Abstract
Aims
To determine whether cannabinoid-positive urine specimens in heroin-dependent outpatients predict other drug use or impairments in psychosocial functioning, and whether such outcomes are better predicted by cannabis-use disorders than by cannabis use itself.
Design
Retrospective analyses of three clinical trials; each included a behavioral intervention (contingency management) for cocaine or heroin use during methadone maintenance. Trials lasted 25–29 weeks; follow-up evaluations occurred 3, 6, and 12 months posttreatment. For the present analyses, data were pooled across trials where appropriate.
Setting
Urban outpatient methadone clinic.
Participants
408 polydrug abusers meeting methadone-maintenance criteria.
Measurements
Participants were categorized as nonusers, occasional users, or frequent users of cannabis based on thrice-weekly qualitative urinalyses. Cannabis-use disorders were assessed with the Diagnostic Interview Schedule III-R. Outcome measures included proportion of cocaine- and opiate-positive urines and the Addiction Severity Index (at intake and follow-ups).
Findings
Cannabis use was not associated with retention, use of cocaine or heroin, or any other outcome measure during or after treatment. Our analyses had a power of .95 to detect an r2 of .11 between cannabis use and heroin or cocaine use; the r2 we detected was less than .03 and nonsignificant. A previous finding that cannabis use predicted lapse to heroin use in heroin-abstinent patients did not replicate in our sample. However, cannabis-use disorders were weakly associated with psychosocial problems at posttreatment follow-up.
Conclusions
Cannabinoid-positive urines need not be a major focus of clinical attention during treatment for opiate dependence, unless patients report symptoms of cannabis-use disorders.
Keywords: cannabis, methadone maintenance, treatment outcome
A heroin-dependent patient in a maintenance program submits a urine sample that indicates recent use of cannabis. Is the patient at especially great risk of being unable to sustain abstinence from heroin or other drugs? This question has been addressed in a small body of literature; summarized with a simple “vote counting” approach, the literature appears to say no—by a slim margin of four studies (Saxon et al., 1993; Nirenberg et al., 1996; Budney, Bickel & Amass, 1998; Church et al., 2001) to two studies (Dupont & Saylor, 1989; Wasserman et al., 1998). Given the practical importance of the question, and especially given that one recent study (Wasserman et al., 1998) raises concerns not addressed in any of the others, there is a need for more studies with larger patient samples. Here we review the literature in some detail, and report urinalysis data from 408 participants in three clinical trials conducted over six years.
An early report concluded that cannabis use in methadone-maintained patients “can interfere with treatment goals” (DuPont & Saylor, 1989). The authors tested more than 300 urine specimens (from an unstated number of patients) from two clinics, at which the respective rates of cannabinoid-positive urine specimens were 27% and 51%. The authors stated that all but one of the cannabinoid-positive specimens was also positive for another drug. However, their own data appear to contradict this, since at least 75% of the cannabinoid-positive specimens seem unaccounted for in the breakdown of other-drug positives (11% of the cannabinoid-positive specimens were positive for cocaine, 9.2% for opiates, 3% for benzodiazepines, and 1.8% for barbiturates). In addition, no base rates were given for other-drug positives in cannabinoid-negative urines (except benzodiazepines, for which the base rates—4% and 7% at the respective clinics—were slightly higher than the rate in cannabinoid-positive urines). Thus, despite the authors’ conclusion, it is not clear that cannabis use was associated with increased likelihood of other drug use.
Four subsequent reports have shown no association between cannabis use and other drug use among heroin-dependent patients in treatment.
Saxon et al. (1993) reported data from all male patients who had been in treatment for at least six months in 1989–1990 at a Seattle methadone clinic. Of the 98 men in the sample, 54 had tested positive for cannabinoids at least once in weekly urinalyses. They did not differ from the other 44 in their rates of other-drug positives. The cannabinoid-positive patients were then subdivided into consistent cannabis users (all tests positive, n=22) and intermittent cannabis users (not all tests positive, n=32). Other-drug positives were actually less frequent in the consistent cannabis users than in the intermittent cannabis users. In a superset (N=117) of the same patient sample, with no minimum treatment duration, results were essentially the same, and there was no association between cannabis use and treatment retention (Saxon et al., 1991).
Nirenberg et al. (1996) reported data from 70 patients (69 male) in an urban methadone-maintenance program; urine was tested weekly for 14 to 45 weeks. The sample was divided into cannabis abstainers (n=15), intermittent users (up to 33.3% positive; n=25); moderate users (33.3–66.6% positive; n=16); and consistent users (66.6–100% positive; n=14). Groups did not differ in their rates of other-drug positives (whereas patients positive for either cocaine or benzodiazepines did show higher rates of opiate positives).
Budney et al. (1998) examined 107 patients (68 male) maintained on buprenorphine in a treatment-research clinic in Vermont; urine was tested thrice weekly for up to 32 weeks. Seventy-one patients tested positive for cannabinoids at least once (or reported use in the 30 days before enrollment). To test associations with treatment outcome, the authors examined a subset of patients (n=79) who had received buprenorphine plus behavior therapy; 51 tested positive for cannabinoids at least once, but this was not associated with differences on any measure of treatment outcome (retention; opiate, cocaine, or benzodiazepine use; or changes in ASI scores from intake to 12 months later). Percentage of cannabinoid-positive urines did not correlate with weeks of heroin abstinence or with percentage of treatment weeks completed.
Church et al. (2001) examined 47 patients (36 male) maintained on methadone and naltrexone in a treatment-research clinic in New York City; urine was tested twice weekly for 6 months. 28 patients tested positive for cannabinoids at least once. Patients were trichotomized as cannabis-abstinent (n=13), intermittent users (n=18), and heavy users (n=10). The intermittent users actually had fewer opiate-positive urines and better naltrexone compliance than the cannabis-abstinent patients; the heavy users showed no differences from the abstinent patients. The meaningfulness of the trichotomy is questionable, since any patient with fewer than 100% of urines positive for cannabinoids was classified as an intermittent user. (The classification was based on an exaggeration of cannabinoids’ detection time in urine, which, at the standard cutoff of 50 ng/mL, should be only 2–3 days after one use [Huestis, Mitchell & Cone, 1995], and roughly 5 days in weekly users and 16–17 days in daily users [Smith-Kielland, Skuterud & Mørland, 1999; inferred from graphs of time-course data].) But across the whole sample, the percentage of cannabinoid-positive urines did not correlate with treatment retention, naltrexone compliance, or the percentage of opiate-, cocaine-, or benzodiazepine-positive urines.
Even before the publication of most of these negative findings, one group concluded that testing for cannabinoids in methadone clinics is not necessary or justified (Saxon et al., 1991, 1993), a conclusion that appears to have influenced practice at some clinics (Budney, personal communication, 2000).
Wasserman et al. (1998) took a different approach, and obtained more discouraging results. Their sample was drawn from four methadone-maintenance programs in the San Francisco area in 1992–1995; analyses were restricted to 74 patients (out of 528) who had stopped using heroin for at least three weeks (two weeks by self-report, then a baseline week of twice-weekly urinalyses). Thirty-five patients tested positive for cannabinoids at least once during the study. The goal of the analysis was to find predictors of subsequent lapses to heroin use (as indicated by at least one heroin-positive urine) during seven further weeks of twice-weekly urinalyses. Lapses occurred in 30 patients (and were followed by recurrent opiate positives in 21 of those patients). Lapses were significantly predicted by only two variables out of 20: not having a goal of absolute heroin abstinence, and testing positive for cannabinoids. Cannabinoid positives were predictive in either of two analyses—one in which cannabinoid positives during the baseline week were treated as a baseline predictor, and one in which cannabinoid positives throughout the study were treated as a time-dependent predictor. No contingency table, effect size, or hazard ratio was given, but using the χ2 value (7.62 for the second analysis) and the marginals, we estimated a relative risk of (21/35)/(9/39) = 2.6. The findings of Wasserman et al. (1998) suggest that for patients who have stopped using heroin, cannabinoid-positive urine is a robust predictor of resumption of heroin use.
Two more questions have received relatively little attention. First, is cannabis use among methadone patients problematic in its own right? That is, even if it does not predict increased use of other drugs, does it predict poorer outcomes in general psychosocial functioning at posttreatment follow-up? This was tested in only one of the studies cited above (Budney et al., 1998), and the answer appeared to be no. Second, should the main object of concern be cannabis abuse/dependence rather than use? That is, will differences in treatment outcome be greater if patients are categorized by whether they meet criteria for a cannabis-use disorder? This approach was used in a report on cocaine-dependent patients (Budney, Higgins & Wong, 1996), but to our knowledge, has not yet been used in any reports on methadone-maintained patients.
We set out first to determine whether the patterns of findings in previous studies would be replicated in our larger sample. (For the previous studies, the mean sample size was 79 [range 47–107], and the mean number of cannabinoid-positive patients was 49 [range 28–71].) We pooled data from three of our completed clinical trials, with sample sizes of 120, 95, and 193, in which the respective numbers of cannabinoid-positive patients were 55, 71, and 94.) We then examined data from follow-up evaluations conducted 3, 6, and 12 months posttreatment, to determine whether cannabis use during treatment predicts subsequent unemployment, percent of income spent on drugs, days of illegal activity, days of imprisonment, and interpersonal conflict. Finally, we tested whether the outcomes of interest, during and after treatment, were more closely associated with cannabis abuse/dependence than with cannabis use.
Methods
Participants
Participants were 408 methadone-maintained outpatients (243 male) in any of three studies at Archway Clinic, the treatment-research program of the National Institute on Drug Abuse Intramural Research Program in Baltimore, MD. Eligibility criteria for the studies were: age 18–65, qualification for methadone maintenance according to Food and Drug Administration guidelines, self-reported history of intravenous opiate use, and continued use of heroin or cocaine during a 5-week baseline treatment phase. Exclusion criteria were: current psychotic, bipolar, or major depressive disorders; current physical dependence on alcohol or sedatives; unstable serious medical illness; current pregnancy or breastfeeding of a child; estimated IQ below 80 (Zachary, 1986); and urologic conditions precluding urine collection. Participants’ mean age was 38.5±0.3 (mean± SEM), with a range of 21 to 57; 59% were African-American and 41% were White; 52% were unemployed; 15% were married.
The intervention common to all three studies was contingency management, in which participants could earn vouchers (redeemable for goods and services) whose value increased with each consecutive drug-negative urine specimen. In the first study (“Opiate Study,” n=120), contingency management targeted toward illicit opiates was combined with a methadone dose increase (Preston, Umbricht & Epstein, 2000). In the second study (“Cocaine Study 1”), two contingencies with different response requirements were each targeted toward cocaine (Preston et al, 2001). In the third study (“Cocaine Study 2,” n=193), contingency management targeted toward cocaine use was combined with cognitive-behavioral therapy (Epstein et al., in press). In the Opiate Study and Cocaine Study 2, approximately half the participants were randomized to a control condition in which vouchers were not contingent on urinalysis results.
In each study, a 5-week Baseline treatment phase with thrice-weekly urine collection was used to assess participants’ initial levels of drug use while they received daily methadone and weekly individual counseling. This was followed immediately by an Intervention phase (8 weeks in the Opiate Study and Cocaine Study 1, and 12 weeks in Cocaine Study 2) in which vouchers were available. This was followed immediately by a 12-week Maintenance phase, essentially a return to baseline conditions, during which urine specimens continued to be collected thrice weekly. The Opiate Study and Cocaine Study 1 ran concurrently from 1994 through 1996. Cocaine Study 2 ran from 1997 through 1999.
In the Opiate Study, methadone dose was 50 mg/day for 57 participants and 70 mg/day for the other 63. In Cocaine Study 1, methadone dose was 50 mg/day for all participants. In Cocaine Study 2, methadone dose was 80 mg/day for 81 participants, 70 mg/day for 111, and 50 mg/day for one participant who could not tolerate higher doses.
Intake measures
Assessments at study intake included the Addiction Severity Index (ASI; McLellan et al., 1985), the Shipley Institute of Living Scale (Zachary, 1986), the Beck Depression Inventory (Beck et al., 1987), and the Diagnostic Interview Schedule (DIS-IIIR; Helzer, Croughan, Robins, & Ratcliff, 1981). The DIS-IIIR is a structured interview based on the Diagnostic and Statistical Manual, Third Edition - Revised (DSM-IIIR) (APA, 1987) for assessment of psychiatric diagnoses, including substance-use disorders. (There was no requirement that participants meet DSM-IIIR criteria for Heroin or Cocaine Dependence, though nearly all participants did meet criteria for Heroin Dependence.) Due to the archival nature of the data, all that was available for most participants was a summary sheet in which any history of a cannabis-use disorder was listed as present or absent, with Abuse and Dependence not differentiated. In most cases, under DSM-IIIR criteria, individuals are more likely to meet criteria for Dependence than for Abuse (Helzer, 1994). Participants in Cocaine Study 2 also filled out an HIV-risk questionnaire that included questions on needle sharing.
Measures of drug use during treatment
Every Monday, Wednesday, and Friday, urine specimens were collected under the observation of laboratory technicians. Assays were conducted with an Enzyme Multiplied Immunoassay Technique (EMIT; Syva Corp., Palo Alto, California) system that gave qualitative results for cannabinoids (11-nor-9-carboxy-Δ9-tetrahydrocannabinol, THCCOOH), cocaine (benzoylecgonine equivalents; BZE), and opiates (morphine). Cutoffs for positive specimens were 300 ng/ml for cocaine and opiates, and 50 ng/ml for cannabinoids. The mean number of specimens collected was 64.3±1.1 (range 16–75), reflecting an average of 22.8±0.3 weeks of treatment (range 5.3–29).
Measure of posttreatment outcome
All patients, including those who dropped out of treatment, were asked to return to the clinic for follow-up ASI interviews approximately 3, 6, and 12 months after the last day of their regular maintenance dose of methadone.
Data analysis
Patients were categorized as nonusers of cannabis (n=188) if they had no cannabinoid-positive urine specimens. Among the remaining 220 patients, the median percentage of cannabinoid-positive specimens was 13.5%, or 1 out of every 7.4 specimens. These patients were dichotomized near the median at what seemed a clinically relevant cutoff point: they were classified as occasional users (n=125) if 1/6 or fewer of their urine specimens were cannabinoid-positive (reflecting one positive specimen every two weeks on average), or as frequent users (n=95) if more than 1/6 of their specimens were positive. The proportion of patients in each group did not differ significantly across studies, χ2 (4) = 6.06, p = .19 (Table 1).
Table 1.
Distribution of nonusers, occasional users, and frequent users of cannabis across studies
| Cocaine Study 1 | Opiate Study | Cocaine Study 2 | TOTAL | |
|---|---|---|---|---|
| nonusers | 40 (42%) | 49 (41%) | 99 (51%) | 188 (46%) |
| occasional users | 31 (33%) | 36 (30%) | 58 (30%) | 125 (31%) |
| frequent users | 24 (25%) | 35 (29%) | 36 (19%) | 95 (23%) |
| TOTAL | 95 | 120 | 193 | 408 |
Intake measures were compared by analysis of variance (ANOVA), χ2 tests, and Fisher exact tests, as appropriate. Data were pooled across all three studies.
Treatment retention was analyzed separately within each study with log-rank χ2 tests from the survival-analysis procedure Lifereg in SAS 8.0. A pooled analysis would have been inappropriate because one study was four weeks longer than the other two.
Use of the targeted drug during the Intervention phase was analyzed separately within each study using analysis of covariance (ANCOVA), with two categorical between-subject factors (cannabis-use category and experimental treatment group) and one continuous covariate (each patient’s baseline percentage of urine specimens positive for the targeted drug, arcsine-transformed to maintain homogeneity of variance). To determine whether we had lost power by treating cannabis use as a categorical predictor, we also performed these analyses in the form of multiple regressions, with the percentage of cannabis-positive urines (arsine-transformed) as a continuous predictor.
For participants who were randomized to a contingent-voucher condition, a repeated-measures ANCOVA was conducted separately within each study to test whether cannabis use was associated with a differential loss of treatment effect—that is, a tendency not to maintain initial levels of abstinence from the targeted drug (cocaine or opiates). Each ANCOVA had one categorical between-subject factor (cannabis-use category), one categorical within-subject factor (study phase: Intervention and the subsequent Maintenance phase), and one continuous covariate (baseline percent positive, arcsine-transformed). Differential loss of treatment effect would be reflected in an interaction between cannabis-use category and study phase.
To assess whether cannabinoid-positive urine predicted lapse to heroin use among patients who had achieved heroin abstinence, we replicated the two Cox proportional-hazard regression analyses used by Wasserman et al. (1998). Participants were included in these analyses only if they had provided opiate-negative urine specimens for at least three consecutive weeks. In the first analysis, cannabis use during the third week of opiate abstinence (corresponding to Wasserman’s “baseline” week) was used as a baseline predictor of time to lapse to opiate use. In the second analysis, cannabis use throughout the period of opiate abstinence was used as a lagged (t minus 1) time-dependent predictor of subsequent lapse to opiate use. As in Wasserman et al. (1998), premature study dropout was coded as resumption of heroin use. However, patients who were still testing negative for opiates at the end of the study were coded as censored. Because Cocaine Study 2 was longer than the other two studies, it was analyzed separately. These analyses were performed with the PHreg procedure in SAS 8.0.
To test whether cocaine and opiate use during intervention would be more strongly associated with lifetime history of Cannabis Abuse/Dependence (assessed at treatment intake by DSM-IIIR criteria) than with any cannabis use during treatment (assessed by cannabinoid-positive urines), we performed two multiple linear regression analyses (Reg procedure, SAS 8.0)—one in which the dependent variable was percentage of cocaine-negative urines, and one in which it was percentage of opiate-negative urines. The predictors were: lifetime diagnosis of Cannabis Abuse or Dependence (dummy-coded), detection of any cannabinoid-positive urines during treatment (dummy-coded), availability of vouchers (for cocaine or opiate abstinence, as appropriate; dummy-coded), and amount of baseline use (of cocaine or opiates, as appropriate). All predictors were entered simultaneously into each regression model. The analyses combined data from all three studies (n=408).
To test whether general psychosocial functioning after treatment would be more strongly associated with lifetime history of Cannabis Abuse/Dependence than with any cannabis use during treatment, we used posttreatment follow-up data, which were available for 262 patients. When compared to the total sample, the follow-up sample (118 nonusers, 80 occasional users, and 64 frequent users) showed no differential loss across cannabis-use categories. Because many participants had missing data (the mean number of follow-ups per patient was 2.6 out of a possible 3), data were analyzed with mixed regressions (Mixed procedure, SAS 8.0). Preliminary analyses showed that continuing methadone treatment was strongly associated with most outcome indices, so this was included as a time-varying covariate. Each model thus contained three between-subject factors (cannabis-use category, presence/absence of a cannabis-use diagnosis, and whether the patient was currently on methadone) and one within-subject factor (posttreatment month: 3, 6, or 12).
In all analyses, to minimize the chance of overlooking effects that were missed in prior studies, no corrections were made for multiple tests of significance. Significance level was set at .05, with trends noted at .10. All tests were two-tailed.
Because the findings were generally negative, we performed post hoc power calculations for selected analyses. For the ANCOVAs on intervention-phase data, we used power tables for the f effect-size measure in Cohen (1988). For the proportional-hazard regressions on lapse to heroin use, we used NCSS software (Hintze, 2001).
Results
Cannabis Use
Among all participants, the mean±SEM percentage of cannabinoid-positive urine specimens was 15±1%; the range was 0 to 100%. Among those classified as occasional users, the mean was 6±0.4% and the range was 1–17%; among those classified as frequent users, the mean was 57±3% and the range was 17–100%.
Cannabis use as a predictor
Were cannabis users demographically different from other patients?
Despite a liberal approach to the analyses (no correction for multiple tests of significance), only two significant differences were found among occasional, frequent, and nonusers of cannabis, and both of the differences were on cannabis-related variables: years of cannabis use (nonusers, 2.9±0.4; occasional users, 4.5±0.7; frequent users, 9.4±1.0; F[2,405]= 24.3, p<.01) and likelihood of ever having met criteria for Cannabis Abuse or Dependence (nonusers, 5%; occasional users, 6%; frequent users, 16; χ2=11.1, df=2, p<.01). On other demographic variables, the groups were essentially indistinguishable, except for a trend toward higher estimated IQs in both groups of cannabis users. Findings were similar when demographics were compared separately within each of the three studies (data not shown).
Did cannabis users drop out of treatment sooner?
Cannabis-use category was not associated with differences in treatment retention in any of the three studies; p values from survival analyses ranged from .62 to .79.
Were cannabis users more likely to use the targeted drug during the study intervention phase?
Cannabis-use category was not associated with any difference in use of the targeted drug during the intervention phase (reflected in the absence of a significant main effect in ANCOVAs controlling for baseline use of the targeted drug). In Cocaine Study 2, there was a trend toward a positive association of cannabis use with cocaine use; the mean ±SEM percentage of cocaine-negative urines was 33±4% in nonusers, 25±4% in occasional users, and 22±6% in frequent users, F(2,125)=2.6, p=.078. However, cannabis-use category accounted for less than 3% of the variance in cocaine use. Also, in that study as well as the other two, there was no interaction of cannabis-use category with treatment group. The absence of such an interaction suggests that cannabis users were not refractory to contingency management or to any of the other experimental interventions used (e.g. cognitive-behavioral therapy in Cocaine Study 2).
When we performed these analyses in the form of multiple regressions, with the percentage of cannabis-positive urines (arsine-transformed) as a continuous predictor rather than a categorical one, its effects (and its interactions with treatment group) failed to approach significance in any of the studies.
Among patients who underwent contingency management, were cannabis users more likely to resume other drug use afterwards?
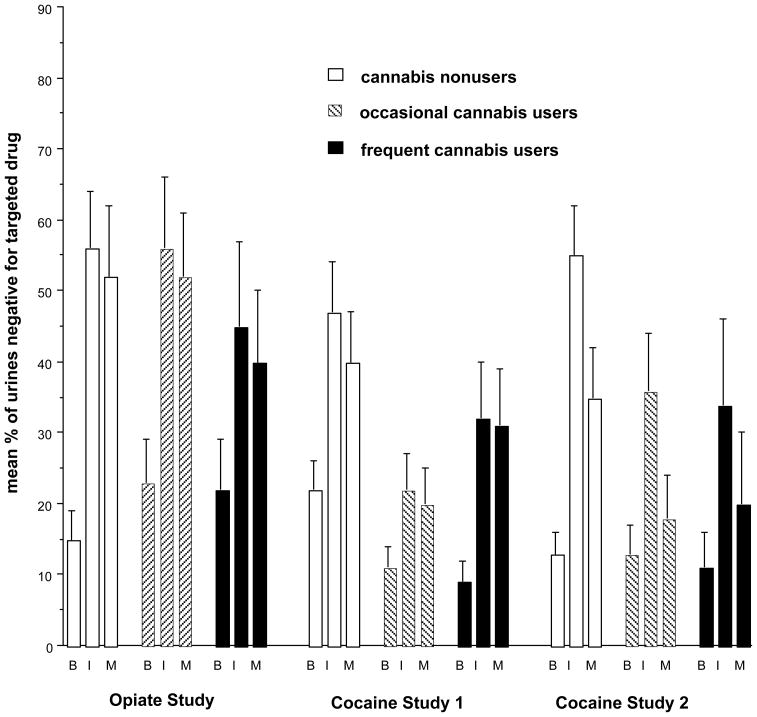
Figure 1 shows the mean percentages of urine specimens negative for the targeted drug (illicit opiates or cocaine) during each study. Repeated-measures ANCOVAs controlling for baseline use of the targeted drug showed no main effect of cannabis-use category and no interaction of cannabis-use category with study phase. The absence of such an interaction suggests that cannabis users showed no special propensity to resume the use of the targeted drug (heroin or cocaine) after contingency management was discontinued.
Figure 1.
Mean (SEM) percentages of urine specimens negative for the targeted drug (illicit opiates or cocaine) during the Baseline (B), Intervention (I), and maintenance (M) phase of each study. Vouchers were available during the Intervention phase only. Data are shown only for participants whose voucher earnings were contingent on abstinence, not for control participants who received vouchers noncontingently. For the Intervention and Maintenance phases, repeated-measures ANCOVAs controlling for Baseline use of the targeted drug showed no main effect of cannabis-use category and no interaction of cannabis-use category with time.
Among patients who achieved abstinence from heroin, did cannabis users show an increased risk of lapse?
To address this question, we replicated the two Cox proportional-hazard regression analyses used by Wasserman et al. (1998).
In the first two studies (Opiate Study and Cocaine Study 1), 113 patients achieved at least three weeks of verified abstinence from illicit opiates. Among these patients, the median duration of abstinence was 6.3 weeks (95% CL 5.7–7.7 weeks). Cannabis use during the third week of abstinence did not predict subsequent lapse to opiate use, although there was a trend in that direction (hazard ratio 1.54, 95% CL 0.93–2.56; χ2 [1]=2.78, p=.095). When cannabis use was treated as a time-dependent predictor, the effect did not approach significance (hazard ratio 1.20, 95% CL 0.69–2.09; χ2 [1]=.41, p=.52).
In Cocaine Study 2, 94 patients achieved at least three weeks of verified abstinence from illicit opiates. Among these patients, the median duration of abstinence was 5.7 weeks (95% CLs 5.3–7.3 weeks). Cannabis use during the third week of abstinence did not predict subsequent lapse to opiate use (hazard ratio 0.90, 95% CL 0.48–1.65; χ2 [1]=.13, p=.72). The same was found when cannabis use was treated as a time-dependent predictor, (hazard ratio 0.95, 95% CL 0.51–1.77, χ2[1]=.02, p=.88).
Did cannabis use predict worse general psychosocial functioning at posttreatment follow-up?
In mixed regressions, cannabis-use category was not associated with any differences in any of the ASI variables tested (days paid for working, legal income, illegal income, days of illegal activity, days in jail, days of drug-related problems, percentage of income spend on drugs, days of family conflict, days of other interpersonal conflict, or days on methadone).
Cannabis abuse/dependence
At treatment intake, 32 participants (8% of the total sample, 15% of the users) met DSM-IIIR criteria for Cannabis Abuse or Dependence. During treatment, they were more likely to be frequent users (n=15) than occasional users (n=8), or nonusers (n=9), χ2[2]=11.08, p=<.004. Due to the archival nature of the data, only summary diagnostic information was available for 21 of the 32 participants. In the 11 participants with more complete data, the mean number of lifetime symptoms reported was 4, and the symptoms most frequently reported were: spending a great deal of time on cannabis-related activities (n=9); experiencing tolerance (n=9); using more or longer than intended (n=8); being unable to refrain or cut down (n=8); and being high on cannabis when expected to perform role obligations or when hazardous (n=8). Eight of the 11 participants reported that no symptoms had occurred within the past year (see Discussion).
Cannabis abuse/dependence as a predictor
Did cannabis abuse/dependence predict use of other drugs during treatment?
In multiple linear regressions on cocaine and heroin use during treatment, lifetime DSM cannabis-use diagnoses were a stronger predictor than urine cannabinoids. However, the direction of the association was not as expected. During treatment, patients who had met criteria for a cannabis-use diagnosis used less heroin and less cocaine (Table 2) than those who had not met criteria.
Table 2.
regression analyses: opiate and cocaine abstinence during Intervention phase (all 3 studies)
| Variable | Parameter estimate ± SEM | t | P | interpretation of parameter estimate |
|---|---|---|---|---|
| Opiates: | ||||
| Percentage of opiate-negative specimens during Baseline | 0.74 ± 0.05 | 14.89 | <.0001* | each 1% increase in baseline opiate negatives predicted a 0.74% increase in opiate negatives during Intervention. |
| Availability of vouchers for opiate-negative specimens (0, 1) | 17.30 ± 4.69 | 3.69 | 0.0003* | opiate-contingent vouchers were associated with a 17% increase in opiate negatives during Intervention. |
| Lifetime diagnosis of Cannabis Abuse or Dependence (0,1) | 15.91 ± 5.73 | 2.78 | 0.0058* | lifetime Cannabis Abuse or Dependence on the DIS was associated with a 15.9% increase in opiate negatives during Intervention. |
| Availability of vouchers for cocaine-negative specimens (0,1) | 3.97 ± 3.60 | 1.10 | 0.2716 | cocaine-contingent vouchers had no effect on opiate negatives during Intervention. |
| Any cannabinoid-positive urine specimens during treatment (0,1) | −0.12 ± 3.06 | −0.04 | 0.9699 | cannabis use during treatment was not associated with any difference in opiate negatives during Intervention. |
| Cocaine: | ||||
| Percentage of cocaine-negative specimens during Baseline | 0.95 ± 0.07 | 13.52 | <.0001* | Each 1% increase in Baseline cocaine negatives predicted a 0.95% increase in cocaine negatives during Intervention. |
| Availability of vouchers for cocaine-negative specimens (0, 1) | 13.35 ± 3.49 | 3.83 | 0.0002* | Cocaine-contingent vouchers were associated with a 13% increase in cocaine negatives during Intervention. |
| Lifetime diagnosis of Cannabis Abuse or Dependence (0,1) | 11.49 ± 5.68 | 2.02 | 0.0438* | Lifetime Cannabis Abuse or Dependence was associated with an 11.5% increase in opiate negatives during Intervention. |
| Any cannabinoid-positive urine specimens during treatment (0,1) | −5.02 ± 3.03 | −1.66 | 0.0987 | Cannabis use during treatment was associated with 5% fewer cocaine negatives during Intervention (p < .10). |
| Availability of vouchers for opiate-negative specimens (0, 1) | −2.31 ± 4.82 | −0.48 | 0.6324 | opiate-contingent vouchers had no effect on cocaine negatives during Intervention. |
Among patients who achieved abstinence from heroin, did cannabis abuse/dependence predict lapse during treatment?
In patients who achieved at least three weeks of heroin abstinence during treatment, lifetime cannabis-use diagnosis did not predict lapse to opiate use in the first two studies (hazard ratio 1.16, 95% CL 0.63–2.13; χ2 [1]=0.22, p=.64) or in the third study (hazard ratio 2.09, 95% CL 0.76–5.76; χ2 [1]=1.66, p=.19).
Did cannabis abuse/dependence predict worse general psychosocial functioning at posttreatment follow-up?
In mixed-regression analyses, lifetime DSM cannabis-use diagnosis at treatment intake were associated with more days in jail at posttreatment follow-up (1.3 ± 0.3 days out of 30 versus 0.2 ± 0.1; F[1,258] = 8.58, p<.0037) and with more days of family conflict: (4.5± 1.0 days out of 30 versus 2.0 ± 0.3; F[1,258] = 6.60, p<.0187).
In the same analyses (i.e. controlling for cannabis-use diagnosis), cannabis-use category showed a trend toward an association with days in jail (F[2,258] = 2.74, p<.066), but the association was not linear (0.6 ± 0.2 for nonusers, 1.1 ± 0.2 for occasional users; 0.6 ± 0.2 for frequent users).
Neither predictor was associated with any differences in: days paid for working, legal income, illegal income, days of illegal activity, percentage of income spent on drugs, days of drug problems, days of nonfamily interpersonal conflict, or days on methadone.
Post hoc power analyses
Power of ANCOVAs on use of other drugs during Intervention phase
Taking into account the presence of other factors in the ANCOVAs, the three studies (Opiate, Cocaine 1, and Cocaine 2) each had power of .95 to detect effect sizes of f = .40, .44, and .35, respectively, for cannabis-use category. These are large-to-medium effects, equivalent to r2 values of .14, .15, and .11. In other words, if cannabis-use category had accounted for 11% or more of the variance in use of the targeted drug (cocaine or illicit opiates), then the probability of our having failed to detect an association would be less than .05.
Power of survival analyses on risk of lapse
The survival analysis in which we pooled data from our first two studies had an N of 113 (of whom 20 used cannabis early in their heroin abstinence) and a lapse rate of 80% in nonusers. This provided us with power of .95 to detect a hazard ratio of 2.22 or more. Thus, the analysis was well-powered to replicate the findings of Wasserman et al., who had found a relative risk (which should be roughly comparable to a hazard ratio) of approximately 2.6.
The survival analysis from our third study had an N of 94 (of whom 14 used cannabis early in their heroin abstinence) and a lapse rate of 90% in nonusers. The lower N and the high rate of lapse in the control group combined to reduce power; this analysis had power of only .58 to detect a hazard ratio of 2.6 or more.
Discussion
In agreement with previous studies (Saxon et al., 1993; Budney et al., 1998; Nirenberg et al., 1996), cannabis use showed little relation to use of heroin or cocaine. Specifically, there was no linear dose-response relationship from nonuse to intermittent use to frequent use of cannabis, nor was there an inverted-U-shaped relationship (as in Church et al., 2001). Obviously, it is not possible to prove the absence of an association between two variables. However, our analyses (with statistical power of .95 to detect an r2 as low as .11) indicate that cannabis use is unlikely to account for more than 11% of the variance in heroin or cocaine use in our population—and may account for much less, since in our sample it accounted for under 3%.
The statistical power of our analyses also indicates that if cannabis use predicts lapse to heroin use among patients who have become heroin-abstinent, the hazard ratio is unlikely to be greater than 2.22. A hazard ratio of that magnitude may be clinically significant, but the actual hazard ratio may be considerably lower; in our sample it was 1.54 at most and not statistically significant. This finding contradicts that of Wasserman et al. (1998). Interpretation of the discrepancy is complicated by a difference in the overall rate of lapse (more than 80% in our patients versus 41% in Wasserman et al.’s patients), which might have obscured any influence of cannabis use in our sample. The difference in overall rate of lapse may have been due to other sample differences (Wasserman et al.’s patients were given a wider, presumably more individualized range of methadone doses, and had generally been at their maintenance dose longer than our patients), and those differences themselves may have moderated any effect of cannabis use. It is also worth noting that our sample, though comparable to that of Wasserman et al. in terms of sex, age, education, and income, had a greater proportion of African-Americans, a slightly lower rate of unemployment, and, moreover, a far lower lifetime rate of psychiatric disorders (43% vs. 1% for Major Depressive Disorder, 49% vs. 6% for PTSD, and 43% vs. 13% for Antisocial Personality Disorder). Psychiatric diagnoses did not predict lapse in Wasserman et al.’s patients, but they did not test for an interaction of cannabis use with psychiatric diagnoses. Thus, it is possible that cannabis use differentially predicts lapse to heroin use in patients with psychiatric comorbidity. Finally, it is possible that the finding of Wasserman et al. (1998) was due to chance; cannabis use was one of 20 predictors tested in that study, and with a Bonferroni correction, its p value increases from .004 to .08.
Our finding that cannabis use was not associated with treatment retention is consistent with others’ findings (Saxon et al., 1991; Budney et al., 1998; Church et al., 2001). In fact, one report on 120 patients in a drug-free treatment program for heroin addiction suggested that marijuana use was a favorable prognostic factor for treatment retention (Ellner, 1977).
Our findings could be taken to suggest that if cannabis use in methadone patients is to be discouraged, it should be for its own sake, not for the sake of a presumed causal relationship with treatment dropout or heroin or cocaine use. But even this interpretation may be too broad, because, in our posttreatment follow-ups, cannabis use during treatment failed to predict any of the psychosocial problems assessed by the ASI. Two of the psychosocial problems (number of recent days in jail and number of recent days of family conflict) were predicted, albeit weakly, by lifetime history of Cannabis Abuse or Dependence. Thus, the negative prognostic signs of interest may be symptoms of Cannabis Abuse or Dependence.
Counterintuitively, Cannabis Abuse or Dependence predicted less use of heroin and cocaine during treatment itself. This seems to replicate a prior finding among cocaine-dependent patients: durations of abstinence from cocaine were significantly longer in patients with comorbid Cannabis Dependence (Budney et al., 1996). It is unlikely that this could be explained by patients’ substituting cannabis for heroin or cocaine; if substitution were occurring, one would expect to see an association with frequency of cannabis use, not with symptoms of Cannabis Abuse or Dependence. (In any case, behavioral-economic work has shown that cannabis is at best a weak substitute for heroin; Petry & Bickel, 1998.) Since criteria were met through self-report via a structured interview, meeting criteria might be a marker of greater insight into drug-associated problems. This is consistent with our own recent finding that cocaine use during treatment was lower in methadone patients who had met criteria for Cocaine Dependence at intake (Epstein et al., in press).
However, even if meeting DSM criteria for Cannabis Abuse or Dependence were a marker of greater insight, we found that patients who met criteria were continuing to use more cannabis than those who did not. This combination of findings—self-reported adverse consequences of cannabis use, plus continued use—is reason enough for cannabis use to be a focus of clinical attention within this subgroup of patients. In our sample, patients reported symptoms such as using more cannabis than intended and being high on cannabis when expected to perform role obligations or when hazardous. These self-reported symptoms seem to provide a good basis for encouraging the patient to reduce or discontinue cannabis use.
This finding is complicated by the fact that, in the 11 patients for whom recency data were available, eight reported that no symptoms had occurred within the past year. There is insufficient evidence to say what level of concern should be generated by continued cannabis use in a patient who endorses a past history of Cannabis Abuse or Dependence. Studies of the natural history of these disorders (Rosenberg & Anthony, 2001; von Sydow et al., 2001) have not addressed the issue, and have not been done in methadone-maintained heroin addicts. In clinical practice, changes in the number or severity of DSM-IV symptoms of Cannabis Abuse or Dependence can be assessed through a semistructured interview (although reliability is not as good as for other diagnostic categories) (Miele et al., 2000). Formal or informal use of such an instrument might serve as a barometer for reemergence of past symptoms.
The present study has limitations that should be considered. The most important is that, except when the DIS was given at study intake, we did not directly ask participants about the consequences of their cannabis use. We also limited the sensitivity of our analyses by categorizing cannabis use rather than treating it as a continuous variable. (However, it is our impression that most methadone-clinic staff and administrators view cannabis use as a behavior categorically distinct from abstinence. By categorizing levels of use, we struck a balance between having more sensitive analyses and having results expressible in terms that would be meaningful to most clinicians. Moreover, when we repeated some of our analyses after coding cannabis use as a continuous predictor, the results did not change.) After the first five weeks of treatment, all of our participants either underwent contingency management or a control condition (with vouchers given noncontingently); we do not have data from patients in more conventional long-term methadone maintenance. (This limitation carries one analytic advantage: our patients were not discharged for using heroin or cocaine, so rates of use were not artificially low.) Although we tried to enroll participants representative of the urban area our clinic serves, we excluded study applicants with symptoms of psychosis, mania, or active suicidal ideation, or with physical dependence on alcohol or sedatives; thus, our findings may not generalize to patients with such comorbidities. Due to the time-limited nature of our studies, no patient was enrolled in our clinic for more than 39 weeks, and our posttreatment follow-up for each patient was limited to three appointments in 12 months; therefore, we may have failed to detect long-term adverse outcomes. Our diagnoses of substance-use disorders were not based on the most recent edition of the DSM, and due to the archival nature of the data, we had only diagnostic summaries for most participants.
Another possible limitation is that our cannabinoid-positive participants did not test positive as frequently, on average, as those in other studies. Overall, 54% tested positive at least once. (For comparison, the corresponding figures from prior studies are 47% [Wasserman et al., 1998], 55% [Saxon et al., 1993], 65% [Budney, Bickel & Amass, 1998] and 79 % [Nirenberg et al., 1996].) However, among the subgroup who tested positive, only 11 (5%) of them tested positive every time. For comparison, the corresponding figure from another study is 41% (Saxon et al., 1993). Similarly, if we use the classification chosen by Nirenberg et al. (1996), the percentages of intermittent, moderate, and consistent users (up to 1/3 positive, 1/3 to 2/3 positive, and 2/3 to all positive) among our users were 68%, 17%, and 15%; among the users described in Nirenberg et al. (1996), the percentages were 45%, 29%, and 25%. The percentage of our users who met DSM criteria for a cannabis-use disorder (15%) was similar to the 17% reported by Budney, Bickel & Amass (1998).
It might be argued that another limitation of the present study was the possibility of a ceiling effect: if patients’ drug problems were already at their maximum, it would be impossible to detect contributions from cannabis use. Mathematically, this was not the case. (For example, at posttreatment follow-up, participants reported having experienced drug-related problems on 13.5±0.9 out of the past 30 days, with a median of 5 and a range of 0–30.) It may be instructive to note that in another sample of methadone-maintained patients, ceiling effects did not prevent detection of problems associated with smoking tobacco (McCarthy et al., 2002).
The present findings add to the evidence that cannabinoid-positive urines in methadone-maintained patients need not be a major focus of clinical attention, unless the patient meets criteria for a cannabis-use disorder by reporting cannabis-associated problems. If the patient reports no such problems, our data (and most of the prior published data) offer little empirical justification for disbelieving the patient. Future work should address the question of long-term outcome in cannabinoid-positive patients who report only a past history of cannabis-associated problems. Future work should also determine whether interventions that decrease cannabis use in methadone-maintained patients (Calsyn & Saxon, 1999) will be effective in patients who meet criteria for a cannabis-use disorder.
References
- AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, D.C: American Psychiatric Association; 1987. Revised. [Google Scholar]
- BECK AT, STEER RA. Beck Depression Inventory Manual. New York: The Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1987. [Google Scholar]
- BUDNEY AJ, HIGGINS ST, WONG CJ. Marijuana use and treatment outcome in cocaine-dependent patients. Experimental and Clinical Psychopharmacology. 1996;4(4):396–403. doi: 10.1037//1064-1297.6.4.419. [DOI] [PubMed] [Google Scholar]
- BUDNEY AJ, BICKEL WK, AMASS L. Marijuana use and treatment outcome among opioid-dependent patients. Addiction. 1998;93(4):493–503. doi: 10.1046/j.1360-0443.1998.9344935.x. [DOI] [PubMed] [Google Scholar]
- CALSYN DA, SAXON AJ. An innovative approach to reducing cannabis use in a subset of methadone maintenance clients. Drug and Alcohol Dependence. 1999;53:167–169. doi: 10.1016/s0376-8716(98)00121-5. [DOI] [PubMed] [Google Scholar]
- CHURCH SH, ROTHENBERG JL, SULLIVAN MA, BORNSTEIN G, NUNES EV. Concurrent substance use and outcome in combined behavioral and naltrexone therapy for opiate dependence. American Journal on Drug and Alcohol Abuse. 2001;27(3):441–452. doi: 10.1081/ada-100104511. [DOI] [PubMed] [Google Scholar]
- COHEN J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- DORE GM, WALKER JD, PAICE JR, CLARKSON S. Methadone maintenance treatment: outcomes from the Otago Methadone Programme. New Zealand Medical Journal. 1999;112:442–445. [PubMed] [Google Scholar]
- ELLNER M. Marijuana use by heroin abusers as a factor in program retention. Journal of Consulting & Clinical Psychology. 1977;45(4):709–710. doi: 10.1037//0022-006x.45.4.709. [DOI] [PubMed] [Google Scholar]
- EPSTEIN DH, HAWKINS W, COVI L, UMBRICHT A, PRESTON KL. Cognitive-Behavioral Therapy Plus Contingency Management for Cocaine Use in Methadone-Maintenance Patients: Findings During Treatment and Across 12-Month Follow-Up. Psychology of Addictive Behaviors. doi: 10.1037/0893-164X.17.1.73. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELZER JE. Psychoactive substance abuse and its relation to dependence. In: Widiger TA, Frances AJ, Pincus HA, Ross R, First MB, Davis W, Kline M, editors. DSM-IV Sourcebook. Washington, DC: American Psychiatric Association; 1994. pp. 21–32. [Google Scholar]
- HINTZE J. NCSS and PASS: Number Cruncher Statistical Systems (Kaysville, Utah) 2001 Downloaded from www.ncss.com, February 15, 2002.
- HUESTIS MA, MITCHELL JM, CONE EJ. Detection times of marijuana metabolites in urine by immunoassay and GC-MS. Journal of Analytical Toxicology. 1995;19:443–449. doi: 10.1093/jat/19.6.443. [DOI] [PubMed] [Google Scholar]
- MCCARTHY WJ, ZHOU Y, HSER Y, COLLINS C. To smoke or not to smoke: impact on disability, quality of life, and illicit drug use in baseline polydrug users. Journal of Addictive Diseases. 2002;21(2):35–54. doi: 10.1300/J069v21n02_04. [DOI] [PubMed] [Google Scholar]
- MIELE GM, CARPENTER KM, COCKERHAM MS, TRAUTMAN KD, BLAINE J, HASIN DS. Substance Dependence Severity Scale (SDSS): reliability and validity of a clinician-administered interview for DSM-IV substance use disorders. Drug and Alcohol Dependence. 2000;59:63–75. doi: 10.1016/s0376-8716(99)00111-8. [DOI] [PubMed] [Google Scholar]
- NIRENBERG TD, LIEPMAN MR, CELLUCCI T, SWIFT RM, SIROTA AD. Cannabis versus other illicit drug use among methadone maintenance patients. Psychology of Addictive Behaviors. 1996;10(4):222–227. [Google Scholar]
- PETRY NM, BICKEL WK. Polydrug abuse in heroin addicts: a behavioral economic analysis. Addiction. 1998;93(3):321–335. doi: 10.1046/j.1360-0443.1998.9333212.x. [DOI] [PubMed] [Google Scholar]
- PRESTON KL, UMBRICHT A, EPSTEIN DH. Methadone dose increase and abstinence reinforcement for treatment of continued heroin use during methadone maintenance. Archives of General Psychiatry. 2000;57:395–404. doi: 10.1001/archpsyc.57.4.395. [DOI] [PubMed] [Google Scholar]
- PRESTON KL, UMBRICHT A, WONG CJ, EPSTEIN DH. Shaping cocaine abstinence by successive approximation. Journal of Consulting & Clinical Psychology. 2001;69(4):643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- ROSENBERG MF, ANTHONY JC. Early clinical manifestations of cannabis dependence in a community sample. Drug and Alcohol Dependence. 2001;64(2):123–131. doi: 10.1016/s0376-8716(00)00229-5. [DOI] [PubMed] [Google Scholar]
- SAXON AJ, CALSYN DA, BLAES PA, HAVER VM, GREENBERG DM. Marijuana use by methadone maintenance patients. NIDA Research Monograph. 1991:105. [PubMed] [Google Scholar]
- SAXON AJ, CALSYN DA, GREENBERG D, BLAES P, HAVER VM, STANTON V. Urine screening for marijuana among methadone-maintained patients. American Journal on Addictions. 1993;2(3):207–211. [Google Scholar]
- SMITH-KIELLAND A, SKUTERUD B, MØRLAND J. Urinary excretion of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol and cannabinoids in frequent and infrequent drug users. Journal of Analytical Toxicology. 1999;23:323–332. doi: 10.1093/jat/23.5.323. [DOI] [PubMed] [Google Scholar]
- VON SYDOW K, LIEB R, PFISTER H, HOFLER M, SONNTAG H, WITTCHEN HU. The natural course of cannabis use, abuse and dependence over four years: a longitudinal community study of adolescents and young adults. Drug and Alcohol Dependence. 2001;64(3):347–361. doi: 10.1016/s0376-8716(01)00137-5. [DOI] [PubMed] [Google Scholar]
- WASSERMAN DA, WEINSTEIN MG, HAVASSY BE, HALL SM. Factors associated with lapses to heroin use during methadone maintenance. Drug and Alcohol Dependence. 1998;52:183–192. doi: 10.1016/s0376-8716(98)00092-1. [DOI] [PubMed] [Google Scholar]