Abstract
HIV-infected persons are at risk for HBV co-infection which is associated with increased morbidity and mortality. Unfortunately, protective immunity following HBV vaccination in HIV-infected persons is poor. This randomized, phase II, open label study aimed to evaluate efficacy and safety of 40 mcg HBV vaccine with or without 250 mcg GM-CSF administered at day 0, weeks 4 and 12. HIV-infected individuals ≥18 years of age, CD4 count ≥200 cells/mm3, seronegative for HBV and HCV, and naïve to HBV vaccination were eligible. Primary endpoints were quantitative HBsAb titers and adverse events. The study enrolled 48 subjects. Median age and baseline CD4 were 41 years and 446 cells/mm3, 37 were on ART, and 26 subjects had undetectable VL. Vaccination was well tolerated. Seven subjects in the GM-CSF group reported transient Grade ≥2 signs/symptoms (six Grade 2, one Grade 3), mostly aches and nausea. GM-CSF had no significant effect on VL or CD4. Four weeks after vaccination, 26 subjects (59%) developed a protective antibody response (HBsAb ≥10mIU/mL; 52% in the GM-CSF arm and 65% in the control arm) without improved Ab titer in the GM-CSF versus control arm (median 11 mIU/mL vs. 92 mIU/mL, respectively). Response was more frequent in those with CD4 ≥350 cells/mm3 (64%) than with CD4 <350 cells/mm3 (50%), though not statistically significant. GM-CSF as an adjuvant did not improve the Ab titer or the development of protective immunity to HBV vaccination in those receiving an accelerated vaccine schedule. Given the common routes of transmission for HIV and HBV, additional HBV vaccine research is warranted.
Keywords: HIV, HBV vaccination, GM-CSF, adjuvant
Background
Worldwide, hepatitis B virus (HBV) is the leading cause of chronic liver disease and accounts for 1 to 2 million deaths annually [1]. HIV and HBV share routes of transmission, with 30%-90% of HIV-infected patients having evidence of prior HBV infection and 10% having chronic HBV infection [2,3]. In the current era of highly active antiretroviral therapy (HAART), liver disease has become a leading cause of mortality [4,5]. Data from the Multicenter AIDS Cohort Study (MACS) illustrate that co-infection with HIV and HBV was associated with a 15-fold increase in mortality when compared with mono-infection with HBV [6].
Prevention of HBV infection is therefore essential for HIV-infected patients [3,7]. Vaccination with HBV vaccine has proven to yield protective levels of antibodies in over 90% of immunocompetent adults [8,9]. Unfortunately, HIV-infected patients respond poorly to HBV vaccination, at rates ranging from 17.5% to 56% [10–13]. To improve immunogenicity of the HBV vaccine, it is adjuvanted to alum which allows for slow release of the vaccine antigen at the injection site, increasing the time for initiation of the immune response by antigen presenting cells and lymphocytes [14]. Due to the fact that response to this adjuvanted HBV vaccine remains poor, particularly in immunocompromised populations, successful strategies that include the use of alternative adjuvants such as GM-CSF have improved responses [15–17].
GM-CSF is a cytokine produced primarily by activated lymphocytes that has been used extensively as a hematopoietic growth factor. It increases neutrophil count, improves antigen-presenting cell (APC) function, and is involved in the development and perpetuation of cellular immune responses [18]. GM-CSF has been studied in HIV-infected patients with opportunistic diseases. GM-CSF was safe and effective at increasing neutrophil count, preventing treatment interruption, and improving malignancy-related outcomes [18–22]. GM-CSF has been studied as an adjuvant to HBV vaccination in patients with end stage renal disease with improved immunogenicity in those persons receiving GM-CSF [15,16,23–25]. One published study to date in HIV-infected persons evaluated the efficacy of a single 20 mcg dose of GM-CSF to augment response to HBV vaccine [26]. Eighty HIV-infected persons received a three-dose series of 40 mcg HBV vaccine and were randomized to receive either placebo or 20 mcg GM-CSF. A significant increase in the development of protective HBsAb (≥10 IU/L) was noted in the GM-CSF group (62%) versus the placebo group (30%) after the second vaccine dose (P <0.0074). One month after vaccination, 72% in the GM-CSF group and 60% in the placebo group had protective titers. This trend was not statistically significant, but average titers were significantly higher in the GM-CSF group (645 versus 375 IU/L, P <0.01). These results suggest promise for the role of GM-CSF to augment immune response to vaccination. Hence, this study was developed to evaluate the use of GM-CSF as an adjuvant to HBV vaccine to improve immunogenicity in HIV-infected subjects.
Methods
Study Design
AIDS Clinical Trials Group (ACTG) A5220 (ClinicalTrials.gov number, NCT00272493) was a multi-site two-arm, randomized, phase II, open-label pilot study in HIV-infected, HBV-uninfected subjects naïve to HBV vaccination with CD4+ T-cell counts ≥200 cells/mm3. The aim was to evaluate efficacy and safety of HBV vaccine, with and without GM-CSF as an adjuvant. All study subjects completed written informed consent before participation. All participating sites had local Institutional Review Board approval.
After completion of screening, subjects were randomized to one of two arms. Arm A subjects received 40 mcg HBV vaccine at day 0, week 4, and week 12. Arm B subjects received 40 mcg HBV vaccine plus 250 mcg GM-CSF at day 0, week 4, and week 12. The study was stratified by screening plasma HIV-1 RNA levels (<1000 and ≥1000 copies/mL) to balance the number of individuals with high HIV-1 viral loads in the two arms. This stratification was based on previous work illustrating the impact of uncontrolled viremia on response to HBV vaccine [13]. After the vaccination series was completed, follow-up study visits were completed at weeks 16, 36, and 60. Data were collected to assess the durability of the antibody response to vaccination and to evaluate the vaccine and GM-CSF effects on CD4+ T-cell count, HIV viral load, and absolute neutrophil count (ANC). Signs and symptoms of grade ≥2, fever of grade ≥1, all ANC and platelet counts regardless of grade, and other laboratory abnormalities grade ≥2 were also collected.
The primary objectives of the study were to evaluate quantitative HBsAb titers 4 weeks after completing HBV vaccination series (week 16), and to assess the safety of GM-CSF as an adjuvant to HBV vaccine. Secondary objectives were to examine the proportion of subjects in each arm who achieve protective immunity (defined as HBsAb ≥10 mIU/mL) at 4, 24, and 48 weeks after vaccination completion (study weeks 16, 36, and 60) and to evaluate the effect of these HBV vaccination strategies on HIV-related measures. HBsAb titers were measured using the commercially available Vitros Immunodiagnostics Products Anti-HBs Quantitative Assay (Ortho-Clinical Diagnostics, Amersham, Bucks, UK).
Study Subjects
Eligible subjects were HIV-infected men and women ≥18 years old, with CD4+ T-cell count ≥200 cells/mm3, with negative serologies for hepatitis: hepatitis C antibody, HBV core total antibody (HBcAb total), qualitative HBV surface antibody (HBsAb), and HBV surface antigen (HBsAg). Subjects were either on HAART (for at least 8 weeks) or not on HAART within 8 weeks prior to study with no plans to start HAART during the study. Required laboratory parameters within 30 days prior to study entry included absolute neutrophil count (ANC) ≥750/mm3 and <20,000/mm3; hemoglobin ≥7.0 g/dL; platelet count ≥50,000/mm3 and <500,000/mm3; calculated creatinine clearance of ≥30 mL/min using the Cockcroft-Gault equation; AST (SGOT), ALT (SGPT), and alkaline phosphatase ≤5 × ULN; and total bilirubin ≤2.5 × ULN (except for subjects taking indinavir or atazanavir). Exclusion criteria included: pregnancy or breast feeding; Karnofsky score <70; previous receipt of any HBV vaccination; use of any systemic antineoplastic or immunomodulatory treatment, systemic corticosteroids, vaccines, interleukins, interferons, growth factors, or IVIG within 30 days prior to study entry; known allergy/sensitivity to any component of the study drugs, including yeast or yeast products; active drug, alcohol use or dependence that would interfere with adherence to study requirements; serious illness requiring systemic treatment and/or hospitalization; and body weight <50 kg.
Study Regimen
Hepatitis B vaccine (Recombivax® HB 40 mcg/mL) was provided by Merck & Co., Inc in single dose vials. GM-CSF (Leukine®) was provided by Berlex Laboratories, Inc. (acquired by Bayer HealthCare Pharmaceuticals) as 500-mcg/mL, 1-mL vials, from which the 250-mcg dose was drawn and the remaining medication was discarded. Both products were refrigerated between 2° and 8° C (36° and 46° F) before use. The 250 mcg dose of GM-CSF was selected based on the results from the trials in ESRD patients and after discussion with the manufacturer regarding optimal dosing [15,16,23].
Study drug injections were administered using aseptic technique during preparation and administration. The HBV vaccine and GM-CSF were administered sequentially in the same deltoid muscle to allow for any local effect on antigen presentation. Subjects remained at the clinic for a 20-minute post-injection observation period following vaccination. Injection site reactions and acute systemic allergic reactions were graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (http://rcc.tech-res.com/safetyandpharmacovigilance). Local reactions were evaluated after the post-injection observation period following each vaccination in follow-up phone calls and during targeted physical examinations performed through week 16. All signs and symptoms of grade >=2, fever of grade >= 1, all ANC and platelet counts of any grade were also collected, regardless of the site opinion of their relation to the study injections. The study was reviewed annually by an Independent Study Monitoring Committee and more frequently by the Independent Toxicity Monitoring Board to assess event relationship to the study injections.
Statistical Methods
The primary analysis on quantitative HBsAb titer comparison between the two arms at week 16 was conducted with a one-sided exact Wilcoxon rank-sum test at a significance level of 5%, and Van Elteren’s test was used to account for study stratification factor on HIV RNA levels. The study was designed for 90% power to detect at least one standard deviation improvement with the addition of GM-CSF using a rank-based test. Measurements below the limit of detection were assigned the lowest rank with an averaged rank for ties. The target sample size of 24 subjects per arm accounted for up to 10% drop out. The HBsAb titers were log10 transformed for the plots.
To test for improvement with GM-CSF in the proportion of subjects who achieved protective immunity, a one-sided Fisher’s exact test was used. Two-sided 5% significance levels were used in other secondary analyses. Wilcoxon rank-sum tests were used to compare continuous variables and Fisher’s exact tests for comparisons of binary and categorical variables between the two arms. Associations of protective immunity at week 16 with binary and categorical measures were assessed using Cochran-Mantel-Haenszel tests, and Van Elteren’s tests were used to investigate associations with continuous measures, stratified by the study arm. All available data were used in the analyses, except where it is noted otherwise.
Results
Baseline Characteristics and Study Follow-up
A5220 enrolled 48 subjects between January and July 2007. The last subject completed the study in September 2008, and data entry was completed in January 2009. Baseline demographic and clinical characteristics for the 48 subjects were well balanced between arms (Table 1). Thirty eight subjects (79%) were male; the median age was 41 years; and the median CD4+ T-cell count was 446 cells/mm3. At study entry, 37 subjects were on HAART. Overall, 26 (54%) subjects had undetectable (<50 cp/mL) HIV-1 RNA at baseline. Two subjects (one in each study arm) received only one vaccination; one study subject in Arm B received only 2 vaccinations, the remaining 45 subjects (94%) received all three vaccinations. One subject inadvertently received GM-CSF in Arm A at week 12. Five subjects (10%) discontinued the study prematurely: 2 subjects in Arm A (one withdrew at week 1 and the other was not able to come to clinic and discontinued at week 66) and 3 subjects in Arm B (at weeks 18, 35 and 49, two due to out-of-state move).
Table 1.
Baseline Demographic and Clinical Characteristic by Study Arm
| Characteristic | Total (n=48) | Arm A (n=24) | Arm B (n=24) | p-value |
|---|---|---|---|---|
| Vaccine | Vaccine plus GM-CSF |
|||
| Median age (IQR) | 41 (33–50) | 40 (29–50) | 41 (35–47) | 0.79a |
| Male gender | 38 (79%) | 18 (75%) | 20 (83%) | 0.72b |
| Race/ethnicity | ||||
| White | 26 (54%) | 10 (42%) | 16 (67%) | 0.23b |
| Black | 15 (31%) | 10 (42%) | 5 (21%) | |
| Hispanic | 7 (15%) | 4 (17%) | 3 (13%) | |
| IVDU | 2 (4%) | 0 (0%) | 2 (8%) | 0.49b |
| Median CD4 cell count (IQR) | ||||
| Week 0 | 445 (273–569) | 461 (307–631) | 445 (271–536) | 0.41a |
| Nadir | 157 (55–343) | 179 (54–337) | 157 (71–343) | 0.99a |
| On HAART at week 0 | 37 (77%) | 19 (79%) | 18 (75%) | 1.00b |
| Week 0 HIV RNA | ||||
| Median log10 (IQR) | 1.74 (1.70–2.55) | 1.73 (1.70–2.39) | 1.76 (1.70–2.57) | 0.70a |
| <1000 cp/mLc | 39 (81%) | 20 (83%) | 19 (79%) | 1.00b |
| <50 cp/mL | 26 (54%) | 13 (54%) | 13(54%) | 1.00b |
Column percentages presented.
Wilcoxon Rank Sum Test.
Fisher’s Exact Test.
Study stratification factor.
Efficacy
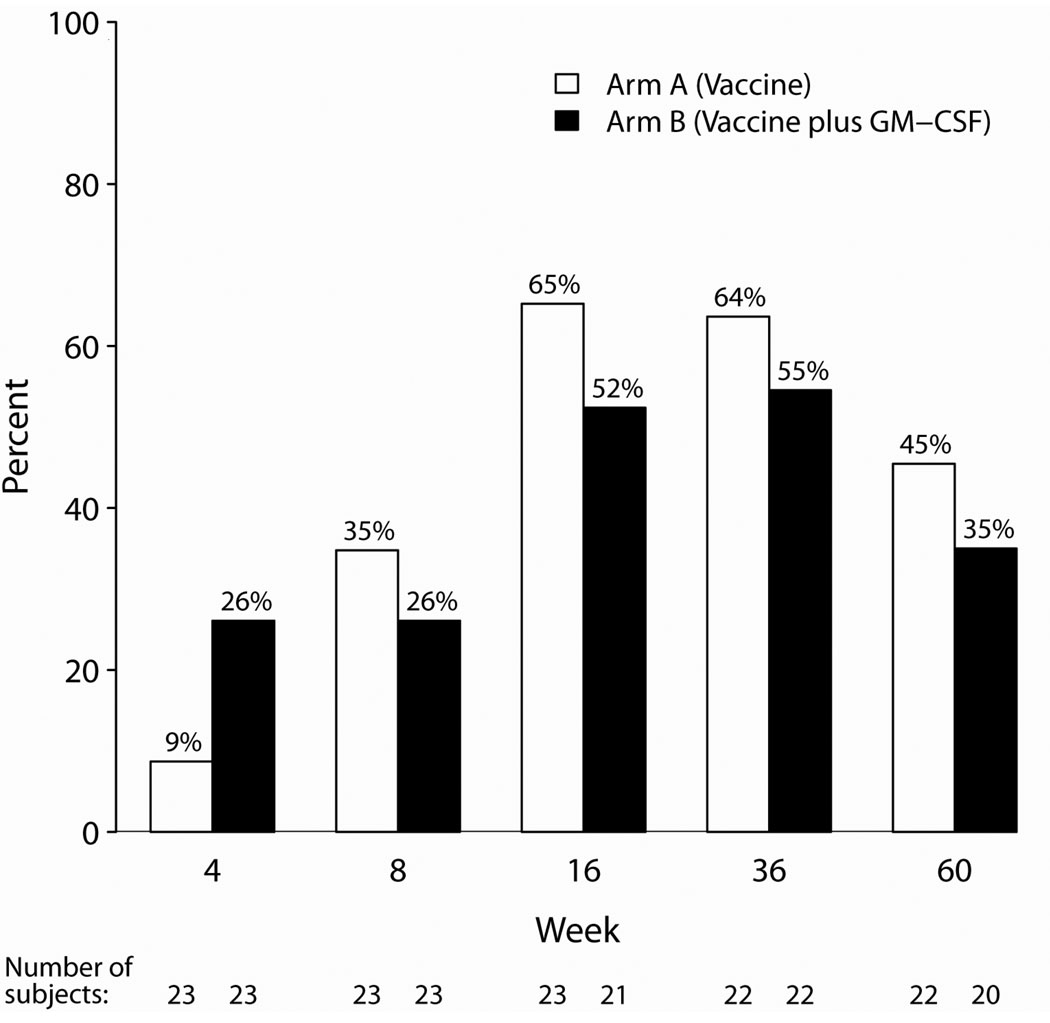
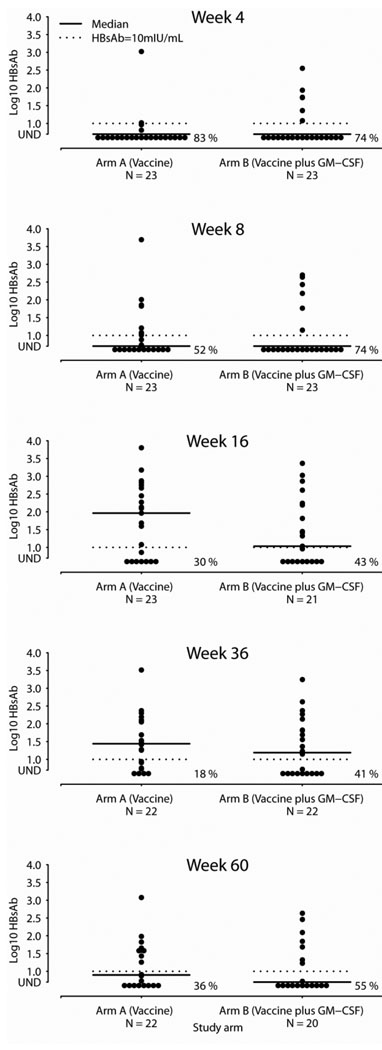
The primary efficacy endpoint was the quantitative HBsAb titer 4 weeks after completion of HBV vaccination series (at week 16). A total of 44 subjects (23 in Arm A and 21 in Arm B) had week 16 specimens available for evaluation. The median HBsAb titer was 27 mIU/mL (range <5 – 6340 mIU/mL), and the titers were not statistically different between arms (p=0.86; 92 mIU/mL in Arm A and 11 mIU/mL in Arm B). One subject in Arm B received only one vaccination, and the results are similar when restricted to 43 subjects who received all 3 vaccinations (median of 16 mIU/mL in Arm B). While there were more responders in the GM-CSF arm at the earliest time point (week 4), this difference was not statistically significant, and the trend was reversed at subsequent study evaluations. Overall, protective immunity (HBsAb ≥10 mIU/mL) developed in 26/44 subjects (59%) at weeks 16 and 36 and subsequently declined to 17/42 (40%) at week 60. Four weeks after completion of the vaccination series, the proportion of responders was higher without GM-CSF (65% vs. 52%). Unfortunately, the proportion of responders waned to 40% (45% vs. 35% respectively) by week 60, suggesting that the response was not durable. (See Figure 1) There were no statistically significant differences between the study arms in response rates at any visit. Log-transformed antibody titers at the study visits are illustrated in Figure 2. The results were similar when stratified by the study stratification factor on HIV RNA levels.
Figure 1. Subjects Developing Protective Ab (HBsAb ≥ 10 mIU/mL).
Proportion of subjects by study arm who developed protective immunity (HBsAb ≥ 10 mIU/mL) at various time points during the study.
Figure 2. HBsAb Titer by Arm at Study Visits.
Log10 HBV sAb titer by study arm at various time points during the study.
Safety
There were no injection site reactions identified in either the 20-minute post-vaccination observation periods or in the follow-up telephone contact (48–96 hours post-vaccination). No hypersensitivity reactions (HSR) were identified. Grade 1 fevers were reported by two subjects, one in each study arm. Seven subjects had signs or symptoms of grade ≥ 2 through week 16: six subjects had grade 2 events and 1 subject had a grade 3 event. All of these events occurred in Arm B, and most of the signs and symptoms were expected from GM-CSF administration. While the difference in the number of events was statistically significant (p=0.01), all resolved subsequently.
Seventeen subjects experienced laboratory abnormalities of Grade ≥ 2: 10 had Grade 2 (5 on each study arm), 4 had grade 3 (3 in Arm A, 1 in Arm B), and 3 had grade 4 (decreased ANCs on Arm B). These differences in laboratory parameters were not statistically significant (p=0.29). No subject had GM-CSF withheld due to elevated ANC or platelet count. A listing of the signs, symptoms, and laboratory abnormalities is given in Tables 2 and 3.
Table 2.
Development of Clinical Adverse Events (New Signs, Symptoms of Grade ≥2) through Week 16
| Study Arm | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm A (n=24) | Arm B (n=24) | Total (n=48) | ||||||||||||||
| Vaccine | Vaccine plus GM-CSF | |||||||||||||||
| Toxicity Grade |
Toxicity Grade |
Toxicity Grade |
||||||||||||||
| Toxicities | 2 | 3 | 4 | 5 | Number Subjects |
2 | 3 | 4 | 5 | Number Subjects |
2 | 3 | 4 | 5 | Number subjects |
|
| Any General Body | 0 | 5 | 5 | |||||||||||||
| Ache/pain/discomfort | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | ||||
| Any Gastrointestinal | 0 | 4 | 4 | |||||||||||||
| Diarrhea | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | ||||
| Nausea | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | ||||
| Nausea and Vomiting | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| Any Renal | 0 | 1 | 1 | |||||||||||||
| Renal Dysfunction | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| Any Reproductive | 0 | 1 | 1 | |||||||||||||
| Contraction/Cramp | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | ||||
| Any Skin | 0 | 1 | 1 | |||||||||||||
| Allergic rash | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| Any Neurological | 0 | 2 | 2 | |||||||||||||
| Headache | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | ||||
| Numbness/tingling | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| Any Other | 0 | 2 | 2 | |||||||||||||
| Dizziness | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| Incontinence | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| ANY SIGN/SYMPTOM | 0 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | 7 | 6 | 1 | 0 | 0 | 7 | |
Some subjects had more than one sign or symptom.
Table 3.
Laboratory Adverse Events (New Lab Abnormalities of Grade ≥2) through Week 16
| Study Arm | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm A (n=24) | Arm B (n=24) | Total (n=48) | ||||||||||||||
| Vaccine | Vaccine plus GM-CSF | |||||||||||||||
| Toxicity Grade |
Toxicity Grade |
Toxicity Grade |
||||||||||||||
| Toxicities | 2 | 3 | 4 | 5 | Number Subjects |
2 | 3 | 4 | 5 | Number Subjects |
2 | 3 | 4 | 5 | Number Subjects |
|
| Any Chemistry, general | 1 | 4 | 5 | |||||||||||||
| Phosphorous | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | ||||
| Potassium | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| Any Endocrine | 1 | 1 | 2 | |||||||||||||
| Fasting blood glucose | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ||||
| Any Hematology | 0 | 3 | 3 | |||||||||||||
| ANC | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | ||||
| White blood cells | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | ||||
| Any Metabolic | 4 | 2 | 6 | |||||||||||||
| Glucosea | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | ||||
| Total Cholesterola | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| Any Liver/Hepatic | 3 | 0 | 3 | |||||||||||||
| Total bilirubin | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | ||||
| Any renal | 1 | 0 | 1 | |||||||||||||
| Serum creatinine | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||||
| ANY TOXICITY | 5 | 3 | 0 | 0 | 8 | 5 | 1 | 3 | 0 | 9 | 10 | 4 | 3 | 0 | 17 | |
Non-fasting.
At entry, 11 subjects (23%) were not on HAART; two subjects in Arm A subsequently initiated therapy. Of the 37 on HAART at week 0, five subjects (14%; 4 in Arm A, 1 in Arm B) changed their regimen during the study. There were no significant differences in the change in either CD4+ T-cell count or HIV-1 RNA levels between the study arms throughout the course of the study (data not shown).
Development of Protective Immunity
Secondary analyses to explore the relationship between protective immunity at week 16 and baseline factors were also performed. At week 16, 26/44 subjects (59%) developed protective immunity (HBsAb ≥ 10 mIU/mL). Subjects who developed immunity were significantly younger than those who did not (median ages 37 and 45 years; p=0.03). There was a trend for higher baseline median CD4+ T-cell count in responders (503 vs. 364 cells/mm3), although this was not statistically significant (p=0.15). As the large majority of subjects were on HAART, baseline viral load parameters were similar between responders and nonresponders. Other parameters were also not significantly different between the responders and non-responders (see Table 4).
Table 4.
Association with Protective Immunity at Week 16
| Characteristic | HBsAb ≥ 10 mIU/mL (n=26) |
HBsAb <10 mIU/mL (n=18) |
p-value |
|---|---|---|---|
| Median age (IQR) | 37 (29, 45) | 45 (40, 54) | 0.03a |
| Gender | 0.46b | ||
| Male (n=34) | 21 (62%) | 13 (38%) | |
| Female (n=10) | 5 (50%) | 5 (50%) | |
| Race/ethnicity | |||
| White (n=24) | 13 (54%) | 11 (46%) | 0.81b |
| Black (n=13) | 9 (69%) | 4 (31%) | |
| Hispanic (n=7) | 4 (57%) | 3 (43%) | |
| Median CD4 cell count (IQR) | |||
| Week 0 | 503 (333, 586) | 364 (246, 463) | 0.15a |
| Nadir | 127 (49, 353) | 143 (93, 250) | 0.75a |
| Baseline CD4 | 0.46b | ||
| <350c/mm3 (n=16) | 8 (50%) | 8 (50%) | |
| ≥350c/mm3 (n=28) | 18 (64%) | 10 (36%) | |
| Nadir CD4 | 0.66b | ||
| <200c/mm3 (n=27) | 15 (56%) | 12 (44%) | |
| ≥200c/mm3 (n=17) | 11 (65%) | 6 (35%) | |
| On HAART at week 0 (n=36) | 21 (58%) | 15 (42%) | 0.89b |
| Week 0 HIV RNA | |||
| Median log10 (IQR) | 1.71 (1.70, 2.02) | 1.78 (1.70, 2.61) | 0.42a |
| <1000 cp/mL (n=37) | 23 (62%) | 14 (38%) | 0.33b |
| <50 cp/mL (n=24) | 15 (63%) | 9 (38%) | 0.59b |
Row percentages presented.
Van Elteren’s Test stratified by study arm.
Cochran-Mantel-Haenszel Test stratified by study arm.
Conclusions
The results of this study showed that expedited high dose HBV vaccination series with GM-CSF as an adjuvant did not improve development of HBsAb titers. The data confirm previous findings that GM-CSF is safe and well tolerated in HIV-infected individuals, albeit with some transient, anticipated side effects. However, co-administration of GM-CSF with HBV vaccine failed to improve HBsAb titer. In fact, the observed response rate was somewhat higher in the vaccine only arm as compared to the GM-CSF arm (65% vs. 52%, not statistically significant), yielding an overall response rate of 59% at 4 weeks after completion of the vaccination series. Unfortunately, the proportion of responders waned to 40% by week 60, suggesting that the response was not durable.
GM-CSF failed to serve as an effective adjuvant to HBV vaccination in this study. This is somewhat surprising given previously published data in patients with end stage renal disease and in HIV-infected patients. A four-dose regimen of HBV vaccine with either GM-CSF (3 mcg/kg) or placebo with the first injection in unvaccinated end stage renal disease subjects yielded positive results [15]. One month after completion of the vaccination series, 44% of subjects in the placebo arm versus 100% in the GM-CSF arm developed protective immunity (P<0.02). A subsequent study evaluating the efficacy of GM-CSF as an adjuvant in subjects on long term dialysis who were non-responders to 40 mcg recombinant HBV vaccination given at an accelerated schedule showed similar benefit. They received one additional dose of HBV vaccine and were randomized to receive either 300 mcg GM-CSF (n=12) or placebo (n=7) [16]. In that study, 92% in the GM-CSF arm versus 0% in the placebo arm responded with a protective HBsAb titer (p=0.001). As noted in the Sasaki trial of HIV-infected subjects, there was a trend toward improved protective immunity among those who received GM-CSF one month after completing the vaccine series, but the durability of vaccine responses was not assessed [26]. While those results suggested promise for the role of GM-CSF to augment immune response to vaccination in HIV-infected persons, our results failed to confirm their findings. Given that we used a much higher dose than in the Sasaki study, there may be a threshold dose of GM-CSF above which response is mitigated [27]. However, the studies in ESRD patients illustrated efficacy with even higher doses of GM-CSF [15,16,23].
Use of higher dose HBV vaccine (40 mcg dose) was selected in this study based on ACIP recommendations and previous research illustrating that this approach offers better responses among HIV-infected persons [26,28–31]. Fonseca et al randomized 210 HIV-infected persons to standard (20 mcg dose) vs. double dose (40 mcg dose) and reported a better seroconversion with the higher dose (47 vs. 34%, p=0.07). This improvement was most noticeable for those with CD4+ T-cell counts ≥350 c/mm3 (64 vs. 39%) and HIV viral loads <10,000 cp/mL (58 vs. 37%). However, another trial comparing 10 mcg vs. 40 mcg dosing of HBV vaccine reported no differences in seroconversion rates between the two strategies (61% and 62%, respectively) [32]. Still, more subjects who received the 40 mcg dose developed HBsAb titers >100 mIU/mL. Sasaki et al. reported a seroconversion rate of 66% in 80 HIV-infected age 18–35 years with CD4+ T-count >350 c/mm3 who received the 40 mcg dose of vaccine. Our results are similar to these reports and confirm that the higher dose of HBV vaccine yields better seroconversion rates than reports from retrospective cohorts [10–13]. Baseline CD4+ T cells and HIV viral load were not found to be statistically significant in our evaluation of protective immunity, but our study was not powered to assess predictive factors. Nonetheless, age was statistically significant in its association with protective immunity at week 16.
We also elected to give HBV vaccine on an expedited schedule (0, 4, and 12 weeks) rather than (0, 4, and 60 weeks). Additional studies in other immunocompromised patient populations have shown that an accelerated schedule yields improved results. Rosman et al. administered an accelerated and high dose of vaccine to a cohort of 100 alcoholic patients [33]. The high-dose and accelerated regimen group responded at a higher rate than the standard vaccine schedule patients (75% versus 46%, p<0.005) with the mean HBsAb titer higher in the accelerated group (76.4 versus 39.6 mIU/mL, p<0.01). Similar results were reported in a study of a high-dose (40 mcg), modified schedule vaccination regimen (0, 1, and 2 months with a booster dose at 6 months) in a study performed in HIV-infected persons [34]. In their study, 89% of the subjects developed protective antibody with 60% developing an HBsAb >1000mIU/mL, and response to vaccine was strongly correlated with controlled viremia. Another study subsequent to ours was recently presented that assessed an accelerated (0,1 and 3 weeks) versus a standard (0,4, and 24 weeks)schedule among 1330 HIV-infected subjects [35]. Completion rate of the three dose series was higher with the accelerated schedule (92% vs. 83%), but development of a protective response with the accelerated schedule was similar to standard schedule only for persons with high CD4 cell counts (>500 c/mm3). Several additional studies illustrate that the conventional vaccine series is difficult to complete. In a recent large study from the military with highly regimented health care, only 62% of 626 HIV-infected persons received 3 doses of HBV vaccine [36]. Given this data, we elected to use an expedited vaccine schedule of 0, 4, and 12 weeks. While this generated a 59% overall response rate at week 16, only 40% of vaccinees had durable protection 1 year after vaccination. Additional research is needed to determine the optimal schedule to generate a protective immune response.
Our trial was subject to limitations of small-scale studies. The study size limited our ability to determine the factors associated with the development of a protective immune response (notably CD4+ T-cell count and plasma HIV RNA). Longer follow up would also provide more information regarding durability, but the decline in subjects with protective antibody at 60 weeks suggests that an additional booster dose of vaccine may be required to maintain protective antibodies. And while our study aimed to optimize response in all subjects by using high dose vaccine and an expedited schedule, it is not possible to distinguish the two effects in the overall response rate. Specifically, the use of an accelerated schedule may have mitigated the benefit reported in previous trials with GM-CSF. Nonetheless, there was no evidence that addition of GM-CSF improves response in this setting. Finally, the durability of the response may have been affected negatively by the accelerated schedule.
However, our study suggests that the vaccine responses to the strategies utilized in this study still fall short of the responses in HIV-uninfected individuals. While the use of adjuvants offers the potential to boost immune response, we failed to find a significant effect from GM-CSF in this study. Given the shared routes of transmission for HIV and HBV, there remains an urgent need for research to further explore the underlying mechanism limiting immune responses to this vaccine and to develop better strategies to induce protective immunity.
Acknowledgements
The authors also wish to gratefully acknowledge all of the study participants who have devoted their time and effort to this research endeavor.
Financial Support. This research was supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (Grants: AI 68636 and AI 68634) and by the institutions listed below. HBV vaccine was donated by Merck and GM-CSF by Bayer. Neither pharmaceutical company was involved in the design, conduct or analysis of data from this study, or in the writing of this paper; however we thank Rafael Munoz, Bayer for his scientific guidance during the design of this study. Grant support was provided, in part, to Dr. Overton from NIH AI069495; Dr. Kang from NIH AI038855 and AI068634; and Dr. Aberg from NIH AI027665 and AI069532.
The following persons and institutions participated in the conduct of this trial: Teresa Spitz, RN and Geyoul Kim, RN (Washington University (Site 2101) CTU Grant #AI 69495); Janet Forcht, RN and Karen Cavanagh, RN (New York University/NYC HHC at Bellevue Hospital Center (Site 0401) CTU Grant # AI 69532); William E. Maher, MD and Laura Laughlin, RN (Ohio State University (Site 2301) CTU Grant# AI 69474); Babafemi Taiwo, M.B.B.S., M.D. and Karen Coleman, R.N. (Northwestern University (Site 2701) CTU Grant# AI 69471); Benigno Rodriguez, MD and Trisha Walton, RN (Case Western Reserve University (Site 2501) CTU Grant # AI 69501); Shelia Dunaway, MD and Sheryl Storey, PA-C (University of Washington (Site 1401) CTU Grant # AI 69434); Judith Feinberg MD and Tammy Mansfield RN ACRN (University of Cincinnati College of Medicine (Site 2401) CTU Grant # AI 69513); Allan R. Tenorio, MD and Beverly E. Sha, MD (Rush University Medical Center (Site 2702) CTU Grant # AI 69471); Jane Reid RN, MS, APN-BC and Carol Greisberger RN BSN CCRC (Rochester (Site 1101) CTU Grant# AI 69511; GCRC grant # RR 024160); Nathan M. Thielman, MD and Martha Silberman, RN (Duke University Medical Center (Site 1601) CTU Grant # AI 69484-02); Ann Marie Anderson, R.N. and Robert C Kalayjian, M.D. (MetroHealth Medical Center (Site 2503) CTU Grant # AI 69501).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest. ETO received research grants from Merck, GlaxoSmithKline, Gilead, Abbott, Tibotec, and Boehinger Ingelheim through Washington University; served as a consultant for Tibotec and GlaxoSmithKline; and served on speakers’ bureau or received honoraria from Merck, Tibotec, GlaxoSmithKline, Bristol-Myers Squibb, GlaxoSmithKline, Monogram Sciences, and Gilead. JA has received honoraria, research support and/or served on the advisory boards of Abbott Laboratories, Boehringer Ingelheim, Bristol Myers Squib, Gilead Sciences, Glaxo-Smith Kline, Merck & Co., Inc., Pfizer, Inc, Schering-Plough and Tibotec Therapeutics. MP has served as a consultant for Merck, Pharmasset, Cinical Care Options and Hoffman LaRoche. All other authors: no conflicts.
References
- 1.Mahoney FJ. Update on diagnosis, management, and prevention of hepatitis B virus infection. Clin Microbiol Rev. 1999;12:351–366. doi: 10.1128/cmr.12.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter M. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Soriano V, Puoti M, Peters M, Benhamou Y, Sulkowski M, Zoulim F, Mauss S, Rockstroh J. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS. 2008 Jul 31;22(12):1399–1410. doi: 10.1097/QAD.0b013e3282f8b46f. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32(3):492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Mocroft A, Soriano V, Rockstroh J, Reiss P, Kirk O, de Wit S, Gatell J, Clotet B, Phillips AN, Lundgren JD EuroSIDA Study Group. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV/AIDS. 2005 Dec 2;19(18):2117–2125. doi: 10.1097/01.aids.0000194799.43799.ea. [DOI] [PubMed] [Google Scholar]
- 6.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B, and risk of liver-related mortality in the Multicenter AIDS Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 7.Rockstroh JK, Bhagani S, Benhamou Y, Bruno R, Mauss S, Peters L, Puoti M, Soriano V, Tural C EACS Executive Committee. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008 Feb;9(2):82–88. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 8.Bloom BS, Hillman AL, Fendrick AM, et al. A reappraisal of hepatitis B vaccination strategies using cost effectiveness analysis. Ann Intern Med. 1993;118:298–306. doi: 10.7326/0003-4819-118-4-199302150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Van Herck K, Van Damme P, Thoelen S, et al. Long-term persistence of antiHBs after vaccination with a recombinant DBA yeast-derived hepatitis B vaccine: 8 year results. Vaccine. 1998;16:1933–1935. doi: 10.1016/s0264-410x(98)00126-1. [DOI] [PubMed] [Google Scholar]
- 10.Keet IPM, van Doornum G, Safary A, et al. Insufficient response to hepatitis B vaccination in HIV-positive homosexual men. AIDS. 1992;6:509–510. [PubMed] [Google Scholar]
- 11.Tayal SC, Sankar KN. Impaired response to recombinant hepatitis B vaccine in asymptomatic HIV infected individuals. AIDS. 1994;8:558–559. doi: 10.1097/00002030-199404000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Bruguera M, Cremades M, Salinas R, et al. Impaired response to recombinant hepatitis B vaccine in asymptomatic HIV infected persons. J Clin Gastroenterol. 1992;14:27–30. doi: 10.1097/00004836-199201000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis. 2005;41:1045–1048. doi: 10.1086/433180. [DOI] [PubMed] [Google Scholar]
- 14.Baylor NW, Egan W, Richman P. Aluminum salts in vaccines-US perspective. Vaccine. 2002;20:S18–S23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 15.Kapoor D, Aggarwal SR, Singh NP, et al. Granulocyte-macrophage colony-stimulating factor enhances the efficacy of hepatitis B virus vaccine in previously unvaccinated haemodialysis patients. J Viral Hepat. 1999;6:405–409. doi: 10.1046/j.1365-2893.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- 16.Jha R, Lakhtakia S, Jaleel MA, et al. Granulocyte-macrophage colony-stimulating factor induced sero-protection in end stage renal failure patients to hepatitis B in vaccine non-responders. Ren Fail. 2001;23:629–636. doi: 10.1081/jdi-100107359. [DOI] [PubMed] [Google Scholar]
- 17.Stärkel P, Stoffel M, Lerut J, Horsmans Y. Response to an experimental HBV vaccine permits withdrawal of HBIg prophylaxis in fulminant and selected chronic HBV-infected liver graft recipients. Liver Transpl. 2005 Oct;11(10):1228–1234. doi: 10.1002/lt.20464. [DOI] [PubMed] [Google Scholar]
- 18.Deresinki SC. Granulocyte-macrophage colony-stimulating factor: potential therapeutic, immunologic and antiretroviral effects in HIV infection. AIDS. 1999;13:633–643. doi: 10.1097/00002030-199904160-00003. [DOI] [PubMed] [Google Scholar]
- 19.Miles S. The use of hematopoietic growth factors in treating HIV infection. Curr Opin Hematol. 1995;2:227–233. doi: 10.1097/00062752-199502030-00012. [DOI] [PubMed] [Google Scholar]
- 20.Hardy WD. Combined ganciclovir and recombinant human granulocyte-macrophage colony-stimulating factor in the treatment of cytomegalovirus retinitis in AIDS patients. J Acquir Immune Defic Syndr. 1991;4 Suppl 1:S22–S28. [PubMed] [Google Scholar]
- 21.Kaplan LD, Kahn JO, Crowe S, et al. Clinical and virological effects of recombinant human granulocyte-macrophage colony-stimulating factor in patients receiving chemotherapy for human immunodeficiency virus-associated non-Hodgkin's lymphoma: results of a randomized trial. J Clin Oncol. 1991;9:929–940. doi: 10.1200/JCO.1991.9.6.929. [DOI] [PubMed] [Google Scholar]
- 22.Gill PS, Mitsuyasu RT, Montgomery T, et al. AIDS Clinical Trials Group Study 094: a phase I/II trial of ABV chemotherapy with zidovudine and recombinant human GM-CSF in AIDS-related Kaposi’s sarcoma. Cancer J Sci Am. 1997;3(5):278–283. [PubMed] [Google Scholar]
- 23.Hess G, Kreiter F, Kosters W, et al. The effect of granulocyte-macrophage colony-stimulating factor (GM-CSF) on hepatitis B vaccination in haemodialysis patients. J Viral Hepat. 1996;3(3):149–153. doi: 10.1111/j.1365-2893.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 24.Evans TG, Schiff M, Graves B, et al. The safety and efficacy of GM-CSF as an adjuvant in hepatitis B vaccination of chronic hemodialysis patients who have failed primary vaccination. Clin Nephrol. 2000;54:138–142. [PubMed] [Google Scholar]
- 25.Cruciani M, Mengoli C, Serpelloni G, Mazzi R, Bosco O, Malena M. Granulocyte macrophage colony-stimulating factor as an adjuvant for hepatitis B vaccination: a meta-analysis. Vaccine. 2007 Jan 8;25(4):709–718. doi: 10.1016/j.vaccine.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki M, Foccacia R, deMessias-Reason IJ. Efficacy of granulocyte-macrophage colony-stimulating factor (GM-CSF) as a vaccine adjuvant for hepatitis B virus in patients with HIV infection. Vaccine. 2003;21:4545–4549. doi: 10.1016/s0264-410x(03)00500-0. [DOI] [PubMed] [Google Scholar]
- 27.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004 Sep 1;64(17):6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 28.Pickering LK, Baker CJ, Freed GL, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(6):817–840. doi: 10.1086/605430. [DOI] [PubMed] [Google Scholar]
- 29.Aberg JA, Gallant JE, Anderson J, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(5):651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009 Apr 10;58(RR-4):1–207. [PubMed] [Google Scholar]
- 31.Fonseca MO, Pang LW, de Paula Cavalheiro N, Barone AA, Heloisa Lopes M. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine. 2005;23:2902–2908. doi: 10.1016/j.vaccine.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 32.Cornejo-Juárez P, Volkow-Fernández P, Escobedo-López K, Vilar-Compte D, Ruiz-Palacios G, Soto-Ramírez LE. Randomized controlled trial of Hepatitis B virus vaccine in HIV-1-infected patients comparing two different doses. AIDS Res Ther. 2006;3:9. doi: 10.1186/1742-6405-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosman AS, Basu P, Galvin K, et al. Efficacy of a high and accelerated dose of hepatitis B vaccine in alcoholic patients: a randomized clinical trial. Am J Med. 1997;103(3):217–222. doi: 10.1016/s0002-9343(97)00132-0. [DOI] [PubMed] [Google Scholar]
- 34.Potsch DV, Oliveira ML, Ginuíno C, Miguel JC, Oliveira SA, Silva EF, Moreira RB, Cruz GV, Oliveira AL, Camacho LA, Barroso PF. High rates of serological response to a modified hepatitis B vaccination schedule in HIV-infected adults subjects. Vaccine. 2009 Dec 6; doi: 10.1016/j.vaccine.2009.11.066. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.de Vries-Sluijs TEMS, Hansen BE, van Doornum GJJ, et al. Accelerated Hepatitis Vaccine Schedule in HIV-Infected Patients; San Francisco, CA. From Prgram and Abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; February 16–19, 2010; Abstract # 624. [Google Scholar]
- 36.Landrum ML, Huppler Hullsiek K, Ganesan A, et al. Hepatitis B Vaccine Responses in a Large U.S. Military Cohort of HIV-infected Individuals: Another Benefit of HAART in those with Preserved CD4 Count. Vaccine. 2009;27(34):4731–4738. doi: 10.1016/j.vaccine.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]