Abstract
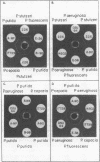
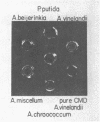
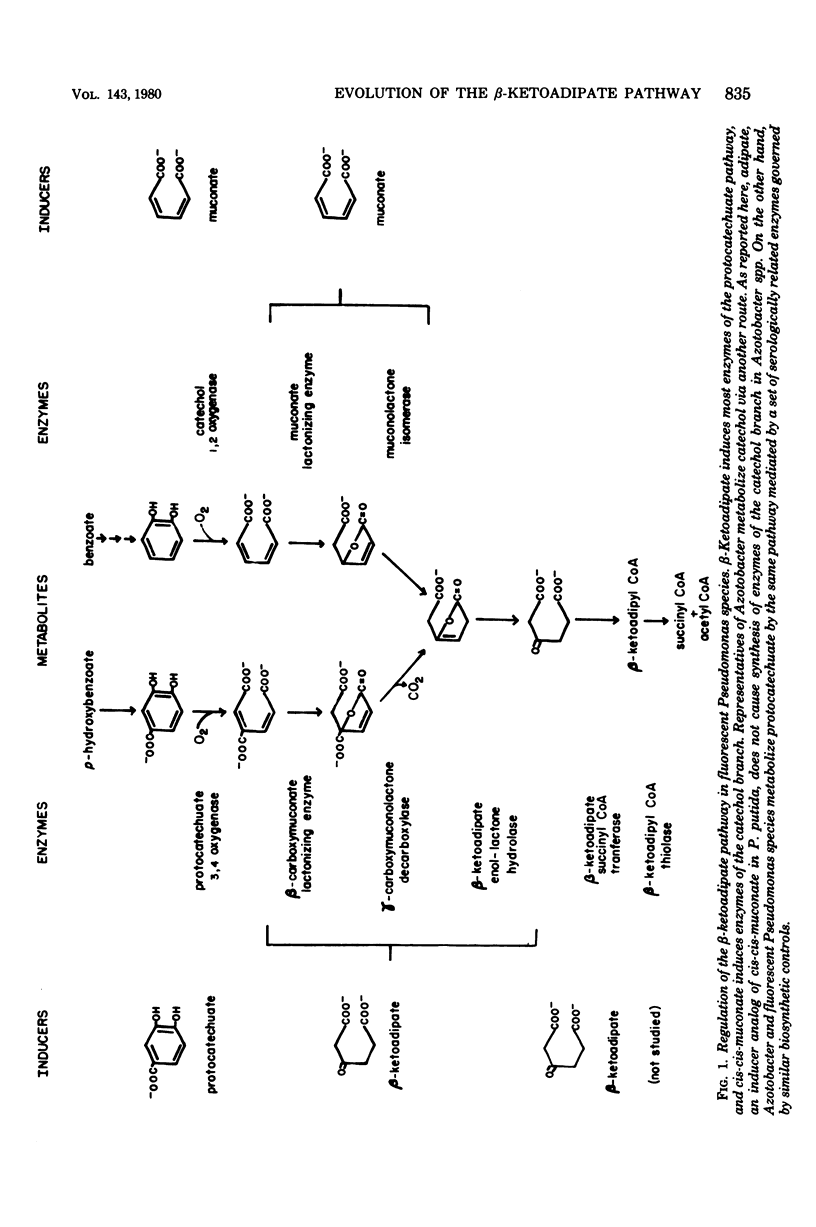
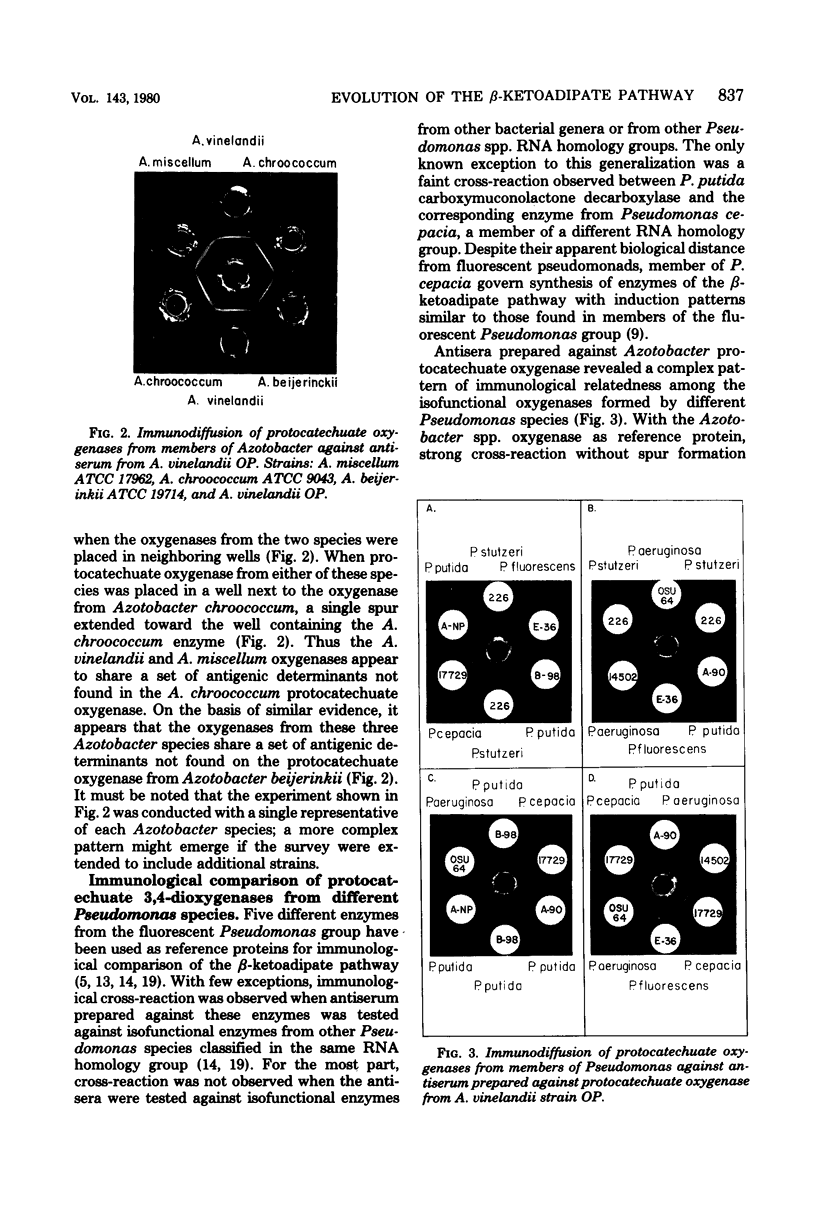
Intergeneric comparison of the three enzymes that initiate metabolism of protocatechuate in Azotobacter and Pseudomonas species revealed close immunological relatedness of isofunctional proteins. Furthermore, beta-ketoadipate induces all of the enzymes of the protocatechuate pathway (except protocatechuate oxygenase) in Azotobacter and in Pseudomonas species of the "fluorescent" and "cepacia" groups. This regulatory property sets the organisms apart from other bacteria. Protocatechuate oxygenase from Pseudomonas cepacia, like the enzyme from fluorescent Pseudomonas species, cross-reacts strongly with antiserum prepared against protocatechuate oxygenase from Azotobacter vinelandii. Double-diffusion experiments conducted with the antiserum revealed relatedness of Azotobacter spp. Protocatechuate oxygenases in the following order: A. vinelandii = Azotobacter miscellum greater than Azotobacter chroococcum greater than Azotobacter beijerinkii. The antiserum also revealed serological heterogeneity among Pseudomonas spp. protocatechuate oxygenases which were serologically indistinguishable in earlier studies using Pseudomonas aeruginosa protocatechuate oxygenase as reference protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bull C., Ballou D. P., Salmeen I. Raman spectrum of protocatechuate dioxygenase from Pseudomonas putida. New low frequency bands. Biochem Biophys Res Commun. 1979 Apr 13;87(3):836–841. doi: 10.1016/0006-291x(79)92033-3. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Durham D. R., Stirling L. A., Ornston L. N., Perry J. J. Intergeneric evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980 Jan 8;19(1):149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- Hardisson C., Sala-Trepat J. M., Stanier R. Y. Pathways for the oxidation of aromatic compounds by Azotobacter. J Gen Microbiol. 1969 Nov;59(1):1–11. doi: 10.1099/00221287-59-1-1. [DOI] [PubMed] [Google Scholar]
- Hou C. T., Lillard M. O. Immunological properties of protocatechuate 3, 4-dioxygenase isofunctional enzymes. J Bacteriol. 1976 Apr;126(1):516–519. doi: 10.1128/jb.126.1.516-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meagher R. B., McCorkle G. M., Ornston M. K., Ornston L. N. Inducible uptake system for -carboxy-cis, cis-muconate in a permeability mutant of Pseudomonas putida. J Bacteriol. 1972 Aug;111(2):465–473. doi: 10.1128/jb.111.2.465-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. The evolution of induction mechanisms in bacteria: insights derived from the study of the beta-ketoadipate pathway. Curr Top Cell Regul. 1977;12:209–262. doi: 10.1016/b978-0-12-152812-6.50011-1. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Ornston L. N., Yeh W. K. Origins of metabolic diversity: evolutionary divergence by sequence repetition. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3996–4000. doi: 10.1073/pnas.76.8.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D., Ornston L. N. Constitutive synthesis of enzymes of the protocatechuate pathway and of the beta-ketoadipate uptake system in mutant strains of Pseudomonas putida. J Bacteriol. 1976 Apr;126(1):272–281. doi: 10.1128/jb.126.1.272-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. IV. Muconolactone isomerase from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1974 Dec 10;249(23):7410–7419. [PubMed] [Google Scholar]
- Patel R. N., Orston L. N. Immunological comparison of enzymes of the beta-ketoadipate pathway. Arch Microbiol. 1976 Oct 11;110(1):27–36. doi: 10.1007/BF00416965. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Yeh W. K., Davis G., Fletcher P., Ornston L. N. Homologous amino acid sequences in enzymes mediating sequential metabolic reactions. J Biol Chem. 1978 Jul 25;253(14):4920–4923. [PubMed] [Google Scholar]
- Yoshida R., Hori K., Fujiwara M., Saeki Y., Kagamiyama H. Nonidentical subunits of protocatechuate 3,4-dioxygenase. Biochemistry. 1976 Sep 7;15(18):4048–4053. doi: 10.1021/bi00663a020. [DOI] [PubMed] [Google Scholar]