Abstract
Introduction
Resveratrol may provide protection against coronary artery disease. We hypothesized that supplemental resveratrol will improve cardiac perfusion in the ischemic territory of swine with hypercholesterolemia and chronic myocardial ischemia.
Methods and Results
Yorkshire swine were fed either a normal diet (control, n=7), a hypercholesterolemic diet (HCC, n=7), or a hypercholesterolemic diet with supplemental resveratrol (100 mg/kg/day orally, HCRV, n=7). Four weeks later, an ameroid constrictor was placed on the left circumflex artery. Animals underwent cardiac magnetic resonance imaging and coronary angiography 7 weeks later, prior to sacrifice and tissue harvest. Total cholesterol was lowered about 30% in HCRV animals (p<0.001). Regional wall motion analysis demonstrated a significant decrease in inferolateral function from baseline to 7 weeks in HCC swine (p=0.04). There was no significant change in regional function in HCRV swine from baseline to 7 weeks (p=0.32). Tissue blood flow during stress was 2.8 fold greater in HCRV swine when compared to HCC swine (p=0.04). Endothelial dependent microvascular relaxation response to Substance P was diminished in HCC swine which was rescued by resveratrol treatment (p=0.004). Capillary density (PECAM-1 staining) demonstrated fewer capillaries in both HCC and HCRV swine v. control swine (p=0.02). Immunoblot analysis demonstrated significantly greater expression in HCRV v. HCC swine of the following markers of angiogenesis: VEGF (p=0.002), peNOS(ser1177)(p=0.04), NFkB (p=0.004), and pAkt(thr308)(p=0.001).
Conclusion
Supplemental resveratrol attenuates regional wall motion abnormalities, improves myocardial perfusion in the collateral dependent region, preserves endothelial dependent coronary vessel function, and upregulates markers of angiogenesis associated with the VEGF signaling pathway.
Keywords: Coronary disease, Hypercholesterolemia, Ischemia, Perfusion, Angiogenesis
Introduction
Resveratrol, a polyphenol found in red wine, has been implicated in protection against coronary artery disease 1. Potential mechanisms include enhancing angiogenesis, reducing oxidant stress and mimicking effects of caloric restriction. Resveratrol has also been implicated in protection from ischemic injury. The mechanism is likely due to the vasorelaxation, anti-apoptotic and pro-angiogenic properties of the supplement 2.
We previously demonstrated that hypercholesterolemia can significantly blunt the endogenous angiogenic response to chronic myocardial ischemia. In that study the effects of hypercholesterolemia on the chronically ischemic myocardium resulted in decreased vessel density, increased levels of anti-angiogenic mediators, depressed microvascular function and decreased perfusion in the ischemic territory relative to those animals fed a normal diet 3. These detrimental changes, along with atherosclerosis, likely combine to lead to cardiovascular disease.
Resveratrol is thought to activate sirtuins, a family of highly conserved proteins with deacetylase activity 4. The most well studied sirtuin is SIRT1, a nicotinamide adenine dinucleotide (NAD)-dependent deacetylase, which acts at the DNA and protein levels to exert its effects 5. In the cardiovascular system activation of SIRT1 is thought to lead to decreased inflammation, platelet aggregation, and apoptosis 6, 7. Furthermore, activation of SIRT1 may promote native angiogenesis and has been shown to increase VEGF and NO production 8. However, these findings have not been validated in large animal models, and the role of resveratrol in the setting of chronic myocardial ischemia, hypercholesterolemia and endothelial dysfunction remains to be examined.
Given the considerable potential for cardioprotective and pro-angiogenic effects of resveratrol, we examined the effects of this agent in a clinically relevant porcine model of hypercholesterolemia and chronic myocardial ischemia. We hypothesized that supplemental resveratrol will improve cardiac perfusion and vascular function in the ischemic territory.
Methods
Animal Model
Yorkshire mini-swine (Parsons Research, Amherst, MA) were fed one of three diets daily throughout the 11 weeks of the experiment. The first group was given 500 grams of a hypercholesterolemic diet daily (HCC, n= 7) composed of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow. A second group was fed the same hypercholesterolemic diet supplemented with 100 mg/kg/day resveratrol (HCRV, n= 7) (Chromadex, Irvine, CA). The third group of swine was fed regular chow (control, n= 7).
After 4 weeks of dietary modification, all animals underwent functional cardiac magnetic resonance imaging (CMR) and ameroid constrictor placement on the proximal left circumflex coronary artery (LCx). For all surgical procedures, anesthesia was induced with ketamine (10 mg/kg IM) and thiopental 2.5%, and maintained with a gas mixture of oxygen at 1.5-2 L/min and 3.0% isoflurane. The animals were intubated and mechanically ventilated at 12-20 breaths/min.
During the first procedure (ameroid placement), through a left mini-thoracotomy, gold-labeled microspheres (Biophysics Assay laboratory, Worcester, MA) were injected into the left atrium during temporary occlusion of the LCx to determine the exact myocardial territory at risk. Next, a titanium ameroid constrictor (1.75 mm internal diameter) was placed around the proximal LCx.
Seven weeks after ameroid placement, swine were anesthetized and CMR and x-ray coronary angiography were completed. The heart was then exposed and microspheres were injected both at rest and during ventricular pacing (150 beats per minute), followed by euthanasia. The heart was harvested and two 1 cm thick transverse slices were cut at the midventricular level and sectioned into 8 segments. Samples were divided and rapidly frozen in liquid nitrogen (molecular studies), placed in 4°C Krebs solution (microvessel reactivity studies), 10% formalin (immunohistochemistry studies), or weighed and dried (microsphere perfusion analyses).
All experiments were approved by the Beth Israel Deaconess Medical Center animal care and use committee. Animals were cared for in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and in accordance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication no. 5377-3 1996).
Cardiovascular Magnetic Resonance (CMR) Imaging
Animals in the HCC and HCRV groups underwent a CMR study before placement of the ameroid (pre) and prior to sacrifice (post) 7 weeks later. All animals were scanned using a 1.5T Philips Achieva scanner (Philips Healthcare, Best, Netherlands) with a five-element cardiac phased-array receiver coil. MR tagging was performed to evaluate dyssynchrony and local myocardial function using three short axis slices with a spiral complementary spatial modulation of magnetization sequence 9. Images were analyzed using a MATLAB (The Mathworks, Natick, MA) program 10. Each myocardial slice was divided into 6 segments according to the AHA/ACC 17 segment model and the circumferential strain of each segment was analyzed. The values obtained represent the amount of regional contraction contributing to the overall ejection fraction. Thus, a more negative number represents greater contraction of the myocardial segment.
X-ray coronary angiography
X-ray coronary angiography was carried out in order to ensure occlusion of the LCx and assess collateral formation. Recorded images were interpreted by a blinded interventional cardiologist. Angiographic collateral formation was assessed according to the Rentrop grading system of 0–3, depending on the presence and extension of the collateral filling of coronary epicardial vessels 11.
Microvessel Studies
After cardiac harvest, coronary arterioles (80-180μm in diameter) from the ischemic territory were dissected from the surrounding tissue and placed in isolated microvessel chambers as described previously 12. The microvascular responses to sodium nitroprusside (SNP, 10-9 to 10-4 mol/L, endothelium independent cGMP-mediated vasodilator), and substance P (SubP, 10-12 to 10-7 mol/L, an endothelium dependent vasodilator) were evaluated. All drugs were applied extraluminally. Relaxation responses were defined as the percent relaxation of the preconstricted diameter. All reagents were obtained from Sigma-Aldrich (St Louis, MO).
Myocardial Perfusion analysis
Myocardial perfusion was determined during each procedure with isotope-labeled microspheres (ILMs), 15 μm diameter (BioPAL Worcester, MA) using previously reported methods 13. Briefly, 1.5×107 gold-labeled microspheres were injected during temporary LCx occlusion at the time of ameroid placement to identify the area at risk. Lutetium (rest) and Europium (pace) labeled ILMs were injected at the final procedure. Following euthanasia, 10 transmural left ventricular sections were collected for ILM assays. The samples were exposed to neutron beams and microsphere densities were measured using a gamma counter.
Molecular studies
Western blotting was performed as previously described 14. Sixty micrograms of total protein was fractionated by 4-20% gradient, SDS polyacrylamide gel electrophoresis (Invitrogen, San Diego, CA) and transferred to PVDF membranes (Millipore, Bedford, MA). Each membrane was incubated with one of the following specific antibodies: anti-VEGF (Calbiochem, San Diego, CA), anti-FGF-2 (US Biological, Swampscott, MA), anti-NFkB, anti-peNOS (ser1177), anti-pAkt (ser473), anti-pAkt (thr473), anti-pERK (thr202/tyr 204) (Cell Signaling, Beverly, MA, USA), anti-eNOS antibody (BD Biosciences, San Jose, CA), and anti-myleoperoxidase (MPO) antibody (Abcam, Inc, Cambridge, MA). Immune complexes were visualized with an enhanced chemiluminescence detection system (Amersham, Piscataway, NJ). Bands were quantified by densitometry of autoradiograph films. Ponceau staining was used to ensure equal protein loading.
Immunohistochemistry
Formalin fixed tissue samples were processed as previously described 15. Antibodies against PECAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA) were applied to the sections for 2 hours at room temperature. Detection was obtained using a biotinylated goat anti-mouse secondary antibody and the avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA). Color was developed using diaminobenzidine substrate (1mg/ml in PBS and 0.03% H2O2). Sections were then counterstained with hematoxilin, dehydrated and mounted. Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY) equipped with a digital camera (Photodoc, Upland, CA) and capillaries were counted in a blinded fashion.
Immunofluorescence Double Staining for Dividing Endothelial Cells
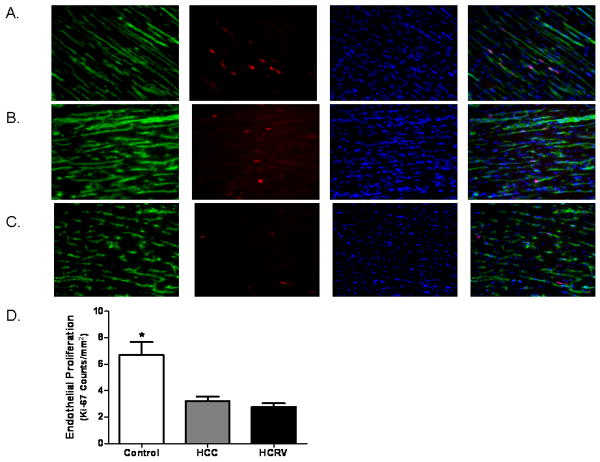
Frozen sections (12μm in thickness) of myocardium from the ischemic territories were formalin fixed for 10 minutes. Antibodies against Ki-67 (Abcam Inc., Cambridge, MA) and PECAM-1 (CD-31, R&D Systems, Minneapolis, MN) were simultaneously applied to the sections and incubated overnight at 4°C. Detection was obtained using appropriate secondary antibodies (Jackson ImmunoReaserch, West Grove, PA). Sections were then mounted in Vectashield with 4′,6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA). Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY) equipped with a digital camera and 200× magnification (Photodoc, Upland, CA). Dividing endothelial cells were counted in a blinded fashion. Structures that were DAPI positive, Ki-67 positive, and within an endothelial cell (CD-31 positive) were considered dividing endothelial cells. Data is presented as number of Ki-67 positive endothelial cells/mm2.
Data Analysis
All results are expressed as mean ± standard error of the mean (SEM). Microvessel responses are expressed as percent relaxation of the preconstricted diameter and were analyzed using two way, repeated measures analysis of variance with a post-hoc Bonferroni test, which was applied to interactions of treatment and dose. Western blots were analyzed after digitalization (ScanJet 4c; Hewlett-Packard, Palo Alto, CA) and NIH ImageJ 1.33 software (National Institute of Health, Bethesda, MD). For data analysis, levels of phosphorylated proteins were normalized to total expression levels. Comparisons between the three groups for blood flow, endothelial proliferation and western blot data were analyzed by one way analysis of variance with Newman-Keuls Multiple Comparison post-hoc test, with the exception of the circumferential strain data which was analyzed by two tailed t-test, using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA). Probability values less than 0.05 were considered significant.
Results
Experimental Model
There was one animal death each in the control and HCRV groups. Necropsy did not identify a clear cause of death but was most likely related to ventricular arrhythmia. There was no mortality in the HCC group. There was no difference in mean heart rate. The HCC group had a higher mean arterial pressure and mean diastolic pressure than the control or HCRV group. Total cholesterol levels were elevated in the HCC and HCRV groups, though the levels in the HCRV group were significantly lower than the HCC group (Table 1).
Table 1.
Comparison of physiological measures in animals fed either regular chow (Control), high cholesterol diet only (HCC), or high cholesterol diet with supplemental resveratrol (HCRV). Blood Flow- amount of myocardial blood flow to collateral dependent region at a resting heart rate, HR- heart rate, MAP- Mean arterial blood pressure, DBP- Diastolic blood pressure.
| Control | HCC | HCRV | p-value | |
|---|---|---|---|---|
|
Total Cholesterol (mg/dL) |
79.9 ± 0.1 | 390 ± 53 | 261 ± 54 | <0.001 |
|
Mean HR (beats/min) |
129 ± 16 | 122 ± 11 | 115 ± 33 | 0.8 |
|
MAP (mmHg) |
69 ± 7 | 73 ± 10 | 65 ± 7 | 0.03 |
|
Mean DBP (mmHg) |
46 ± 2.1 | 71 ± 4.7 | 53 ± 9 | 0.01 |
|
Blood Flow- Rest (mL/min/g) |
0.07 ± 0.03 | 0.13 ± 0.003 | 0.08 ± 0.003 | 0.25 |
Cardiac Magnetic Resonance Imaging (CMR)
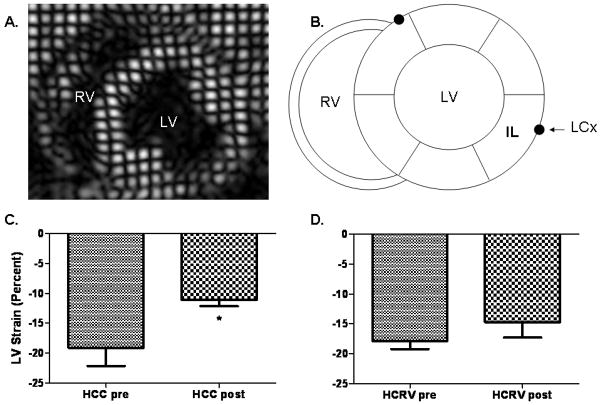
Comparisons of regional function were made by analyzing the differences in the amount of muscle contraction in each region prior to placement of the ameroid with the amount of contraction at the end of the experiment within each group. Regional wall motion analysis using CMR circumferential strain demonstrated a significant decrease in inferolateral regional function (region of measurement shown in Figure 1A) from baseline (pre) to 7 weeks of chronic ischemia (post) in HCC swine in the basal myocardial slice (-19.1±4% to -11.1±1%) (Figure 1B). Regional function in HCRV swine from baseline (pre) to 7 weeks chronic ischemia (post) was preserved, without a significant worsening in inferolateral wall motion despite LCx ischemia (-17.8±7% to -14.7%±3%)(Figure 1C).
Figure 1.
Coronary magnetic resonance imaging (CMR) analysis. (A) Axial CMR circumferential strain image. (B) Schematic cross-sectional representation of the heart depicting the proximity of the left circumflex coronary artery (LCx) to the inferolateral (IL) wall of the left ventricle (LV). RV- Right ventricle. (C) Regional wall motion analysis using circumferential strain demonstrates a significant decrease in IL wall function in the HCC group post (*p= 0.04) (D) and no change in the HCRV group (p= 0.32).
X-ray Coronary Angiography
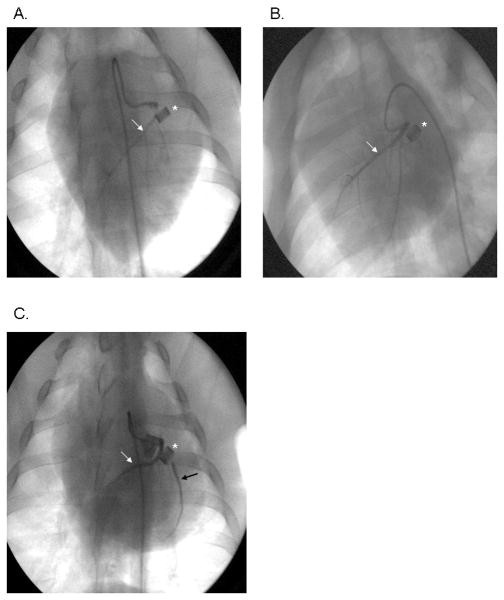
All animals were found to have ≥99% occlusion of the proximal LCx by the ameroid constrictors. Rentrop collateral scores were not significantly different among the groups (control 0.9±0.3, HCC 1.3±0.3, HCRV 1.3±0.4, p= 0.68)(Figure 2 A-C).
Figure 2.
X-ray coronary angiograms demonstrating presence of flow in the left circumflex coronary artery (LCx) in (A) the control, (B) the HCC, and (C) the HCRV groups. The HCRV animal demonstrates brisk filling of the LCx through proximal collaterals from the LAD. *represents the ameroid constrictor, white arrow denotes the LAD, black arrow depicts antegrade flow in the LCx.
Microvessel Analysis
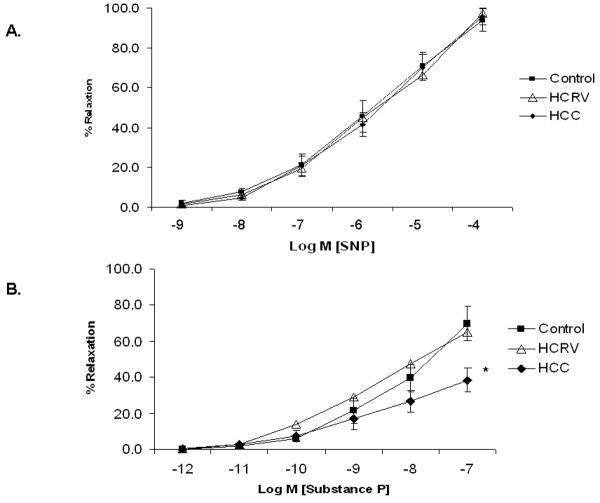
Microvascular response to the endothelial independent drug SNP demonstrated no differences between groups (control 93.9±5.7%, HCRV 97.4±3.4%, HCC 93.9±5.7% at 10-4 M)(Figure 3A). Endothelial dependent microvascular relaxation to Substance P (10-7M) was diminished in HCC swine, but was normalized in the HCRV group (HCC 38.5±6.7%, HCRV 65.1±10.2%, control 69.6±9.4%)(Figure 3B).
Figure 3.
Coronary microvascular responses in vitro. (A) Microvessel response to SNP, an endothelial independent vasodilator (p= 0.95). (B) Microvessel response to Substance P, an endothelial dependent vasodilator (*p= 0.004).
Myocardial Flow Analysis
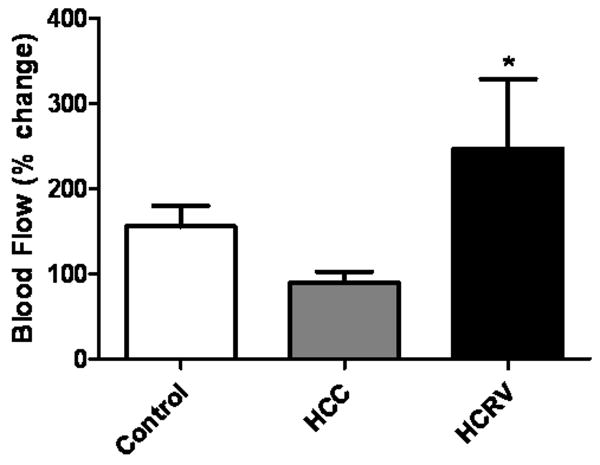
At 7 weeks after ameroid placement, myocardial perfusion at rest was similar between groups (control 0.07±0.03 mL/min/g, HCC 0.13±0.03 mL/min/g, HCRV 0.08±0.03 mL/min/g, p= 0.25). When the heart was stressed with ventricular pacing at 150 beats/minute, there was a significantly greater increase in baseline-adjusted myocardial flow in the ischemic territory of the HCRV group (HCRV 247±124%, control 155±27%, HCC 89±14%)(Figure 4).
Figure 4.
Myocardial blood flow analysis. Percentage change in myocardial blood flow from resting state to a stress state was significantly increased in the HCRV group. (*p= 0.04).
Immunohistochemistry
Staining of fixed sections from the ischemic territory for PECAM-1 (CD31) demonstrated that the HCC and HCRV groups had significantly fewer capillaries (PECAM-1 positive vessels/200× field) than control (control 96±17.1, HCC 61±5.4, HCRV 65±5.4, p= 0.02). Double staining for endothelium (CD31) and cellular proliferation (Ki-67) demonstrated fewer dividing endothelial cells in the HCC and HCRV groups as compared to the control (Figure 5).
Figure 5.
Endothelial cell proliferation. Immunofluorescence double staining for endothelium (CD-31, green) and dividing cells (Ki-67, red) with DAPI (blue) allow for the visualization of dividing endothelial cells in the ischemic territory which appear pink in the combined image. This demonstrates significantly fewer vessels in the HCC and HCRV groups as compared to the control group, (A) control, (B) HCC and (C) HCRV (B) Quantification of dividing endothelial cells further shows fewer dividing endothelial cells in the HCC and HCRV groups (*p= 0.02)(Magnification 200×).
Protein Expression
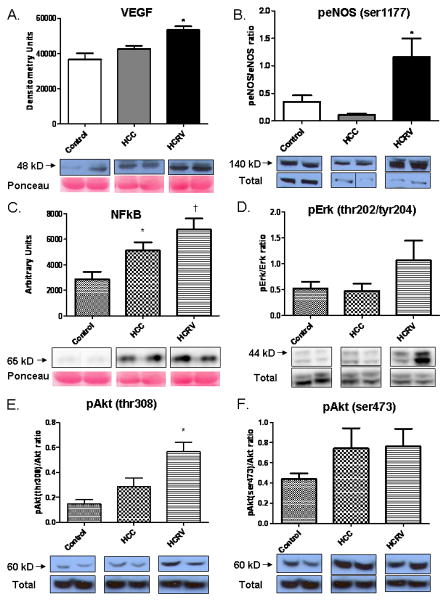
Immunoblot analysis of proteins associated with angiogenesis demonstrated significant increases in angiogenic protein levels in the ischemic territory of the HCRV group. VEGF expression increased 1.45 fold in the HCRV group v. control and 1.26 fold v. HCC (Figure 6A). Phospho-eNOS (ser1177) increased 3.33 fold in the HCRV group v. control and 2.18 fold v. HCC (Figure 6B). Protein expression of NFkB showed a 2.4 fold increase in the HCRV group v. Control and 1.3 fold v. HCC (Figure 6C). The expression of MPO was not different between the groups (p= 0.80). There was a trend towards increased phosopho-ERK (thr202/tyr 204) in the HCRV group, however this was not statistically significant (2.36 fold in HCRV v. Control and 2.35 fold v. HCC) (Figure 6D). There was increased phosphorylation of Akt on both threonine 308 (2.8 fold v. Control and 1.7 fold v. HCC) and serine 473 (Figure 6E and F). There was no significant difference between groups in the expression of total eNOS (p= 0.3) and FGF-2 (p= 0.2). Additionally, there was no difference in the expression of MPO between the groups (p=0.22).
Figure 6.
Increased expression of angiogenic signaling proteins and mediators in the HCRV group. (A) VEGF (*p= 0.002). (B) peNOS (ser1177)(*p= 0.04). (C) NFkB expression is increased in the HCC group and to a greater extent in the HCRV group (*p< 0.05 v. control, † p< 0.05 v. HCC). (D) Phospho-ERK (thr202/tyr 204)(p= 0.15). (E) Phospho-Akt (thr308)(*p< 0.05). (F) Phospho-Akt (ser473)(p=0.29). All blots were normalized to total protein stain with Ponceau.
Relationship between total cholesterol and phospho-eNOS (ser1177)
Linear regression was used to explore a correlation between total cholesterol and phospho-eNOS (ser1177). There was a significant inverse relationship between total cholesterol and phospho-eNOS (ser1177) levels (R2= 0.42, p= 0.02)(Table 1).
Discussion
The principle findings of the current study demonstrate that administration of supplemental resveratrol improves myocardial and coronary vascular function but have limited effect on vessel density in the setting of chronic ischemia coupled with hypercholesterolemia.
Evidence for improved perfusion in resveratrol treated hypercholesterolemic swine include: 1) preserved regional wall motion in the ischemic territory of HCRV swine that was similar to baseline measurements obtained prior to induction of ischemia, 2) flow augmentation to the ischemic territory during ventricular pacing (myocardial stress). Resveratrol increased collateral dependent blood flow compared to that in the HCC and control groups, 3) a direct increase in endothelial-dependent microvascular relaxation, facilitating vasodilation in the ischemic myocardium. Supplemental resveratrol decreased the cholesterol level by about 30%, but improved the endothelial response to substance P by 94%. Linear regression demonstrated a significant inverse relationship between total cholesterol and phospho-eNOS (ser1177) levels.
Resveratrol also increased expression of phospho-eNOS (ser1177), which likely provides more endothelial NO. Increased NO would facilitate a more robust vasorelaxation response to increased oxygen demand during pacing, and may explain why the HCRV group had a marked increase in myocardial blood flow. Previous studies have demonstrated improved microvascular relaxation with resveratrol. In a study by Mattagajasingh et al endothelial relaxation responses in control rat aortas were improved compared to dominant negative SIRT1 mutant rats. SIRT1 was found to co-localize with eNOS in the endothelium. The result is increased concentrations of NO in the endothelium 16. A second study demonstrated that eNOS also activates SIRT1 creating a positive feedback loop 8. In addition, in isolated in vitro swine coronary arteries, resveratrol induced an endothelial-dependent relaxation response. This response was attenuated in the presence of L-NNA, an inhibitor of NO synthesis 17. Collectively, these reports support our findings that resveratrol may improve endothelium dependent relaxation at least in part via increased activation of eNOS. The increased expression of eNOS and improvement in endothelial dependent relaxation likely contributed to the preservation of regional myocardial function seen in the HCRV group. Additionally, the HCRV animals displayed large increases in VEGF expression. VEGF is not only involved with angiogenesis, but is also a potent vasodilator and its expression may also drive increased myocardial blood flow in vivo 12.
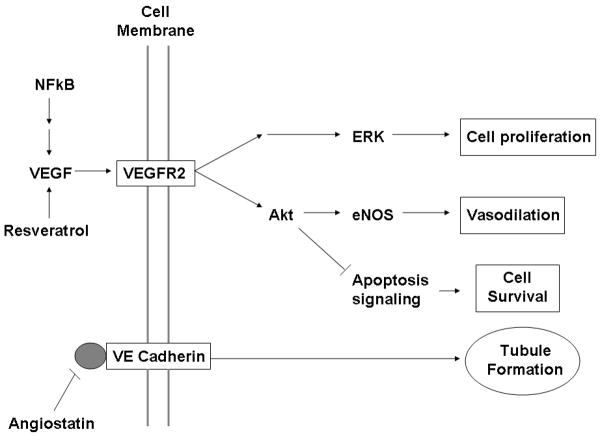
Numerous studies in vitro and in small animals have demonstrated the angiogenic properties of resveratrol 18, 19, though few have been done in large animals. In the current study we noted increased molecular markers associated with the VEGF pathway, but did not demonstrate an increase in FGF-2 expression or the quantity of vessels. It may be that seven weeks after the ameroid placement did not allow sufficient time to visualize vessel formation in a hypercholesterolemic model. Another possible explanation for this lack of neovascularization may be the presence of unmeasured inhibitors of angiogenesis such as endostatin or angiostatin. Endostatin inhibits endothelial cell proliferation and migration and decreases vascular tube formation 20 (Figure 7). Previous work from our lab and others has demonstrated that inhibitors of the angiogenic pathway are increased in the setting of chronic illnesses such as hypercholesterolemia and diabetes 14. A recent study from our group examining the effects of atorvastatin on angiogenesis in a porcine model of hypercholesterolemia and chronic myocardial ischemia with exogenously administered VEGF demonstrated no increase in new vessel formation, and this was associated with a significantly elevated expression of endostatin in the myocardial tissue 21. A prolonged increase in expression of phospho-Akt (ser473), observed in the present study, has also been implicated as an inhibitor of angiogenesis 22. Therefore, we feel that while resveretrol may mediate increased vascular function, possibly via direct vasodilatory effects of VEGF and eNOS, it is unable to rescue the hypercholesterolemia-induced impaired angiogenic response even in the face of elevated VEGF.
Figure 7.
Schematic drawing demonstrating the relevant proteins of the angiogenic pathway influenced by resveratrol, and a potential site of inhibition by angiostatin.
Interestingly, we also demonstrated an increase in NFkB expression in the HCC group and an even greater increase in the HCRV group. Immunoblotting for myeloperoxidase, a marker of neutrophil infiltration, demonstrated no difference between the groups. This suggests there is an alternate stimulus for NFkB expression. Hypoxia is known to induce local inflammation (TNFα, IL-8, NFkB), and NFkB is known to upregulate VEGF. This local inflammatory response is thought to be involved not only in the initiation of angiogenesis, but also with the remodeling necessary for formation of new vasculature. Mediators of inflammation lead to vascular permeability and increase factors involved with extracellular matrix remodeling 23.
There are limitations to the current study. Due to the paucity of large animal resveratrol studies, there is little data available on the optimum dosage in large animals and humans. There have been concerns about the bioavailablity of orally delivered resveratrol, and some studies have shown low systemic concentrations 24. Early studies in animal models and humans demonstrated no adverse effects at doses up to 5 grams 25. Thus we chose a higher dose in an attempt to achieve adequate cardiac tissue levels. Though the current study was not designed to examine side effects of resveratrol, no adverse events were found.
Although the observed changes were sufficient to allow determination of statistical significance, the number of animals in each group was relatively small and therefore our findings should be interpreted in this context. Also, in addition to coronary microvessels, it would be interesting to explore resveratrol's effects on larger coronary vessels as well as peripheral arteries. In addition molecular markers were examined at only a single time point and changes in molecular signaling are likely to take place over time. Finally, although a number of significant changes were found in the HCRV group, we did not demonstrate that these changes were wholly or in part secondary to SIRT1 activation.
Conclusion
Supplemental resveratrol in the setting of hypercholesterolemia and chronic myocardial ischemia attenuates regional wall motion abnormalities, improves collateral dependent perfusion, preserves endothelial dependent coronary vessel function, and upregulates markers of angiogenesis associated with the VEGF signaling pathway. This may represent a possible agent for study in humans with risk factors for ischemic heart disease, and may result in clinically significant primary prevention or protection during an acute ischemic myocardial event.
Clinical Significance Statement
Currently resveratrol and its analogs are being developed and evaluated as therapeutic agents for treatment of a wide number of illnesses, such as diabetes mellitus, hypercholesterolemia, and inflammatory bowel disease. In the current study we demonstrated numerous beneficial effects of oral resveratrol treatment in swine fed a hypercholesterolemic diet. These include decreasing total cholesterol, lowering systemic blood pressures, improved endothelial microvascular function, increased blood flow to ischemic myocardium during stress, and preservation of regional ventricular function. Improved perfusion to the ischemic territory was not associated with an improvement in angiogenesis or increased vascular density, but due to improved vascular reactivity and elevations in myocardial levels of the vasoactive proteins VEGF and phospho-eNOS. While resveratrol may provide a number of benefits, it is important to fully characterize the pleotropic effects of this supplement before it reaches large scale clinical trials in humans. These findings are significant as millions of patients in the US suffer from the effects of illnesses that reservatrol may benefit. While there appear to be numerous potential benefits of resveratrol, extensive study in clinically relevant large animal models and human clinical trials will be required to fully evaluate the potential effects, both detrimental and beneficial.
Acknowledgments
The authors wish to acknowledge Shu-Hua Xu for assistance with molecular studies and Kraig V. Kissinger for performing CMR studies.
FUNDING
Funding for this study was through NIH HL grants RO169024, RO146716, and T32 HL07734-16 and 5T32-HL0074.
Footnotes
DISCLOSURES
The authors do not report any conflicts of interest related to this study.
References
- 1.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 3.Boodhwani M, Nakai Y, Mieno S, Voisine P, Bianchi C, Araujo EG, Feng J, Michael K, Li J, Sellke FW. Hypercholesterolemia impairs the myocardial angiogenic response in a swine model of chronic ischemia: role of endostatin and oxidative stress. Ann Thorac Surg. 2006;81:634–641. doi: 10.1016/j.athoracsur.2005.07.090. [DOI] [PubMed] [Google Scholar]
- 4.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 5.Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillarisetti S. A review of Sirt1 and Sirt1 modulators in cardiovascular and metabolic diseases. Recent Pat Cardiovasc Drug Discov. 2008;3:156–164. doi: 10.2174/157489008786263989. [DOI] [PubMed] [Google Scholar]
- 7.Stef G, Csiszar A, Lerea K, Ungvari Z, Veress G. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J Cardiovasc Pharmacol. 2006;48:1–5. doi: 10.1097/01.fjc.0000238592.67191.ab. [DOI] [PubMed] [Google Scholar]
- 8.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 9.Ryf S, Kissinger KV, Spiegel MA, Bornert P, Manning WJ, Boesiger P, Stuber M. Spiral MR myocardial tagging. Magn Reson Med. 2004;51:237–242. doi: 10.1002/mrm.10688. [DOI] [PubMed] [Google Scholar]
- 10.Haber I, Metaxas DN, Axel L. Three-dimensional motion reconstruction and analysis of the right ventricle using tagged MRI. Med Image Anal. 2000;4:335–355. doi: 10.1016/s1361-8415(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 11.Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986;74:469–476. doi: 10.1161/01.cir.74.3.469. [DOI] [PubMed] [Google Scholar]
- 12.Tofukuji M, Metais C, Li J, Franklin A, Simons M, Sellke FW. Myocardial VEGF expression after cardiopulmonary bypass and cardioplegia. Circulation. 1998;98:II242–246. discussion II247-248. [PubMed] [Google Scholar]
- 13.Boodhwani M, Voisine P, Ruel M, Sodha NR, Feng J, Xu SH, Bianchi C, Sellke FW. Comparison of vascular endothelial growth factor and fibroblast growth factor-2 in a swine model of endothelial dysfunction. Eur J Cardiothorac Surg. 2008;33:645–650. doi: 10.1016/j.ejcts.2007.12.016. discussion 251-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, Clements RT, Sellke FW. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I31–37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, Clements RT, Sellke FW. Functional, Cellular, and Molecular Characterization of the Angiogenic Response to Chronic Myocardial Ischemia in Diabetes. Circulation. 2007;116:I-31–37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li HF, Tian ZF, Qiu XQ, Wu JX, Zhang P, Jia ZJ. A study of mechanisms involved in vasodilatation induced by resveratrol in isolated porcine coronary artery. Physiol Res. 2006;55:365–372. doi: 10.33549/physiolres.930826. [DOI] [PubMed] [Google Scholar]
- 18.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sodha NR, Clements RT, Boodhwani M, Xu SH, Laham RJ, Bianchi C, Sellke FW. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296:H428–434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boodhwani M, Mieno S, Voisine P, Feng J, Sodha N, Li J, Sellke FW. High-dose atorvastatin is associated with impaired myocardial angiogenesis in response to vascular endothelial growth factor in hypercholesterolemic swine. J Thorac Cardiovasc Surg. 2006;132:1299–1306. doi: 10.1016/j.jtcvs.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 22.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 24.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 25.Elliott PJ, Jirousek M. Sirtuins: novel targets for metabolic disease. Curr Opin Investig Drugs. 2008;9:371–378. [PubMed] [Google Scholar]