 in good diastereoselectivity and with a preference of diastereoisomer (SS,SC)-39 (Table 3, entry 13).46
Table 3.
Addition of the salt of alkyl phosphites to imines (S)-37.
| Entry | R | R' | M | Yield (%) | 38 : 39 | Ref. |
|---|---|---|---|---|---|---|
| 1 | OEt | C6H5 | Li | 85 | 92 : 08 | 43a |
| 2 | OEt | C6H5 | Na | 80 | 96 : 04 | 43a |
| 3 | OEt | 4-MeOC6H4 | Li | 50 | 92 : 08 | 43a |
| 4 | OEt | 4-MeOC6H4 | Na | 50 | 95 : 05 | 43a |
| 5 | OEt | n-Pr | Li | 78 | 92 : 08 | 43b |
| 6 | Oi-Pr | C6H5 | Li | 82 | 74 : 26 | 46b |
| 7 | Oi-Pr | 4-MeOC6H4 | Li | 55 | 93 : 07 | 46b |
| 8 | Oi-Pr | n-Pr | Na | 86 | 99 : 01 | 43b |
| 9 | OMe | C6H5 | Na | a | 88 : 12 | 46a |
| 10 | OMe | C6H5 | Li | a | 94 : 06 | 46a |
| 11 | OMe | 2-furyl | Li | a | 94 : 06 | 46b |
| 12 | OMe | 2-thienyl | Li | a | 95 : 05 | 46b |
| 13 | NEt2 | C6H5 | Li | a | 10 : 90 | 46a |
75-80% yield.
The high diasteroselectivity obtained in the addition of the lithium salt of alkyl phosphites to p-toluenesulfinyl imine (S)-37 may be rationalized by assuming a coordination of lithium to the nitrogen lone pair, facilitating the delivery of the phosphorus atom to the prochiral trigonal carbon center from the face opposite to the sulfinyl oxygen atom (Figure 4).43 However, a model to explain the opposite configuration observed in the addition of bis(diethylamido) phosphite to p-toluenesulfinyl imine (S)-37 has not yet been elucidated.46
Figure 4.
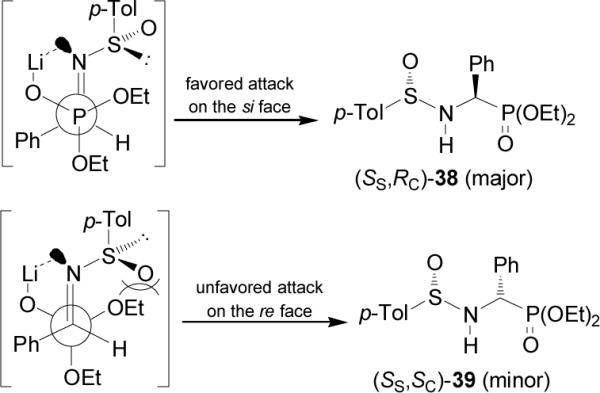
Addition of LiP(O)(OEt)2 to imine (S)-37.
Removal of N-sulfinyl auxiliary in the diastereoisomer (SS,RC)-38 (R' = Ph, and R = OEt) by acidic hydrolysis with TFA in methanol gave the enantiomerically pure α-aminophosphonic diethyl esther (R)-28, whereas the hydrolysis with hydrochloric acid in acetic acid at reflux produced the enantiomerically pure (R)-phosphophenylglycine 8. In a similar way, hydrolysis of (SS,SC)-39 afforded the (S)-phosphophenylglycine 8 (Scheme 9).
Scheme 9.
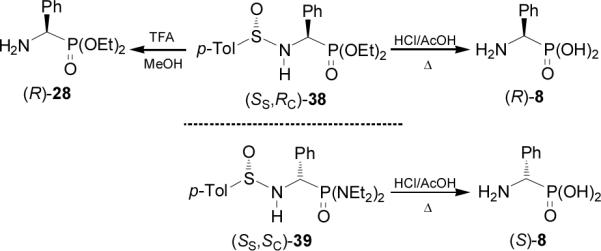
On the other hand, addition of lithium salt of bis(diethylamido) phosphine borane complex to enantiopure p-toluenesulfinyl imines (S)-37a-e in THF at -78°C, afforded the corresponding derivatives (SS,SC)-40a-e as principal diastereoisomers in high yield. Treatment of (SS,SC)-40a-e with hydrochloric acid in AcOH at reflux led to enantiomerically pure (S)-α-aminophosphonic acids 8a-e in good yield. In a similar way, the imines (R)-37a-b were transformed into (R)-α-aminophosphonic acids 8a-b (Scheme 10).47
Scheme 10.
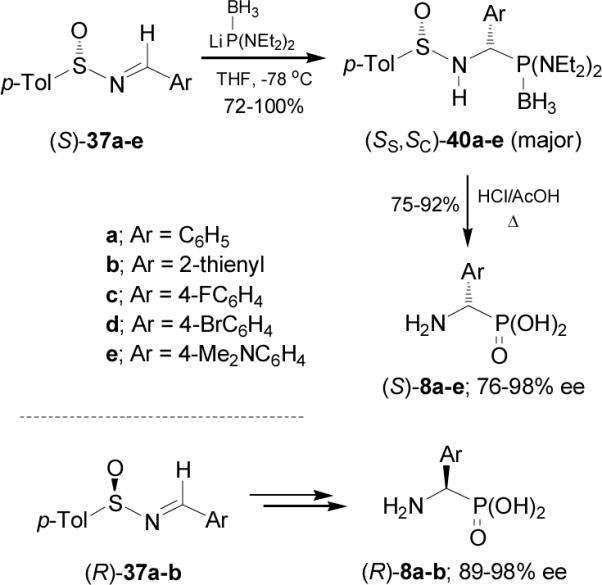
The diastereoselectivity obtained in the addition of lithium salt of bis(diethylamido) phosphine to p-toluenesulfinyl imines (S)-37a-e is opposite to that observed with lithium dialkyl phosphites. These results have been explained in terms of the transition state model D, in which the lithium cation is coordinated to the nitrogen lone pair, facilitating the delivery of the phosphorous atom to the prochiral trigonal carbon center from the less hindered face occupied by the lone pair of electrons at sulfur (Figure 5).
Figure 5.
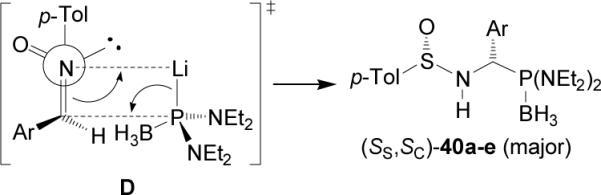
Transition state for the formation of (SS,SC)-40.
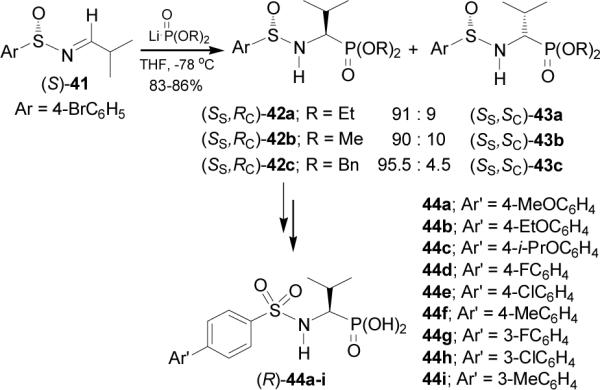
Recently, Gallina et al.48 have described the preparation of N-arylsulfonylaminophosphonic acids (R)-44a-i using the addition of lithium dialkyl phosphites to enantiopure and conformationally restricted sulfinimines 41 as key step. In this context, the addition of the lithium salt of dialkyl phosphites to imine (S)-41, obtained from condensation of isobutyraldehyde with (S)-p-bromobenzene-sulfinamide,49 afforded the mixture of (SS,RC)-42a-c and (SS,SC)-43a-c in good yield and diastereoselectivity (Scheme 11). Diastereoisomerically pure (SS,RC)-42a-c were transformed into (R)-α-aminophosphonic acids 44a-i, which showed a selective inhibition of matrix metalloproteinases (MMPs).
Scheme 11.
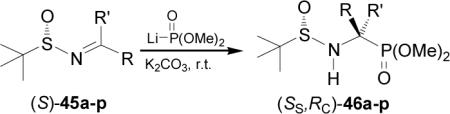
Recently, Chen and Yuan50 reported the nucleophilic addition of dialkyl phosphites to N-tert-butylsulfinyl imines in order to obtain enantiomerically pure α-aminophosphonic acids. The N-tert-butylsulfinyl group activates the imines for the nucleophilic addition and serves as a powerful chiral directing group and, after the addition reaction, is readily cleavaged upon treatment of the product with acid. Competitive nucleophilic attack at sulfur atom is minimized in the addition to N-tert-butylsulfinyl imines versus N-p-tolylsulfinyl imines due to the greater steric hindrance and reduced electronegativity of the tert-butyl group relative to the p-tolyl moiety.51 Thus, the nucleophilic addition of the lithium salt of dimethyl phosphite to N-tert-butylsulfinyl imines (S)-45a-p in the presence of K2CO3 in dichloromethane or ethyl ether52 at room temperature provided the phosphonates (SS,RC)-46a-p in good yield and with moderated to excellent diastereoselectivity (Table 4).
Table 4.
Addition of lithium dimethyl phosphite to (S)-45a-p.
| Product | R | R' | Solvent | Yield (%) | de (%) |
|---|---|---|---|---|---|
| 46a | C6H5 | H | CH2Cl2 | 81 | 81.8 |
| 46b | 4-MeOC6H4 | H | CH2Cl2 | 78 | 85.2 |
| 46c | 4-MeC6H4 | H | CH2Cl2 | 81 | 80.2 |
| 46d | 4-ClC6H4 | H | CH2Cl2 | 82 | 72.4 |
| 46e | Et | H | CH2Cl2 | 80 | 77.0 |
| 46f | i-Pr | H | CH2Cl2 | 79 | 85.1 |
| 46g | t-Bu | H | CH2Cl2 | 77 | 86.9 |
| 46h | C6H5 | Me | Et2O | 85 | >95 |
| 46i | 4-MeC6H4 | Me | Et2O | 82 | >95 |
| 46j | 4-ClC6H4 | Me | Et2O | 85 | >95 |
| 46k | 4-NO2C6H4 | Me | Et2O | 81 | >95 |
| 46l | 1-Naphtyl | Me | Et2O | 83 | >95 |
| 46m | 4-PhC6H4 | Me | Et2O | 80 | >95 |
| 46n | Et | Me | CH2Cl2 | 73 | 72.4 |
| 46o | n-Bu | Me | CH2Cl2 | 75 | >95 |
| 46p | t-Bu | Me | CH2Cl2 | 73 | >95 |
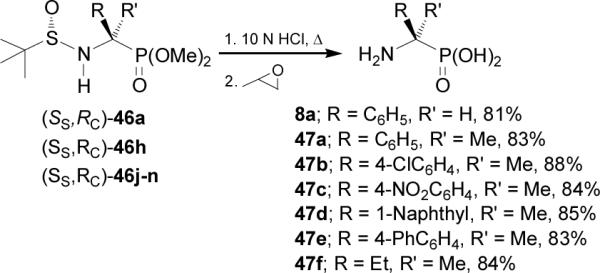
Acidic hydrolysis of diastereomerically pure (SS,RC)-46a, 46h, and 46j-n, with 10 N HCl under reflux followed by treatment with propylene oxide led to enantiomerically pure α-aminophosphonic acids (R)-8a and quaternary (R)-47a-f, analogues of α-methyl α-amino acids, which are of considerable interest because their incorporation into peptides results in a improvement in their rigidity,53 resistance to protease enzymes, and often enhancement of the bioactivity54 (Scheme 12).
Scheme 12.
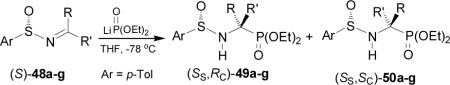
Davis et al.45 reported that the addition of lithium salt of diethyl phosphite to enantiopure imines (S)-48a-g, readily obtained by condensation of (S)-p-toluenesulfinamide with the appropriate ketone in the presence of Ti(OEt)4, 55 4 gave the α-aminophosphonates (SS,RC)-49a-g in good yield and excellent diasteroselectivity, except for the imine (S)-48g derived from 2-hexanone, where the α-aminophosphonates (SS,RC)-49g and (SS,SC)-50g were obtained with 82:18 dr. (Table 5).56
Table 5.
Addition of lithium diethyl phosphite to (S)-48a-g.
| Sulfinimine | R | R' | Yield (%) | 49 : 50 |
|---|---|---|---|---|
| 48a | Me | 4-MeOC6H4 | 73 | >99 : 1 |
| 48b | Me | 4-MeC6H4 | 91 | >99 : 1 |
| 48c | Me | C6H5 | 92 | >99 : 1 |
| 48d | Et | C6H5 | 93 | >99 : 1 |
| 48e | Me | 4-NO2C6H4 | 93 | >99 : 1 |
| 48f | Me | t-Bu | 97 | >99 : 1 |
| 48g | Me | n-Bu | 71 | 82 : 18 |
The high diastereoselectivity obtained in the addition of the lithium salt of diethyl phosphite to enantiopure p-toluenesulfinyl imines (S)-48a-g has been explained in terms of the transition state model E, in which the lithium cation is coordinated to both sulfinyl and phosphonate oxygens in a seven-membered twisted chairlake transition state, and assuming that the sulfinyl imine has the favored geometry, a plausible rationalization for the preferential formation of (SS,RC)-49a-g. By contrast the twisted-chair transition state F leading to the minor product (SS,SC)-50a-g has the bulky aryl and p-tolyl groups in the energetically unfavorable axial positions (Figure 6).
Figure 6.
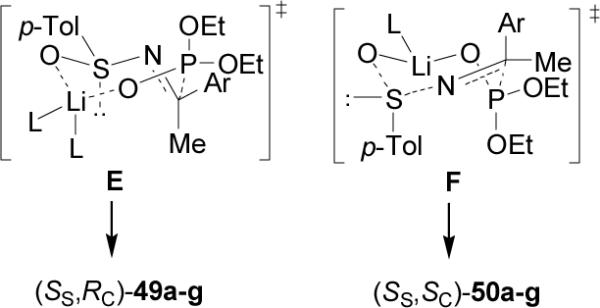
Rationalization for the formation of (SS,RC)-49a-g.
Removal of N-sulfinyl auxiliary in the diastereoisomerically pure (SS,RC)-49b,e,f with TFA in methanol produced the enantiomerically pure α-aminophosphonates (R)-51a-c, whereas the acidic hydrolysis of (SS,RC)-49b,e,f with 10 N HCl at reflux followed by treatment with propylene oxide gave the enantiomerically pure (R)-α-aminophosphonic acids 47c,g,h (Scheme 13).
Scheme 13.
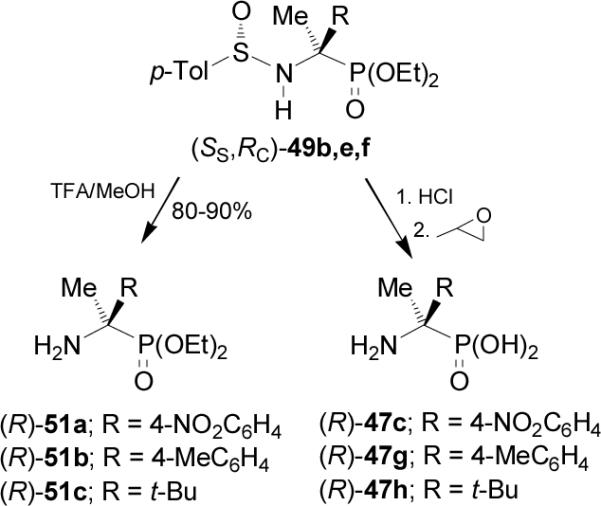
Aza-Darzens reaction of (S)-37 with the lithium anion of diethyl chloromethylphosphonate 52 in THF at -78 °C, afforded the α-chloro-β-amino derivatives (SS,1S,2R)-53 and (SS,1R,2R)-54 in good yield (72-98%) and with moderated to excellent diastereoisomeric ratio (54:46 to 92:8).57 Identical results were obtained using diethyl bromo-, iodo- or tosylphosphonates. Reaction of diastereoisomerically pure (SS,1S,2R)-53 with sodium hydride gave the aziridines (SS,2S,3R)-55 in 64-85% yield via SN2 inversion α to phosphorus, which by treatment with TFA provided to (2S,3R)-56 in 70-82% yield. Catalytic hydrogenation under (Pd/C-HCO2NH4) conditions produced the (S)-α-aminophosphonates 28a-f in 67-98% yield (Scheme 14).58,59
Scheme 14.
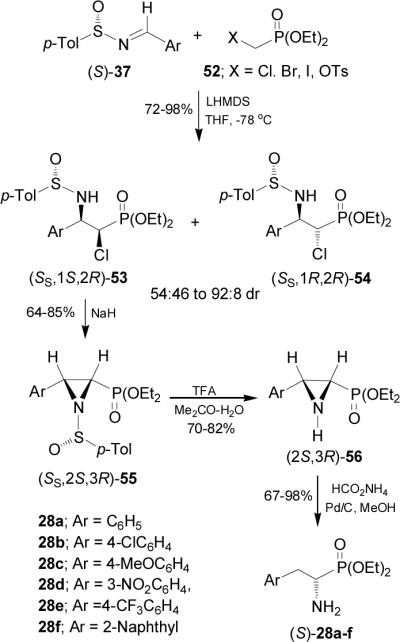
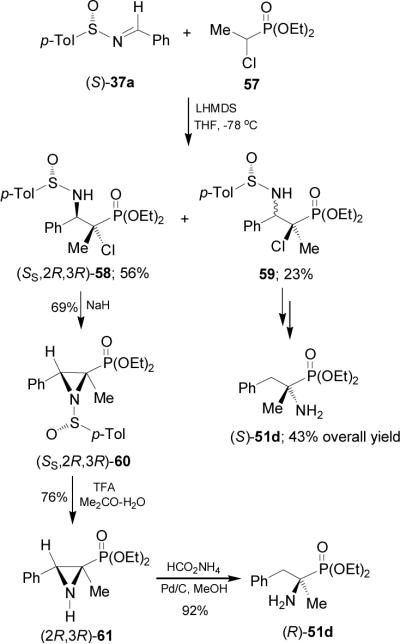
On the other hand, aza-Darzens reaction of imine (S)-37a with the lithium anion of diethyl 1-chloroethylphosphonate 57 in THF at -78 °C afforded the α-chloro-β-amino derivative (SS,2R,3R)-58 in 56% yield, and the unseparable mixture of (SS,2S,3R)-59 and (SS,2S,3S)-59 in 23% yield. Reaction of diastereoisomerically pure (SS,2R,3R)-58 with NaH gave the aziridine (SS,2R,3R)-60 in 69% yield, which by treatment with TFA led to (2R,3R)-61 in 76% yield. Finally, catalytic hydrogenation under (Pd/C-HCO2NH4) conditions gave the diethyl (R)-α-methylphosphophenylalanine diethyl esther 51d in 92% yield. In a similar way, the mixture of 59 was converted into (S)-51d in 43% overall yield, after three steps (Scheme 15).60
Scheme 15.
The formation of (SS,2R,3R)-58 was explained through of a transition state model G, in which the lithium anion derived from 57 attacks the sulfiminine (S)-37a for the si face, whereas the re face is sterically shielded by the sulfinyl oxygen in the six-membered transition state (Figure 7).
Figure 7.
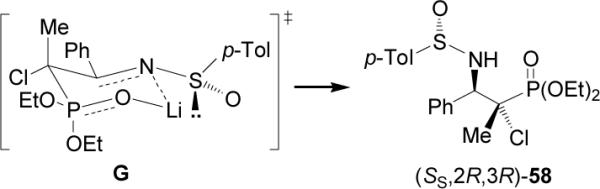
Proposed predominant transition state for phosphonate addition to sulfinimine 37a.
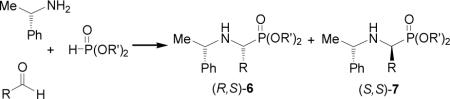
Other important methodology used in the synthesis of α-aminophosphonates is the Kabachnik-Fields reaction,61 which is an efficient three-component reaction of aldehydes or ketones, amines and phosphites under solvent free conditions.62 The first asymmetric synthesis of α-amino-phosphonates via one-pot three-component reaction was reported by Heydari et al.63 They carried out the reaction of dimethyl phosphite to imines derived from (S)-α-MBA prepared in situ, in the presence of lithium perchloratediethyl eter (LPDE), obtaining the α-aminophosphonates (R,S)-6 and (S,S)-7 in good yield and moderated diastereoselectivity (Table 6, entries 1-4). In a similar way, one-pot three-component reaction of dimethyl phosphite, with arylaldehydes and (S)-α-MBA in the presence of LPDE and trimethylsilyl chloride (TMSCl), gave the phosphonates (R,S)-6 and (S,S)-7 in good yield and moderated diastereoselectivity (Table 6, entries 5-8).64
Table 6.
One-pot three-component synthesis of (R,S)-6 and (S,S)-7.
| Entry | R | R' | Conditions | Yield (%) | 6 : 7 | Ref |
|---|---|---|---|---|---|---|
| 1 | i-Pr | Me | A | 95 | 79 : 21 | 63 |
| 2 | t-Bu | Me | A | 96 | 82 : 18 | 63 |
| 3 | c-Hexyl | Me | A | 92 | 80 : 20 | 63 |
| 4 | Bn | Me | A | 90 | 83 : 17 | 63 |
| 5 | Ph | Et | B | 95 | 75 : 25 | 64 |
| 6 | 4-ClC6H4 | Et | B | 97 | 78 : 22 | 64 |
| 7 | 4-NO2C6H4 | Et | B | 96 | 80 : 20 | 64 |
| 8 | PhCH=CH2 | Et | B | 93 | 70 : 30 | 64 |
| 9 | Ph | Et | C | 92 | 57 : 43 | 67 |
| 10 | 4-MeOC6H4 | Et | C | 88 | 57 : 43 | 67 |
| 11 | Ph | Et | D | 90 | 83 : 17 | 68 |
| 12 | 2-Py | Et | D | 90 | 78 : 22 | 68 |
Conditions: A. LPDE, B. LPDE/TMSCl, C. Yb(OTf)3/MgSO4, D. InCl3
Nucleophilic addition of phosphites to imines catalyzed by base or acids under three-component conditions is the most convenient methodology for the synthesis of α-amino-phosphonates. Lewis acids such as SnCl2, SnCl4, BF3.Et2O, ZnCl2, and MgBr2 have been used.65 However, these reactions cannot be carried out in one-pot reaction with carbonyl compounds, amine and dialkyl phosphite, because the imines and water that exist during the imine formation can decompose or deactivate the Lewis acids.66 This disadvantage has been overcome by a recent procedure describe by Qian and Huang,67 by using a combination of lanthanide triflate as catalyst in the presence of 4 Å molecular sieves or magnesium sulphate in dichloromethane as the best solvent. However, although this procedure afford excellent yields for aromatic aldehydes, only low to moderated yields were obtained for aliphatic aldehydes, which is atributed to that aromatic aldehydes have higher reactivity than aliphatic aldehydes. For example three component reaction of benzaldehyde, (S)-α-MBA and diethyl phosphite in the presence of catalytic amount of ytterbium triflate (10% mol) and anhydrous MgSO4 at room temperature, gave the α-aminophosphonates (R,S)-6 and (S,S)-7 in excellent yield and 57:43 diastereoisomeric ratio. Similar results were obtained when p-methoxybenzaldehyde was used (Table 6, entries 9-10). The α-aminophosphonates (R,S)-6 and (S,S)-7 were obtained with better diastereoisomeric ratio (83:17) when three component reaction of benzaldehyde, (S)-α-MBA and diethyl phosphite was carried out in the presence of catalytic amount of indium(III) chloride (10% mol) in dry THF at reflux or under sonication (Table 6, entry 11).68 The reaction of 2-formylpyridine under identical conditions led to the α-amino-phosphonates (R,S)-6 and (S,S)-7 in 90% yield and 78:22 diastereoisomeric ratio (Table 6, entry 12). This methodology afforded excellent yields for aliphatic and aromatic aldehydes as well as with open-chain, cyclic, and aromatic ketones.
One-pot reaction of methylcyclopropanone acetal (2S)-62 obtained in two steps from commercially available methyl (S)-3-hydroxy-2-methylpropionate,69 with (S)-α-MBA hydro-chloride and triethyl phosphite in the presence of catalytic amount of TMSCl in ethanol at 55 °C, afforded the α-aminophosphonates (1S2S)-63 and (1R2S)-64 in 80% yield and 87:13 diastereoisomeric ratio. The selectivity was not altered when (R)-α-MBA or (S)-1-(1-naphthyl)ethylamine were used as chiral auxiliary. Hydrogenolysis of diastereoisomerically pure (1S2S)-63 in the presence of Pearlman's catalyst, afforded the α-aminophosphonate (1S2S)-65 in 82% yield, which by hydrolysis with trimethylsilyl iodide (TMSI) followed by the treatment with propylene oxide gave the (1S,2S)-1-amino-2-methylcyclopropanephosphonic acid 66 in 86% yield (Scheme 16).70
Scheme 16.
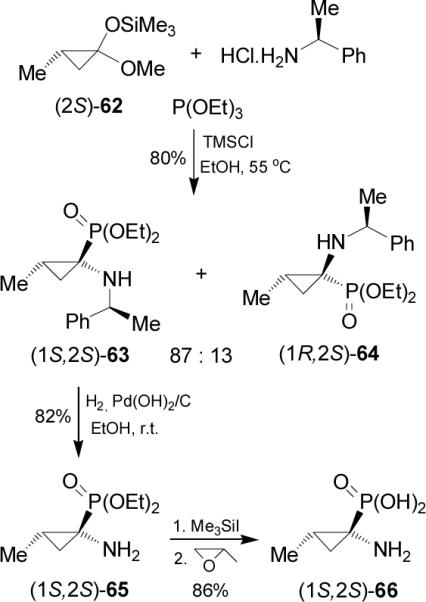
The diastereoselectivity obtained in the nucleophilic addition of triethyl phosphite to the iminium intermediate 67 takes place from the less hindered face (si-face) opposite to the methyl group on the cyclopropane with a relative like approach, affording (1S2S)-63 as the principal product (Figure 8).
Figure 8.
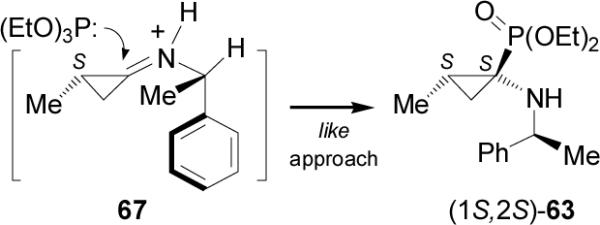
Model proposed for addition of (EtO)3P to 67.
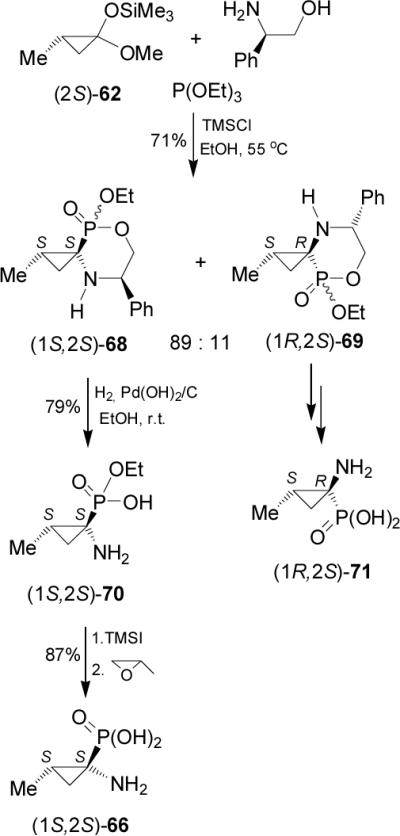
In a similar way, one-pot reaction of methylcyclopropanone acetal (2S)-62 with (R)-phenylglycinol and triethyl phosphite in the presence of catalytic amount of TMSCl in ethanol at 55 °C afforded the spirophosphonates 68 and 69 in good yield, and with the trans isomer as the major product (ratio 89:11). Reaction of (2S)-62 with (-)-norephedrine and triethyl phosphite under the same conditions gave the spirophosphonates in low yield and diastereoisomeric ratio. Hydrogenolysis of diastereoisomerically pure 68 in the presence of catalytic amount of Pearlman's catalyst, afforded the cyclic α-aminophosphonate 70 in 79% yield, which by hydrolysis with TMSI followed by the treatment with propylene oxide gave the cyclic α-aminophosphonic acid (1S,2S)-66 in 87% yield. Under identical conditions 69 led to (1R,2S)-1-amino-2-methylcyclopropanephosphonic acid 71 (Scheme 17).71
Scheme 17.
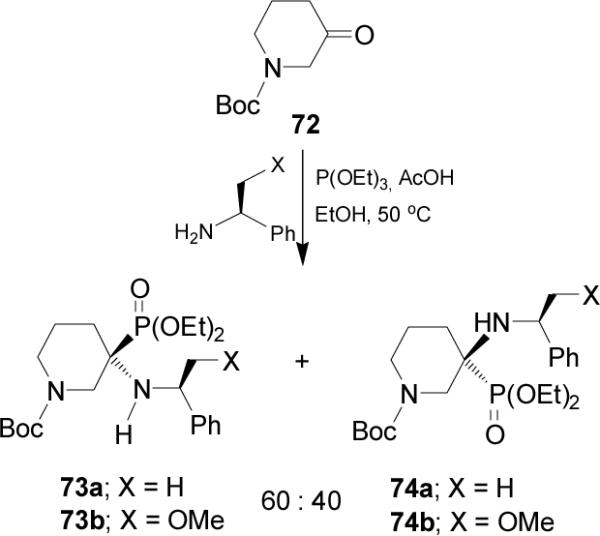
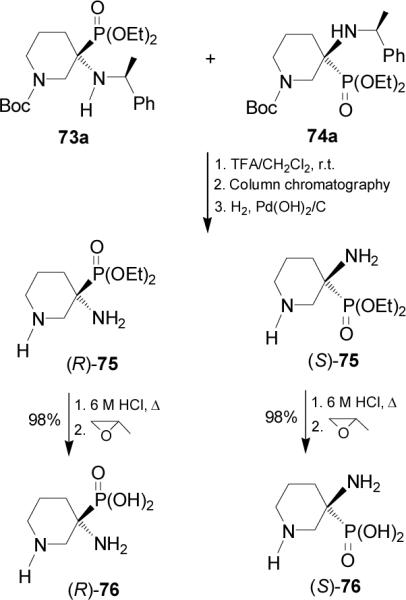
Recently Fadel et al.72 described that the three-component reaction of N-Boc-3-piperidinone 72, (S)-α-MBA (χ = H) and trimethyl phosphite in the presence of AcOH and anhydrous MgSO4 at 50 °C, gave the α-aminophosphonates (R,S)-73a and (S,S)-74a in 75% yield and 60:40 dr. Similar results were obtained when (S)-α-methoxymethylbenzylamine (χ = OMe) was used, obtaining the α-aminophosphonates (R,S)-73b and (S,S)-74b (Scheme 18).
Scheme 18.
Cleavage of the N-Boc protecting group in 73a/74a with TFA at room temperature followed by chromatographic separation and hydrogenolysis over Pd(OH)2 of each diastereoisomer, gave the phosphonates (R)-75 and (S)-75 in good yield, which by hydrolysis with aqueous HCl solution followed by treatment with propylene oxide provided the enantiomerically pure α,β-diaminophosphonic acids (R)-76 and (S)-76 in quantitative yield (Scheme 19).73
Scheme 19.
On the other hand, three component reaction of several aliphatic aldehydes with (R)-2-phenylglycinol and dimethyl phosphite in the presence of 5 M LPDE, afforded the α-aminophosphonates (R,R)-25 and (S,R)-26 in excellent yield and good diastereoselectivity, predominating the diastereoisomer (R,R)-25 (Table 7, entries 1-3).63 In a similar way, reaction of aromatic aldehydes, methoxymethyl ether of (S)-2-phenylglycinol or (S)-1-methoxy-3-methyl-2-butylamine and diethyl phosphite in the presence of catalytic amount of ytterbium triflate (10% mol) and anhydrous MgSO4 at room temperature, gave the α-aminophosphonates (S,S)-25 and (R,S)-26 in good yield and moderate diastereoselectivity in favor of diastereoisomer (S,S)-25 (Table 7, entries 4-7).67
Table 7.
One-pot three-component synthesis of α-aminophosphonates (R,S)-25 and (S,S)-26.
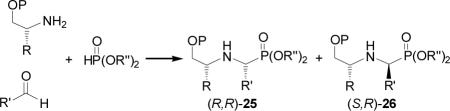
| Entry | P | R | R' | R” | Conditions | Yield (%) | 25 : 26 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | H | Ph | i-Pr | Me | 2.0 M, LPDE, -15 °C | 90 | 88 : 12 | 63 |
| 2 | H | Ph | t-Bu | Me | 2.0M,LPDE, -15 °C | 95 | 91 : 09 | 63 |
| 3 | H | Ph | c-Hexyl | Me | 2.0 M, LPDE, -15 °C | 94 | 90 : 10 | 63 |
| 4 | Me | Ph | Ph | Et | Yb(OTf)3/MgSO4 | 95 | 78 : 22 | 67 a |
| 5 | Me | Ph | 4-MeOC6H4 | Et | Yb(OTf)3/MgSO4 | 91 | 78 : 22 | 67 a |
| 6 | Me | i-Bu | Ph | Et | Yb(OTf)3/MgSO4 | 82 | 74 : 26 | 67 a |
| 7 | Me | i-Bu | 4-MeOC6H4 | Et | Yb(OTf)3/MgSO4 | 81 | 74 : 26 | 67 a |
The configuration of chiral auxiliary was (S) and the principal product was the diastereoisomer (S,S)-25.
The high diasteroselectivity obtained using (R)-2-phenylglycinol has been explained on the basis of the aza analogue of the Anh-Eisenstein hypothesis,74 where the nucleophilic attack on the imine 77 should take place antiperiplanar to the phenyl group to give the diastereoisomer (S,R)-25 as principal product.63 Whereas, the addition of diethyl phosphite to the imines derived from methoxymethyl ether of (S)-2-phenylglycinol or (S)-1-methoxy-3-methyl-2-butylamine in the presence of catalytic amount of ytterbium triflate, the authors suggest the model 78 transition state in which the Yb(OTf)3 is chelated by the nitrogen and the methoxy group, and the nucleophilic attack on the imine should take place antiperiplanar to the α-i-Bu group (Figure 9).67
Figure 9.
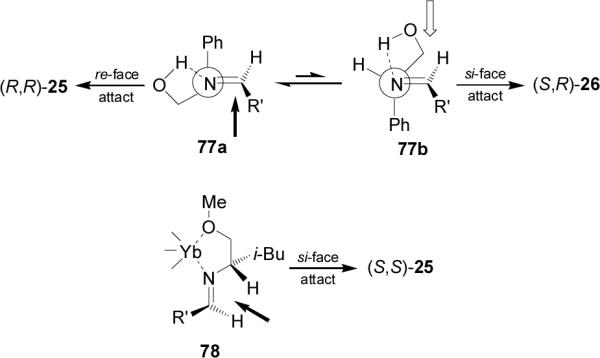
transition state in the formation of 25 and 26.
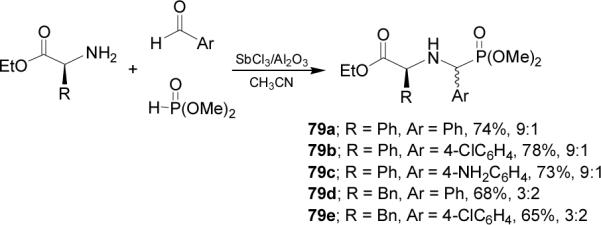
Very recently Kapoor et al.75 reported the synthesis of α-aminophosphonates 79a-e from (S)-phenylglycine and (S)-phenylalanine. In this context, reaction of aryl aldehydes with amino acid esters and dimethyl phosphite in the presence of antimony trichloride adsorbed on alumina as an efficient and recyclable catalyst, gave the mixture of α-aminophosphonates 79a-e in moderated yield and diastereoisomeric ratio (Scheme 20).
Scheme 20.
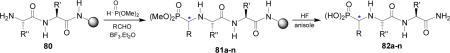
Houghten et al.76 have reported the preparation of α-aminophosphonates bearing peptides under three components reaction. In this context, reaction of aldehyde, resin-bound peptides 80 and dimethyl phosphite in the presence of catalytic amount of BF3.Et2O (10% mol) gave the α-aminophosphonates 81a-n, which by hydrolysis with HF and anisole gave the α-aminoalkyl phosphonopeptides 82a-n. The results are showed in the Table 8.
Table 8.
One-pot preparation of α-aminophosphonopeptides 82a-n.
 | |||||
|---|---|---|---|---|---|
| Compound | R | R' | R” | Yield (%) | ratioa |
| 82a | Ph | Bn | Bn | 85 | 80 : 20 |
| 82b | Ph | Bn | HO2CCH2 | 79 | 40 : 60 |
| 82c | Ph | Bn | -(CH2)3- | 82 | 90 : 10 |
| 82d | Ph | Bn | Me | 83 | 34 : 66 |
| 82e | Pr | Bn | Me | 88 | 20 : 80 |
| 82f | Pr | Bn | HO2CCH2 | 86 | n.d. |
| 82g | Pr | Bn | Bn | 92 | n.d. |
| 82h | Bu | Bn | i-Pr | 80 | 40 : 60 |
| 82i | Bu | Me | Bn | 71 | n.d. |
| 82j | Ph | Me | Bn | 53 | n.d. |
| 82k | Pr | Me | Bn | 65 | n.d. |
| 82l | Bu | H | Bn | 88 | 63 : 37 |
| 82m | i-PrC6H4 | Bn | -(CH2)3- | 75 | 20 : 80 |
| 82n | i-PrC6H4 | Bn | Me | 70 | 44 : 56 |
Stereochemistry was not assigned (n.d. = not determined).
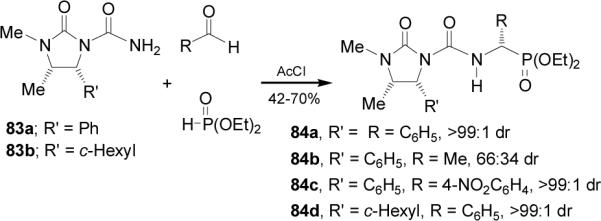
Three-component reaction of chiral amides 83a-b with aldehydes and dimethyl phosphite in the presence of acetyl chloride at 0 °C, afforded the α-aminophosphonates 84a-d in moderate yield and excellent diastereoselectivity,77 which is consistent with frontside attack of the phosphorous nucleophile on the s-cis/E conformation of the N-acylimine intermediate (Scheme 21). Hydrolysis of 84a gave the (S)-phosphophenylglycine 8.78
Scheme 21.
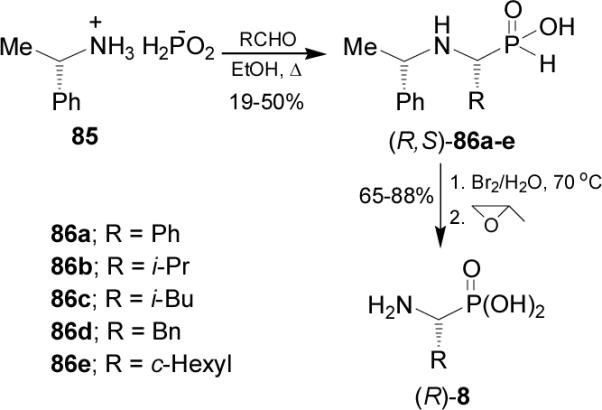
On the other hand, reaction of chiral hypophosphorous acid salt 85 obtained by addition of (S)-α-MBA to anhydrous hypophosphorous acid, with aldehydes at reflux in ethanol gave the corresponding N-protected α-aminophosphonous acids 86a-e as a single diastereoisomer, which by treatment with bromine-water solution at 70 °C followed by addition of propylene oxide afforded the (R)-α-aminophosphonic acids 8 (Scheme 22). (S)-α-Aminophosphonic acids 8 were obtained using (R)-α-MBA.79
Scheme 22.
2.1.2. Addition of alkyl phosphites to imines derived from chiral aldehydes and ketones
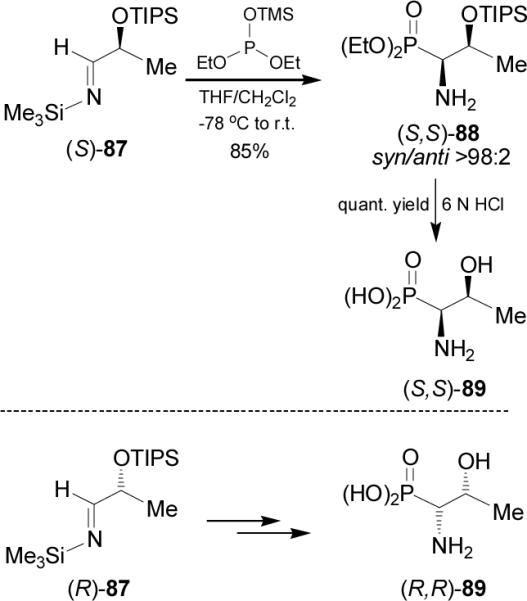
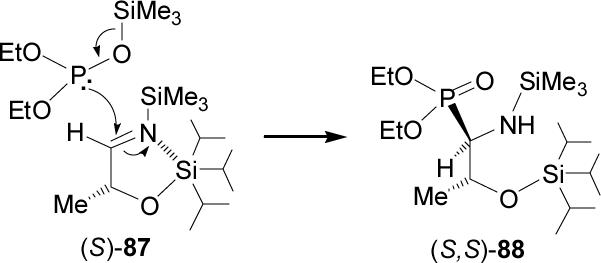
In order to obtain diasteroisomeric pure phosphotreonine 89, Bongini et al.80reported that the nucleophilic addition of trimethylsilyldiethyl phosphite to the imine (S)-87 readily obtained by condensation of (S)-2-triisopropylsilyoxy lactaldehyde with N-trimethylsilylamine, provided the β-silyloxy-β-aminophosphonate (S,S)-88 in 85% yield and >98:2 syn/anti diastereoisomeric ratio. Acidic hydrolysis of (S,S)-88 with 6 N HCl at reflux gave the phosphotreonine (S,S)-89. In a similar way, addition of trimethylsilyldiethyl phosphite to the imine (R)-87, followed by acidic hydrolysis afforded the (R,R)-phosphotreonine 89 (Scheme 23).
Scheme 23.
The high diasteroselectivity in the addition of trimethylsilyldiethyl phosphite to the imine (S)-87 is characterized by two important features: (1) the α-silyloxy group induces high degree of syn diastereoselectivity, without chelating Lewis acid; (2) with increasing bulkiness of the silicon protecting group, an enhance of the syn diastereoselectivity was observed. Computational studies showed that a pentacoordinate silicon group may be involved in the determination of the diastereoselectivity of the reaction. It should be noted that the reaction proceeds at -78 °C, at this temperature the (EtO)2P-OSiMe3 tautomeric structure is stabilized and strongly promotes nucleophilic reactivity via a concerted [2 + 3] cycloaddition reaction (Figure 10).
Figure 10.
Transition state prosed for addition of (EtO)2POSiMe3 to (S)-87.
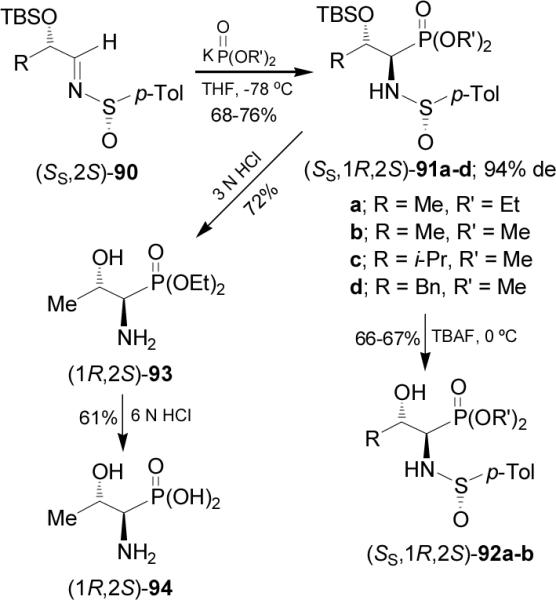
Recently, Davis and Prasad reported81 that the addition of potassium salt of diethyl- or dimethyl phosphite to enantiopure O-protected α-hydroxy sulfinimine (SS,2S)-90 readily obtained by condensation of (S)-p-toluenesulfinamide with the appropriate O-protected α-hydroxy aldehyde in the presence of Ti(OEt)4, afforded the α-aminophosphonates (SS,1R,2S)-91a-d in good yield and 94% de.82 Low diastereoselectivity was obtained when the lithium or sodium salt of alkyl phosphites were used. Treatment of (SS,1R,2S)-91a-b with tetrabutylamonium fluoride (TBAF) at 0 °C gave the β-hydroxy derivatives 92a-b in 66-67% yield. Whereas, hydrolysis of (SS,1R,2S)-91a with 3 N HCl at reflux led to α-amino-β-hydroxyphosphonate (1R,2S)-93 in 72% yield, which by hydrolysis with 6 N HCl at reflux produced the α-amino-β-hydroxyphosphonic acid (1R,2S)-94 in 61% yield (Scheme 24).
Scheme 24.
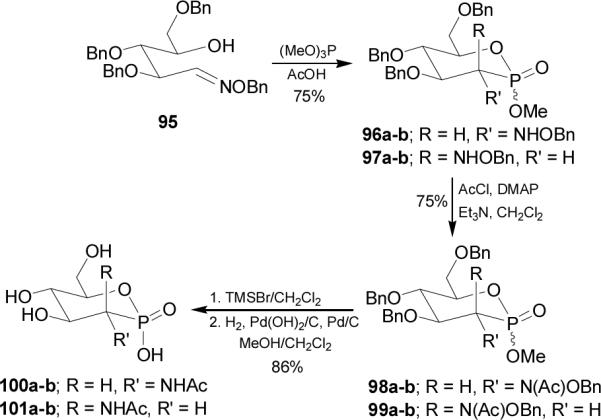
Addition of trimethyl phosphite to the oxime 95 readily obtained from condensation of O-benzyl hydroxylamine and 2,3,5-tri-O-benzyl D-arabinose,83 afforded a mixture of D-gluco and D-manno isomers 96 and 97, each consisting of a pair of two isomeric phosphonates (a and b). The cyclization into the phosphonates was spontaneous under these reaction conditions. Acetylation of 96a gave the corresponding N-acetyl D-gluco derivative 98a, whereas acetylation of the remaining mixture of isomers led to the other D-glucophosphonates 98b and D-mannose analogues 99a-b. Hydrolysis of methyl esters 98a-b and 99a-b and chromatographic separation followed by the hydrogenolysis of each isomer gave the N-acetyl-D-glucosamine phosphonate 100 and N-acetyl-D-mannosamine phosphonate 101 as free acids (Scheme 25).84
Scheme 25.
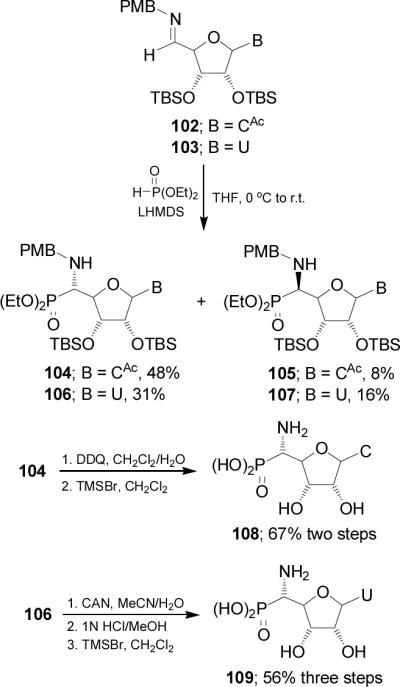
Nucleophilic addition of lithium salt of diethyl phosphite to nucleosyl imines 102 and 103 prepared from condensation of protected cytidine85 and uridine,86 respectively, with pmethoxybenzylamine, for cytidine series gave the α-aminophosphonates 104 and 105 in 6:1 ratio, and for uridine series afforded 106 and 107 in 2:1 ratio. Oxidation of the pmethoxybenzyl (PMB) protective group in the α-aminophosphonate 104 with DDQ followed by the hydrolysis of phosphono esters with TMSBr provided the α-aminophosphonic acid 108 directly in good yield. Presumably, HBr generated in situ from excess of TMSBr was sufficient to remove the TBS group. On the other hand, treatment of 106 under oxidative conditions with cerium amonium nitrate (CAN) to remove the PMB-protective group, followed by the hydrolysis of phosphono ester with TMSBr afforded the α-aminophosphonic acid 109 in good yield (Scheme 26).87
Scheme 26.
Addition of diethyl phosphite to N-benzyl nitrones derived from chiral α-alcoxy aldehydes has been a methodology used for the synthesis of polihydroxylated α-aminophosphonates. For example, Pollini et al.88 reported that the nucleophilic addition of diethyl phosphite to N-benzyl nitrones 110a-c readily obtained from D-glyceraldehyde, Ltreose and D-galactose, in the presence of tert-butyldimethylsilyl triflate (TBDMSOTf) afforded exclusively the syn-adducts 111a-c in good yield, which by catalytic hydrogenation over Pd(OH)2/C in the presence of di(tert-butyl)dicarbonate (Boc)2O gave the α-N-Boc-aminophosphonates 112a-c in moderated yield (Scheme 27).
Scheme 27.
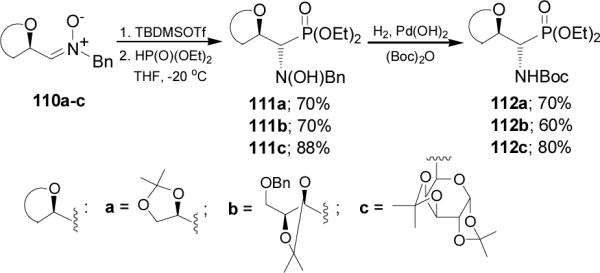
The high diastereoselectivity obtained in the addition of diethyl phosphite to 110a has been explained in terms of the two transition state structures 113 and 114, in which the silicon atom of the trialkylsilyl group coordinates to both the nitrone oxygen atom and one of the oxygen atoms of the dioxolane ring (α-chelation) or (β-chelation), respectively (Figure 11). The formation of anti diastereoisomer suggests that the addition of diethyl phosphite occurs preferentially by the si face of the nitrone in the β-chelate model 114.
Figure 11.
Models for the addition of HP(O)(OEt)2 to 110a.
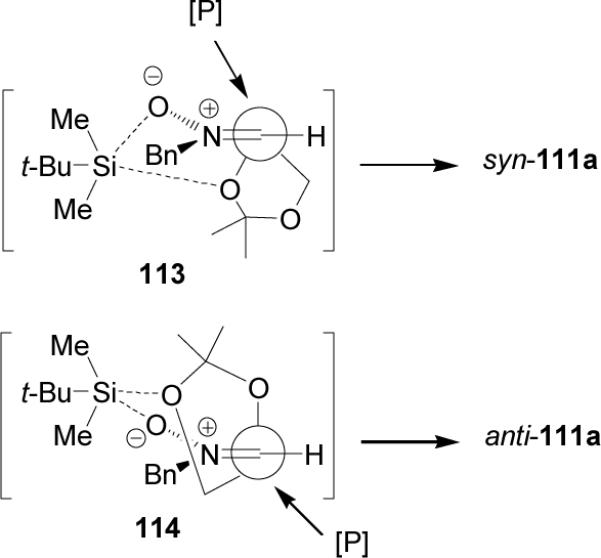
On the other hand, treatment of TBDMSOTf-precomplexed nitrones 115a-c with diethyl phosphite in THF at 20 °C, afforded the α,β-diaminophosphonates 116a-c and 117a-c in good yield and 95:5 dr. Catalytic hydrogenation of 116ac over Pd(OH)2/C in the presence of (Boc)2O gave the N,N-diprotected α,β-diaminophosphonates 118a-c in moderated yields (Scheme 28).88
Scheme 28.
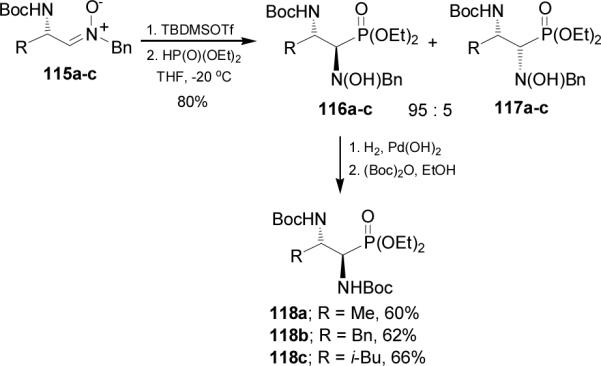
In a similar way, treatment of N,N-diprotected α-amino nitrones 119a-d with TBDMSOTf followed by nucleophilic addition of diethyl phosphite afforded exclusively the syn adducts 120a-d in good yield. Catalytic hydrogenation of 120a-d over Pd(OH)2/C in the presence of (Boc)2O provided the N,N-diprotected α,β-diaminophosphonates 121a-d in moderated yield (Scheme 29).88
Scheme 29.
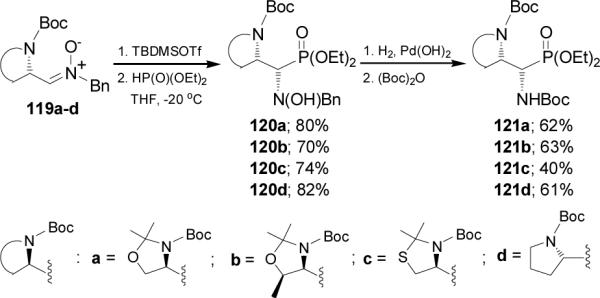
To explain the observed syn/anti stereoselectivity in the nucleophilic addition reaction of diethyl phosphite to the nitrones 115a-c and 119a-d, the authors have postulated two transition state structures 122 and 123 (Figure 12), in which the silicon atom of trialkylsilyl group coordinates to both the nitrone oxygen atom and the carbamate group. The difference between these conformations exists on the outside and inside positions of the medium-sized substituent. The addition of diethyl phosphite to N-monosubstituted derivatives 115a-c should occur from the less-hindered side of the cyclic chelate 122 leading the anti products 116a-c, whereas the addition to N,N-disubstituted α-nitrones 119a-d should take place from the less-hindered side of the cyclic chelate 123 (re attack) to give exclusively the syn adducts 120a-d.
Figure 12.
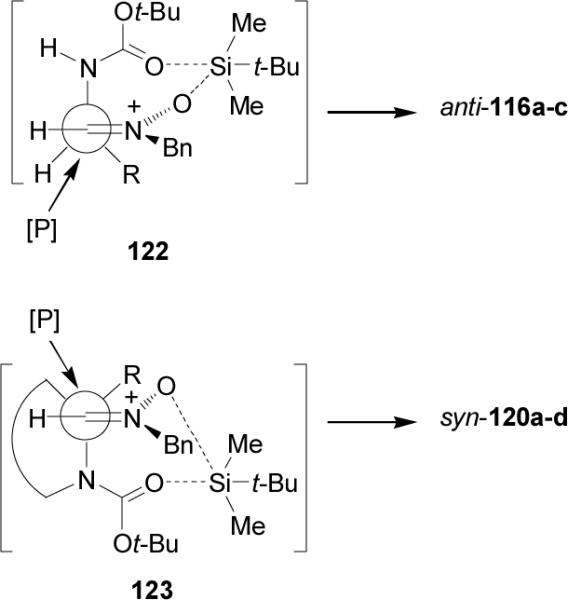
Models for the addition of HP(O)(OEt)2 to nitrones.
2.1.3. Addition of chiral alkyl phosphites to nonchiral and chiral imines
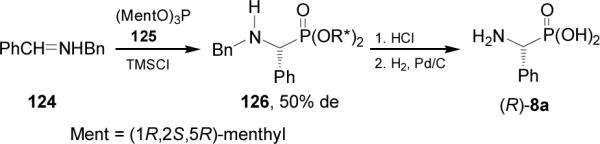
The chiral auxiliary can be attached not only to the imine fragment but also to the phosphite residue. In this context, chiral C3-symmetric trialkyl phosphites have been studied as starting reagents for the preparation of chiral organophosphorous compounds. For example, nucleophilic addition of tris[(1R,2S,5R)-menthyl] phosphite 125 readily obtained from reaction of (1R,2S,5R)-menthol with phopshorous trichloride, to the imine 124 in the presence of trimethylsilyl chloride afforded the α-aminophosphonates 126 in good yield and moderated diastereoselectivity (de = 50%). Hydrolysis of diastereoisomerically pure 126 with HCl in dioxane, followed by catalytic hydrogenolysis over Pd/C gave the (R)-phosphophenylglycine 8a with ca. 95% ee (Scheme 30).89
Scheme 30.
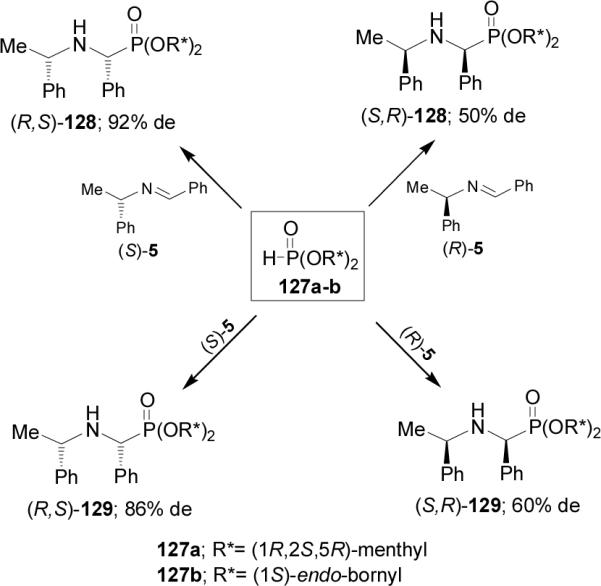
Recently, Kolodiazhnyi et al.90 reported that the addition of chiral dialkyl phosphites 127a-b [R* = (1R,2S,5R)-menthyl and (1S)-endo-bornyl] to chiral imines derived from (S)-and (R)-α-MBA, is accompanied by a double asymmetric induction at the α-carbon atom. Thus, addition of 127a to imine (S)-5 at 80 °C gave the α-aminophosphonate (R,S)-128 in 60% yield and 92% de, whereas addition of 127a to imine (R)-5 led to α-aminophosphonate (S,R)-128 in 50% yield and 50% de.91 In a similar way, nucleophilic addition of chiral di-[(1S)-endo-bornyl] phosphite 127b to (S)-5 provided the α-aminophosphonate (R,S)-129 in 60% yield and 86% de, whereas addition of 127b to (R)-5 afforded the α-aminophosphonate (S,R)-129 in 70% yield and 60% de (Scheme 31). Hydrolysis of diastereoisomerically pure (R,S)-128 and (S,R)-128 with HCl in dioxane, followed by catalytic hydrogenolysis over Pd/C gave the (S)- and (R)-phosphophenylglycine 8a, respectively.
Scheme 31.
On the other hand, addition of tris[(1R,2S,5R)-menth-2-yl] phosphite 125 to the imine 124 in the presence of BF3.OEt2 afforded the α-aminophosphonate 126 with 30% de. In a similar way, addition of 125 to imine (S)-5 in the presence of BF3.OEt2 gave the α-aminophosphonate (S,S)-130 with 70% de (Scheme 32).
Scheme 32.
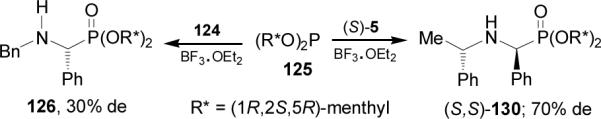
It is noteworthy that the reaction of tris[(1R,2S,5R)-menthyl] phosphite 125 and di-[(1R,2S,5R)-menthyl] phosphite 127a with Schiff bases differ in steric results and led to the diastereoisomers with opposite absolute configuration at the α-carbon atom.
Reaction of chiral P-H spirophosphoranes 131 with longchain aldimines 132 is a methodology used for the synthesis of α-aminophosphonic acid amphiphiles 135 in both enantiopure forms.92 In this context, reaction of aldimine 132 (R' = C18H37) with the spirophosphorane 131a readily obtained from (S)-α-hydroxyisovaleric acid,93 followed by the selective hydrolysis of 133 afforded the derivative 134a with 15:85 dr. In a similar way, nucleophilic addition of spirophosphoranes 131b-d obtained from tartaric acid esters, to the aldimines 132 (R' = C18H37, R' = C16H31, R' = C12H23) followed by the selective hydrolysis of 133 gave the derivatives 134b-f with 55:45 dr in all cases. The lack of diastereoselectivity obtained for 134b-f, compared with 134a, might be due to the structural lability of spirophosphoranes 131b-f. The absence of the carbonyl intracyclic group may also reduce the acidity and the reactivity of the P-H bond. Finally, acidic hydrolysis of diastereosiomerically pure 134a-f afforded the α-aminophosphonic acid amphiphiles 135a-f in both enantiopure forms (Scheme 33).
Scheme 33.
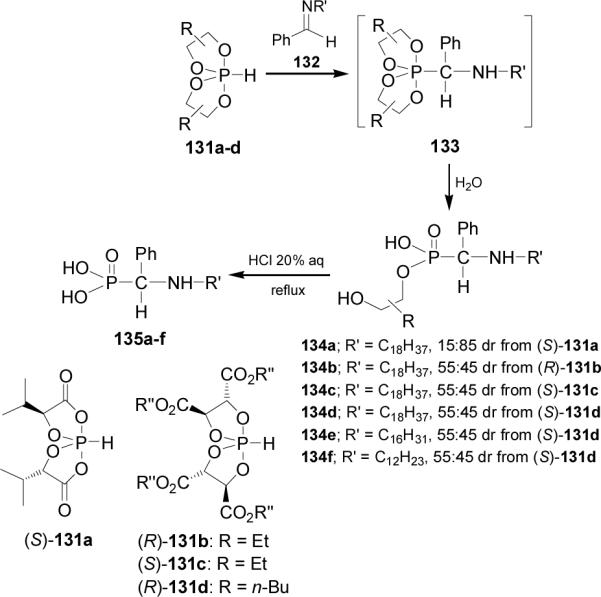
Since the chiral auxiliary might be easily removed by hydrolysis of the phosphonic ester, Martens et al.94 carried out the addition of chiral BINOL-phosphite 136 to achiral 3-thiazolines 137a-e in the presence of BF3.OEt2 obtaining tthe corresponding thiazolidinyl phosphonates 138a-e in moderated yield and excellent diastereoselectivity. It is noteworthy that stereoselectivity of the BINOL-phosphite 136 seems to be independent of steric demands of the nearby substituents R. In contrast, the nature of more distant substituent R' of the N,S-acetalic carbon atom influences the diastereoselectivity to a larger extent (Table 9).95
Table 9.
Addition of chiral BINOL-phosphite 136 to achiral 3-thiazolines 137a-e.
| Product | R/R | R'/R' | Yield (%) | dr |
|---|---|---|---|---|
| 138a | Me/Me | Me/Me | 47 | 83 : 17 |
| 138b | Me/Me | -(CH2)5- | 47 | >95 : 5 |
| 138c | -(CH2)5- | Me/Me | 37 | 80 : 20 |
| 138d | -(CH2)5- | -(CH2)5- | 68 | >95 : 5 |
| 138e | H/H | -(CH2)5- | 30 | >95 : 5 |
Removal of the chiral auxiliary and cleavage of the N,S-acetal94 might be performed by acidic hydrolysis as has benn described in the literature, thus maintaining the chiral information of the released α-aminophosphonic acid.
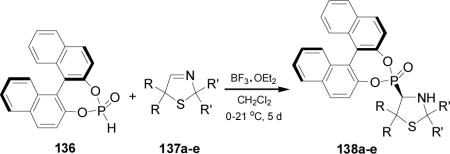
Swamy et al.96 reported the utility of chiral cyclic chlorophosphites derived from BINOL as scaffolds for the onepot synthesis of α-aminophosphonates under solvent-free conditions. In this context, treatment of the chlorophosphite (R)-139 with urethane and arylaldehydes at 80 °C, yielded the α-aminophosphonates 140a-c in good yield and 60:40 dr, with the (R,S) diastereoisomers as principal products (Scheme 34).
Scheme 34.
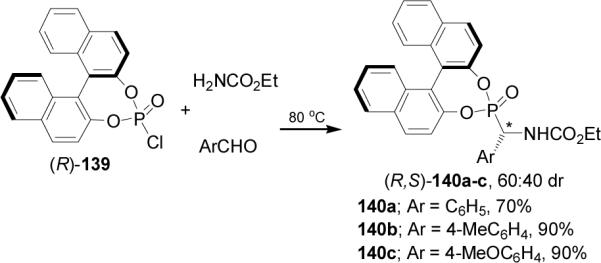
In a similar way, reaction of (R)-139 with benzylcarbamate and arylaldehydes, afforded the α-aminophosphonates 141a-d and 142a-d in good yield and diastereoisomeric ratios from 1.1:1 to 1.8:1, with the (R,S)-141a-d diastereoisomers as principal products (Scheme 35).97
Scheme 35.
On the other hand, treatment of diethyl (R,R)-2-chloro-1,3,2-dioxaphospholane-4,5-dicarboxylate 143 readily prepared from diethyl L-tartrate and phosphorus trichloride, with benzyl carbamate and arylaldehydes followed by the addition of H2O, led to the α-aminophosphonates 144a-f and 145a-f in good yield, and diastereoisomeric ratios from 1.7:1 to 2.5:1, with the (R,R,R)-144a-d diastereoisomers as principal products. Saponification of diastereoisomerically pure 144a and 145a afforded the (R)- and (S)-N-Cbzphosphophenylglycine 146a, respectively (Scheme 36).97
Scheme 36.
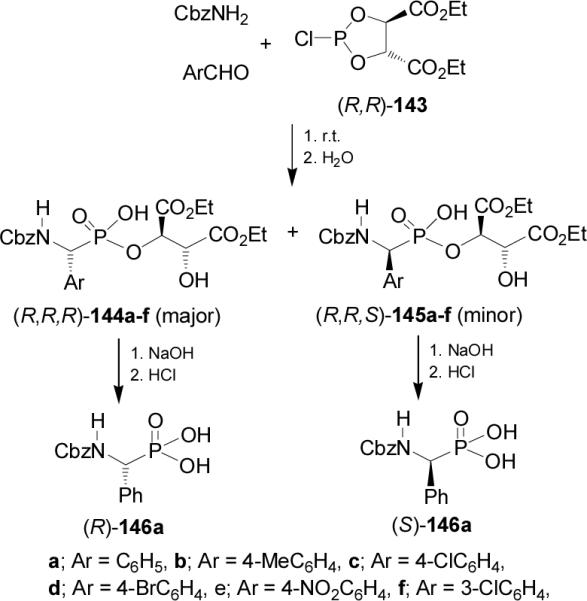
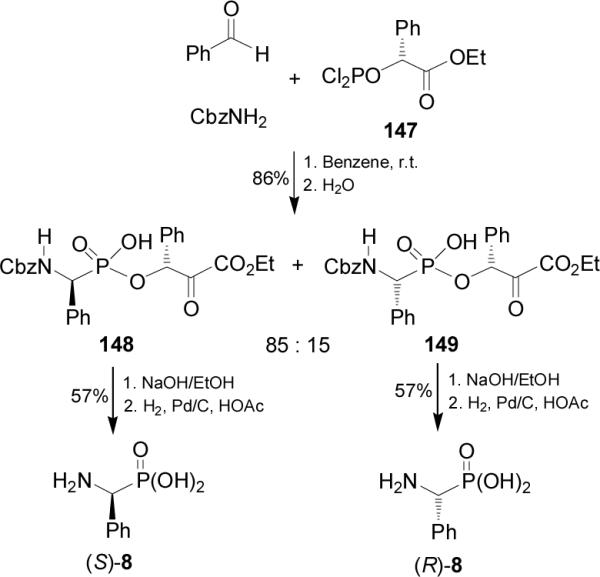
Using this Mannich type multicomponent reaction, Xu and Gao98 prepared the depsiphosphonopeptides 148 and 149, which are analogues of naturally occurring peptides. Thus, reaction of 1-carboethoxy phosphorodichloridites 147 with benzyl carbamate and benzaldehyde in anhydrous benzene, followed by the hydrolysis afforded the depsiphosphonopeptides 148 and 149 in 86% yield and 85:15 dr. Saponification of diastereoisomerically pure 148 and 149 followed by cleavage of Cbz protective group provided the enantiomerically pure (S)- and (R)-phosphophenylglycine 8, respectively (Scheme 37).
Scheme 37.
2.1.4. Catalytic asymmetric addition of alkyl phosphites to non-chiral imines
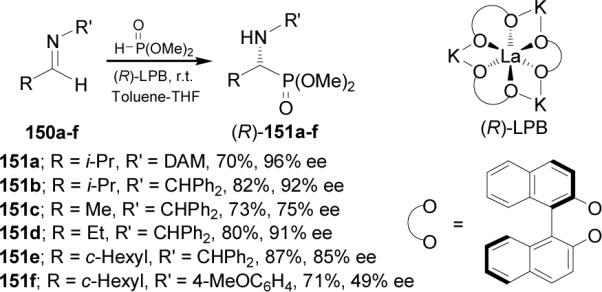
Catalytic enantioselective synthesis is one of the most important topics in modern synthetic chemistry because it provides the most efficient methodology to approach in the preparation of enantiomerically pure compounds.99 In this context, Shibasaki et al.100 reported the first catalytic hydrophosphonylation of imines. Thus, addition of dimethyl phosphite to imines 150a-f in the presence of lanthanoid-potassium-BINOL complex [(R)-LPB] gave the (R)-α-aminophosphonates 151a-f in moderated to high enantiomeric excess (Scheme 38).
Scheme 38.
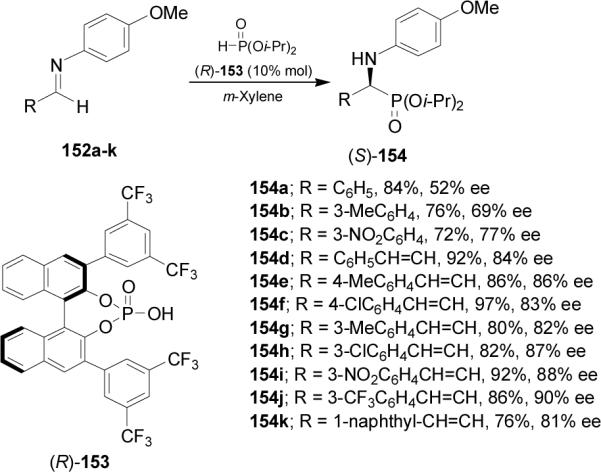
Another approach for the synthesis of chiral α-amino-phosphonates is the chiral Brønsted acid catalyzed enantioselective hydrophosphonylation of non chiralimines. For example, Akiyama et al.101 reported that hydrophosphonylation of imines 152a-k catalyzed by the cyclic phosphoric acid 153, derived from (R)-BINOL, afforded the (S)-α-aminophosphonates 154a-k in good yield and enantioselectivity (Scheme 39).
Scheme 39.
To explain the high chiral induction, the authors propose a nine-membered transition state (Figure 13), wherein phosphonic acid (R)-153 plays two roles: (1) the phosphonic acid hydrogen activates the imine as a Brønsted acid, and (2) phosphoryl oxygen activates the nucleophile by coordinating with the hydrogen of the phosphite as a Brønsted base, thereby promoting re facial attack to the imine and increasing the enantioselectivity by proximity effect.
Figure 13.
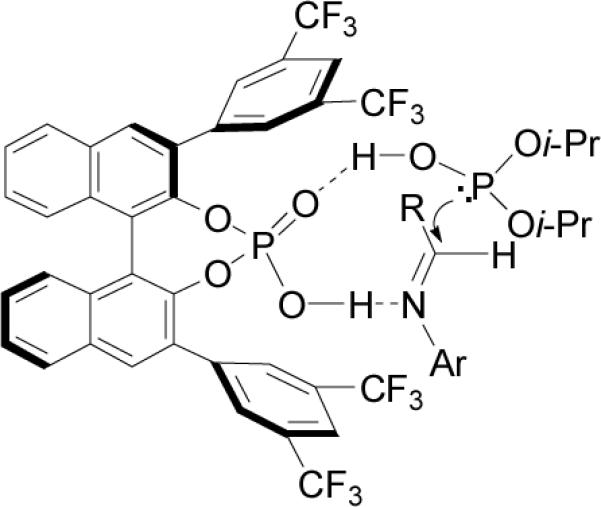
Plausible reaction mechanism of 152a-k.
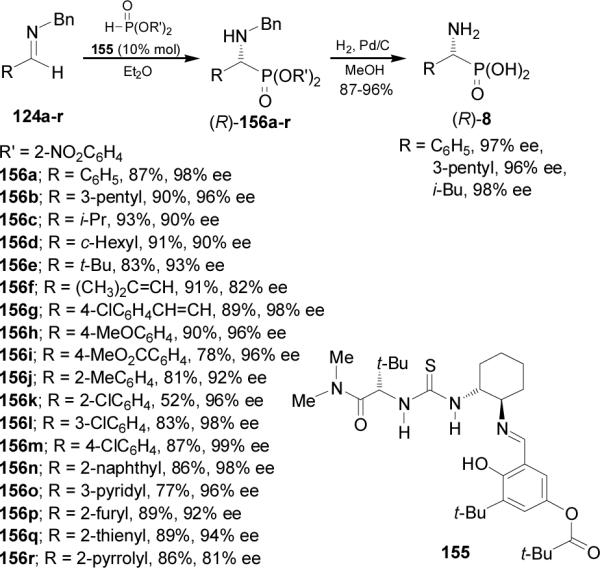
On the other hand, Joly and Jacobsen102 found that the nucleophilic addition of di(o-nitrobenzyl) phosphite to N-benzyl imines 124a-r in the presence of chiral urea 155 as catalyst, gave the (R)-α-aminophosphonates 156a-r in excellent both yield and enantioselectivity. Hydrogenolysis of 156a, 156b and 156f afforded the enantiomerically enriched (R)-α-aminophosphonic acids 8 (Scheme 40).
Scheme 40.
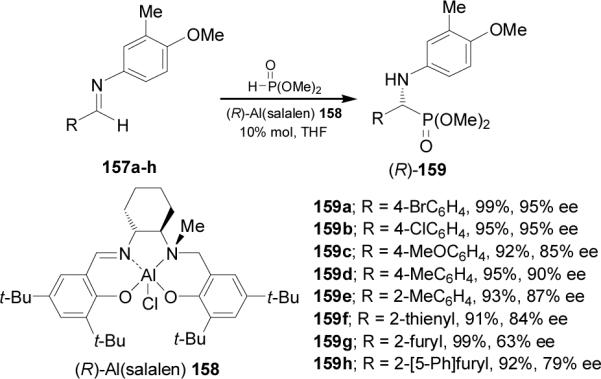
Recently, Katsuki et al.103 reported that the asymmetric hydrophosphonylation of aromatic aldimines 157a-h bearing 4-methoxy-3-methylphenyl group as N-protecting group in the presence of complex (R)-Al(salalen) 158 as catalyst, gave the (R)-α-aminophosphonates 159a-h in excellent yield and good enantioselectivity. When the imine carried an electron-withdrawing p-substituent, the enantioselectivity was improved up to 95% ee; however, the presence of an electron-donating p-substituent decreased to 85% ee (Scheme 41).104
Scheme 41.
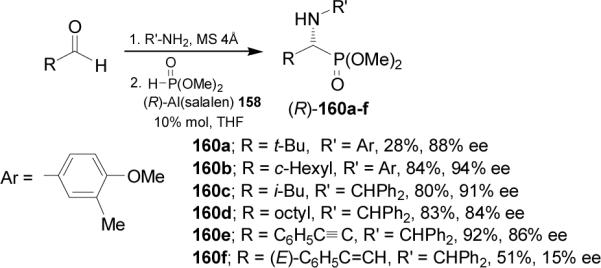
One-pot hydrophosphonylation of aldehydes, 4-methoxy-3-methylaniline or diphenylmethylamine and dimethyl phosphite in the presence of complex (R)-Al(salalen) 158 as catalyst, afforded the (R)-α-aminophosphonates 160a-f in good enantioselectivity (Scheme 42).103,105
Scheme 42.
On the other hand, addition of diethyl phosphite to N-Boc protected imines 161a-i at 20 °C in the presence of quinine 162 as chiral catalyst, provided the (R)-α-aminophosphonates 163a-i in moderated yield and enantioselectivity. An enhanced in the enantioselectivity was observed when the reaction was carried out at -20 °C (Scheme 43).106
Scheme 43.
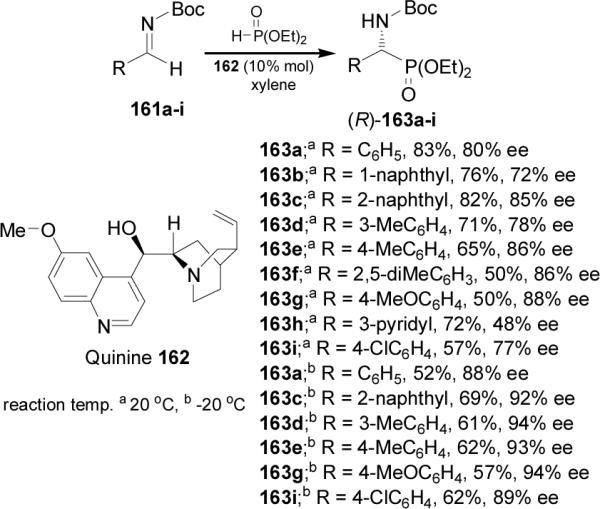
To explain the high chiral induction, the authors proposed that the imine is activated by a hydrogen bonding with the acidic hydroxyl group in the quinine, and that the phosphite-phosphonate equilibrium toward the phosphite form could attack to the eletrophilic azomethine carbon (Figure 14).106
Figure 14.
Proposed mechanism for the addition of (EtO)2P(O)H to 161 catalyzed by quinine.
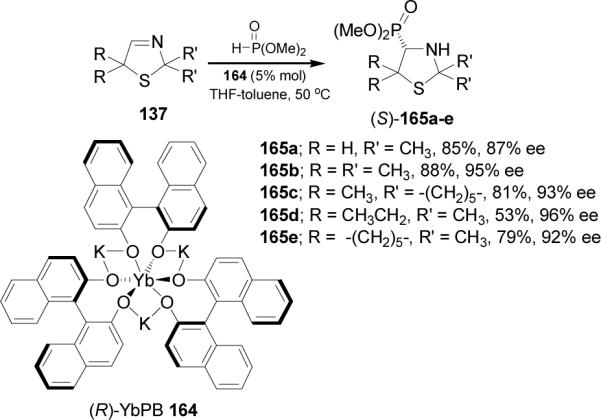
In 1998 Shibasaki et al.107 described for the first time the catalytic and enantioselective hydrophosphonylation of cyclic imines. In this context, nucleophilic addition of dimethyl phosphite to thiazolines 137 catalyzed by (R)-YbPB 164, afforded the corresponding 4-thiazolidinyl phosphonates (S)-165a-e in good yield and enantiomeric excess (Scheme 44).
Scheme 44.
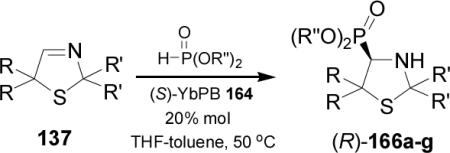
In a similar way, enantioselective hydrophosphonylation of cyclic imines 137 using cyclic phosphites, catalyzed by (S)-YbPB 164, provided the 4-thiazolidinyl phosphonates (R)-166a-g in excellent enantiomeric excess and high chemical yields (Table 10).108
Table 10.
Catalytic and enantioselective hydrophosphonylation of 137.
| Product | R/R | R'/R' | R”/R” | Yield (%) | ee (%) |
|---|---|---|---|---|---|
| 166a | Me/Me | Me/Me | -(CH2)3- | 99 | 97 |
| 166b | Me/Me | Me/Me | -CH2C(CH3)2CH2- | 90 | 92 |
| 166c | Me/Me | Me/Me | -CH2CH=CHCH2- | 87 | 93 |
| 166d | Me/Me | -(CH2)5- | -(CH2)3- | 61 | 97 |
| 166e | Me/Me | -(CH2)5- | -CH2C(CH3)2CH2- | 82 | 98 |
| 166f | -(CH2)5- | Me/Me | -(CH2)3- | 85 | 97 |
| 166g | -(CH2)5- | Me/Me | -CH2C(CH3)2CH2- | 69 | 96 |
2.2. Stereoselective C-C bond formation
2.2.1. Alkylation of phosphoglycine derivatives
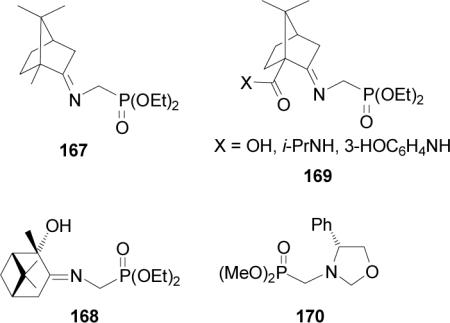
The chiral Schiff bases formed from esters of glycine and chiral carbonyl compounds are one of the most popular approaches for the asymmetric synthesis of α-amino acids.109 In a similar way, the chiral Schiff bases prepared from phosphoglycine have been also used in the asymmetric synthesis of α-aminophosphonic acids. For example, the Schiff base 167 derived from (R)-camphor and phosphoglycine diethyl ester was used by Schöllkopf110 for the asymmetric synthesis of α-aminophosphonic acids. On the other hand, the Schiff base 168 derived from (1S,2S,5S)-2-hydroxypinan-3-one and phosphoglycine diethyl ester has been used by Roumestant et al.111 for the stereoselective synthesis of α-aminophosphonic acids. Jommi et al.112 reported that the chiral Schiff base 169 obtained from condensation of (+)-ketopinic acid and phosphoglycine diethyl ester is an important compound for the asymmetric synthesis of α-aminophosphonic acids. Alkylation of the oxazolidine 170 derived from (R)-phenylglycinol has been also used in the enantioselective synthesis of α-aminoalkylphosphonic acids.113
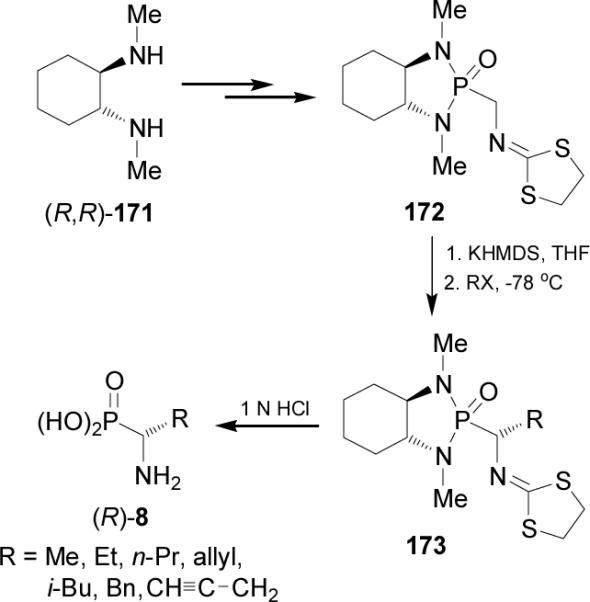
In 1990 Hanessian and Bennani114 reported the enantio-selective synthesis of α-aminoalkylphosphonic acids (R)-8 via the diastereoselective alkylation of bicyclic phosphono-amide 172 which was easily prepared from (R,R)-diamine 171 (Scheme 45).
Scheme 45.
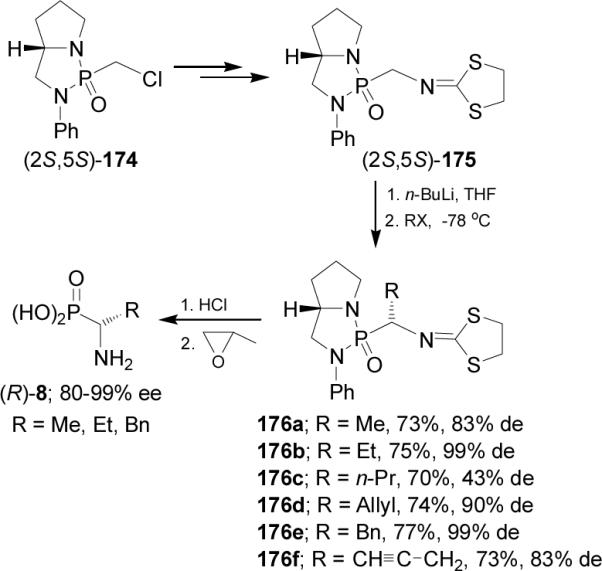
Recently, Cheng-Ye and Qian-Yi115 reported a facile and efficient asymmetric synthesis of α-aminophosphonic acids (R)-8 via the diastereoselective alkylation of bicyclic phosphonoamide 175. Thus, treatment of (2S,5S)-175 readily obtained from (2S,5S)-174 derived from (S)-2-anilinomethylpyrrolidine, with n-BuLi in THF at -78 °C followed by the addition of alkyl halide, afforded the alkylated products 176a-f in moderated yield and with moderated to excellent diastereoselectivity (43-99%). Acidic hydrolysis of 176a-b and 176e gave the (R)-α-aminoalkylphosphonic acids 8 with excellent enantiomeric excess (Scheme 46).116
Scheme 46.
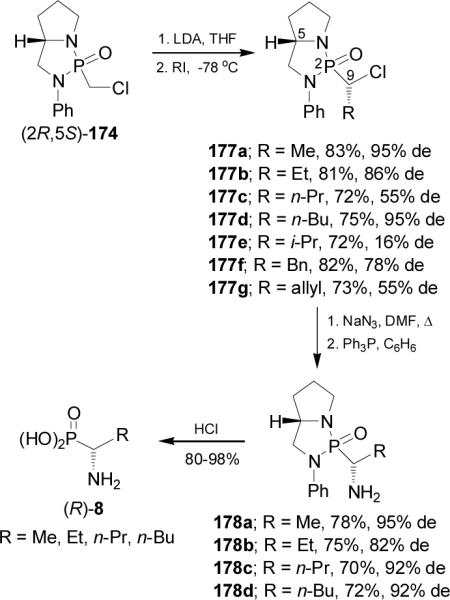
On the other hand, treatment of bicyclic chloromethylphosphonoamide (2R,5S)-174 with lithium diisopropylamide (LDA) in THF at -78 °C followed by addition of alkyl iodide, afforded the alkylated products (2R,5S,9S)-177a-g in good yield (72-83%) and with low to good levels of diastereoselectivity (16-95% de). Nucleophilic displacement on chloro derivatives 177a-d by the azide ion and subsequent treatment under Staudinger reaction117 conditions, gave the α-aminophosphonoamides 178a-d in good yield and diastereoselectivity (82-95% de). Acidic hydrolysis of 178a-d led to (R)-α-aminophosphonic acids 8 (Scheme 47).118,119
Scheme 47.
Recently, Yokomatsu et al.120 reported the diastereoselective synthesis of α-aminophosphonate 184 by a highly diastereoselective alkylation of phosphoglycine derivative (RP)-179. In this context, treatment of (RP)-179 with LHMDS in THF at -78 °C followed by addition of benzyl bromide afforded the benzylated product (R,RP)-180 in 73% yield and 10:1 diastereoisomeric ratio,121 which by hydrogenolysis over Pd(OH)2/C and subsequent tosylation of free amine (R,RP)-181 gave the N-tosyl derivative (R,RP)-182 in 93% yield. Deprotection of ketal moiety in (R,RP)-182 with TMSCl and EtOH provided the compound (R,RP)-183 in 73% yield.122 Finally, oxidation of (R,RP)-183 with DMSO and I2 followed by the esterification with diazoethane led to enantiomerically pure (R)-α-aminophosphonate 184 in 37% yield (Scheme 48).
Scheme 48.
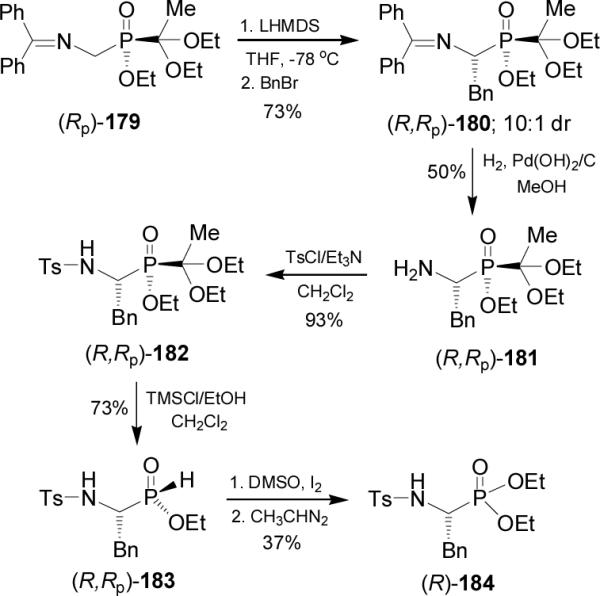
Recently, we have reported the first stereochemical reversal in the benzylation reaction of the phosphonoamide 185123 changing the LDA equivalents. In this context, the enolization of 185 with freshly LDA 2.0 equiv in THF at -78 °C, followed by the addition of benzyl bromide afforded the quaternary β-phosphonoamides (R,S)-186 and (S,S)-187 in 77% yield and 90:10 diastereoisomeric ratio, with predominance of (R,S)-186. Whereas, when the enolate of 185 was generated with LDA 2.5 equiv at -78 °C followed by the addition of benzyl bromide, gave also the quaternary β-phosphonoamides (R,S)-186 and (S,S)-187 in 83% yield and 20:80 dr, but now with a predominance of (S,S)-187 (Scheme 49).124 Quaternary β-phosphonoamides (R,S)-186 and (S,S)-187 could be transformed into quaternary α-aminophosphonic acids after several reactions including the Curtius rearrangement.125
Scheme 49.
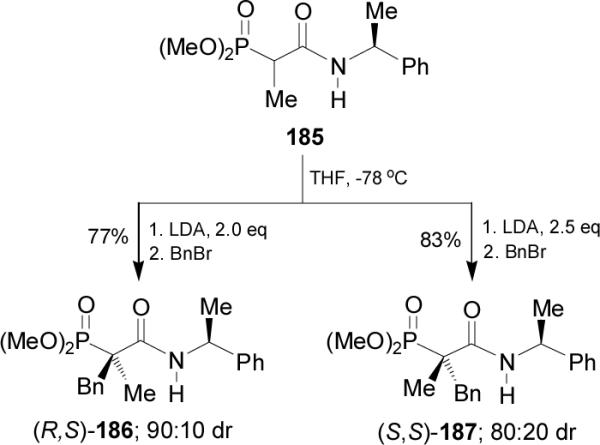
Asymmetric Michael addition of diethyl (1-cyanoethyl)-phosphonate 188 to acrylaldehyde in the presence of Rh(acac)(CO)2 and (R,R)-(S,S)-PhTRAP 189 in dry benzene, afforded the quaternary optically active (4-oxoalkyl)-phosphonate 190 in 80% yield and 92% ee, which by treatment with benzyltriphenylphosphonium ylide and subsequent hydrogenation of new formed carbon-carbon double bond led to cyano derivative 191 in 86% yield. Complete hydrolysis of 191 using 47% HBr followed by the esterification with diazomethane led to dimethyl ester 192 in 40% yield. Selective hydrolysis of carbomethoxy group in 192 and subsequent Curtius rearrangement125 followed by the treatment with benzyl alcohol gave the quaternary α-aminophosphonate 193 in 81% yield (Scheme 50).126
Scheme 50.
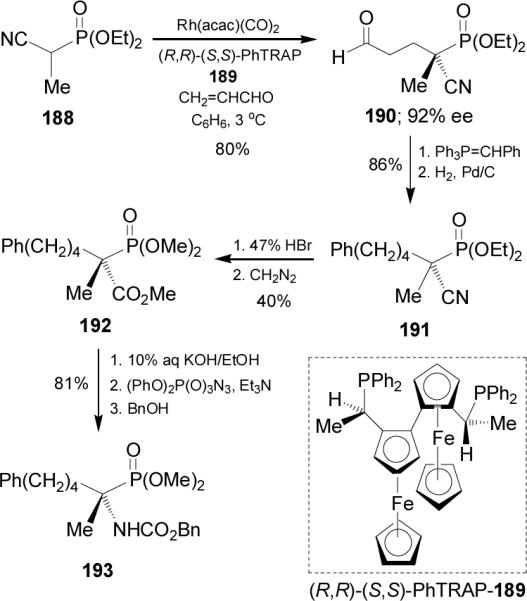
On the other hand, asymmetric allylation of α-acetamido β-ketophosphonate 194 at -30°C with allyl acetates 195 using potassium tert-butoxide as base in the presence of 1 mol % of chiral catalyst prepared in situ from (R)-BINAP and [Pd(π-allyl)(cod)]BF4, afforded the α-allyl α-aminophosphonates 196a-e in 78-87% ee. However, the allylation reaction of α-acetamido β-carbomethoxyphosphonate gave 196f with only low enantioselectivity (Scheme 51).127
Scheme 51.
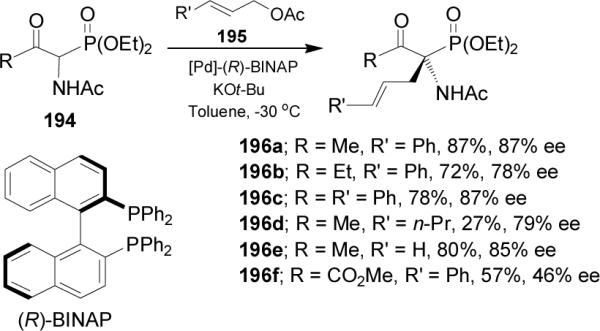
Recently, Jászay et al.128 reported the synthesis of (S)-phosphoglutamic acid 200 via a catalytic enantioselective Michael addition. In this context, treatment of the achiral Schiff base 197 derived from phosphoglycine, with sodium tert-butoxide followed by the addition of tert-butyl acrylate in the presence of TADDOL 198, produced the α-aminophosphonate 199 in 95% yield and 72% ee.129 Acidic hydrolysis of 199 using 6 N HCl gave the (S)-phosphoglutamic acid130 200 (Scheme 52).
Scheme 52.
2.2.2. Nucleophilic addition to iminophosphonates
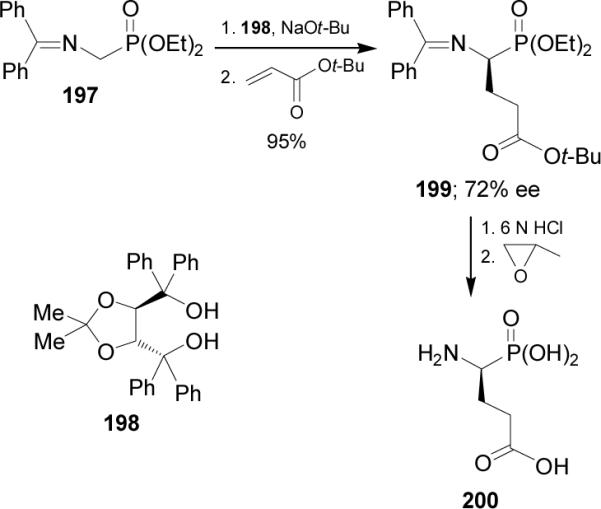
Catalytic enantioselective carbon-carbon bond-forming reactions have been used for the asymmetric synthesis of α-aminophosphonates. In this context, addition of silicon enolates 202a-k derived from aromatic and aliphatic ketones, to N-acyl-α-iminophosphonate 201 catalyzed by the chiral copper(II) complex derived from Cu(OTf)2 and diamine 203 in the presence of hexafluoroisopropyl alcohol (HFIP) at 0 °C, gave the γ-keto-α-aminophosphonates 204a-k in good yield (70-88%) and enantioselectivity (76-94% ee), (Scheme 53).131
Scheme 53.
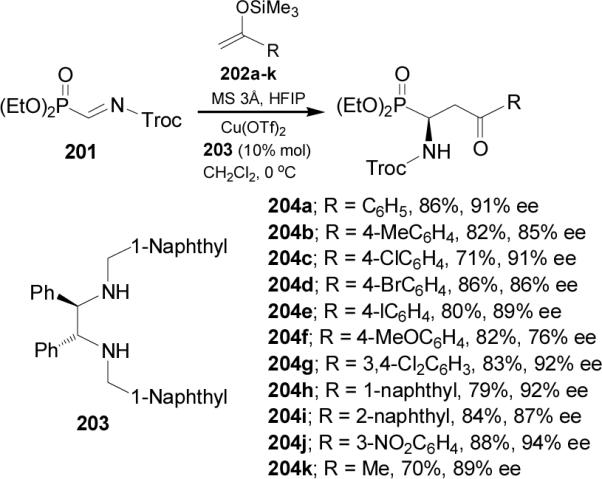
Treatment of 204g and 204j with concentrate HCl at reflux followed by recrystallization afforded the γ-keto-α-aminophosphonic acids 205g and 205j, respectively, with excellent enantioselectivity. Whereas, reaction of 204a with zinc powder in acetic acid, followed by the hydrogenolysis over Pd/C in acetic acid and methanosulfonic acid provided the α-aminophosphonate 206 with good enantioselectivity (Scheme 54).
Scheme 54.
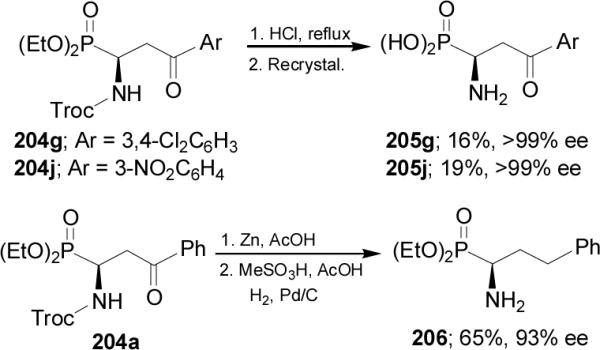
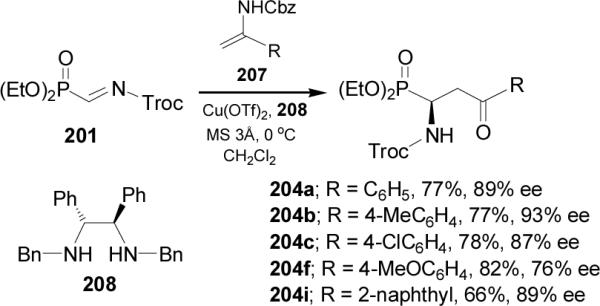
In a similar way, reaction of α-iminophosphonate 201 with the enamines 207 in the presence of Cu(OTf)2 and chiral diamine 208 in dichloromethane at 0 °C followed by hydrolysis, provided the corresponding γ-keto-α-aminophosphonates 204 in good yield (66-82%) and enantioselectivity (76-93% ee), (Scheme 55).132,133
Scheme 55.
Kobayashi et al.134 reported the first example of catalytic enantioselective allylations of α-iminophosphonates for the synthesis of α-allyl α-aminophosphonates 210. In this context, allylation reaction of N-acyl-α-iminophosphonate 201 with the allylsilanes 209a-e in the presence of Cu(OTf)2 and the chiral diamine 203 in dichloromethane at 0 °C, led to the α-aminophosphonates 210 in good yield (66-86%) and enantioselectivity (79-89% ee), (Scheme 56).
Scheme 56.
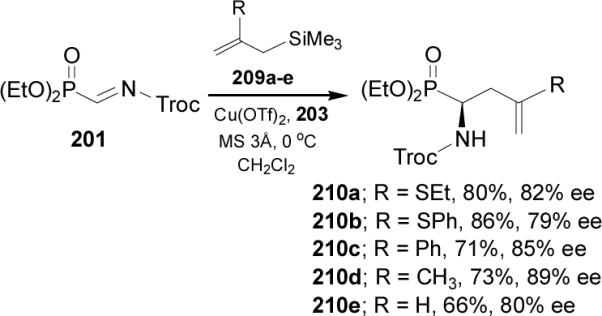
Recently, Dodda and Zhao135 reported the first enantioselective synthesis of α-aminopropargylphosphonates 214am through the direct addition of terminal alkynes 212 to α-iminophosphonate 211 in the presence of a copper(I)-bisoxazoline 213 complex as chiral catalyst. In general, high yields (56-92%) and good levels of asymmetric induction (60-81% ee) were obtained (Scheme 57).
Scheme 57.
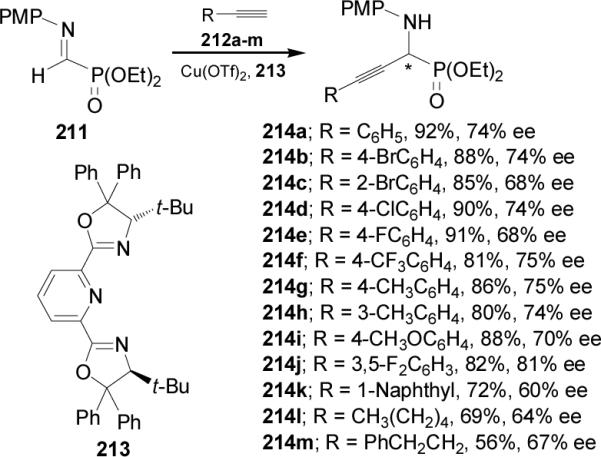
Carbon-carbon bond-forming via 1,3-dipolar cycloaddition reaction has been used for the synthesis of (R)- and (S)-phosphohomoserine 220.136 In this context, 1,3-dipolar cycloaddition of the nitrone (S)-215 with allyl alcohol in the presence of ZnCl2 or MgBr2 gave the unseparable mixture of cis-isomers (3S,5S,1'S)-216 and (3R,5R,1'S)-217 in a 50:50 ratio.137,138 Hydrogenation of diastereoisomerically pure (3S,5S,1'S)-216 in the presence of (Boc)2O led to NBoc-aminodiol (1S,3S)-218 in 75% yield, which by treatment with sodium metaperiodate followed by the reduction of aldehyde generated with NaBH4 provided the corresponding alcohol (S)-219. Finally, acidic hydrolysis of (S)-219 with 6 M HCl and subsequent treatment with propylene oxide afforded the enantiomerically pure (S)-phosphohomoserine 220 in 94% yield. In a similar fashion, diastereoisomerically pure (3R,5R,1'S)-217 was transformed into (R)-phosphohomoserine 220 (Scheme 58).
Scheme 58.
2.3. Stereoselective C-N bond formation
2.3.1. Stereoselective electrophilic amination
Other approach for the asymmetric synthesis of α-aminophosphonic acids is the stereoselective electrophilic amination of chiral α-phosphonates carbanions. In this context, several chiral oxazaphosphorinanes and oxazaphospholanes and diazophospholanes, derived from alkylphosphonic dichlorides and appropiated chiral amino alcohols or diamines, have been used as key substrates in the electrophilic amination. For example, Denmark et al.139 reported the asymmetric synthesis of α-aminophosphonic acids from 221 via stereoselective electrophilic amination. On the other hand, chiral oxazaphospholanes 222140 and 223141 have been also used in the synthesis of α-aminophosphonic acids. Similar results have been obtained using the diazaphospholane 224.142
Recently, Jørgensen et al.143 reported that the enantioselective α-amination of α-ketophosphonates 225 with dibenzyl azodicarboxylate in the presence of a catalyst formed by combination of chiral bisoxazoline 226 and Zn(OTf)2, afforded the corresponding aminated products 227 in good yield (75-98%) and excellent enantioselectivity (85-95% ee) (Table 11).
Table 11.
Catalytic enantioselective α-amination of 225.
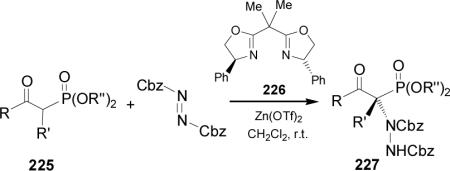
| Entry | R | R' | R” | Yield (%) | ee (%) |
|---|---|---|---|---|---|
| 1 | Ph | Me | Et | 85 | 92 |
| 2 | 2-Naph | Me | Et | 93 | 92 |
| 3 | Bn | Me | Et | 60 | 95 |
| 4 | Me | Me | Et | 75 | 85 |
| 5 | Ph | Allyl | Et | 85 | 98 |
| 6 | Ph | Me | Me | 97 | 94 |
| 7 | -(CH2)3- | Et | 98 | 95 | |
| 8 | -(CH2)4- | Et | 98 | 94 | |
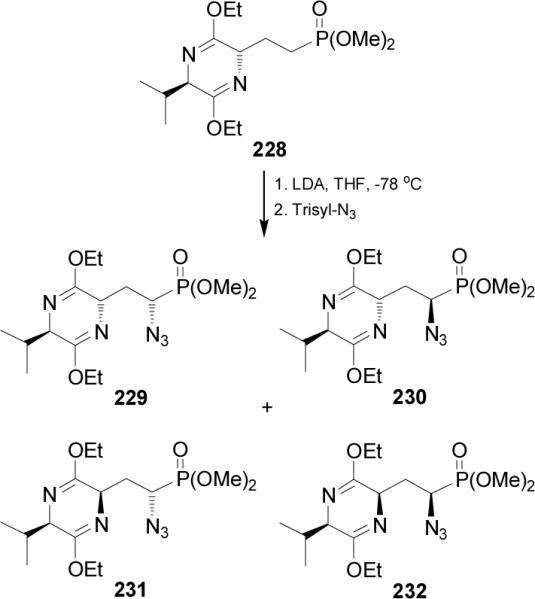
Recently, Ruiz et al.144 reported that the treatment of bislactim ether 228 with LDA in THF at -78 °C followed by the addition of trisyl-N3, gave the phosphonates 229 and 230 derived from azidation by a complete retention of 2,5-trans configuration of the bis-lactim ether, and the phosphonates 231 and 232 products of the racemization of bis-lactim 229 at position 2. The mixture of the phosphonates 229, 230, 231 and 232 was obtained in 80% yield and 7:7:1:1 ratio (Scheme 59).145
Scheme 59.
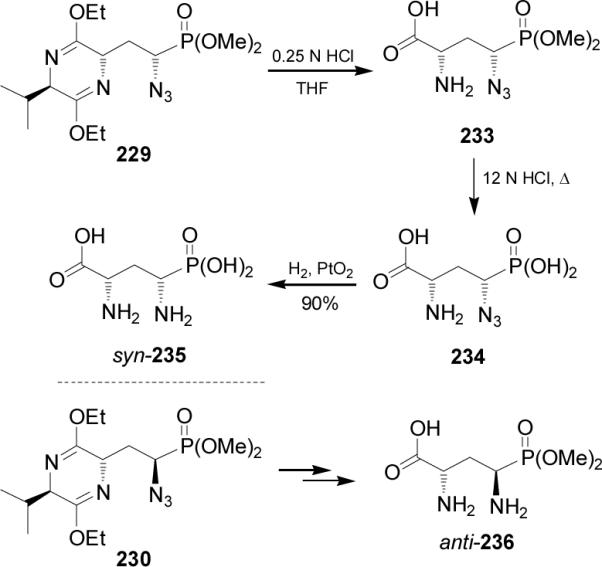
Mild acid hydrolysis of diastereoisomerically pure 229 with 0.25 N HCl provided the compound 233, which by acid hydrolysis with 12 N HCl at reflux led to α-azidophosphonic acid 234. Finally, catalytic hydrogenation of 234 over PtO2 gave the α-aminophosphonic acid syn-235 in 90% yield, an AP4 derivative.146 In a similar way, 230 was transformed into anti-236 (Scheme 60).
Scheme 60.
2.3.2. Addition of amines to enaminophosphonates
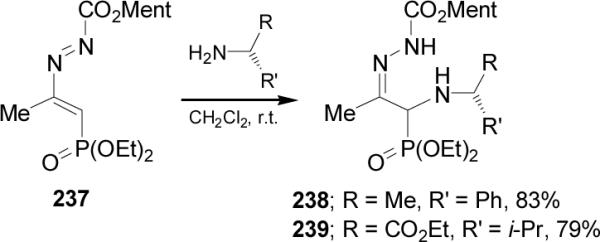
Palacios et al.147 reported that the addition of (R)-α-MBA (R = Me, R' = Ph) and ethyl (S)-valinate (R = CO2Et, R' = i-Pr) to 1,2-diaza-1,3-butadiene 237 afforded the α-aminophosphonates 238 and 239, respectively, in good yield but with very low diastereoselectivity (<10% ds), (Scheme 61). Similar results were obtained in the addition of non-chiral amines to 237.
Scheme 61.
The same authors reported that the Michael addition of benzylamine to 1,2-diaza-1,3-butadiene 240 derived from L-lactic acid, gave the corresponding α-aminophosphonate 241 in 75% yield and 40% de. In a similar way, addition of (S)-valine methyl ester to 240 afforded the non-separable diastereoisomeric mixture 242 in 57% yield and moderated diastereoselectivity (Scheme 62).
Scheme 62.
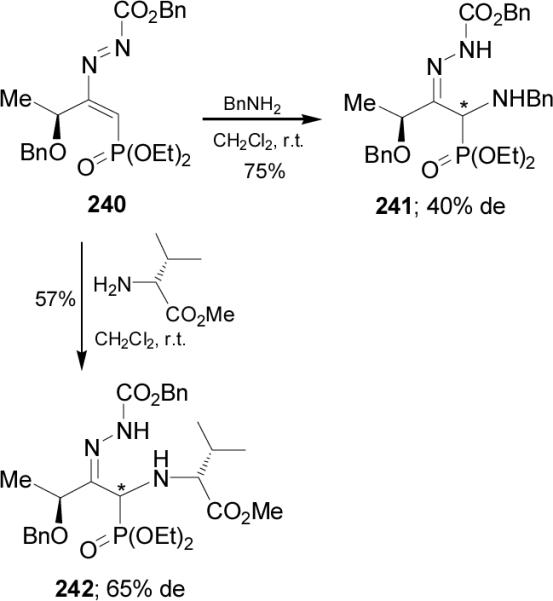
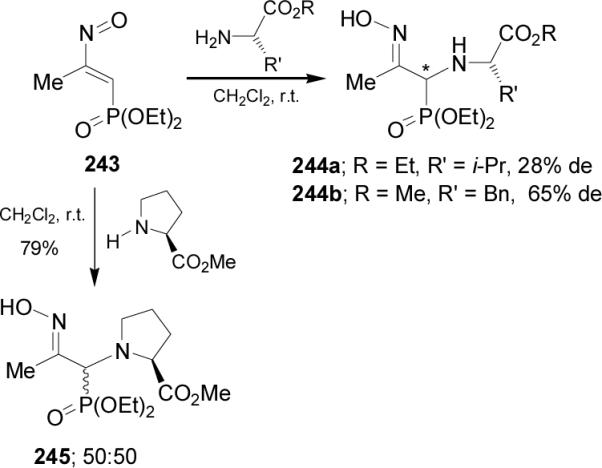
Phosphonyl nitrosoalkenes are reactive intermediates as Michael aceptors toward nucleophilic reagents such as ammonia, amines and enantiomerically pure α-amino esters. For example, addition of L-valine ethyl ester hydrochloride to nitrosoalkene 243 afforded the α-aminophosphonate 244a as nonseparable diastereoisomeric mixture in 82% yield and 28% de. In a similar way, Michael addition of L-phenylalanine methyl ester hydrochloride to 243 gave the α-aminophosphonate 244b in 80% yield and 65% de. However, no diastereoselection was observed when L-proline methyl ester hydrochloride was added to 243, and both diastereoisomers of 245 were obtained as an equimolecular mixture (Scheme 63).148
Scheme 63.
2.4. Stereoselective C-H bond formation
2.4.1. Catalytic hydrogenation of dehydroaminophosphonates
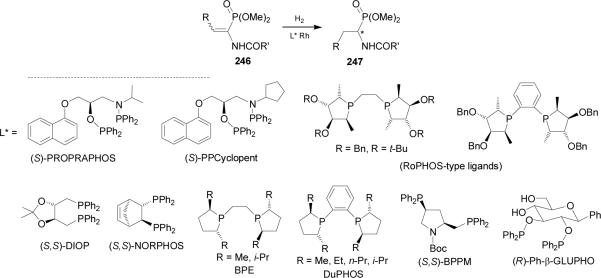
Catalytic asymmetric hydrogenation of dehydroaminophosphonates149 type 246 is other methodology available for synthesis of optically pure α-aminophosphonic acids and derivatives. In this context, from 1985 to 1999 several catalyst have been used in the hydrogenation of 246 obtaining the α-aminophosphonates 247 in good yield and excellent levels of enantioselectivity (Scheme 64).150
Scheme 64.
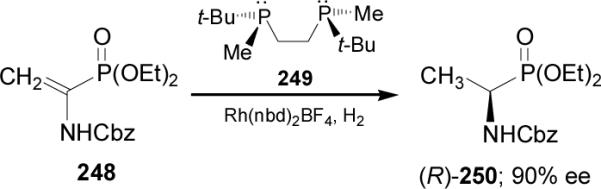
In 2004, Imamoto et al.151 described that the catalytic hydrogenation of dehydroaminophosphonate 248 in the presence of rhodium complex (R,R)-t-BuBisP* 249 gave the (R)-α-aminophosphonate 250 in 90% ee (Scheme 65).
Scheme 65.
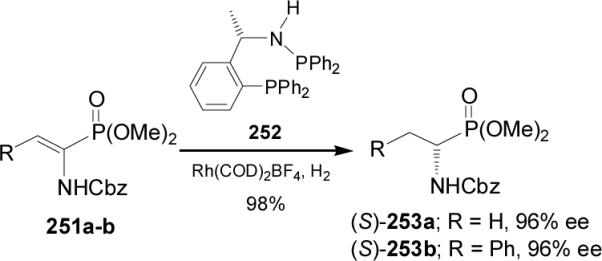
Recently, Hu et al.152 reported that the chiral phosphineaminophosphine (PEAphos) 252 readily obtained from (S)-MBA promoted an excellent enantioselectivities in the Rhcatalyzed asymmetric hydrogenation of dehydroaminophosphonates 251a-b, and the corresponding (S)-α-amino-phosphonates 253a-b were obtained in excellent yield and enantioselectivity (96% ee), (Scheme 66).
Scheme 66.
On the other hand, asymmetric hydrogenation of dehydroaminophosphonate 251b in the presence of a rhodium complex derived of bis(phospholanes) 254 or BASPHOS 255, gave the (R)-α-aminophosphonate 253b in moderated enantioselectivity (20.8-78.8% ee), (Scheme 67).153
Scheme 67.
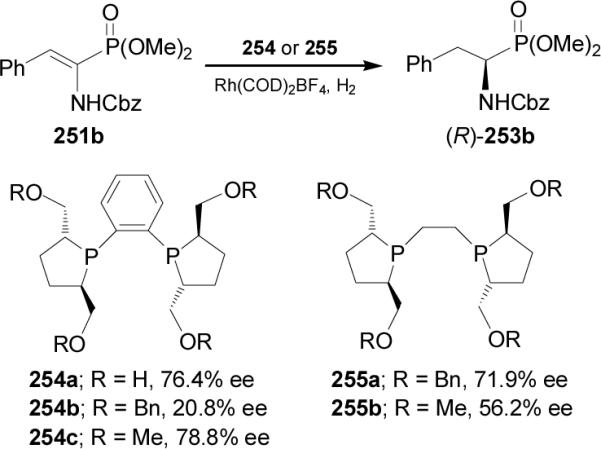
1-Amino-2,2,2-trifluoroethanephosphonic acid (R)-260 has been obtained by a base-catalyzed [1,3]-proton shift reaction of diisobutyl 1-N-α-methylbenzylimino-2,2,2-trifluoroethanephosphonate 257.154 In this context, the reaction of chloroimine (S)-256 with triisobutyl phosphite at 80 °C gave the (S)-α-iminophosphonate 257 in 79% yield, which by [1,3]-proton shift induced by triethylamine afforded the α-aminophosphonate (R)-258 in 73% yield and 67% ee. Acidic hydrolysis of imine group in (R)-258 with 2 N HCl produced the α-aminophosphonate (R)-259 in 85% yield. Finally, treatment of (R)-259 with concentrated hydro-chloric acid, followed by the addition of propylene oxide afforded the (R)-α-aminophosphonic acid 260 in 90% yield (Scheme 68).155
Scheme 68.
2.5. Resolutions
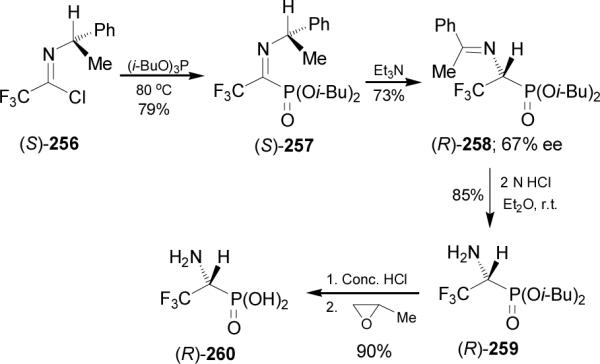
Optically active α-aminophosphonic acids can be also obtained by resolution. For example, reaction of dibenzoyl L-tartaric anhydride 261 with diphenyl α-aminophosphonates 262a-e provided the amides 263a-e. Hydrolysis of diastereoisomerically pure 263a-e obtained by crystallization gave both enantiomers of (S)- and (R)-α-aminophosphonic acids 8 in high yields (Scheme 69).156
Scheme 69.
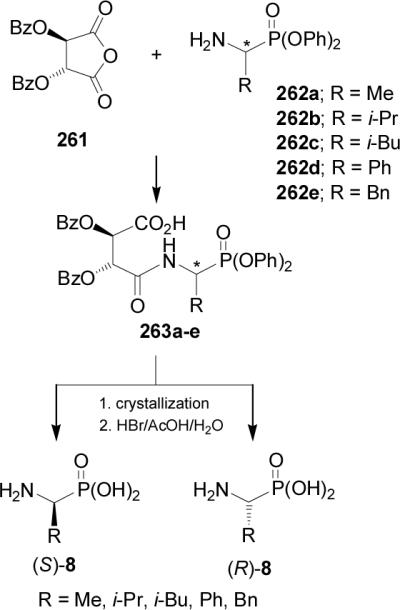
On the other hand, resolution of (±)-264 on a 500 g-scale using simulated moving-bed chromathography on Chiracel OJ, gave the (R)-α-aminophosphonate 265 in 35% yield and 99% ee (Scheme 70).157
Scheme 70.
Biocatalytic resolution of racemic molecules has attracted the interest of synthetic chemists for several decades.158 In this context, Yuan et al.159 reported that CALB-catalyzed acylation of 266a-e using ethyl acetate as acetylating reagent, produced the optically enriched 267a-e and 268a-e in good yield and enantioselectivity (Table 12).
Table 12.
CALB-catalyzed enantioselective acetylation of 266a-e.
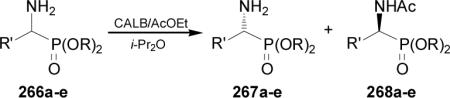
| 267 | 268 | |||||
|---|---|---|---|---|---|---|
| Substrate | R | R' | Yield (%) | ee (%) | Yield (%) | ee (%) |
| 266a | Et | Me | 41 | 99.7 | 48 | 90 |
| 266b | n-Pr | Me | 42 | 90 | 42 | 98 |
| 266c | i-Pr | Me | 44 | 96 | 43 | 98 |
| 266d | Et | Et | 73 | 18 | 10 | 100 |
| 266e | Et | CF3 | a | -- | -- | -- |
No reaction
2.6. Chiral pool
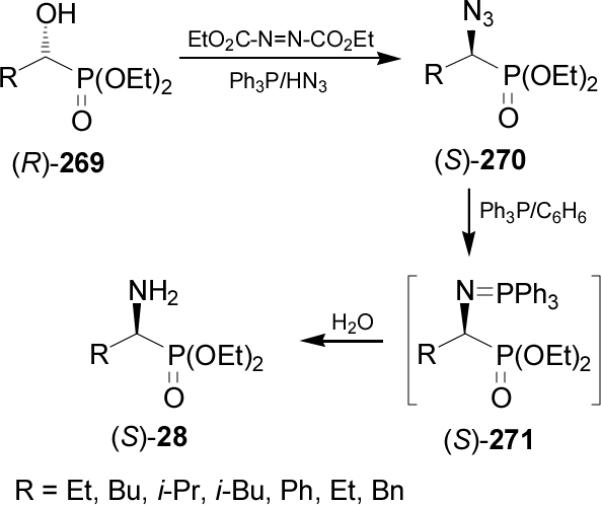
The hydroxy group in enantiomerically pure α-hydroxyalkylphosphonates can be replaced by an amino function using the Mitsunobu reaction.160 For example, treatment of (R)-α-hydroxyphosphonates 269 under Mitsunobu conditions using triphenylphosphine (Ph3P), diethyl azodicarboxylate (DEAD) and hydrazoic acid, afforded the (S)-α-azidophosphonates 270 with complete inversion of configuration.161 Reduction of azido group in 270 under Staudinger reaction117 with Ph3P followed by the hydrolysis of the iminophosphoranes 271 gave the (S)-α-aminophosphonates 28 in good yield (50-88%) and enantioselectivity (40-82% ee) (Scheme 71).162
Scheme 71.
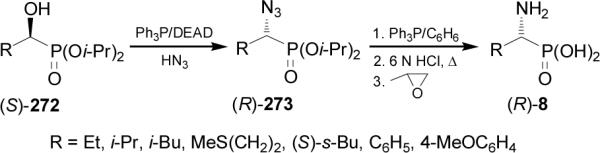
In a similar way, treatment of (S)-α-hydroxyphosphonates 272 (92-99% ee) with Ph3P/DEAD/HN3, gave the (R)-α-azidophosphonates 273 with good yield and 68-90% ee, which by reduction of azido group with Ph3P followed by acidic hydrolysis led to the (R)-α-aminophosphonic acids 8 in 59-85% yield (Scheme 72).163
Scheme 72.
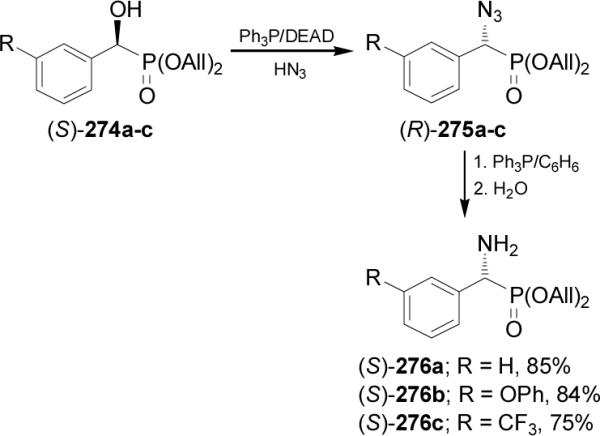
On the other hand, treatment of (S)-α-hydroxyphosphonates 274a-c with Ph3P/DEAD/HN3 gave the (R)-α-azidophosphonates 275a-c in 88-98% yield, which by reduction of azido group with PPh3 led to the (R)-α-amino-phosphonates 276a-c in 75-85% yield (Scheme 73).164
Scheme 73.
α-Amino-β-hydroxyphosphonic acids can be obtained from α,β-dihydroxyphosphonates via Mitsunobu azidation. For example, reaction of 277a-b with Ph3P/DEAD/HN3 afforded the α-azidophosphonates 278a-b in moderated yield, which by catalytic hydrogenation over Pd/C in the presence of (Boc)2O provided to the N-Boc-α-aminophosphonates 279a and 279b in 85 and 83% yield, respectively (Scheme 74).165
Scheme 74.
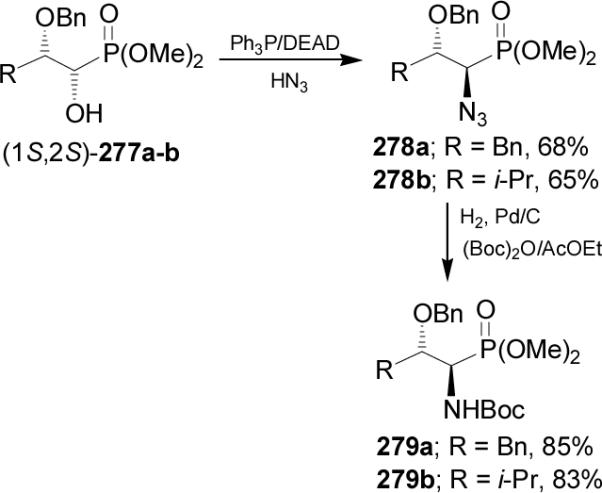
In a similar way, treatment of (1S,2S)-280 with Ph3P/DEAD and HN3 led to α-azidophosphonate 281, which by catalytic hydrogenation over PtO2, followed by acidic hydrolysis of 282 gave the (1R,2S)-phosphotreonine 283. Under identical conditions (1R,2R)-280 was transformed into (1S,2R)-283 (Scheme 75).166
Scheme 75.
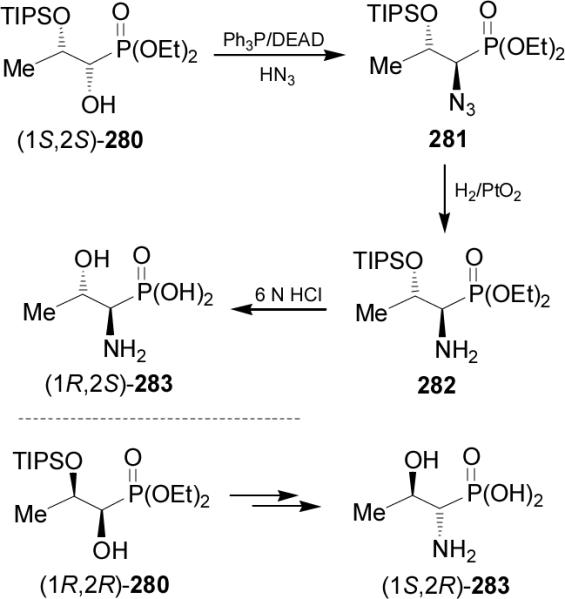
On the other hand, reaction of (S)-284 with p-nitrobenzenesulfonyl chloride furnished the corresponding nosylate (S)-285 in 94% yield, which under Staudinger reaction gave the corresponding aziridine (R)-286. Regioselective opening of the aziridine (R)-286 with TFA followed by acidic hydrolysis with hydrochloric acid and subsequent ion exchange, afforded the (R)-phosphoserine 287 in 59% yield (Scheme 76).167
Scheme 76.
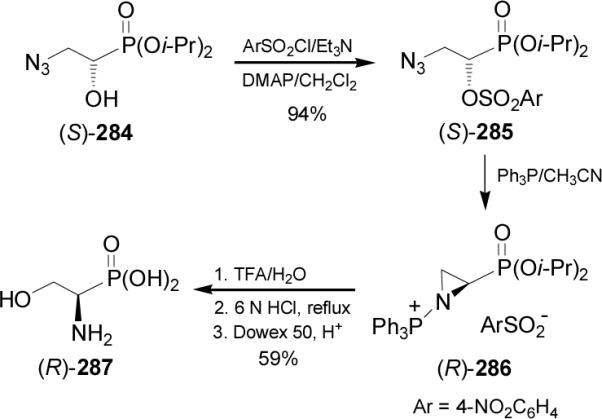
Treatment of phosphoserine diethyl esther (R)-288 with tosylchloride afforded the corresponding N-tosylate (R)-289 in 74% yield, which by reaction with mesylchloride afforded the O-mesylate derivative (R)-290 in 75% yield. Reaction of (R)-290 with NaH in THF gave the aziridine-2-phosphonate (R)-291 in 88% yield, which by reaction with several nucleophiles gave the α-aminophosphonates (R)-292a-j in 36-87% yield. In a similar way, the α-aminophosphonates (S)-292a-j were obtained from (S)-288 (Scheme 77).168
Scheme 77.
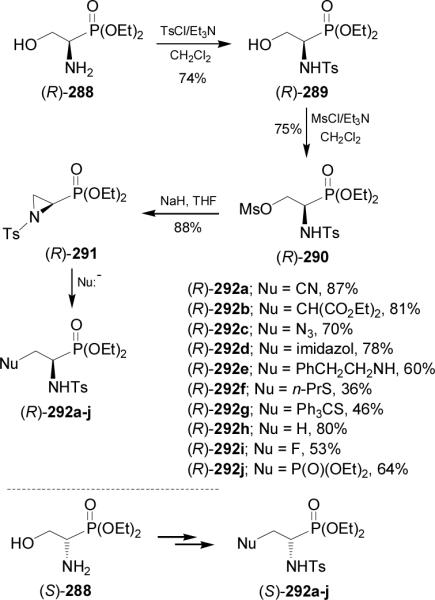
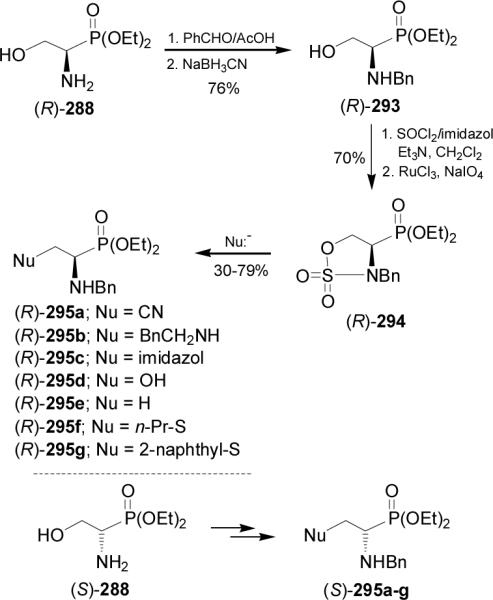
On the other hand, reaction of phosphoserinate (R)-288 with benzaldehyde followed by the reduction with sodium cyanoborohydride in acetic acid afforded the N-benzyl aminophosphonate (R)-293 in 76% yield. Treatment of (R)-293 with thionyl chloride and subsequent oxidation with sodium periodate in the presence of ruthenium chloride gave the sulfonamide (R)-294 in 70% yield, which by reaction with several nucleophiles provided the α-aminophosphonates (R)-295a-g. In a similar way, (S)-295a-g were obtained from (S)-288 (Scheme 78). 169
Scheme 78.
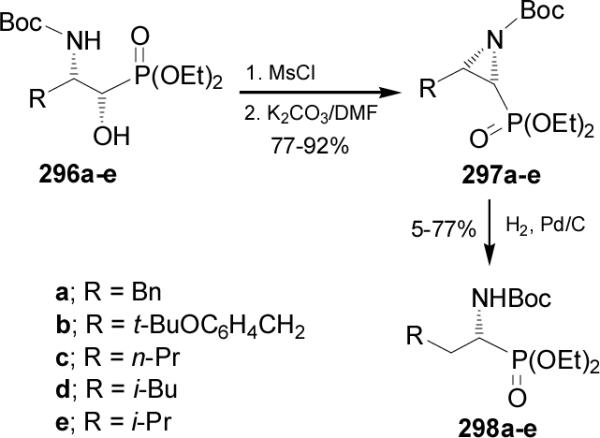
Pousset and Larchevêque170 reported that the catalytic hydrogenation of N-Boc-aziridine-2-phosphonates 297a-e readily obtained from 3-amino-2-hydoxyphosphonates 296a-e in 77-92% yield, furnished the N-Boc-α-aminophosphonates 298a-e in moderated yield (5-77%) and high enantioselectivity (Scheme 79).
Scheme 79.
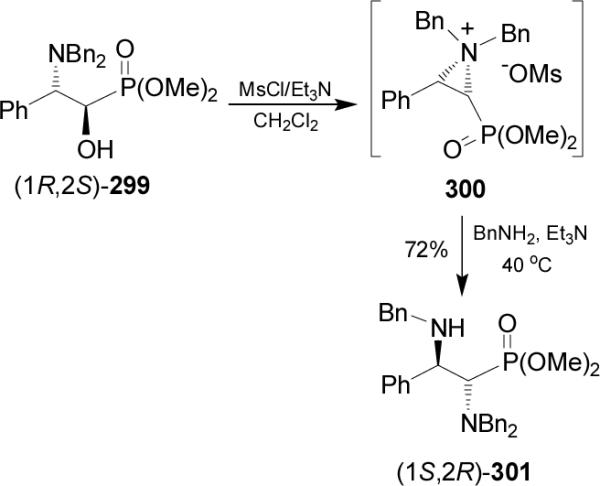
On the other hand, treatment of α-hydroxy phosphonate (1R,2S)-299 with mesyl chloride in the presence of triethylamine followed by the addition of benzylamine gave the α,β-diaminophosphonate (1S,2R)-301 in 72% yield. Transformation of (1R,2S)-299 into (1S,2R)-301 with inversion of configuration takes place through the participation of the aziridium ion 300 (Scheme 80).171
Scheme 80.
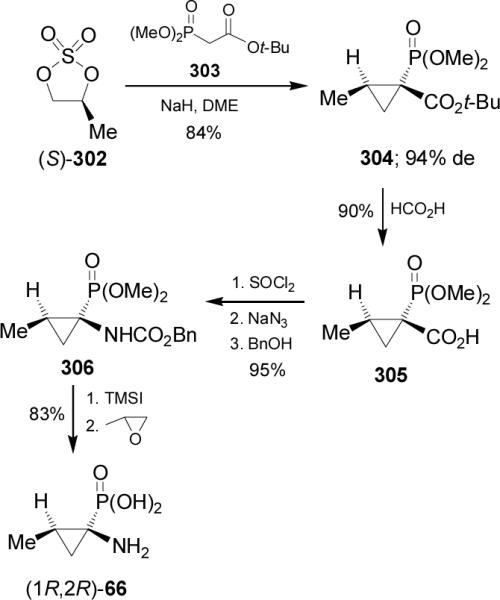
Treatment of sulfate (S)-302 readily obtained by reaction of (S)-1,2-propanediol, with dimethyl t-butoxycarbonylmethylphosphonate 303 and NaH gave the cyclopropane derivative 304 in 84% yield and 94% de. Acidic hydrolysis of 304 with formic acid afforded the carboxylic acid derivative 305 in 90% yield, which by treatment with thionyl chloride, followed by Curtius rearrangement using sodium azide and subsequent addition of benzyl alcohol, furnished the N-protected aminophosphonate 306 in 95% yield. Finally, hydrolysis of 306 with TMSI followed by treatment with propylene oxide led to enantiomerically pure (1R,2R)-1-amino-2-methylcyclopropanephosphonic acid 66 in 83% yield (Scheme 81).172 The aminophosphonic acid 66 is an analogue of (1S,2R)-allo-norcoronamic acid.173
Scheme 81.
2.7. Stereoselective synthesis of azaheterocyclic phosphonic acids and derivatives
Azaheterocyclic phosphonates are considered as one of the most biologically important class of heterocyclic. In this context, in 2004 Stevens et al.174 published a review about synthetic methods for azaheterocyclic phosphonates and their biological activity, and recently De Kimpe et al.175 published other review on the synthesis and reactivity of C-heteroatoms-ubsituted aziridines, including C-phosphorus-substituted aziridines. Now we describe herein only some examples and an update over the stereoselective synthesis of azaheterocyclic phosphonic acids and derivatives.
2.7.1. Aziridin-2-ylphosphonic acids and derivatives
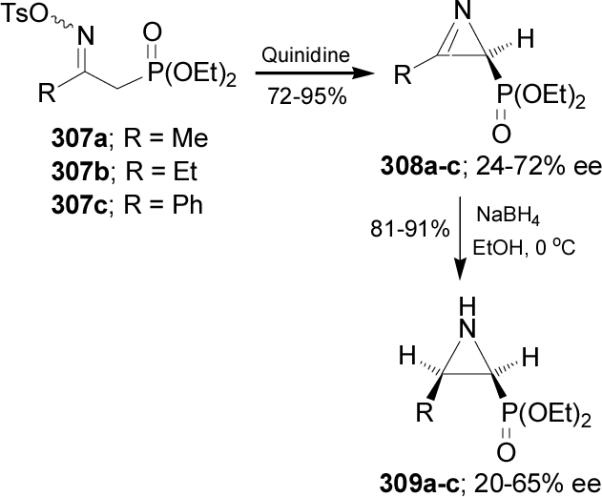
Palacios et al.176,177 reported that the treatment of O-tosyl oximes 307a-c with quinidine (QN) afforded the 2H-azirine-2-phosphonates 308a-c in good yield (72-95%) and moderated enantioselectivity (24-72% ee). When (-)-sparteine, hydroquinidine or quinine were used as a base, 308a-c were obtained with low enantioselectivity. Reduction of 308a-c with NaBH4 in ethanol gave the 2-phosphorylated cis-aziridines 309a-c in 81-91% yield and with 20-65% ee (Scheme 82).
Scheme 82.
2.7.2. Azetidin-2-ylphosphonic acids and derivatives
Stereoselective synthesis of azetidin-2-ylphosphonates has been scarcely explored. The first asymmetric synthesis of azetidine-2-phosphonates of type 313, 316 and 317 was reported by Couty et al.178 In this context, treatment of aminophosphonate 310 derived from (S)-N-benzyl phenylglycinol,179 with thionyl chloride in dichloromethane followed by the addition of NaHCO3 gave the chloro derivative 311 in 92% yield. Reaction of 311 with LHMDS in THF afforded only the 1,3-trans azetidine 312 in 75% yield, which by hydrolysis of the phosphonate moiety with TMSBr followed by purification by ion-exchange chromatography led to azetidin-2-ylphosphonic acid 313 in 86% yield. In a similar way, aminophosphonates 314 and 315 derived from (1R,2S)-ephedrine and (1R,2S)-pseudo-ephedrine, respectively, afforded the aminophosphonic acids 316 and 317 in good yield (Scheme 83).
Scheme 83.
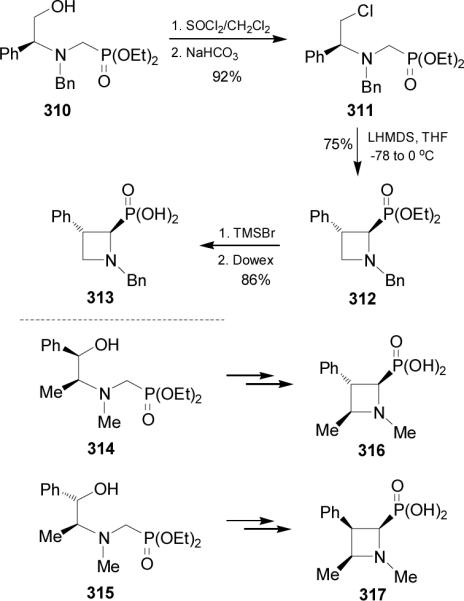
2.7.3. Pyrrolidin-2-ylphosphonic acids and derivatives
Reaction of 2,5-dimethoxytetrahydrofurane 318 with (R)-phenylglycinol and benzotriazole (BtH) via a double Robinson-Schopf condensation,180 afforded the bicyclic derivative (3R,5S,7aS)-319 in 80% yield, which by an Michaelis-Arbuzov reaction with triethyl phosphite in the presence of ZnCl2 gave the corresponding phosphonate (3R,5S,7aS)-320 as a single diastereoisomer in 77% yield. Hydrogenolysis of 320 followed by acidic hydrolysis of phosphonate moiety with 6 M HCl and subsequent treatment with propylene oxide led to (S)-phosphoproline 321 in 89% yield (Scheme 84).181
Scheme 84.
On the other hand, treatment of (3R,5S,7aS)-320 with n-BuLi followed by the addition of MeI afforded the methylated product (3R,5S,7aS)-322 in 95% yield, high diastereoselectivity, and with retention of the configuration. Hydrogenolysis of 322 over Pd/H2 gave the quaternary phosphorproline diethyl esther (S)-323 in 83% yield (Scheme 85).182
Scheme 85.
Addition of trimethyl phosphite to the bicyclic lactam 324 readily obtained from (R)-phenylglycinol, in the presence of TiCl4 gave the phosphonylated pyrrolidinone 325 in 86% yield and 62% de (Scheme 86).183
Scheme 86.
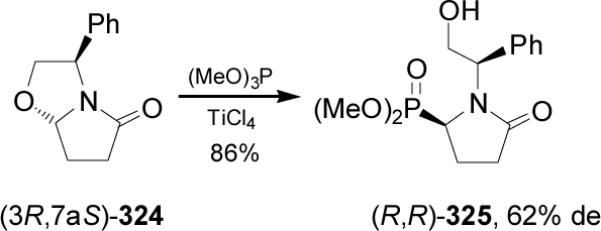
Treatment of chiral sulfinyl imines 326a-b with lithium salt of diethyl phosphite gave the α-aminophosphonates 327a-b in good yield and moderated diastereoselectivity. Cleavage of sulfinyl group and hydrolysis of acetal gave the aminocarbonyl derivative, which cyclized to afford the iminophosphonates 328a-b. Finally, catalytic hydrogenation of 328a-b led to the cyclic α-aminophosphonates 329a-b. In a similar way, chiral sulfinyl mine 330 furnished to α-aminophosphonate (2R,5S)-331 (Scheme 87).184
Scheme 87.
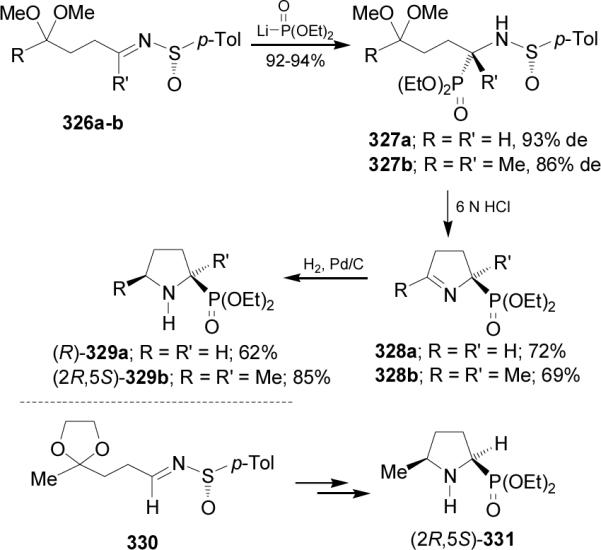
Davies et al.185 reported the synthesis of cis-5-substituted pyrrolidine-2-phosphonates 339a-d using metal carbenoid NH insertion. In this context, reaction of β-amino esters 332a-d with lithium dimethyl methylphosphonate gave the corresponding δ-amino-β-ketophosphonates 333a-d, which by treatment with TFA followed by the reaction with (Boc)2O afforded the derivatives 334a-d in 80-90% yield. Reaction of 334a-d with NaH and 4-acetamidobenzenesulfonyl azide (4-ABSA) furnished the diazo derivatives 335a-d in excellent yield (83-91%), which by treatment with Rh2(OAc)4 led to the 3-oxo-pyrrolidine phosphonates 336a-d. Removal of the 3-oxo group in 336a-d by treatment with NaH followed by the addition of diethyl chlorophosphonate, and subsequent hydrogenation of 337ad provided the cyclic phosphonates 338a-d in good yield. Finally, cleavage of Boc protective group in 338a-d with TFA afforded the cis-5-substituted pyrrolidine 2-phosphonates 339a-d in 68-86% yield (Scheme 88).186
Scheme 88.
Reaction of (2R,5R)-336a with NaH followed by the addition of allyl bromide187 in the presence of 18-crown-6 gave the quaternary phosphonate (2R,5R)-340 in 35% yield, which by treatment with TFA afforded the pyrrolidine phosphonate (2R,5R)-341 in 76% yield as a single diastereoisomer, product derived from the retention of the configuration. Catalytic hydrogenation of 341 gave the acyclic α-amino-β-ketophosphonate (R)-342 in 95% yield (Scheme 89).188
Scheme 89.
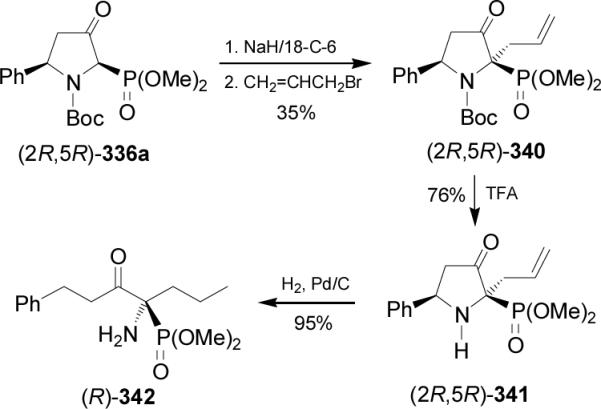
Reduction of L-pyroglutamic acid derivative 343 with super hydride in THF at -78 °C followed by acetylation and subsequent treatment with trimethyl phosphite in the presence of BF3.OEt2, gave the cyclic phosphonates 344 and 345 in moderated to good yield (45-80%) and diastereoselectivities from 1:1.5 to 1:1.9 (Scheme 90).189
Scheme 90.
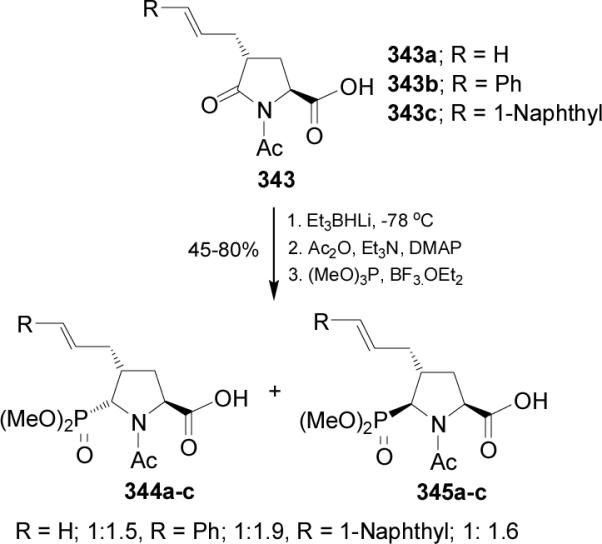
Decarboxylation-phosphorylation reaction of α-amino acids afford α-aminophosphonates in good yield.190 For example, treatment of (4R)-acetoxyproline derivative 346 with PhI(OAc)2-I2 under sunlight followed by the reaction with (MeO)3P in the presence of BF3.OEt2 afforded the α-aminophosphonates 347 and its epimer 348 in 64 and 15% yield, respectively (Scheme 91).191
Scheme 91.
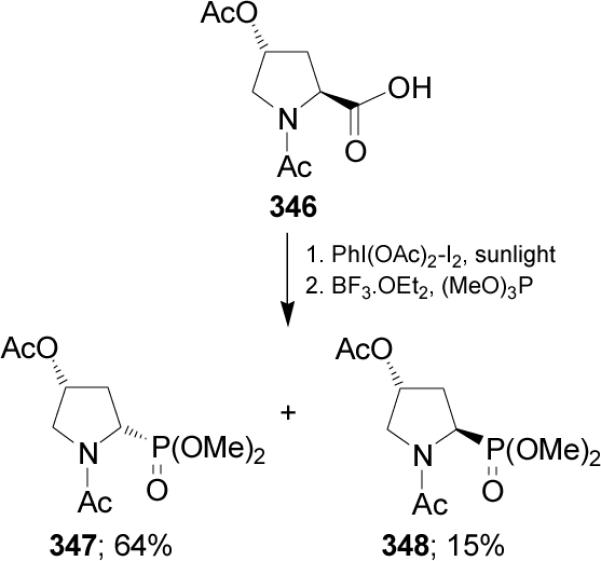
A better result was obtained in the oxidative decarboxylation of 349. In this context, anodic oxidation of 349 afforded a 1:1 mixture of 350 in 90% yield, which by treatment with trimethyl phosphite in the presence of TiCl4, gave the cyclic phosphonate 351 in 80% yield and 96% de (Scheme 92).192
Scheme 92.
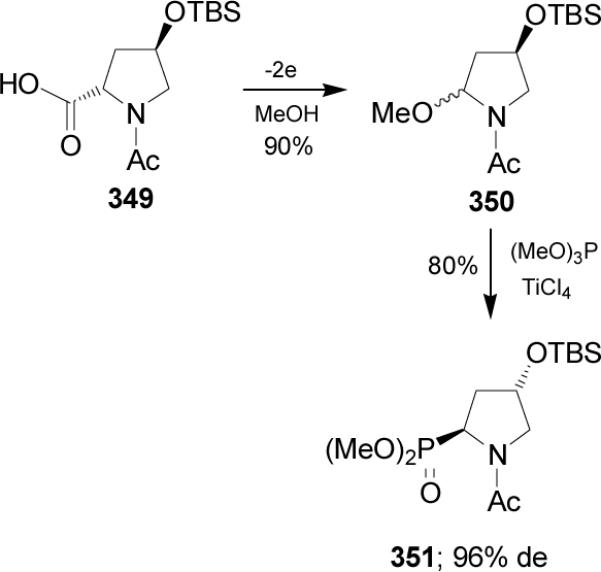
On the other hand, reaction of (3S,5S,1'S)-216 with mesyl chloride in the presence of triethylamine gave the mesylated derivative (3S,5S,1'S)-352 in 96% yield, which by hydrogenolysis followed by treatment of product obtained 353 with K2CO3 furnished (2S,4S)-354 in 75% yield. In a similar way, reaction of (3R,5R,1'S)-217 gave (2R,4R)-354 (Scheme 93).137a
Scheme 93.
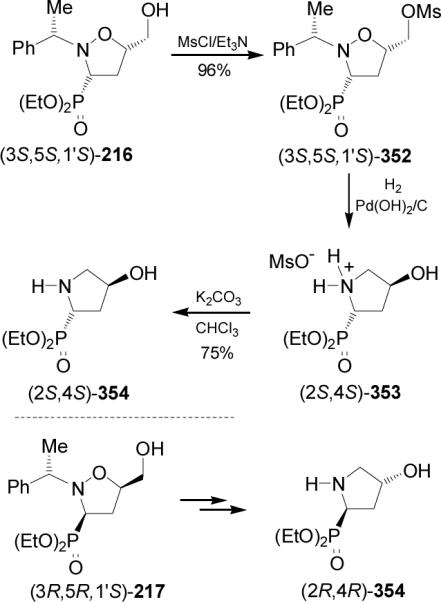
On the other hand, addition of dimethyl or diethyl phosphite to the nitrone 355 at 40 °C gave the corresponding N-hydroxy phosphonates 356a-b in quantitative yield. O,NBis-deprotection in 356a-b by hydrogenolysis over Pd/C in EtOH and aqueous 1 N HCl afforded the pyrrolidinephosphonates 357a-b as hydrochlorides in 43 and 61% yield, respectively (Scheme 94).193
Scheme 94.
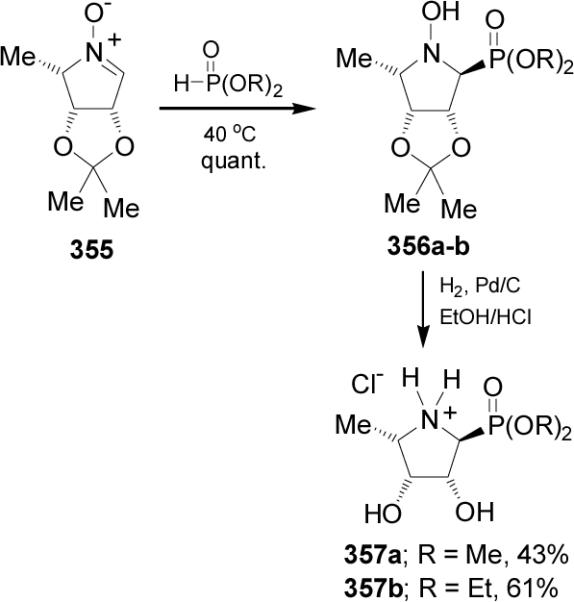
2.7.4. Piperidin-2-ylphosphonic acids and derivatives
Davis et al.184 described the stereoselective synthesis of piperidin-2-yl-phosphonates 361a-b from chiral sulfinyl imines 358a-b. In this context, reaction of the imines 358ab with lithium salt of diethyl phosphite afforded the α-aminophosphonates 359a-b in good yield and excellent diastereoselectivity. Cleavage of sulfinyl group and acidic hydrolysis of ketal in 359a-b gave the amino-carbonyl derivative, which by cyclization afforded the iminophosphonates 360a-b. Finally, catalytic hydrogenation of 360a-b led to cyclic α-aminophosphonates (2R,7S)-361a and (2R,7R)-361b (Scheme 95).194
Scheme 95.
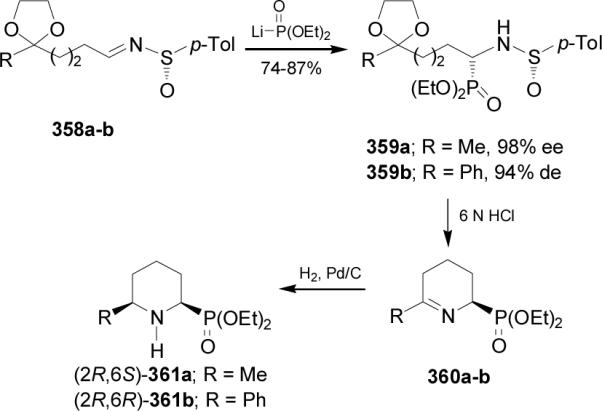
In a similar way, the cyclic α-aminophosphonate (2R,7S)-363 was obtained in moderated yield and diastereoselectivity from chiral sulfinyl imine 362 (Scheme 96). 194
Scheme 96.
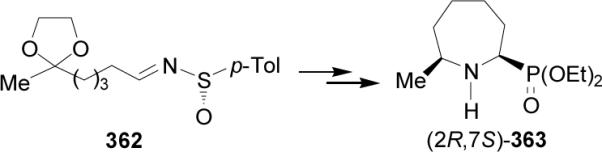
3. Conlusion
In spite of the recognised relevance of the α-aminophosphonic acids it is obvious that there is a very important gap between the possibilities that offer these compounds in relationship with the corresponding counterparts, the α-amino acids. Nevertheless, during the last years these α-aminophosphonic acids are gaining importance step by step so, in this way, numerous papers have been published on their stereoselective synthesis. Authors of this report have covered all advances related to the synthesis of these important compounds and, although there is a long way to cover many of these procedures are already competitive in such a way that many of them can be applied to the synthesis on these compounds in enantiomerically pure form and in a multigram scale, being persuaded that all these efforts will contribute to the future advance in the interest of these important compounds.
4. Acknowledgments
This work was carried out with the financial support of CONACYT-MEXICO (Projects 62271) and financial support from the Ministerio de Educación y Ciencia-FEDER (project CTQ2007-62245) and Gobierno de Aragón (group E40 and project MI041/2007) is gratefully acknowledged. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract number N01-CO-12400. The content of this publication does not necessarily reflect the view of the policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank to Ana Isabel Jiméenez and Victoria Labastida for their valuable help.
5. References
- 1.(a) Horiguchi M, Kandatsu M. Nature. 1959;184:901–902. doi: 10.1038/184901b0. [DOI] [PubMed] [Google Scholar]; (b) Bayer E, Gugel KH, Hägele K, Hagenmaier H, Jessipow S, Konig WA, Zähner H. Helv. Chim. Acta. 1972;55:224–239. doi: 10.1002/hlca.19720550126. [DOI] [PubMed] [Google Scholar]; (c) Kamiya T, Hemmi K, Takeno H, Hashimoto M. Tetrahedron Lett. 1980;21:95–98. [Google Scholar]
- 2.(a) Vassiliou S, Xeilari M, Yiotakis A, Germbecka J, Pawełczak M, Kafarski P, Mucha A. Bioorg. Med. Chem. Lett. 2007;15:3187–3200. doi: 10.1016/j.bmc.2007.02.042. [DOI] [PubMed] [Google Scholar]; (b) El Kaïm L, Grimaud L, Hadrot S. Tetrahedron Lett. 2006;47:3945–3947. [Google Scholar]; (c) Cristau H-J, Coulombeau A, Genevois-Borella A, Pirat J-L. Tetrahedron Lett. 2001;42:4491–4494. [Google Scholar]; (d) Kukhar VP, Hudson HR, editors. Aminophosphonic and Aminophosphinic Acids. John Wiley & Sons; 2000. [Google Scholar]; (e) De Lombaert S, Blanchard L, Stamfort LB, Tan J, Wallace EM, Satoh Y, Fitt J, Hoyer D, Simonsbergen D, Moliterni J, Marcopoulos N, Savage P, Chou M, Trapani AJ, Jeng AY. J. Med. Chem. 2000;43:488–504. doi: 10.1021/jm990507o. [DOI] [PubMed] [Google Scholar]; (f) De Lombaert S, Stamfort LB, Blanchard L, Tan J, Hoyer D, Diefenbacher CG, Wei D, Wallace EM, Moskal MA, Savage P, Jeng AY. Bioorg. Med. Chem. Lett. 1997;7:1059–1064. [Google Scholar]; (g) Lloyd J, Schmidt JB, Hunt JT, Barrish JC, Little DK, Tymiak AA. Bioorg. Med. Chem. Lett. 1996;6:1323–1326. [Google Scholar]; (h) Kafarsky P, Lejczak B. Phosphorous, Sulfur, Silicon Relat. Elem. 1991;63:193–215. For comprehensive review, see: [Google Scholar]; (i) McLeod DA, Brinkworth RI, Ashley JA, Janda KD, Wirsching P. Bioorg. Med. Chem. Lett. 1991;1:653–658. [Google Scholar]
- 3.(a) Hirschmann R, Smith AB, III, Taylor CM, Benkovic PA, Taylor SD, Yager KM, Sprengler PA, Benkovic SJ. Science. 1994;265:234–237. doi: 10.1126/science.8023141. [DOI] [PubMed] [Google Scholar]; (b) Allen MC, Fuhrer W, Tuck B, Wade R, Wood JM. J. Med. Chem. 1989;32:1652–1661. doi: 10.1021/jm00127a041. [DOI] [PubMed] [Google Scholar]; (c) Logusch EW, Walker DM, McDonald JF, Leo GC, Franz JE. J. Org. Chem. 1988;53:4069–4074. [Google Scholar]; (d) Giannousis PP, Bartlett PA. J. Med. Chem. 1987;30:1603–1609. doi: 10.1021/jm00392a014. [DOI] [PubMed] [Google Scholar]
- 4.(a) Emgenbroich M, Wulff G. Chem. Eur. J. 2003;9:4106–4117. doi: 10.1002/chem.200304783. [DOI] [PubMed] [Google Scholar]; (b) Sikorski JA, Miller MJ, Braccolino DS, Cleary DG, Corey SD, Font JL, Gruys KJ, Han CY, Lin K-C, Pajengraa PD, Ream JE, Schnur D, Shah A, Walker MC. Phosphorous, Sulfur, Silicon Relat. Elem. 1993;76:375–378. [Google Scholar]
- 5.(a) Senten K, Daniëls L, Var der Veken P, De Meester I, Lambeir A-M, Scharpé S, Haemers A, Augustyns K. J. Comb. Chem. 2003;5:336–344. doi: 10.1021/cc020096o. [DOI] [PubMed] [Google Scholar]; (b) Stowasser B, Budt K-H, Jian-Qi L, Peyman A, Ruppert D. Tetrahedron Lett. 1992;33:6625–6628. [Google Scholar]
- 6.Patel DV, Rielly-Gauvin K, Ryono DE. Tetrahedron Lett. 1990;31:5587–5590. [Google Scholar]
- 7.Beers SA, Schwender CF, Loughney DA, Malloy E, Demarest K, Jordan J. Bioorg. Med. Chem. 1996;4:1693–1701. doi: 10.1016/0968-0896(96)00186-1. [DOI] [PubMed] [Google Scholar]
- 8.(a) Burke TR, Jr., Barchi JJ, Jr., George C, Wolf G, Shoelson SE, Yan X. J. Med. Chem. 1995;38:1386–1396. doi: 10.1021/jm00008a017. [DOI] [PubMed] [Google Scholar]; (b) Bruke TR, Jr., Kole HK, Roller PP. Biochem. Biophys. Res. Commun. 1994;204:129–134. doi: 10.1006/bbrc.1994.2435. [DOI] [PubMed] [Google Scholar]; (c) Atherton FR, Hassall CH, Lambert RW. J. Med. Chem. 1986;29:29–40. doi: 10.1021/jm00151a005. [DOI] [PubMed] [Google Scholar]
- 9.(a) Atherton FR, Hassall CH, Lambert RW. J. Med. Chem. 1989;29:29–40. doi: 10.1021/jm00151a005. [DOI] [PubMed] [Google Scholar]; (b) Kametani T, Kigasawa K, Hiiragi M, Wakisaka K, Haga S, Sugi H, Tanigawa K, Suzuki Y, Fukawa K, Irino O, Saita O, Yamabe S. Heterocycles. 1981;16:1205–1242. [Google Scholar]; (c) Baylis EK, Campbell CD, Dingwall JG. J. Chem. Soc., Perkin Trans. 1. 1984:2845–2853. [Google Scholar]; (d) Lejczak B, Kafarski P, Sztajer H, Mastalerz P. J. Med. Chem. 1986;29:2212–2217. doi: 10.1021/jm00161a014. [DOI] [PubMed] [Google Scholar]; (e) Okada Y, Iguchi S, Mimura M, Yagyu M. Chem. Pharm. Bull. 1980;28:1320–1323. [Google Scholar]
- 10.(a) Grembecka J, Mucha A, Cierpicki T, Kafarski P. J. Med. Chem. 2003;46:2641–2655. doi: 10.1021/jm030795v. [DOI] [PubMed] [Google Scholar]; (b) Moore JD, Sprott KT, Hanson PR. J. Org. Chem. 2002;67:8123–8129. doi: 10.1021/jo0262208. [DOI] [PubMed] [Google Scholar]; (c) Liu W, Rogers CJ, Fisher AJ, Toney MD. Biochemistry. 2002;41:12320–12328. doi: 10.1021/bi026318g. [DOI] [PubMed] [Google Scholar]; (d) Osipov SN, Artyushin OI, Kolomiets AF, Bruneau C, Dixneuf PH. Synlett. 2000:1031–1033. [Google Scholar]; (e) Ranu BC, Hajra A, Jana U. Org. Lett. 1999;1:1141–1143. [Google Scholar]; (f) Kim KS, Hurh EY, Youn JN, Park JI. J. Org. Chem. 1999;64:9272–9274. [Google Scholar]; (g) Bird J, De Melo RC, Harper GP, Hunter DJ, Karran EH, Markwell RE, Miles-Williams AJ, Rahman SS, Ward RW. J. Med. Chem. 1994;37:158–169. doi: 10.1021/jm00027a020. [DOI] [PubMed] [Google Scholar]; (h) Pratt RF. Science. 1989;246:917–919. doi: 10.1126/science.2814513. [DOI] [PubMed] [Google Scholar]; (i) Atherton FR, Hall MJ, Hassall CH, Lambert RW, Ringrose PS. Antimicrob. Agents Chemother. 1979;15:677–683. doi: 10.1128/aac.15.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Allen JG, Atherton FR, Hall MJ, Hassall CH, Holmes SW, Lambert RW, Nisbet LJ, Ringrose PS. Antimicrob. Agents Chemother. 1979;15:684–695. doi: 10.1128/aac.15.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Atherton FR, Hall MJ, Hassall CH, Lambert RW, Lloyd WJ, Ringrose PS. Antimicrob. Agents Chemother. 1979;15:696–705. doi: 10.1128/aac.15.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Allen JG, Atherton FR, Hall MJ, Hassall CH, Holmes SW, Lambert RW, Nisbet LJ, Ringrose PS. Nature. 1978;272:56–58. doi: 10.1038/272056a0. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Chen R. Heteroat. Chem. 2000;11:480–492. [Google Scholar]
- 12.(a) Maier L, Diel PJ. Phosphorous, Sulfur, Silicon Relat. Elem. 1994;90:259–279. [Google Scholar]; (b) Maier L, Diel PJ. Phosphorous, Sulfur, Silicon Relat. Elem. 1991;57:57–64. [Google Scholar]
- 13.(a) Yager KM, Taylor CM, Smith AB., III J. Am. Chem. Soc. 1994;116:9377–9378. [Google Scholar]; (b) Natchev IA. Liebigs Ann. Chem. 1988:861–867. [Google Scholar]
- 14.Lavielle G, Hautefaye P, Schaeffer C, Boutin JA, Cudennec CA, Pierré A. J. Med. Chem. 1991;34:1998–2003. doi: 10.1021/jm00111a012. [DOI] [PubMed] [Google Scholar]
- 15.Smith AB, III, Taylor CM, Bencovic SJ, Hirschmann R. Tetrahedron Lett. 1994;35:6853–6856. [Google Scholar]
- 16.(a) Cowell SM, Lee YS, Cain JP, Hruby VJ. Curr. Med. Chem. 2004;11:2785–2798. doi: 10.2174/0929867043364270. [DOI] [PubMed] [Google Scholar]; (b) Toniolo C, Crisma M, Formaggio F, Peggion C. Biopolymers (Pept. Sci.) 2001;60:396–419. doi: 10.1002/1097-0282(2001)60:6<396::AID-BIP10184>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]; (c) Hruby VJ, Li G, Haskell-Luevano C, Shenderovich M. Biopolymers (Pept. Sci.) 1997;43:219–266. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]; (d) Gante J. Angew. Chem. Int. Ed. Engl. 1994;33:1699–1720. [Google Scholar]; (e) Giannis A, Kolter T. Angew. Chem. Int. Ed. Engl. 1993;32:1244–1267. [Google Scholar]
- 17.Jagodic V, Herak MJ. J. Inorg. Nucl. Chem. 1970;32:1323–1332. [Google Scholar]
- 18.Bligh SWA, Choi N, Green D. St. C., Hudson HR, McGrath CM, McPartlin M, Pianka M. Polyhedron. 1993;12:2887–2890. references therein. [Google Scholar]
- 19.(a) Bulman RA. In: Trace Metals and Fluoride in Bones and Teeth. Priest NO, van de Vyver FL, editors. CRC Press; Boca Raton, FL: 1990. p. 271. [Google Scholar]; (b) Engel R. Chem. Rev. 1977;77:349–367. [Google Scholar]
- 20.(a) Drag M, Pawelczak M, Kafarski P. Chirality. 2003;15:S104–S107. doi: 10.1002/chir.10261. [DOI] [PubMed] [Google Scholar]; (b) Baylis EK, Campbell CD, Dingwall JG. J. Chem. Soc., Perkin Trans 1. 1984:2845–2853. [Google Scholar]; (c) Atherton FR, Hall MJ, Hassall CH, Lambert RW, Lloyd WJ, Ringrose PS. Antimicrob. Agents. Chemother. 1979;15:696–705. doi: 10.1128/aac.15.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Stamper C, Bennett B, Edwards T, Holz RC, Ringe D, Petsko G. Biochemistry. 2001;40:7035–7046. doi: 10.1021/bi0100891. [DOI] [PubMed] [Google Scholar]; (b) Kafarski P, Lejczak B. Can. J. Chem. 1983;61:2425–2430. [Google Scholar]
- 22.(a) Solodenko VA, Kukhar VP. Tetrahedron Lett. 1989;32:66–75. [Google Scholar]; (b) Polyak MS. Antibiot. Med. Biotekhnol. 1987;32:66–75. [PubMed] [Google Scholar]
- 23.(a) Uziel J, Genêt JP. Russ. J. Org. Chem. 1997;33:1521–1542. [Google Scholar]; (b) Kukhar VP, Soloshonok VA, Solodenko VA. Phosphorous, Sulfur, Silicon Relat. Elem. 1994;92:239–264. [Google Scholar]; (c)Ref. 2a
- 24.(a) Pudovik AN. Dokl. Akad. Nauk SSSR. 1952;83:865–869. [Google Scholar]; (b) Romanenko VD, Kukhar VP. Chem. Rev. 2006;106:3868–3935. doi: 10.1021/cr051000q. [DOI] [PubMed] [Google Scholar]
- 25.(a) Gilmore WF, McBride HA. J. Am. Chem. Soc. 1972;94:4361–4361. doi: 10.1021/ja00767a065. [DOI] [PubMed] [Google Scholar]; (b) Failla S, Finocchiaro P, Hägele G, Kalchenko VI. Phosphorous, Sulfur, Silicon Relat. Elem. 1997;128:63–77. For the addition of sodium salt of diethyl phosphite to imines, see: [Google Scholar]
- 26.The diastereosiomeric ratio of this reaction was determinated by Glowiak T, Sawka-Dobrowolska W, Kowalik J, Mastalerz P, Soroka M, Zon J. Tetrahedron Lett. 1977;18:3965–3968.
- 27.Smith AB, III, Taylor CM, Benkovic SJ, Hirschmann R. Tetrahedron Lett. 1994;35:6853–6856. [Google Scholar]
- 28.Cherenok S, Vovk A, Muravyova I, Shivanyuk A, Kukhar V, Lipkowski J, Kalchenko V. Org. Lett. 2006;8:549–552. doi: 10.1021/ol052469a. [DOI] [PubMed] [Google Scholar]
- 29.Tongcharoensirikul P, Suarez AI, Voelker T, Thompson CM. J. Org. Chem. 2004;69:2322–2326. doi: 10.1021/jo035707t. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Wang G, Li Y, Yuan CY. Acta Chim. Sin. 1993;51:1195–1202. [Google Scholar]
- 31.(a) Hoffmann RW. Chem. Rev. 1989;89:1841–1860. [Google Scholar]; (b) Broeker JL, Hoffmann RW, Houk KN. J. Am. Chem. Soc. 1991;113:5006–5017. [Google Scholar]; (c) Kraus GA, Jeon I. Org. Lett. 2006;8:5315–5316. doi: 10.1021/ol0621194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabó A, Jászay ZM, Hegedűs L, Tőke L, Petneházy I. Tetrahedron Lett. 2003;44:4603–4606. [Google Scholar]
- 33.For the application of phosphinic acids in biochemistry, biology and medicine, see: Collinsová M, Jirácek J. Curr. Med. Chem. 2000;7:629–647. doi: 10.2174/0929867003374831.
- 34.Smith AB, III, Yager KM, Taylor CM. J. Am. Chem. Soc. 1995;117:10879–10888. [Google Scholar]; (b) Ref. 12a
- 35.Alonso E, Alonso E, Solís A, Del Pozo C. Synlett. 2000:698–700. [Google Scholar]
- 36.Smith AB, III, Yager KM, Phillips BW, Taylor CM. Org. Synth. 1998;75:19–29. [Google Scholar]
- 37.Neither sodium nor potassium salt of diethyl phosphite prepared by treatment of diethyl phosphite with NHMDS or KHMDS in THF gave any detectable α-aminophosphonates (R,R)-25 and (S,R)-26 even after several days at room temperature
- 38.Dimukhametov MN, Bajandina EV, Davydova EY, Litvinov IA, Gubaidullin AT, Dobrynin AB, Zyablikova TA, Alfonsov VA. Heteroat. Chem. 2003;14:56–61. [Google Scholar]
- 39.Bhattacharya AK, Thyagarajan G. Chem. Rev. 1981;81:415–430. [Google Scholar]
- 40.Shang Z-Q, Chen R-Y, Huang Y. Heteroat. Chem. 2007;18:230–235. [Google Scholar]
- 41.Davis FA, Reddy RE, Szewczyk JM, Portonovo PS. Tetrahedron Lett. 1993;34:6229–6232. [Google Scholar]
- 42.(a) Ordoñez M, Guerrero-de la Rosa V, Labastida V, Llera JM. Tetrahedron: Asymmetry. 1996;7:2675–2686. [Google Scholar]; (b) Han Z, Krishnamurthy D, Pflum D, Grover P, Wald SA, Sennayake CH. Org. Lett. 2002;4:4025–4028. doi: 10.1021/ol026699q. [DOI] [PubMed] [Google Scholar]; (c) Fernández I, Khiar N. Chem. Rev. 2003;103:3651–3705. doi: 10.1021/cr990372u. [DOI] [PubMed] [Google Scholar]; (d) Mikolajczyk M, Drabowicz J, Kielbasinski P, editors. Chiral Sulfur Reagents: Applications in Asymmetric and Stereoseletive Synthesis. CRC Prees; New York: 1997. [Google Scholar]; (e) Page P, editor. Organosulfur Chemistry: Synthetic and Stereochemical Aspects. Vol. 2. Academic Press; London: 1998. [Google Scholar]; (f) Gnas Y, Glorius F. Synthesis. 2006. pp. 1899–1930. [Google Scholar]
- 43.(a) Lefebvre IM, Evans SA., Jr. J. Org. Chem. 1997;62:7532–7533. [Google Scholar]; (b) Lefebvre IM, Evans SA., Jr. Phosphorous, Sulfur, Silicon Relat. Elem. 1999:144–146. 397–400. [Google Scholar]
- 44.For the synthesis of chiral sulfinimines, see: Davis FA, Reddy RE, Szewczyk JM, Reddy GV, Portonovo PS, Zhang H, Fanelli D, Reddy RT, Zhou P, Carroll PJ. J. Org. Chem. 1997;62:2555–2563. doi: 10.1021/jo970077e. references therein.
- 45.Davis FA, Lee S, Yan H, Titus DD. Org. Lett. 2001;3:1757–1760. doi: 10.1021/ol015945f. [DOI] [PubMed] [Google Scholar]
- 46.(a) Mikolajczyk M, Lyzwa P, Drabowicz J. Tetrahedron: Asymmetry. 1997;8:3991–3994. [Google Scholar]; (b) Mikolajczyk M. J. Organomet. Chem. 2005;690:2488–2496. [Google Scholar]
- 47.Mikolajczyk M, Lyzwa P, Drabowicz J. Tetrahedron: Asymmetry. 2002;13:2571–2576. [Google Scholar]
- 48.(a) Biasone A, Tortorella P, Campestre C, Agamennone M, Preziuso S, Chiappini M, Nuti E, Carelli P, Rossello A, Mazza F, Gallina C. Bioorg. Med. Chem. 2007;15:791–799. doi: 10.1016/j.bmc.2006.10.047. [DOI] [PubMed] [Google Scholar]; (b) Pochetti G, Gavuzzo E, Campestre C, Agamennone M, Tortorella P, Consalvi V, Gallina C, Hiller O, Tschesche H, Mazza F. J. Med. Chem. 2006;49:923–931. doi: 10.1021/jm050787+. [DOI] [PubMed] [Google Scholar]
- 49.Capozzi MA, Cardellicchio C, Naso F, Spina G, Tortorella P. J. Org. Chem. 2001;66:5933–5936. doi: 10.1021/jo010334m. [DOI] [PubMed] [Google Scholar]
- 50.Chen Q, Yuan C. Synthesis. 2007:3779–3786. [Google Scholar]
- 51.Ellman JA, Owens TD, Tang TP. Acc. Chem. Res. 2002;35:984–995. doi: 10.1021/ar020066u. [DOI] [PubMed] [Google Scholar]
- 52.Addition of dimethyl phosphite to N-tert-butylsulfinyl aldimines (S)-45a-g in the presence the other bases as: LHMDS, CaH2, Et3N, KF, CsF, Li2CO3, and Na2CO3 gave the phosphonates (SS,RC)-46a-g with low diastereoselectivity
- 53.For the synthesis of αα-disubstituted amino acids, see: Cativiela C, Díaz-de-Villegas MD. Tetrahedron: Asymmetry. 2007;18:569–623. Cativiela C, Díaz-de-Villegas MD. Tetrahedron: Asymmetry. 1998;9:3517–3599.. Cativiela C, Díaz-de-Villegas MD. Tetrahedron: Asymmetry. 2000;11:645–732.. Alias M, Cativiela C, Díaz-de-Villegas MD, Gálvez JA, Lapena Y. Tetrahedron. 1998;54:14963–14974.. Avenoza A, Cativiela C, Corzana F, Peregrina JM, Zurbano MM. J. Org. Chem. 1999;64:8220–8225. doi: 10.1021/jo990957o.
- 54.See, for example: Colson P-J, Hegedus LS. J. Org. Chem. 1993;58:5918–5924.. Shao H, Zhu Q, Goodman M. J. Org. Chem. 1995;60:790–791.. Roos EL, López MC, Brook MA, Hiemstra H, Speckamp WN. J. Org. Chem. 1993;58:3259–3268.. Chinchilla R, Galindo N, Nájera C. Synthesis. 1999:704–717.. Belokon YN, Davies RG, North M. Tetrahedron Lett. 2000;41:7245–7248.. Nájera C, Abellán T, Sansano JM. Eur. J. Org. Chem. 2000;15:2809–2820.. Belokon YN, Fuentes J, North M, Steed JW. Tetrahedron. 2004;60:3191–3204..
- 55.(a) Davis FA, Lee S, Zhang H, Fanelli DL. J. Org. Chem. 2000;65:8704–8708. doi: 10.1021/jo001179z. [DOI] [PubMed] [Google Scholar]; (b) Davis FA, Zhang Y, Andemichael Y, Fang T, Fanelli DL, Zhang H. J. Org. Chem. 1999;64:1403–1406. [Google Scholar]; (c) Fanelli DL, Szewczyk JM, Zhang Y, Reddy GV, Burns DM, Davis FA. Org. Synth. 1999;77:50–56. [Google Scholar]
- 56.Addition of bis(N,N-diethylamino)phosphite to 48b afforded the α-aminophosphonate with opposite configuration; however, this compound was unstable under hydrolysis conditions, and decomposition occurred.
- 57.Reaction of (S)-N-tert-butylsulfinyl or (S)-2,4,6-trimethylphenylsulfynil imines with the anion of 52 gave directly the corresponding aziridines in excellent diastereoselectivity
- 58.(a) Davis FA, McCoull W. Tetrahedron Lett. 1999;40:249–252. [Google Scholar]; (b) Kim DY, Suh KH, Choi S, Mang JY, Chang SK. Synth. Commun. 2000;30:87–95. [Google Scholar]; (c) Davis FA, Zhang Y, Rao A, Zhang Z. Tetrahedron. 2001;57:6345–6352. [Google Scholar]; (d) Davis FA, Wu Y, Yan H, McCoull W, Prasad KR. J. Org. Chem. 2003;68:2410–2419. doi: 10.1021/jo020707z. [DOI] [PubMed] [Google Scholar]; (e) Davis FA, Ramachandar T, Wu Y. J. Org. Chem. 2003;68:6894–6898. doi: 10.1021/jo030142m. [DOI] [PubMed] [Google Scholar]
- 59.For the synthesis of enantiomerically pure α-aminophosphonates from aziridines, see: Hanessian S, Bennani YL, Herve Y. Synlett. 1993:35–36.
- 60.Davies FA, McCoull W, Titus DD. Org. Lett. 1999;1:1053–1055. doi: 10.1021/ol990855k. [DOI] [PubMed] [Google Scholar]
- 61.Kabachnik MI, Medved TY. Dokl. Akad. Nauk SSSR. 1952:689–691. [Google Scholar]
- 62.For the recent synthesis of non-chiral α-aminophosphonates via one-pot three-component reaction, see: Bhagat S, Chakraborti AK. J. Org. Chem. 2007;72:1263–1270. doi: 10.1021/jo062140i.. Kaboudin B, Jafari E. Synthesis. 2007:1823–1826.. Bhattacharya AK, Kaur T. Synthesis. 2007:745–748.. Prasad GS, Krishna JR, Manjunath M, Reddy OVS, Krishnaiah M, Reddy CS, Puranik VG. Arkivoc. 2007;(xiii):133–141.. Kaboudin B, Moradi K, Sardarian AR. Arkivoc. 2007;(xiii):210–217.. Kasthuraiah M, Kumar KA, Reddy CS, Reddy CD. Heteroat. Chem. 2007;18:2–8.. Hua F, Meijuan F, Xiaoxia L, Guo T, Yufen Z. Heteroat. Chem. 2007;18:9–15.. Wu J, Sun W, Xia H-G, Sun X. Org. Biomol. Chem. 2006;4:1663–1666. doi: 10.1039/b602536f.. Mu XJ, Lei M-Y, Zou J-P, Zhang W. Tetrahedron Lett. 2006;47:1125–1127.. Kudrimoti S, Bommena VR. Tetrahedron Lett. 2005;46:1209–1210.. Zahouily M, Elmakssoudi A, Mezdar A, Rayadh A, Sebti S. J. Chem. Res. 2005:324–327.. Reddy KR, Reddy KS, Reddy CV, Mahesh M, Raju PVK, Reddy VVN. Chem. Lett. 2005;34:444–445.. Akiyama T, Matsuda K, Fuchibe K. Synthesis. 2005:2606–2608.. Kabachnik MM, Zobnina EV, Beletskaya IP. Synlett. 2005:1393–1396.. Van der Veken P, El Sayed I, Joossens J, Stevens CV, Augustyns K, Haemers A. Synthesis. 2005:634–638.. Zhan Z-P, Yang R-F, Li J-P. Chem. Lett. 2005;34:1042–1043.. Kaboudin B, Moradi K. Tetrahedron Lett. 2005;46:2989–2991.. Akiyama T, Matsuda K, Fuchibe K. Synthesis. 2005:2606–2608..
- 63.Heydari A, Karimian A, Ipaktschi J. Tetrahedron Lett. 1998;39:6729–6732. [Google Scholar]
- 64.Saidi MR, Azizi N. Synlett. 2002:1347–1349. [Google Scholar]
- 65.Laschat S, Kunz H. Synthesis. 1992:90–95. [Google Scholar]
- 66.Zon J. Pol. J. Chem. 1981;55:643–646. [Google Scholar]
- 67.Qian C, Huang T. J. Org. Chem. 1998;63:4125–4128. [Google Scholar]
- 68.Ranu BC, Hajra A, Jana U. Org. Lett. 1999;1:1141–1143. [Google Scholar]
- 69.(a) Fadel A, Canet J-L, Salaün J. Synlett. 1990:89–91. [Google Scholar]; (b) Fadel A, Khesrani A. Tetrahedron: Asymmetry. 1998;9:305–320. [Google Scholar]
- 70.(a) Fadel A, Tesson N. Eur. J. Org. Chem. 2000:2153–2159. [Google Scholar]; (b) Tesson N, Dorigneux B, Fadel A. Tetrahedron: Asymmetry. 2002;13:2267–2276. For the reaction of (±)-62, see: [Google Scholar]
- 71.Fadel A, Tesson N. Tetrahedron: Asymmetry. 2000;11:2023–2031. [Google Scholar]
- 72.Louaisil N, Rabasso N, Fadel A. Synthesis. 2007:289–293. [Google Scholar]
- 73.For the synthesis of quaternary acyclic α,β-diaminophosphonic acids, see: Studer A, Seebach D. Heterocycles. 1995;40:357–378..
- 74.(a) Anh NT, Eisenstein O. Nouv. J. Chim. 1977;1:61–70. [Google Scholar]; (b) Wu Y-D, Houk KN. J. Am. Chem. Soc. 1987;109:908–910. [Google Scholar]; (c) Broeker JL, Hoffmann RW, Houk KN. J. Am. Chem. Soc. 1991;113:5006–5017. [Google Scholar]; (d) Chakraborty TK, Azhar HK, Venkat RG. Tetrahedron. 1995;51:9179–9190. [Google Scholar]
- 75.Satish-Kumar A, Taneja SC, Hundal MS, Kapoor KK. Tetrahedron Lett. 2008;49:2208–2212. [Google Scholar]
- 76.Rinnová M, Nefzi A, Houghten RA. Tetrahedron Lett. 2002;43:4103–4106. [Google Scholar]
- 77.For the synthesis of α-aminophosphonates using chiral amides, see: Chung S-K, Kang D-H. Tetrahedron: Asymmetry. 1996;7:21–24.. Oshikawa T, Yamashita M. Bull. Chem. Soc. Jpn. 1989;62:3177–3181.. Huber JW, III, Gilmore WF. Tetrahedron Lett. 1979;33:3049–3052..
- 78.Ross GHP, Balasubramaniam S. Synth. Commun. 1998;28:3877–3884. [Google Scholar]
- 79.Hamilton R, Walker B, Walker BJ. Tetrahedron Lett. 1995;36:4451–4454. [Google Scholar]; Also, see: Drag M, Grembecka J, Pawelczak M, Kafarski P. Eur. J. Med. Chem. 2005;40:764–771. doi: 10.1016/j.ejmech.2005.02.011. Drag M, Pawelczak M, Kafarski P. Chirality. 2003;15:S104–S107. doi: 10.1002/chir.10261.. Drag M, Grembecka J, Kafarski P. Phosphorous, Sulfur, Silicon Relat. Elem. 2002;177:1591–1595..
- 80.(a) Bongini A, Camerini R, Panunzio M. Tetrahedron: Asymmetry. 1996;7:1467–1476. [Google Scholar]; (b) Bongini A, Camerini R, Hofman S, Panunzio M. Tetrahedron Lett. 1994;35:8045–8048. [Google Scholar]
- 81.Davis FA, Prasad KR. J. Org. Chem. 2003;68:7249–7253. doi: 10.1021/jo0301649. [DOI] [PubMed] [Google Scholar]
- 82.For the synthesis of α-amino-β-hydroxyphosphonates, see: Sawamura M, Ito Y, Hayashi T. Tetrahedron Lett. 1989;30:2247–2250.. Kitamura M, Tokunaga M, Pham T, Lubell WD, Noyori R. Tetrahedron Lett. 1995;36:5769–5772.. Bongini A, Camerini R, Panunzio M. Tetrahedron: Asymmetry. 1996;7:1467–1476.. Davis FA, McCoull W, Titus DD. Org. Lett. 1999;1:1053–1055. doi: 10.1021/ol990855k.. Renaud P, Seebach D. Angew. Chem. Int. Ed. Engl. 1986;25:843–844.. De Risi C, Perrone D, Dondoni A, Pollini GP, Bertolasi V. Eur. J. Org. Chem. 2003:1904–1914..
- 83.Finch P, Iskander GM, Siriwardena AH. Carbohydr. Res. 1991;210:319–325. [Google Scholar]
- 84.Hanessian S, Rogel O. J. Org. Chem. 2000;65:2667–2674. doi: 10.1021/jo991696l. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, Wiemer AJ, Hohl RJ, Wiemer DF. J. Org. Chem. 2002;67:9331–9339. doi: 10.1021/jo020483k. [DOI] [PubMed] [Google Scholar]
- 86.Zhu XF, Williams HJ, Scott AI. J. Chem. Soc., Perkin Trans. 1. 2000:2305–2306. [Google Scholar]
- 87.Chen X, Wiemer DF. J. Org. Chem. 2003;68:6108–6114. doi: 10.1021/jo030050x. [DOI] [PubMed] [Google Scholar]
- 88.(a) De Risi C, Perrone D, Dondoni A, Pollini GP, Bertolasi V. Eur. J. Org. Chem. 2003:1904–1914. [Google Scholar]; (b) De Risi C, Dondoni A, Perrone D, Pollini GP. Tetrahedron Lett. 2001;42:3033–3036. [Google Scholar]
- 89.Kolodiazhnyi OI, Sheiko S, Grishkun EV. Heteroat. Chem. 2000;11:138–143. [Google Scholar]
- 90.(a) Kachkovskii GA, Andrushko NV, Sheiko SY, Kolodiazhnyi OI. Russ. J. Gen. Chem. 2005;75:1735–1743. [Google Scholar]; (b) Kolodiazhnyi OI. Phosphorous, Sulfur, Silicon Relat. Elem. 2002;177:2111–2114. [Google Scholar]; (c) Sergei S, Irina G, Eugeni G, Kolodiazhnyi OI. Phosphorous, Sulfur, Silicon Relat. Elem. 2002;177:2269–2270. [Google Scholar]; (d) Kolodiazhnyi OI, Sheiko SY. Russ. J. Gen. Chem. 2001;71:1155–1156. [Google Scholar]; (e) Kolodiazhnyi OI, Sheiko SY. Russ. J. Gen. Chem. 2001;71:977–978. [Google Scholar]
- 91.Similar results were obtained in the addition of 127a to chiral imines obtained by condensation from (S)- and (R)-α-MBA with p-Me2NC6H4CHO, p-NO2C6H4CHO, p-FC6H4CHO and p-BrC6H4CHO. Electron-acceptor substituents in aromatic ring reduced the stereoselectivity, whereas electron donors increases it
- 92.(a) Déjugnat C, Etemad-Moghadam G, Rico-Lattes I. Chem. Commun. 2003:1858–1859. doi: 10.1039/b304420c. [DOI] [PubMed] [Google Scholar]; (b) Vercruysse K, Déjugnat C, Munoz A, Etemad-Moghadam G. Eur. J. Org. Chem. 2000:281–289. [Google Scholar]
- 93.(a) Munoz A, Garriges B, Koenig M. Tetrahedron. 1980;36:2467–2482. [Google Scholar]; (b) Koenig M, Munoz A, Garriges B, Wolf R. Phosphorous, Sulfur, Silicon Relat. Elem. 1979;6:435–451. [Google Scholar]
- 94.Schlemminger I, Willecke A, Maison W, Koch R, Lützen A, Martens J. J. Chem. Soc., Perkin Trans. 1. 2001:2804–2816. [Google Scholar]
- 95.For the preliminary asymmetric addition of chiral cyclic phosphites to achiral 3-thiazolines, see: Hoppe I, Schöllkopf U, Nieger M, Egert E. Angew. Chem. Int. Ed. Engl. 1985;24:1067–1068.
- 96.Swamy KCK, Kumaraswamy S, Kumar KS, Muthiah C. Tetrahedron Lett. 2005;46:3347–3351. [Google Scholar]
- 97.Liu H, Cai S, Xu J. J. Pept. Sci. 2006;12:337–340. doi: 10.1002/psc.731. [DOI] [PubMed] [Google Scholar]
- 98.Xu J, Gao Y. Synthesis. 2006:783–788. [Google Scholar]
- 99.(a) Noyori R. Angew. Chem. Int. Ed. 2002;41:2008–2022. [PubMed] [Google Scholar]; (b) Zhu J. Angew. Chem. Int. Ed. 2007;46:8938–8940. references therein. [Google Scholar]; (c) Ojima I. Catalytic Asymmetric Synthesis. 2nd ed. John Wiley & Sons; 2000. [Google Scholar]
- 100.Sasai H, Arai S, Tahara Y, Shibasaki M. J. Org. Chem. 1995;60:6656–6657. [Google Scholar]
- 101.Akiyama T, Morita H, Itoh J, Fuchibe K. Org. Lett. 2005;7:2583–2585. doi: 10.1021/ol050695e. [DOI] [PubMed] [Google Scholar]
- 102.Joly GD, Jacobsen EN. J. Am. Chem. Soc. 2004;126:4102–4103. doi: 10.1021/ja0494398. [DOI] [PubMed] [Google Scholar]
- 103.Saito B, Egami H, Katsuki T. J. Am. Chem. Soc. 2007;129:1978–1986. doi: 10.1021/ja0651005. [DOI] [PubMed] [Google Scholar]
- 104.The addition of diethyl phosphite to imines catalyzed by bisgluco-18-crown-6 or monoaza-15-crown-5 gave the α-aminophosphonates with only low enantioselectivity, see: Jaszay ZM, Nagy E, Bako P, Petnehazy I, Töke L. Supplement to Chimica Oggi. Chemistry Today. 2006;24:24–26.
- 105.For the one-pot synthesis of α-aminophosphonates using β-cyclodextrin (β-CD) in water at reflux, see: Kaboudin B, Sorbiun M. Tetrahedron Lett. 2007;48:9015–9017.
- 106.Pettersen D, Marcolini M, Bernardi L, Fini F, Herrera RP, Sgarzani V, Ricci A. J. Org. Chem. 2006;71:6269–6272. doi: 10.1021/jo060708h. [DOI] [PubMed] [Google Scholar]
- 107.Gröger H, Saida Y, Sasai H, Yamaguchi K, Martens J, Shibasaki M. J. Am. Chem. Soc. 1998;120:3089–3103. [Google Scholar]
- 108.(a) Schlemminger I, Saida Y, Gröger H, Maison W, Durot N, Sasai H, Shibasaki M, Martens J. J. Org. Chem. 2000;65:4818–4825. doi: 10.1021/jo991882r. [DOI] [PubMed] [Google Scholar]; (b) Yamakoshi K, Harwood SJ, Kanai M, Shibasaki M. Tetrahedron Lett. 1999;40:2565–2568. [Google Scholar]
- 109.Chen F-Y, Uang B-J. J. Org. Chem. 2001;66:3650–3652. doi: 10.1021/jo015569c.. Laue KW, Muck-Lichtenfeld C, Haufe G. Tetrahedron. 1999;55:10413–10424.. Meyer L, Poirier J-M, Duhamel P, Duhamel L. J. Org. Chem. 1998;63:8094–8095.. Yeh T-L, Liao C-C, Uang B-J. Tetrahedron. 1997;53:11141–11152.. Tabcheh M, El Achqar A, Pappalardo A, Roumestant M-L, Viallefont P. Tetrahedron. 1991;47:4611–4618.. Oppolzer W, Moretti R, Thomi S. Tetrahedron Lett. 1989;30:6009–6010.. McIntosh JM, Cassidy KC. Can. J. Chem. 1988;66:3116–3119..
- 110.(a) Shöllkopf U, Schütze R. Liebigs Ann. Chem. 1987:45–49. [Google Scholar]; (b) Groth U, Richter L, Shöllkopf U. Liebigs Ann. Chem. 1992:903–909. [Google Scholar]; (c) Groth U, Richter L, Shöllkopf U. Tetrahedron. 1992;48:117–122. [Google Scholar]; (d) Song Y, Niederer D, Lane-Bell PM, Lam LKP, Crawley S, Palcic MM, Pickard MA, Pruess DL, Vederas JC. J. Org. Chem. 1994;59:5784–5793. For the synthesis of phosphonic analogue of diaminopimelic acid (P-DAP) using 167, see: [Google Scholar]
- 111.(a) Ouazzani F, Roumestant M-L, Viallefont P, El Hallaoui A. Tetrahedron: Asymmetry. 1991;2:913–917. [Google Scholar]; (b) Hannour S, Roumestant M-L, Viallefont P, Riche C, Martinez J, El Hallaoui A, Ouazzani F. Tetrahedron: Asymmetry. 1998;9:2329–2332. [Google Scholar]
- 112.(a) Jommi G, Miglierini G, Pagliarin R, Sello G, Sisti M. Tetrahedron: Asymmetry. 1992;3:1131–1134. [Google Scholar]; (b) Ferrari M, Jommi G, Miglierini G, Pagliarin R, Sisti M. Synth. Commun. 1992;22:107–123. [Google Scholar]; (c) Cabella G, Jommi G, Pagliarin R, Sello G, Sisti M. Tetrahedron. 1995;51:1817–1826. [Google Scholar]
- 113.(a) Maury C, Royer J, Husson H-P. Tetrahedron Lett. 1992;33:6127–6130. [Google Scholar]; (b) Maury C, Gharbaoui T, Royer J, Husson HP. J. Org. Chem. 1996;61:3687–3693. doi: 10.1021/jo960020c. Also, see: [DOI] [PubMed] [Google Scholar]
- 114.Hanessian S, Bennani YL. Tetrahedron Lett. 1990;31:6465–6468. [Google Scholar]
- 115.Cheng-Ye Y, Qian-Yi C. Chin. J. Chem. 2005;23:1671–1676. [Google Scholar]
- 116.Diastereoselective alkylation of (2R,5S)-175 followed by the acidic hydrolysis led to (S)-α-aminophosphonic acids 8
- 117.(a) Gololobov YG, Kasukhin LF. Tetrahedron. 1992;48:1353–1406. [Google Scholar]; (b) Tian WQ, Wang YA. J. Org. Chem. 2004;69:4299–4308. doi: 10.1021/jo049702n. references therein. [DOI] [PubMed] [Google Scholar]
- 118.(a) Yuan C, Li S, Wang G. Heteroat. Chem. 2000;11:528–535. [Google Scholar]; (b) Yuan C, Li S, Cui S, Wang G. Phosphorous, Sulfur, Silicon Relat. Elem. 2002;177:1731–1734. [Google Scholar]
- 119.Diastereoselective alkylation of (2R,5R)-174 and subsequent reactions gave the (S)-α-aminophosphonic acids 8
- 120.(a) Haruki T, Yamagishi T, Yokomatsu T. Tetrahedron:Asymmetry. 2007;18:2886–2893. [Google Scholar]; (b) Yamagishi T, Haruki T, Yokomatsu T. Tetrahedron. 2006;62:9210–9217. [Google Scholar]
- 121.Alkylation of (SP)-179 afforded (S,SP)-180 in 74% yield and with 10:1 diastereoisomeric ratio
- 122.For the synthesis of α-amino-α'-hydroxyphosphinates from reaction of (R,RP)-183 with several alkyl and aryl aldehydes, see: Kaboudin B, Haruki T, Yamagishi T, Yokomatsu T. Synthesis. 2007:3226–3232.
- 123.For the practical and efficient synthesis of (E)-α,β-unsaturated amides bearing (S)-α-MBA from phosphonoamides type 185 via Horner-Wadsworth-Emmons reaction, see: Ordóñez M, Hernández-Fernández E, Montiel-Pérez M, Rojas-Cabrera H, Fernández-Zertuche M, García-Barradas O. Tetrahedron: Asymmetry. 2007;18:2427–2436. Hernández-Fernández E, Fernández-Zertuche M, García-Barradas O, Muñoz-Muñiz O, Ordóñez M. Synlett. 2006:440–444..
- 124.Ordóñez M, Hernández-Fernández E, Xahuentitla J, Cativiela C. Chem. Commun. 2005:1336–1338. doi: 10.1039/b416616g. [DOI] [PubMed] [Google Scholar]
- 125.(a) Lebel H, Leogane O. Org. Lett. 2006;8:5717–5720. doi: 10.1021/ol0622920. [DOI] [PubMed] [Google Scholar]; (b) Lebel H, Leogane O. Org. Lett. 2005;7:4107–4110. doi: 10.1021/ol051428b. [DOI] [PubMed] [Google Scholar]; (c) Marinescu L, Thinggaard J, Thomsen IB, Bols M. J. Org. Chem. 2003;68:9453–9455. doi: 10.1021/jo035163v. [DOI] [PubMed] [Google Scholar]; (d) Scriven EF, Turnbull K. Chem. Rev. 1988;88:297–368. [Google Scholar]; (e) Smith PAS. Org. React. 1946;3:337–449. [Google Scholar]
- 126.(a) Sawamura M, Hamashima H, Ito Y. Bull. Chem. Soc.Jpn. 2000;73:2559–2562. [Google Scholar]; (b) Sawamura M, Ito Y, Hayashi T. Tetrahedron Lett. 1989;30:2247–2250. Also, see: [Google Scholar]
- 127.Kuwano R, Nishio R, Ito Y. Org. Lett. 1999;1:837–839. [Google Scholar]
- 128.Jászay ZM, Németh G, Pham TS, Petneházy I, Grün A, Tőke L. Tetrahedron: Asymmetry. 2005;16:3837–3840. [Google Scholar]
- 129.For the catalytic enantioselective synthesis of α-aminophosphonates from 197, see: Baldwin IC, Williams JMJ, Beckett RP. Tetrahedron: Asymmetry. 1995;6:679–682.
- 130.(a) Ma D, Ma Z, Jian J, Yang Z, Zheng C. Tetrahedron: Asymmetry. 1997;8:889–893. [Google Scholar]; (b) Lejczak B, Kafarski P, Mastalerz P. J. Chromatography. 1985;324:455–461. [Google Scholar]; (c) Antczak K, Szewczyk J. Phosphorous, Sulfur, Silicon Relat. Elem. 1985;22:247–51. [Google Scholar]
- 131.Kobayashi S, Kiyohara H, Nakamura Y, Matsubara R. J. Am. Chem. Soc. 2004;126:6558–6559. doi: 10.1021/ja048791i. [DOI] [PubMed] [Google Scholar]
- 132.Kiyohara H, Matsubara R, Kobayashi S. Org. Lett. 2006;8:5333–5335. doi: 10.1021/ol062144+. [DOI] [PubMed] [Google Scholar]
- 133.For the synthesis of non-chiral α-aminophosphonates via addition of enamines to α-iminophosphonates, see: Risch N, Piper S, Winter A, Lefarth-Risse A. Eur. J. Org. Chem. 2005:387–394.
- 134.Kiyohara H, Nakamura Y, Matsubara R, Kobayashi S. Angew. Chem. Int. Ed. 2006;45:1615–1617. doi: 10.1002/anie.200504196. [DOI] [PubMed] [Google Scholar]
- 135.(a) Dodda R, Zhao C-G. Tetrahedron Lett. 2007;48:4339–4342. doi: 10.1016/j.tetlet.2007.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dodda R, Zhao C-G. Org. Lett. 2007;9:165–167. doi: 10.1021/ol0627810. For the synthesis of non-chiral α-iminopropargylphosphonates, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Piotrowska DG, Głowacka IE. Tetrahedron: Asymmetry. 2007;18:2787–2790. [Google Scholar]
- 137.(a) Piotrowska DG, Głowacka IE. Tetrahedron:Asymmetry. 2007;18:1351–1363. [Google Scholar]; (b) Vasella A, Voeffray R. Helv. Chim. Acta. 1982;65:1953–1964. For the preparation of isoxazolidines from N-glycosyl-C-dialkoxyphosphorylated nitrones, see: [Google Scholar]
- 138.For the cycloaddition reactions of nonchiral phosphonitrones, see: Piotrowska DG. Tetrahedron Lett. 2006;47:5363–5366. Piotrowska DG. Tetrahedron. 2006;62:12306–12317..
- 139.Denmark SE, Chatani N, Pansare SV. Tetrahedron. 1992;48:2191–2208. [Google Scholar]
- 140.Jommi G, Miglierini G, Pagliarin R, Sello G, Sisti M. Tetrahedron. 1992;48:7275–7288. [Google Scholar]
- 141.Pagliarin R, Papeo G, Sello G, Sisti M. Tetrahedron. 1996;52:13783–13794. [Google Scholar]
- 142.(a) Hanessian S, Bennani YL. Synthesis. 1994:1272–1274. [Google Scholar]; (b) Maffre D, Dumy P, Vidal J-P, Escale R, Girard J-P. J. Chem. Res. (S) 1994:30–31. [Google Scholar]
- 143.Bernardi L, Zhuang W, Jørgensen KA. J. Am. Chem Soc. 2005;127:5772–5773. doi: 10.1021/ja050989v. [DOI] [PubMed] [Google Scholar]
- 144.Fernández MC, Díaz A, Guillín JJ, Blanco O, Ruiz M, Ojea V. J. Org. Chem. 2006;71:6958–6974. doi: 10.1021/jo061072x. [DOI] [PubMed] [Google Scholar]
- 145.For the stereoselective azidation of chiral α-alkyl phosphonoamides, see: ref. 142a
- 146.(a) Jiao X, Chen W, Hu B. Synth. Commun. 1992;22:1179–1186. [Google Scholar]; (b) Fernández MC, Quintela JM, Ruiz M, Ojea V. Tetrahedron: Asymmetry. 2002;13:233–237. [Google Scholar]; (c) Reyes-Rangel G, Marañon V, Avila-Ortiz CG, Anaya de Parrodi C, Quintero L, Juaristi E. Tetrahedron. 2006;62:8404–8409. [Google Scholar]; (d) Fernández MC, Díaz A, Guillín JJ, Blanco O, Ruiz M, Ojea V. J. Org. Chem. 2006;71:6958–6974. doi: 10.1021/jo061072x. references therein. [DOI] [PubMed] [Google Scholar]; (e) Sibille P, López S, Brabet I, Valenti O, Oueslati N, Gaven F, Goudet C, Bertrand H-O, Neyton J, Marino MJ, Amalric M, Pin J-P, Acher FC. J. Med. Chem. 2007;50:3585–3595. doi: 10.1021/jm070262c. [DOI] [PubMed] [Google Scholar]
- 147.(a) Palacios F, Aparicio D, López Y, de los Santos JM. Tetrahedron. 2005;61:2815–2830. [Google Scholar]; (b) Palacios F, Aparicio D, López Y, de los Santos JM. Tetrahedron Lett. 2004;45:4345–4348. [Google Scholar]
- 148.de los Santos JM, Ignacio R, Aparicio D, Palacios F. J. Org. Chem. 2007;72:5202–5206. doi: 10.1021/jo0705521. [DOI] [PubMed] [Google Scholar]
- 149.For the preparation of dehydroaminophosphonates type 246, see: Quiclet-Sire B, Zard SZ, Zhang H. J. Organomet. Chem. 2002:643–644. 404–408. Błażewska K, Gajda T. Tetrahedron. 2004;60:11701–11707..
- 150.For catalytic asymmetric synthesis of α- and β-amino-phosphonic acid derivatives, see: Ma J-A. Chem. Soc. Rev. 2006;35:630–636. doi: 10.1039/b517100h.. For related references, see: Schöllkopf U, Hoppe I, Thiele A. Liebigs Ann. Chem. 1985:555–559. Schmidt U, Oehme G, Krause H. Synth. Commun. 1996;26:777–781.. Holz J, Quirmbach M, Schmidt U, Heller D, Stürmer R, Börner A. J. Org. Chem. 1998;63:8031–8034.. Schmidt U, Krause HW, Oehme G, Michalik M, Fischer C. Chirality. 1998;10:564–572.. Grassert I, Schmidt U, Ziegler S, Fischer C, Oehme G. Tetrahedron: Asymmetry. 1998;9:4193–4202.. Burk MJ, Stammers TA, Straub JA. Org. Lett. 1999;1:387–390..
- 151.Gridnev ID, Yasutake M, Imamoto T, Beletskaya IP. Procedings of the National Academic of Sciences (PNAS) 2004;101:5385–5390. doi: 10.1073/pnas.0306993101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang D-Y, Huang J-D, Hu X-P, Deng J, Yu S-B, Duan Z-C, Zheng Z. J. Org. Chem. 2008;73:2011–2014. doi: 10.1021/jo702488j. [DOI] [PubMed] [Google Scholar]
- 153.Holz J, Stürmer R, Schmidt U, Drexler H-J, Heller D, Krimmer H-P, Börner A. Eur. J. Org. Chem. 2001:4615–4624. doi: 10.1021/jo010445l. [DOI] [PubMed] [Google Scholar]
- 154.For the synthesis of non-chiral 1-amino-2,2,2-trifluoroethanephosphonates, see: Osipov SN, Artyushin OI, Kolomiets AF, Bruneau C, Picquet M, Dixneuf PH. Eur. J. Org. Chem. 2001:3891–3897. Rassukana YV, Kolotylo MV, Sinitsa OA, Pirozhenko VV, Onys'ko PP. Synthesis. 2007:2627–2630..
- 155.Xiao J, Zhang X, Yuan C. Heteroat. Chem. 2000;11:536–540. [Google Scholar]
- 156.(a) Kafarzki P, Lejczak B, Szewczyk J. Can. J. Chem. 1983;61:2425–2030. [Google Scholar]; (b) Wagner J, Lerner RA, Barbas CF., III Bioorg. Med. Chem. 1996;4:901–916. doi: 10.1016/0968-0896(96)00085-5. [DOI] [PubMed] [Google Scholar]; (c) Kowalik J, Sawka-Dobrowolska W, Głowiak T. J. Chem. Soc., Chem. Commun. 1984:446–447. Also, see: [Google Scholar]
- 157.(a) Auberson YP, Acklin P, Bischoff S, Moretti R, Ofner S, Schmutz M, Veenstra SJ. Bioorg. Med. Chem. Lett. 1999;9:249–254. doi: 10.1016/s0960-894x(98)00720-3. [DOI] [PubMed] [Google Scholar]; (b) Lämmerhofer M, Hebenstreit D, Gavioli E, Lindner W, Mucha A, Kafarski P, Wieczorek P. Tetrahedron: Asymmetry. 2003;14:2557–2565. see also: [Google Scholar]; (c) Caccamese S, Failla S, Finocchiaro P, Hägele G, Principato G. J. Chem. Res. (S) 1992:242–243. [Google Scholar]
- 158.(a) Ou L, Xu Y, Ludwig D, Pan J, Xu JH. Org. Process Res. Dev. 2008;12:192–195. [Google Scholar]; (b) Snyder PW, Johannes MS, Vogen BN, Clark RL, Toone EJ. J. Org. Chem. 2007;72:7459–7461. doi: 10.1021/jo0711541. [DOI] [PubMed] [Google Scholar]; (c) Adam W, Lukacs Z, Saha-Moller CR, Schreier P. J. Am. Chem. Soc. 2000;122:4887–4892. [Google Scholar]; (d) Burgess K, Jennings LD. J. Org. Chem. 1990;55:1138–1139. [Google Scholar]
- 159.Yuan C, Xu C, Zhang Y. Tetrahedron. 2003;59:6095–6102. [Google Scholar]
- 160.(a) Mitsunobu O. Synthesis. 1981:1–28. [Google Scholar]; (b) Castro BR. Org. React. 1983;29:1–162. [Google Scholar]; (c) Hughess DL. Org. React. 1992;42:335–356. [Google Scholar]; (d) Hughess DL. Org. Prep. Proced. Int. 1996;28:127–164. [Google Scholar]; (e) Dodge JA, Jones SA. Rec. Res. Dev. Org. Chem. 1997;1:273–283. [Google Scholar]; (f) Dembinski R. Eur. J. Org. Chem. 2004:2763–2772. [Google Scholar]; (g) Dandapani S, Curran DP. Tetrahedron. 2002;58:3855–3864. [Google Scholar]; (h) Lipshutz BH, Chung DW, Rich B, Corral R. Org. Lett. 2006;8:5069–5072. doi: 10.1021/ol0618757. [DOI] [PubMed] [Google Scholar]
- 161.For the synthesis of non-chiral α-aminophosphonates from α-hydroxyphosphonates under solvent-free conditions using microwave irradiation, see: Kaboudin B. Tetrahedron Lett. 2003;44:1051–1053.
- 162.(a) Yokomatsu T, Shibuya S. Tetrahedron: Asymmetry. 1992;3:377–378. [Google Scholar]; (b) Gajda T. Tetrahedron: Asymmetry. 1994;5:1965–1972. [Google Scholar]; (c) Hammerschmidt F, Hanbauer M. J. Org. Chem. 2000;65:6121–6131. doi: 10.1021/jo000585f. [DOI] [PubMed] [Google Scholar]
- 163.(a) Hammerschmidt F, Wuggenig F. Tetrahedron: Asymmetry. 1999;10:1709–1721. [Google Scholar]; (b) Drescher M, Li Y-F, Hammerschmidt F. Tetrahedron. 1995;51:4933–4946. [Google Scholar]
- 164.Skropeta D, Schwörer R, Schmidt RR. Bioorg. Med. Chem. Lett. 2003;13:3351–3354. doi: 10.1016/s0960-894x(03)00672-3. [DOI] [PubMed] [Google Scholar]
- 165.(a) Yokomatsu T, Yoshida Y, Shibuya S. J. Org. Chem. 1994;59:7930–7933. [Google Scholar]; (b) Yokomatsu T, Suemune K, Yamagishi T, Shibuya S. Synlett. 1995:847–849. [Google Scholar]
- 166.(a) Bongini A, Camerini R, Panunzio M. Tetrahedron: Asymmetry. 1996;7:1467–1476. [Google Scholar]; (b) Simov BP, Wuggenig F, Lämmerhofer M, Lindner W, Zarbl E, Hammerschmidt F. Eur. J. Org. Chem. 2002:1139–1142. [Google Scholar]
- 167.Hammerschmidt F, Lindner W, Wuggenig F, Zarbl E. Tetrahedron: Asymmetry. 2000;11:2955–2964. [Google Scholar]
- 168.Dolence EK, Roylance JB. Tetrahedron: Asymmetry. 2004;15:3307–3322. [Google Scholar]
- 169.Dolence EK, Mayer G, Kelly BD. Tetrahedron: Asymmetry. 2005;16:1583–1594. [Google Scholar]
- 170.Pousset C, Larchevêque M. Tetrahedron Lett. 2002;43:5257–5260. [Google Scholar]
- 171.Piotrowska DG, Wróblewski AE. Tetrahedron. 2003;59:8405–8410. [Google Scholar]
- 172.Hercouet A, Le Corre M, Carboni B. Tetrahedron Lett. 2000;41:197–199. [Google Scholar]
- 173.(a) Gaucher A, Olliver J, Marguerite J, Paugam R, Salaün J. Can. J. Chem. 1994;72:1312–1327. [Google Scholar]; (b) Cativiela C, Díaz-de-Villegas MD, Jiménez AI. Tetrahedron: Asymmetry. 1995;6:177–182. [Google Scholar]; (c) Hercouet A, Bessieres B, Le Corre M. Tetrahedron: Asymmetry. 1996;7:283–284. [Google Scholar]
- 174.Moonen K, Laureyn I, Stevens CV. Chem. Rev. 2004;104:6177–6125. doi: 10.1021/cr030451c. [DOI] [PubMed] [Google Scholar]
- 175.Singh GS, D'hooghe M, De Kimpe N. Chem. Rev. 2007;107:2080–2135. doi: 10.1021/cr0680033. [DOI] [PubMed] [Google Scholar]
- 176.(a) Palacios F, Ochoa de Retana AM, Gil JI. Tetrahedron Lett. 2000;41:5363–5366. [Google Scholar]; (b) Palacios F, Aparicio D, Ochoa de Retana AM, de los Santos JM, Gil JI, López de Munain R. Tetrahedron: Asymmetry. 2003;14:689–700. [Google Scholar]
- 177.For the synthesis and application of racemic 2-phosphorylated aziridines, see: Palacios F, Ochoa de Retana AM, Alonso JM. J. Org. Chem. 2005;70:8895–8901. doi: 10.1021/jo051404i. Palacios F, Ochoa de Retana AM, Alonso JM. J. Org. Chem. 2006;71:6141–6148. doi: 10.1021/jo060865g..
- 178.Agami C, Couty F, Rabasso N. Tetrahedron Lett. 2002;43:4633–4636. [Google Scholar]
- 179.For the preparation of diethyl phosphonate 310, see: Groth U, Schöllkopf U. Tetrahedron. 1992;48:117–122.
- 180.(a) Robinson R. J. Chem. Soc. 1917;111:762–768. [Google Scholar]; (b) Schopf C. Angew. Chem. 1937;50:779–787. [Google Scholar]; (c) Stevens RV, Lee AWM. J. Am. Chem. Soc. 1979;101:7032–7035. [Google Scholar]; (d) Bonin M, Grierson DS, Royer J, Husson H-P. Org. Synth. Coll. 1998;9:176–180. [Google Scholar]
- 181.(a) Katritzky AR, Cui X-L, Yang B, Steel PJ. J. Org. Chem. 1999;64:1979–1985. doi: 10.1021/jo9821426. [DOI] [PubMed] [Google Scholar]; (b) Dinér P, Amedjkouh M. Org. Biomol. Chem. 2006;4:2091–2096. doi: 10.1039/b605091c. [DOI] [PubMed] [Google Scholar]
- 182.Amedjikouh M, Westerlund K. Tetrahedron Lett. 2004;45:5175–5177. [Google Scholar]
- 183.Baussanne I, Chiaroni A, Royer J. Tetrahedron: Asymmetry. 2001;12:1219–1224. [Google Scholar]
- 184.Davis FA, Lee SH, Xu H. J. Org. Chem. 2004;69:3774–3781. doi: 10.1021/jo040127x. [DOI] [PubMed] [Google Scholar]
- 185.Davis FA, Wu Y, Xu H, Zhang J. Org. Lett. 2004;6:4523–4525. doi: 10.1021/ol048157+. [DOI] [PubMed] [Google Scholar]
- 186.For the synthesis of substituted pyrrolidine phosphonates, see: Dondas HA, Durust Y, Grigg R, Slater MJ, Sarker MAB. Tetrahedron. 2005;61:10667–10682.
- 187.The alkylation reaction of 336a with iodomethane and benzylbromide gave complex mixtures
- 188.Davis FA, Xu H, Wu Y, Zhang J. Org. Lett. 2006;8:2273–2276. doi: 10.1021/ol060521c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hanessian S, Gauchet C, Charron G, Marin J, Nakache P. J. Org. Chem. 2006;71:2760–2778. doi: 10.1021/jo052649y. [DOI] [PubMed] [Google Scholar]
- 190.For the conversion of racemic cyclic α-amino acids into cyclic α-aminophosphonic acids, see: Kaname M, Mashige H, Yoshifuji S. Chem. Pharm. Bull. 2001;49:531–536. doi: 10.1248/cpb.49.531..
- 191.Boto A, Gallardo JA, Hernández R, Saavedra CJ. Tetrahedron Lett. 2005;46:7807–7811. [Google Scholar]
- 192.Zhao Y, Cao S, Li Y, Ma Y. Faming Zhuanli Shenqing Gongkai Shoumingshu, CN 10797226. Chem. Abst. 1993;1995;122 183469. [Google Scholar]
- 193.Chevrier C, Le Nouën D, Defoin A, Tarnus C. Eur. J. Org. Chem. 2006:2384–2392. [Google Scholar]
- 194.For the stereoselective synthesis of piperidin-2-yl-phosphonic acids and derivatives, see: Maury C, Wang Q, Gharbaoui T, Chiadmi M, Tomas A, Royer J, Husson HP. Tetrahedron. 1997;53:3627–3636.. Davis FA, Wu Y, Yan H, Prasad KR, McCoull W. Org. Lett. 2002;4:655–658. doi: 10.1021/ol017289p.. Kaname M, Arakawa Y, Yoshifuji S. Tetrahedron Lett. 2001;42:2713–2716.. Kaname M, Yoshinaga K, Arakawa Y, Yoshifuji S. Tetrahedron Lett. 1999;40:7993–7994.. Kaname M, Yoshinaga K, Arakawa Y, Yoshifuji S. Chem. Pharm. Bull. 2004;52:160–162. doi: 10.1248/cpb.52.160..