Abstract
With the exception of lymphoma involving the spleen, other primary and secondary neoplasms are rare and infrequently encountered. Primary malignant neoplasms involving the spleen are lymphoma and angiosarcoma. Primary benign neoplasms involving the spleen include hemangioma, lymphangioma, littoral cell angioma and splenic cyst and solid lesions such as hamartoma and inflammatory pseudotumor.
Keywords: Spleen, neoplasms, CT, MRI, FDG-PET
Introduction
Primary splenic neoplasms can be broadly categorized into lymphoid neoplasms arising from the white pulp and vascular neoplasms, which arise from the red pulp[1]. Primary tumors arising from vascular elements include benign lesions such as hemangioma, lymphangioma and hamartoma, intermediate lesions such as hemangioendothelioma, hemangiopericytoma and littoral cell angioma as well as the frankly malignant hemangiosarcoma[2].
Primary malignant neoplasms of the spleen
Lymphoma
Primary involvement of the spleen by lymphoma is much less common than secondary involvement, accounting for less than 1% of all lymphomas[3]. Primary splenic lymphoma usually represents non-Hodgkin lymphoma of B cell origin. Secondary involvement of the spleen is much more common and is seen in association with enlarged lymph nodes in the rest of abdomen[4].
Radiologically four different patterns of involvement of splenic lymphoma have been described, which correspond to patterns of pathological involvement[5]: diffuse infiltration, which manifests as splenomegaly; small, focal or miliary nodules; multiple large nodular lesions; and bulky solid masses. Lymphomatous nodules and masses are seen as low-density lesions on contrast-enhanced computed tomography (CT) (Fig. 1). On magnetic resonance imaging (MRI) these lesions are isointense on precontrast T1- and T2-weighted sequences, but hypointense on post-contrast sequences. Diffuse infiltration or tiny (<1 cm) nodular lesions are seen in 45–70% of splenic lymphoma and can sometimes be difficult to visualize on CT and MRI[6]. [18F]Fluorodeoxyglucose (FDG)-positron emission tomography (PET) imaging can help in identifying lymphomatous involvement in these cases, thereby improving overall accuracy in staging of disease. Treated lymphomatous deposits may appear necrotic and eventually can show areas of calcification within them (Fig. 2).
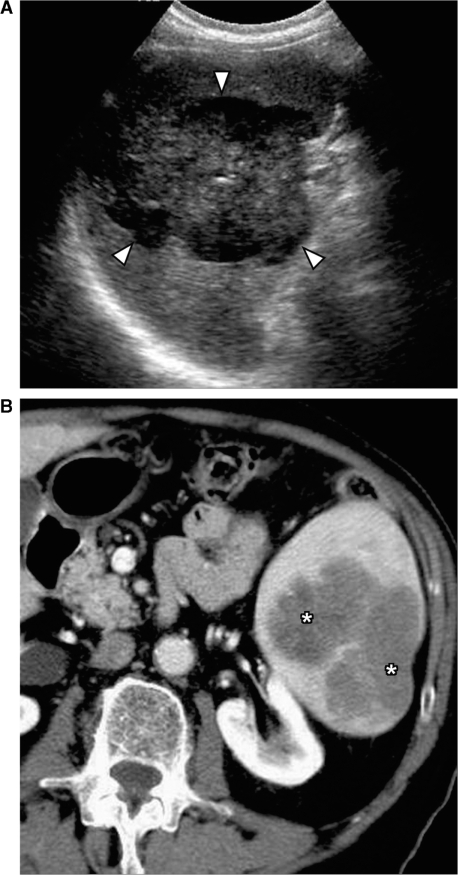
Figure 1.
Primary splenic lymphoma. (A) Ultrasound shows a large heterogeneous mass in the spleen (arrowheads). (B) Contrast-enhanced CT shows a lobulated hypodense mass in the spleen (*). Biopsy confirmed it to be non-Hodgkin lymphoma of B-cell origin.
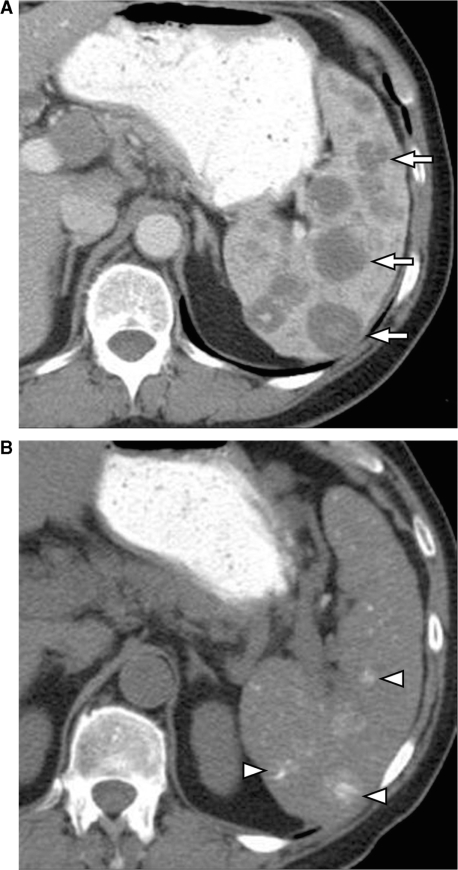
Figure 2.
Calcification in the spleen following treatment for lymphoma. (A) Contrast-enhanced CT shows multiple hypodense lesions in the spleen (arrows) secondary to lymphomatous deposits. (B) Unenhanced CT scan obtained 2 years after chemotherapy shows multiple foci of calcification representing treated lymphoma (arrowheads).
Angiosarcoma
Although rare, angiosarcoma is the most common primary non-hematopoietic malignant tumor of the spleen[7]. This is a highly aggressive tumor with poor prognosis. Spenomegaly is commonly found and spontaneous splenic rupture has been reported in approximately 25% of patients[7]. Metastatic disease is usually found at presentation with the liver being the most common site of metastasis.
The radiological appearance of the tumor reflects its aggressive nature seen on pathology. Splenic angiosarcoma appears as an aggressive splenic mass or masses with associated splenomegaly[8]. On sonography, it is seen as multiple complex heterogeneous masses involving the spleen. On contrast-enhanced CT, angiosarcomas are usually seen as multiple hypervascular masses in the spleen (Fig. 3). The lesions have heterogeneous appearance due to internal areas of hemorrhage and necrosis. Calcifications have been rarely reported in these lesions. Their appearance on MRI reflects the heterogeneity of these tumors, with areas of mixed high and low signal on T1- and T2-weighted images and heterogeneous enhancement noted in the solid portions of tumor on contrast-enhanced sequences. Intra- or perisplenic hemorrhage or frank hemoperitoneum can be seen on CT or MRI[8]. In all suspected cases of splenic angiosarcoma, special care should be taken before performing percutaneous biopsy due to the high risk of massive hemorrhage[9].
Figure 3.
Splenic angiosarcoma. Contrast-enhanced CT shows a hypodense mass in the spleen (arrow) with focal areas of enhancement secondary to the vascular nature of tumor (arrowhead).
Primary benign splenic neoplasms
Hemangioma
Hemangioma is the most common benign neoplasm of spleen and most splenic hemangiomas are found incidentally on imaging[10]. Rarely they can be multiple or diffuse as seen in hemangiomatosis or be associated with generalized angiomatosis syndromes.
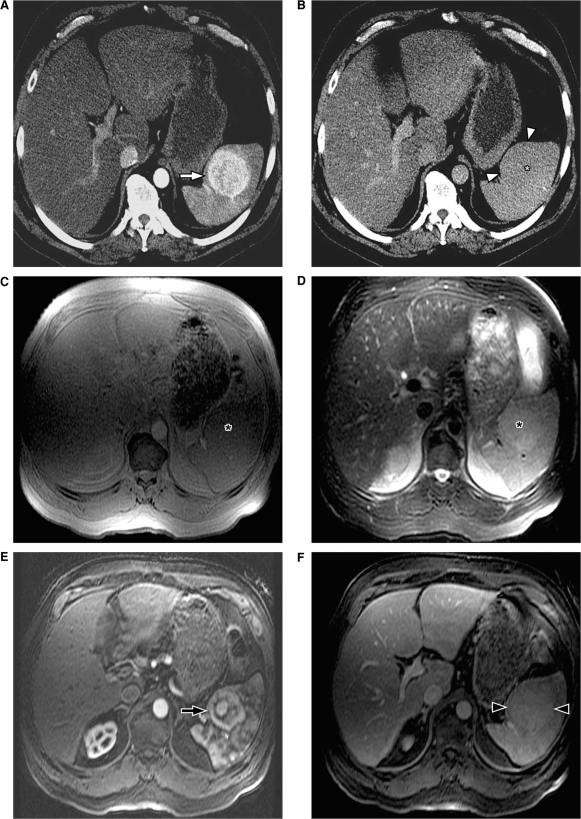
Splenic hemangiomas have varied radiological appearances depending on the capillary or cavernous components of the hemangioma. On ultrasound the smaller hemangiomas can be seen as discrete echogenic lesions and the larger lesions may have a more complex appearance[11]. Punctate peripheral calcifications may be noted on CT. On MRI, splenic hemangiomas are typically iso- to hypointense on T1-weighted images and hyperintense on T2-weighted images. Following contrast administration, splenic hemangiomas show varying patterns of enhancement with most lesions showing peripheral enhancement in the arterial phase with progressive centripetal fill-in and persistent retention of contrast on the delayed phase (Fig. 4)[12]. However, unlike hepatic cavernous hemangiomas, the discontinuous peripheral puddles or globules of contrast enhancement are uncommonly seen in splenic hemangiomas, with a more continuous solid rim of enhancement being seen more often in the arterial phase[10]. Other patterns of enhancement that can be seen are immediate homogeneous enhancement with persistent delayed enhancement and peripheral enhancement with persistent lack of central enhancement on delayed images. Cavernous hemangiomas typically show heterogeneous enhancement due to presence of cystic non-enhancing areas within the lesion. Technetium-99m labeled red blood cell (RBC) scans are also helpful in characterizing atypical lesions by demonstrating increased activity on delayed images[13].
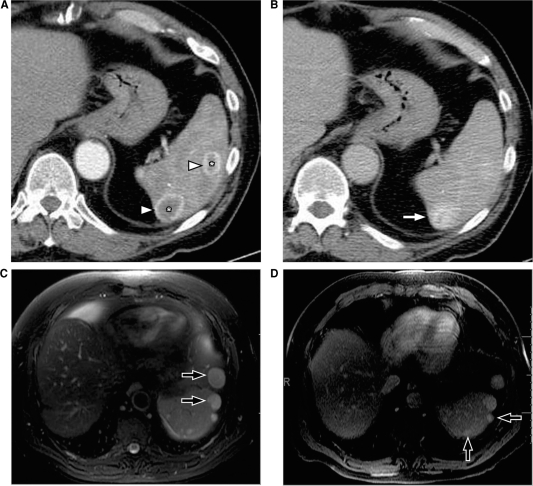
Figure 4.
Splenic hemangiomas. (A) Arterial phase of contrast-enhanced CT shows two lesions in the spleen (*), which demonstrate the peripheral continuous rim of enhancement (arrowheads). (B) Venous phase shows progressive centripetal enhancement in both of the lesions, which is better seen in the posterior lesion (arrow). MRI in a different patient shows multiple hemangiomas (arrows) which are hyperintense on T2-weighted images (C) and show retention of contrast on delayed post contrast T1-weighted images (D).
Lymphangioma
Splenic lymphangiomas are rare, benign, slow-growing neoplasms usually seen in childhood[14]. Similar to hemangiomas, splenic lymphangiomas may be isolated or may be part of rare lymphangiomatous syndrome involving multiple organs. Large splenic lymphangiomas may present as an abdominal mass in children, but in the adult population, they are often noted as an incidental asymptomatic finding[15].
Splenic lymphangiomas are usually seen as multiloculated cysts of varying size that are predominantly subcapsular in location[14]. They are hypoechoeic on ultrasound and hypodense on CT, with few enhancing septa and occasional peripheral rim calcification (Fig. 5). Most of them follow the signal intensity of simple fluid on MRI, but few cysts may demonstrate a high T1 signal due to proteinaceous or hemorrhagic content[16].
Figure 5.
Splenic lymphangioma. Contrast-enhanced CT shows a large multicystic lesion (arrows) with thin septations (arrowheads) in the spleen extending to the capsular margin.
Hamartoma
Splenic hamartoma is a rare benign lesion composed of malformed splenic red pulp elements without organized lymphoid follicles, the exact cause of which is uncertain[17]. Hamartomas are usually discovered incidentally or rarely as a large mass or splenomegaly.
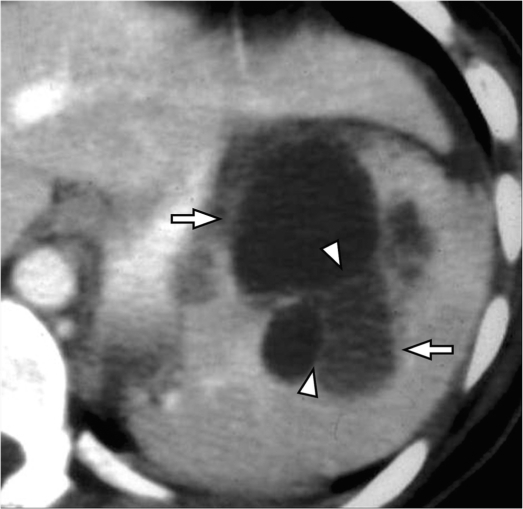
On ultrasound, hamartomas are usually seen as well-circumscribed homogeneous solid masses[18]. Typical hamartomas are isodense on non-contrast CT and are usually isointense on T1-weighted images and heterogeneously hyperintense on T2-weighted images[12,19]. Following contrast administration, hamartomas show diffuse early enhancement and uniform delayed enhancement appearing isodense/isointense to the remainder of the spleen and hence on the delayed phase a contour abnormality may be the only visible finding (Fig. 6)[19]. In larger lesions, heterogeneous enhancement may be observed with uniform and prolonged retention of contrast noted on delayed images. The persistent delayed enhancement is thought be due to stagnant contrast material within the sinusoids of the red pulp in the hamartoma[19,20].
Figure 6.
Splenic hamartoma. (A) Arterial phase of contrast-enhanced CT shows an enhancing mass in the spleen (arrow). (B) The mass is isodense on the venous phase (asterisk) and can be appreciated only as a capsular bulge (arrowheads). On MRI, the mass is isointense on the T1-weighted inphase (C) and is slightly hyperintense on the T2-weighted fat-saturated sequence (asterisk) (D). After gadolinium administration, heterogeneous enhancement is noted in the arterial phase (arrow) (E) with uniform delayed enhancement noted on the delayed phase (arrowheads) (F).
Although imaging findings may suggest the possibility of splenic hamartoma, a definitive diagnosis based on imaging or percutaneous biopsy may not be possible and more often, splenectomy is needed for a definitive diagnosis[21].
Littoral cell angioma
Littoral cell angioma is a rare vascular tumor arising from the littoral cell, which lines the sinuses of splenic red pulp[22]. It involves the spleen diffusely with multiple nodular masses of red pulp elements. Most patients present with splenomegaly and laboratory findings of hypersplenism[23].
Littoral cell angioma is usually seen as multiple masses of varying sizes involving the entire spleen, which are isodense on non-contrast CT, are hypodense on the early portal phase following contrast administration and can be isodense on the delayed phase (Fig. 7)[23]. On MRI, littoral cell angiomas may show low signal intensity on all sequences similar to siderotic nodules due to hemosiderin accumulation within neoplastic littoral cells[24]. The diagnosis of littoral cell angioma should be suspected when multiple hypodense nodules are seen in a patient with splenomegaly and hypersplenism[2].
Figure 7.
Littoral cell angioma of the spleen. (A,B) Contrast-enhanced axial CT images of the spleen show multiple well-defined hypodense lesions (arrowheads) ranging in size from 5 mm to 3 cm. Pathology showed littoral cell angioma of the spleen.
Inflammatory pseudotumor
Splenic inflammatory pseudotumor is a rare benign lesion of uncertain cause, likely resulting from an unusual inflammatory reparative response to injury such as infection[25]. It is usually asymptomatic and incidentally detected, but may present with symptoms of mass effect.
On CT, usually a well-circumscribed mass, which may show areas of calcification, is seen. Enhancement can be homogeneous or heterogeneous and delayed enhancement patterns have been described[26]. A definitive imaging diagnosis is not possible and diagnosis is established only after surgery.
Uncommon primary splenic neoplasms
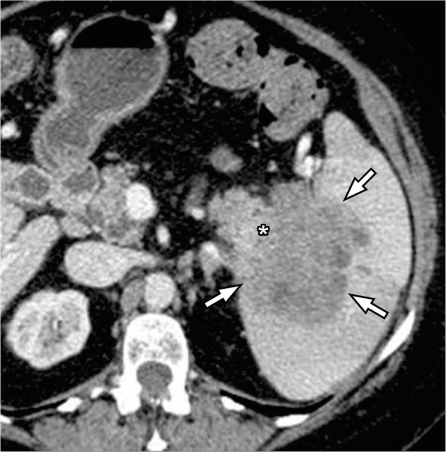
Primary mesenchymal tumors such as lipoma, angiomyolipoma, fibroma, fibrosarcoma, leiomyosarcoma and malignant fibrous histocytoma have been reported to occur in the splenic parenchyma but are quite rare (Fig. 8). In the few published reports, except for lipoma, which is characterized by the presence of fat, no pathognomonic features are noted in the remainder of the lesions[27].
Figure 8.
Splenic sarcoma. Contrast-enhanced CT shows a large exophytic mass arising from the spleen (arrows). Large necrotic areas (*) and few foci of calcification (arrowhead) are noted within the lesion.
Metastatic lesions
Splenic involvement by metastasis is relatively uncommon and is thought to be secondary to lack of afferent lymphatics[28]. Hematogeneous spread is considered the most probable route of spread resulting in splenic metastases. Splenic metastases are seen in only 2–9% of untreated cancer patients and are seen as isolated splenic lesions in 5.2% of patients[28,29]. Tumors that most commonly metastasize to the spleen include melanoma, and tumors of the breast, lung, ovary, colon, stomach and pancreas.
Most metastases to the spleen are seen as solitary or multiple masses and diffuse infiltration is a rare phenomenon[28]. The sonographic appearance of splenic metastasis is variable with most appearing hypoechoeic. On contrast-enhanced CT they are seen as hypodense lesions that are best appreciated on the portal venous phase (Fig. 9). On MRI they may be difficult to identify on the precontrast T1- and T2 weighted sequences and are seen as hypointense lesions on T1-weighted images following contrast administration[30]. Cystic or necrotic degeneration can occur in metastasis; this is usually seen in metastasis from melanoma and the lesions appear as unilocular or multilocular cystic lesions with septations[16]. Calcification in splenic metastasis is rare unless the primary tumor is a mucinous adenocarcinoma (Fig. 10)[31].
Figure 9.
Splenic metastasis from melanoma. Contrast-enhanced CT shows multiple hypodense masses in the liver (arrowheads) and a large mass in the spleen (arrow) demonstrating central necrosis (*) in a patient with known melanoma representing hepatic and splenic metastasis from melanoma.
Figure 10.
Calcified splenic metastasis from ovarian cancer. Contrast-enhanced CT shows a heterogeneously calcified lesion in the spleen (arrow) in a patient with treated ovarian cancer metastasis.
Perisplenic neoplasms infiltrating spleen
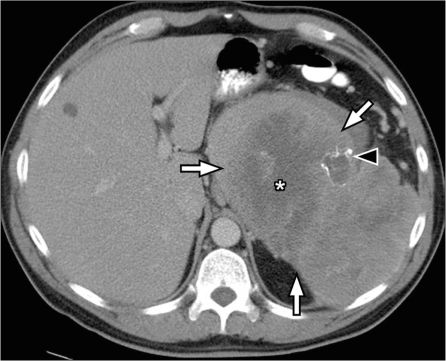
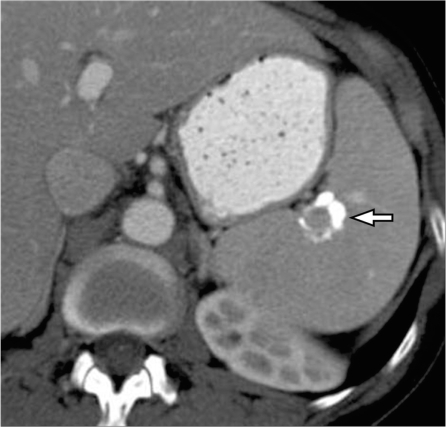
Implants on the serosal surface of the spleen are seen in patients with peritoneal carcinomatosis, commonly from ovarian or gastrointestinal primary neoplasms. These implants are seen on CT to cause indentation and scalloping of the surface of the spleen. Pseudomyxoma peritonei also often involves the spleen and is seen as scalloping of the splenic margin (Fig. 11). Direct tumor invasion of the spleen is uncommon, but can be seen in tumors originating from the pancreas, stomach, colon or left kidney and retroperitoneum (Fig. 12)[32].
Figure 11.
Perisplenic deposits in pseudomyxoma peritonei. Contrast-enhanced CT scan shows multiple well-defined cystic lesions in the perisplenic region invaginating into the spleen (arrows) in a patient with known pseudomyxoma peritonei. Similar cystic deposits are also noted in the perigastric region (*).
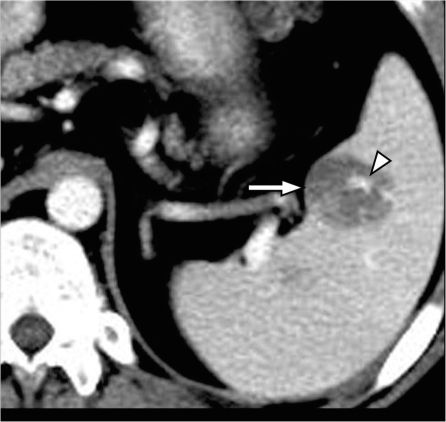
Figure 12.
Pancreatic cancer invading through the splenic hilum. Contrast-enhanced CT shows a large heterogeneous mass centered on the splenic hilum (arrows) and inseparable from the tail of the pancreas (*) representing pancreatic adenocarcinoma of the pancreatic tail with splenic invasion.
Non-neoplastic splenic lesions mimicking splenic neoplasms
Granulomatous diseases and sarcoidosis
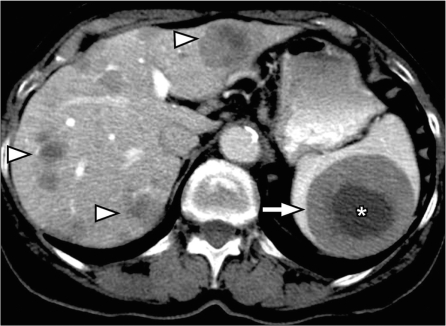
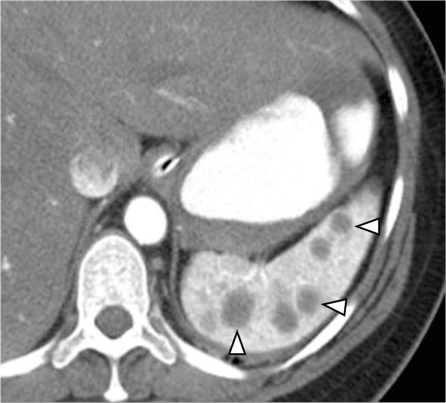
Sarcoidosis is a multisystem disease with 5–15% of patients having abdominal involvement[33]. Splenic involvement in sarcoidosis is commonly seen as splenomegaly and less commonly as multiple nodules (Fig. 13). There can be associated hepatic involvement and lymphadenopathy in some cases. The nodules are hypodense on CT, and are typically hypointense on all MRI sequences showing minimal and delayed enhancement following contrast administration[34]. The nodular pattern of involvement can mimic lymphoma or metastatic disease to the spleen. The presence of larger, confluent lymph nodes suggests the possibility of lymphoma, and the presence of a primary malignancy suggests metastatic disease.
Figure 13.
Splenic sarcoidosis. Contrast-enhanced CT shows splenomegaly with innumerable hypodense nodules in the spleen (white arrowheads). Multiple tiny nodules in the liver (black arrowheads) and few enlarged lymph nodes along the celiac axis (arrow) are noted in a patient with known sarcoidosis representing hepatosplenic involvement and lymph node enlargement.
Peliosis of spleen
Peliosis of the spleen is a rare condition caused by sinusoidal dilation leading to formation of multiple cyst-like blood-filled cavities within the splenic parenchyma[35]. This is usually an incidental finding noted in an asymptomatic individual, however spontaneous splenic rupture has been reported[36]. On CT, multiple small well-defined, hypodense cyst-like lesions are seen (Fig. 14). The cystic spaces contain blood components within them that might lead to the presence of fluid-fluid levels and variable signal intensity on MRI[37]. The lesions may or may not enhance after contrast administration. The cystic nature of the lesions should be helpful in distinguishing peliosis from lymphoma or metastasis, which are typically solid in nature.
Figure 14.
Peliosis of spleen. Contrast-enhanced CT shows multiple hypodense lesions (arrowheads) in a normal-sized spleen. Pathology showed peliosis of the spleen.
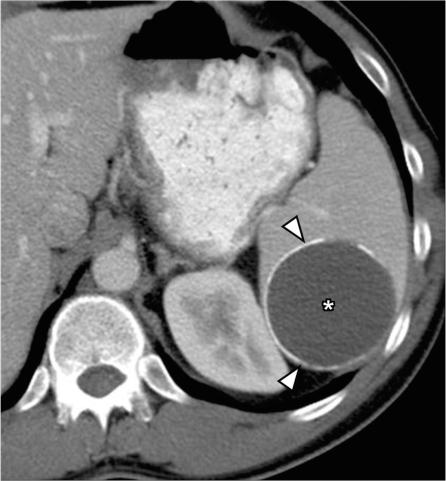
Splenic cyst
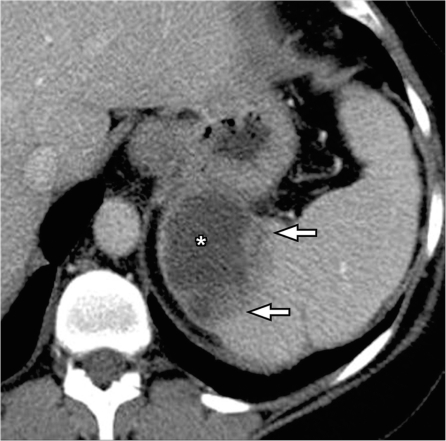
Most splenic cystic lesions are non-neoplastic lesions and include congenital cysts, post-traumatic pseudocysts (Fig. 15), pancreatic pseudocyst and parasitic (echinococcal) cysts[27]. Most of these are easily distinguishable from cystic metastasis, which usually have associated solid components.
Figure 15.
Splenic pseudocyst. Contrast-enhanced CT shows a well-defined cyst in the spleen (*) with peripheral rim of calcification (arrowheads) and no internal septations, presumed to be a post-traumatic pseudocyst.
Splenic abscess
Pyogenic splenic abscess can be seen as an isolated involvement of the spleen or as part of a systemic involvement. They are seen as heterogeneous hypodense lesions with smooth or irregular margins that can show variable enhancement (Fig. 16)[38]. The clinical history is usually corroborative of an infectious cause in these cases.
Figure 16.
Splenic abscess. Contrast-enhanced CT shows a heterogeneous cystic lesion (*) with thick irregular enhancing margins (arrows) in a patient with septicemia representing a splenic abscess.
Mycobacterial and fungal involvement of the spleen is usually seen as multiple small 5–10 mm nodules involving the spleen diffusely. There can also be associated hepatic involvement. These are usually seen in immunocompromised patients which can help suggest this diagnosis.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Dachman A. Radiology of the spleen. St. Louis, MO: Mosby; 1993. [Google Scholar]
- 2.Kamaya A, Weinstein S, Desser TS. Multiple lesions of the spleen: differential diagnosis of cystic and solid lesions. Semin Ultrasound CT MR. 2006;27:389–403. doi: 10.1053/j.sult.2006.06.004. doi:10.1053/j.sult.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Spier CM, Kjeldsberg CR, Eyre HJ, Behm FG. Malignant lymphoma with primary presentation in the spleen. A study of 20 patients. Arch Pathol Lab Med. 1985;109:1076–80. [PubMed] [Google Scholar]
- 4.Bhatia K, Sahdev A, Reznek RH. Lymphoma of the spleen. Semin Ultrasound CT MR. 2007;28:12–20. doi: 10.1053/j.sult.2006.10.010. doi:10.1053/j.sult.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Ahmann DL, Kiely JM, Harrison EG, Jr, Payne WS. Malignant lymphoma of the spleen. A review of 49 cases in which the diagnosis was made at splenectomy. Cancer. 1966;19:461–9. doi: 10.1002/1097-0142(196604)19:4<461::aid-cncr2820190402>3.0.co;2-x. doi:10.1002/1097-0142(196604)19:4<461::AID-CNCR2820190402>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Rabushka LS, Kawashima A, Fishman EK. Imaging of the spleen: CT with supplemental MR examination. Radiographics. 1994;14:307–32. doi: 10.1148/radiographics.14.2.8190956. [DOI] [PubMed] [Google Scholar]
- 7.Falk S, Krishnan J, Meis JM. Primary angiosarcoma of the spleen. A clinicopathologic study of 40 cases. Am J Surg Pathol. 1993;17:959–70. doi: 10.1097/00000478-199310000-00001. doi:10.1097/00000478-199310000-00001. PMid:8372948. [DOI] [PubMed] [Google Scholar]
- 8.Thompson WM, Levy AD, Aguilera NS, Gorospe L, Abbott RM. Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology. 2005;235:106–15. doi: 10.1148/radiol.2351040308. doi:10.1148/radiol.2351040308. PMid:15749977. [DOI] [PubMed] [Google Scholar]
- 9.Lucey BC, Boland GW, Maher MM, Hahn PF, Gervais DA, Mueller PR. Percutaneous nonvascular splenic intervention: a 10-year review. AJR Am J Roentgenol. 2002;179:1591–6. doi: 10.2214/ajr.179.6.1791591. [DOI] [PubMed] [Google Scholar]
- 10.Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137–63. doi: 10.1148/rg.244045006. doi:10.1148/rg.244045006. PMid:15256634. [DOI] [PubMed] [Google Scholar]
- 11.Goerg C, Schwerk WB, Goerg K. Sonography of focal lesions of the spleen. AJR Am J Roentgenol. 1991;156:949–53. doi: 10.2214/ajr.156.5.2017957. [DOI] [PubMed] [Google Scholar]
- 12.Ramani M, Reinhold C, Semelka RC, et al. Splenic hemangiomas and hamartomas: MR imaging characteristics of 28 lesions. Radiology. 1997;202:166–72. doi: 10.1148/radiology.202.1.8988207. [DOI] [PubMed] [Google Scholar]
- 13.Wijaya J, Kapoor R, Roach P. Tc-99m-labeled RBC scintigraphy and splenic hemangioma. Clin Nucl Med. 2001;26:1022–3. doi: 10.1097/00003072-200112000-00006. doi:10.1097/00003072-200112000-00006. PMid:11711705. [DOI] [PubMed] [Google Scholar]
- 14.Wadsworth DT, Newman B, Abramson SJ, Carpenter BL, Lorenzo RL. Splenic lymphangiomatosis in children. Radiology. 1997;202:173–6. doi: 10.1148/radiology.202.1.8988208. [DOI] [PubMed] [Google Scholar]
- 15.Morgenstern L, Bello JM, Fisher BL, Verham RP. The clinical spectrum of lymphangiomas and lymphangiomatosis of the spleen. Am Surg. 1992;58:599–604. [PubMed] [Google Scholar]
- 16.Urrutia M, Mergo PJ, Ros LH, Torres GM, Ros PR. Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics. 1996;16:107–29. doi: 10.1148/radiographics.16.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Morgenstern L, McCafferty L, Rosenberg J, Michel SL. Hamartomas of the spleen. Arch Surg. 1984;119:1291–3. doi: 10.1001/archsurg.1984.01390230057013. [DOI] [PubMed] [Google Scholar]
- 18.Chou YH, Chiou HJ, Tiu CM, Chiou SY, Hsia CY, Tsay SH. Splenic hamartoma: presentation on contrast-enhanced sonography. J Clin Ultrasound. 2004;32:425–8. doi: 10.1002/jcu.20061. doi:10.1002/jcu.20061. PMid:15372453. [DOI] [PubMed] [Google Scholar]
- 19.Yu RS, Zhang SZ, Hua JM. Imaging findings of splenic hamartoma. World J Gastroenterol. 2004;10:2613–15. doi: 10.3748/wjg.v10.i17.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohtomo K, Fukuda H, Mori K, Minami M, Itai Y, Inoue Y. CT and MR appearances of splenic hamartoma. J Comput Assist Tomogr. 1992;16:425–8. doi: 10.1097/00004728-199205000-00015. doi:10.1097/00004728-199205000-00015. PMid:1592926. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH. Fine-needle aspiration cytology of splenic hamartoma. Diagn Cytopathol. 2003;28:82–5. doi: 10.1002/dc.10230. doi:10.1002/dc.10230. PMid:12561026. [DOI] [PubMed] [Google Scholar]
- 22.Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023–33. doi:10.1097/00000478-199111000-00001. PMid:1928554. [PubMed] [Google Scholar]
- 23.Levy AD, Abbott RM, Abbondanzo SL. Littoral cell angioma of the spleen: CT features with clinicopathologic comparison. Radiology. 2004;230:485–90. doi: 10.1148/radiol.2302030196. doi:10.1148/radiol.2302030196. PMid:14752189. [DOI] [PubMed] [Google Scholar]
- 24.Oliver-Goldaracena JM, Blanco A, Miralles M, Martin-Gonzalez MA. Littoral cell angioma of the spleen: US and MR imaging findings. Abdom Imaging. 1998;23:636–9. doi: 10.1007/s002619900420. doi:10.1007/s002619900420. PMid:9922201. [DOI] [PubMed] [Google Scholar]
- 25.Inada T, Yano T, Shima S, et al. Inflammatory pseudotumor of the spleen. Intern Med. 1992;31:941–5. doi: 10.2169/internalmedicine.31.941. doi:10.2169/internalmedicine.31.941. PMid:1450507. [DOI] [PubMed] [Google Scholar]
- 26.Irie H, Honda H, Kaneko K, et al. Inflammatory pseudotumors of the spleen: CT and MRI findings. J Comput Assist Tomogr. 1996;20:244–8. doi: 10.1097/00004728-199603000-00014. doi:10.1097/00004728-199603000-00014. PMid:8606231. [DOI] [PubMed] [Google Scholar]
- 27.Warshauer DM, Hall HL. Solitary splenic lesions. Semin Ultrasound CT MR. 2006;27:370–88. doi: 10.1053/j.sult.2006.06.003. doi:10.1053/j.sult.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Lam KY, Tang V. Metastatic tumors to the spleen: a 25-year clinicopathologic study. Arch Pathol Lab Med. 2000;124:526–30. doi: 10.5858/2000-124-0526-MTTTS. [DOI] [PubMed] [Google Scholar]
- 29.Schon CA, Gorg C, Ramaswamy A, Barth PJ. Splenic metastases in a large unselected autopsy series. Pathol Res Pract. 2006;202:351–6. doi: 10.1016/j.prp.2005.12.008. doi:10.1016/j.prp.2005.12.008. PMid:16488085. [DOI] [PubMed] [Google Scholar]
- 30.Hahn PF, Weissleder R, Stark DD, Saini S, Elizondo G, Ferrucci JT. MR imaging of focal splenic tumors. AJR Am J Roentgenol. 1988;150:823–7. doi: 10.2214/ajr.150.4.823. [DOI] [PubMed] [Google Scholar]
- 31.Williams L, Kumar A, Aggarwal S. Calcified splenic metastasis from gastric carcinoma. Abdom Imaging. 1995;20:312–14. doi: 10.1007/BF00203360. doi:10.1007/BF00203360. PMid:7549732. [DOI] [PubMed] [Google Scholar]
- 32.Taylor AJ, Dodds WJ, Erickson SJ, Stewart ET. CT of acquired abnormalities of the spleen. AJR Am J Roentgenol. 1991;157:1213–19. doi: 10.2214/ajr.157.6.1950868. [DOI] [PubMed] [Google Scholar]
- 33.Warshauer DM, Lee JK. Imaging manifestations of abdominal sarcoidosis. AJR Am J Roentgenol. 2004;182:15–28. doi: 10.2214/ajr.182.1.1820015. [DOI] [PubMed] [Google Scholar]
- 34.Warshauer DM. Splenic sarcoidosis. Semin Ultrasound CT MR. 2007;28:21–7. doi: 10.1053/j.sult.2006.10.004. doi:10.1053/j.sult.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Tsokos M, Erbersdobler A. Pathology of peliosis. Forensic Sci Int. 2005;149:25–33. doi: 10.1016/j.forsciint.2004.05.010. doi:10.1016/j.forsciint.2004.05.010. PMid:15734106. [DOI] [PubMed] [Google Scholar]
- 36.Shimono T, Yamaoka T, Nishimura K, et al. Peliosis of the spleen: splenic rupture with intraperitoneal hemorrhage. Abdom Imaging. 1998;23:201–2. doi: 10.1007/s002619900323. doi:10.1007/s002619900323. PMid:9516517. [DOI] [PubMed] [Google Scholar]
- 37.Maves CK, Caron KH, Bisset, 3rd GS, Agarwal R. Splenic and hepatic peliosis: MR findings. AJR Am J Roentgenol. 1992;158:75–6. doi: 10.2214/ajr.158.1.1727362. [DOI] [PubMed] [Google Scholar]
- 38.Ng KK, Lee TY, Wan YL, et al. Splenic abscess: diagnosis and management. Hepatogastroenterology. 2002;49:567–71. [PubMed] [Google Scholar]