Abstract
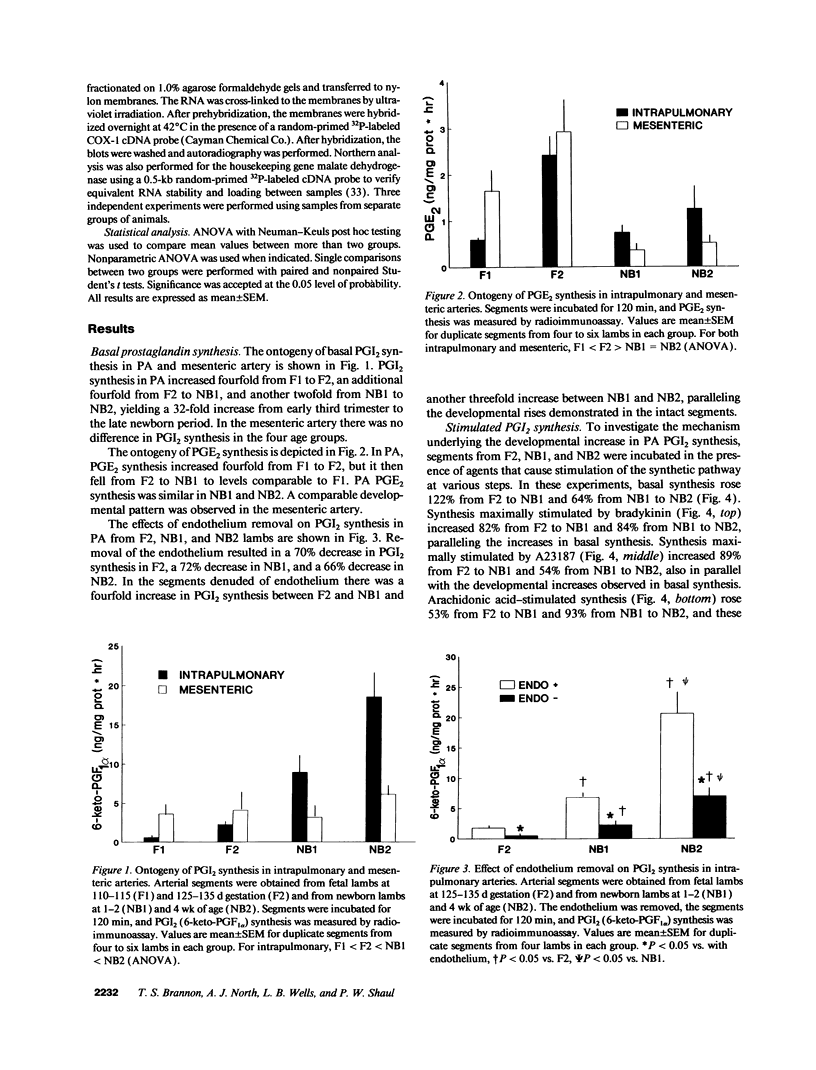
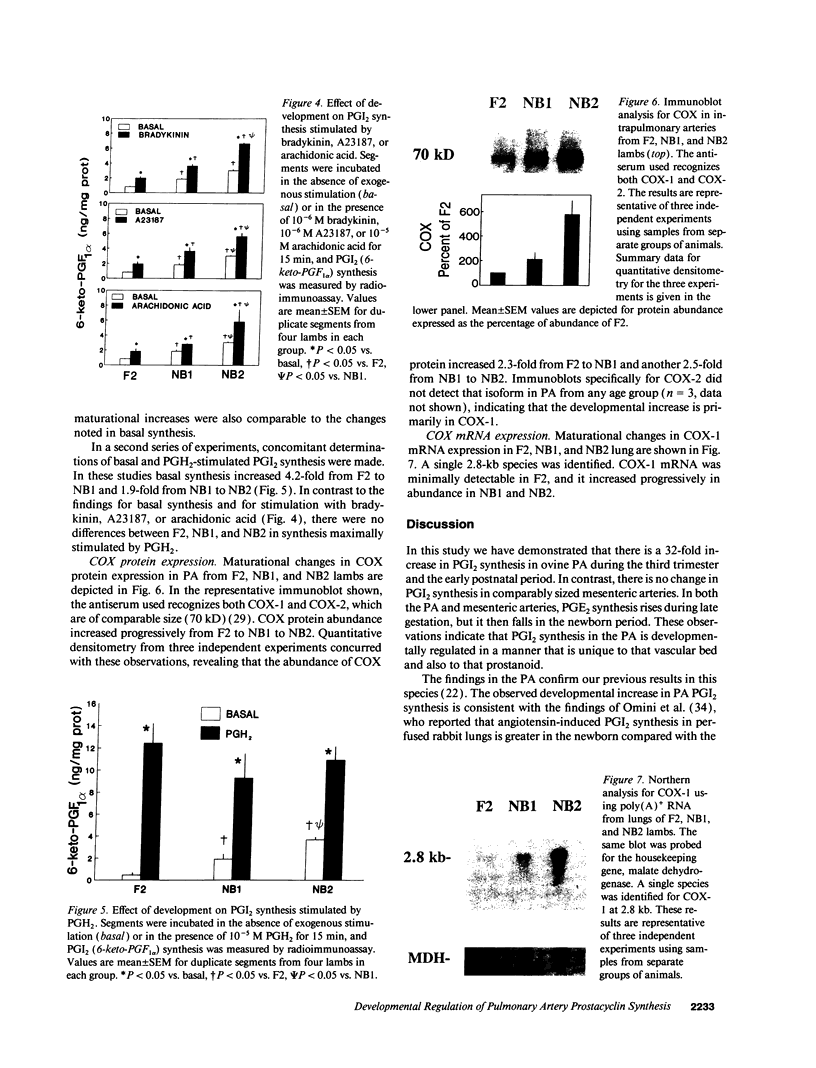
Prostacyclin (PGI2) is a key mediator of pulmonary vasomotor tone during late gestation and in the newborn, and its production in whole lung increases during that period. We investigated the developmental regulation of PGI2 synthesis in ovine intrapulmonary artery (PA) segments from 110 to 115 d (F1) and 125 to 135 d gestation fetal lambs (F2, term = 144 d) and 1- and 4-wk-old newborn lambs (NB1 and NB2). Basal PGI2 rose fourfold from F1 to F2, fourfold from F2 to NB1, and twofold from NB1 to NB2. In all age groups 66-72% of PGI2 was derived from the endothelium. Similar fold increases in PGI2 were observed with maturation in intact and endothelium-denuded segments. In intact PA from F2, NB1, and NB2, basal PGI2 synthesis and synthesis maximally stimulated by bradykinin, A23187, or arachidonic acid rose with development in a comparable manner. In contrast, PGI2 synthesis stimulated by exogenous PGH2, the product of cyclooxygenase, was similar at all ages. Immunoblot analyses of PA from F2, NB1, and NB2 revealed that there is a sixfold maturational increase in cyclooxygenase-1 protein; the cyclooxygenase-2 isoform was not detectable. Cyclooxygenase-1 mRNA abundance in whole lung also rose with development. Thus, PGI2 synthesis in ovine PA endothelium and vascular smooth muscle increases markedly during late fetal and early newborn life; the increase is due to a rise in cyclooxygenase activity related to enhanced expression of cyclooxygenase-1. We conclude that there is developmental regulation of PA cyclooxygenase-1 gene expression, and that this may be critical to successful cardiopulmonary transition and function in the newborn.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abman S. H., Accurso F. J. Acute effects of partial compression of ductus arteriosus on fetal pulmonary circulation. Am J Physiol. 1989 Aug;257(2 Pt 2):H626–H634. doi: 10.1152/ajpheart.1989.257.2.H626. [DOI] [PubMed] [Google Scholar]
- Abman S. H., Stenmark K. R. Changes in lung eicosanoid content during normal and abnormal transition in perinatal lambs. Am J Physiol. 1992 Feb;262(2 Pt 1):L214–L222. doi: 10.1152/ajplung.1992.262.2.L214. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badesch D. B., Orton E. C., Zapp L. M., Westcott J. Y., Hester J., Voelkel N. F., Stenmark K. R. Decreased arterial wall prostaglandin production in neonatal calves with severe chronic pulmonary hypertension. Am J Respir Cell Mol Biol. 1989 Dec;1(6):489–498. doi: 10.1165/ajrcmb/1.6.489. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davidson D. Pulmonary hemodynamics at birth: effect of acute cyclooxygenase inhibition in lambs. J Appl Physiol (1985) 1988 Apr;64(4):1676–1682. doi: 10.1152/jappl.1988.64.4.1676. [DOI] [PubMed] [Google Scholar]
- Fagan J. M., Goldberg A. L. Inhibitors of protein and RNA synthesis cause a rapid block in prostaglandin production at the prostaglandin synthase step. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2771–2775. doi: 10.1073/pnas.83.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Grant P. M., Tellam J., May V. L., Strauss A. W. Isolation and nucleotide sequence of a cDNA clone encoding rat mitochondrial malate dehydrogenase. Nucleic Acids Res. 1986 Aug 11;14(15):6053–6066. doi: 10.1093/nar/14.15.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R., Rojas J., Sundell H. Pulmonary vascular response to prostacyclin in fetal lambs. Prostaglandins. 1979 Dec;18(6):927–934. doi: 10.1016/0090-6980(79)90129-1. [DOI] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner J. J., Gwebu E. T., Panganamala R. V., Milo G. E., Cornwell D. C., Sharma H. M., Geer J. C. Fatty acids and their prostaglandin derivatives: inhibitors of proliferation in aortic smooth muscle cells. Science. 1977 Jul 15;197(4300):289–291. doi: 10.1126/science.877555. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffler C. W., Hessler J. R. Pulmonary and systemic vascular effects of exogenous prostaglandin I2 in fetal lambs. Eur J Pharmacol. 1979 Feb 15;54(1-2):37–42. doi: 10.1016/0014-2999(79)90405-9. [DOI] [PubMed] [Google Scholar]
- Leffler C. W., Tyler T. L., Cassin S. Effect of indomethacin on pulmonary vascular response to ventilation of fetal goats. Am J Physiol. 1978 Apr;234(4):H346–H351. doi: 10.1152/ajpheart.1978.234.4.H346. [DOI] [PubMed] [Google Scholar]
- Levin D. L., Rudolph A. M., Heymann M. A., Phibbs R. H. Morphological development of the pulmonary vascular bed in fetal lambs. Circulation. 1976 Jan;53(1):144–151. doi: 10.1161/01.cir.53.1.144. [DOI] [PubMed] [Google Scholar]
- Lock J. E., Olley P. M., Coceani F. Direct pulmonary vascular responses to prostaglandins in the conscious newborn lamb. Am J Physiol. 1980 May;238(5):H631–H638. doi: 10.1152/ajpheart.1980.238.5.H631. [DOI] [PubMed] [Google Scholar]
- Lock J. E., Olley P. M., Soldin S., Coceani F. Indomethacin-induced pulmonary vasoconstriction in the conscious newborn lamb. Am J Physiol. 1980 May;238(5):H639–H651. doi: 10.1152/ajpheart.1980.238.5.H639. [DOI] [PubMed] [Google Scholar]
- Marshall P. J., Kulmacz R. J., Lands W. E. Constraints on prostaglandin biosynthesis in tissues. J Biol Chem. 1987 Mar 15;262(8):3510–3517. [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Satoh K., Prescott S. M. Cultured endothelial cells synthesize both platelet-activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest. 1985 Jul;76(1):271–280. doi: 10.1172/JCI111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Niedermeyer M. E., Ogletree M. L., Brigham K. L. Pulmonary hypertension and increased vasoreactivity caused by repeated indomethacin in sheep. J Appl Physiol (1985) 1985 Aug;59(2):443–452. doi: 10.1152/jappl.1985.59.2.443. [DOI] [PubMed] [Google Scholar]
- O'Banion M. K., Winn V. D., Young D. A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki S., Ogino N., Yamamoto S., Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979 Feb 10;254(3):829–836. [PubMed] [Google Scholar]
- Omini C., Viganò T., Marini A., Pasargiklian R., Fano M., Maselli M. A. Angiotensin II: a releaser of PGI2 from fetal and newborn rabbit lungs. Prostaglandins. 1983 Jun;25(6):901–910. doi: 10.1016/0090-6980(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R. Prostaglandin biosynthesis and catabolism in the developing fetal sheep lung. Prostaglandins. 1977 Apr;13(4):649–660. doi: 10.1016/0090-6980(77)90235-0. [DOI] [PubMed] [Google Scholar]
- Powell W. S., Solomon S. Biosynthesis of prostaglandins and thromboxane B2 by fetal lung homogenates. Prostaglandins. 1978 Feb;15(2):351–364. doi: 10.1016/0090-6980(78)90175-2. [DOI] [PubMed] [Google Scholar]
- Printz M. P., Skidgel R. A., Friedman W. F. Studies of pulmonary prostaglandin biosynthetic and catabolic enzymes as factors in ductus arteriosus patency and closure. Evidence for a shift in products with gestational age. Pediatr Res. 1984 Jan;18(1):19–24. [PubMed] [Google Scholar]
- Rabinovitch M., Boudreau N., Vella G., Coceani F., Olley P. M. Oxygen-related prostaglandin synthesis in ductus arteriosus and other vascular cells. Pediatr Res. 1989 Oct;26(4):330–335. doi: 10.1203/00006450-198910000-00009. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Mullen M., Rosenberg H. C., Maruyama K., O'Brodovich H., Olley P. M. Angiotensin II prevents hypoxic pulmonary hypertension and vascular changes in rat. Am J Physiol. 1988 Mar;254(3 Pt 2):H500–H508. doi: 10.1152/ajpheart.1988.254.3.H500. [DOI] [PubMed] [Google Scholar]
- Reid L. M. The pulmonary circulation: remodeling in growth and disease. The 1978 J. Burns Amberson lecture. Am Rev Respir Dis. 1979 Apr;119(4):531–546. doi: 10.1164/arrd.1979.119.4.531. [DOI] [PubMed] [Google Scholar]
- Shaul P. W., Campbell W. B., Farrar M. A., Magness R. R. Oxygen modulates prostacyclin synthesis in ovine fetal pulmonary arteries by an effect on cyclooxygenase. J Clin Invest. 1992 Dec;90(6):2147–2155. doi: 10.1172/JCI116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul P. W., Farrar M. A., Magness R. R. Oxygen modulation of pulmonary arterial prostacyclin synthesis is developmentally regulated. Am J Physiol. 1993 Aug;265(2 Pt 2):H621–H628. doi: 10.1152/ajpheart.1993.265.2.H621. [DOI] [PubMed] [Google Scholar]
- Shaul P. W., Farrar M. A., Magness R. R. Prostacyclin synthesis and stimulation of cyclic AMP production in ovine fetal vasculature: heterogeneity in pulmonary and systemic arteries. Dev Pharmacol Ther. 1992;18(1-2):89–99. [PubMed] [Google Scholar]
- Shaul P. W., Farrar M. A., Zellers T. M. Oxygen modulates endothelium-derived relaxing factor production in fetal pulmonary arteries. Am J Physiol. 1992 Feb;262(2 Pt 2):H355–H364. doi: 10.1152/ajpheart.1992.262.2.H355. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Friedman W. F., Printz M. P. Prostaglandin biosynthetic activities of isolated fetal lamb ductus arteriosus, other blood vessels, and lung tissue. Pediatr Res. 1984 Jan;18(1):12–18. [PubMed] [Google Scholar]
- Smith W. L. Prostaglandin biosynthesis and its compartmentation in vascular smooth muscle and endothelial cells. Annu Rev Physiol. 1986;48:251–262. doi: 10.1146/annurev.ph.48.030186.001343. [DOI] [PubMed] [Google Scholar]
- Tyler T., Wallis R., Leffler C., Cassin S. The effects of indomethacin on the pulmonary vascular response to hypoxia in the premature and mature newborn goat. Proc Soc Exp Biol Med. 1975 Dec;150(3):695–698. doi: 10.3181/00379727-150-39108. [DOI] [PubMed] [Google Scholar]
- Van der Ouderaa F. J., Buytenhek M., Nugteren D. H., Van Dorp D. A. Purification and characterisation of prostaglandin endoperoxide synthetase from sheep vesicular glands. Biochim Biophys Acta. 1977 May 25;487(2):315–331. doi: 10.1016/0005-2760(77)90008-x. [DOI] [PubMed] [Google Scholar]
- van Grondelle A., Worthen G. S., Ellis D., Mathias M. M., Murphy R. C., Strife R. J., Reeves J. T., Voelkel N. F. Altering hydrodynamic variables influences PGI2 production by isolated lungs and endothelial cells. J Appl Physiol Respir Environ Exerc Physiol. 1984 Aug;57(2):388–395. doi: 10.1152/jappl.1984.57.2.388. [DOI] [PubMed] [Google Scholar]