Abstract
Background
We sought to reduce local recurrence for retroperitoneal sarcomas by using a coordinated strategy of advanced radiation techniques and aggressive en-bloc surgical resection.
Methods
Proton-beam radiation therapy (PBRT) and/or intensity-modulated radiation therapy (IMRT) were delivered to improve tumor target coverage and spare selected adjacent organs. Surgical resection of tumor and adjacent organs was performed to obtain a disease-free anterior margin. Intraoperative electron radiation therapy (IOERT) was delivered to any close posterior margin.
Results
Twenty patients had primary tumors and eight had recurrent tumors. Tumors were large (median size 9.75 cm), primarily liposarcomas and leiomyosarcomas (71%), and were mostly of intermediate or high grade (81%). PBRT and/or IMRT were delivered to all patients, preferably preoperatively (75%), to a median dose of 50 Gy. Surgical resection included up to five adjacent organs, most commonly the colon (n = 7) and kidney (n = 7). Margins were positive for disease, usually posteriorly, in 15 patients (54%). IOERT was delivered to the posterior margin in 12 patients (43%) to a median dose of 11 Gy. Surgical complications occurred in eight patients (28.6%), and radiation-related complications occurred in four patients (14%). After a median follow-up of 33 months, only two patients (10%) with primary disease experienced local recurrence, while three patients (37.5%) with recurrent disease experienced local recurrence.
Conclusions
Aggressive resection of retroperitoneal sarcomas can achieve a disease-negative anterior margin. PBRT and/or IMRT with IOERT may possibly deliver sufficient radiation dose to the posterior margin to control microscopic residual disease. This strategy may minimize radiation-related morbidity and reduce local recurrence, especially in patients with primary disease.
Soft tissue sarcomas (STS) are uncommon, heterogeneous tumors that arise from tissues of mesodermal origin.1 Approximately 15% of STS occur in the retroperitoneum, and thus there are only approximately 1600 retroperitoneal sarcomas (RPS) in the United States per year.2 Given the very low incidence of RPS, there have been few completed prospective, randomized trials examining the optimal treatment of these tumors, and there are marked institutional biases regarding optimal local therapy.
The primary therapy for RPS is complete surgical resection. There has been much recent controversy over the aggressiveness of surgical resection that should be performed for retroperitoneal STS. A group from Milan, Italy, recently advocated a policy of liberal en-bloc resection of adjacent organs, and a retrospective comparison of patients before and after the adoption of this policy revealed improved local recurrence (58% vs. 28%) with the more aggressive strategy.3 At the same time, a multicenter retrospective review of surgical patients in France found that compartmental resection of contiguous organs was associated on multivariate analysis with a 3.29-fold lower rate of local recurrence compared to only complete gross tumor resection.4 An accompanying editorial pointed out that the strategy of resecting some contiguous organs, such as the kidney and colon, while leaving other contiguous organs, such as the inferior vena cava and aorta, may be flawed, and that the Milan and French study results may be due to case selection bias and other confounding variables.5
There are also contrasting opinions regarding the role of adjuvant radiotherapy for RPS. For extremity STS, surgical resection and adjuvant radiation can result in local recurrence rates of <10%.6 Even with a positive microscopic margin, adequate doses of radiation (>64 Gy) can often sterilize residual microscopic disease and result in local recurrence rates of <20%, although some studies have not found such high control rates even with higher radiation doses.7,8 In contrast to extremity STS, RPS are more difficult to irradiate because they arise adjacent to radiation-sensitive organs such as the kidney, liver, and small bowel. Given the difficulty of delivering adequate doses of radiation to RPS, many institutions treat these tumors with surgery alone, and local recurrence rates are generally ≥40%.9–11 Furthermore, local recurrence rather than distant metastasis is the primary cause of death for patients with RPS.9
The past decade has seen the development and evolution of radiotherapy techniques that allow for more accurate delivery of radiation to tumors situated in difficult anatomic locations including the retroperitoneum. Intensity-modulated radiation therapy (IMRT) is a method of delivering external-beam radiotherapy that uses photon radiation beams with varying fluences across multiple radiation fields.12 These radiation fields are designed by sophisticated computer planning programs with inverse planning methods, such that radiation dose is delivered to the planning treatment volume (PTV) in three dimensions while limiting dose to prescribed limits for radiation-sensitive organs. Proton-beam radiation therapy (PBRT) involves the use of protons (or the nuclei of hydrogen atoms) rather than traditional photons.13 Protons are particles (in contrast to traditional photons, which are best characterized as electromagnetic waves) that reduce the radiation dose to normal tissues (i.e., integral dose) by approximately 60% because of a lower entrance dose and the near absence of any exit dose. This allows delivery to the prescription dose to the tumor with much greater sparing of adjacent organs and structures than is possible with photons. Intraoperative electron radiation therapy (IOERT) involves the use of a radiotherapy machine in the operating room to deliver high doses of electron radiation to the tumor bed with most radiation-sensitive organs and structures retracted away.14 A few series have reported on the use of IOERT and IMRT for RPS, but to our knowledge, PBRT for RPS has not been previously reported.15–18
Our institution's results with traditional external-beam photon radiation and IOERT for RPS were published in 2001.16 Local control for patients receiving total gross resection, external-beam radiation, and IOERT was 74%, and local control for patients receiving total gross resection and external-beam radiation without IOERT was 61%. In 2003, we began a strategy for the treatment of RPS that incorporated IMRT, PBRT, and/or IOERT with a synchronized surgical strategy. Patients were treated preoperatively with either PBRT or IMRT to a dose of 45–50.4 Gy. Aggressive surgical resection with removal of contiguous organs was then performed with a goal of removing all gross residual disease and obtaining a microscopically negative margin along the anterior surface of the tumor. The best margin possible was attempted along the posterior margin (retroperitoneal musculature and fat) and along any contiguous organ/structure left in situ. The tumor specimen was then analyzed by frozen section to determine the adequacy of the surgical margin. IOERT was delivered to the any close or positive posterior margin or margin along an unresected contiguous organ or vital structure. For patients in whom a diagnosis of STS could not be obtained before surgery or patients who were too symptomatic to tolerate preoperative radiation, aggressive surgery was initially performed and IOERT was delivered to the tumor bed. An omental flap was then placed along the tumor bed and postoperative radiation was then delivered by PBRT.
Here we describe a series of 28 patients treated at our institution with aggressive surgical resection along with IMRT, PBRT, and/or IOERT.
Methods
Patients
Patients with either primary or isolated recurrent retroperitoneal or pelvic STS were considered for this treatment strategy. Patients with tumors that could not be irradiated within the dose constraints of adjacent normal tissues were excluded. Patients who had multifocal disease or whose disease was deemed unresectable were also excluded. Twenty-eight patients treated with the described strategy between September 2003 and October 2008 were added to the Massachusetts General Hospital institutional review board-approved sarcoma database and followed prospectively. All patients were evaluated by a complete history and physical examination. Radiological imaging included abdomen/pelvis computed tomographic (CT) scan and sometimes a magnetic resonance imaging scan of the primary site, along with a chest CT scan. Cases, radiological imaging, and pathology slides were reviewed at a weekly Massachusetts General Hospital multidisciplinary sarcoma conference. The treatment plan was individualized for each patient by consensus after open-format discussion.
External-Beam Radiation Therapy
For patients with RPS who could tolerate preoperative therapy, an image-guided biopsy avoiding any transperitoneal approach was performed to establish the diagnosis. Once the diagnosis was established, patients received PBRT, IMRT, or both. Because normal tissue sparing with PBRT is greatest for large tumors (i.e., the larger the tumor, the larger the volume of normal tissue beyond it that will be spared by the exit dose), PBRT was used preferentially in patients with large tumors that were adjacent to liver, both kidneys, or large volumes of small bowel. There was often a waiting list for PBRT, given the limited availability of treatment slots. Hence, many of the patients slotted for treatment with PBRT would start their treatment with IMRT (18–30.6 Gy in 10–17 fractions over 2–3.5 weeks) to avoid a delay in initiation of radiotherapy and then transition to PBRT. Some patients, particularly those with smaller tumors away from the liver, could be treated to the planned preoperative radiation dose with IMRT alone. For both IMRT and PBRT, patients were planned and treated supine with legs placed in a foam immobilizer and the torso on a wing board fitted with posts that patients would grip with their arms extended above the head. All patients underwent radiation-planning CT scans, which were performed with oral and intravenous contrast with the patient in the treatment position.
For preoperative radiotherapy, the gross tumor volume (GTV) was contoured on each axial slice. For mobile tumors near the diaphragm, respiratory gating with the RPM system (Varian Medical, Palo Alto, CA) was used beginning in 2005 for patients with >1 cm target motion. The clinical target volume (CTV) was considered the tissues adjacent to the GTV judged to be involved on a subclinical basis. This volume was generated by expanding the GTV by 1.5 cm and then editing the CTV to eliminate tissues thought to be at low risk for involvement, such as those with an intervening fascial or bony barrier interposed between that tissue and the tumor. The expansion for the PTV for IMRT was 0.5 cm; for PBRT, the apertures were designed such that the prescription isodoses were 3 mm wider than the CTV and PTV. The prescription doses were to deliver 45 Gy in 25 fractions to the CTV and a 5.4-Gy boost to the GTV if permitted by normal tissue. For PBRT, doses were prescribed to the isodose lines that encompassed the target volumes. For IMRT, the aim was for the prescription isodose to encompass >95% of the planning target volumes. For both IMRT and PBRT, no more than 10% of the PTV was to receive >110% of the prescription dose. If any postoperative boost radiation was provided, this was administered exclusively with PBRT.
For postoperative radiotherapy, the preoperative GTV was constructed by manual review of the preoperative CT and by clips placed at surgery or generated by fusing the postoperative planning CT scan with the preoperative diagnostic CT scan. The expansion for the CTV was generated as described above for preoperative CTV with a 1.5-cm expansion, but the editing of the CTV was much greater, in general excluding much of the volume previously occupied by tumor, but now often just filled with bowel or other viscera not really at risk for subclinical disease. The expansions for planning target volumes for IMRT were also 0.5.cm, and the proton apertures were designed to treat the CTVs with 0.3-cm lateral margins. Dose prescriptions were the same as those for preoperative external-beam radiotherapy. For patients who had received intraoperative radiation, the postoperative external-beam radiation dose was 45–50.4 Gy in 25 to 28 fractions. Only six patients did not receive preoperative radiation. Of these patients, three received intraoperative radiation to doses of 10, 12.5, and 15 Gy, respectively. The postoperative radiation dose in these six patients was a median of 50.2 Gy with a range of 37.5–66.6 Gy. Except for one patient who received 37.5 Gy postoperatively, the remaining patients received 45 Gy to the initial CTV, and a boost to the areas of margin positivity (particularly if bowel could be excluded by use of interposed omentum) of 0–21.6 Gy with a median of 5.4 Gy.
Organs at risk were also contoured. These included the liver, the spinal cord, both kidneys, small and large bowel, and the stomach. No more than 20% of the liver was to receive the prescription dose; only ≤40% of the liver volume was to receive a dose of ≥30 Gy, and no more than 50% of the liver could receive more than 25 Gy. At least two-thirds of the volume of one functioning kidney was to receive a radiation dose of <20 Gy. Ipsilateral kidney sparing was not a planning aim if the ipsilateral kidney was located in the target volume, provided the opposite kidney had been demonstrated to be normal. No segment of the spinal cord could receive more than 48 Gy. In general, the small bowel dose limit was 50.4 Gy, although focal areas of one wall of the small bowel could receive 57.6 Gy if the dose was falling off across the bowel with protons or IMRT. Stomach irradiation dose was limited to 55 Gy, and the colon dose was limited to 60 Gy; generally the posterior wall of a portion of the colon was in the boosted volume.
Surgery
Five to 7 weeks after preoperative radiotherapy, patients underwent surgical resection of their tumors. The surgical approach usually included positioning patients in a modified lateral decubitus position (except for midline tumors) and making generous incisions. Thoracoabdominal incisions were performed for upper abdominal tumors abutting the diaphragm. Tumors and adjacent organs were then circumferentially dissected from anterior to posterior with the goal of aggressively resecting adjacent organs en bloc to obtain a microscopically negative anterior margin. The posterior margin was then sharply dissected under direct vision with the goal of obtaining the best possible posterior margin. The surgeon then took the resected specimen to the operating room pathology laboratory, the specimen was oriented for the sarcoma pathologist, and the closest margin or margins as assessed by the surgeon were analyzed by frozen section.
Patients who were too symptomatic to tolerate preoperative radiotherapy or in whom image-guided biopsy results could not establish a diagnosis underwent initial surgical resection. A frozen section was performed on the surgical specimen to establish the diagnosis, and IOERT was then delivered to the tumor bed. An omental flap was then mobilized and placed into the tumor bed, and postoperative proton-beam radiation was delivered.
Intraoperative Radiotherapy
For patients considered for IOERT, the radiation oncologist reviewed the operative findings and joined the surgeon in the operating room pathology laboratory to review the surgical margins. IOERT was delivered to any close or positive posterior margin when technically feasible with a dedicated linear accelerator with up to 18 meV built into one of institution's operating rooms. The IOERT treatment volume was determined jointly by the radiation oncologist and surgeon to cover the regions at highest risk. Applicators (circular, ellipsoid, or rectangular) in various sizes were used to direct the IOERT beam; in some patients with large tumors, two adjacent fields were abutted. Customized shielding (multiple layers of 1/64-in. lead, with thickness determined by the electron energy used) was available to further shape the field and shield normal tissues not thought to be at risk. Bolus (0.5–1.0 cm) was used where necessary to increase the surface dose as required by the configuration of disease. After selection and immobilization of the applicator with a Buckwalter retractor and customized attachments, the patient on the operating room table was positioned underneath the linear accelerator, and the applicator was docked with the electron cone. The correct position of the applicator was confirmed by periscope before the delivery of IOERT. During IOERT, patients were ventilated with 100% oxygen and monitored by video camera. Typically a single dose of 10 Gy was provided for completely resected tumors, 12.5–15 Gy was delivered for microscopic disease, and 15–20 Gy was delivered for macroscopic residual disease. Electron energies in the range of 6–15 meV were prescribed to the 90% isodose.
Clinical Data
Clinical information regarding patients was obtained from the Massachusetts General Hospital's medical records and sarcoma database. Recorded patient data included age, sex, symptoms, and physical examination findings. Characteristics of the primary tumor were recorded and included size, site, and grade. All biopsy and surgical specimens were analyzed by experienced sarcoma pathologists (A.P.R. or G.P.N.), who determined grade (I, low; II, intermediate; III, high) and margin of resection (microscopically negative, microscopically positive, grossly positive). Grossly positive margin was defined as a resection in which the surgeon thought gross tumor was left unresected. Microscopically positive was defined as the presence of tumor cells at the inked specimen margin. Information regarding radiotherapy was obtained from the MGH Department of Radiation Oncology sarcoma database. Operative reports and the operating room logs were reviewed for all patients.
Median follow-up was 33 months. For follow-up, patients were followed every 3 months for the first 2 years and every 6 months from year 3 through year 5. Patients with low-grade tumors were followed through year 10. Chest, abdomen, and pelvis CT scans were obtained at every visit for high-grade tumors. Chest X-ray and abdomen/pelvis CT scans were obtained at every visit or every other visit for low-grade tumors. The location and time of initial local and distant recurrences were recorded.
Statistical Analysis
Local recurrence-free survival, distant recurrence-free survival, and disease-specific survival were determined for all patients. Survival curves were estimated by the Kaplan–Meier method.19 Clinical variables were associated with survival by the univariate Cox proportional hazard model.20
Results
Disease Manifestation and Tumor Characteristics
This study analyzes 28 patients with a confirmed pathologic diagnosis of RPS treated at one institution between September 2003 and October 2007 with the treatment strategy described above. Twenty patients had primary disease, and eight patients had isolated local recurrence (Table 1). The median age of all patients was 56 years (range 33–85 years). Slightly more than half of patients were women. Fifty-seven percent of patients manifested symptoms due to tumor, while the remaining patients had their tumors discovered incidentally. The most common symptom exhibited when the patient sought care was abdominal pain or discomfort (n = 11); other symptoms included those consistent with the mass effect within the abdomen (e.g., early satiety, anorexia, weight loss, n = 4), compression of nerves or blood vessels (e.g., lower extremity pain, numbness, or edema; n = 4), intratumoral bleeding (n = 2), mass effect on the lungs (e.g., shortness of breath; n = 1), and hematuria (n = 1). For those with symptoms, the median length of time with symptoms was 4 months, and six patients had symptoms for ≥12 months. Six patients (21%) had a palpable abdominal mass that was found at physical examination.
TABLE 1. Patient and tumor characteristics.
| Characteristic | All patients | Patients with primary tumors | Patients with local recurrence |
|---|---|---|---|
| Number of patients | 28 | 20 | 8 |
| Median age, years (range) | 56 (33–85) | 54 (51–85) | 60 (33–84) |
| Female sex, n (%) | 15 (53.5%) | 12 (60.0%) | 3 (37.5%) |
| Symptoms, n (%) | |||
| Yes | 16 (57.1%) | 14 (70.0%) | 2 (25.0%) |
| No | 12 (42.9%) | 6 (30.0%) | 6 (75.0%) |
| Median tumor size, cm (range) | 9.75 (3.7–30) | 9.75 (4.5–30) | 9.65 (3.7–23) |
| Histology, n (%) | |||
| Liposarcoma | 14 (50.0%) | 8 (40.0%) | 6 (75%) |
| Leiomyosarcoma | 6 (21.4%) | 6 (30.0%) | 0 (0%) |
| Other | 8 (28.6%) | 6 (30.0%) | 2 (25.0%) |
| Grade, n (%) | |||
| Low | 5 (17.9%) | 3 (15.0%) | 2 (25%) |
| Intermediate | 9 (32.1%) | 5 (25.0%) | 4 (50%) |
| High | 12 (42.9%) | 11 (55.0%) | 1 (12.5%) |
| Not specified | 2 (7.1%) | 1 (5.0%) | 1 (12.5%) |
The median size of all tumors was 9.75 cm (Table 1). There was no marked size difference between primary tumors and recurrent tumors. In terms of histological subtype, half of tumors were liposarcomas. This included four well-differentiated liposarcomas, eight well-differentiated/dedifferentiated liposarcomas, and two myxoid liposarcomas. Six tumors were leiomyosarcomas (including two tumors arising with the inferior vena cava), two were malignant fibrous histiocytomas, two were carcinosarcomas, two were malignant peripheral nerve sheath tumors, one was a pleomorphic fibrosarcoma, and one was a myxofibrosarcoma. Two-thirds of tumors were intermediate- or high-grade.
Pretreatment Biopsy
Four patients did not undergo biopsy before the initiation of treatment. Two of these patients underwent initial surgery without preoperative biopsy as a result of severe symptoms, and the other two patients had presumed local recurrence of their original tumor. The remaining 24 patients underwent pretreatment biopsy, and results of 23 (96%) of these biopsies established the diagnosis of sarcoma. The one patient who had an incorrect diagnosis on the basis of pretreatment biopsy was a patient with underlying neurofibromatosis who had a biopsy sample of a 13.5-cm retroperitoneal mass that showed neurofibroma. The tumor was marginally resected, and frozen section analysis still showed neurofibroma. However, the final pathological diagnosis was malignant peripheral nerve sheath tumor arising within a neurofibroma. This patient subsequently underwent a repeat exploration along with IOERT followed by postoperative radiotherapy. The correct histological subtype of STS was diagnosed in 19 patients (76%).
Surgical Therapy
Twenty-five of 28 operations were performed by one surgeon (S.S.Y). Twenty-five patients (89.3%) had complete gross resection of their tumor, while three patients (10.7%) had a small amount of gross tumor left unresected (Table 2). Six tumors (21.4%) were resected without requiring removal of adjacent organs. The remaining 22 tumors required resection of adjacent organs to clear the anterior surgical margin, with a median of two adjacent organs resected (range 0–5 organs). Resected organs or vital structures included colon (n = 7), kidney (n = 7), major vein (e.g., inferior vena cava [IVC], iliac vein) (n = 7), adrenal gland (n = 5), distal pancreas (n = 3), spleen (n = 3), gallbladder (n = 3), small bowel (n = 2), major artery (e.g., aorta, iliac artery) (n = 3), diaphragm, liver, major nerve, iliac bone, and testicle (all n = 1). Resection of muscle (e.g., abdominal wall, psoas, or iliacus muscles) was not counted as an adjacent organ or vital structure resection. Median operating time was 4 h and 17 min. Eight patients (28.6%) had one or more postoperative complication within the first 30 days after operation. These included postoperative ileus (n = 5), bleeding requiring reoperation (n = 2), urinary tract infection (n = 2), wound infection, pneumonia, and arrhythmia (all n = 1). Median length of stay was 6 days (range 3–12 days). There were no postoperative deaths.
TABLE 2. Surgical therapy.
| Characteristic | All patients | Patients with primary tumors | Patients with local recurrence |
|---|---|---|---|
| Number of patients | 28 | 20 | 8 |
| Median number of adjacent organs resected (range) | 1 (0–5) | 2 (0–5) | 0.5 (0–3) |
| Median operating time, min (range) | 257 (80–552) | 274.5 (138–552) | 240.5 (80–420) |
| Margin, n (%) | |||
| Microscopically negative | 13 (46.4%) | 11 (55.0%) | 2 (25.0%) |
| Microscopically positive | 12 (42.9%) | 7 (35.0%) | 5 (62.5%) |
| Grossly positive | 3 (10.7%) | 2 (10.0%) | 1 (12.5%) |
| Median length of stay, days (range) | 6 (3–12) | 6.5 (3–12) | 4 (3–6) |
For each case, the resection specimen was taken to the operating room pathology laboratory, and the specimen was oriented for the sarcoma pathologist by the attending surgeon. The attending surgeon identified the closest surgical margin for the pathologist, and this margin was analyzed by frozen section. Frozen section assessment of the closest margin was microscopically negative in 13 specimens and microscopically close or positive in 12 patients. Three specimens that were assessed intraoperatively as microscopically negative were ultimately assessed as microscopically positive. Thus, on final pathology, margins were microscopically negative in 13 patients, microscopically positive in 12 patients, and grossly positive in 3 patients. Microscopically negative margins were achieved more frequently in patients with primary tumors (11 of 20, 55%) as opposed to recurrent tumors (2 of 8, 25%).
Radiotherapy
Table 3 summarizes the radiotherapy treatment for patient in this series. Twenty patients received preoperative radiotherapy (median dose 50 Gy, range 37.5–54 Gy), and six patients received postoperative radiotherapy (median dose 50.2 Gy, range 37.5–66.6 Gy) because of either an inability to obtain a preoperative diagnosis or the presence of severe symptoms. Two patients received both preoperative and postoperative radiotherapy totaling 50.4 and 66.4 Gy. Eighteen patients received some component of PBRT, and 10 patients received IMRT. IOERT was delivered to any close or positive microscopic margin whenever feasible. Twelve patents received IOERT to a median dose of 11 Gy (range 6–15 Gy). Two patients received doses lower than those specified in the protocol as a result of normal tissue constraints. One patient's tumor was adjacent to liver. The other patient's tumor was adjacent to one kidney, and his contralateral kidney had been previously removed.
TABLE 3. Radiotherapy.
| Characteristic | All patients | Patients with primary tumors | Patients with local recurrence |
|---|---|---|---|
| Number of patients | 28 | 20 | 8 |
| External-beam radiation median dose, Gy (range) | 50.0 (37.5–66.6) | 50.0 (45–66.6) | 50.2 (37.5–54) |
| Type of external-beam radiation, n (%) | |||
| Proton beam | 10 (35.7%) | 6 (30.0%) | 4 (50.0%) |
| Intensity modulated | 11 (39.3%) | 8 (40%) | 3 (37.5%) |
| Both proton beam and intensity modulated | 7 (25%) | 6 (30.0%) | 1 (12.5%) |
| Timing of external-beam radiation, n (%) | |||
| Preoperative | 20 (71.4%) | 14 (70%) | 6 (75%) |
| Postoperative | 6 (21.4%) | 5 (25%) | 1 (12.5%) |
| Both preoperative and postoperative | 2 (7.1%) | 1 (5.0%) | 1 (12.5%) |
| Intraoperative radiation | |||
| Patients, n (%) | 12 (42.9%) | 8 (40%) | 5 (62.5%) |
| Median dose, Gy (range) | 11 (6–15) | 11.75 (7.5–15) | 10 (6–12.5) |
The availability of PBRT allowed for the delivery of preoperative radiotherapy to very large tumors. The largest tumor in this series was in 71-year-old woman with a 30 cm in maximal dimension well-differentiated and dedifferentiated liposarcoma located in the left retroperitoneum (Fig. 1a). A comparison of dose distribution for IMRT and PBRT therapy demonstrated marked sparing of the spinal cord, liver, right kidney, small bowel, and stomach (data not shown). After 45 Gy of PBRT, this patient underwent surgical resection of the tumor along with the spleen, distal pancreas, left kidney and adrenal gland, left colon, and sigmoid colon followed by 12.5 Gy IOERT to the tumor bed. She remains free of disease >4 years after surgery (Fig. 1b).
FIG. 1.
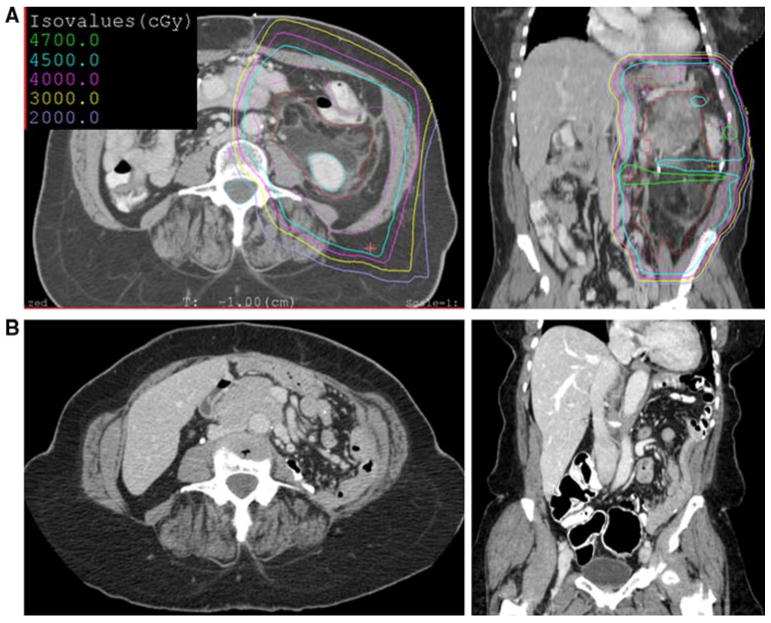
a Axial and coronal images of right retroperitoneal well-differentiated and dedifferentiated liposarcoma along with isodose lines that indicate the percentage of the proton-beam radiation prescription. b Follow-up computed tomographic scan 4 years after surgery and radiation
In circumstances where a preoperative diagnosis could not be obtained, a diagnosis was established by frozen section analysis at the time of surgical resection, and IOERT was delivered to the tumor bed. The greater omentum was then mobilized off the transverse colon and placed into the tumor bed to displace small bowel away from the tumor bed. Proton-beam radiation was then delivered postoperatively to the tumor bed. For example, one 51-year-old man sought care for severe abdominal pain and early satiety, and a 17-cm left retroperitoneal mass was found on abdominal CT scan (Fig. 2a). Results of a CT-guided biopsy suggested gastrointestinal stromal tumor. Imatinib was administered for 6 weeks, but the patient's symptoms progressed. The patient thus underwent surgical resection of the tumor along with the distal pancreas, spleen, left kidney and adrenal gland, and left colon. Intraoperative frozen section analysis of the surgical specimen suggested this tumor was not a gastrointestinal stromal tumor but rather a grade III/III malignant fibrous histiocytoma. IOERT (15 Gy) was delivered to each of two abutting fields designed to encompass the posterior margin of the tumor, and an omental flap was placed over the tumor bed. The patient then received postoperative PBRT to 45 Gy, which allowed sparing of the spinal cord, right kidney, colon, stomach, small bowel, and liver compared to IMRT (Fig. 2b). The patient remains free of disease nearly 4 years after surgery.
FIG. 2.
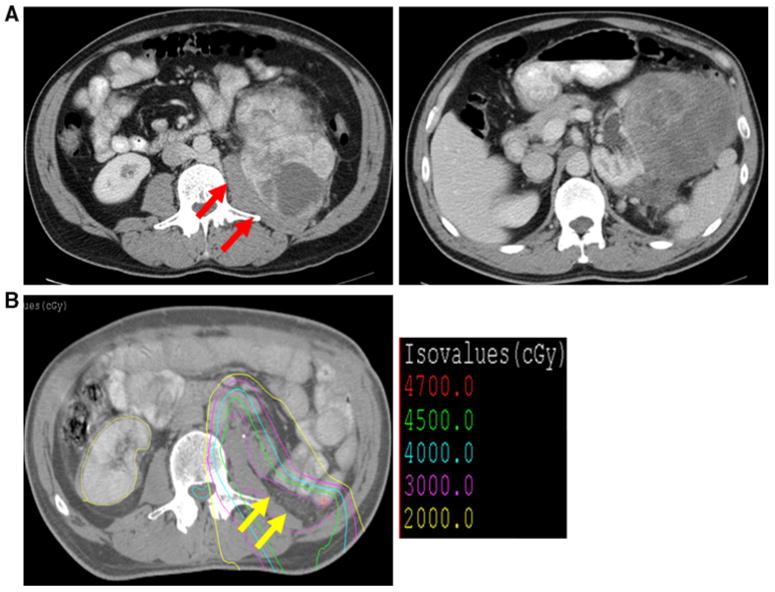
a Axial images of abdominal computed tomographic (CT) scan demonstrating right malignant fibrous histiocytoma retroperitoneal sarcomas abutting psoas muscle (red arrows). b Proton-beam radiotherapy planning CT scan demonstrating omental flap (yellow arrows) and isodose lines
Several patients experienced minor acute radiotherapy toxicities including skin erythema, nausea, and bowel habit changes. Complications that may be related to radiotherapy occurred in four patients (14.3%). One patient received preoperative IMRT and PBRT 54 Gy and IOERT 7.5 Gy and developed a ureteral stricture. Another patient received preoperative PBRT 45 Gy and no IOERT and developed severe bleeding 2 weeks after surgery requiring reoperation, during which no obvious source of bleeding was identified. The third patients received preoperative IMRT 54 Gy and developed a late enterocutaneous fistula. The final patient received IOERT 10 Gy followed by postoperative IMRT 50 Gy and developed an infected seroma several weeks after surgery.
Medical Therapy
Only three patients received adjuvant or neoadjuvant chemotherapy (two with primary disease and one with recurrent disease). One of these patients was a 23-year-old man who sought care 8 years after resection of a myxoid liposarcoma with a 15-cm local recurrence encasing the celiac axis. He received ET-743 (Trabectedin) for 9 months at another institution with a partial response. ET-743 was discontinued as a result of toxicity, and the tumor grew to 6.7 cm in maximum axial diameter (Fig. 3a), so he was referred to our institution for PBRT. For this central tumor in a young patient, PBRT allowed marked sparing of liver, spinal cord, stomach/duodenum, kidneys, and colon compared to IMRT (Fig. 3b, c). He received PBRT to 50.4 Gy with a decrease in tumor size to 4.9 cm such that the tumor no longer encased the celiac axis. Surgical resection was then performed followed by 10 Gy of IOERT. He received no further therapy and has been free of disease for 5½ years.
FIG. 3.
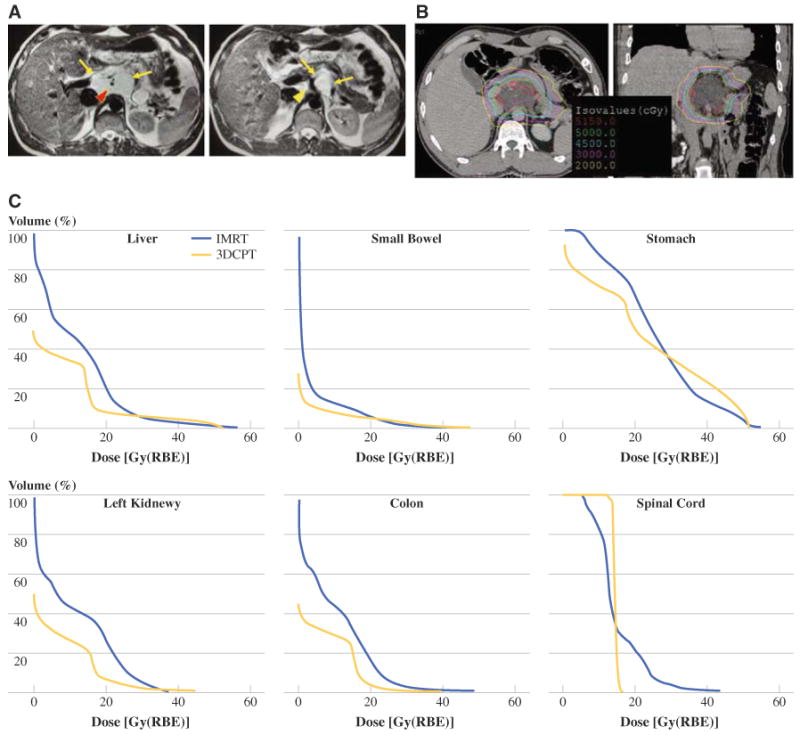
a Axial images of abdominal magnetic resonance imaging demonstrating myxoid liposarcoma (outlined in yellow arrows) encasing left gastric vessels (red arrowhead) and celiac axis (yellow arrowhead). b Proton-beam radiotherapy planning computed tomographic scan demonstrating isodose lines. c Dose volume graphs comparing IMRT versus 3D conformal protons (3DCPT) for liver, small bowel, stomach, kidney, colon, and spinal cord
Another patient was a 56-year-old woman who had a 6.9 cm intermediate-grade leiomyosarcoma of the retrohepatic inferior vena cava. This tumor extended superiorly beyond the hepatic veins and into the right atrium and inferiorly to below the level of the renal veins (Fig. 4a). Given that this tumor was thought to be unresectable, the patient received one cycle of mesna, adriamycin, ifosfamide, and dacarbazine (MAID) chemotherapy followed by a combination of ifosfamide and PBRT, which greatly spared radiation to the adjacent liver (Fig. 4b). The tumor shrank to 5.4 cm such that the superior extent was at the level of the hepatic veins and the inferior extent was above the renal veins. Thus, the tumor was resected on venovenous bypass along with resection of the retrohepatic IVC, right adrenal gland, and portion of the right diaphragm. The IVC was reconstructed with a 20-mm ringed polytetrafluoroethylene graft with the cuff of IVC around the hepatic veins sewn into the polytetrafluoroethylene graft (Fig. 4c). No evidence of disease remains 3 years after surgery.
FIG. 4.
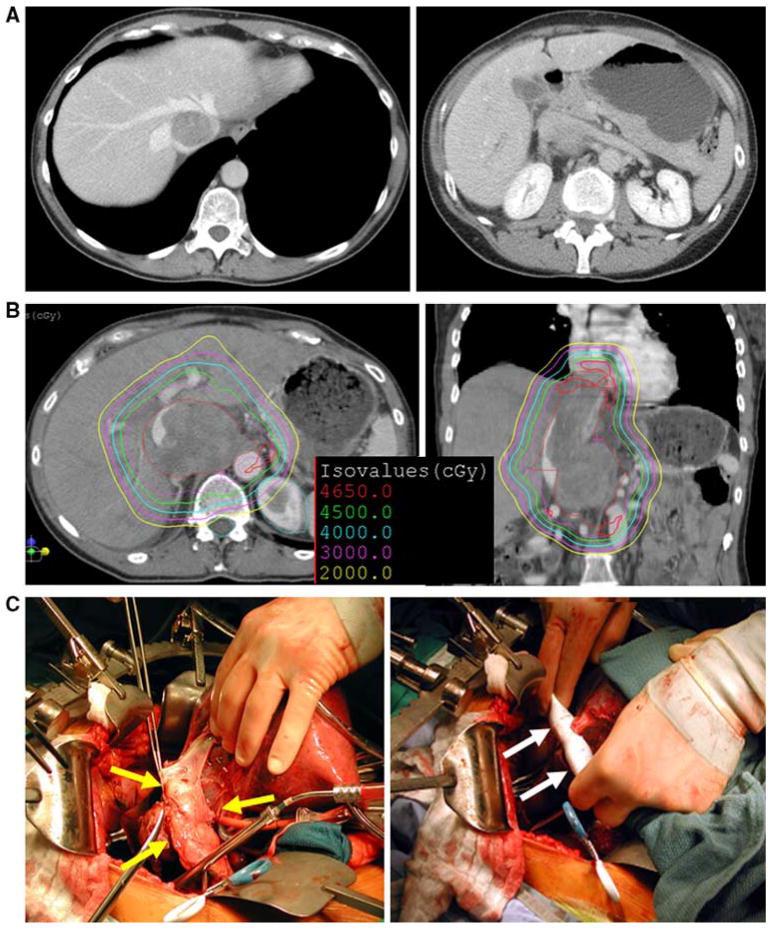
a Axial images of abdominal computed tomographic (CT) scan at the level of hepatic veins and the level of renal veins demonstrating retrohepatic inferior vena cava (IVC) leiomyosarcoma. b Proton-beam radiotherapy planning CT demonstrating isodose lines. c Intraoperative photographs demonstrating IVC with tumor (yellow arrows) and IVC replacement with ringed polytetrafluoroethylene graft with IVC cuff around hepatic veins sewn into graft (white arrows)
Recurrence and Survival in Patients with Primary Disease
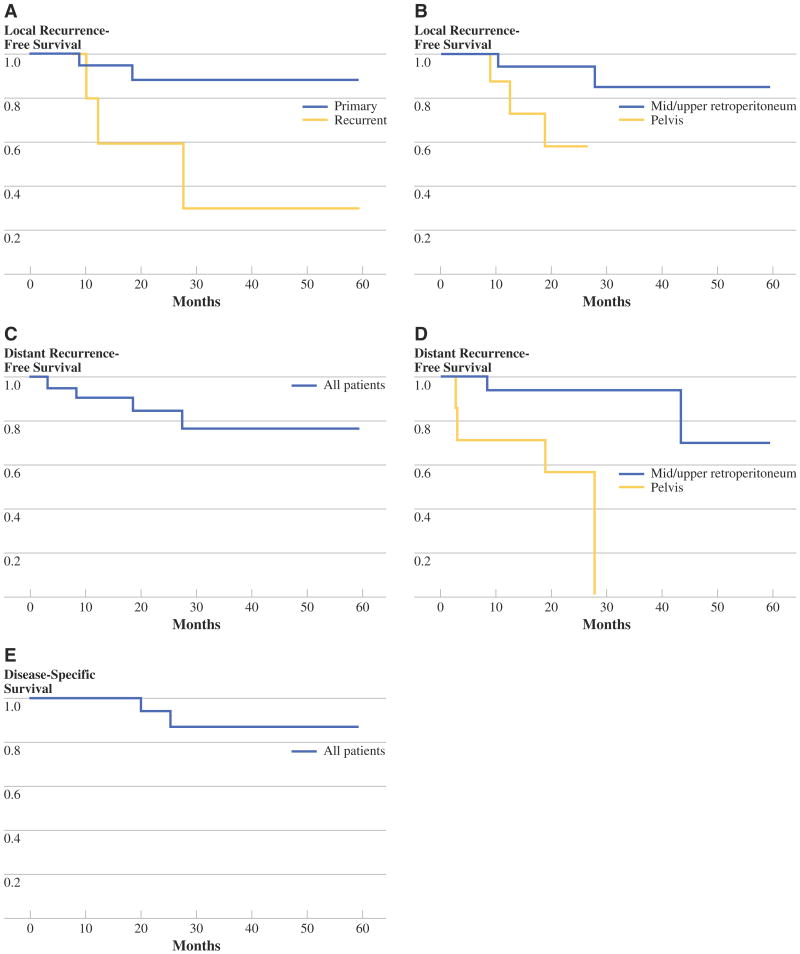
Median follow-up for all patients was 33 months. Three-year local recurrence-free survival for primary and recurrent tumors was 90% and 30%, respectively (Fig. 5a). There were two local recurrences in the 20 patients with primary tumors and three local recurrences in the 8 patients with recurrent tumors. By univariate analysis, initial presentation with local recurrence and pelvis site of tumor were both associated with an increased risk of local recurrence (Table 4, Fig. 5b). The association between pelvis location and increased local recurrence persisted when only patients with primary disease were analyzed (P = 0.038). Of note, margin status was not a statistically significant prognostic factor for local recurrence (P = 0.633). Median time to local recurrence was 12.3 months and ranged from 9 to 23 months.
FIG. 5.
Local recurrence-free survival stratified by primary versus recurrent tumor a or by retroperitoneum versus pelvis site b. Distant recurrence-free survival for all patients c or stratified by tumor location d. e Disease-specific survival for all patients
TABLE 4. Univariate analysis of local recurrence.
| Factor | All patients | Patients with primary disease | ||||
|---|---|---|---|---|---|---|
| n | Mean LRFS, months | P | n | Mean LRFS, months | P | |
| Age | 0.984 | 0.223 | ||||
| ≤56 years | 14 | 48.7 | 11 | 37.2 | ||
| >56 years | 14 | 50.5 | 9 | NA | ||
| Sex | 0.121 | 0.062 | ||||
| Female | 15 | 55.4 | 12 | NA | ||
| Male | 13 | 41.2 | 8 | 34.2 | ||
| Presentation | 0.025 | NA | ||||
| Primary disease | 20 | 54.2 | NA | NA | ||
| Recurrent disease | 8 | 30.6 | NA | NA | ||
| Site | 0.036 | 0.038 | ||||
| Retroperitoneum | 20 | 53.4 | 14 | NA | ||
| Pelvis | 8 | 21 | 6 | 22.1 | ||
| Tumor size | 0.8297 | 0.967 | ||||
| <10 cm | 14 | 47.6 | 10 | 54.2 | ||
| ≥10 cm | 14 | 37.5 | 10 | 39.8 | ||
| Grade | 0.422 | 0.552 | ||||
| I, low | 5 | NA | 3 | NA | ||
| II, intermediate, or III, high | 21 | 35.2 | 16 | 39.2 | ||
| Histology | 0.761 | 0.281 | ||||
| Liposarcoma | 14 | 49.1 | 11 | NA | ||
| Nonliposarcoma | 14 | 48.6 | 9 | 51.3 | ||
| Number of adjacent organs resected | 0.127 | 0.758 | ||||
| 0–1 | 15 | 40.8 | 9 | 51.1 | ||
| >1 | 13 | 40.7 | 11 | 40.2 | ||
| Margin | 0.633 | 0.83 | ||||
| Microscopically negative | 13 | 49.6 | 11 | 54.7 | ||
| Microscopically or grossly positive | 15 | 47 | 9 | 39.2 | ||
| Timing of radiation | 0.506 | 0.435 | ||||
| Preop or preop and postop | 22 | 51.4 | 15 | 41 | ||
| Only postop | 6 | 42.7 | 5 | 49.1 | ||
| Total dose of radiation | 0.563 | 0.962 | ||||
| <64 Gy | 14 | 45.7 | 10 | 54.2 | ||
| ≥64 Gy | 14 | 48.1 | 10 | 40.2 | ||
| Adjuvant chemotherapy | 0.287 | 0.58 | ||||
| No | 23 | 45.6 | 16 | 52.6 | ||
| Yes | 3 | NA | 2 | NA | ||
LRFS local recurrence-free surviva, preop preoperative, postop postoperative, NA not applicable
Six patients experienced distant recurrence. Three patients had intraperitoneal recurrences, one had lung and brain metastases, one had lung only metastases, and one had subcutaneous metastases. The overall 3-year distant recurrence-free survival was 78% (Fig. 5c).
On univariate analysis, pelvic tumor site was associated with distant recurrence (P = 0.002) (Fig. 5d and Table 5), and nonliposarcoma histology trended toward increased distant recurrence (P = 0.055) (Table 5). Median time to distant recurrence was 8.1 months and ranged from 4 to 43 months. Overall 3-year disease-specific and overall survival was 87% (Fig. 5e).
TABLE 5. Univariate analysis of distant recurrence.
| Factor | All patients | Patients with primary disease | ||||
|---|---|---|---|---|---|---|
| n | Mean DRFS, months | P | n | Mean DRFS, months | P | |
| Age | 0.593 | 0.129 | ||||
| ≤56 | 13 | 41.2 | 10 | 32.8 | ||
| >56 | 13 | 45.1 | 8 | NA | ||
| Sex | 0.320 | 0.373 | ||||
| Female | 13 | 50.5 | 10 | 54.2 | ||
| Male | 13 | 40.4 | 8 | 33.5 | ||
| Presentation | 0.267 | NA | ||||
| Primary disease | 18 | 50.5 | NA | NA | ||
| Recurrent disease | 8 | 30.6 | NA | NA | ||
| Site | 0.002 | 0.126 | ||||
| Retroperitoneum | 19 | 53.4 | 13 | NA | ||
| Pelvis | 7 | 21 | 5 | 20.1 | ||
| Size | 0.151 | 0.427 | ||||
| <10 cm | 12 | 38.8 | 8 | 45.8 | ||
| ≥10 cm | 14 | 41.2 | 10 | 40.7 | ||
| Grade | 0.316 | 0.422 | ||||
| I, low | 5 | NA | 3 | NA | ||
| II, intermediate, or III, high | 19 | 34.3 | 14 | 36.8 | ||
| Histology | 0.055 | 0.129 | ||||
| Liposarcoma | 14 | 54 | 8 | NA | ||
| Nonliposarcoma | 12 | 38.7 | 10 | 44.4 | ||
| Adjacent organs resected | 0.628 | 0.495 | ||||
| 0–1 | 15 | 41.8 | 9 | 46.3 | ||
| >1 | 11 | 36.9 | 9 | 39.8 | ||
| Margin | 0.276 | 0.572 | ||||
| Microscopically negative | 11 | 38.6 | 9 | 47.3 | ||
| Microscopically or grossly positive | 15 | 50 | 9 | 40.1 | ||
| Timing of radiation | 0.287 | 0.435 | ||||
| Preop or preop and postop | 20 | 50.1 | 13 | 41.2 | ||
| Only postop | 6 | 38.6 | 5 | 49.1 | ||
| Total dose of radiation | 0.214 | 0.373 | ||||
| <64 Gy | 12 | 39.2 | 8 | 45.8 | ||
| ≥64 Gy | 14 | 51.6 | 10 | 40.2 | ||
| Adjuvant chemotherapy | 0.246 | 0.507 | ||||
| No | 23 | 41.3 | 16 | 49.3 | ||
| Yes | 3 | NA | 2 | NA | ||
DRFS distant recurrence-free survival
Discussion
The aggressiveness of surgery and delivery of radiotherapy for RPS are highly debatable topics. Local recurrence for RPS after surgical resection alone is >40% in large series.9–11 Some have recently argued that more aggressive surgery leads to lower recurrence rates, while others have argued that sacrificing some dispensable organs (e.g., colon and kidney) while leaving other less easily resected organs (e.g., duodenum and aorta) may not be sufficient to clear all margins of microscopic disease.3–5 In addition, some favor adjuvant radiation, while others argue that one cannot safely deliver enough radiotherapy to RPS to reliably control microscopic residual disease.21 In this study, we used a treatment strategy for RPS that involved (1) aggressive surgical resection of the tumor and contiguous organs aimed at obtaining a negative anterior margin and (2) advanced radiation modalities (PBRT, IMRT, and IOERT) aimed at delivering adequate radiation doses to microscopic residual disease (often at the posterior margin) and minimizing radiation dose to adjacent radiation-sensitive organs. In a series of 28 patients treated with this strategy, radiation-related complications (14.3%) and surgical morbidity (28.6%) were acceptable, and 3-year local recurrence-free survival was 90% for patients with primary disease. Local control was much lower for patients with recurrent disease.
Most radiation oncologists currently agree that if radiation is delivered to RPS, it is best delivered preoperatively. There are several advantages in the delivery of preoperative external-beam radiotherapy to RPS compared to postoperative therapy: the tumor acts as a tissue expander displacing radiation-sensitive organs away from the radiation field, there is a decreased risk of tumor seeding if there is tumor cut-through, and better oxygenation of the periphery of the tumor results in increased radiosensitivity.21
The data on the efficacy of preoperative radiotherapy for RPS are retrospective and equivocal. Several studies have demonstrated lower local recurrence rates with the addition of adjuvant radiotherapy to surgical resection, but these studies may have selection bias and other confounding variables.21–27 Other studies have found local recurrence rates of up to 60% even with the addition of radiotherapy.10,28,29 Radiotherapy to a dose of 45–50 Gy may result in lower local recurrence rates when tumors are resected with a negative microscopic margin, but doses >55 Gy may be required to control tumors resected with a positive microscopic margin.23,24 Radiation doses >55 Gy often exceed the normal tissue tolerance of radiation-sensitive organs such as the kidney, small bowel, and liver that are often adjacent to retroperitoneal tumors. More recent studies have used IMRT to deliver higher doses of external-beam radiotherapy to RPS. The University of Alabama group reported on 16 RPS that received up to 57.5 Gy to the highest risk posterior margin.18 Two-year recurrence-free survival was 80%. Bossi et al. from Belgium used IMRT to deliver radiation only to the posterior margin of retroperitoneal liposarcomas followed by surgical resection, and there were only two local recurrences in 18 patients.15
The advantages of delivering photon radiation via IMRT have been reviewed elsewhere.12 There are several theoretical advantages of PBRT. Although radiation doses of 45–50 Gy can be delivered to retroperitoneal tumors without PBRT, PBRT offers additional advantages over IMRT and 3D conformal radiotherapy.30 Protons have a reduced entrance dose compared to traditional photons as well as almost no exit dose (Fig. 6). Thus, PBRT reduces the radiation of adjacent normal organs and tissues by approximately 60% and allows delivery of the prescription dose to the tumor with much greater sparing of adjacent organs and structures than is possible with photons. Whether PBRT offers a clinical advantage for any given patient depends on the location of the tumor and the adjacent normal tissues. PBRT is most advantageous for tumors near the liver, where much greater hepatic sparing is often achievable, but may also allow important reductions in dose to other organs such as kidney, small bowel, and stomach.
FIG. 6.
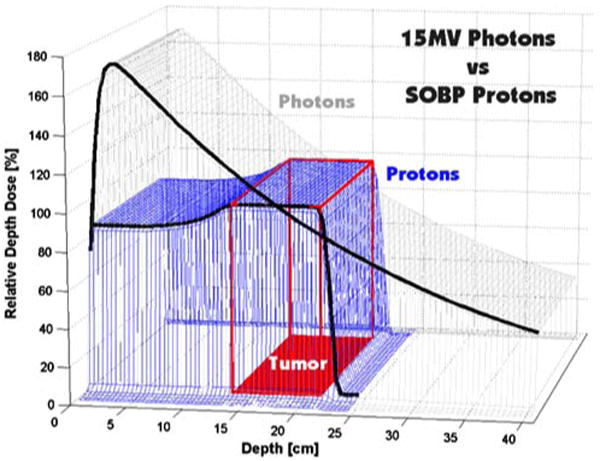
Depth-dose distributions for a single field of 15 MV photons and a spread-out Bragg peak (SOBP) 23 cm in range and 12 cm in modulation. Modified from Suit and Chu31
IOERT combined with external-beam radiotherapy is one approach to safely deliver high radiation doses to RPSs. The Mayo Clinic retrospectively analyzed patients with RPS who received IOERT and external-beam radiotherapy. In the 43 patients with primary disease, 5-year local recurrence-free survival ranged from 60% to 100%, depending on whether margins were grossly positive, microscopically positive, or microscopically negative.17 Similarly, a report from our institution in 2001 demonstrated a 5-year local recurrence-free survival of 83% when external-beam radiotherapy combined with intraoperative radiation was used.16
To our knowledge, this study is the first to report on the use of PBRT for RPS and is the first to examine the combination of IMRT or PBRT with IOERT for these tumors. We learned several lessons during the conduct of this treatment strategy. First, there must be good coordination between the radiation oncologist and surgeon in order for the described strategy to be effective. This surgeon must determine in advance which contiguous organs will likely be resected and which organs will likely be left in situ. The radiation oncologist must adjust the radiation planning in response to the surgical plan. For example, if a nephrectomy or partial hepatectomy is planned, radiation to the contralateral kidney or residual liver must be strictly avoided. For well-differentiated liposarcomas, the exact extent of disease may be difficult to ascertain on the basis of imaging studies. For the patients in this study, the treating radiation oncologist and surgeon usually reviewed the radiation target volumes jointly to come to a consensus on the extent of disease and areas of highest risk for microscopic residual disease. Second, our strategy of radiotherapy and surgical resection was much more successful for patients with primary disease compared to patients with recurrent disease. For recurrent tumors, the visible macroscopic recurrence often did not encompass the entire field at risk of microscopic residual disease. Thus, targeting the recurrent macroscopic tumor with radiation and surgery left residual microscopic disease that was neither resected or radiated. Thus, in the three patients with recurrent disease who experienced local recurrence, the recurrence was outside the surgery and radiation fields. Perhaps a better strategy for these tumors is to carefully analyze the original primary tumor bed and target surgery and radiation to the entire field at risk rather than merely the visible local recurrence. Third, many patients could not be treated with this strategy. Some patients were too symptomatic or had tumors that were too large for preoperative radiotherapy, and adequate intraoperative and postoperative radiotherapy was not feasible. Many patients sought care at our institution immediately after surgical resection, and thus preoperative radiotherapy or IOERT could not be performed.
There are several limitations to this study including a heterogeneous patient population, heterogeneity in the administration of radiation, and small sample size. This report describes a somewhat favorable group of patients compared to other series of RPS; given that 43% of patients were asymptomatic, most patients were able to tolerate preoperative therapy, and median tumor size was 9.75 cm. In addition, PBRT is not widely available, making broad application of this strategy difficult.
The addition of chemotherapy or targeted biological agents may improve the efficacy of radiotherapy against RPS and increase local control. Pisters et al. in a phase I study, treated 35 patients preoperatively with 4 to 5 weeks of doxorubicin and escalating doses of radiation followed by surgical resection and IOERT in selected patients.32 Pisters et al. determined this was a feasible strategy and obtained acceptable morbidity at the highest preoperative radiation dose of 50.4 Gy. Our group is currently conducting a phase II trial combining neoadjuvant bevacizumab, an antiangiogenic agent, and radiotherapy for STS (including RPS) at risk of local recurrence (http://clinicaltrials.gov/ct2/show/NCT00356031).
In conclusion, the most common cause of death for patients with RPS is local recurrence rather than distant metastases. Aggressive surgical resection to obtain a complete gross resection (and negative margins if possible) is the mainstay of treatment. A strategy of preoperative PBRT and/or IMRT followed by aggressive surgical resection and IOERT may possibly allow for the delivery of adequate radiation doses to control microscopic residual disease and minimizes radiation dose to adjacent organs. For patients unable to tolerate preoperative therapy, IOERT to the tumor bed followed by an omental flap and postoperative PBRT is another option. This strategy may reduce local recurrence rates, especially for patient with primary tumors. The role of chemotherapy and biologically targeted agents to improve the efficacy of radiotherapy in this setting requires further investigation.
Acknowledgments
Thomas F. DeLaney received a speaker's honorarium from IBA Proton Therapy, and David G. Kirsch is a consultant for Guidepoint Global.
Footnotes
Presented in part at the 62nd Annual Meeting of the Society of Surgical Oncology, March 4–8, 2008, Phoenix, AZ, and at the 14th Annual Meeting of the Connective Tissue Oncology Society, November 13–15, 2008, London, UK.
References
- 1.Brennan MF, Lewis JL. Diagnosis and management of soft tissue sarcoma. London: Martin Dunitz; 2002. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Gronchi A, Lo VS, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 4.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–7. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 5.Pisters PW. Resection of some—but not all—clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. J Clin Oncol. 2009;27:6–8. doi: 10.1200/JCO.2008.18.7138. [DOI] [PubMed] [Google Scholar]
- 6.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 7.DeLaney TF, Kepka L, Goldberg SI, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67:1460–9. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Zagars GK, Ballo MT. Significance of dose in postoperative radiotherapy for soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2003;56:473–81. doi: 10.1016/s0360-3016(02)04573-x. [DOI] [PubMed] [Google Scholar]
- 9.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–65. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–68. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.van Dalen T, Plooij JM, Van Coevorden F, et al. Long-term prognosis of primary retroperitoneal soft tissue sarcoma. Eur J Surg Oncol. 2007;33:234–8. doi: 10.1016/j.ejso.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 12.DeLaney TF, Trofimov AV, Engelsman M, Suit HD. Advanced-technology radiation therapy in the management of bone and soft tissue sarcomas. Cancer Control. 2005;12:27–35. doi: 10.1177/107327480501200104. [DOI] [PubMed] [Google Scholar]
- 13.Brada M, Pijls-Johannesma M, De RD. Proton therapy in clinical practice: current clinical evidence. J Clin Oncol. 2007;25:965–70. doi: 10.1200/JCO.2006.10.0131. [DOI] [PubMed] [Google Scholar]
- 14.Willett CG, Czito BG, Tyler DS. Intraoperative radiation therapy. J Clin Oncol. 2007;25:971–7. doi: 10.1200/JCO.2006.10.0255. [DOI] [PubMed] [Google Scholar]
- 15.Bossi A, De Wever I, Van Limbergen E, Vanstraelen B. Intensity modulated radiation-therapy for preoperative posterior abdominal wall irradiation of retroperitoneal liposarcomas. Int J Radiat Oncol Biol Phys. 2007;67:164–70. doi: 10.1016/j.ijrobp.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Gieschen HL, Spiro IJ, Suit HD, et al. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001;50:127–31. doi: 10.1016/s0360-3016(00)01589-3. [DOI] [PubMed] [Google Scholar]
- 17.Petersen IA, Haddock MG, Donohue JH, et al. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2002;52:469–75. doi: 10.1016/s0360-3016(01)02595-0. [DOI] [PubMed] [Google Scholar]
- 18.Tzeng CW, Fiveash JB, Popple RA, et al. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107:371–9. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 20.Cox D. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 21.Pawlik TM, Ahuja N, Herman JM. The role of radiation in retroperitoneal sarcomas: a surgical perspective. Curr Opin Oncol. 2007;19:359–66. doi: 10.1097/CCO.0b013e328122d757. [DOI] [PubMed] [Google Scholar]
- 22.Catton CN, O'Sullivan B, Kotwall C, et al. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1994;29:1005–10. doi: 10.1016/0360-3016(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 23.Tepper JE, Suit HD, Wood WC, et al. Radiation therapy of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1984;10:825–30. doi: 10.1016/0360-3016(84)90383-3. [DOI] [PubMed] [Google Scholar]
- 24.Fein DA, Corn BW, Lanciano RM, et al. Management of retroperitoneal sarcomas: does dose escalation impact on locoregional control? Int J Radiat Oncol Biol Phys. 1995;31:129–34. doi: 10.1016/0360-3016(94)E0302-Z. [DOI] [PubMed] [Google Scholar]
- 25.van Doorn RC, Gallee MP, Hart AA, et al. Resectable retroperitoneal soft tissue sarcomas. The effect of extent of resection and postoperative radiation therapy on local tumor control. Cancer. 1994;73:637–42. doi: 10.1002/1097-0142(19940201)73:3<637::aid-cncr2820730322>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 26.White JS, Biberdorf D, DiFrancesco LM, Kurien E, Temple W. Use of tissue expanders and pre-operative external beam radiotherapy in the treatment of retroperitoneal sarcoma. Ann Surg Oncol. 2007;14:583–90. doi: 10.1245/s10434-006-9139-0. [DOI] [PubMed] [Google Scholar]
- 27.Zlotecki RA, Katz TS, Morris CG, Lind DS, Hochwald SN. Adjuvant radiation therapy for resectable retroperitoneal soft tissue sarcoma: the University of Florida experience. Am J Clin Oncol. 2005;28:310–6. doi: 10.1097/01.coc.0000158441.96455.31. [DOI] [PubMed] [Google Scholar]
- 28.Ballo MT, Zagars GK, Pollock RE, et al. Retroperitoneal soft tissue sarcoma: an analysis of radiation and surgical treatment. Int J Radiat Oncol Biol Phys. 2007;67:158–63. doi: 10.1016/j.ijrobp.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 29.Youssef E, Fontanesi J, Mott M, et al. Long-term outcome of combined modality therapy in retroperitoneal and deep-trunk soft-tissue sarcoma: analysis of prognostic factors. Int J Radiat Oncol Biol Phys. 2002;54:514–9. doi: 10.1016/s0360-3016(02)02942-5. [DOI] [PubMed] [Google Scholar]
- 30.DeLaney TF, Kirsch DG. Bone and soft tissue. In: DeLaney TF, Kooy HM, editors. Proton and charged particle radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 172–85. [Google Scholar]
- 31.Suit HD, Chu W. History of charged particle radiotherapy. In: DeLaney TF, Kooy HM, editors. Proton and charged particle radiotherapy. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 1–7. [Google Scholar]
- 32.Pisters PW, Ballo MT, Fenstermacher MJ, et al. Phase I trial of preoperative concurrent doxorubicin and radiation therapy, surgical resection, and intraoperative electron-beam radiation therapy for patients with localized retroperitoneal sarcoma. J Clin Oncol. 2003;21:3092–7. doi: 10.1200/JCO.2003.01.143. [DOI] [PubMed] [Google Scholar]