Abstract
To test whether a combination of contingency management and methadone dose increase would promote abstinence from heroin and cocaine, we conducted a randomized controlled trial using a 2 X 3 (Dose X Contingency) factorial design in which dose assignment was double-blind. Participants were 252 heroin- and cocaine-abusing outpatients on methadone maintenance. They were randomly assigned to methadone dose (70 or 100 mg/day, double blind) and voucher condition (noncontingent, contingent on cocaine-negative urines, or “split”). The “split” contingency was a novel contingency that reinforced abstinence from either drug while doubly reinforcing simultaneous abstinence from both: the total value of incentives was “split” between drugs to contain costs. The main outcome measures were percentages of urine specimens negative for heroin, cocaine, and both simultaneously; these were monitored during a 5-week baseline of standard treatment (to determine study eligibility), a 12-week intervention, and a 10-week maintenance phase (to examine intervention effects in return-to-baseline conditions). DSM-IV criteria for ongoing drug dependence were assessed at study exit. Urine-screen results showed that the methadone dose increase reduced heroin use but not cocaine use. The Split 100mg group was the only group to achieve a longer duration of simultaneous negatives than its same-dose Noncontingent control group. The frequency of DSM-IV opiate and cocaine dependence diagnoses decreased in the active intervention groups. For a split contingency to promote simultaneous abstinence from cocaine and heroin, a relatively high dose of methadone appears necessary but not sufficient; an increase in overall incentive amount may also be required.
Keywords: contingency management, polydrug dependence, methadone dose, DSM diagnoses
1. Introduction
Opiate-dependent patients in methadone-maintenance programs often abuse both heroin and cocaine (ONDCP, 2004). Ongoing abuse of either drug can be effectively decreased with contingency management (CM), in which a desired behavior is monitored (e.g. by frequent urine testing for drug use) and reinforced (e.g. by vouchers with monetary value). However, CM is less effective when targeted toward multiple drugs simultaneously (Peachey, 1987; Griffith et al., 2000; Prendergast et al., 2006).
Accordingly, some attempts to target CM toward heroin and cocaine simultaneously have had negative or only modest results. When methadone-maintained patients were offered $1155 in vouchers for heroin and cocaine abstinence over 12 weeks, most never earned a voucher (Downey et al., 2000). In a similar study in an outpatient drug-free program, with $1087 in vouchers available, the rate of drug-free urines was only 20%, with no significant difference between the CM condition and a control condition (Katz et al., 2002). Another study tested a stepwise approach in which the initial requirement was abstinence from cocaine and heroin, then from all illicit drugs for a possible $755 in vouchers over 16 weeks. The effect of CM was small (almost 50% of CM patients never provided a negative specimen), slow to develop (significant differences during treatment weeks 12–16 only), and dissipated immediately upon cessation of the contingency (Piotrowski et al., 1999).
A few studies targeting dual drug contingencies have yielded positive results, but only in the context of an intensive psychosocial intervention (Silverman et al., 2001), in the absence of a control group (Katz et al., 2001), or with high-magnitude vouchers ($3369 over 9 weeks) (Dallery et al., 2001). The last finding supports the argument that voucher-based CM can almost always be efficacious, but does little to address concerns about its cost. (See Discussion for other relevant studies.) Although widespread implementation of voucher-based CM may be hindered as much by philosophical objections as by cost concerns (Kirby et al., 2006), cost is nonetheless worth addressing.
In the present study, we explored a new way to target interventions toward cocaine and heroin use simultaneously without increasing the overall cost of the vouchers beyond that typically used in studies of this type (e.g. $1155 in vouchers for 12 weeks of abstinence from a targeted drug—note that this refers to the maximum amount available, not to the amount that every participant will earn). We were also interested in continuing a previous line of research in which we systematically assessed the interaction between methadone dose and voucher incentive effects. (Preston et al., 2000), using a higher range of doses than we had previously tested.
In a between-subjects, 2 X 3 design, we tested the interaction between methadone dose (70 vs 100 mg/day) and two abstinence incentive strategies, with a noncontingent-voucher condition serving as control. The first strategy was to target all voucher earnings on cocaine. Our rationale was that this would allocate all available voucher resources toward a problem for which there is no pharmacological intervention, while taking full advantage of the effectiveness of methadone for heroin use: higher doses of methadone are associated with lower rates of heroin use (Strain et al., 1993; Ling et al., 1996; Schottenfeld et al., 1997). It also seemed possible that reinforcement of cocaine abstinence would generalize to increases in heroin abstinence (Silverman et al., 1998). However, such “carryover” of effects is not seen consistently, and may be most reliable when methadone doses are lower than those in the present study (Epstein and Preston, 2008). The second, novel strategy was to split the contingent earnings and across the two drugs of interest (heroin and cocaine) and offer half the amount for abstinence from each drug independent of abstinence from the other drug. Thus, a participant who had used one drug would still have an incentive to abstain from the other drug before the next clinic visit. We hypothesized that through this mechanism, the “split contingency” would increase abstinence from each drug, and, on the general principle that “abstinence begets abstinence” (Higgins et al., 2000a; Kirshenbaum et al., 2009), would ultimately increase the frequency of simultaneous abstinence from both drugs without costing more than a contingency aimed at one drug alone. We also hypothesized that this effect would be more prominent when abstinence from illicit opiates was facilitated by the use of a higher dose of methadone.
2. Method
2.1 Participants
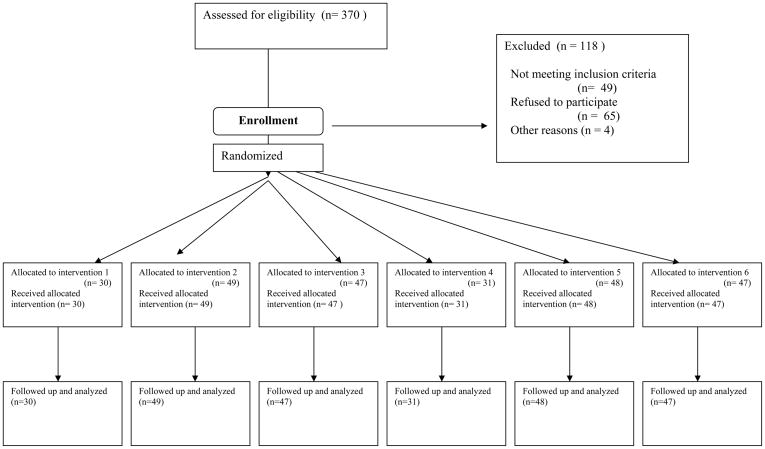
Participants were selected from 370 outpatients admitted for methadone maintenance at a research clinic in Baltimore, MD. Screening included medical, psychiatric, and drug-use histories, physical examination, standard laboratory screens, and a battery of assessment instruments, including the Addiction Severity Index (ASI) (McLellan et al., 1985) and the Diagnostic Interview Schedule (DIS-IV) (Robins et al., 1995). Eligibility criteria for initial enrollment were: age 18–65, cocaine and opiate use (by self-report and urine screen), and physical dependence on opiates. Direct transfer from community methadone programs was permitted, but did not occur in this sample. Exclusion criteria were: current psychotic, bipolar, or major depressive disorders; current physical dependence on alcohol or sedatives; unstable serious medical illness; estimated IQ below 80, per the Shipley Institute of Living Scale (Zachary, 1986); and conditions precluding urine collection. Eligibility for randomization to a group was based on subsequent heroin and cocaine use during a five-week baseline (see below). DSM-IV diagnoses of heroin or cocaine dependence were not required. Of 370 patients enrolled, 49 failed to meet drug-use criteria for randomization, 65 dropped out before being randomized, and four had serious medical events (e.g. emergence of previously undisclosed bipolar disorder; severe stabbing injury) that prevented randomization. The remaining 252 were randomized to an experimental group. Figure 1 shows participants’ flow through the study.
Figure 1.
Flowchart of participants’ progress through the study. Data in this report are from the 252 participants who were randomized to a treatment group for the 12-week intervention phase. Of those, 194 completed the intervention and progressed immediately to an 8-week maintenance phase (data from that phase are not included in this report).
This study was approved by the Institutional Review Board of the NIDA Intramural Research Program; each participant gave written informed consent.
2.2. Standard treatment
All participants received daily methadone and weekly individual counseling for 25 weeks. For individual-counseling sessions, counselors completed a semistructured psychosocial assessment and treatment plan for each participant. Reduction of substance use was the primary goal. Methadone HCl (Mallinckrodt, Inc., St. Louis, MO) was administered orally in 35 ml of cherry-flavored solution. Dose was stabilized at 70 mg/day within one week.
2.3. Urine and breath toxicology
Mondays, Wednesdays, and Fridays, urine specimens were collected under observation. Testing was conducted with an Enzyme Multiplied Immunoassay Technique (EMIT; Syva Corp., Palo Alto, California) system that provided qualitative results for cocaine (benzoylecgonine equivalents; BZE), opiates (morphine), marijuana, and benzodiazepines (oxazepam). Cutoffs were 300 ng/ml for cocaine, opiates, and benzodiazepines, and 50 ng/ml for marijuana. Breath alcohol was determined with an Alco-Sensor III (Intoximeters, Inc., St. Louis, MO). Use of alcohol, benzodiazepines, and nonheroin opiates was rarely detected or reported, use of cannabis was detected in approximately 20% of urine screens, and use of any of these drugs was not associated with methadone dose or contingency (data not shown).
BZE concentrations were also assayed quantitatively by fluorescence polarization immunoassay (FPIA) using TDx Cocaine Metabolite Assay reagents (Abbott Laboratories, Abbott Park, IL) (cross-reactivity: 100% for BZE and ≤1% for cocaine, ecgonine methyl ester, and ecgonine: linear range: 30 to 5000 ng/ml; specimens with higher concentrations were diluted to concentrations within this range and reanalyzed).
2.4. Self-reported measures
Immediately after each urine collection, participants were asked how many times they had used any drug on each day since the previous collection. At baseline and every two weeks through week 34, participants filled out the Social Adjustment Scale – Self-Report (SAS-SR) (Weissman and Bothwell, 1976), a measure of quality of life with acceptable psychometric properties (Larson, 1993).
2.5. Study timeline and groups
Baseline began upon enrollment and continued until the participant had provided 15 urine specimens (at least 5 weeks; actual mean length was 5.4 weeks, with a range of 4.6 to 7.4 weeks, and did not differ across experimental groups, p = .90). Participants were then eligible for randomization if they had tolerated 70 mg/day and tested positive for heroin and cocaine at least four times each (not necessarily on the same days). Participants not meeting these criteria (n = 49) were excluded from randomization but permitted to remain in treatment for the rest of the study; their data are not reported here. Participants were not told about the randomization criteria.
For the 12-week Intervention, participants were randomized to one of six experimental groups (described below). For the 8-week Maintenance, standard treatment was resumed. Thereafter, participants were encouraged to transfer to other community treatment programs; those who did not were offered a 10-week methadone taper. Upon departing the program or beginning the taper (whichever came first), participants were reassessed at exit interviews that included urine collection and the Substance Dependence Severity Scale (SDSS), a semistructured interview assessing DSM-IV substance-dependence symptoms over the preceding 30 days, with acceptable reliability and validity (Miele et al., 2000). The SDSS was intended to complement our urine-based outcome measures with information about the consequences of substance use. To avoid participant burden, the SDSS was not administered at intake (when DSM-IV substance-dependence symptoms were assessed as part of a lengthier DIS-IV interview), so results are available from exit only.
We used a 2 × 3 design, with each participant assigned to one of two methadone-dose conditions and one of three CM conditions, as shown in Figure 1. Assignment was unequal to maximize power for pairwise comparisons of interest (Woods et al., 1998; Dumville et al., 2006). A priori power calculations indicated that an N of roughly 45 per group in groups 2–6 would provide 80% power to detect a medium-large effect size (h=0.58; Cohen, 1988) in terms of overall proportions of drug-negative urine specimens.
Blinding of CM conditions was not possible, but dose conditions were double-blind. Participants were first randomized to CM conditions by a study technician who used a Microsoft Excel macro that stratified randomization by race, sex, employment status, probation status, and frequency of opiate- and cocaine-positive urine specimens during baseline. Participants were then randomized to a dose condition using a similar Excel macro with identical stratification variables; this was done by an investigator (K.L.P.) who had no contact with participants. Dose assignments were known only to her and to in-house pharmacy staff, who also had no contact with participants.
2.6. CM conditions
In each of the three CM conditions, participants had the opportunity to receive vouchers exchangeable for goods and services (such as bus passes, clothing, or bill payments). Cash was not given for vouchers. The maximum total value of vouchers was $1155.
2.6.1. Noncontingent (Groups NCLow and NCHigh)
In this control condition, each participant received vouchers independent of urine results on a schedule yoked to the performance of a participant in one of the other conditions. Participants were not informed of the yoking, but told that they would receive vouchers on a “completely unpredictable schedule.”
2.6.2. Cocaine contingency (Groups CocLow and CocHigh)
Participants received a voucher immediately upon provision of each cocaine-negative urine. The value of the vouchers began at $2.50 and increased by $1.50 for each consecutive cocaine-negative urine. Upon provision of a cocaine-positive urine or failure to provide a urine, the voucher was withheld, and the value of the next earned voucher was reset to $2.50. An additional voucher worth $10.00 was given for every three consecutive cocaine-negative urines.
2.6.3. Split contingency (Groups SplitLow and SplitHigh)
Two independent contingencies were in place—one for opiates, one for cocaine. Each contingency was as described above, except that all the values were halved (initial voucher $1.25; each consecutive negative urine increased the value $0.75; every three consecutive negative urines reinforced with an extra $5.00).
2.7. Methadone-Dose Conditions
2.7.1. No dose increase (70 mg) (Groups NCLow, CocLow, and SplitLow)
Participants assigned to this condition remained at the 70 mg/day dose established during baseline.
2.7.2. Dose increase (100 mg) (Groups NCHigh, CocHigh, and SplitHigh)
Participants assigned to this condition received dose increases to a target of 100 mg/day, given in increments of 10 mg at two-day intervals. One participant in the Cocaine contingency tolerated only 80 mg/day, and one participant in the Split contingency tolerated only 90 mg/day; the other 124 received 100 mg/day. All were analyzed on an intent-to-treat basis.
2.8. Data analysis
Analyses in this report focus on the Intervention phase. For all analyses, the alpha level was p ≤ .05 (two-tailed), with trends noted at p ≤ .10.
Intake measures were analyzed by ANOVA (for continuous variables), Pearson χ2 (for categorical variables), or Fisher’s exact test (for categorical variables with expected cell sizes under 5).
Study retention was analyzed with a log-rank test (SAS LIFETEST procedure) of time until provision of the final urine sample; participants who left before the final week of the intervention phase were considered dropouts.
Qualitative urine results from Intervention were analyzed by repeated-measures logistic regression (SAS GLIMMIX macro). In three sets of analyses, the dependent variables were opiate-negative urines, cocaine-negative urines, and urines simultaneously negative for opiates and cocaine. We entered dose and contingency into each model to test main effects; we also performed planned comparisons regardless of main effects. To test the effect of contingency independent of methadone dose, each of the four treatment groups (CocLow, CocHigh, SplitLow, and SplitHigh) was compared to the same-dose noncontingent group (NCLow or NCHigh). Pairwise planned comparisons were also conducted among the four contingent groups. Each model controlled for baseline drug use (percentage of urine specimens negative for the drug or drugs being analyzed, arcsine transformed); the nonrandomness of the missing data was addressed by including a term for dropout (Hedeker and Gibbons, 1997). GLIMMIX analysis penalizes for missing data points, which occurred slightly more often in the low-dose groups. This could result in discrepancies between F values and effect sizes (e.g. a smaller F value having the larger effect size.) A first-order autoregressive error structure was used.
Longest duration of simultaneous abstinence was analyzed by ANOVA followed by planned t-tests.
Quantitative urinalyses for cocaine were analyzed in models identical to those described above, using mixed regressions (SAS MIXED procedure). Values below the laboratory reporting cutoff (100) were coded as 0. Because the data were right-skewed, all nonzero values were log-transformed (Delucchi et al., 1997).
Self-reported drug use was collapsed into periods matching the urine days; mean uses per day were calculated for each period. The data were analyzed with mixed regressions as described above. Data were missing for four participants (one from group NCLow, one from group CocLow, and two from group SplitHigh).
SAS-SR quality-of-life data were also analyzed by mixed regressions as described above.
DSM-IV substance dependence at study exit was analyzed by chi-square.
Effect sizes for differences between percentages are expressed in terms of Cohen’s h; h values of .2, .5, and .8 denote small, medium, and large effect sizes, corresponding roughly to R2 values of .01, .06, and .14 (Cohen, 1988). Effect sizes for differences between means are expressed in terms of Cohen’s d; d values of .2, .5, and .8 denote small, medium, and large effect sizes, corresponding roughly to R2 values of .01, .06, and .14 (Cohen, 1988).
3. Results
3.1. Participant Characteristics
Demographic characteristics of the 252 randomized participants did not differ significantly across the six experimental groups (Table 1). At treatment entry, most (96%) participants met DSM-IV criteria for opiate dependence while 67% met criteria for cocaine dependence, and another 7% met criteria for cocaine abuse. These proportions did not differ significantly (p values >.90) across the six experimental groups. Rates of other current psychiatric diagnoses (such as phobias, PTSD, and depression) were under 3% each, except for antisocial personality disorder (12%).
Table 1.
Participant Characteristics
| Total N | 252 participants |
| Women | 52% |
| African American | 66% |
| Age | 37.8 years (SD = 7.6, range 19–57 |
| Education | 11.4 years of (SD = 1.8, range 6–18), |
| Estimated IQ | 92.6 (SD = 8.6, range 80–113) |
| Employment | |
| Unemployed | 42% |
| employed part time | 18% |
| employed full time | 38% |
| Marital status | |
| never married | 53% |
| separated or divorced | 33% |
| married | 12% |
| Drug History | |
| Cocaine | |
| Lifetime use | 7.6 years (SD = 6.2, range 0–30); |
| Use in past 30 days | 18.3 days (SD = 9.7, range 3–30). |
| Route | injected intravenously -50%, smoked - 42%, insufflated - 8%. T |
| Heroin | |
| Lifetime use | 10.3 years (SD = 7.5, range 1–35) |
| Use in past 30 days | 29.4 of the past 30 days (SD = 3.0, range 4–30) |
| Route | injected intravenously - 61%, insufflated - 39% |
| Baseline drug use | |
| Urines testing opiate positive only (mean %) | 11% (SD 18, range 0–80%) |
| Urines testing cocaine positive only (mean %) | 13% (SD 20, range 0–73%) |
| Urines testing both opiate and coc positive (mean %) | 71% (SD 27, range 13–100%) |
| Urines testing neither opiate nor coc positive (mean %) | 5% (SD 11, range 0–67%) |
3.2. Retention
Mean retention was 15.1 weeks (SD 8.4) out of 17 for Baseline and Intervention only and 20.8 weeks (SD 5.9) out of 27 for the whole study; 23% of participants dropped out before the end of Intervention, 44% before the end of Maintenance. Retention did not differ by dose (log-rank χ2 = 0.44, df=1, p=.51) or contingency (log-rank χ2 = 0.68, df=2, p=.71) in the Baseline and Intervention or for the whole study [dose (log-rank χ2 = 1.73, df=1, p=.19); contingency (log-rank χ2 = 2.51, df=2, p=.29)].
3.3. Voucher Earnings
Across all six groups, the mean amount of voucher income was $416.87 (SD 26.26, range $0 – 1155), with no significant differences among groups. Among the four contingent groups, 81% of participants contacted the reinforcer (i.e. earned at least one voucher), again with no significant differences among groups.
3.4. Main effect of methadone dose
3.4.1
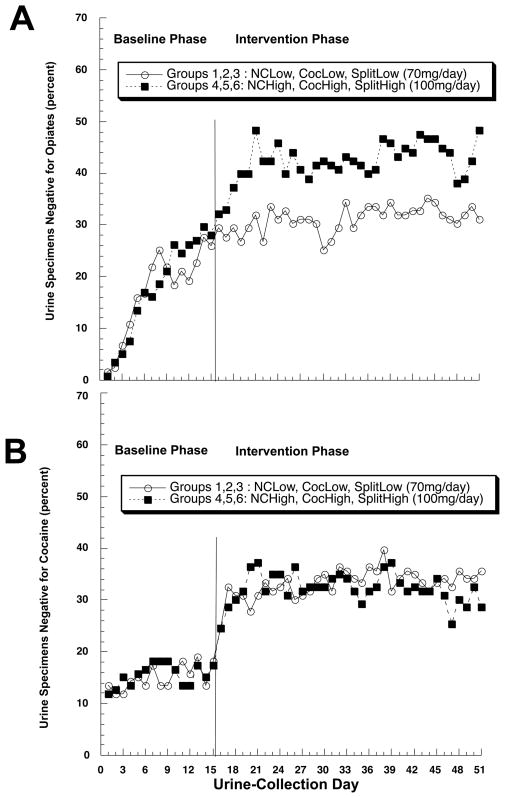
The percentage of urines negative for illicit opiates was greater in the 100mg groups than in the 70mg groups, F(1,244) = 6.08, p = .0143 (figure 2A). The overall percentages, adjusted for baseline use, were 20% (95% CL 12–33%) in the 70mg groups and 42% (95% CL 28–57%) in the 100mg groups; the effect size for this difference is h = 0.35 (small to medium). The percentage of urines negative for cocaine (Figure 2B), or for opiates and cocaine simultaneously (data not shown), did not differ by dose.
Figure 2.
Effect of methadone dose on abstinence from (A) illicit opiates and (B) cocaine (B) in methadone-maintained outpatients. Data are collapse across contingency-management groups. NCLow: noncontingent vouchers, lower dose of methadone. CocLow: cocaine-contingent vouchers, lower dose of methadone. SplitLow: cocaine- and opiate-contingent vouchers, lower dose of methadone. NCHigh, CocHigh, SplitHigh: corresponding voucher conditions with higher dose of methadone.
3.4.2. Current DSM-IV dependence at study exit
Higher methadone dose was associated with a lower likelihood of continued DSM-IV heroin dependence as assessed by the SDSS on the last day on which the full maintenance dose was administered. Criteria for current heroin dependence were still met by 43 out of 82 (52%) participants in the 70mg groups, versus 34/96 (35%) in the 100mg groups, X2 = 5.22, df = 1, p = .022, h = .35 (small to medium). There was no differential effect of methadone dose on current DSM-IV cocaine dependence: criteria were met by 48 out of 82 (59%) participants in the 70mg groups and 53/96 (55%) in the 100mg groups, X2 = 0.20, df = 1, p = .66.
3.5. Main effect of contingencies
There was a main effect of contingency on cocaine-negative urines, F(2,244) = 7.36, p = .0008, and on urines simultaneously negative for opiates and cocaine, F(2,244) = 3.61, p = .0285, but not in opiate-negative urines, F(2,244) = 2.51, p = .0830.
3.6. Pairwise contingency effects and interactions with dose
3.6.1. Cocaine
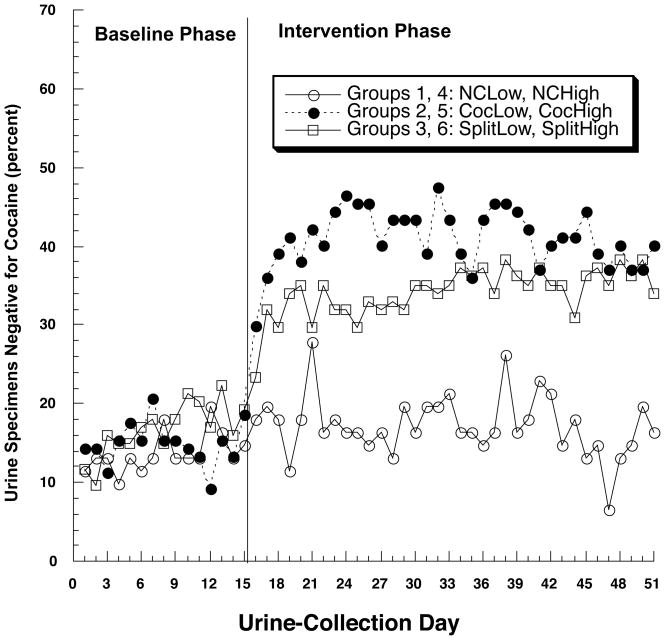
Contingency effects on cocaine use are shown in Figure 3 with data collapsed across methadone dose, as results were very similar across the two doses. The CocLow and CocHigh group each had a higher percentage of cocaine-negative urines than its same-dose Noncontingent control group [CocLow vs. NCLow, F(1,76) = 5.15, p=.026, h = 1.14; CocHigh vs. NCHigh, F(1,76) = 6.71, p = .011, h = 0.88]. The corresponding comparisons for the Split-contingency groups only approached significance [SplitLow vs. NCLow, F(1,74) = 3.29, p = .074, h = 0.64; SplitHigh vs. NCHigh, F(1,75) = 3.92, p = .051, h = 0.62], suggesting that splitting the vouchers reduced their effectiveness in maintaining abstinence from cocaine, although the Split and Cocaine contingency groups did not differ significantly from each other at either dose. A similar pattern was seen in quantitative urinalyses for the cocaine metabolite benzoylecgonine (Table 2).
Figure 3.
Effect of contingency management on abstinence from cocaine, collapsed across methadone dose due to absence of a dose effect. Abbreviations as in Figure 2.
Table 2.
Effects of methadone dose and contingency group
| Means and SD for Six Groups | Statistical Analysis Results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 70 mg groups |
100 mg groups |
contrasts, 70 mg groups |
contrasts, 100 mg groups |
|||||||||
| NC | Coc | Split | NC | Coc | Split | NC vs C | C vs S | NC vs S | NC vs C | C vs S | NC vs S | |
| Quantitative benzoylecgonine | ||||||||||||
| Mean BZE ng/mL | 55,842 | 44,183 | 42,981 | 66,313 | 42,397 | 56,717 | p = .02 | n.s. | n.s. | p<.003 | n.s. | p<.04 |
| SD | 47,258 | 46,550 | 47,316 | 45,735 | 47,264 | 46,786 | ||||||
| Longest duration of simultaneous abstinence | ||||||||||||
| Mean weeks | 0.91 | 1.26 | 2.00 | 0.71 | 2.17 | 2.71 | n.s. | n.s. | n.s. | p<.04 | n.s. | p<.02 |
| SD | 2.33 | 1.93 | 3.36 | 1.25 | 3.59 | 4.21 | ||||||
| Self-reported cocaine use | ||||||||||||
| Mean uses/day | 0.39 | 0.20 | 0.32 | 0.40 | 0.25 | 0.29 | p=.003 | p=.033 | n.s. | p=.012 | n.s. | p<.009 |
| SD | 0.27 | 0.27 | 0.27 | 0.27 | 0.28 | 0.29 | ||||||
| Quality of life - SAS-SR | ||||||||||||
| Mean score, family | 1.79 | 1.51 | 1.39 | 1.54 | 1.54 | 1.34 | p=.082 | n.s. | p=.014 | n.s. | n.s. | n.s. |
| SD | 0.68 | 0.67 | 0.69 | 0.69 | 0.70 | 0.71 | ||||||
| Mean score, financial | 2.39 | 1.99 | 2.18 | 2.22 | 1.89 | 1.09 | p=.09 | n.s. | n.s. | n.s. | n.s. | n.s. |
| SD | 1.04 | 1.03 | 1.02 | 1.05 | 1.09 | 1.01 | ||||||
NC - noncontingent; C - cocaine contingency; S - split contingency; BZE - benzoylecgonine; n.s. - not significant, p > .10.
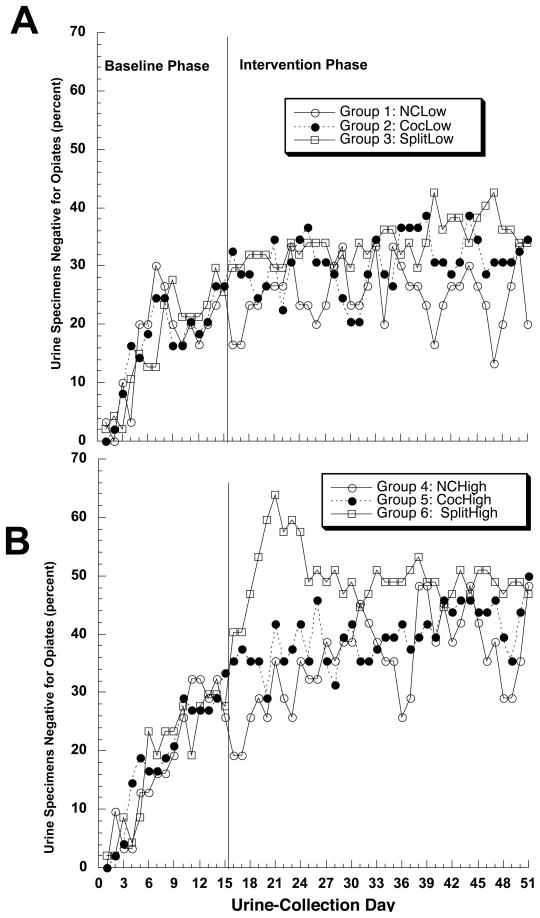
3.6.2. Opiates
Within the 70mg dose condition, contingency had no effect on the frequency of opiate-negative urines (figure 4a). Within the 100mg dose condition, the split contingency appeared to increase the frequency of opiate-negative urines during the second week of intervention (figure 4b), but this effect quickly dissipated, and pairwise comparisons showed no significant effect of the split or cocaine contingency on opiate-negative urines at either dose of methadone.
Figure 4.
Effect of contingency management on abstinence from illicit opiates, at methadone doses of (A) 70 mg/day or (B) 100 mg/day. Abbreviations as in Figure 2.
3.6.3. Simultaneous negatives
In pairwise comparisons, the SplitHigh group was the only one with a higher percentage of opiate- and cocaine-negative urines compared to its same-dose noncontingent control group [SplitHigh vs. NCHigh, F(1,75) = 3.97, p = .049, h = 0.45], (Figure 5b). However, trends toward significance were observed for CocHigh [CocHigh vs. NCHigh, F(1,76) = 3.21, p = .077, h = 0.39] (Figure 5b), and CocLow [CocLow vs. NCLow, F(1,76) = 3.12, p=.081, h = 0.37] (Figure 5a), and the split- and cocaine-contingency groups did not differ significantly from each other at either dose.
Figure 5.
Effect of contingency management on simultaneous abstinence from illicit opiates and cocaine, at methadone doses of (A) 70 mg/day or (B) 100 mg/day. Abbreviations as in Figure 2.
There was a main effect of contingency, F(2,246) = 4.70, p = .01, on longest duration of urines negative for both opiates and cocaine. There was no contingency-by-dose interaction, but in pairwise comparisons, differences were significant only within the 100-mg groups (Table 2); effect sizes were d = .49 (CocHigh vs. NCHigh) and d = .67 (SplitHigh vs. NCHigh).
3.6.4
Self-reported cocaine use generally supported urinalysis findings (Table 2), with more uses per day in the noncontingent groups (least-squares mean = 0.39, SD = 0.28) than in the cocaine contingency groups (least-squares mean = 0.23, SD = 0.29) or the split groups (least-squares mean = 0.28, SD = 0.28), resulting in a significant effect of contingency, F(2,240) = 7.60, p = .0006. There was no effect of contingency on self-reported use of opiates.
3.6.5. Current DSM-IV heroin dependence at study exit
The main effect of contingency was not significant, X2 (2) = 4.18, p = .12 (Table 3). However, planned comparisons showed that heroin dependence was less frequent in the split-contingency groups than in the noncontingent groups (h = .39), independent of methadone dose.
Table 3.
Substance Dependence symptoms at exit
| Group |
Contrasts |
|||||
|---|---|---|---|---|---|---|
| NC | Cocaine | Split | NC vs Cocaine | NC vs Split | Split vs Cocaine | |
| % still cocaine dependent | 76% | 55% | 45% | X2(1)=5.02, p=.025 | X2(1)=9.99, p<.002 | n.s. |
| % still heroin dependent | 53% | 45% | 34% | n.s. | X2(1)=4.05, p=.044 | n.s. |
| symptoms of cocaine dep. | 2.6 | 2.1 | 1.6 | n.s. | Tukey-adjusted p<.05 | n.s. |
| symptoms of heroin dep. | 1.7 | 1.6 | 1.3 | n.s. | n.s. | n.s. |
3.6.6. Current DSM-IV cocaine dependence at study exit
The main effect of contingency was significant, X2(2) = 9.97, p = .0068. Planned comparisons showed that cocaine dependence was less frequent in each of the contingency groups than in the noncontingent groups (Table 3); effect sizes were h = .65 (split groups vs. noncontingent groups) and h = .45 (cocaine groups vs. noncontingent groups). The mean number of dependence symptoms was also lower in the split-contingency groups than in the noncontingent groups.
4. Discussion
This study evaluated two strategies for combining methadone maintenance and contingency management to promote abstinence in polydrug users. Our findings support three general conclusions:
1. In contingency management, the magnitude of the incentive matters
By splitting a finite amount of voucher reinforcement across two target drugs, we elicited some abstinence from the second drug (heroin), but at the cost of slightly less abstinence from the first (cocaine). Our findings accord well with general principles of behavior (Nevin, 1999), but are disappointing as regards the possibility of getting more from each dollar spent on CM.
To our knowledge, our splitting of the contingency between two primary drugs of abuse was a novel approach to polydrug abuse when the study was begun. A variant of the split contingency has now been used in two other studies (Petry and Martin, 2002; Petry et al., 2005), but was not tested against other contingencies. As implemented in one of those studies (Petry et al., 2005), the main contingency required simultaneous abstinence from a subset of substances (cocaine, amphetamines, and alcohol), with additional “split” reinforcement for abstinence from cannabis and opioids, not all of which were primary drugs of abuse. Crucially, the comparison group was “usual care” rather than noncontingent vouchers; attrition over 12 weeks was higher in the “usual care” group (65%) than in the contingent group (51%), and outcome differences were significant only when all missing samples were considered positive. Similarly, in the other study (Petry and Martin, 2002), the comparison group was “usual care” rather than noncontingent vouchers, and both groups had relatively low rates of baseline use. Thus, the results of those studies are consistent with those of other studies that do not strongly support the effectiveness of targeting multiple drugs simultaneously (Piotrowski et al., 1999; Downey et al., 2000; Katz et al., 2002).1
2. In methadone maintenance, dose matters—but not for cocaine use
Despite findings to the contrary (Strain et al., 1996), it is still claimed that high-dose methadone increases abstinence from cocaine (Peles et al., 2006). These claims have generally been based on case series. We found that a double-blind, randomly assigned increase from 70 to 100 mg/day increased heroin abstinence, but not cocaine abstinence (Figure 2). To our knowledge, this is one of the clearest demonstrations of that point to date. One caveat is that patients in the cited case series received a mean dose of 171 mg/day whereas we did not test doses higher than 100 mg/day. Still, at the doses we tested, there was no sign of a dose-response effect of methadone on cocaine use.
The split contingency did elicit a statistically significant (though modest) increase in simultaneous negatives—but this increase reached statistical significance only when combined with a methadone dose increase. This finding is one that we had sought to obtain in an earlier study using lower doses of methadone (50 and 70 mg/day) (Preston et al., 2000); perhaps the higher doses in the present study were needed to elicit it. Our findings are similar to those of a recent study in which a contingency requiring simultaneous absence from cocaine and opiates was (modestly) effective only in patients receiving 100 mg of LAAM, not in patients receiving 30 mg of LAAM (Oliveto et al., 2005).
3. Effective treatment has clinically significant effects beyond merely decreasing drug use
This study was one of a series of CM studies at our clinic that, to our knowledge, were the first to include an outcome measure directly reflecting DSM-IV diagnoses (Ghitza et al., 2007). We found that rates of opioid dependence were significantly lower at the end of treatment in participants given a higher methadone dose or split contingency, while rates of cocaine dependence were significantly lower in participants given either of the CM interventions. These findings complement and extend the urine data; they reflect group differences not only in ongoing drug use, but in the psychosocial problems directly attributed to it by participants. (One limitation of the study is that, for reasons related to participant burden, we assessed DSM substance-dependence diagnosis at intake with a different instrument, the DIS, rather than the SDSS. Thus, we did not analyze substance dependence in terms of change over time. However, there were no baseline differences in rates of drug dependence across groups.) The contingency interventions were also associated with improvements in quality of life as assessed on the SAS-SR. These findings speak to the clinical significance of our treatment effects: combining targeted treatments can decrease drug use and the problems associated with it. The benefits are dose related: higher-intensity treatments, in the form of methadone dose and value of incentives, are more effective than lower-intensity treatments. Nevertheless, further work is needed to determine optimal combinations of treatments.
Limitations
The high dose used in the current study, while higher than the average dose prescribed in methadone clinics in the US, was lower than the daily dose received by 20% of respondents to a national survey in 2003 (Addiction Treatment Forum, 2003). Thus, stronger or different interactions may have been observed if different doses had been employed. In addition, to facilitate assessment of dose/CM interaction, doses were fixed within the study protocol and not individualized, as is the case in usual care practice. Participants did not report that their doses were failing to “hold” them. Nonetheless, in ongoing research, we are moving toward more flexible and individualized dosing procedures.
Another limitation is that the study included no comparison group in which the response requirement was simultaneous abstinence from cocaine and opioids. Therefore, we cannot discern whether the split contingency had any advantages or disadvantages compared to a condition that simply required simultaneous abstinence from both drugs. A direct comparison of the two contingencies would be worthwhile, as a simultaneous abstinence contingency may be easier to manage and more consistent with clinical philosophy and practice (Petry and Martin, 2002).
Finally, our design deliberately confounded two parameters: dual reinforcement of heroin and cocaine abstinence, and a reduction in the amount of reinforcement available for each form of abstinence. It seems likely that higher rates of abstinence from both drugs could be induced if a higher magnitude of reinforcement was offered for each; this would an important finding in terms of its theoretical implications about the responsiveness of polydrug abuse to CM—but it would not address the cost-containment issued that we had hoped to address with this study.
Policy implications
What do our findings imply for the dissemination of CM to nonresearch settings? Innovative, locally based approaches to financing CM will continue to be important (Amass and Kamien, 2004), but a case still needs to be made for large-scale public financing. Formal cost-benefit analyses of CM are few, and findings of cost offsets at individual sites have failed to reach statistical significance due partly to small sample sizes (Hartz et al., 1999). A broader view may provide a better perspective. The costs of heroin dependence alone in the US have been estimated at $21.9 billion per year (Mark et al., 2001), which averages to $19,380-$53,676 per year for each user, depending how users are counted (varying prevalence estimates taken from ref. (Mark et al., 2001)). If CM can produce enduring abstinence in some patients, as has been shown with cocaine dependence (Higgins et al., 2000a; Higgins et al., 2000b), then a one-time expenditure of several thousand dollars per patient—which is what a high-magnitude incentive could entail—might be economically justifiable.
In sum, the present study showed that simultaneous reduction of dual cocaine and heroin abuse is possible and that, although it probably requires deployment of considerable therapeutic resources, the costs are likely offset by broad, clinically significant improvements in functioning.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH National Institute on Drug Abuse.
Footnotes
A preliminary report of these findings was presented at the 65th annual scientific meeting of the College of Problems on Drug Dependence, Bal Harbour, FL, 2003.
A recent multisite study by some of the same investigators (Peirce et al., 2006) might initially seem comparable to the present study; however, no split contingency was used: the contingency required simultanous abstinence from cocaine, amphetamines, and alcohol, with bonus draws for opiate negatives given only if the participant was negative for the primary-target drugs. Again, the comparison group was “usual care” rather than noncontingent vouchers, and there was no baseline phase. Encouragingly, opiate use did decrease in the CM group in that study. One possible reason for the decrease is that it reflected generalization of CM effects to a drug that was not the primary target. Such generalization seems more likely to occur at lower doses of methadone (Epstein and Preston, 2008), and the methadone doses at most of the study sites were lower than those used in the present study.
References
- Addiction Treatment Forum. [Accessed August 7, 2008];ATF Newsletter. 2003 < http://www.atforum.com/SiteRoot/pages/current_pastissues/spring2003.shtml#anchor1221360>.
- Amass L, Kamien J. A tale of two cities: financing two voucher programs for substance abusers through community donations. Exp Clin Psychopharm. 2004;12:147–155. doi: 10.1037/1064-1297.12.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Dallery J, Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcer magnitude. Exp Clin Psychopharm. 2001;9(3):317–325. doi: 10.1037//1064-1297.9.3.317. [DOI] [PubMed] [Google Scholar]
- Delucchi K, Jones R, Batki S. Measurement properties of quantitative urine benzoylecgonine in clinical trials research. Addiction. 1997;92:297–302. [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharm. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Dumville JC, Hahn S, Miles JN, Torgerson DJ. The use of unequal randomisation ratios in clinical trials: a review. Contemporary Clinical Trials. 2006;27:1–12. doi: 10.1016/j.cct.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. Contingency management in the treatment of opiate-use disorders. In: Higgins ST, Silverman K, Heil SH, editors. Contingency Management in the Treatment of Substance Use Disorders: A Science-Based Treatment Innovation. Guilford; New York: 2008. pp. 42–60. [Google Scholar]
- Ghitza UE, Epstein DH, Schmittner J, Vahabzadeh M, Lin JL, Preston KL. Randomized trial of prize-based reinforcement density for simultaneous abstinence from cocaine and heroin. J Consult Clin Psychol. 2007;75:765–774. doi: 10.1037/0022-006X.75.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Rowan-Szal GA, Roark RR, Simpson DD. Contingency management in outpatient methadone treatment: a meta-analysis. Drug Alcohol Depen. 2000;58:55–66. doi: 10.1016/s0376-8716(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Hartz DT, Meek P, Piotrowski NA, Tusel DJ, Henke CJ, Delucchi K, Sees K, Hall SM. A cost-effectiveness and cost-benefit analysis of contingency contracting-enhanced methadone detoxification treatment. Am J Drug Alcohol Ab. 1999;25:207–218. doi: 10.1081/ada-100101856. [DOI] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Exp Clin Psychopharm. 2000a;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1-year follow-up. J Consult Clin Psychol. 2000b;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Katz EC, Gruber K, Chutuape MA, Stitzer ML. Reinforcement-based outpatient treatment for opiate and cocaine abusers. J Subst Abuse Treat. 2001;20:93–98. doi: 10.1016/s0740-5472(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Katz EC, Chutuape MA, Jones HE, Stitzer ML. Voucher reinforcement for heroin and cocaine abstinence in an outpatient drug-free program. Exp Clin Psychopharm. 2002;10:136–143. doi: 10.1037//1064-1297.10.2.136. [DOI] [PubMed] [Google Scholar]
- Kirby KC, Benishek LA, Dugosh KL, Kerwin ME. Substance abuse treatment providers’ beliefs and objections regarding contingency management: implications for dissemination. Drug Alcohol Depend. 2006;85:19–27. doi: 10.1016/j.drugalcdep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AP, Olsen DM, Bickel WK. A quantitative review of the ubiquitous relapse curve. J Subst Abuse Treat. 2009;20:8–17. doi: 10.1016/j.jsat.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson JS. The measurement of social well-being. Social Indicators Research. 1993;28:285–296. [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiat. 1996;53:401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depen. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- McLellan A, Luborsky L, Cacciola J, Griffith J, Evans F, Barr H, O’Brien C. New data from the Addiction Severity Index: reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Miele G, Carpenter K, Smith Cockerham M, Trautman K, Blaine J, Hasin D. Substance Dependence Severity Scale (SDSS): reliability and validity of a clinician-administered interview for DSM-IV substance use disorders. Drug Alcohol Depen. 2000;59:63–75. doi: 10.1016/s0376-8716(99)00111-8. [DOI] [PubMed] [Google Scholar]
- Nevin JA. Analyzing Thorndike’s Law of Effect: the question of stimulus-response bonds. Journal of the Experimental Analysis of Behavior. 1999;72:447–450. doi: 10.1901/jeab.1999.72-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveto A, Poling J, Sevarino KA, Gonsai KR, McCance-Katz EF, Stine SM, Kosten TR. Efficacy of dose and contingency management procedures in LAAM-maintained cocaine-dependent patients. Drug Alcohol Depen. 2005;79:157–165. doi: 10.1016/j.drugalcdep.2005.01.007. [DOI] [PubMed] [Google Scholar]
- ONDCP. [Accessed August 7, 2008];Pulse Check: Trends in Drug Abuse. 2004 http://www.whitehousedrugpolicy.gov/publications/drugfact/pulsechk/january04/index.html.
- Peachey JE. Clinical observations of agonist-antagonist analgesic dependence. Drug Alcohol Depen. 1987;20:347–365. doi: 10.1016/0376-8716(87)90008-1. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Schwartz M, Krasnansky J, Pencer E, Silva-Vazquez L, Kirby KC, Royer-Malvestuto C, Roll JM, Cohen A, Copersino ML, Kolodner K, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiat. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Peles E, Kreek MJ, Kellogg S, Adelson M. High methadone dose significantly reduces cocaine use in methadone maintenance treatment (MMT) patients. J Addict Dis. 2006;25:43–50. doi: 10.1300/J069v25n01_07. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Obert J, Killeen T, Saladin ME, Cowell M, Kirby KC, Sterling R, Royer-Malvestuto C, Hamilton J, Booth RE, Macdonald M, Liebert M, Rader L, Burns R, DiMaria J, Copersino M, Stabile PQ, Kolodner K, Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiat. 2005;62:1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Piotrowski NA, Tusel DJ, Sees KL, Reilly PM, Banys P, Meek P, Hall SM. Contingency contracting with monetary reinforcers for abstinence from multiple drugs in a methadone program. Exp Clin Psychopharm. 1999;7:399–411. doi: 10.1037//1064-1297.7.4.399. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Epstein DH. Methadone dose increase and abstinence reinforcement for treatment of continued heroin use during methadone maintenance. Arch Gen Psychiat. 2000;57:395–404. doi: 10.1001/archpsyc.57.4.395. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM., III . The Diagnostic Interview Schedule, version IV. Washington University; St. Louis, MO: 1995. [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Zeidonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiat. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Robles E, Stitzer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: six-month abstinence outcomes. Exp Clin Psychopharm. 2001;9:14–23. doi: 10.1037/1064-1297.9.1.14. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Methadone dose and treatment outcome. Drug Alcohol Depen. 1993;33:105–117. doi: 10.1016/0376-8716(93)90052-r. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: self-reports, urinalysis and addiction severity index. J Clin Psychopharmacol. 1996;16:58–67. doi: 10.1097/00004714-199602000-00010. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiat. 1976;33:1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Woods SW, Sholomskas DE, Shear MK, Gorman JM, Barlow DH, Goddard AW, Cohen J. Efficient allocation of patients to treatment cells in clinical trials with more than two treatment conditions. Am J Psychiatry. 1998;155:1446–1448. doi: 10.1176/ajp.155.10.1446. [DOI] [PubMed] [Google Scholar]
- Zachary R. Revised Manual. Western Psychological Services; Los Angeles: 1986. Shipley Institute of Living Scale. [Google Scholar]