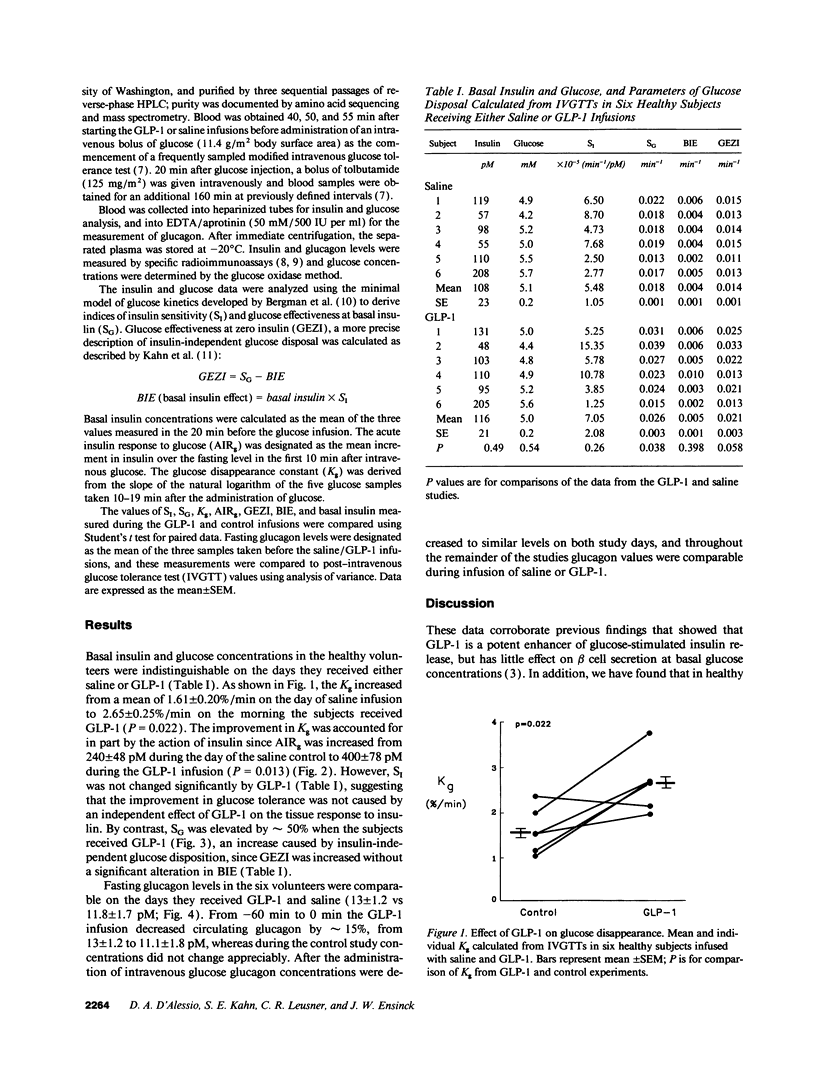
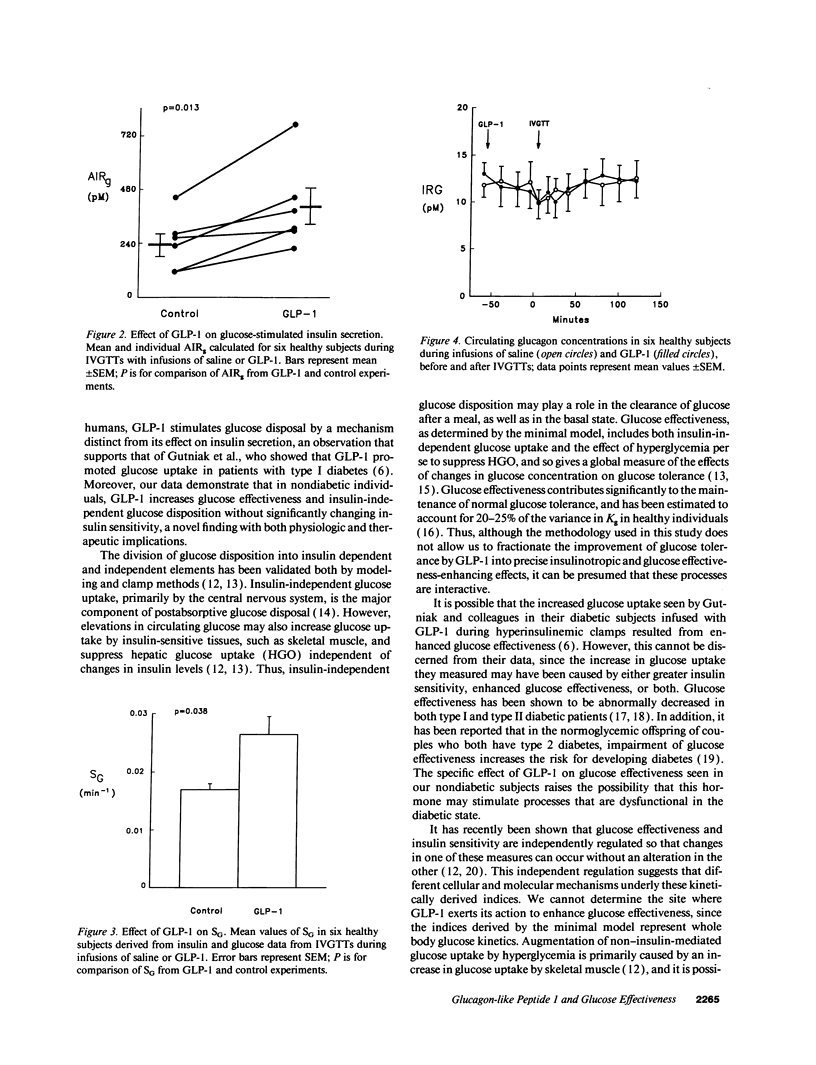
Abstract
Glucagon-like peptide 1 [7-36 amide] (GLP-1) has been shown to enhance insulin secretion in healthy and type II diabetic humans, and to increase glucose disposal in type I diabetic patients. To further define its action on glucose kinetics, we studied six healthy subjects who received either GLP-1 (45 pmol/kg per h) or 150 mM saline on two mornings during which a modified intravenous glucose tolerance test was performed. Plasma insulin and glucose levels were analyzed using Bergman's minimal model of glucose kinetics to derive indices of insulin sensitivity (SI) and glucose effectiveness at basal insulin (SG), the latter a measure of glucose disposition independent of changes in insulin. In addition, basal insulin concentrations, the acute insulin response to glucose (AIRg), plasma glucagon levels, and the glucose disappearance constant (Kg) were measured on the days that subjects received GLP-1 or saline. Compared with saline infusions, GLP-1 increased the mean Kg from 1.61 +/- 0.20 to 2.65 +/- 0.25%/min (P = 0.022). The enhanced glucose disappearance seen with GLP-1 was in part the result of its insulinotropic effect, as indicated by a rise in AIRg from 240 +/- 48 to 400 +/- 78 pM (P = 0.013). However, there was also an increase in SG from 1.77 +/- 0.11 to 2.65 +/- 0.33 x 10(-2).min-1 (P = 0.038), which was accounted for primarily by insulin-independent processes, viz glucose effectiveness in the absence of insulin. There was no significant effect of GLP-1 on SI or basal insulin, and glucagon levels were not different during the glucose tolerance tests with or without GLP-1. Thus, GLP-1 improves glucose tolerance both through its insulinotropic action and by increasing glucose effectiveness. These findings suggest that GLP-1 has direct effects on tissues involved in glucose disposition. Furthermore, this peptide may be useful for studying the process of insulin-independent glucose disposal, and pharmacologic analogues may be beneficial for treating patients with diabetes mellitus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ader M., Pacini G., Yang Y. J., Bergman R. N. Importance of glucose per se to intravenous glucose tolerance. Comparison of the minimal-model prediction with direct measurements. Diabetes. 1985 Nov;34(11):1092–1103. doi: 10.2337/diab.34.11.1092. [DOI] [PubMed] [Google Scholar]
- Baron A. D., Brechtel G., Wallace P., Edelman S. V. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988 Dec;255(6 Pt 1):E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Beard J. C., Bergman R. N., Ward W. K., Porte D., Jr The insulin sensitivity index in nondiabetic man. Correlation between clamp-derived and IVGTT-derived values. Diabetes. 1986 Mar;35(3):362–369. doi: 10.2337/diab.35.3.362. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Ider Y. Z., Bowden C. R., Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979 Jun;236(6):E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- Bergman R. N. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989 Dec;38(12):1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- Drucker D. J. Glucagon and the glucagon-like peptides. Pancreas. 1990 Jul;5(4):484–488. doi: 10.1097/00006676-199007000-00018. [DOI] [PubMed] [Google Scholar]
- Finegood D. T., Hramiak I. M., Dupre J. A modified protocol for estimation of insulin sensitivity with the minimal model of glucose kinetics in patients with insulin-dependent diabetes. J Clin Endocrinol Metab. 1990 Jun;70(6):1538–1549. doi: 10.1210/jcem-70-6-1538. [DOI] [PubMed] [Google Scholar]
- Gutniak M., Orskov C., Holst J. J., Ahrén B., Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992 May 14;326(20):1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- Göke R., Fehmann H. C., Göke B. Glucagon-like peptide-1(7-36) amide is a new incretin/enterogastrone candidate. Eur J Clin Invest. 1991 Apr;21(2):135–144. doi: 10.1111/j.1365-2362.1991.tb01802.x. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Malvaux P., Lambert A. E. Glucagon immunoassay using polyethylene glycol to precipitate antibody-bound hormone. Diabetologia. 1974 Feb;10(1):61–68. doi: 10.1007/BF00421415. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Bergman R. N., Schwartz M. W., Taborsky G. J., Jr, Porte D., Jr Short-term hyperglycemia and hyperinsulinemia improve insulin action but do not alter glucose action in normal humans. Am J Physiol. 1992 Apr;262(4 Pt 1):E518–E523. doi: 10.1152/ajpendo.1992.262.4.E518. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Klaff L. J., Schwartz M. W., Beard J. C., Bergman R. N., Taborsky G. J., Jr, Porte D., Jr Treatment with a somatostatin analog decreases pancreatic B-cell and whole body sensitivity to glucose. J Clin Endocrinol Metab. 1990 Oct;71(4):994–1002. doi: 10.1210/jcem-71-4-994. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Prigeon R. L., McCulloch D. K., Boyko E. J., Bergman R. N., Schwartz M. W., Neifing J. L., Ward W. K., Beard J. C., Palmer J. P. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes. 1994 Apr;43(4):587–592. doi: 10.2337/diab.43.4.587. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Mueller G. L., Cherrington A. D., Perry J. M., Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979 Jan;48(1):171–175. doi: 10.1210/jcem-48-1-171. [DOI] [PubMed] [Google Scholar]
- Martin B. C., Warram J. H., Krolewski A. S., Bergman R. N., Soeldner J. S., Kahn C. R. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992 Oct 17;340(8825):925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- Nauck M. A., Heimesaat M. M., Orskov C., Holst J. J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993 Jan;91(1):301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov C., Jeppesen J., Madsbad S., Holst J. J. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991 Feb;87(2):415–423. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr Brain metabolism during fasting. J Clin Invest. 1967 Oct;46(10):1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacca L., Hendler R., Sherwin R. S. Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab. 1978 Nov;47(5):1160–1163. doi: 10.1210/jcem-47-5-1160. [DOI] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde I., Mérida E., Delgado E., Trapote M. A., Villanueva-Peñacarrillo M. L. Presence and characterization of glucagon-like peptide-1(7-36) amide receptors in solubilized membranes of rat adipose tissue. Endocrinology. 1993 Jan;132(1):75–79. doi: 10.1210/endo.132.1.8380388. [DOI] [PubMed] [Google Scholar]
- Welch S., Gebhart S. S., Bergman R. N., Phillips L. S. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab. 1990 Dec;71(6):1508–1518. doi: 10.1210/jcem-71-6-1508. [DOI] [PubMed] [Google Scholar]
- Zaharko D. S., Beck L. V. Studies of a simplified plasma insulin immunoassaay using cellulose powder. Diabetes. 1968 Jul;17(7):444–459. doi: 10.2337/diab.17.7.444. [DOI] [PubMed] [Google Scholar]



