Abstract
Two different methodologies for the synthesis of monotelechelic poly(oxa)norbornenes prepared by living ring-opening metathesis polymerization (ROMP) are presented. The first method, termed direct end-capping, is carried out by adding an internal cis-olefin terminating agent (TA) to the reaction mixture immediately after the completion of the living ROMP reaction. The second method relies on cross metathesis (CM) between a methylene-terminated poly(oxa)norbornene and a cis-olefin TA mediated by the ruthenium olefin metathesis catalyst (H2IMes)(Cl)2Ru(CH-o-OiPrC6H4) (H2IMes = 1,3-dimesitylimidazolidine-2-ylidene). TAs containing various functional groups, including alcohols, acetates, bromides, a-bromoesters, thioacetates, N-hydroxysuccinimidyl esters and Boc-amines, as well as fluorescein and biotin groups, were synthesized and tested. The direct end-capping method typically resulted in >90% end-functionalization efficiency, while the CM method was nearly as effective for TAs without polar functional groups or significant steric bulk. End-functionalization efficiency values were determined by 1H NMR spectroscopy.
Introduction
Ring-opening metathesis polymerization (ROMP) has become a widely used method for the synthesis of both industrially relevant and academically interesting polymers.1 Among the many types of interesting polymer architectures accessible by ROMP are block,2 hyperbranched,3 dendronized,4 brush,5 and cyclic polymers.6,4c Many of these architectures can be produced with a high degree of molecular weight control and with narrow polydispersities. Control of both polymer architecture and molecular weight is a result of developments in olefin metathesis catalyst design, using mainly ruthenium and molybdenum catalysts.7 With the wide variety of polymers that olefin metathesis catalysts have enabled, it is remarkable that narrow polydispersity, monotelechelic ROMP polymers remain difficult to prepare.
Telechelic polymers are linear polymers in which a desired functionality is placed at one (monotelechelic) or both (ditelechelic) of the chain ends.8 Functionalized chain ends can then be used for a number of applications, including growing another polymer block using a different polymerization mechanism,9 attaching biomolecules,10 or cyclizing to form cyclic polymers.11 A number of strategies for making low polydispersity, monotelechelic ROMP polymers have been employed to produce hybrid block copolymers,12 fluorescent polymers13 and graft polymers.14 However, the synthetic methods currently available for producing narrow polydispersity, monotelechelic ROMP polymers all either result in less than quantitative end-capping or are limited in the types of functional groups that can be appended to the chain end.
Ditelechelic ROMP polymers are traditionally synthesized by reacting a small, strained olefin such as norbornene or cyclooctene with an internal olefin chain transfer agent (CTA) in the presence of an olefin metathesis catalyst.15 At thermodynamic equilibrium, every CTA molecule is incorporated into the polymer chain, and the molecular weight is controlled by controlling the monomer to CTA molar ratio. The resulting polymers have high degrees of chain-end functionality, but because the polymerization mechanism is dominated by chain transfer, the products have polydispersity indexes (PDIs) of around 2.0. High PDIs are acceptable for many purposes, but low PDIs are required for several applications, such as when specific morphologies of block copolymers are desired. Additionally, this method is incapable of placing the desired functionality only at one end of the polymer.
Methods for synthesizing low PDI, telechelic ROMP polymers have been developed by several groups over the past decade. The custom initiator method is one such process, by which a ROMP initiator is synthesized and isolated and then used to initiate polymerization, forming a monotelechelic polymer.13,16 The custom initiator method can be effective, but it has the drawback that a new catalyst must be made for each new chain-end functionality. Synthesis of new olefin metathesis catalysts can be difficult and low yielding, especially when complex functionality is desired. In a different strategy, custom terminating agents have been used to produce monotelechelic ROMP polymers.16c,17 In the case of ruthenium-mediated ROMP, functionalized vinyl ethers are most commonly used to simultaneously end-cap a growing polymer chain and deactivate the metathesis catalyst. Typically end-capping is not complete with functionalized vinyl ethers, as we have shown previously.12f Other terminating agents include acrylates,18 as well as vinyl lactones and vinyl carbonates.19 Aldehydes can be used very effectively as terminators in molybdenum-mediated ROMP,12d,20 but the lack of air, moisture, and functional group tolerance of molybdenum metathesis catalysts limits this process to polymers with minimal functionality. Another more recent end-functionalization strategy is the sacrificial monomer method.21 The sacrificial monomer method requires the synthesis of a block copolymer with one block comprised of the desired monomer and the other block comprised of a readily degradable monomer. Subsequent degradation of the sacrificial block yields an ω-end-functionalized polymer. The sacrificial monomer method is quite effective, but it is limited to only a few functional groups, all of which must be further derivatized after polymerization to add any additional functionality.
We recently described a direct method of monotelechelic ROMP polymer synthesis utilizing an internal cis-olefin as a terminating agent (TA).12f Because the backbone olefins of substituted poly(oxa)norbornenes are too sterically hindered to undergo metathesis, the TA adds only to the ω-end of the polymer. Any further metathesis reactions are degenerate. Using a TA containing α–bromoester groups, we observed complete end-capping to form a low polydispersity, monotelechelic polymer with an α–bromoester group. Subsequent atom-transfer radical polymerization (ATRP) using the end-capped polynorbornene as a macroinitiator showed a complete shift in the GPC peaks from homopolymer to block copolymer, indicating that end-capping was quantitative and occurred only on the ω end of the polymer. We also recently reported an extension of this method to α,ω-end-functionalized polymers by reacting the catalyst with the desired symmetrical cis-olefin before addition of monomer.22 Now we report on the development of this direct end-capping method to include other functional groups. In addition, we describe a post-polymerization method of end-functionalization of the ω-end of ROMP polymers using cross metathesis.
Results and Discussion
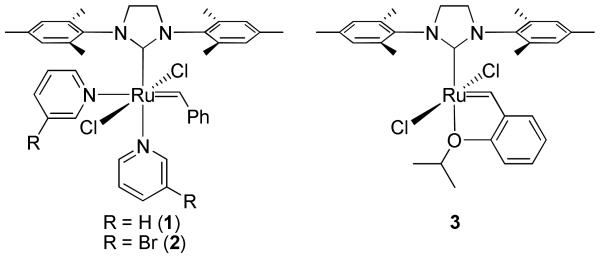
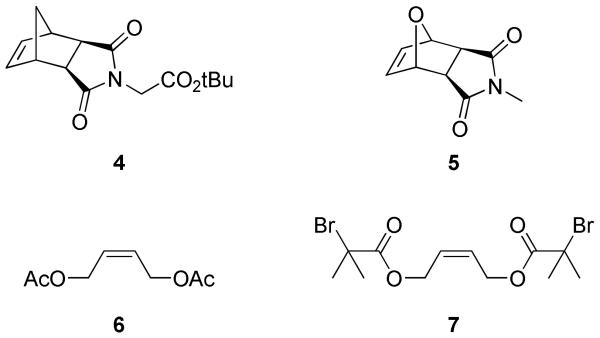
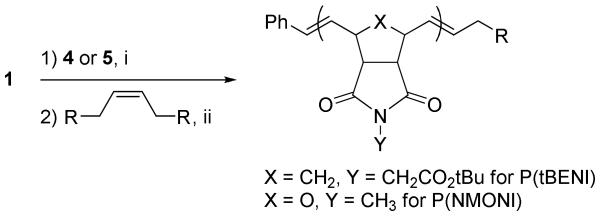
Recently, pyridine-containing ruthenium olefin metathesis catalysts 1 and 2 (Figure 1) have found use as mediators of ROMP due to their fast-initiation rates and high functional group tolerance.2d,17c,23 Catalysts 1 and 2 have been shown to effect the living ROMP of a wide variety of strained cyclic olefin monomers, including many with high levels of functionality, such as saccharides,23b,24 peptides,25 and charged groups.23d,26 Our lab currently uses catalyst 1 for most living ROMP reactions because it initiates quickly enough to afford low polydispersity polymers while maintaining longer benchtop stability than catalyst 2. tert-Butyl ester norbornene imide (tBENI) (4), was selected as the monomer in this study because of the ease of synthesizing large quantities of the material in high purity (Figure 2). Monomer 5, N-methyloxanorbornene imide (NMONI) was also used in this work in cases where peak overlap prevented accurate end-group quantification by 1H NMR spectroscopy.
Figure 1.
Ruthenium olefin metathesis catalysts used in this study.
Figure 2.
Monomers (4 and 5) and previously reported TAs (6 and 7) used in this study.
We first sought to examine the substrate scope of our previously-developed method of end-capping polynorbornenes, which requires adding a symmetrical cis-olefin TA to the reaction mixture after the completion of a living ROMP reaction. To this end, we synthesized several new, symmetrical cis-olefin terminating agents. The end-capping of a ROMP polymer chain can be thought of as a single-turnover cross metathesis (CM) reaction between the active metal center on the end of the polymer chain and the TA. Previous work from our group on cross metathesis indicates that unhindered, electron-rich olefins are reactive cross metathesis substrates, as are many olefins with allylic substitution.27 All new TAs were designed with these criteria in mind. In addition, TAs 6 and 7 (Figure 2), which are known to be active cross metathesis substrates, were included in this study.
TA Syntheses
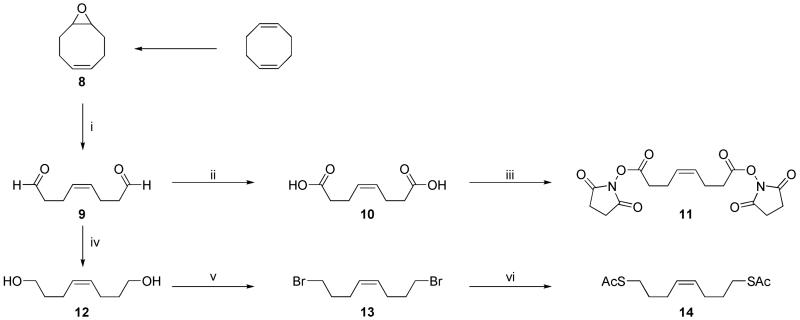
To quickly synthesize a variety of TAs with varying functionality, we began with previously reported epoxide 8.28 Oxidation of 8 using periodic acid afforded dial 9, which was further oxidized using Jones reagent to diacid 10. Diacid 10 was derivatized to NHS ester-containing TA 11 by an EDC coupling reaction to enable amine conjugation to a polynorbornene terminus. Reduction of dial 9 to diol 12 was accomplished using NaBH4. Diol 12 was then converted to dibromide 13, with potential use in copper-catalyzed azide-alkyne cycloaddition when converted to the azide after end-capping, a process that has been demonstrated using a similar TA.29 A diazide TA was also synthesized from dibromide 13, but it was found to quickly deactivate the metathesis catalyst. Finally, dibromide 13 was converted to dithioacetate 14 using potassium thioacetate. Removal of the acetate groups using LiAlH4 to afford a dithiol was successful, but the resulting TA appeared to be incompatible with the metathesis catalyst.
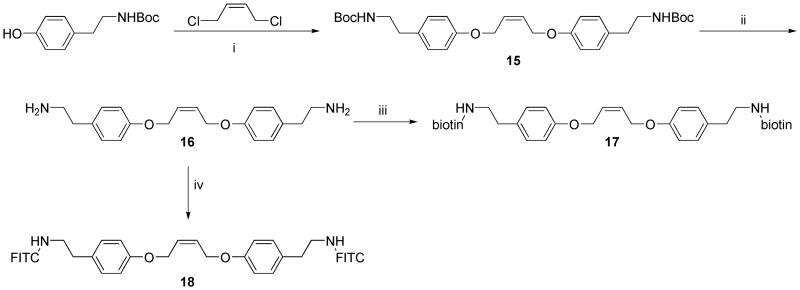
In order to incorporate olefins with allylic substitution into this study, we also synthesized a Bocamine-containing TA (15) by the reaction of 1,4-dichloro-cis-2-butene with Boc-tyramine (Scheme 2). Removal of the Boc-groups using trifluoroacetic acid afforded diamine 16. Coupling of diamine 16 with biotin using EDC afforded the biotin-containing TA, 17. Biotin end-capped polynorbornenes have been reported once before using 30 equiv of a functionalized vinyl ether;10a a method that requires less of the expensive and difficult to remove biotin terminating agent could be useful for biological applications of ROMP polymers. A fluorescein-containing TA (18) was also synthesized from diamine 16 by addition of fluorescein isothiocyanate (FITC). With a set of symmetrical cis-olefins with varied functionality in hand, we set out to examine their reactivity in end-functionalization reactions.
Scheme 2.
Synthesis of NHBoc, FITC, and Biotin-Containing TAs.a
aReagents and conditions: (i) K2CO3, DMF, 90 °C; (ii) TFA, CH2Cl2, rt; (iii) biotin, EDC, DPTS, DMF, rt; (iv) FITC, DMF, rt.
End functionalization by direct end-capping
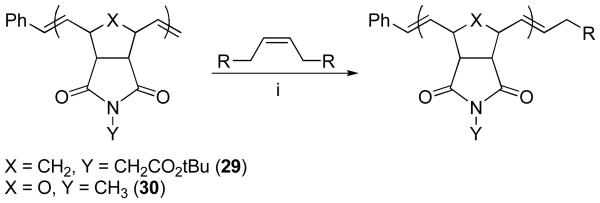
Using our previously described method,12f end-capping of P(tBENI) or P(NMONI) chains with TAs 6, 7, 9, 11-15, 17 and 18 was carried out (Scheme 3). Briefly, a solution of catalyst 1 was injected into a rapidly stirring vial of monomer 4 (25 eqiuv) in CH2Cl2 under argon on the benchtop. After three minutes, the desired TA (5 equiv) was added as a solution in CH2Cl2 or MeOH (for TAs 17 and 18). Biotin-containing TA 17 was not soluble in MeOH at the concentrations used, so it was added as a slurry. The reaction mixture was stirred at room temperature under argon for an additional 6 h, at which point ethyl vinyl ether was added to quench the reaction. The polymer products were isolated in high purity and yield in most cases by precipitation of the reaction mixture into a large volume of diethyl ether/hexanes (or isopropanol/hexanes) (1:1), followed by filtration and washing with diethyl ether. In cases where the TA was not soluble in the precipitation solvent mixture, the precipitated polymer products were further purified by dialyzing the reaction mixture against DMSO or DMF and then H2O, followed by lyophilization. NMR spectroscopy was used to confirm complete removal of the excess TA. Carboxylic acid-containing TA 10 and primary amine-containing TA 16 were found to deactivate the catalyst, presumably due to coordination to the metal center. All polymers were characterized by gel permeation chromatography (GPC) and showed the expected narrow polydispersity and molecular weights of approximately 7000 Daltons.
Scheme 3.
End-functionalization of P(tBENI) or P(NMONI) using the direct end-capping method.a
aReagents and conditions: (i) CH2Cl2, rt, 3 min; (ii) CH2Cl2 or MeOH, rt, 6 h.
1H NMR spectroscopy was used to evaluate the percentage of end-capping by comparing the integral values of the phenyl end groups derived from catalyst 1 with the integral values of the appended functional group. A relaxation delay of 10 s was used to ensure precise integral values, and NMR spectra were taken on a 500 MHz spectrometer for a high level of resolution. The results, summarized in Table 1, show that most TAs are capable of near-quantitative end-capping. Notably, FITC-containing TA 18 showed a high degree of end-capping, providing a simple, direct method to fluorescent, monotelechelic polynorbornenes. Biotin-containing TA 17, which is only sparingly soluble in CH2Cl2/MeOH mixtures, reached 93% end-capping efficiency, indicating that the direct end-capping methodology is sufficiently robust to compensate for poorly soluble TAs.
Table 1.
End-capping Efficiency of P(tBENI) or P(NMONI) Polymers using the Direct End-Capping Method.
| Product | TA | R = | % efficiencya |
|---|---|---|---|
| 19 | 6 | OAc | 97 |
| 20 | 7 | OC(O)C(CH3)2Br | >98 |
| 21 | 9 | CH2C(O)H | 59 |
| 22 | 11 | CH2C(O)NHS | 80 |
| 23 | 12 | CH2CH2OH | 97 |
| 24 | 13 | CH2CH2Br | >98 |
| 25 | 14 | CH2CH2SAc | 91 |
| 26 | 15 |
|
>98 |
| 27 | 17 |
|
93 |
| 28 | 18 |
|
>98 |
Efficiency determined from the 1H NMR integral ratio of the phenyl end group protons derived from catalyst 1 to the newly-installed end group protons.
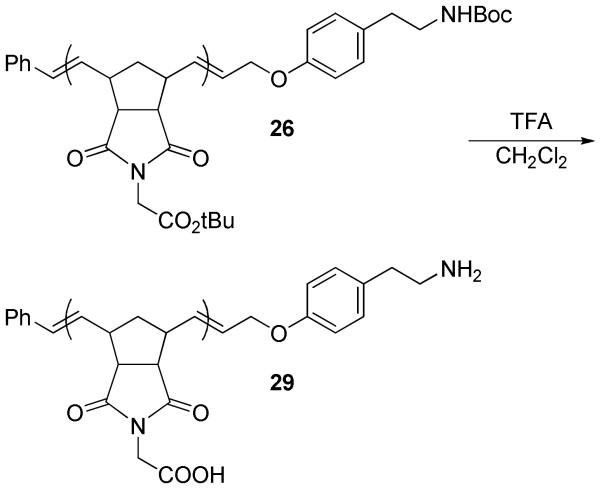
To synthesize an amine-terminated polynorbornene, polymer 26 was treated with trifluoroacetic acid in CH2Cl2 to remove the Boc protecting group as well as the tert-butyl ester groups on the repeating units (Scheme 4). 1H NMR spectroscopy showed complete removal of both the Boc and the tert-butyl ester groups, affording amine-terminated poly (carboxymethylene norbornene imide) (P(CMNI)) 29. This simple route to amine-terminated ROMP polymers may prove useful for appending biomolecules or other large groups to polymer termini using standard amine-carboxylic acid coupling chemistry.
Scheme 4.
Removal of Boc and tert-Butyl Ester Groups to Afford Amine-Terminated Polynorbornene.
End-functionalization using CM
As an alternative to direct end-capping, we sought to develop a post-polymerization method for the synthesis of monotelechelic poly(oxa)norbornenes. Post-polymerization end-functionalization would provide an alternative route to monotelechelic polynorbornenes that might be useful in cases where direct end-capping is not feasible due to solubility or other concerns. Noting that most ROMP reactions are quenched with ethyl vinyl ether, we thought CM would be an effective end-capping reaction. Uses of CM in polymer synthesis remain almost exclusively limited to acyclic-diene metathesis polymerization (ADMET).1a Considering the capability of CM to make highly-functional olefins in small molecules, we sought to extend the use of CM in polymer synthesis to polymer end-functionalization.
Previous studies in our group have shown that CM between a terminal olefin and a disubstituted olefin is a highly effective method for olefin homologation and functionalization.30 In fact, CM between a terminal olefin and an internal, disubstituted olefin is considerably more effective than CM between two terminal olefins. This result is attributed to the greater stability of a propagating ruthenium alkylidene versus a propagating ruthenium methylidene.31 In a CM reaction between a terminal olefin and a disubstituted olefin, the propagating ruthenium alkylidene is greatly favored over the methylidene. The TAs described earlier in this study represent a group of internal olefins that would be expected to perform well in CM and showed high reactivity in direct end-capping. We used these same TAs in the CM portion of this study for the sake of convenience and to compare the efficiencies of the two methods, but it should be noted that trans-olefins or mixtures of cis- and trans-olefins would be expected to perform similarly because in CM cis-olefins are often quickly isomerized to their trans forms, indicating that the initial stereochemistry of the olefin is irrelevant.
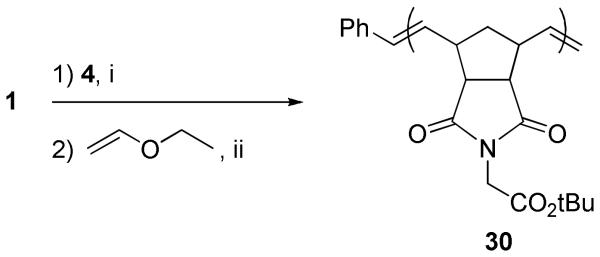
To prepare an olefin-terminated polymer to act as the terminal olefin cross partner, the ROMP of monomer 4 was again carried out using catalyst 1 (Scheme 5). P(tBENI) polymer 30 was prepared by injecting catalyst 1 from a stock solution into a vigorously stirring solution of monomer 4 (25 equiv). After 3 min polymerization was terminated using ethyl vinyl ether to end-cap the polymer with a methylene end group. Polymer 30 showed the low PDI and controllable molecular weight expected from catalyst 1. As noted earlier, previous work from our group has indicated that end-capping using vinyl ethers does not result in 100% end-capping with a terminal olefin.12f The remaining end groups are believed to be vinyl ethers derived from ethyl vinyl ether termination. We assumed that the vinyl ether-capped portion of polymer 30, which is not visible by 1H NMR spectroscopy due to overlapping signals with backbone protons, would also be active in CM when run at the temperatures and with catalyst loadings described here.
Scheme 5.
Synthesis of methylene-capped P(tBENI).a
aReagents and conditions: (i) CH2Cl2, 3 min, rt; (ii) rt, 15 min.
We chose to use the chelating-ether ruthenium olefin metathesis catalyst (H2IMes)(Cl)2Ru(CH-o-OiPrC6H4) (H2IMes = 1,3-dimesitylimidazolidine-2-ylidene) (3) in this study due to its longer lifetime and higher efficiency in CM than catalysts 1 and 2. A solvent screen showed that toluene was the best solvent for this reaction, with common metathesis solvents THF and CH2Cl2 performing considerably worse. The optimal temperature was found to be 40 °C, at which the reaction was complete in 12 h and there was no substantial loss of the phenyl end group on the α end of the polymer. Higher temperatures led to some cross metathesis of the phenyl-norbornenyl internal olefin. Under no circumstances, including higher temperatures, higher catalyst loadings and longer reaction times, was there any indication that the backbone norbornenyl-norbornenyl olefins underwent metathesis, as indicated by GPC before and after CM. Additionally, our group previously reported that CM between two methylene-capped polymer chains can be used dimerize the polymer;32 we observed no polymer-polymer CM when TA was present.
Using the optimized conditions of catalyst 3 (50 mol% relative to polymer) in toluene at 40 °C for 12 h with 5 equiv of TA, we examined the CM of polymers 19 and 20 on TAs 6, 7, 9, 11-15, 17 and 18 (Scheme 6). All reactions were run under argon on the benchtop, and end-capping reactions using 17 and 18 were run in 4:1 toluene/methanol to aid in solubility. The polymer products were isolated using the same procedures as described in the direct end-capping section. The results from the CM reactions are summarized in Table 2. TAs 6, 7, 13 and 15 showed high degrees of end-capping efficiency (>85%), as was seen using the direct end-capping method, indicating that polymer end-functionalization using the CM method is highly efficient when sterically unencumbered TAs and TAs without polar functionalities are used. TAs containing polar functional groups were generally less effective, with dial TA 9 and diol TA 12 showing only 36 and 60 % end-functionalization efficiency, respectively. The polar functional groups on TAs 9, 11 and 12 may limit the efficiency of CM only for these particular TAs due to coordination of the Lewis basic functionality to the metal center after the initial metathesis event, but further studies on TAs with longer spacer groups between the olefin and the functional group are warranted.
Scheme 6.
End-functionalization of P(tBENI) or P(NMONI) using cross metathesis
aReagents and conditions: (i) catalyst 3 (50 mol% relative to polymer), toluene or toluene/MeOH, 40 °C, 12 h.
Table 2.
End-capping Efficiency of P(tBENI) or P(NMONI) Polymers using Cross Metathesis
| Product | TA | R = | % efficiencya |
|---|---|---|---|
| 31 | 6 | OAc | 89 |
| 32 | 7 | OC(O)C(CH3)2Br | >90 |
| 33 | 9 | CH2C(O)H | 36 |
| 34 | 11 | CH2C(O)NHS | 44 |
| 35 | 12 | CH2CH2OH | 60 |
| 36 | 13 | CH2CH2Br | >90 |
| 37 | 14 | CH2CH2SAc | 70 |
| 38 | 15 |
|
>90 |
| 39 | 17 |
|
69 |
| 40 | 18 |
|
40 |
Efficiency determined from the 1H NMR integral ratio of the phenyl end group protons derived from catalyst 1 to the newly-installed end group protons.
We attribute the higher efficiency of the direct end-capping method compared with the CM method to the necessity for only a single metathesis turnover in the case of direct end-capping. In the case of CM, several metathesis steps need to occur to effect end-functionalization, including reaction of the TA with the catalyst, reaction of the functionalized catalyst with the polymer chain end, and productive metathesis to release the catalyst and functionalized polymer. A low concentration of polymer chain ends is also expected to limit the effectiveness of the CM method. However, while generally not as effective as the direct end-capping method, the CM method may prove useful in cases where a TA is not soluble in CH2Cl2 or in cases where the study of a single batch of polymer with varying end groups is needed. Moreover, this method facilitates the synthesis and use of poly(oxa)norbornenes as pivotal macromolecular building blocks which can be prepared on large scale, stored indefinitely, and then functionalized as desired. Additionally, it should be noted that this methodology is not limited to polynorbornenes; theoretically CM would be capable of end-functionalizing many olefin-terminated polymers.
Conclusions
We have presented two methods for the end-functionalization of poly(oxa)norbornenes to afford monotelechelic ROMP polymers. The direct end-capping method involves a single metathesis turnover between the ruthenium metal center on a living polymer chain and an internal olefin TA. This method was found to be highly effective for a wide variety of TAs, including fluorescein and biotin-containing TAs, demonstrating the versatility of this approach. We expect that the direct end-capping methodology will find use in a variety of areas, including bioconjugation, surface attachment, and in the synthesis of mechanistically incompatible block copolymers. The CM method is a two-step procedure requiring termination of ROMP with ethyl vinyl ether, followed by cross metathesis of the methylene-capped polymer with an internal olefin TA. Though generally less effective than direct end-capping, end-functionalization by CM may also find use in cases where direct end-capping is not feasible or with olefin-terminated polymers made by methods other than ROMP.
Experimental Section
General Information
NMR spectra of small molecules were measured in CDCl3 on Varian Mercury 300 MHz spectrometers unless otherwise noted. NMR spectra of polymers were measured on Varian Inova 500 or 600 MHz spectrometers with a relaxation delay of 10 s in CD2Cl2 unless otherwise noted. 1H and 13C NMR chemical shifts are reported in ppm relative to proteosolvent resonances. Flash column chromatography of organic compounds was performed using silica gel 60 (230-400 mesh). High-resolution mass spectra (EI and FAB) were provided by California Institute of Technology Mass Spectrometry Facility. Gel permeation chromatography (GPC) was carried out in THF on two PLgel 10 μm mixed-B LS columns (Polymer Labs) connected in series with a DAWN EOS multiangle laser light scattering (MALLS) detector and an Optilab DSP differential refractometer (both from Wyatt Technology). No calibration standards were used, and the dn/dc values used were 0.109 for tBENI polymers and 0.135 for NMONI polymers, as calculated by averaging several runs assuming 100% mass elution from the columns.
Materials
CH2Cl2, Et2O and toluene were purified by passage through solvent purification columns and degassed.33 MeOH was dried over Mg and distilled. Anhydrous DMF was obtained from Acros Chemical Company and used as received. (H2IMes)(PCy3)(Cl)2RuCHPh and (H2IMes)(Cl)2RuCH(o-OiPrC6H4) (3) were provided by Materia. (H2IMes)(pyr)2(Cl)2RuCHPh (1) was prepared from (H2IMes)(PCy3)(Cl)2RuCHPh according to a literature procedure.34 tert-Butyl ester norbornene imide (4) was prepared as described previously.12f cis-2-Butene-1,4-diyl bis(2-bromo-2-methylpropanoate) (6) was prepared similarly to a procedure described previously.12f cis-1,4-Dichloro-2-butene was obtained from Aldrich Chemical Company and distilled prior to use. cis-1,2-Epoxy-5-cyclooctene (8) and cis-4-octene-1,8-diol (12) were prepared according to a literature procedure.28 Deuterated solvents were obtained from Cambridge Isotope Labs. Dimethylaminopyridinium p-toluene sulfonate (DPTS) was prepared according to a literature procedure.35 All other materials, including N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) were obtained from Aldrich Chemical Company and used as received.
cis-4-Octene-1,8-dial (9)
To a solution of cis-1,2-epoxy-5-cyclooctene (8) (1.00 g, 1 equiv) in p-dioxane (10 mL) at 0 °C was added periodic acid (2.12 g, 1.15 equiv) dropwise in 10 mL H2O. The reaction mixture was allowed to warm to room temperature. After 2.5 h, the reaction mixture was extracted with Et2O (3 × 30 mL). The combined organic layers were dried over Na2SO4, and the residue was purified by Kugelrohr distillation to afford 9 as a clear oil in 58% yield (650 mg). 1H NMR: δ 2.38 (m, 4H), 2.56 (m, 4H), 5.28 (t, J = 4.5 Hz, 2H), 9.76 (m, 2H). 13C NMR: δ 201.99, 120.04, 43.69, 20.11. HRMS: calculated: 140.0837; found: 140.0832.
cis-4-Octene-1,8-dioic acid (10)
To a solution of dial 9 (400 mg, 1 equiv) in acetone (20 mL) at 0 °C was added Jones reagent dropwise until the orange color persisted. The reaction mixture was then allowed to stir at room temperature. After 90 min the acetone was removed in vacuo and the green residue was taken up in H2O (20 mL). The H2O layer was extracted with EtOAc (3 × 25 mL), and the combined organic layers were washed with brine (10 mL) and dried over Na2SO4. Removal of the solvent in vacuo yielded a white powder that was recrystallized from EtOAc/hexanes to afford 10 as a white solid in 60% yield (293 mg). 1H NMR (10% CD3OD in CDCl3): δ 2.30-2.39 (m, 8H), 4.96 (br s, 2H), 5.37 (t, J = 4.5 Hz, 2H). 13C NMR: δ 177.11, 129.12, 34.08. 22.75. HRMS: calculated: 172.0736; found: 172.0738.
cis-4-Octene-1,8-bis(N-hydroxysuccinimidyl) ester (11)
An oven-dried, 2-necked, round-bottom flask under argon was charged with diacid 10 (100 mg, 1 equiv). CH2Cl2 (5 mL) was added to the flask, followed by EDC (657 mg, 5.9 equiv) and DPTS (30 mg, 0.2 equiv). N-hydroxysuccinimide (348 mg, 5.2 equiv) was added, and the reaction mixture was allowed to stir at room temperature. After 20 h H2O (10 mL) was added, and the CH2Cl2 layer was separated off, washed with brine and dried over Na2SO4. The crude product was purified by passage through a plug of silica (eluting with EtOAc) followed by recrystallization from toluene or toluene/hexanes to afford 11 as a white powder in 39% yield (84 mg). 1H NMR (acetone-d6): δ 2.52 (m, 4H), 2.72 (t, J = 6.9 Hz, 4H), 2.80-2.95 (m, 8H), 5.54 (t, J = 4.8 Hz, 2H). 13C NMR (acetone-d6): δ 170.59, 169.33, 129.63, 31.30, 26.34, 23.11. HRMS: calculated: 367.1141; found: 367.1139.
cis-4-Octene-1,8-dibromide (13)
A round-bottom flask was charged with diol 7 (763 mg, 1 equiv) in CH2Cl2 (20 mL). CBr4 (3.70 g, 2.1 equiv) was added, and the reaction mixture was cooled to 0 °C. Once cool, PPh3 (3.09 g, 2.2 equiv) was added, and the reaction mixture was allowed to warm to room temperature. After 16 h the solvent was removed in vacuo, affording a pale yellow oil. Hexanes (30 mL) was added, causing white solids to crash out. The suspension was stirred and sonicated, and the solids were filtered off. The filtrate was concentrated in vacuo, affording a pale yellow oil. The product was purified by silica gel chromatography (5% EtOAc in hexanes) to yield 13 as a clear oil in 78% yield (1.12 g). 1H NMR: δ 1.86 (quintet, J = 7.2 Hz, 4H), 2.16 (m 4H), 3.35 (t, J = 6.6 Hz, 4H), 5.33 (m, 2H). 13C NMR: δ 129.43, 33.48, 32.68, 25.87. HRMS: calculated: 269.9442; found: 269.9473.
S,S′-cis-4-octene-1,8-diyl diethanethioate (14)
A round-bottom flask was charged with dibromide 13 (584 mg, 1 equiv) and DMF (10 mL). Potassium thioacetate (720 mg, 2.9 equiv) was added, and the reaction mixture was heated at 65 °C. After 30 min the solvent was removed in vacuo. The residue was taken up in CH2Cl2 and filtered, and the solvent was removed in vacuo. The crude product was purified by silica gel chromatography (5% EtOAc in hexanes) to yield 14 as a pale orange oil in 79% yield (442 mg). 1H NMR: δ 1.63 (quintet, J = 7.2 Hz, 4H), 2.12 (dd, J = 7.2 Hz, 5.7 Hz, 4H), 2.33 (s, 6H), 2.87 (t, J = 7.2 Hz, 4H), 5.37 (t, J = 4.5 Hz, 2H). 13C NMR: δ 196.06, 129.61, 30.85, 29.57, 28.82, 26.52. HRMS(M+H): calculated: 261.0983; found: 261.0994.
Boc-amine-containing TA 15
A round-bottom flask was charged with Boc-tyramine (2.30 g, 2.2 equiv), K2CO3 (2.02 g, 3.3 equiv) and DMF (30 mL). 1,4-Dichloro-cis-2-butene (500 μL, 1 equiv) was added, and the reaction mixture was heated at 90 °C. After 3 h the solvent was removed in vacuo, and the residue was taken up in CH2Cl2, washed with H2O and brine, and dried over Na2SO4. The product was purified by silica gel chromatography (2% MeOH in CH2Cl2) to yield 15 as a white powder in 45% yield (1.07 g). 1H NMR: δ 1.43 (s, 18H), 2.73 (t, J = 7.2 Hz, 4H), 3.33 (q, J =6.6 Hz, 4H), 4.52 (s, 2H), 4.65 (d, J = 4.5 Hz, 4H), 5.93 (t, J = 4.5 Hz, 2H), 6.85 (m, 4H), 7.25 (m, 4H). 13C NMR: δ 157.17, 156.03, 131.59, 129.92, 128.75, 114.90, 79.28, 64.34, 42.10, 35.44, 28.56. HRMS: calculated: 527.3121; found: 527.3115.
Amine-containing TA 16
A round-bottom flask was charged with bis(Boc-amine) 15 (1.07 g, 1 equiv) in CH2Cl2 (20 mL). TFA (1.5 mL, 10 equiv) was added, and the flask was capped with a septum with a needle through it. After 24 h the reaction was quenched with 5% aqueous NH4OH and then diluted with H2O (20 mL). The CH2Cl2 layer was removed, and the aqueous layer was washed with CH2Cl2 (3 × 15 mL). The organic layers were combined and dried over Na2SO4. The solvent was removed in vacuo to afford 16 as a pale yellow oil in 98% yield (651 mg) in sufficient purity for future reactions. 1H NMR: δ 2.66 (t, J = 6.9 Hz, 4H), 2.89 (t, J = 6.9 Hz, 4H), 4.63 (d, J = 3.9 Hz, 4H), 5.90 (t, J = 3.3 Hz, 2H), 6.85 (m, 4H), 7.09 (m, 4H). 13C NMR: δ 156.98, 132.19, 129.89, 128.70, 114.79, 64.30, 43.58, 38.99. HRMS: calculated: 327.2073; found: 327.2067.
Biotin-containing TA 17
An oven-dried, 2-necked, round-bottom flask under argon was charged with D-biotin (173 mg, 2.6 equiv). DMF (3 mL) was added, followed by EDC (160 mg, 3.1 equiv) and DPTS (15 mg, 0.2 equiv). Diamine 16 (88 mg, 1 equiv) was then added as a solution in DMF (3 mL). The colorless reaction mixture was allowed to stir at room temperature under argon. After 40 h the DMF was removed in vacuo, and the residue was triturated with H2O. Recrystallization of the crude product from toluene/MeOH (4:1) afforded a white powder in 45% yield (95 mg). 1H NMR (DMSO-d6): δ 1.20-1.59 (m, 12H), 2.01 (t, J = 7.2 Hz, 4H), 2.53-2.63 (m, 6H), 2.77-2.83 (m, 2H), 3.00-3.08 (m, 4H), 3.15-3.22 (m, 4H), 4.08-4.11 (m, 4H), 4.26-4.30 (m, 2H), 4.65 (d, J = 3.6 Hz, 4H), 5.81 (t, J = 3.2 Hz, 2H), 6.34 (s, 2H), 6.40 (s, 2H), 6.84 (d, J = 8.4 Hz, 4H), 7.07 (d, J = 8.4 Hz, 4H), 7.80 (t, J = 5.4 Hz, 2H). 13C NMR (DMSO-d6): δ 171.88, 162.70, 156.48, 131.69, 129.57, 128.46, 114.49, 63.77, 61.03, 59.18, 55.42, 40.29, 35.19, 34.34, 28.18, 28.04, 25.30. HRMS (M+H): calculated: 779.3625; found: 779.3629.
FITC-containing TA 18
An oven-dried, 2-necked, round-bottom flask under argon was charged with FITC (180 mg, 2.1 equiv). Diamine 10 (70 mg, 1 equiv) was then added in DMF (2 mL). The reaction vessel was covered with foil and allowed to stir at room temperature. After 16 h the solvent was removed in vacuo. The crude product was taken up in MeOH (1 mL) and precipitated into Et2O (60 mL). The product was recovered by filtration to yield 12 as a bright orange powder in 80% yield (202 mg). 1H NMR (CD3OD): δ 2.83 (s, 4H), 2.95 (s, 2H), 3.76 (s, 4H), 4.54 (d, J = 3.3 Hz, 4H), 5.72 (t, J = 3.3 Hz, 2H), 6.48-7.09 (m, 22H), 7.56 (dd, J = 8.4 Hz, 1.8 Hz, 1.7 H, major isomer), 7.83 (d, J = 8.4 Hz, 0.3 H, minor isomer), 8.02 (s, 1.7 H, major isomer), 8.22 (s, 0.3 H, minor isomer) . 13C NMR: δ 182.34, 171.05, 166.59, 164.82, 161.43, 158.48, 154.15, 149.63, 142.28, 141.92, 132.54, 131.96, 130.92, 130.36, 129.60, 129.07, 125.84, 119.35, 120.18, 116.24, 115.92, 113.74, 111.43, 103.57, 47.09. HRMS: calculated 1105.2788, found 1105.2795.
Typical synthesis of monotelechelic poly(oxa)norbornenes using the direct end-capping method
To a stirring solution of monomer (0.069 mmol, 25 equiv) in CH2Cl2 (0.6 mL) under argon was added catalyst 1 (0.00275 mmol, 1 equiv) as a solution in CH2Cl2 (0.1 mL) quickly via syringe. After 3 min TA (0.0137 mmol, 5 equiv) was added to the reaction mixture as a solution in CH2Cl2 (or MeOH for TAs 17 and 18) (0.2 mL). The vial was sealed under argon and stirred for 6 h, at which point ethyl vinyl ether (0.3 mL) was added. The reaction mixture was then precipitated into Et2O/hexanes (1:1) (20 mL), and the products were recovered by filtration and dried under vacuum. Yields were typically > 90 %. In the case of TAs 17 and 18, the reaction mixture was diluted with DMSO or DMF to 4 mL, and this solution was dialyzed against DMSO or DMF (8000 MWCO) for three days, followed by H2O for one day. The aqueous polymer mixture was then lyophilized to afford the clean polymer product. Yields were typically 60-80 % in this case. Larger scale reactions using the direct end-capping method, as reported previously, show similar results.12f
P(tBENI)=CHCH2OAc by direct end-capping (19)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 4.51-4.58 (m, 2H), 5.40-5.80 (d, 2n H), 7.20-7.45 (m, 5H). GPC: Mn = 7400, Mw/Mn = 1.06.
P(tBENI)=CHCH2OC(O)C(CH3)2Br by direct end-capping (20)
1H NMR: δ 1.20-1.80 (m, 10n H), 1.91 (s, 6H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 4.65-4.75 (m, 2H), 5.40-5.80 (d, 2n H) 7.20-7.45 (m, 5H). GPC: Mn = 8700, Mw/Mn = 1.02.
P(tBENI)=CHCH2CH2C(O)H by direct end-capping (21)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 7.20-7.45 (m, 5H), 9.78 (s, 1H). GPC: Mn = 5300, Mw/Mn = 1.06.
P(NMONI)=CHCH2CH2C(O)NHS by direct end-capping (22)
1H NMR: δ 2.76-2.85 (m, 4H), 2.90-3.00 (s, 3n H), 3.20-3.45 (s, 2n H), 4.30-4.60 (m, n H), 4.70-5.00 (m, n H), 5.70-6.10 (m, 2n H) 7.30-7.50 (m, 5H). GPC: Mn = 5600, Mw/Mn = 1.02.
P(tBENI)=CHCH2CH2CH2OH by direct end-capping (23)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 3.58-3.65 (m, 2H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 7.20-7.45 (m, 5H). GPC: Mn = 6600, Mw/Mn = 1.06.
P(tBENI)=CHCH2CH2CH2Br by direct end-capping (24)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 3.42-3.52 (m, 2H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 7.20-7.45 (m, 5H). GPC: Mn = 7400, Mw/Mn = 1.04.
P(NMONI)=CHCH2CH2CH2SAc by direct end-capping (25)
1H NMR: δ 1.68-1.75 (m, 2H), 2.33 (s, 3H), 2.90-3.00 (s, 3n H), 3.20-3.45 (s, 2n H), 4.30-4.60 (m, n H), 4.70-5.00 (m, n H), 5.70-6.10 (m, 2n H) 7.30-7.50 (m, 5H). GPC: Mn = 5200, Mw/Mn = 1.03.
P(tBENI)=CHCH2O-p-CH2CH2NHBoc-C6H4 by direct end-capping (26)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 6.91-6.96 (m, 2H), 7.20-7.45 (m, 5H), 7.12-7.17 (m, 2H). GPC: Mn = 5400, Mw/Mn = 1.06.
P(tBENI)=CHCH2O-p-CH2CH2NHbiotin-C6H4 by direct end-capping (27)
1H NMR (DMSO-d6): δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 4.27 (t, J = 3.6 Hz, 1H), 4.45 (s, 2H), 5.40-5.80 (d, 2n H), 6.31 (s, 1H), 6.38 (s, 1H), 6.82 (m, 2H), 7.07 (m, 2H), 7.20-7.45 (m, 5H), 7.78 (s, 1H). GPC: Mn = 8600, Mw/Mn = 1.01.
P(tBENI)=CHCH2O-p-CH2CH2NHFITC-C6H4 by direct end-capping (28)
1H NMR (DMSO-d6): δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 6.48-6.80 (m, 10H), 6.85-7.00 (m, 2H), 7.10-8.30 (m, 8H). Note that because the peaks from the initiator-derived phenyl end group overlapped with the peaks of the terminating agent, the value for the end-functionalization efficiency in Table 1 was determined by comparing the initial monomer/catalyst ratio to the integral ratio of the backbone olefin protons at 5.40-5.80 ppm to the protons derived from the TA at 6.85-7.00 ppm. GPC: Mn = 10000, Mw/Mn = 1.06.
P(CMNI)=CHCH2O-p-CH2CH2NH2-C6H4 (29)
Polymer 26 (37.5 mg, 1 equiv) was dissolved in CH2Cl2 (1 mL), and trifluoroacetic acid (105 μL, 10 equiv relative to OtBu and NHBoc groups) was added. The vial was capped with a septum with a needle through it, and the reaction mixture was allowed to stir at room temperature for 2 days. The reaction mixture was then precipitated into a large volume of Et2O/hexanes (1:1), and the polymer product was recovered by filtration (29.2 mg). 1H NMR (DMSO-d6): δ 1.40-1.60 (s, n H), 1.90-2.05 (s, n H), 2.60-2.70 (s, n H), 2.76 (m, 2H), 2.90-3.10 (m, 3n H), 3.90-4.05 (s, 2n H), 5.35-5.70 (m, 2n H), 6.88 (m, 2H), 7.13 (m, 2H), 7.18-7.42 (m, 5H), 12.80-13.20 (s, n H).
P(tBENI)=CH2 (30)
To a stirring solution of monomer 4 (507 mg, 25 equiv) in CH2Cl2 (5 mL) under argon was added catalyst 1 (53.2 mg, 1 equiv) as a solution in CH2Cl2 (0.3 mL) quickly via syringe. The reaction mixture was allowed to stir under argon for 3 min, at which point ethyl vinyl ether (1 mL) was added. After an additional 10 min, the reaction mixture was precipitated into Et2O/hexanes (1:1) (200 mL). Polymer 19 was recovered by filtration as a light brown powder in 93% yield (480 mg). 1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 5.05-5.25 (m, 2H), 5.40-5.80 (d, 2n H) 7.20-7.45 (m, 5H). GPC: Mn = 7500, Mw/Mn = 1.02.
Typical synthesis of monotelechelic poly(oxa)norbornenes using CM
Methylene-terminated polymer 19 or 20 (0.00159 mmol, 1 equiv) was dissolved in toluene (0.3 mL) in a septum-capped vial under argon. TA (0.00795 mmol, 5 equiv) was added as a solution in toluene or MeOH (for TAs 17 and 18) (0.1 mL), and catalyst 3 (0.000795 mmol, 0.5 equiv) was added as a solution in toluene (0.1 mL). The vial was sealed under argon and allowed to stir for 12 h at 40 °C (or 60 °C for TAs 15, 17 and 18). Products were recovered using the same methods described for the direct end-capping procedure. Larger scale CM reactions (0.03 mmol in polymer) showed similar results.
P(tBENI)=CHCH2OAc by CM (31)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 4.51-4.58 (m, 2H), 5.40-5.80 (d, 2n H), 7.20-7.45 (m, 5H). GPC: Mn = 7500, Mw/Mn = 1.03.
P(tBENI)=CHCH2OC(O)C(CH3)2Br (32)
1H NMR: δ 1.20-1.80 (m, 10n H), 1.91 (s, 6H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 4.65-4.75 (m, 2H), 5.40-5.80 (d, 2n H) 7.20-7.45 (m, 5H). GPC: Mn = 7300, Mw/Mn = 1.04.
P(tBENI)=CHCH2CH2C(O)H by CM (33)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 7.20-7.45 (m, 5H), 9.78 (s, 1H). GPC: Mn = 7200, Mw/Mn = 1.04.
P(tBENI)=CHCH2CH2C(O)NHS by CM (34)
To minimize overlapping peaks in the NMR spectrum, the tert-butyl ester group was removed by treatment with trifluoroacetic acid as in polymer 29. The product was characterized as P(CMNI)=CHCH2CH2C(O)NHS. 1H NMR(DMSO-d6): δ 1.40-1.60 (s, n H), 1.90-2.05 (s, n H), 2.60-2.70 (s, n H), 2.80 (s, 2H), 2.90-3.10 (m, 3n H), 3.90-4.05 (s, 2n H), 5.35-5.70 (m, 2n H), 6.88 (m, 2H), 7.13 (m, 2H), 7.18-7.42 (m, 5H), 12.80-13.20 (s, n H). GPC was not performed due to low solubility in THF.
P(tBENI)=CHCH2CH2CH2OH by CM (35)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 3.58-3.65 (m, 2H), 5.40-5.80 (d, 2n H), 7.20-7.45 (m, 5H). GPC: Mn = 7100, Mw/Mn = 1.08.
P(tBENI)=CHCH2CH2CH2Br by CM (36)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 3.42-3.52 (m, 2H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 7.20-7.45 (m, 5H). GPC: Mn = 7600, Mw/Mn = 1.03.
P(tBENI)=CHCH2CH2CH2SAc by CM (37)
To minimize overlapping peaks in the NMR spectrum, the tert-butyl ester group was removed by treatment with trifluoroacetic acid as in polymer 29. The product was characterized as P(CMNI)=CHCH2CH2CH2SAc. 1H NMR(DMSO-d6): δ 1.40-1.60 (s, n H), 1.90-2.05 (s, n H), 2.32 (s, 3H) 2.60-2.70 (s, n H), 2.82 (m, 2H), 2.90-3.10 (m, 3n H), 3.90-4.05 (s, 2n H), 5.35-5.70 (m, 2n H), 6.88 (m, 2H), 7.13 (m, 2H), 7.18-7.42 (m, 5H), 12.80-13.20 (s, n H). GPC was not performed due to low solubility in THF.
P(tBENI)=CHCH2O-p-CH2CH2NHBoc-C6H4 by CM (38)
1H NMR: δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 6.91-6.96 (m, 2H), 7.20-7.45 (m, 5H), 7.12-7.17 (m, 2H). GPC: Mn = 6900, Mw/Mn = 1.04.
P(tBENI)=CHCH2O-p-CH2CH2NHbiotin-C6H4 by CM (39)
1H NMR (DMSO-d6): δ 1.20-1.80 (m, 10n H), 2.00-2.30 (m, n H), 2.70-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 4.27 (t, J = 3.6 Hz, 1H), 4.45 (s, 2H), 5.40-5.80 (d, 2n H), 6.31 (s, 1H), 6.38 (s, 1H), 6.82 (m, 2H), 7.07 (m, 2H), 7.20-7.45 (m, 5H), 7.78 (s, 1H). GPC: Mn = 8000, Mw/Mn = 1.02.
P(tBENI)=CHCH2O-p-CH2CH2NHFITC-C6H4 by CM (40)
1H NMR (DMSO-d6): δ 1.20-1.80 (m, 10n H), 1.90-2.10 (m, n H), 2.60-3.40 (m, 4n H), 4.00-4.20 (br s, 2n H), 5.40-5.80 (d, 2n H), 6.48-6.80 (m, 10H), 6.85-7.00 (m, 2H), 7.10-8.30 (m, 8H). Note that because the peaks from the initiator-derived phenyl end group overlapped with the peaks of the terminating agent, the value for the end-functionalization efficiency in Table 1 was determined by comparing the initial monomer/catalyst ratio to the integral ratio of the backbone olefin protons at 5.40-5.80 ppm to the protons derived from the TA at 6.85-7.00 ppm. GPC: Mn = 10000, Mw/Mn = 1.06.
Scheme 1.
Synthesis of Several Functionalized Internal cis-Olefins for Poly(oxa)norbornene End-Functionalization.a
aReagents and conditions: (i) I(O)(OH)5, p-dioxane/H2O, 0 °C to rt; (ii) Jones reagent, acetone, 0 °C to rt; (iii) N-hydroxysuccinimide, EDC, DPTS, CH2Cl2, rt; (iv) NaBH4, Et2O/EtOH, 0 °C to rt; (v) CBr4, PPh3, CH2Cl2, 0 °C to rt; (vi) KSAc, DMF, 65 °C.
Acknowledgement
We thank Materia for catalyst as well as the National Science Foundation (CHE-0809418), the National Institutes of Health (R01 GM31332) and the National Cancer Institute (5U54 CA119347) for funding. Yan Xia is acknowledged for a generous donation of TA 7. We also thank Paul G. Clark and Dr. David VanderVelde for NMR assistance.
References
- 1.(a) Grubbs RH. Handbook of Metathesis. Vol. 3. Wiley-VCH; Weinheim: 2003. [Google Scholar]; (b) Bielawski CW, Grubbs RH. Prog. Polym. Sci. 2007;32:1–29. [Google Scholar]
- 2.(a) Bertin PA, Watson KJ, Nguyen ST. Macromolecules. 2004;37:8364–8372. [Google Scholar]; (b) South CR, Burd C, Weck M. Acct. Chem. Res. 2007;40:63–74. doi: 10.1021/ar0500160. [DOI] [PubMed] [Google Scholar]; (c) Kluger C, Binder WH. J. Poly. Sci., Part A: Polym. Chem. 2007;45:485–499. [Google Scholar]; (d) Matson JB, Grubbs RH. J. Am. Chem. Soc. 2008;131:6731–6733. doi: 10.1021/ja802010d. [DOI] [PubMed] [Google Scholar]; (e) Kolonko EM, Pontrello JK, Mangold SL, Kiessling LL. J. Am. Chem. Soc. 2009;131:7327–7333. doi: 10.1021/ja809284s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Gorodetskaya IA, Choi TL, Grubbs RH. J. Am. Chem. Soc. 2007;129:12672–12673. doi: 10.1021/ja0759040. [DOI] [PubMed] [Google Scholar]; (b) Gorodetskaya IA, Gorodetsky AA, Vinogradova EV, Grubbs RH. Macromolecules. 2009;42:2895–2898. [Google Scholar]
- 4.(a) Helms B, Mynar JL, Hawker CJ, Fréchet JMJ. J. Am. Chem. Soc. 2004;126:15020–15021. doi: 10.1021/ja044744e. [DOI] [PubMed] [Google Scholar]; (b) Boydston AJ, Holcombe TW, Unruh DA, Fréchet JMJ, Grubbs RH. J. Am. Chem. Soc. 2009;131:5388–5389. doi: 10.1021/ja901658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia Y, Kornfield JA, Grubbs RH. Macromolecules. 2009;42:3761–3766. [Google Scholar]
- 6.(a) Boydston AJ, Xia Y, Kornfield JA, Gorodetskaya IA, Grubbs RH. J. Am. Chem. Soc. 2008;130:12775–12882. doi: 10.1021/ja8037849. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xia Y, Boydston AJ, Yao Y, Kornfield JA, Gorodetskaya IA, Spiess HW, Grubbs RH. J. Am. Chem. Soc. 2009;131:2670–2677. doi: 10.1021/ja808296a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trnka TM, Grubbs RH. Acc. Chem. Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 8.For recent reviews see: Lo Verso F, Likos CN. Polymer. 2008;49:1425–1434. Hadjichristidis N, Pitsikalis M, Pispas S, Iatrou H. Chem. Rev. 2001;101:3747–3792. doi: 10.1021/cr9901337. Hatada K, Kitayama T. Polym. Int. 2000;49:11–47. For a review on end-capping of ROMP polymers see Hilf S, Kilbinger AFM. Nat. Chem. 2009;1:537–546. doi: 10.1038/nchem.347.
- 9.Yagci Y, Tasdelen MA. Prog. Polym. Sci. 2006;31:1133–1170. [Google Scholar]
- 10.(a) Chen B, Metera K, Sleiman HF. Macromolecules. 2005;38:1084–1090. [Google Scholar]; (b) Boyer C, Liu J, Bulmus V, Davis TP, Barner-Kowollik C, Stenzel MH. Macromolecules. 2008;41:5641–5650. [Google Scholar]
- 11.(a) Laurent BA, Grayson SA. J. Am. Chem. Soc. 2006;128:4238–4239. doi: 10.1021/ja0585836. [DOI] [PubMed] [Google Scholar]; (b) Eugene DM, Grayson SM. Macromolecules. 2008;41:5082–5084. [Google Scholar]; (c) Adachi K, Honda S, Hayaski S, Tezuka Y. Macromolecules. 2008;41:7898–7903. [Google Scholar]; (d) Lepoittevin B, Dourges MA, Masure M, Hemery P, Baran K, Cramail H. Macromolecules. 2000;33:8218–8224. [Google Scholar]
- 12.(a) Amass AJ, Bas S, Gregory D, Mathew MC. Makromol. Chem. 1985;186:325–330. [Google Scholar]; (b) Castle TC, Hutchings LR, Khosravi E. Macromolecules. 2004;37:2035–2040. [Google Scholar]; (c) Tritto I, Sacchi MC, Grubbs RH. J. Mol. Catal. 1993;82:103–111. [Google Scholar]; (d) Coca S, Paik HJ, Matyjaszewski K. Macromolecules. 1997;30:6513–6516. [Google Scholar]; (e) Li MH, Keller P, Albouy PA. Macromolecules. 2003;36:2284–2292. [Google Scholar]; (f) Matson JB, Grubbs RH. Macromolecules. 2008;41:5626–5631. [Google Scholar]
- 13.Burtscher D, Saf R, Slugovc C. J. Poly. Sci., Part A: Polym. Chem. 2006;44:6136–6145. [Google Scholar]
- 14.Hilf S, Kilbinger AFM. Macromol. Rapid Commun. 2007;28:1225–1230. [Google Scholar]
- 15.(a) Cramail H, Fontanille M, Soum A. J. Mol. Catal. 1991;65:193–203. [Google Scholar]; (b) Chung TC, Chasmawala M. Macromolecules. 1991;24:3718–3720. [Google Scholar]; (c) France MB, Grubbs RH, McGrath DV, Paciello RA. Macromolecules. 1993;26:4742–4747. [Google Scholar]; (d) Hillmyer MA, Grubbs RH. Macromolecules. 1993;26:872–874. [Google Scholar]; (e) Maughon BR, Morita T, Bielawski CW, Grubbs RH. Macromolecules. 2000;33:1929–1935. [Google Scholar]
- 16.(a) Katayama H, Urushima H, Ozawa F. J. Organomet. Chem. 2000;1:16–25. [Google Scholar]; (b) Bielawski CW, Louie J, Grubbs RH. J. Am. Chem. Soc. 2000;122:12872–12873. [Google Scholar]; (c) Ambade AV, Yang SK, Weck M. Angew. Chem., Int. Ed. 2009;48:2894–2898. doi: 10.1002/anie.200805116. [DOI] [PubMed] [Google Scholar]
- 17.(a) Katayama H, Fukuse Y, Nobuto Y, Akamatsu K, Ozawa F. Macromolecules. 2003;36:7020–7026. [Google Scholar]; (b) Owen RM, Gestwicki JE, Young T, Kiessling LL. Org. Lett. 2002;4:2293–2296. doi: 10.1021/ol0259239. [DOI] [PubMed] [Google Scholar]; (c) Kolonko EM, Kiessling LL. J. Am. Chem. Soc. 2008;130:5626–5627. doi: 10.1021/ja8001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lexer C, Saf R, Slugovc C. J. Poly. Sci., Part A: Polym. Chem. 2009;47:299–305. [Google Scholar]
- 19.Hilf S, Grubbs RH, Kilbinger AFM. J. Am. Chem. Soc. 2008;130:11040–11048. doi: 10.1021/ja8022863. [DOI] [PubMed] [Google Scholar]
- 20.Murphy JJ, Kawasaki T, Fujiki M, Nomura K. Macromolecules. 2005;38:1075–1083. [Google Scholar]
- 21.(a) Hilf S, Berger-Nicoletti E, Grubbs RH, Kilbinger AFM. Angew. Chem., Int. Ed. 2006;45:8045–8048. doi: 10.1002/anie.200602323. [DOI] [PubMed] [Google Scholar]; (b) Hilf S, Kilbinger AFM. Macromol. Rapid Comm. 2007;28:1225–1230. [Google Scholar]; (c) Hilf S, Hanik N, Kilbinger AFM. J. Poly. Sci., Part A: Polym. Chem. 2008;46:2913–2921. [Google Scholar]; (d) Hilf S, Kilbinger AFM. Macromolecules. 2009;42:1099–1106. [Google Scholar]
- 22.Matson JB, Virgil SC, Grubbs RH. J. Am. Chem. Soc. 2009;131:3355–3362. doi: 10.1021/ja809081h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Choi TL, Grubbs RH. Angew. Chem., Int. Ed. 2003;42:1743–1746. doi: 10.1002/anie.200250632. [DOI] [PubMed] [Google Scholar]; (b) Camm KD, Castro NM, Liu Y, Czechura P, Snelgrove JL, Fogg DE. J. Am. Chem. Soc. 2007;129:4168–4169. doi: 10.1021/ja071047o. [DOI] [PubMed] [Google Scholar]; (c) Kratz K, Breitenkamp K, Hule R, Pochan D, Emrick T. Macromolecules. 2009;42:3227–3229. [Google Scholar]; (d) Colak S, Tew GN. Macromolecules. 2008;41:8436–8440. [Google Scholar]
- 24.Rawat M, Gama CI, Matson JB, Hsieh-Wilson LC. J. Am. Chem. Soc. 2008;130:2959–2961. doi: 10.1021/ja709993p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breitenkamp RB, Ou Z, Breitenkamp K, Muthukumar M, Emrick T. Macromolecules. 2007;40:7617–7624. [Google Scholar]
- 26.Eren T, Som A, Rennie JR, Nelson CF, Urgina Y, Nüsslein K, Coughlin EB, Tew GN. Macromol. Chem. Phys. 2008;209:516–524. [Google Scholar]
- 27.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 28.Raederstorff D, Shu AYL, Thompson JE, Djerassi C. J. Org. Chem. 1987;52:2337–2346. [Google Scholar]
- 29.Dag A, Durmaz H, Sirkecioglu O, Hizal G, Tunca U. J. Poly. Sci., Part A: Polym. Chem. 2009;47:2344–2351. [Google Scholar]
- 30.Blackwell HE, O'Leary DJ, Chatterjee AK, Washenfelder RA, Bussmann DA, Grubbs RH. J. Am. Chem. Soc. 2000;122:58–71. [Google Scholar]
- 31.Ulman M, Grubbs RH. Organometallics. 1998;17:2484–2489. [Google Scholar]
- 32.Bielawski CW, Benitez D, Morita T, Grubbs RH. Macromolecules. 2001;34:8610–8618. [Google Scholar]
- 33.Pangborn AB, Giardello MA, Grubbs RH, Rosen RK, Timmers FJ. Organometallics. 1996;15:1518–1520. [Google Scholar]
- 34.Love JA, Morgan JP, Trnka TA, Grubbs RH. Angew. Chem., Int. Ed. 2002;41:4035–4037. doi: 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 35.Moore JS, Stupp SI. Macromolecules. 1990;23:65–70. [Google Scholar]