Abstract
Background
Although pulmonary valve replacement (PVR) is effective in reducing right ventricular (RV) volume overload in patients with chronic pulmonary regurgitation (PR), persistent RV dysfunction and subsequent adverse clinical outcomes have been reported. This trial was conducted to investigate whether the addition of surgical RV remodeling with exclusion of scar tissue to PVR would result in improved RV function and laboratory and clinical parameters, as compared with PVR alone.
Methods and Results
Between February 2004 and October 2008, 64 patients who underwent RV outflow tract procedures in early childhood, had ≥moderate PR, and fulfilled defined criteria for PVR were randomly assigned to undergo either PVR alone (n = 34) or PVR with surgical RV remodeling (n = 30). No significant difference was observed in the primary outcome (change in RV ejection fraction: -2±7% in the PVR alone group and -1±7% in the PVR with RV remodeling group, P = 0.38) or in any of the secondary outcomes at 6-month postoperative follow-up. Multivariable analysis of the entire cohort identified preoperative RV end-systolic volume index <90 ml/m2 and QRS duration <140 ms to be associated with optimal postoperative outcome (normal RV size and function), and RV ejection fraction <45% and QRS duration ≥160 ms to be associated with suboptimal postoperative outcome (RV dilatation and dysfunction).
Conclusion
The addition of surgical remodeling of the RV to PVR in patients with chronic PR did not result in a measurable early benefit. Referral to PVR based on QRS duration, RV end-systolic volume, or RV ejection fraction may be beneficial.
Keywords: tetralogy of Fallot; heart defect, congenital; remodeling; surgery; magnetic resonance imaging
Severe pulmonary regurgitation (PR) is common in patients with repaired tetralogy of Fallot (TOF) and other congenital heart disease requiring pulmonary valvotomy or outflow reconstruction. The resultant chronic right ventricular (RV) volume overload, as well as myocardial fibrosis and regional wall motion abnormalities, lead to RV dilatation, biventricular dysfunction, heart failure symptoms, arrhythmias, and death.1-5 Pulmonary valve replacement (PVR) is highly effective in eliminating or greatly reducing PR and leads to a substantial decrease and, in some patients, normalization of RV end-diastolic volume.6, 7 However, recovery of RV dysfunction after successful PVR has been inconsistent, with most reports demonstrating either no change or even decline in some patients.8-11 Based on the observation that residual scar tissue often persists in the RV after standard PVR and the experience in adult patients with left ventricular (LV) aneurysms, in whom surgical remodeling with aneurysm resection has been shown to improve LV mechanics,12 it has been suggested that the addition of RV remodeling to PVR will results in improved RV mechanics.13 However, no studies have systematically compared the efficacy of PVR alone to PVR with surgical RV remodeling. The goal of this prospective randomized clinical trial was to compare the effects of two surgical strategies—PVR alone (standard treatment) versus PVR with surgical RV remodeling—on RV mechanics and the incidence of adverse events in patients with repaired TOF or similar physiology.
Methods
Study Design
We conducted a single center, non-blinded, randomized prospective clinical trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. The trial protocol was approved by a Data and Safety Monitoring Board (DSMB) appointed by the NHLBI and by the Children's Hospital Boston Committee on Clinical Investigation. Informed, written consent was obtained from all patients or legal guardians. Oversight of the trial was provided by the DSMB, whose members were unaware of the study group assignments.
Subjects and Randomization
All patients older than 10 years of age with repaired TOF or similar physiology presenting to Children's Hospital Boston for PVR from February 2004 through October 2008 were eligible for enrollment if they had chronic pulmonary regurgitation measured by cardiac magnetic resonance (CMR) (PR fraction ≥25%) and ≥2 of the following criteria: 1) RV end-diastolic volume index (RVEDVi) ≥160 ml/m2; 2) RV end-systolic volume index (RVESVi) ≥70 ml/m2; 3) RV ejection fraction (EF) ≤45%; 4) LVEDVi ≤65 ml/m2; 5) RV outflow aneurysm (defined as focal dilatation with akinetic or dyskinetic wall motion); or 6) clinical criteria (e.g., exercise intolerance, symptoms and signs of heart failure, or cardiac medications). Exclusion criteria included any of the following: 1) severe RV outflow tract obstruction (defined as peak systolic ejection gradient ≥60 mm Hg by cardiac catheterization); 2) severe RV hypertension at systemic or higher level; 3) additional sources of RV volume overload other then pulmonary or tricuspid valve regurgitation (e.g., partially anomalous pulmonary venous connection); and 4) contraindications to pre-operative CMR.
All patients underwent pre-operative diagnostic cardiac imaging according to institutional clinical practice. After determination of eligibility, the patient's cardiologist and cardiovascular surgeon were asked for permission to seek enrollment in the trial. Following informed consent, patients were enrolled and randomized to undergo either PVR alone (standard treatment) or PVR with surgical RV remodeling. Randomization was performed with a computerized random number generator (Harvard School of Public Health) in permuted blocks and stratified by surgeon. Treatment crossover after randomization based on the surgeon's intraoperative judgment was allowed, but primary analyses were performed on an intention-to-treat basis. Data collection began at the time of enrollment and continued prospectively through the pre-operative evaluation, operative and postoperative hospitalization, and clinical assessment 6 months after surgery.
Surgical Procedure
The techniques for PVR alone and for PVR with RV remodeling have been described in detail previously.13 Briefly, surgery was performed through median sternotomy on cardiopulmonary bypass with mild hypothermia (core temperature 30-34° C) with the heart beating, except for cases with an interatrial communication. Once cardiopulmonary bypass was established, a longitudinal incision was made parallel to the axis of the RV outflow tract, extending across the RV infundibulum to the proximal main pulmonary artery. When present, aneurysms of the outflow patch were resected (considered a standard part of PVR regardless of treatment group assignment). A bioprosthetic valve (gluteraldehyde preserved bovine pericardial valve mounted on a rigid sewing ring; Edwards Lifesciences, Irvine, CA or Sorin Mitroflow, Milan, Italy) was then selected based on the predicted pulmonary annulus size for body surface area. The valve was attached to the RV outflow-pulmonary artery junction by interrupted or continuous non-absorbable sutures placed circumferentially around the sewing ring. Once the valve was fixed in place, the ventriculotomy was closed with a continuous monofilament suture reinforced with pledgets or felt strips to prevent tearing through the RV tissue. Additional surgical procedures (e.g., tricuspid valvuloplasty, Maze procedure) were performed as clinically indicated.
In patients assigned to PVR with RV remodeling, contiguous regions of transmural scar in the RV anterior wall and infundibulum apart from the previous patch were identified by visual inspection and palpation. These regions were characterized by their thin wall, which, in contrast to viable myocardium, collapsed during diastole. The right ventriculotomy was then extended through the scar tissue, confirming its presence until viable myocardium was encountered. Once the bioprosthetic valve was sutured in the RV outflow-pulmonary artery junction using the same technique as in patients with PVR alone, scar tissue was resected and the extended right ventriculotomy was closed using plication to exclude any remaining scar tissue.
Patient Evaluation
The preoperative and 6-month postoperative evaluations included a detailed history and physical examination with emphasis on symptoms and signs of heart failure, a standard 12-lead ECG and a signal average ECG, a 24-hour Holter monitor, an exercise test comprised of symptom-limited progressive bicycle ergometry with a metabolic cart, a transthoracic echocardiogram, a CMR study, and a questionnaire-based assessment of physical and mental status using the SF-36 Physical and Mental Health Summary Scale or the Child Health Questionnaire (CHQ-PF50), depending on age and developmental status. Cardiac catheterization was not a requirement, though data was recorded if it was performed for clinical indications. Intraoperative data included details of the surgical procedure, length of surgery (time from skin incision to skin closure), cardiopulmonary bypass time, cross-clamp time, length of RV incision, and, in the PVR with RV remodeling group, length and width of RV remodeling. Intra- and postoperative adverse events were recorded as well as time on mechanical ventilation, length of stay in the intensive care unit, and length of stay in the hospital. Pre-discharge tests included a 12-lead ECG, a 24-hour Holter monitor, and an echocardiogram. Details of the CMR study protocol and analytical techniques utilized in our laboratory for assessment of patients with repaired TOF have been published previously,3, 14, 15 as was the exercise study protocol.16
Outcomes
The primary outcome was change in RV EF measured by CMR 6 months postoperatively compared with the preoperative RV EF. Secondary outcomes were changes in the following 3 categories 6 months postoperatively compared with the preoperative values: 1) measures of RV and LV mechanics evaluated by CMR, including EDVi, ESVi, ventricular mass index, and mass-to-volume ratio; 2) clinical status score measured by the SF-36 or CHQ-PF50 questionnaires; and 3) laboratory measures of exercise capacity and anaerobic threshold, pulmonary function, QRS duration, QT dispersion, ventricular ectopy, T-wave alternans, and heart rate turbulence on Holter. In addition, we compared the treatment groups in terms of incidence and severity of adverse events.
To further evaluate the effects of PVR with and without RV remodeling on outcomes at 6 months after surgery, we analyzed the associations between preoperative and intraoperative variables and 2 outcome categories—optimal and suboptimal postoperative status. Optimal outcome was defined as normal RV size (end-diastolic volume index ≤114 ml/m2) and function (ejection fraction ≥48%). Suboptimal outcome was defined as RV dilatation (end-diastolic volume index ≥120 ml/m2) and dysfunction (ejection fraction ≤45%).
Statistical Analysis
Sample size calculations were based on a two-sided, two-sample comparison of the primary outcome variable, change in RV ejection fraction. Assuming a standard deviation of 6%,17 60 patients (30 per treatment group) would provide 90% power to detect a mean difference of 5% in change in RV ejection fraction.
All treatment group comparisons were performed on an intention-to-treat basis. Patient and clinical characteristics and surgical variables were compared to ensure comparability of the groups at baseline. Fisher's exact test was used for categorical variables, the Wilcoxon rank sum test for ordinal variables and for continuous variables that were not normally distributed, and the two-sample t test for normally distributed continuous variables. Changes in outcome variables from baseline to 6 months after PVR were compared using the two-sample t test or the Wilcoxon rank sum test as appropriate. Comparisons were first made for only those patients who returned for the 6 month follow-up visit, and then carrying forward the baseline values (implying no change) of those who failed to return. If treatment group differences were not found, the groups were combined to evaluate changes over time using the paired t test or the Wilcoxon signed-rank test. Logistic regression analysis was used to examine the relationships between baseline patient and clinical characteristics and optimal and suboptimal status at 6-month follow-up for the two treatment groups combined. Receiver-operator characteristic curves were used to identify relevant cutoff values for selected continuous predictor variables.
Results
Patients
Between February 1, 2004, and October 15, 2008, 139 patients referred for PVR at our institution were screened for study eligibility, of whom 82 (59%) met inclusion criteria. Of this group, 17 (21%) declined enrollment and 1 (1%) withdrew after initial consent, leaving 64 patients (78%) who enrolled, were randomized, and underwent PVR according to study protocol. Of the study group, 61 patients (95%) completed the 6-month postoperative evaluation. Selected preoperative features of 10 of these patients were included in the study of Wald et al.3 Table 1 summarizes the demographic and preoperative characteristics of the study patients. Demographic and preoperative characteristics were similar among patients who enrolled in the trial and those who declined participation. Associated cardiovascular anomalies included right aortic arch in 11 patients (17%), branch pulmonary artery stenosis in 10 (16%), patent foramen ovale or secundum atrial septal defect in 6 (9%), left superior vena cava to coronary sinus in 2 (3%), and anomalous coronary artery origin in 2 (3%).
Table 1. Demographic and Clinical Characteristics of the Patients.
| Variable | All Patients (N = 64) | PVR Alone (N = 34) | PVR with RV Remodeling (N = 30) | P Value |
|---|---|---|---|---|
| Gender (male:female) | 2.2 | 2.8 | 1.7 | 0.43 |
| Race, n (%) | 0.19 | |||
| White | 54 (84) | 31 (91) | 23 (77) | |
| Black | 5 (8) | 1 (3) | 4 (13) | |
| Hispanic and other | 5 (8) | 2 (6) | 3 (10) | |
| Median age at enrollment (years) | 21 (11 - 58) | 20 (11 - 57) | 21 (12 - 58) | 0.80 |
| Median age at initial RVOT surgery* (years) | 1 (0 - 18) | 1 (0 - 16) | 1 (0 - 18) | 0.36 |
| Median time from RVOT surgery* to PVR (years) | 20 (11 - 47) | 19 (11 - 47) | 20 (11 - 45) | 0.98 |
| Body surface area at time of PVR (m2) | 1.68 ± 0.34 | 1.70 ± 0.34 | 1.66 ± 0.34 | 0.62 |
| Diagnosis, n (%) | 0.42 | |||
| Tetralogy of Fallot** | 51 (80) | 28 (82) | 23 (77) | |
| Pulmonary stenosis or atresia with IVS | 5 (8) | 2 (6) | 3 (10) | |
| Truncus arteriosus | 2 (3) | 2 (6) | 0 (0) | |
| Other | 6 (9) | 2 (6) | 4 (13) | |
| Median number of previous cardiac surgical procedures | 2 (1 - 7) | 2 (1 - 7) | 1 (1 - 7) | 0.06 |
| Cardiac symptoms, n (%) | 44 (69) | 22 (65) | 22 (73) | 0.59 |
| Cardiac medications, n (%) | 27 (42) | 11 (32) | 16 (53) | 0.13 |
| NYHA class, n (%)1 | 0.96 | |||
| I | 34 (54) | 18 (55) | 16 (53) | |
| II | 23 (37) | 12 (36) | 11 (37) | |
| III | 5 (8) | 2 (6) | 3 (10) | |
| IV | 1 (2) | 1 (3) | 0 (0) |
Data presented as median (range) or mean ± SD, as appropriate
RVOT surgery= right ventricular outflow tract procedure resulting in pulmonary regurgitation
Associated pulmonary atresia was present in 8 patients, absent pulmonary valve in 3, and common atrioventricular canal in 3
NYHA class missing for one patient in the PVR Alone group.
IVS= intact ventricular septum; PA= pulmonary artery; PVR= pulmonary valve replacement; RV= right ventricle; RVOT= right ventricular outflow tract
Surgical Procedures
Of the 34 patients who were assigned to undergo PVR alone, 33 (97%) underwent the assigned operation and 1 crossed over to PVR with RV remodeling due to a large, calcified scar. Of the 30 patients who were assigned to undergo PVR with RV remodeling, 28 (93%) underwent the assigned operation and 2 crossed over to PVR alone due to lack of resectable scar tissue.
The treatment groups were similar in age at surgery, duration of operation, total cardiopulmonary bypass time, aortic cross clamp time, lowest core temperature, size of the bioprosthetic pulmonary valve, and use of RV outflow patch (Table 2). The extent of RV plication in the PVR with RV remodeling group is detailed in Table 2. The frequency of associated procedures was also similar among the groups.
Table 2. Surgical and Postoperative Data.
| Variable | All Patients (N = 64) | PVR Alone (N = 34) | PVR with RV Remodeling (N = 30) | P Value |
|---|---|---|---|---|
| Median age at PVR (years) | 21 (11 - 58) | 20 (11 - 57) | 21 (12 - 58) | 0.80 |
| Surgeon, n (%) | 1.0 | |||
| A | 25 (39) | 13 (38) | 12 (40) | |
| B | 17 (27) | 9 (26) | 8 (27) | |
| C | 20 (31) | 11 (32) | 9 (30) | |
| D | 2 (3) | 1 (3) | 1 (3) | |
| Size of bioprosthetic pulmonary valve (mm) | 27 (21 - 29) | 27 (21 - 29) | 27 (25 - 29) | 0.38 |
| Length of operation (minutes) | 254 ± 70 | 245 ± 75 | 264 ± 65 | 0.28 |
| Total cardiopulmonary bypass time (minutes) | 103 ± 35 | 101 ± 37 | 105 ± 35 | 0.63 |
| Aortic cross-clamp time (minutes) | 27 ± 30 | 25 (0 - 103) | 25 (0 - 90) | 0.75 |
| Lowest core temperature (°C) | 30.6 ± 2.4 | 30.4 ± 2.9 | 30.8 ± 1.7 | 0.46 |
| Use of RVOT patch, n (%) | 29 (45) | 14 (41) | 15 (50) | 0.62 |
| Length of RVOT patch (cm) | 4 (2 - 12) | 5 (2 - 12) | 4 (2 - 12) | 0.66 |
| Width of RVOT patch (cm) | 2.5 (1 - 5) | 2.7 (1 - 4) | 2.5 (1.5 - 5) | 0.67 |
| Area of RVOT patch (cm2) | 10 (3 - 60) | 12 (3 - 48) | 9 (3 - 60) | 0.56 |
| Extent of RV plication | ||||
| Length (cm) | — | — | 6 (1.5 - 10) | |
| Width (cm) | — | — | 3 (1 - 5.5) | |
| Area (cm2) | — | — | 21 (1.5 - 41.3) | |
| Additional procedures, n (%) | ||||
| Closure of PFO or ASD | 19 (30) | 10 (29) | 9 (30) | 1.0 |
| Pulmonary artery plasty | 9 (14) | 7 (21) | 2 (7) | 0.15 |
| Tricuspid annuloplasty | 12 (19) | 4 (12) | 8 (27) | 0.2 |
| Cryoablation | 5 (8) | 2 (6) | 3 (10) | 0.66 |
| Maze procedure | 7 (11) | 3 (9) | 4 (13) | 0.7 |
| Closure of VSD | 3 (5) | 1 (3) | 2 (7) | 0.60 |
| AICD | 1 (2) | 0 (0) | 1 (3) | 0.47 |
| Other procedures* | 5 (8) | 4 (12) | 1 (3) | 0.36 |
| Intraoperative complication** | 3 (5) | 1 (3) | 2 (7) | 0.6 |
| Postoperative course | ||||
| Pressor support, n (%) | 23 (36) | 14 (41) | 9 (30) | 0.44 |
| Median length of ICU stay (days) | 2 (1 - 12) | 2 (1 - 9) | 2 (1 - 12) | 0.48 |
| Median length of hospital stay (days) | 6 (4 - 16) | 6 (4 - 13) | 6 (5 - 16) | 0.22 |
Data presented as median (range) or mean ± SD, as appropriate.
Closure of aorta-to-MPA fistula (n= 1); mitral valve prolapse repair (n= 1); tracheal dilatation (n= 1); ascending aorta aneurysm repair (n= 2).
Bleeding during median sternotomy requiring emergent cardiopulmonary bypass in 1 patient and ventricular tachycardia or fibrillation requiring cardioversion in 2 patients.
AICD= automatic implantable cardioverter defibrillator; ASD= atrial septal defect; ICU= intensive care unit; PFO= patent foramen ovale; PVR= pulmonary valve replacement; RVOT= right ventricular outflow tract; VSD= ventricular septal defect
There were no deaths in this trial. Only 1 serious intraoperative complication occurred in a patient assigned to the PVR group—bleeding during median sternotomy that required emergency cannulation for cardiopulmonary bypass. The remainder of this patient's operation and the hospital course were uneventful. There were no significant differences between groups in terms of postoperative course, including rate of complications, frequency of pressor support, durations of mechanical ventilation, length of stay in the cardiac intensive care unit, or time to hospital discharge (Table 2). None of the patients in this cohort had ventricular tachycardia on Holter monitor.
Primary Outcome
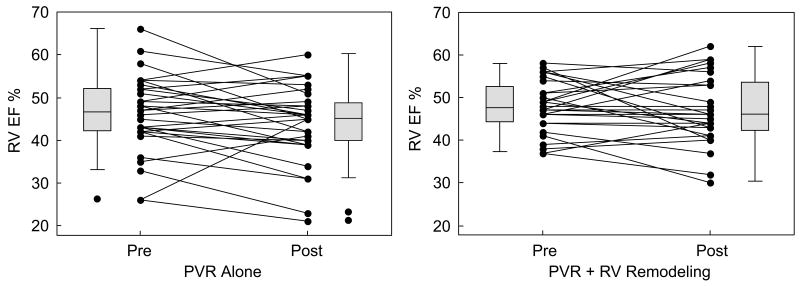
Change in RV EF 6 months postoperatively compared with the preoperative RV EF, measured by CMR, was -2±7% in the PVR alone group and -1±7% in the PVR with RV remodeling group (p = 0.38) (Table 3 and Figure 1).
Table 3. Between-Groups Comparisons of Pre- and Postoperative Findings.
| Preoperative | 6-Month Postoperative | Change from Pre- to 6-Month Postoperative | ||||
|---|---|---|---|---|---|---|
| Variable | PVR Alone (N = 34) | PVR with RV Remodeling (N = 30) | PVR Alone (N = 32) | PVR with RV Remodeling (N = 28) | PVR Alone (N = 34) | PVR with RV Remodeling (N = 30) |
| Cardiac Magnetic Resonance | ||||||
| RV ejection fraction (%) | 46 ± 9 | 48 ± 7 | 44 ± 9 | 47 ± 8 | -2 ± 7 | -1 ± 7 |
| Pulmonary regurgitation (%) | 49 ± 12 | 50 ± 9 | 4 ± 5 | 6 ± 11 | -25 ± 24 | -31 ± 22 |
| RV end-diastolic volume index (ml/m2) | 199± 34 | 202 ± 41 | 126 ± 26 | 119 ± 25 | -68 ± 37 | -78 ± 46 |
| RV end-systolic volume index (ml/m2) | 108 ± 29 | 105 ± 29 | 72 ± 26 | 64 ± 21 | -33 ± 23 | -39 ± 26 |
| RV stroke volume (ml) | 153 ± 41 | 158 ± 37 | 91 ± 21 | 95 ± 25 | -57 ± 36 | -58 ±36 |
| RV mass-to-volume ratio (g/ml) | 0.22 ± 0.05 | 0.21 ± 0.04 | 0.25 ± 0.06 | 0.25 ± 0.05 | 0.03 ± 0.06 | 0.04 ± 0.05 |
| LV end-diastolic volume index (ml/m2) | 92 ± 16 | 85 ± 15 | 95 ± 16 | 92 ± 19 | 3 ± 11 | 6 ± 12 |
| LV end-systolic volume index (ml/m2) | 40 ± 12 | 36 ± 9 | 43 ± 13 | 39 ± 11 | 3 ± 8 | 2 ± 8 |
| LV stroke volume (ml) | 87 ± 21 | 81 ± 21 | 90 ± 20 | 91 ± 25 | 3 ± 13 | 9 ± 15 |
| LV ejection fraction (%) | 57 ± 8 | 58 ± 7 | 56 ± 7 | 58 ± 7 | -1 ± 5 | 0 ± 7 |
| LV mass-to-volume ratio (g/ml) | 0.66 ± 0.11 | 0.66 ± 0.13 | 0.64 ± 0.11 | 0.61 ± 0.11 | -0.01 ± 0.10 | -0.05 ± 0.11 |
| ECG | ||||||
| QRS duration (ms) | 161 (91 - 200) | 150 (82 - 182) | 161 (80 - 202) | 142 (82 - 190) | 0 (-20 - 49) | -1 (-24 - 16) |
| QRS dispersion (ms) | 21 (9 - 54) | 22 (9 - 49) | 23 (12 - 40) | 27 (17 - 40) | 0 (-32 - 15) | 0 (-32 - 14) |
| Heart rate-corrected QT interval (ms) | 450 (301 - 514) | 455 (370 - 523) | 461 (359 - 528) | 460 (381 - 558) | 0 (-96 - 162) | 4 (-84 - 70) |
| QT dispersion (ms) | 51 (17 - 200) | 51 (12 - 171) | 45 (22 - 178) | 62 (29 - 116) | 0 (-119 - 64) | 0 (-69 - 68) |
| JT interval (ms) | 264 (204 - 418) | 285 (213 - 351) | 254 (200 - 359) | 275 (125 - 333) | 0 (-172 - 48) | 0 (-146 -56) |
| JT dispersion (ms) | 53 (13 - 195) | 55 (18 - 200) | 40 (27 - 172) | 60 (34 - 114) | 0 (-108 - 69) | 0 (-102 - 49) |
| Exercise test | ||||||
| Peak VO2 (ml/kg/min) | 27.1 (7.8 - 47.2) | 26.5 (9.8 - 41.4) | 27.0 (9.7 - 47.7) | 27.4 (10 - 43.9) | 0.0 (-8.6 - 6.1) | 0.0 (-10.6 - 8.7) |
| % predicted peak VO2 (%) | 66 (26 - 93) | 62 (42 - 101) | 69 (30 - 95) | 68 (41 - 95) | -1 (-11 - 18) | 1 (-25 - 25) |
| % predicted peak work rate | 71 (14 - 102) | 70 (34 - 106) | 77 (42 - 108) | 78 (30 - 122) | 0 (-8 - 27) | 1 (-9 - 25) |
| VAT (ml/kg/min) | 16.0 (8.0 - 28.8) | 14.5 (6.9 - 26.9) | 14.7 (7.1 - 21.9) | 15.4 (5.9 - 23.7) | 0.0 (-11.1 - 4.8) | 0.8 (-11.5 - 7.1) |
| % predicted VO2 at VAT | 39 (27 - 65) | 38 (22 - 58) | 37 (22 - 53) | 38 (23 - 56) | 0 (-24 - 14) | 1 (-23 - 18) |
| Peak heart rate (beats/min) | 165 (106 - 208) | 153 (87 - 193) | 168 (110 - 201) | 156 (100 - 194) | 0 (-31 - 35) | 1 (-67 - 103) |
| % predicted peak heart rate | 92 (65 - 111) | 83 (59 - 123) | 92 (68 - 109) | 89 (56 - 128) | 1 (-20 - 18) | 2 (-42 - 69) |
| Echocardiogram | ||||||
| ≤mild tricuspid regurgitation, % | 91 | 82 | 100 | 96 | † | ‡ |
| ≥moderate tricuspid regurgitation, % | 9 | 18 | 0 | 4 | † | ‡ |
| RV systolic pressure by tricuspid regurgitation (mm Hg) | 37 (20 - 76) | 30 (16 - 52) | 26 (20 - 57) | 25 (14 - 56) | 0 (-38 - 3) | 0 (-21 - 11) |
| RVOT gradient (mm Hg) | 20 (3 - 70) | 13 (3 - 45) | 12 (6 - 34) | 15 (6 - 40) | -2 (-60 - 24) | 0 (-12 - 8) |
| Functional status | ||||||
| SF-36 physical component | 48.7 ± 9.1 | 49.9 ± 7.3 | 53.6 ± 7.1 | 56.0 ± 2.6 | 5 ± 9 | 6 ± 6 |
| SF-36 mental component | 50.0 ± 7.7 | 49.1 ± 11.4 | 53.7 ± 6.8 | 55.4 ± 7.7 | 3 ± 6 | 6 ± 10 |
| CHQ-PF50 physical component | 43.2 ± 13.9 | 41.2 ± 14.6 | 50.4 ± 9.7 | 52.4 ± 4.6 | 7 ± 11 | 10 ± 14 |
| CHQ-PF50 mental component | 49.8 ± 10.2 | 49.6 ± 7.0 | 50.0 ± 10.5 | 54.5 ± 4.5 | 0 ± 9 | 4 ± 4 |
Data presented as median (range) or mean ± SD, as appropriate. Changes from pre- to 6-month postoperative were calculated carrying forward baseline values of those who failed to return for 6-month follow-up
In the PVR alone group, 4 patients decreased from ≥ moderate regurgitation to ≤ mild regurgitation
In the PVR with RV remodeling group, 3 patients decreased from ≥ moderate regurgitation to ≤ mild regurgitation.
VAT= ventilatory anaerobic threshold; VO2= oxygen consumption
Figure 1.
Changes in RV ejection fraction in patients assigned to PVR alone and to PVR with RV remodeling.
Secondary Outcomes
There was no significant difference between the treatment groups in any of the secondary outcome variables 6 months postoperatively compared with the preoperative values (Table 3). The lack of treatment benefit persisted after controlling for surgeon, and in analyses by treatment received. In addition, the incidence and severity of adverse events was similar in the treatment groups.
Predictors of Postoperative Status
Given that postoperative outcomes were similar in both treatment groups, subsequent post-hoc analyses combined all patients. Table 4 compares the clinical, laboratory, and functional parameters before and 6 months after surgery for all patients.
Table 4. Effects of PVR on Key Outcomes in all Patients.
| Variable | Preoperative | 6-month Follow-Up | Change | P Value |
|---|---|---|---|---|
| NYHA class | ||||
| I | 34 (54) | 56 (92) | † | <0.001 |
| II | 23 (37) | 3 (5) | ||
| III | 5 (8) | 2 (3) | ||
| IV | 1 (2) | 0 (0) | ||
| Cardiac Magnetic Resonance | ||||
| RV ejection fraction (%) | 47 ± 8 | 45 ± 9 | -2 ± 7 | 0.07 |
| Pulmonary regurgitation (%) | 49 ± 11 | 5 ± 9 | -44 ± 12 | <0.001 |
| RV end-diastolic volume index (ml/m2) | 201 ± 37 | 123 ± 25 | -78 ± 38 | <0.001 |
| RV end-systolic volume index (ml/m2) | 107 ± 29 | 68 ± 24 | -39 ± 24 | <0.001 |
| RV stroke volume (ml) | 155 ± 39 | 93 ± 23 | -61 ± 33 | <0.001 |
| RV mass-to-volume ratio (g/ml) | 0.22 ± 0.04 | 0.25 ± 0.06 | 0.03 ± 0.06 | <0.001 |
| LV end-diastolic volume index (ml/m2) | 89 ± 15 | 94 ± 17 | 5 ± 12 | 0.002 |
| LV ejection fraction (%) | 58 ± 8 | 57 ±7 | -1 ± 6 | 0.52 |
| ECG | ||||
| QRS duration (ms) | 154 (82-200) | 150 (80-202) | -2 (-24 - 49) | 0.12 |
| QRS dispersion (ms) | 22 (9-54) | 23 (12 – 40) | 4 (-32 - 15) | 0.84 |
| QTc (ms) | 450 (301-523) | 460 (359-558) | 4 (-96 - 162) | 0.53 |
| JT interval (ms) | 274 (204-418) | 259 (125-359) | -5 (-172 - 56) | 0.05 |
| Exercise | ||||
| Peak VO2 (ml/kg/min) | 26.5 (7.8-47.2) | 27 (9.7-47.7) | 0 (-10 - 8.7) | 0.89 |
| % predicted peak VO2 (%) | 65 (26-101) | 68 (30-95) | 1 (-25 - 25) | 0.57 |
| % predicted VO2 at VAT | 38 (22-65) | 38 (22-56) | 0 (-24 - 18) | 0.86 |
| % predicted peak heart rate (%) | 88 (59-123) | 92 (56-128) | 2 (-42 - 69) | 0.23 |
| Echocardiogram | ||||
| ≥ moderate tricuspid regurgitation, n (%) | 8 (13) | 1 (2) | ‡ | 0.02 |
| RV systolic pressure by tricuspid regurgitation (mm Hg) | 31 (16-76) | 25 (14-57) | -3 (-38 - 11) | 0.02 |
| RVOT gradient (mm Hg) | 16 (3-70) | 14 (6-40) | -4 (-60 - 24) | 0.04 |
| Functional status | ||||
| SF-36 physical component | 49.2 ± 8.3 | 54.6 ± 5.7 | 5 ± 8 | <0.001 |
| SF-36 mental component | 49.6 ± 9.4 | 54.4 ± 7.2 | 5 ± 8 | <0.001 |
| CHQ-PF50 physical component | 42.3 ± 13.9 | 51.3 ± 7.9 | 8 ± 12 | 0.002 |
| CHQ-PF50 mental component | 49.7 ± 8.8 | 51.9 ± 8.7 | 2 ± 7 | 0.29 |
VAT= ventilatory anaerobic threshold; VO2= oxygen consumption
24 patients had a decrease in NYHA class at 6-month follow-up relative to preoperative; no patients demonstrated increased NYHA class.
7 patients with ≥ moderate tricuspid regurgitation preoperatively decreased to ≤ mild tricuspid regurgitation at the 6-month follow-up; no patients demonstrated increased tricuspid regurgitation grade.
Multiple preoperative variables were associated with an optimal postoperative outcome (normal RV size and systolic function) (Table 5). Of the 60 patients with complete 6-month follow-up data, 11 (18%) had an optimal outcome based on the defined criteria. Preoperative RV EDVi was not associated with an optimal postoperative outcome. Multivariable analysis considering all preoperative parameters identified preoperative QRS duration <140 ms as the only independent predictor of optimal outcome (OR 8.22, 95% CI (1.88, 36.0), p = 0.005). Considering CMR variables only, preoperative RV ESVi <90 ml/m2 was an independent predictor of optimal outcome (OR 5.40, 95% CI (1.34, 21.7), p = 0.02). A separate multivariable analysis of CMR parameters associated with a normal postoperative RV size (without consideration of RV RF) identified preoperative RV ESVi <90 ml/m2 (OR 3.67, 95% CI (1.00, 13.5), p = 0.05) and RV EDVi <190 ml/m2 (OR 3.45, 95% CI (0.96, 12.4), p = 0.06) as independent predictors.
Table 5. Preoperative Predictors of Outcomes in all Patients.
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Univariate Predictors of Optimal Postoperative Outcome* | |||
| RV ESVi (OR per ↓ 25 ml/m2) | 2.44 | (0.99, 6.03) | 0.05 |
| RV ESVi <90 ml/m2 | 5.40 | (1.34, 21.7) | 0.02 |
| RV EF (OR per ↑ 10%) | 3.85 | (1.26, 11.81) | 0.02 |
| RV mass-to-volume ratio (OR per ↓ 0.05) | 2.49 | (1.00, 6.17) | 0.05 |
| QRS duration (OR per ↓ 25 ms) | 2.46 | (1.34, 4.49) | 0.004 |
| QRS duration <140 ms | 8.22 | (1.88, 36.0) | 0.005 |
| % predicted peak VO2 (OR per ↑ 10%) | 1.80 | (1.02, 3.18) | 0.04 |
| % predicted peak VO2 ≥70% | 5.91 | (1.09, 32.0) | 0.04 |
| % predicted VO2 at VAT (OR per ↑ 10%) | 2.37 | (1.03, 5.42) | 0.04 |
| Mutivariable Predictors of Optimal Postoperative Outcome: All Variables | |||
| QRS duration <140 ms | 8.22 | (1.88, 36.0) | 0.005 |
| Mutivariable Predictors of Optimal Postoperative Outcome: CMR Variables | |||
| RV ESVi <90 ml/m2 | 5.40 | (1.34, 21.7) | 0.02 |
| Univariate Predictors of Suboptimal Postoperative Outcome** | |||
| RV ESVi (OR per ↑ 25 ml/m2) | 3.06 | (1.53, 6.12) | 0.002 |
| RV ESVi ≥100 ml/m2 | 16.4 | (3.31, 81.3) | 0.001 |
| RV EF (OR per ↓ 10%) | 13.0 | (3.08, 54.6) | <0.001 |
| RV EF <45% | 31.1 | (6.89, 140.4) | <0.001 |
| LV ESVi (OR per ↑ 10 ml/m2) | 2.06 | (1.16, 3.63) | 0.01 |
| LV ESVi ≥50 ml/m2 | 12.4 | (2.25, 68.5) | 0.004 |
| LV EF (OR per ↓ 10%) | 3.36 | (1.37, 8.23) | 0.008 |
| LV EF <55% | 3.88 | (1.2, 12.5) | 0.02 |
| QRS duration (OR per ↑ 25 ms) | 5.08 | (1.91, 13.5) | <0.001 |
| QRS duration ≥160 ms | 26.3 | (5.17, 134.4) | <0.001 |
| % predicted peak VO2 (OR per ↓ 10%) | 1.53 | (1.00, 2.34) | 0.05 |
| % predicted VAT (OR per ↓ 10%) | 2.89 | (1.19, 6.98) | 0.02 |
| % predicted VO2 at VAT <40% | 5.31 | (1.29, 21.9) | 0.02 |
| Mutivariable Predictors of Suboptimal Postoperative Outcome: All Variables | |||
| RV EF ≤45% | 14.5 | (2.80, 75.2) | 0.001 |
| QRS duration ≥160 ms | 11.1 | (1.81, 67.8) | 0.009 |
OR, odds ratio
Optimal outcome was defined as RV EDVi ≤114 ml/m2 and RV EF ≥48%
Suboptimal outcome was defined as RV EDVi ≥120 ml/m2 and RV EF ≤45%
Multiple preoperative variables were associated with a suboptimal postoperative outcome (dilated RV and systolic dysfunction) (Table 5). Of the 60 patients with complete 6-month follow-up data, 19 (32%) had a suboptimal outcome based on the defined criteria. Preoperative RV EDVi was not associated with a suboptimal postoperative outcome. Multivariable analysis considering all preoperative parameters identified preoperative RV EF ≤45% (OR 14.5, 95% CI (2.80, 75.2), p = 0.001) and QRS duration ≥160 ms (OR 11.1, 95% CI (1.81, 67.8), p = 0.009) as independent predictors of suboptimal outcome.
Discussion
This clinical trial compared the postoperative outcomes of PVR alone with those of PVR with remodeling of the RV in patients with severe chronic pulmonary regurgitation and RV dilatation. The elimination of PR was associated with marked reduction in RV end-diastolic and end-systolic volumes, unchanged RV ejection fraction, lower right ventricular outflow tract gradient and RV systolic pressure, increased LV end-diastolic volume, unchanged exercise parameters, and significant improvements in measures of symptoms and functional status. However, both the primary and the secondary outcomes of the two study groups were similar and the addition of RV remodeling to PVR was not associated with a measurable benefit 6 months after surgery. The lack of treatment benefit persisted after controlling for surgeon and for treatment received. Notably, the rates of intra- and postoperative complications were similar in the two treatment groups.
Several possible reasons might explain the negative outcome of this trial. The amount of scar tissue excluded from the RV free wall in the experimental group may have been insufficient to remodel the chamber to a degree that significantly alters wall stress and translates into mechanical or clinical benefit. It is also conceivable that modifications of the geometry and/or location of the remodeling may be required to reduce wall stress. Another possibility is that the time interval between surgery and postoperative evaluation was too short and that some benefits may manifest later. However, van Straten et al., in a study that examined the time course of changes in RV size and function after PVR in 25 patients, found no further changes from 7 months after surgery to 19 months.10 Lastly, we cannot exclude the possibility that by the time PVR was performed irreversible myocardial damage with myocytes loss and diffuse fibrosis resulted in lack of improvement in RV function. Notably, similar failure to show clinical benefit from surgical remodeling of the LV in patients undergoing coronary bypass was recently reported by Jones et al.18
Given the lack of a measurable treatment effect, we combined the study groups and analyzed preoperative factors associated with optimal and suboptimal outcomes 6 months after PVR. In contrast to previous studies that focused on normalization of RV end-diastolic volume after PVR,9, 11, 19, 20 we required both normal RV size and function to satisfy the requirement for an optimal postoperative outcome and, similarly, both persistent dilatation and dysfunction for a suboptimal postoperative outcome. Moreover, to clearly separate between optimal and suboptimal outcomes, the criteria for RV size and function did not overlap, thus excluding patients with mild postoperative RV dilatation and dysfunction. RV EF ≤45% was selected because it was previously found to be an independent risk factor for major adverse clinical outcomes in patients with repaired TOF.4 That analysis found that preoperative QRS duration <140 ms and RV ESVi <90 ml/m2 were associated with normal postoperative RV size and function whereas preoperative QRS duration ≥160 ms and RV EF ≤45% were associated with persistent postoperative RV dilatation and dysfunction. Given that preservation of RV mechanics is an important goal of PVR, these findings suggest that earlier surgical or transcatheter valve implantation in patients with pulmonary regurgitation may be beneficial. These observations are different from those in studies that identified threshold values of RV end-diastolic volume as an indication for PVR,9, 11, 19, 20 but are in agreement with the findings of Henkens et al., who found that preoperative RV ESVi and RV EF (corrected for PR and other shunts) best predicted postoperative RV size and systolic function.8 Importantly, although it remains unknown whether pulmonary valve implantation based on the findings of this cohort translates into survival or quality of life benefits, recent evidence indicates that delaying PVR until overt symptoms manifest, QRS duration reaches or exceeds 180 ms, or preoperative RV size and function are markedly abnormal is associated with excess morbidity and mortality after PVR.21-23
Limitations
We cannot exclude the possibility that some treatment effects may manifest later than 6 months postoperatively. Given that RV mechanics may continue to evolve during late follow-up and that ventricular tachycardia can manifest years after PVR,22 continued follow-up of this cohort is warranted. Furthermore, given that RV remodeling was not designed to achieve a predetermined geometric goal, further research should examine whether patient-specific, CMR-based preoperative computer modeling of the operation may lead to improved postoperative RV mechanics.
Conclusions
In this trial, the addition of surgical RV remodeling to PVR alone in patients with chronic PR did not result in a measurable benefit. Analysis of the entire cohort found that preoperative RV end-systolic volume index <90 ml/m2 and QRS duration <140 ms were associated with normal postoperative RV size and function, whereas preoperative RV ejection fraction ≤45% and QRS duration ≥160 ms were associated with persistent postoperative RV dilatation and dysfunction. These criteria suggest that use of QRS duration and end-systolic volume index threshold values for surgical or transcatheter valve implantation in patients with pulmonary regurgitation may be beneficial. Finally, given the large number of patients with persistent RV dysfunction after PVR, exploration of new techniques aimed at restoring ventricular function is warranted.
Acknowledgments
We thank the members of the Data and Safety Monitoring Board for their diligent monitoring and helpful suggestions throughout the study. We are indebted to the patients participating in this trial.
Source of Funding: This work was supported by the National Institutes of Health (NIH/NHLBI 1P50 HL074734-01).
Footnotes
Clinical Trials Registration Information: http://www.clinicaltrials.gov: NCT00112320
Conflict of Interest Disclosures: None
References
- 1.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 2.Therrien J, Siu SC, McLaughlin PR, Liu PP, Williams WG, Webb GD. Pulmonary valve replacement in adults late after repair of tetralogy of fallot: are we operating too late? J Am Coll Cardiol. 2000;36:1670–1675. doi: 10.1016/s0735-1097(00)00930-x. [DOI] [PubMed] [Google Scholar]
- 3.Wald RM, Haber I, Wald R, Valente AM, Powell AJ, Geva T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation. 2009;119:1370–1377. doi: 10.1161/CIRCULATIONAHA.108.816546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knauth AL, Gauvreau K, Powell AJ, Landzberg MJ, Walsh EP, Lock JE, del Nido PJ, Geva T. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart. 2008;94:211–216. doi: 10.1136/hrt.2006.104745. [DOI] [PubMed] [Google Scholar]
- 5.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 6.Oosterhof T, Meijboom FJ, Vliegen HW, Hazekamp MG, Zwinderman AH, Bouma BJ, van Dijk AP, Mulder BJ. Long-term follow-up of homograft function after pulmonary valve replacement in patients with tetralogy of Fallot. Eur Heart J. 2006;27:1478–1484. doi: 10.1093/eurheartj/ehl033. [DOI] [PubMed] [Google Scholar]
- 7.Vliegen HW, Van Straten A, De Roos A, Roest AA, Schoof PH, Zwinderman AH, Ottenkamp J, Van Der Wall EE, Hazekamp MG. Magnetic resonance imaging to assess the hemodynamic effects of pulmonary valve replacement in adults late after repair of tetralogy of fallot. Circulation. 2002;106:1703–1707. doi: 10.1161/01.cir.0000030995.59403.f8. [DOI] [PubMed] [Google Scholar]
- 8.Henkens IR, van Straten A, Schalij MJ, Hazekamp MG, de Roos A, van der Wall EE, Vliegen HW. Predicting outcome of pulmonary valve replacement in adult tetralogy of Fallot patients. Ann Thorac Surg. 2007;83:907–911. doi: 10.1016/j.athoracsur.2006.09.090. [DOI] [PubMed] [Google Scholar]
- 9.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 10.van Straten A, Vliegen HW, Hazekamp MG, Bax JJ, Schoof PH, Ottenkamp J, van der Wall EE, de Roos A. Right ventricular function after pulmonary valve replacement in patients with tetralogy of Fallot. Radiology. 2004;233:824–829. doi: 10.1148/radiol.2333030804. [DOI] [PubMed] [Google Scholar]
- 11.Buechel ER, Dave HH, Kellenberger CJ, Dodge-Khatami A, Pretre R, Berger F, Bauersfeld U. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur Heart J. 2005;26:2721–2727. doi: 10.1093/eurheartj/ehi581. [DOI] [PubMed] [Google Scholar]
- 12.Athanasuleas CL, Stanley AW, Jr, Buckberg GD, Dor V, DiDonato M, Blackstone EH. Surgical anterior ventricular endocardial restoration (SAVER) in the dilated remodeled ventricle after anterior myocardial infarction. RESTORE group. Reconstructive Endoventricular Surgery, returning Torsion Original Radius Elliptical Shape to the LV. J Am Coll Cardiol. 2001;37:1199–1209. doi: 10.1016/s0735-1097(01)01119-6. [DOI] [PubMed] [Google Scholar]
- 13.del Nido PJ. Surgical management of right ventricular dysfunction late after repair of tetralogy of fallot: right ventricular remodeling surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006;9:29–34. doi: 10.1053/j.pcsu.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging. 2008;28:67–73. doi: 10.1002/jmri.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samyn MM, Powell AJ, Garg R, Sena L, Geva T. Range of ventricular dimensions and function by steady-state free precession cine MRI in repaired tetralogy of Fallot: right ventricular outflow tract patch vs. conduit repair. J Magn Reson Imaging. 2007;26:934–940. doi: 10.1002/jmri.21094. [DOI] [PubMed] [Google Scholar]
- 16.Meadows J, Powell AJ, Geva T, Dorfman A, Gauvreau K, Rhodes J. Cardiac magnetic resonance imaging correlates of exercise capacity in patients with surgically repaired tetralogy of Fallot. Am J Cardiol. 2007;100:1446–1450. doi: 10.1016/j.amjcard.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Pattynama PM, Lamb HJ, Van der Velde EA, Van der Geest RJ, Van der Wall EE, De Roos A. Reproducibility of MRI-derived measurements of right ventricular volumes and myocardial mass. Magn Reson Imaging. 1995;13:53–63. doi: 10.1016/0730-725x(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 18.Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O'Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dave HH, Buechel ER, Dodge-Khatami A, Kadner A, Rousson V, Bauersfeld U, Pretre R. Early insertion of a pulmonary valve for chronic regurgitation helps restoration of ventricular dimensions. Ann Thorac Surg. 2005;80:1615–1620. doi: 10.1016/j.athoracsur.2005.04.058. discussion 1620-1611. [DOI] [PubMed] [Google Scholar]
- 20.Frigiola A, Tsang V, Bull C, Coats L, Khambadkone S, Derrick G, Mist B, Walker F, van Doorn C, Bonhoeffer P, Taylor AM. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation. 2008;118:S182–190. doi: 10.1161/CIRCULATIONAHA.107.756825. [DOI] [PubMed] [Google Scholar]
- 21.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, Mulder BJ. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 22.Harrild DM, Berul CI, Cecchin F, Geva T, Gauvreau K, Pigula F, Walsh EP. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009;119:445–451. doi: 10.1161/CIRCULATIONAHA.108.775221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meijboom FJ, Roos-Hesselink JW, McGhie JS, Spitaels SE, van Domburg RT, Utens LM, Simoons ML, Bogers AJ. Consequences of a selective approach toward pulmonary valve replacement in adult patients with tetralogy of Fallot and pulmonary regurgitation. J Thorac Cardiovasc Surg. 2008;135:50–55. doi: 10.1016/j.jtcvs.2007.07.030. [DOI] [PubMed] [Google Scholar]