Abstract
Sphingosine-1-phosphate (S1P) is an important regulator of cellular functions via interaction with its receptors S1P1–5. To date, nothing is known about the S1P receptor expression and the effects of S1P signaling in Wilms tumor. In this study, we found ubiquitous expression of S1P receptors in Wilms tumor specimens and cell lines. We demonstrated that S1P1 acted as a promigratory modulator by employing S1P1 antagonist VPC44116, S1P1 siRNA and adenoviral transduction in Wilms tumor cells. Further, we clarified that S1P1-mediated migration occurred via Gi coupling and activation of PI3K and Rac1. In addition, S1P stimulated WiT49 cell invasion through S1P1/Gi signaling pathway. We consider that targeting S1P1 may be a point of therapeutic intervention in Wilms tumor.
Keywords: migration, invasion, sphingosine 1-phosphate, WiT49, Wilms tumor
Introduction
Wilms tumor is the most common malignant renal tumor in children. In the last few decades there have been major advances in our knowledge of the molecular basis of this tumor and in clinical outcomes. Despite this progress, our understanding of the events leading to Wilms tumor progression and metastasis is still limited. Children at risk for adverse outcome, such as those with nuclear anaplasia, a marker for chemoresistance, or those with loss of heterozygosity for chromosomes 1p and 16q, for example, must rely on adjuvant chemotherapy and/or radiotherapy for treatment [1]. Unfortunately, these therapies are associated with significant late sequelae, such as radiation-related scoliosis, gonadal failure, adverse effects on pregnancy, cardiomyopathy, and increased long-term risk of second malignancies [2–8]. Clearly, a better understanding of the stimuli and signaling pathways involved in Wilms tumor progression would be a substantial advancement.
Wilms and other renal tumors are typically angiogenic, invasive and potentially metastatic. Mortality is usually associated with a metastatic phenotype. Essential to the process of metastasis are cellular proliferation, migration and invasion. Recent studies indicate that the bioactive lipid sphingosine-1-phosphate (S1P) may play an important role in carcinogenesis by regulating angiogenesis, tumor cell proliferation, migration, invasion and metastasis [9]. The diverse and at times opposing actions of S1P are mediated by its coupling to different isotypes of the endothelial differentiation gene family of G protein-coupled receptors (GPCRs). Currently, five S1P receptors (S1P1–5) are known that bind S1P. Studies performed in human glioblastoma cell lines have shown that S1P potently stimulated tumor cell migration through Gi coupling while mediated inhibition via S1P2 [10,11], which was in accordance with the findings in B16 mouse melanoma cells [12,13]. Therefore, we hypothesized that in Wilms tumor, S1P receptor isotypic expression determines the migratory phenotype. That is, the relative expression of S1P1 to S1P2 determines the ability of S1P to promote or inhibit migration.
Herein, we detailed the results of our studies for the first time showing the expression of different S1P receptors in Wilms tumor specimens as well as Wilms tumor cell lines. Further, in WiT49 cells, a well-characterized Wilms tumor cell line derived from a primary lung metastasis [14], we demonstrated that the relative ratio of S1P1/S1P2 could determine the ability of Wilms tumor cells to migrate. We explored the pathways responsible for cell motility downstream of S1P/S1P1 signaling and found both PI3K and Rac1 were involved. Finally we found that Wilms tumor cells invaded in response to S1P, and that this activity is also driven by S1P1/Gi signaling.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS) and penicillin-streptomycin were purchased from Gibco (Grand Island, NY). Fatty-acid free BSA was purchased from Sigma (Saint Louis, MO). S1P was purchase from Biomol (Plymouth Meeting, PA) and JTE-013 was from Tocris Bioscience (Ellisville, MO). Pertussis toxin (PTX), wortmannin, and NSC23766 were purchased from Calbiochem (La Jolla, CA). Primary antibody for Rac1 was from BD (Becton Dickinson Labware, MA) and β-Actin antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). S1P1 (E49) monoclonal antibody was developed and characterized as described [15].
Cell culture, siRNA transfection and adenoviral transduction
The WiT49 cell line was kindly provided by Dr. Herman Yeger (Hospital for Sick Children, Toronto, Canada) and cultured as described previously [14]. The G401 and SK-NEP-1 cell lines were obtained from ATCC and cultured in DMEM supplemented with 15% FBS and antibiotics. Transfection of small interfering RNA (siRNA) oligonucleotide duplexes to block S1P1 expression was done using Oligofectamine reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Briefly, WiT49 cells were transfected with 100 nM S1P1 siRNA or non-specific (NS) siRNA (Dharmacon Lafayette, CO; S1P1 siRNA: GCAGCUCGGUCUCUGACUA; NS siRNA: UUCUCCGAACGUGUCACGUUU), and harvested 48 h later for different assays. For adenoviral transduction, cells were infected with adenovirus containing GFP, S1P1, S1P2, or dominant-negative Rac1 for 16–24 h (100–200 multiplicity of infection) before migration assay was done.
Quantitative real-time PCR
Total RNA was isolated from cells using RNA-STAT 60 following the manufacturer’s instructions and cDNA was generated from 1 μg RNA in the presence of random hexamer primers, deoxynucleoside triphosphates and Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA). PCR primers were designed using Primer Express™ 2.0 (Applied Biosystems) according to the software guidelines [10]. Sequences were as follows: 5′-GCAGCAGCAAGATGCGAAG-3′ (forward) and 5′-CGATGAGTGATCCAGGCTTTT-3′ (reverse) for the S1P1 gene, 5′-GGCCTAGCCAGTTCTGAAAGC-3′ (forward) and 5′-GCGTTTCCAGCGTCTCCTT-3′ (reverse) for the S1P2 gene, 5′-CTGGTGACCATCGTGATCCTC-3′ (forward) and 5′-ACGCTCACCACAATCACCAC-3′ (reverse) for the S1P3 gene, 5′-CCCTCTACTCCAAGCGCTACA-3′ (forward) and 5′-CCATAGAGGCCCATGATGGT-3′ (reverse) for the S1P4 gene, 5′-AGCAGAAGAGGGACTTGCCC-3′ (forward) and 5′-TCTGTGGTTTTCAACCTCCCAT-3′ (reverse) for the S1P5 gene, 5′-GACAGGATGCAGAAGGAGATTACT-3′(forward) and 5′-TGATCCACATCTGCTGGAAGGT-3′ (reverse) for the β-Actin gene and 5′-TGCACCACCAACTGCTTAGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGAG-3′ (reverse) for the GAPDH gene. Real-time PCR was performed using SYBR Green I DNA binding dye technology on an ABI Prism 7900 HT Sequence Detection System (PE Applied Biosystems, Foster City, CA). Results were expressed relative to the internal control gene GAPDH or β-Actin.
Immunohistochemistry analysis
Paraffin-embedded Wilms tumor specimens were deparaffized in xylene, rehydrated in ethanol and incubated with Tris-EDTA Buffer (10mM Tris, 1mM EDTA, 0.05% Tween 20, pH 9.0) in a steamer for 25 min, followed by incubation with 3% hydrogen peroxide at room temperature (RT) for 10 min to quench endogeneous peroxidase. After blocked with Power Block™ (BioGenex, San Ramon, CA) for 30 min at RT, slides were incubated with E49 monoclonal antibody in 1% BSA in PBS overnight at 4 °C in a humidified chamber. Then they were washed with PBS twice followed by incubation with biotinylated secondary antibody for 30 min at RT. Slides were then incubated in ABC reagent (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) for 30 min and color was developed in DAB substrate (DAB substrate kit, Vector Laboratories, Burlingame, CA) for 5–10 min. Cells were counterstained with hematoxylin, dehydrated with ethanol and xylene and mounted for analysis.
Cell migration assay
Migration assay was done in 96-well chemotaxis microchamber (Neuroprobe, Gaithersburg, MD), as described previously [16]. Briefly, polycarbonate filter with a pore size of 8 μm was coated with 50 μg/ml fibronectin. S1P or FTY720-P was diluted with 0.1% fatty acid-free BSA into appropriate concentrations and added into the lower chamber at 85 μl per well. Cells were serum starved in medium containing 0.1% fatty acid-free BSA for 2 h prior to trypsinizing and pretreated with or without different inhibitors for 10 min. Then they were placed in the upper compartment at 5 × 104 per well in 0.39 ml medium and allowed to migrate 5 h at 37°C in a humidified incubator with 5% CO2. After the incubation period the filter was taken out and nonmigrated cells were removed with a cotton swab. The filter was then fixed overnight with 4% paraformaldehyde in PBS at 4°C. Attached cells were stained with 0.1% crystal violet and eluted with 10% acetic acid in 96-well plates. Quantification was done based on absorbance at 600 nm by a Spectramax340 plate reader (Molecular Devices).
Cell invasion assay
Invasion assay was performed in 24-well modified Boyden chambers (Corning Costar, Cambridge, MA), which was coated with 1 mg/ml Matrigel (Becton Dickinson Labware, MA) and allowed to gel for 30 min at 37°C. In the standard assay, After 2 h serum starvation, cells were trypsinized, pretreated with or without different inhibitors for 10 min and added to the upper compartment of the chamber at 1 × 105 per well in 0.1 ml medium. The lower compartment contained 0.6 ml of serum-free DMEM supplemented with different concentrations of S1P. After 24 h incubation at 37°C, all non-invaded cells were removed from the upper face of the transwell membrane with a cotton swab; the invaded cells were fixed with 90% ethanol and stained with 0.1% crystal violet. The stained cells were then extracted with 10% acetic acid and the OD values were determined at 600 nm.
Western blot analysis
The treated WiT49 cells were washed with ice-cold PBS and homogenized in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl pH7.5, 500 mM NaCl, 10 mM MgCl2, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 1 mM sodium orthovanadate, 1 mM sodium fluoride, and 1 × protease inhibitor mixture). Samples were centrifuged at 14,000 g for 20 min at 4 °C, and protein concentrations of supernatants were determined by BCA protein assay Kit (Pierce, Rockford, IL). Equal amounts of protein were separated on SDS-PAGE and blotted to nitrocellulose membranes. The membranes were incubated with the indicated primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Immunoreactivity was visualized by exposure to X-ray film using Pierce ECL Western Blotting Substrate (Pierce Inc, Rockford, IL), according to the manufacturer’s instructions.
Affinity precipitation of Rac1-GTP
WiT49 cells were lysed in RIPA buffer after S1P stimulation and 500 μg of cell lysates were incubated with GST-PAK beads, as described previously [10,17]. Bound proteins were resolved on 12% SDS-PAGE and detected by western blot analysis.
Statistical Analysis
All experiments on cell lines were performed at least twice on separate occasions. The data are presented as means ± SD from a representative experiment. The statistical significance of differences between two groups was determined by Student’s t test using Microsoft Excel software.
Results
S1P receptors expression in Wilms tumor
The bioactive lipid S1P has been implicated in tumorigenesis through the regulation of critical steps including tumor cell proliferation, migration and invasion as a result of interaction with its cognate receptors [9,12,13]. To date, nothing is known about S1P receptors expression in Wilms tumor. Therefore, we examined S1P receptors expression in 10 fresh frozen Wilms tumor specimens from Children’s Oncology Group (COG) by quantitative real-time PCR analysis (Table SI). The result showed that S1P1, S1P2, S1P3 and S1P5 were variably expressed in all of them, but not S1P4. Interestingly, the level of S1P1 mRNA was much higher than all the others (Fig. 1A). Using purified E49 monoclonal antibody which is specific for human S1P1 [15] (Fig. S1), we confirmed that S1P1 was consistently expressed in all Wilms tumor specimens evaluated by immunohistochemistry analysis. The staining was most consistently and prominently visualized in vascular endothelial cells and in the blastemal component of tumors (Fig. 1B). However, epithelial component on average exhibited a similar staining intensity to that of the blastemal component while expression in the stromal component was minimal (Table I).
Figure 1.
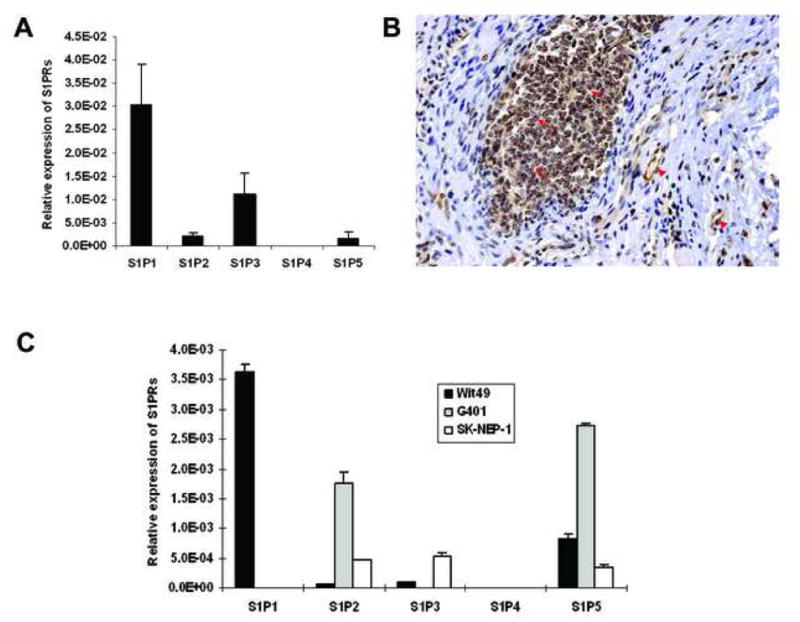
The ubiquitous expression of S1P receptors in Wilms tumor specimens and cell lines. (A) Quantitative real-time PCR for S1P receptors mRNA expression in 10 Wilms tumor samples from COG. Expression was normalized to the expression of the housekeeping gene β-Actin. Data are the mean±SE, n=10. (B) Immunohistochemistry analysis was done to detect S1P1 protein and photos were taken under the light microscope (×20). A typical picture from nine slides is shown. (Arrow, blastemal cells; arrowhead, vascular endothelial cells). (C) Quantitative real-time PCR for S1P receptors mRNA expression in Wilms tumor cells. Expression was normalized to the expression of the housekeeping gene GAPDH. Data are the mean±SD of triplicates.
Table I.
Staining intensity of S1P1 in different compartments of Wilms tumor
| Tumor compartment | # of specimens expressing S1P1 | Average staining intensity |
|---|---|---|
| Vascular endothelial cells | 9/9 | 2.3 |
| Blastemal | 7/8* | 2 |
| Epithelial | 7/7 | 2 |
| Stromal | 6/6 | 1.3 |
NOTE: All slides were reviewed in a blinded fashion by a renal pathologist (Dr. Harold Yamase). Staining was evaluated by tumor histologic category (blastemal, epithelial, stromal or vascular endothelial cells) and intensity scored as 0, 1, 2 or 3.
The specimen has no S1P1 expression is from a patient that had undergone preoperative chemotherapy.
To determine which S1P receptors are expressed in Wilms tumor cells, we also performed relative quantification of mRNA for each receptor by quantitative real-time PCR. All cell lines studied expressed several S1P receptors at varying levels, with S1P4 showing barely detectable levels (Fig. 1C). Specifically, WiT49 cells, a cell line derived from a primary lung metastasis of Wilms tumor, had comparatively high level of S1P1 and low S1P2. By contrast, G401 cells expressed high level of S1P2 but no S1P1. Another suspended pediatric renal tumor cell line SK- NEP-1 also showed relatively high expression of S1P2 and very low S1P1. On the basis of these results, we chose WiT49 and G401 cell lines for further studies evaluating the roles of S1P1 and S1P2 in cellular migration and invasion.
S1P regulates Wilms tumor cell migration
S1P may either stimulate or inhibit cellular migration depending on the cell type examined [12,18–20]. We therefore tested the effect of S1P on cell migration in these two Wilms tumor cell lines and found that S1P had a differential effect on them. The addition of S1P to the lower chamber markedly induced WiT49 cell migration in a concentration-dependent manner. This effect began at as low as 1 nM, with the maximal effect observed at 100 nM and reduced migration seen at higher concentration of 1 μM, giving a typical bell-shaped concentration-response curve (Fig. 2A). Using S1P analogue FTY720-phosphate (FTY720-P), which is an agonist for all S1P receptors except S1P2, we also found a similar migration effect (Fig. 2B). However, neither S1P nor FTY720-P could stimulate cell migration in G401 cells that had high expression of S1P2 and no S1P1 (data not shown).
Figure 2.
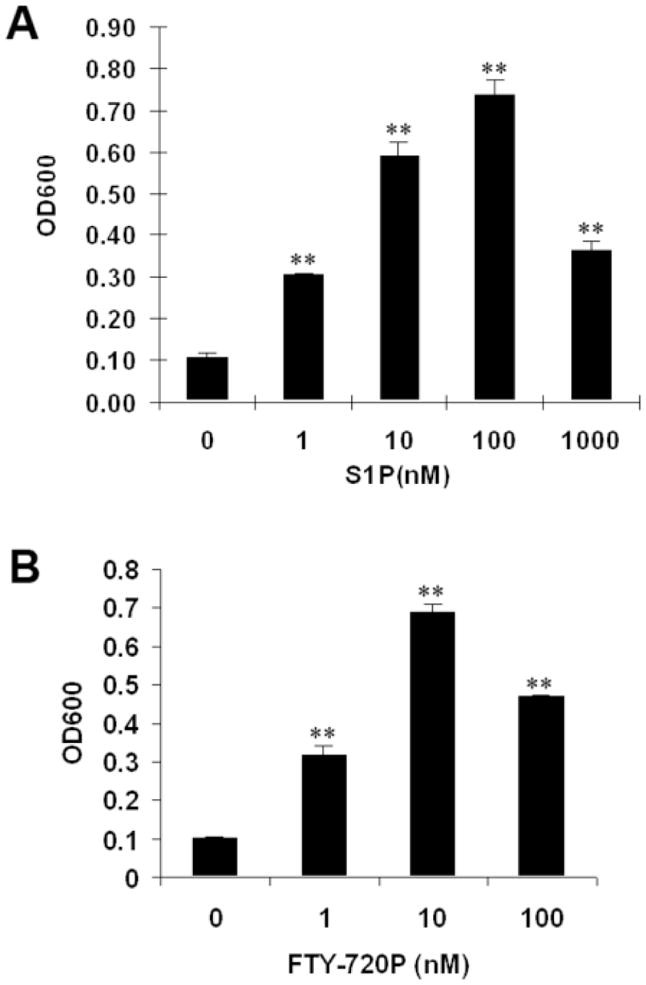
Effects of S1P and FTY720-P on WiT49 cell migration. Migration assays were done in WiT49 cells using S1P (A) and FTY720-P (B) at the indicated concentrations, separately. **, P < 0.01 versus without S1P (A) or FTY720-P (B).
S1P1 is promigratory while S1P2 is anti-migratory in Wilms tumor cells
To explore the unique effects of S1P receptors on cell migration, we employed a series of techniques in Wilms tumor cells. First, we used the S1P1 antagonist VPC44116 [21] and found it potently inhibited S1P-induced WiT49 cell migration in a concentration-dependent manner (Fig. 3A), which suggested that S1P-induced migration may occur via S1P1 signaling pathway.
Figure 3.
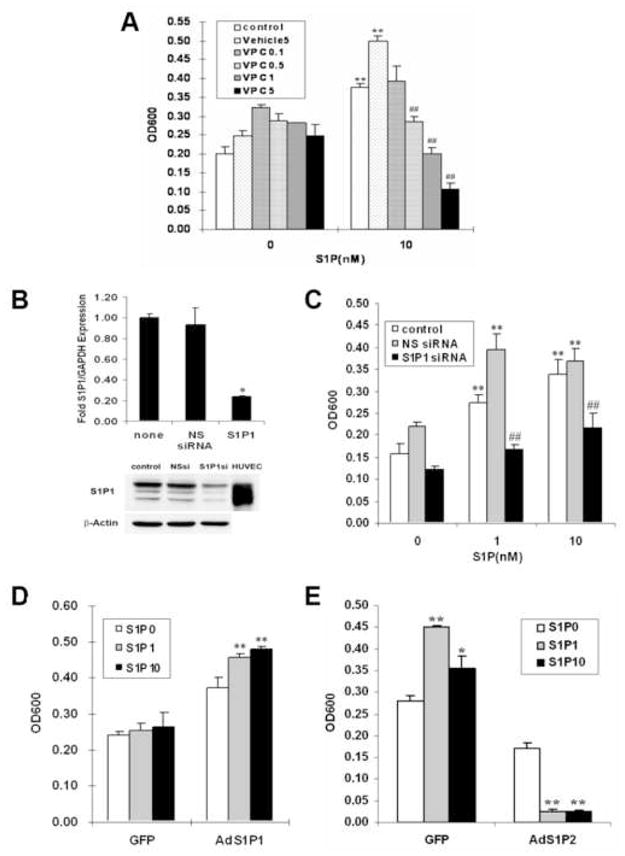
S1P1 is promigratory while S1P2 is antimigratory in Wilms tumor cells. (A) S1P1antagonist VPC44116 (0.1, 0.5, 1, 5 μM) blocked 10 nM S1P-induced migration in WiT49 cells. **, P< 0.01 versus without S1P; ##, P < 0.01 VPC versus vehicle control (5 μM) in S1P treatment group. (B) WiT49 cells were transfected with 100 nM S1P1 siRNA or NS siRNA, harvested 48 h later and assayed for the expression levels of S1P1 by quantitative real-time PCR (top) and western blot analysis (bottom). Columns in top of B, fold over untransfected (none). *, P< 0.05 versus NS siRNA. HUVEC in bottom of B is the positive control for S1P1 band. (C) Migration assay was done using the WiT49 cells transfected with 100 nM S1P1 siRNA or NS siRNA. **, P < 0.01 versus without S1P; ##, P < 0.01 versus NS siRNA in S1P treatment group. (D) G401 cells were infected with adenovirus overexpressing S1P1 or GFP as a control. After 16–24 h, cells were harvested and subjected to the migration assay with S1P (0, 1, 10 nM) stimulation. **, P < 0.01 versus without S1P. (E) Migration assay was done using the WiT49 cells overexpressing S1P2 or GFP with S1P (0, 1, 10 nM) stimulation. *, P < 0.05, **, P < 0.01 versus without S1P.
To substantiate this notion, we used siRNA technology to downregulate S1P1 expression in WiT49 cells. To validate this approach, we measured the mRNA and protein levels of S1P1 in cells treated with S1P1 siRNA at 48 h time point. The siRNA against S1P1 was extremely effective at reducing the expression levels of S1P1 by quantitative real-time PCR and western blot analysis (Fig. 3B), whereas the non-specific (NS) siRNA had no such effect. Treatment of WiT49 cells with this S1P1 siRNA effectively downregulated S1P-mediated migration while the NS siRNA did not (Fig. 3C).
Alternatively, we changed the balance of S1P1/S1P2 expression by adenoviral transduction in pediatric renal tumor cells. Introduction of S1P1 conferred migration upon G401 cells which previously did not migrate (Fig. S2A and 3D). This further confirmed that S1P1 is indeed responsible for S1P-induced migration in pediatric renal tumor cells. On the other hand, experiments were also done with WiT49 cells overexpressing S1P2, this resulted in significant inhibition, not stimulation, of migration in response to S1P (Fig. S2B and 3E). In agreement with our previous work on glioblastoma cells [10], these data demonstrate that S1P1 has a promigratory effect while S1P2 has anti-migratory effect in Wilms tumor cells.
S1P-induced migration is Gi dependent, and both PI3K and Rac1 are involved in this process
To investigate the pathways downstream of S1P1 in Wilms tumor cells, we tested the effect of S1P alone or in combination with PTX, a known inhibitor of Gi protein on WiT49 cells. PTX completely blocked S1P as well as FTY720-P mediated migration (Fig. 4A and B), which suggested that S1P-induced migration in WiT49 cells was Gi dependent.
Figure 4.
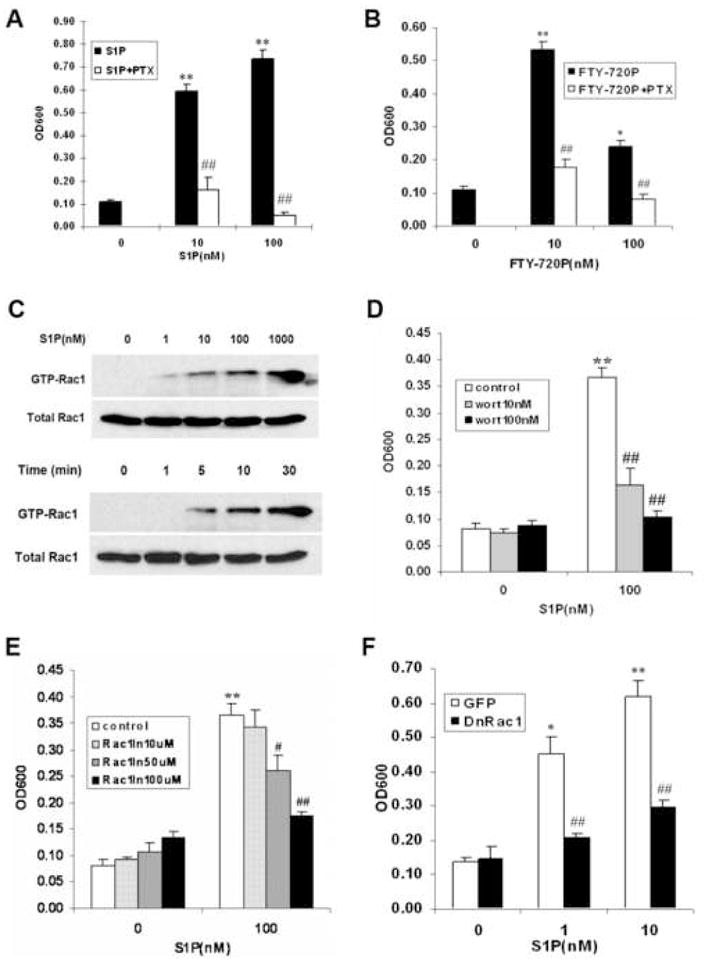
S1P-induced migration is Gi dependent, and both PI3K and Rac1 are involved in this process. (A and B) WiT49 cells migrated in the presence or absence of different concentrations of S1P (A), FTY720-P (B) and 400 ng/ml PTX. *, P < 0.05, **, P < 0.01 versus without S1P or FTY720-P; ##, P < 0.01 versus cells without PTX. (C) Rac1 was activated in response to S1P concentration-dependently (stimulation for 10 min) or time-dependently (with 100 nM S1P) by pull-down assay as described in Materials and Methods. (D and E) WiT49 cells were pretreated with PI3K inhibitor wortamannin (D) or Rac1 inhibitor NSC23766 (E) at the indicated concentrations for 10 min after serum starvation and migration assay was done with or without 100 nM S1P. **, P < 0.01 versus without S1P; #, P < 0.05, ##, P < 0.01 versus cells without inhibitors in S1P treatment groups. (F) Migration assay was done using the WiT49 cells overexpressing dominant-negative Rac1 or GFP with S1P (0, 1, 10 nM) stimulation. *, P < 0.05, **, P < 0.01 versus without S1P; ##, P < 0.01 versus GFP in S1P treatment groups.
Signaling through PI3K and Rac1 as well as MAPK pathways has been shown to mediate S1P-induced migration [22–24]. Pull-down assays showed that Rac1 was rapidly activated by S1P in a time- and concentration-dependent manner (Fig. 4C). Treatment of these cells with the PI3K inhibitor wortmannin and Rac1 inhibitor NSC23766 strongly blocked S1P-mediated migration (Fig. 4D and E), which suggested that both PI3K and Rac1 were involved in this process. The pivotal role of Rac1 in S1P-induced migration of WiT49 cells was further confirmed by using the dominant negative mutant, N19 Rac. Migration of WiT49 cells overexpressing dominant negative Rac1 was significantly inhibited compared to control cells overexpressing GFP (Fig. S3 and 4F), indicating that Rac1 was required for S1P-induced WiT49 cell migration. However, inhibition of three MAPK pathways by using p38 inhibitor SB203580, ERK inhibitor U0126 and JNK inhibitor SP600125 did not change the effects of S1P on cell migration (data not shown), which suggested that MAPK pathways were not involved in this process.
Taken together, these data indicate that S1P induces WiT49 cell migration through a Gi/PI3K/Rac1 dependent pathway.
S1P potently stimulates Wilms tumor cell invasion via S1P1/Gi signaling
Having shown that S1P can induce cell migration in Wilms tumor cells, we were interested in knowing whether S1P could induce cell invasion, a critical step for tumor metastasis. Results showed that S1P stimulated WiT49 cell invasion concentration-dependently (Fig. 5A), and this effect was also Gi-protein linked (Fig. 5B). Using S1P1 antagonist VPC44116 and S1P2 antagonist JTE-013 [25] we further found that blockade of S1P1 signaling completely abolished S1P-induced invasion while blockade of S1P2 signaling did not (Fig. 5B). These data suggest that S1P1 is responsible for S1P-induced invasion while S1P2 inhibits invasion in Wilms tumor, in a similar pattern to cell migration.
Figure 5.
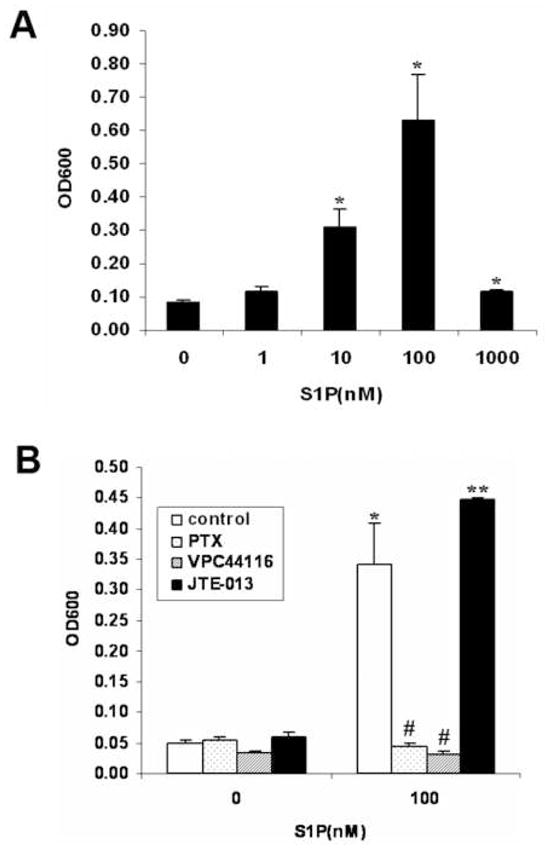
S1P stimulated WiT49 cell invasion via S1P1/Gi signaling. (A) S1P stimulated WiT49 cell invasion in a concentration dependent manner. *, P < 0.05 versus without S1P. (B) WiT49 cells were pretreated with S1P1 antagonist VPC44116 (1 μM), S1P2 antagonist JTE-013 (1 μM) or PTX (100 ng/ml) for 10 min after serum starvation and invasion assay was done in the presence or absence of 100 nM S1P. *, P < 0.05, **, P < 0.01 versus without S1P; #, P < 0.05 versus control cells without inhibitors in S1P treatment group.
Discussion
The bioactive lipid S1P regulates different cellular functions by binding to its five specific G- protein coupled receptors (S1P1–5) [26]. Interaction between S1P and S1P receptors is known to regulate many important physiologic events. For example, mutation of a subclass of S1P receptors can cause aberrant cardiac development as a consequence of abnormal cell migration among cardiac precursor cells [27]. Liu and coworkers have shown that S1P1 plays an essential role in vascular development. S1P1 knockout mice died in utero owing to defective migration of vascular smooth muscle cells [28]. S1P has also been shown to be an important regulator of cellular crosstalk, causing transactivation of VEGF receptors in endothelial cells [29]. Recently, it has been reported that S1P has a potent immunoregulatory role as evidenced by studies employing the immunosuppressive agent FTY720. Briefly, interaction between FTY720 and S1P1 resulted in a lymphopenic effect [30]. Additional work has revealed a potent and multifaceted role for S1P in CNS function, skeletal system, limb development and the reproductive tract [31]. These discoveries have stimulated tremendous interest in this lipid mediator and its receptors as potential therapeutic targets.
Increasingly, S1P and its receptors are implicated as important regulators of tumor cell proliferation, migration, invasion and metastasis. Work in this area has been done in cancer cells such as glioblastoma cells [10], B16 melanoma cells [12,13], human thyroid [18], gastric [20] and ovarian cancer cells [32]. With the successful use of FTY720 [33] and a biospecific anti-S1P monoclonal antibody [34] to inhibit tumor progression in several murine tumor models, S1P and its receptors, especially S1P1, have come to be regarded as the potential targets in cancer therapy. Currently nothing is known about the role of S1P signaling in Wilms or other pediatric renal tumors. Therefore, first we performed quantitative real-time PCR on 10 fresh frozen Wilms tumor specimens to examine S1P receptors expression. Results show that Wilms tumors expressed multiple S1P receptors while the expression of S1P1 was much higher than those of the others (Fig. 1A). IHC staining for the first time confirmed expression of S1P1 in Wilms tumor (Fig. 1B). Preliminary evaluation suggests that it is mainly expressed in the blood vessels and blastemal components. Interestingly, one specimen that exhibited no or little S1P1 staining in the blastemal compartment was from a patient that had undergone preoperative chemotherapy (Asterisk in Table I) which suggests that S1P1 might be a novel target of chemotherapy in Wilms tumor. Subsequently, we performed quantitative real-time PCR on three cell lines to detect the S1P receptors profile. Two cell lines WiT49, a metastatic Wilms tumor cell line and G401 demonstrated differential expression of S1P receptors, such that WiT49 preferentially expressed S1P1 while G401 expressed high level of S1P2 but no S1P1.
S1P has previously been reported to be a common mitogen for many cell types [24]. The effect of S1P on proliferation was investigated in Wilms tumor cells by MTT assay. However, S1P failed to stimulate cell proliferation in all three cell lines tested. These results indicate the exogenously added S1P did not stimulate proliferation of Wilms tumor cell lines.
However, we found that S1P has a profound and differential effect on pediatric renal tumor cell motility. WiT49 cells migrated in response to S1P, giving a typical bell-shaped dose-response curve while G401 did not migrate at all (Fig. 2). To explain this differential effect of S1P on cell migration, we next proved that S1P1 has promigratory effect while S1P2 mediates inhibition of migration in Wilms tumor cells by employing S1P1 antagonist, S1P1 siRNA and adenovirus overexpressing S1P1 or S1P2 in these cells (Fig. 3). Briefly, antagonism or silencing of S1P1 could shut down S1P-driven migration in WiT49 cells, indicating that S1P1 is a chemoattractant receptor. This finding was confirmed in G401 cells overexpressing S1P1 by adenoviral transduction. Importantly, it clarifies that G401 cells are able to migrate to S1P, and are not lacking of downstream signaling machinery. Conversely, overexpression of S1P2 in WiT49 cells completely reversed the effect of S1P on cell migration. It did not stimulate but inhibited WiT49 cell migration, which parallels other reports that define S1P2 as a chemorepellant receptor [12,20]. This data is also congruous with our own work in human GB cells [10].
S1P regulation of cellular migration occurs through the Rho family of GTPases (Rac1, Rho, CDC42). Insight into the differential effects that S1P has on cell migration was initially derived from experiments performed in non-malignant cells, including CHO cells, Swiss 3T3, and COS7 cells. G12/13 coupling by S1P2 results in inhibition of Rac and activation of Rho with subsequent inhibition of cell motility [35]. Conversely, S1P1 couples exclusively to Gi and activates Rac1 that then stimulates cell migration [23]. In our research, we also studied the downstream effectors of S1P-mediated migration. Our experiments demonstrated that S1P1-mediated migration occurs via GPCR -Gi coupling and activation of downstream PI3K and Rac1 (Fig. 4). Moreover, it seemed that Rac1 did not act as downstream of activated PI3K in WiT49 cells since pretreated WiT49 cells with PI3K inhibitor wortamannin could not block Rac1 activation in the pull-down assay (data not shown).
Notably, our invasion assay found that S1P signaling regulates not only migration, but also invasion in Wilms tumor cells (Fig. 5). Interestingly, this effect was also Gi protein linked and could be blocked by S1P1 antagonist VPC44116, suggesting that S1P1 was responsible for this effect. This is in accordance with recent findings by Yamaguchi et al. that overexpression of S1P1 into mouse B16 melanoma cells could greatly aggravate lung metastasis 3 weeks after injection into mouse tail veins [13]. However, more comprehensive studies on primary tumors are warranted to determine whether S1P1 expression has value in predicting subsequent invasion, tumor sequelae development after surgery, and poor prognosis.
In summary, the bioactive lipid S1P regulates Wilms tumor cell migration by interaction with its specific receptors. Two of them, S1P1 and S1P2, exert opposing effects on cell migration such that the former is promigratory and the latter is antimigratory. In addition, S1P-induced migration appears to occur via Gi coupling, and both P13K and Rac1 are involved in this process. More importantly, S1P also stimulated tumor cell invasion via S1P1/Gi signaling. As cell migration and invasion are the initial critical steps in tumor metastasis, S1P/S1P1 signaling pathway represents a new and potentially important source of prognostic information and therapeutic intervention in Wilms tumor. Given the intense interest in pharmacotherapeutics targeting this pathway [36], further studies evaluating its role in cancer progression are warranted.
Supplementary Material
The specificity of the purified E49 monoclonal antibody. Immunohistochemistry analysis was done as described in Material and Methods using the purified E49 monoclonal antibody and photos were taken under the light microscope (×20). (A) negative control, 293 cells, second antibody alone; (B) control, 293 cells; (C) 293 cells overexpressing S1P1.
The effectiveness of overexpression of S1P1 or S1P2 by adenoviral transduction in pediatric renal tumor cells. (A) G401 cells were infected with adenovirus overexpressing S1P1 or GFP with MOI 100, harvested 24 h later and assayed for the expression of S1P1 by western blot analysis. (B) WiT49 cells were infected with adenovirus overexpressing V5-S1P2 or GFP with MOI 100, harvested 24 h later and assayed for the expression of V5 by western blot analysis.
The effectiveness of overexpression of DnRac1 by adenoviral transduction in WiT49 cells. WiT49 cells were infected with adenovirus overexpressing DnRac1 or GFP with MOI 100, harvested 24 h later and assayed for the expression of Rac1 by western blot analysis.
Acknowledgments
This work was supported by NIH grant k08DK070468A and the Seraph Foundation. Dr. Teresa Sanchez is partially supported by AHA Scientist Development Grant. Wilms tumor specimens were obtained through collaboration with the Children’s Oncology Group Biopathology Center. We thank Dr. Herman Yeger for the WiT49 cell line, Novartis Pharma for FTY720-P, Dr. Martin Schwartz for GST-PAK construct, Dr. William Sessa for dnRac virus, and Xiaohong Wang for technical support.
Footnotes
Conflicts of Interest Statement
None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy PE, Breslow NE, Li S, Perlman E, Beckwith JB, Ritchey ML, Shamberger RC, Haase GM, D’Angio GJ, Donaldson M, Coppes MJ, Malogolowkin M, Shearer P, Thomas PR, Macklis R, Tomlinson G, Huff V, Green DM. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 2.Benoist MR, Lemerle J, Jean R, Rufin P, Scheinmann P, Paupe J. Effects of pulmonary function of whole lung irradiation for Wilm’s tumour in children. Thorax. 1982;37:175–180. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeler RM, Wolff JE, Anderson RA, Coppes MJ. Long-term complications and post-treatment follow-up of patients with Wilms’ tumor. Semin Urol Oncol. 1999;17:55–61. [PubMed] [Google Scholar]
- 4.Green DM, Peabody EM, Nan B, Peterson S, Kalapurakal JA, Breslow NE. Pregnancy outcome after treatment for Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2002;20:2506–2513. doi: 10.1200/JCO.2002.07.159. [DOI] [PubMed] [Google Scholar]
- 5.Othman F, Guo CY, Webber C, Atkinson SA, Barr RD. Osteopenia in survivors of Wilms tumor. Int J Oncol. 2002;20:827–833. [PubMed] [Google Scholar]
- 6.Paulino AC, Wen BC, Brown CK, Tannous R, Mayr NA, Zhen WK, Weidner GJ, Hussey DH. Late effects in children treated with radiation therapy for Wilms’ tumor. Int J Radiat Oncol Biol Phys. 2000;46:1239–1246. doi: 10.1016/s0360-3016(99)00534-9. [DOI] [PubMed] [Google Scholar]
- 7.Scheibel-Jost P, Pfeil J, Niethard FU, Fromm B, Willich E, Kuttig H. Spinal growth after irradiation for Wilm’s tumour. Int Orthop. 1991;15:387–391. doi: 10.1007/BF00186885. [DOI] [PubMed] [Google Scholar]
- 8.Trebo MM, Mann G, Dworzak M, Zoubek A, Gadner H. Wilms tumor and cardiomyopathy. Med Pediatr Oncol. 2003;41:574. doi: 10.1002/mpo.10398. [DOI] [PubMed] [Google Scholar]
- 9.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids—receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 10.Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–3795. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- 11.Van Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi: 10.1016/s0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 12.Arikawa K, Takuwa N, Yamaguchi H, Sugimoto N, Kitayama J, Nagawa H, Takehara K, Takuwa Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor. Requirement of inhibition of cellular RAC activity. J Biol Chem. 2003;278:32841–32851. doi: 10.1074/jbc.M305024200. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi H, Kitayama J, Takuwa N, Arikawa K, Inoki I, Takehara K, Nagawa H, Takuwa Y. Sphingosine-1-phosphate receptor subtype-specific positive and negative regulation of Rac and haematogenous metastasis of melanoma cells. Biochem J. 2003;374:715–722. doi: 10.1042/BJ20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alami J, Williams BR, Yeger H. Derivation and characterization of a Wilms’ tumour cell line, WiT 49. Int J Cancer. 2003;107:365–374. doi: 10.1002/ijc.11429. [DOI] [PubMed] [Google Scholar]
- 15.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- 17.Paik JH, Chae S, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J Biol Chem. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. [DOI] [PubMed] [Google Scholar]
- 18.Balthasar S, Samulin J, Ahlgren H, Bergelin N, Lundqvist M, Toescu EC, Eggo MC, Tornquist K. Sphingosine 1-phosphate receptor expression profile and regulation of migration in human thyroid cancer cells. Biochem J. 2006;398:547–556. doi: 10.1042/BJ20060299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita H, Kitayama J, Shida D, Yamaguchi H, Mori K, Osada M, Aoki S, Yatomi Y, Takuwa Y, Nagawa H. Sphingosine 1-phosphate receptor expression profile in human gastric cancer cells: differential regulation on the migration and proliferation. J Surg Res. 2006;130:80–87. doi: 10.1016/j.jss.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Foss FW, Jr, Snyder AH, Davis MD, Rouse M, Okusa MD, Lynch KR, Macdonald TL. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg Med Chem. 2007;15:663–677. doi: 10.1016/j.bmc.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baudhuin LM, Cristina KL, Lu J, Xu Y. Akt activation induced by lysophosphatidic acid and sphingosine-1-phosphate requires both mitogen-activated protein kinase kinase and p38 mitogen-activated protein kinase and is cell-line specific. Mol Pharmacol. 2002;62:660–671. doi: 10.1124/mol.62.3.660. [DOI] [PubMed] [Google Scholar]
- 23.Taha TA, Argraves KM, Obeid LM. Sphingosine-1-phosphate receptors: receptor specificity versus functional redundancy. Biochim Biophys Acta. 2004;1682:48–55. doi: 10.1016/j.bbalip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Takuwa Y. Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2002;1582:112–120. doi: 10.1016/s1388-1981(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 26.Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem. 1990;265:9308–9313. [PubMed] [Google Scholar]
- 27.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo A, Nagashima K, Kurose H, Mochizuki S, Matsuda M, Mochizuki N. Sphingosine 1-phosphate induces membrane ruffling and increases motility of human umbilical vein endothelial cells via vascular endothelial growth factor receptor and CrkII. J Biol Chem. 2002;277:23747–23754. doi: 10.1074/jbc.M111794200. [DOI] [PubMed] [Google Scholar]
- 30.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 31.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Park KS, Kim MK, Lee HY, Kim SD, Lee SY, Kim JM, Ryu SH, Bae YS. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochem Biophys Res Commun. 2007;356:239–244. doi: 10.1016/j.bbrc.2007.02.112. [DOI] [PubMed] [Google Scholar]
- 33.LaMontagne K, Littlewood-Evans A, Schnell C, O’Reilly T, Wyder L, Sanchez T, Probst B, Butler J, Wood A, Liau G, Billy E, Theuer A, Hla T, Wood J. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–231. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- 34.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The specificity of the purified E49 monoclonal antibody. Immunohistochemistry analysis was done as described in Material and Methods using the purified E49 monoclonal antibody and photos were taken under the light microscope (×20). (A) negative control, 293 cells, second antibody alone; (B) control, 293 cells; (C) 293 cells overexpressing S1P1.
The effectiveness of overexpression of S1P1 or S1P2 by adenoviral transduction in pediatric renal tumor cells. (A) G401 cells were infected with adenovirus overexpressing S1P1 or GFP with MOI 100, harvested 24 h later and assayed for the expression of S1P1 by western blot analysis. (B) WiT49 cells were infected with adenovirus overexpressing V5-S1P2 or GFP with MOI 100, harvested 24 h later and assayed for the expression of V5 by western blot analysis.
The effectiveness of overexpression of DnRac1 by adenoviral transduction in WiT49 cells. WiT49 cells were infected with adenovirus overexpressing DnRac1 or GFP with MOI 100, harvested 24 h later and assayed for the expression of Rac1 by western blot analysis.


