Abstract
The hepatocyte is especially vulnerable to injury due to its central role in xenobiotic metabolism including drugs and alcohol, participation in lipid and fatty acid metabolism, its unique role in the enterohepatic circulation of bile acids, the widespread prevalence of hepatotropic viruses, and its existence within a milieu of innate immune responding cells. Apoptosis and necrosis are the most widely recognized forms of hepatocyte cell death. The hepatocyte displays many unique features regarding cell death by apoptosis. It is quite susceptible to death receptor-mediated injury, and its death receptor signaling pathways involve the mitochondrial pathway for efficient cell killing. Also, death receptors can trigger lysosomal disruption in hepatocytes which further promote cell and tissue injury. Interestingly, hepatocytes are protected from cell death by only two anti-apoptotic proteins, Bcl-xL and Mcl-1, which have nonredundant functions. Endoplasmic reticulum stress or the unfolded protein response contributes to hepatocyte cell death during alterations of lipid and fatty acid metabolism. Finally, the current information implicating RIP kinases in necrosis provides an approach to more fully address this mode of cell death in hepatocyte injury. All of these processes contributing to hepatocyte injury are discussed in the context of potential therapeutic strategies.
I. INTRODUCTION
The liver is an organ of immense complexity that has fascinated mankind since antiquity. The liver is essential for survival as no other organ can compensate for its multiplicity of functions. Multiple phenotypically distinct cell types comprise the liver. The predominant liver cell is the hepatocyte, a polarized epithelial cell. Hepatocytes regulate intermediary metabolism, detoxify endo- and xenobiotics, manufacture critical circulating proteins, and generate bile acid-dependent bile flow. The other polarized epithelial cell type in the liver is the cholangiocyte, which lines the bile ducts and modulates bile flow (242). The vascular structures in the liver are the sinusoids, which are lined by a fenestrated endothelial cell type (62). The sinusoidal pericyte is also termed the hepatic stellate cell and in addition to its pericyte functions can be transformed into a myofibroblast phenotype (79); activated myofibroblasts contribute to the exuberant wound healing response of the liver during chronic disease states. The liver is also enriched in resident tissue macrophages termed Kupffer cells, natural killer (NK), and natural killer-T (NKT) cells, making it a key organ of the innate immune system (83). These cells of the innate immune system often contribute to and amplify liver injury.
Sinusoidal endothelial cells, cholangiocytes, and hepatocytes are each uniquely susceptible to various type of injury and play a role in distinct clinically recognized syndromes of liver injury. For example, cholangiocyte damage results in impairment of bile flow or cholestasis (242), sinusoidal endothelial cell injury is manifest as the sinusoidal obstruction syndrome (62), while hepatocyte injury results in liver dysfunction. Any chronic form of liver damage can result in myofibroblast activation, dys-regulated hepatic fibrosis, and cirrhosis (79). Indeed, cirrhosis is the most nefarious consequence of continuous liver injury, as it results in portal hypertension, liver failure, and death. Continuous cell turnover and hepatic fibrosis are also permissive for the development of hepatocellular carcinoma, a frequent complication of chronic liver diseases (Figs. 1 and 2). Because most forms of liver injury involve hepatocytes as either a primary or secondary target, we focus this review on hepatocyte injury. Also, prior articles in Physiological Reviews have focused on cholestasis that involves cholangiocytes and also on stellate cell biology (79, 257). However, where these overlap, we will also discuss mechanisms of injury to the other cell types.
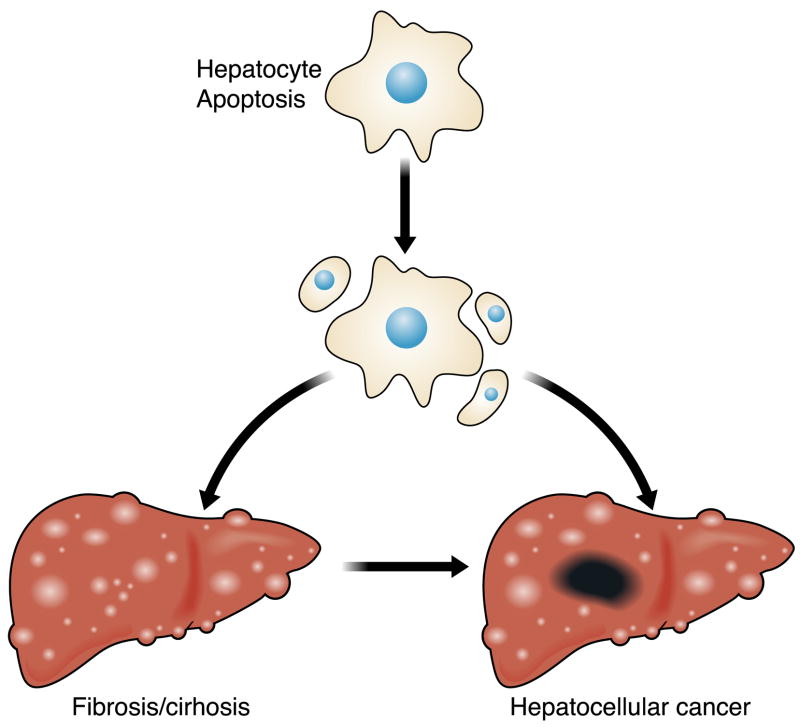
FIG. 1.
Hepatocyte apoptosis as a mechanism of liver injury and carcinogenesis. The precise mechanism(s) by which apoptosis promotes liver inflammation and fibrosis is unclear but well described in the literature. The cell turnover also provides a platform for cancer-initiating mutations, while the proapoptotic pressure is an impetus to develop mechanisms to avoid apoptosis (a hallmark of cancer).
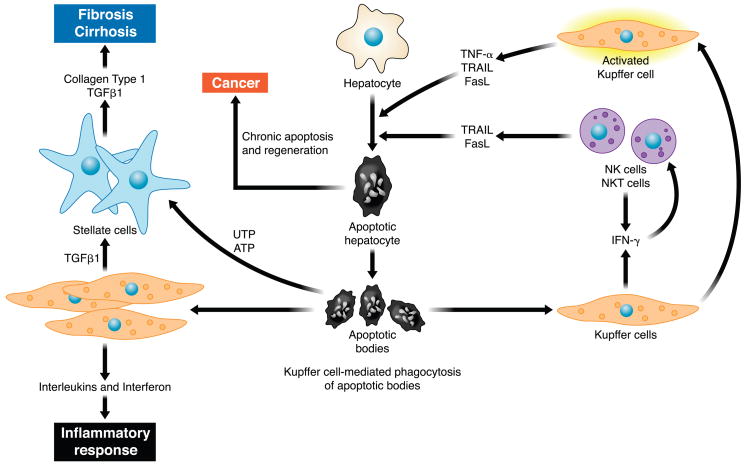
FIG. 2.
Cellular mechanisms of hepatic injury and fibrosis due to hepatocyte apoptosis. Hepatocyte apoptosis results in the formation of apoptotic bodies. Engulfment of apoptotic bodies by liver resident macrophages or Kupffer cells enhances their expression of death ligands such as TRAIL, Fas, and tumor necrosis factor (TNF)-α. These death ligands in a feed-forward loop further promote hepatocyte apoptosis and generation of apoptotic bodies. Engulfment of apoptotic bodies by stellate cells promotes their activation and enhances their development into myofibroblasts, which secrete collagen type 1 and transforming growth factor (TGF)-β1, promoting development of fibrosis and cirrhosis. Recently, apoptotic cells were shown to release the nucleotides UTP and ATP, which bind P2Y2 purinergic receptors on myofibroblasts enhancing their secretion of collagen. The constant cell turnover and proapoptotic pressure are also permissive for the development of hepatocellular carcinoma.
II. MODES OF CELL DEATH
Several modes of cell death have been classified by consensus agreement including apoptosis, necrosis, necroptosis, autophagy, and cornification (Table 1) (144). This classification of cell death is based primarily on morphological criteria, and each form of cell death is described in detail below, except cornification which is limited to skin tissue. Although the term programmed cell death is often used in the literature, this simply refers to a genetically controlled process. However, as genes modulate cell susceptibility to multiple processes including accidental cell death (115), the term lacks specificity and will not be used in this review.
TABLE 1.
Modes of cell death
| Cell Death Mode | Morphology | Inhibitor |
|---|---|---|
| Apoptosis | Cell shrinkage | Caspase inhibitor |
| Blocks within organelles | ||
| Nucleus fragmentation | ||
| Cell fragmentation | ||
| Necrosis | Cellular swelling | Necrostatin-1 |
| Blebs devoid of organelles | ||
| Moderate chromatin condensation | ||
| Rupture of plasma membrane | ||
| Autophagy | Cytoplasmic vacuolization | 3-Methyladenine |
| Double membrane vacuoles | ||
| No chromatin condensation |
A. Apoptosis
1. Definition and hepatic consequences of apoptosis
Apoptosis is derived from a Greek word that describes “leaves falling from a tree” and was first used by Kerr et al. (134) to define a specific morphological aspect of cell death characterized by membrane blebbing, shrinkage of the cell, chromatin condensation (pyknosis), and nuclear fragmentation (karyorrhexis). Scission of the cell into membrane defined bodies termed apoptotic bodies that often contain micronuclei (nuclear fragments) is a hallmark of apoptosis. These apoptotic bodies express “eat me” signals on the external cell membrane, such as phosphatidylserine, resulting in their engulfment by phagocytic cells (256). In the liver, both stellate cells and Kupffer cells, the resident macrophages of the liver, can phagocytose apoptotic bodies (31, 33, 124).
Hepatocyte apoptosis can be considered to be a pivotal step in most forms of liver injury (Fig. 1). Enhanced hepatocyte apoptosis is tightly coupled with inflammation and fibrosis (110, 252, 269). Hepatocyte injury and turnover are also essential for liver cell cancer, and cell deletion by apoptosis stimulates hepatocyte mitogenesis. For example, conditional deletion of a key anti-apoptotic protein in the hepatocyte, Mcl-1, results not only in hepatocyte apoptosis, but in the pressure for constant cell turnover which also promotes hepatocellular carcinogenesis. Apparently, the cell turnover due to apoptosis, the fibrotic extracellular matrix, and constant pro-apoptotic stimulus, are permissive for the development of liver cancer.
The mechanisms by which apoptosis promotes inflammation relate to the activation of the resident macrophages in the liver, the Kupffer cells (Fig. 2). Following engulfment of apoptotic bodies, Kupffer cells express the death ligands tumor necrosis factor (TNF)-α, TNF-related apoptosis-inducing ligand (TRAIL), and Fas ligand (FasL) (31), capable of inducing death receptor-mediated apoptosis in hepatocytes, which may further aggravate liver inflammation and fibrosis. Engulfment of apoptotic bodies by macrophages also induces FasL expression (135), which exerts a proinflammatory activity (41). As a consequence of chronic liver injury, hepatic stellate cells (HSC) undergo a process of activation (Fig. 2). When activated HSC engulf the apoptotic bodies resulting from hepatocyte apoptosis, they produce profibrogenic cytokines (such as transforming growth factor-β 1) and type I collagen (33). Recent information suggests that apoptotic cells also release the nucleotides ATP and UTP, which can bind to purinergic receptors on macrophages and HSC, especially the P2Y2 receptor (67). As P2Y2 receptors are present on HSC (64), release of nucleotides by apoptotic hepatocytes is likely an additional mechanism by which apoptosis promotes hepatic fibrosis. Therefore, emerging concepts implicate excessive apoptosis as a keystone in hepatic inflammation and fibrogenesis.
2. Caspases
Biochemically, apoptosis is commonly initiated and executed by activation of intracellular enzymes termed caspases as a contraction for cysteine-dependent aspartate specific protease (Fig. 3) (213). Like other intracellular proteases, caspases are synthesized as zymogens that must undergo proteolytic cleavage to exert proteolytic activity. Caspases have a dominant specificity for protein substrates containing four- or five-amino acid sequences containing an aspartate residue in the P1 position (the amino acid on the NH2-terminal side of the scissile bond), a unique feature of this class of proteases. Caspases themselves must be cleaved at aspartate residues for activation, and thus caspases are activated by other caspases. These proteases may be classified as initiator caspases, which contain a long prodomain for scaffolding with other proteins, and executioner (effector) caspases, containing a short prodomain. Initiator caspases include caspases 2, 8, 9, and 10. The initiator caspases 8 and 10 are involved in death receptor-mediated apoptosis, whereas caspase 9 initiates apoptosis following mitochondrial dysfunction. The precise role of caspase 2 in apoptosis remains unclear, but it has been implicated in cell death by endoplasmic reticulum stress (the unfolded protein response) and following DNA damage (147, 202, 266). Executioner caspases such as caspases 3, 7, and 6 are activated by cleavage by initiator caspases at aspartate residues and can be activated by either death receptor or mitochondrial pathways of apoptosis. These caspases may activate caspase-activated DNase (CAD) by cleaving ICAD, an inhibitor of this enzyme (256). CAD activation results in DNA cleavage at internucleosomal linker regions of DNA resulting in the ladder pattern of DNA cleavage (multiples of the 180-bp nucleosomal regions) thought to be characteristic of apoptosis. Caspase inhibitors attenuate hepatocyte apoptosis, especially that by death receptors, providing a tool to dissect the role of apoptosis in pathophysiological models (30). Also, caspase inhibitors are attractive agents for the treatment of human disease as they attenuate hepatocyte fibrosis by reducing apoptosis in preclinical models (30, 288).
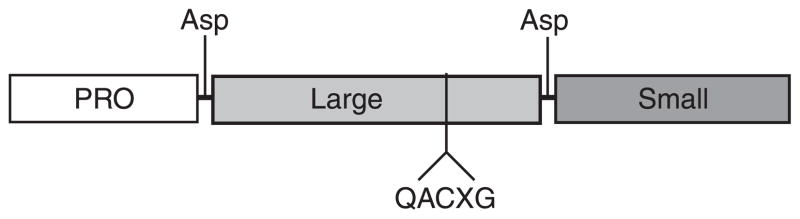
FIG. 3.
Caspase structure. Caspases consist of a catalytic domain organized in a large subunit (~20 kDa), a small subunit (~10 kDa), and a prodomain of variable length. Effector caspases exhibit a short prodomain, whereas initiator caspases have long prodomains that direct binding to protein complexes. Caspase-directed cleavages at aspartate residues in a linker region between the large and the small subunit and between the prodomain and the large subunit generate the formation of the 20- and 10-kDa polypeptides that oligomerize to form the heterotetrameric active form of protease. The catalytic cysteine is embedded in the conserved pentapeptide QACXG motif within the large subunit.
3. Mitochondrial outer membrane permeabilization and Bcl-2 proteins
The mitochondrial pathway of apoptosis is characterized by its regulation by the Bcl-2 protein family (see below) (52, 296). Pro-apoptotic members of this family result in mitochondrial outer membrane permeabilization (MOMP) releasing several activators of apoptosis into the cytosol including cytochrome c, second activator of mitochondrial apoptosis (SMAC), endonuclease G, high temperature requirement A2 (HrtA2), and apoptosis-inducing factor (AIF) (217). Egress of cytochrome c from the mitochondrial intramembrane space into the cytosol promotes formation of a protein complex comprising caspase 9 and apoptosis activating factor-1 (APAF-1) (52, 91). This complex termed the apoptosome results in caspase 9 activation leading to effector caspases 3, 7, and 6 activation and hepatocyte apoptosis.
The Bcl-2 (B-cell lymphoma-2) family is a group of proteins that regulate mitochondrial dysfunction during apoptosis. The family comprises both pro- and anti-apoptotic members interacting with each other and/or with the mitochondria to control the integrity of the outer mitochondrial membrane (OMM) (296). Bcl-2 proteins are divided into three subsets, based on number of Bcl-2 homology domains (BH1–4 domains) comprising the protein: 1) the anti-apoptotic members Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1, containing BH domains 1–4; 2) the pro-apoptotic multidomain effector proteins Bax, Bak, and Bok containing BH domains 1–3; and 3) the BH3-only pro-apoptotic members Bid, Bim, Bad, Bik, Bmf, Hrk, Noxa, and Puma, which function as initial sensors of different apoptotic signals. Despite this accepted classification based on their structure and function, the specific protein-protein interactions between members of the Bcl-2 family are still highly controversial. The pro-apoptotic proteins Bak, which normally resides in the OMM, and Bax, which translocates from the cytosol to the OMM after an apoptotic stimulus, are directly responsible for causing MOMP by inserting in the membrane after a conformational change and forming proteolipid pores (155). These proteins are essential for MOMP, as cells simultaneously lacking Bax and Bak are resistant to multiple pro-apoptotic stimuli (166, 279). However, the mechanism by which Bax and Bax are activated has not been completely clarified. In a model referred to as the “direct activation model,” a subset of BH3-only proteins termed “direct activators” (including Bid, Bim, and Puma) can either be sequestered by the pro-survival proteins, or bind directly to Bax and Bak, inducing their conformational changes and activation (136). All the other BH3-only proteins named “sensitizers” or “derepressors” only bind to the pro-survival proteins, thus lowering the capacity of the pro-survival proteins to sequester the direct activators or dissociating the pro-survival proteins from binding to and preventing activation of Bax and/or Bak (42) (Fig. 4). By whatever mechanism they function, the BH3-only proteins are key initiators of the mitochondrial cell death pathway.
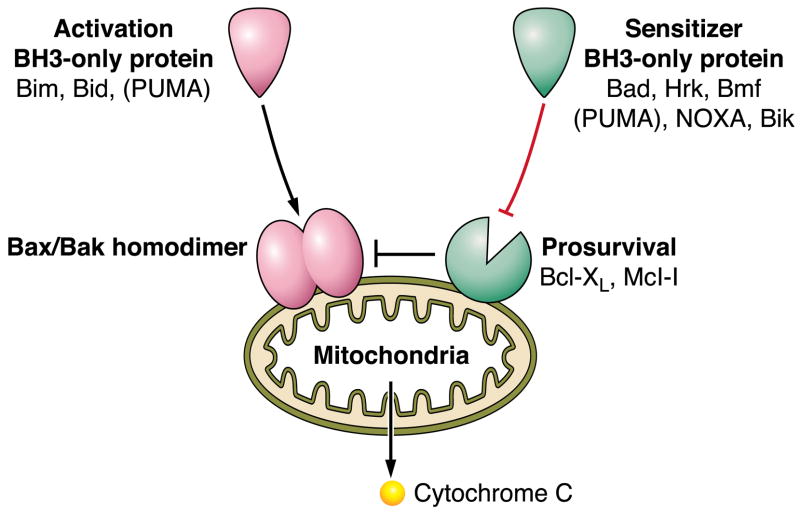
FIG. 4.
Bcl-2 family proteins regulate the mitochondrial pathway of apoptosis. Mitochondrial dysfunction is induced by homodimerization of Bax or Bak within the outer mitochondrial membrane. The homodimerization of these proteins can be directly stimulated by the BH3-only proteins Bim or Bid, and perhaps PUMA; hence, these three proteins are referred to as direct activators. Bax and Bak homodimerization is also kept in check by pro-survival Bcl-2 proteins, which in the hepatocyte are Bcl-xL and Mcl-1. The pro-survival function of these proteins can be repressed by the sensitizer BH3-only proteins Bad, Hrk, Bmf, NOXA, Bik, and PUMA. BH3-only protein repression of Mcl-1 and Bcl-xL then allows activation of Bax and/or Bak by an as yet unclear mechanism.
Bcl-2, the founding member of the Bcl-2 family, is not expressed in hepatocytes (39, 149, 263), while mice genetically deficient for Bcl-w or Bfl-/A1 have no specific liver phenotype under basal conditions (100, 214, 223). In contrast, mice in which either Bcl-xL or Mcl-1 has been conditionally deleted in hepatocytes have a pronounced and similar phenotype characterized by significant hepatocyte apoptosis. Indeed, although Bcl-xL and Mcl-1 are both multidomain anti-apoptotic proteins, hepatocyte deficiency in either one of these anti-apoptotic proteins results in spontaneous caspase 3/7 activation, hepatocyte apoptosis, and elevated levels of serum aminotransferases (252, 269). These observations are consistent with nonredundant, cytoprotective functions for both proteins in the hepatocyte, or with predominant expression of either Bcl-xL or Mcl-1 in individual hepatocytes, an un-likely but testable hypothesis. Alternatively, either Bcl-xL or Mcl-1 is modified in the hepatocyte such that it is incapable of maximal cytoprotection. Evidence for this latter concept exists as Bcl-xL is predominantly deamidated in the hepatocyte, a posttranslational modification which impairs its function (251). Inhibitory posttranslational modifications of Mcl-1 are also likely prevalent in hepatocytes. Thus the liver is subject to basal apoptotic stress which requires Mcl-1 plus Bcl-xL for survival. Liver injury in viral hepatitis may also occur as a result of viral proteins targeting Mcl-1 and/or Bcl-xL; for example, the hepatitis viral core protein may contain a BH3-only like domain which binds and targets Mcl-1 disabling its cytoprotective functions (195).
The death receptors Fas, TNF receptor 1 (TNF-R1), and TRAIL receptor 1/2 (TRAIL-R1/2) are major mediators of the apoptotic pathway in the liver. Upon stimulation by their cognate ligands, FasL, TRAIL, and TNF-α, respectively, the death receptors oligomerize and recruit different adaptor proteins which activate the initiator caspase 8 and likely caspase 10. Active caspase 8 cleaves the BH3-only protein Bid generating truncated Bid (t-Bid), which, following N-myristoylation (298), translocates to mitochondria and, in concert with active Bax and Bak, permeabilizes the OMM (Fig. 5). Bid is essential for Fas-mediated apoptosis in hepatocytes (294) and also appears to mediate hepatocyte apoptosis in Mcl-1 knockout mice (110). Since death receptors are ubiquitously expressed in the liver, hepatocytes may be subject to death receptor-induced Bid cleavage with subsequent Bax/Bak activation and cell death. Adequate cellular levels of the anti-apoptotic proteins Mcl-1 and/or Bcl-xL are, therefore, necessary to counteract the deleterious consequences of constitutive Bid activation.
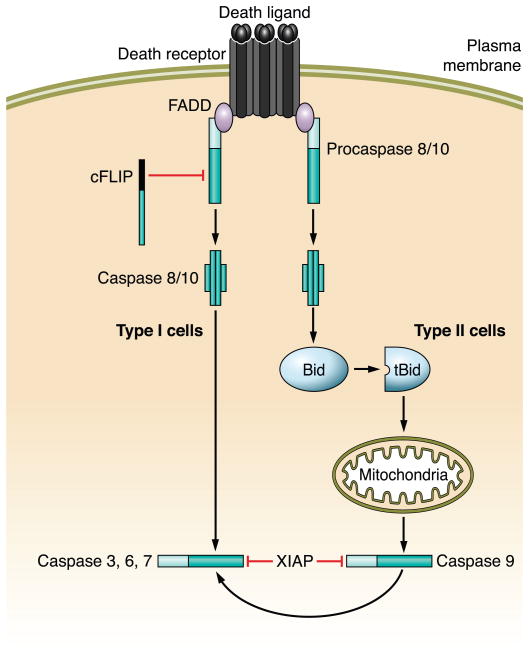
FIG. 5.
Death receptor signaling and its interdigitation with the mitochondrial pathway of apoptosis. Ligation of a death receptor by its ligand results in the recruitment of pro-caspases 8 and 10 to a receptor complex via homotypic interactions with FADD (or the other adaptor protein TRADD). Within this complex, caspases 8 and 10 undergo auto-activation. Active caspase 8/10 can directly activate caspase 3, an executioner caspase (type I cells), or cleave Bid, a BH3-only member of the Bcl-2 family, to generate truncated Bid (tBid) which in turn induces mitochondrial dysfunction (type II cells). X-linked inhibitor of apoptosis (XIAP) determines which pathway results in cell death. In hepatocytes, activation of the mitochondrial pathway is necessary to overcome resistance by XIAP.
B. Oncotic Necrosis
1. Definition and hepatic consequences of necrosis
Unlike apoptosis, which is mediated by well-defined pathways, necrosis is thought to represent in large part an accidental form of cell death with simultaneous disruption of multiple pathways (e.g., anoxic cell death). Necrosis is derived from the Greek “necros” for corpse (115) and is characterized by oncosis (Greek for swelling) and the formation of plasma membrane blebs, which in contrast to the blebs of apoptosis are devoid of organelles (89, 180). In addition to cell swelling, organelle swelling is also a morphological feature of oncotic necrosis. A cellular feature of necrosis is rupture of the plasma membrane which occurs during a “metastable state” resulting in bleb rupture (180). Thus necrosis is accompanied by complete release of cellular constituents into the extracellular environment, a pathological phenomenon that can elicit a significant inflammatory response. Classic examples of oncotic necrosis include the following: 1) ATP depletion such as occurs during ischemia or hypoxic cell injury; 2) excessive formation of reactive oxygen species as takes place during reperfusion of ischemic tissue; 3) toxins including acetaminophen and other xenobiotics; 4) overwhelming tissue injury as occurs in acute fulminant liver failure; and 5) sustained increases in intracellular calcium as defined in multiple experimental conditions (217). The mode of cell death by toxic stimuli is often concentration dependent, with low concentrations more likely to induce apoptosis and high concentrations necrosis. Thus many of the stimuli listed above may induce both modes of cell death dependent on the severity of the insult.
2. RIP kinases
The signaling cascades culminating in necrosis have been more difficult to elucidate compared with the ample information regarding these events in apoptosis. The BH3-only protein Bmf has been implicated in necrosis, suggesting that apoptosis and necrosis may share death mediators in their pathways (112). However, recent data suggest members of the receptor interacting protein (RIP) family contribute to necrosis (76). RIP1 and RIP2 interact with death receptors and caspases through shared death domains (DD) and caspase recruitment domains (CARD). RIP3 interacts with RIP1 via a RIP homotypic interaction motif (RHIM). Activation of RIP3 may be key in cell death by necrosis as this kinase is an energy metabolism regulator and its uncontrolled and sustained activation can deplete the cell of ATP (44, 299). Necrostatin-1 inhibits members of this family and reduces necrotic injury providing a new tool to help identify necrosis in pathophysiological models (60). How RIP3 may trigger the mitochondrial permeability transition remains unclear. No tissue-specific pathways regarding hepatocyte necrosis have been defined to date.
3. Mitochondrial permeability transition
Mitochondrial dysfunction is a cardinal feature of oncotic necrosis and is characterized by permeability of the outer plus the inner mitochondrial membranes (IMM) (180), a phenomenon termed the mitochondrial permeability transition (MPT) (16, 217). This is in contrast to apoptosis which is associated primarily with selective permeabilization only of the OMM (143). The loss of the IMM permeability barrier results in a collapse of ion gradients across the IMM driving the mitochondrial membrane potential to that of the cytosol (16). The loss of the mitochondrial membrane potential (Δψ; ~180 mV) and the pH gradient (alkaline to the cytosol) dissipates the proton-motive force preventing oxidative phosphorylation. In the absence of oxidative phosphorylation, cellular ATP levels fall precluding maintenance of ion pumps and other cellular processes, all culminating in cell blebbing and swelling. The molecular characterization of the permeability transition has remained elusive. Although the voltage-dependent ion channel (VDAC) and the adenine nucleotide transporter (ANT) have been implicated in the formation of a giant megachannel spanning both membranes, knockout of either gene does not preclude development of the MPT (217). In contrast, knockout of cyclophilin D does inhibit development of the MPT and more importantly attenuates ischemic tissue injury (217). Thus mitochondrial dysfunction is a crucial event in both apoptosis and necrosis, although the molecular mechanisms are quite dissimilar.
C. Necroptosis
1. Definition and hepatic consequences of necroptosis
Necroptosis is a form of death receptor-mediated cytotoxicity occurring in cells in which caspase 8 is deleted or inhibited (82). By morphology, necroptosis resembles oncotic necrosis, although it is initiated by a classic apoptotic cue. RIP kinases, which are recruited to death receptor complexes, initiate and help execute the necrotic phenotype (57). However, given that caspase 8-deficient mice are resistant to Fas- and TNF-α-mediated cell death (132), it is unlikely that hepatocytes intrinsically undergo necroptosis, although this mode of cell death may potentially occur in pathophysiological conditions.
D. Autophagy
1. Definition and hepatic consequences of autophagy
Autophagy (self-digestion) is a catabolic cellular process dependent on lysosomes for its execution (193). Catabolism within a cell is heterogeneous, and in this context, many different forms of autophagy have been defined. Microautophagy refers to invagination of the lysosomal membrane with engulfment of its cytosolic contents, followed by pinching off into the lysosome for degradation. In contrast, macroautophagy involves formation of a double membrane-bound structure within the cytoplasm, which encloses cellular organelles or cytoplasm, thus forming the autophagosome. The autophagosome then fuses with the lysosome, its outer membrane becomes part of the lysosome, and the inner membrane-bound cargo is degraded within the lysosome. Chaperone-mediated autophagy involves direct transport of soluble proteins with the consensus pentapeptide sequence KFERQ via the chaperone heat shock cognate 70 (hsc70) to the lysosomal docking protein lysosome-associated membrane protein 2A (LAMP2A) followed by translocation into the lysosome for degradation. Much has been learned about autophagy in the preceding decade, primarily on a cellular level. Advances are being made in understanding its role at an organismal level.
Macroautophagy (referred to as autophagy hereafter) leads to degradation of obsolete organelles and cytosol; however, it also occurs under conditions of nutrient deprivation. In a homeostatic sense, autophagy allows recycling of cellular constituents and, under conditions of starvation, promotes cellular survival by catabolism. Genetic screens in yeast have led to the identification of autophagy-related genes (Atg), starting with Atg1. Subsequently, mammalian autophagy genes have been identified as well. This has been followed by advances in understanding of the formation of autophagosomes within cells, regulation of autophagy, and its roles in pathophysiology. Autophagy has since then been linked to cell survival under nutrient deprivation and growth factor withdrawal (21, 172) and is under the inhibitory control of mTOR (mammalian target of rapamycin) (172). Autophagy has also been linked to cell death, innate immunity, adaptive immunity, and tumorigenesis. Morphologically, autophagic cell death is defined by the massive cytoplasmic accumulation of autophagosomes in dying cells without chromatin condensation, a hallmark of apoptosis (144). Although autophagosomes have been observed in many dying cells, this association does not imply that autophagy is mediating their cell death. Indeed, autophagy is protective in the setting of dying cells (28). In vivo, developmental cell death of the larval salivary gland of Drosophila occurs by autophagy (156). In the liver, autophagy is a prominent survival mechanism under nutrient deprivation (293). Aggregation-prone mutant α1-antitrypsin is also cleared from the liver by autophagy (208). Mice deficient for Atg-7 in the liver demonstrate impaired adaptation to starvation, organelle turnover, accumulation of aggregated proteins and organelles, and hepatomegaly (140). Recent studies have demonstrated a role for autophagy in lipid metabolism and storage (240, 241); however, its role in fatty liver disease is unclear. Thus, overall, autophagy seems to be a pro-survival, protective pathway in the liver, and its role in liver injury, if any, remains to be elucidated.
III. BASIC CONCEPTS OF HEPATOCYTE INJURY
Disturbances of hepatic function and/or accentuated activation of the innate immune system cause liver stress, endangering hepatocyte survival. Indeed, biomarkers of hepatocyte injury such as serum aminotransferases are present in all humans. What constitutes an elevation of these biomarkers and, therefore, the cutoffs for a “healthy liver” are uncertain and a subject of clinical interest (137). However, the mere presence of aminotransferases in the circulation implies basal hepatocyte stress and turnover, and perhaps some level of hepatocyte injury is simply to be expected given the magnitude and variety of hepatocyte functions.
A. The Hepatocyte and Its Hostile Environment
Liver damage is common in medical practice given the worldwide use of alcohol, pharmacological use of drugs and medicinal use of unregulated compounds (e.g., herbal compounds, weight loss supplements, etc.), hepatotropic viruses, attack by free fatty acids in the metabolic syndrome, immune-mediated inflammatory processes afflicting the liver, genetic conditions, and other less common disease states. These pathobiological conditions and the unique features of the liver that render it susceptible to injury are summarized below.
1. Portal blood flow, xenobiotic biotransformation, and hepatotoxicity
Xenobiotics (chemical compounds, such as a drug or ethanol, that are foreign to the body of a living organism) are a common cause of liver injury. The susceptibility of the liver to xenobiotic-mediated injury is due to the unique vascular and metabolic features of the liver. Approximately 75% of hepatic blood flow comes directly from the gastrointestinal viscera and spleen via the portal vein; the other 25% of hepatic blood flow is derived from the hepatic artery. Portal blood is enriched in ingested xenobiotics absorbed by the gut which are delivered at high concentrations to the liver. The liver has evolved to defend the organism from the unintended xenobiotic exposure by developing a complex array of detoxifying enzymes including hydroxylation, oxidation, and conjugation reactions (131). Although this detoxification system renders many xenobiotics harmless and facilitates their elimination from the body, it also has the potential to convert chemical compounds into highly reactive intermediate compounds. These reactive molecules can be quite injurious to the liver. An example of liver injury by this mechanism is acetaminophen (121) (Fig. 6).
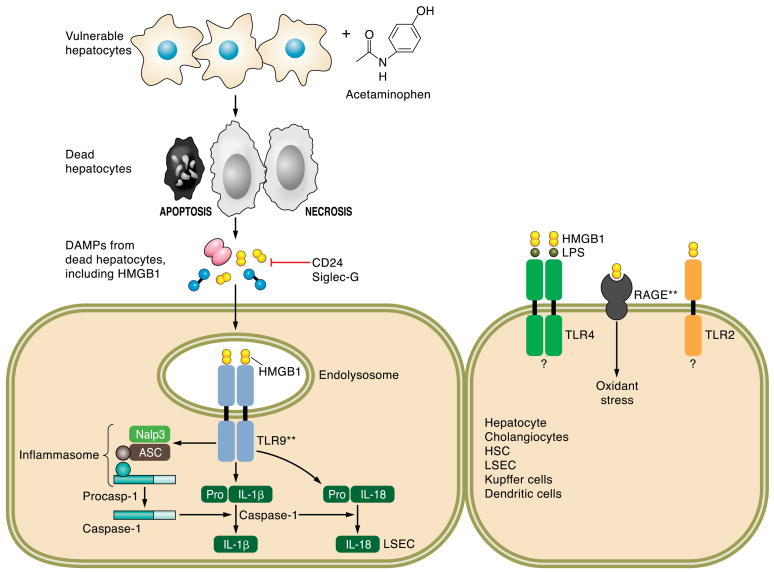
FIG. 6.
Danger associated molecular patterns (DAMPs) and acetaminophen-induced liver injury. DAMPs are intracellular signals released from dying cells, both necrotic and apoptotic cells. Nuclear and cytoplasmic DAMPs including the protein HMGB-1, nucleosides, uric acid, and heat shock proteins are released from dying cells. These DAMPs activate the innate immune system and initiate a secondary cascade of inflammation and injury. In the case of acetaminophen (AAP), animals models have elucidated a role for HMGB-1-initiated signaling events indicated in this figure with double asterisk (**). HMGB-1, in a TLR9-dependent manner, activates the inflammasome consisting of Nalp3 (NACHT, LRR, and pyrin domain-containing protein 3), ASC (apoptosis-associated speck-like protein containing CARD), and procaspase 1. Activation and autoproteolytic cleavage of procaspase 1 yields active caspase 1 in the inflammasome. Independent of the inflammasome, HMGB1-induced TLR9 activation results in enhanced expression of pro-interleukin (IL)-1β and pro-IL-18. Cleavage of pro-IL-1β and pro-IL-18 to active IL-1β and IL-18 is catalyzed by caspase 1. CD24 and Siglec-G are negative regulators of HMGB1-activated innate immune responses. HMGB-1 can bind LPS and enhance TLR4 activation, as well as activate TLR2; however, the contribution of these two pathways to AAP-induced liver injury has not yet been elucidated.
Acetaminophen, when ingested in amounts that overwhelm conjugation reactions, is converted in the hepatocyte to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which results in depletion of hepatocyte glutathione stores rendering the hepatocyte vulnerable to oxidative stress. NAPQI can also react with proteins in a process termed protein arylation. The resultant oxidative stress and protein dysfunction causes mitochondrial dysfunction and activation of other deleterious intracellular cascades culminating in loss of cell viability by both necrosis and apoptosis (141). Aiding the ability of the hepatocyte to generate glutathione by supplying exogenous N-acetylcysteine is a clinical strategy to ameliorate acute acetaminophen intoxication (243). What is most interesting is that the initial wave of hepatotoxicity by acetaminophen is likely not sufficient to cause fatal liver injury; rather, the secondary activation of the innate immune system is what most likely destroys the liver. Hepatocyte death by necrosis releases intracellular constituents such as DNA, uric acid, and nucleotides that are recognized by the innate immune system as danger-associated molecular pattern (DAMPs) signals (169) (Fig. 6). In particular, DAMPs result in activation of the inflammasome which consists of NOD-like receptors (NLR) (185). Acetaminophen hepatoxicity results in activation of the NALP3 inflammasome, a protein complex which serves to proteolytically activate the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 (185). These pro-inflammatory cytokines then induce a second wave of hepatoxicity that results in liver failure (176). Indeed, animals genetically deficient for IL-1β are resistant to death from acute acetaminophen hepatoxicity (117). Acetaminophen toxicity is an example of the complex but deadly interactions between xenobiotic metabolism and the hepatic innate immune system.
Alcohol (ethanol; also a xenobiotic) consumption is common in many societies and can be associated with severe liver injury in a subset of heavy consumers. Alcohol induces expression of the detoxifying enzyme Cyp2E1 which generates reactive oxygen species as it metabolizes its substrates (171). Alcohol induction of Cyp2E1 can also predispose to xenobiotic associated liver injury; for example, chronic alcohol use sensitizes the liver to injury by acetaminophen by enhancing its biotransformation to toxic intermediary compounds (53). Alcohol also modulates the cytokine environment of the liver and evokes the expression of the inflammatory cytokines TNF-α, IL-1, and IL-6 (181). These cytokines are likely generated through Toll-like receptor 4 on Kupffer cells (181) (Fig. 7). Alcohol-associated liver injury also represents a complex, and at this time poorly understood, relationship between biotransformation and the innate immune system. Unfortunately, unlike other human inflammatory diseases, alcohol-induced acute liver injury is not improved by anti-TNF-α directed therapy (24), and thus, considerable more work is necessary to understand this disease process.
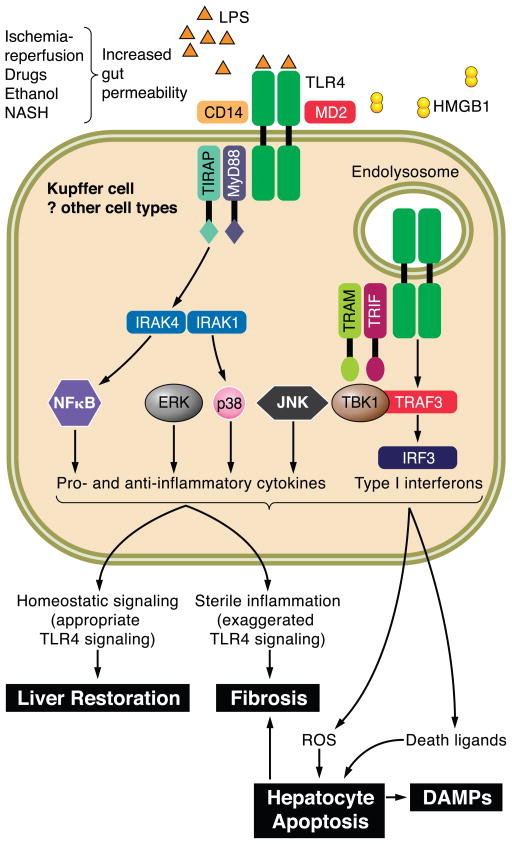
FIG. 7.
Toll-like receptor 4 (TLR4) signaling in Kupffer cells. Shown here is a Kupffer cell with cell surface TLR4 expression. Upon ligation with lipopolysaccharide, a process facilitated by the coreceptors CD14 and MD2 as well as LPS-binding protein, the proximal adaptor molecules MyD88 and TIRAP are recruited, activating kinase cascades that result in activation of NF-κB, and the MAPK ERK1, p38, and JNK. Subsequent TLR4 internalization and recruitment of the adaptor proteins TRIF and TRAM activate type 1 interferon production. Initial TLR4 activation can occur downstream of changes in gut permeability that permit greater translocation of bacterial LPS. TLR4 can also be activated by DAMPs, including HMGB-1 released from dying cells. Upon activation of Kupffer cells, many pro- and anti-inflammatory cytokines are produced, which serve a physiological as well as pathological role in the liver. Further recruitment of leukocytes with amplification of cytokine production occurs. Death ligands (FasL and TRAIL) activate death receptor-mediated hepatocyte apoptosis. Oxidant stress can also lead to cell death. DAMPs released from dying cells further accentuate TLR4 signaling. TGF-β produced by Kupffer cells promotes the activation of hepatic stellate cells, although multiple other signals also govern this complex process, including engulfment of apoptotic hepatocytes and other TLRs.
2. Free fatty acids as toxic endobiotics
Excessive serum concentrations of free fatty acids (FFA) may be classified as potentially toxic nutrients or toxic endobiotics (compounds endogenous to a living organism). The metabolic syndrome, characterized by obesity and insulin resistance, is common in western societies. This medical condition is associated with elevated levels of circulating FFA (150). The excess serum FFA in the context of insulin resistance saturates the transport and storage capacity of adipocytes leading to their uptake by ectopic sites, including the liver. The surfeit of FFA results in their participation in nonoxidative pathways with potential deleterious consequences for the hepatocyte resulting in nonalcoholic fatty liver disease (NAFLD) (179). Indeed, this is currently the most common form of liver injury in Western societies afflicted with a rising incidence of obesity.
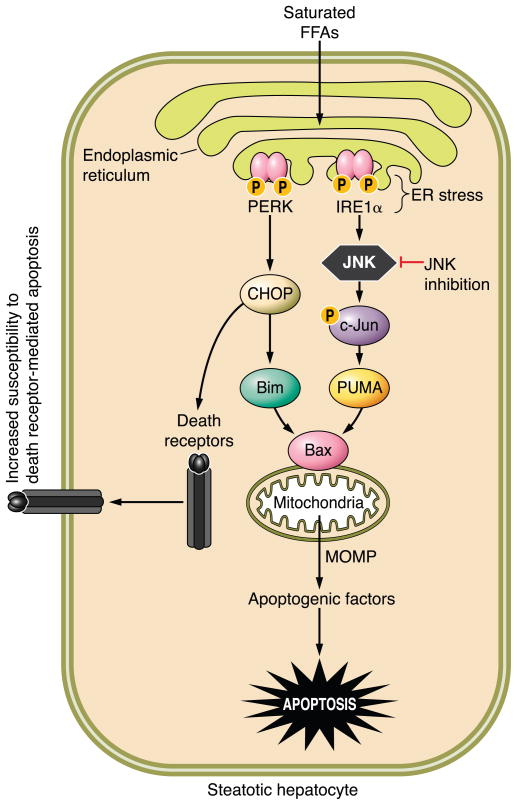
A subset of NAFLD individuals develop severe hepatic lipotoxicity, a syndrome referred to as nonalcoholic steatohepatitis (NASH) (1), which can progress to cirrhosis and its chronic sequela (66, 218). Although the liver in this syndrome is characterized by steatosis, current concepts indicate that FFA, and not their esterified product (triglyceride), mediate hepatic lipotoxicity as inhibitors of triglyceride formation actually potentiate liver injury (167, 290). Elevated serum FFA levels also correlate with liver disease severity (5, 55, 200). In vitro studies suggest saturated FFA, such as palmitic acid or stearic acid, as opposed to unsaturated FFA, such as oleic acid, are more pro-apoptogenic (84, 276). Consistent with FFA-mediated hepatocyte apoptosis as a pathogenic mechanisms in NASH, human NASH is characterized by increased hepatocyte apoptosis (71), and serum biomarkers of apoptosis are useful for identifying NASH in humans (285). Thus NASH appears to be, in part, a disease process due to FFA-mediated hepatocyte apoptosis.
3. Bile acids as toxic endobiotics
Bile acids are amphipathic planar molecules synthesized from cholesterol within the hepatocytes. After their synthesis, these compounds are secreted into the bile and enter the proximal small intestinal lumen where they participate in digestion. Bile acids are reabsorbed in the terminal ileum and are efficiently transported from the portal vein back into the hepatocyte, a physiological process termed the enterohepatic circulation (257). In bile, their concentrations are in the millimolar range, a relatively toxic concentration for these bioactive molecules. Bile acid homeostasis is, therefore, tightly regulated in health, and their cellular and tissue concentrations are restricted. However, in cholestatic conditions, their secretion into bile is impaired, resulting in their hepatic retention and accumulation. In these cholestatic conditions, hepatocytes are exposed to toxic bile acid concentrations that trigger hepatocyte injury. Thus any cholestatic process, regardless of its etiopathogenesis, can be associated with a secondary injury related to bile acid toxicity (107).
Primary bile acids, those synthesized by the liver, include the trihydroxy bile acid cholate and the dihydroxy bile acid chenodeoxycholate. Either bile acid can be conjugated to glycine or taurine (113). Chenodeoxycholate is more toxic than cholate, and glycine conjugates are more toxic than taurine conjugates (95). The toxicity profile mirrors their hydrophobicity with glycine conjugates of dihydroxy bile acids being more hydrophobic than the taurine conjugates of trihydroxy bile acids. Bile acids induce cell toxicity by promoting death receptor-mediated apoptosis by enhancing their expression and trafficking to the plasma membrane (107, 108, 245) (Fig. 8). Ursodeoxycholate, a epimer of chenodeoxycholate, has hydrophilic properties and is able to ameliorate liver injury by toxic bile acids (4). Indeed, ursodeoxcholate is used clinically in the therapy of primary biliary cirrhosis and other cholestatic liver diseases (163).
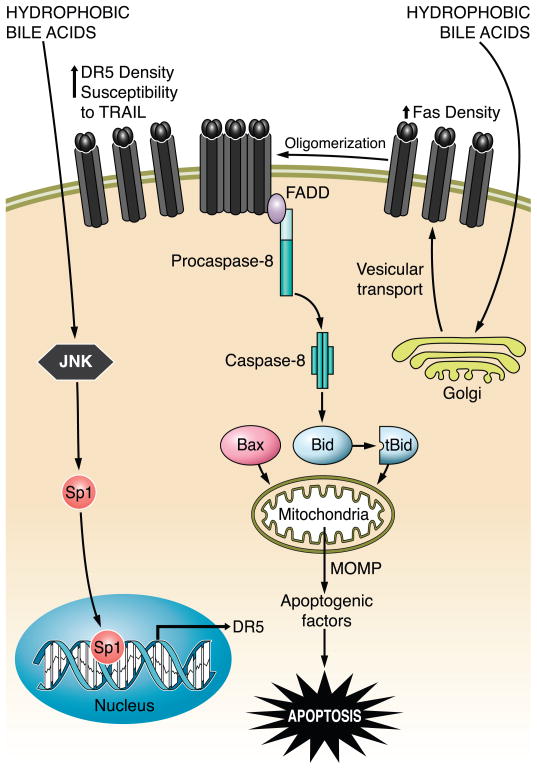
FIG. 8.
The bile acid-induced/death receptor-mediated apoptosis pathway. Hydrophobic bile acid stimulates Golgi-associated and microtubule-dependent Fas trafficking to plasma membrane, resulting in an increased density of cell surface Fas. In a FasL-independent manner, Fas undergoes oligomerization and recruits FADD, which, in turn, binds to pro-caspase 8 and facilitates its activation by autocatalytic processes. Active caspase 8 cleaves Bid, whose truncated form translocates to mitochondria and cooperates with Bax to induce MOMP. This results in release of apoptogenic factors, such as cytochrome c, AIF, and SMAC, into the cytosol, which ultimately promote the activation of effector caspases 3, 6, and 7 and cell death. At the same time, hydrophobic bile acids also cause upregulation of death receptor 5 (DR5) via JNK-mediated activation of the transcription factor Sp1 (specificity protein 1), sensitizing the hepatocytes to TRAIL-induced apoptosis.
4. Hepatotropic viruses
The liver is frequently afflicted by hepatotropic viruses. Acute liver injury can occur with infection due to hepatitis A virus, hepatitis E virus, cytomegalovirus, herpes simplex virus, and Epstein Barr virus. Chronic injury by hepatitis C virus (HCV) and hepatitis B virus (HBV) is even more common (68). HCV, an RNA virus, is usually acquired postnatally by parental exposure and results in chronic hepatitis in the majority of individuals. It frequently leads to liver cirrhosis, hepatic failure, and need for liver transplantation (29). HBV, a DNA virus, is commonly transmitted vertically at the time of birth in endemic areas of the world, but can also be transmitted parentally including sexual transmission (153). Like HCV, it also can chronically infect the host, eliciting chronic liver injury leading to cirrhosis and the sequelae of advanced liver disease. Hepatic injury by HCV and HBV is common worldwide. Liver injury in both HBV and HCV is thought to be mediated by adaptive and innate immune responses, as both immune responses can induce target cell apoptosis, a feature common in both viral liver diseases (25, 73).
B. The Liver as a Component of the Innate Immune System
The liver is the interface of the human biosphere where ingested nutrients and xenobiotics and gut-derived endobiotics borne by the portal vein encounter the internal milieu. Thus for the liver to possess innate immune functions seems teleologically sound. The microanatomy of the hepatic sinusoid is engineered to optimally present portal blood to hepatocytes and other cells of the innate immune system. Cell types that participate in innate immune responses in the liver include hepatocytes, cholangiocytes, HSC, liver sinusoidal endothelial cells (LSEC), dendritic cells, Kupffer cells, NK cells, and NKT cells. These cell types produce immunomodulatory cytokines, chemokines, acute phase proteins, complement, death ligands, and inflammatory mediators. Kupffer cells, HSC, LSEC, cholangiocytes, and dendritic cells can function as antigen presenting cells, and NK cells perform tumor and virus surveillance. Hepatocytes produce and secrete complement, acute phase proteins, and many cytokines including TNF-α and IL-6. LSEC function as efficient scavengers and tolerogenic antigen presenting cells (63, 271). Several subsets of dendritic cells exist in the liver, although in low numbers in normal liver (161). HSC also function as antigen presenting cells for stimulation of NKT cells as well as CD8+ and CD4+ T cells and participate in protective immunity against bacterial infections (287). Kupffer cells are the most numerous antigen-presenting cells in the liver; in addition, they participate in the clearance of apoptotic hepatocytes via phagocytosis, a process that promotes their activation and secretion of inflammatory cytokines (31).
The innate immune system in the liver displays several unique properties. It exhibits tolerance to a vast variety of stimuli, including lipopolysaccharide, while maintaining a rapid response to pathogens. In chronic viral infection, the innate immune system is undermined by viral factors, and after orthotopic liver transplantation, allograft tolerance can develop. Activation of the innate immune system is evident in acute and chronic liver diseases of varied etiology, in keeping with the defining features of innate immunity, i.e., nonspecificity, inborn and initial response. However, in the liver, even in chronic hepatitis, the ongoing activation of the innate immune system plays a key role in perpetuating inflammation. Utilizing dual models of acetaminophen-induced acute liver injury in mice, which results in predominantly necrotic cell death, as well as intraportal apoptotic hepatocyte DNA delivery, the role of toll-like receptor 9 (TLR9) in mediating liver inflammation, presumably via DAMPs released by dead cells, has been demonstrated (117). Furthermore, the release of DAMPs from damaged hepatocytes in acetaminophen-induced liver injury, and the activation of Kupffer cells by these signals has recently been demonstrated (184). The role of NK cells and NKT cells in secretion of interferon-γ (IFN-γ), neutrophil infiltration, chemokine secretion, and death ligand secretion has previously been demonstrated (168). Chronic inflammation is a hallmark of metabolic syndrome-associated nonalcoholic fatty liver disease (151, 159). Ethanol affects many components of the innate immune system, and chronic ethanol exposure promotes a proinflammatory milieu in the liver (152, 249). In chronic hepatitis C infection, NK cells promote inflammation and may facilitate persistence of infection (2). HBV can also activate the innate immune response in infected humans (77).
Recognition of immunogenic signals by the innate immune system is orchestrated via pathogen recognition receptors (PRR) that recognize a series of conserved microbial pathogen-associated molecular patterns (PAMPs). Similarly, damaged cells display DAMPs permitting their recognition by PRR. The PRR can be cytoplasmic or transmembrane. Cell membrane and cytoplasmic toll like receptors (TLRs), some members of the cytoplasmic nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and cytoplasmic retinoic acid-inducible gene-1 (RIG-1)-like helicases (RLHs) recognize bacterial and viral products such as lipoproteins, peptidoglycans, double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), short double-stranded RNA, and hypomethylated DNA (18, 118, 295). Interactions between these diverse pattern recognition receptors and their ligands can be promiscuous or specific, e.g., TLR4 is activated by the bacterial endotoxin lipopolysaccharide (LPS), and RIG-1 serves as a promiscuous sensor for intracellular dsRNA. DAMPs are intracellular molecules within intact cells, distinct from PAMPs, which have an immunomodulatory function. Several intracellular DAMPs, including uric acid, heat shock protein 70, high mobility group box (HMGB)-1, and genomic double-stranded DNA, have been identified (142). DAMP-activated cell signals are distinct from PAMP-activated cell signals, as demonstrated in mice lacking CD-24 (40). These mice develop an exaggerated inflammatory response upon acetaminophen-induced liver injury, without exhibiting enhanced sensitivity to LPS. An integrated example of how these processes interdigitate to produce acetaminophen-related liver injury is depicted in Figure 6.
IV. PATHOBIOLOGY OF HEPATIC APOPTOSIS AND DISEASE RELEVANCE
A. Death Ligands and Receptors
1. Definition
Death receptors are a subgroup of the TNF/nerve growth factor (NGF) receptor superfamily (96). These cytokine receptors are type I transmembrane proteins (single membrane-spanning proteins with an extracellular NH2 terminus) with three distinct regions: an extracellular, NH2-terminal ligand-interacting domain characterized by serial cysteine-rich repeat domains, a membrane-spanning region, and an intracellular COOH-terminal domain characterized by a stretch of ~60 – 80 amino acids known as the “death domain” necessary for the initiation of cytotoxic signals when engaged by cognate ligands. Some death receptors, namely, Fas (CD95/APO1), TNF-R1 (p55/CD120a), TRAIL-R1 (death receptor 4-DR4), and TRAIL-R2 (death receptor 5-DR5/APO-2/KILLER), are ubiquitously expressed in the liver (69). These receptors bind to specific ligands, the majority of which are type II transmembrane proteins (single membrane-spanning proteins with an extracellular COOH terminus) belonging to the TNF family (96). Engagement of death receptors by their corresponding ligands (FasL, TNF-α, and TRAIL, respectively) triggers the so-called extrinsic pathways of apoptosis, a signaling cascade resulting in activation of effector caspases and cell death. In liver cells, the extrinsic pathway often partially overlaps with the intrinsic/mitochondrial pathway via cleavage and activation of Bid, which, in turn, results in MOMP and release of pro-apoptogenic factors (Fig. 5). Because of its high exposure to toxic endobiotics, xenobiotics, and viruses, the liver has developed a sophisticated defense system to ensure the efficient removal of damaged or virus-infected cells in a tightly controlled manner, and the death receptors are a fundamental tool in this process. However, the high level of expression of death receptors can also work as a double-edged sword, as it renders the liver more susceptible to excessive apoptosis by this pathway. Indeed, every death receptor expressed in the liver has been implicated in liver injury in different pathological settings. The signaling pathways activated by these death receptors and their ligands, as well as their role in the pathophysiology of the liver, are discussed in greater detail below.
2. Fas signaling
Fas is constitutively expressed by every cell type in the liver (69). Its ligand, FasL, is mainly expressed as a transmembrane protein on the cell surface of activated cytotoxic T lymphocytes (CTL), which play a pivotal role in the removal of Fas-overexpressing, virus-infected, or mutated liver cells from healthy livers (15, 170). In certain pathological conditions, such as alcoholic hepatitis and Wilson’s disease, FasL can also be expressed by hepatocytes and induce fratricide cell death of Fas-expressing hepatocytes, amplifying the tissue damage (80). Kupffer cells also express FasL in response to engulfment of apoptotic bodies, a process that may, under pathological conditions, exacerbate hepatocyte apoptosis and liver injury and promote liver inflammation and fibrosis (31).
The trimeric FasL binds to preoligomerized Fas on the plasma membrane to initiate the signaling cascade (239). Ligand binding induces a conformational change in the receptor intracellular domain which results in recruitment of the adaptor protein FADD (Fas-associated protein with death domain) and pro-caspase 8 and/or 10, after relocalization of the receptor to lipid rafts (96). Multiple receptors are then recruited to the lipid rafts to form larger aggregates that are subsequently internalized via clathrin-mediated endocytosis and delivered to the early endosomal compartment, a step required for efficient complex formation and amplification of the death signal (157). This complex, comprising Fas, FasL, FADD, and caspase 8, is referred to as death-inducing signaling complex (DISC). The long and short splicing variants of cellular FLICE/caspase 8 inhibitory protein (c-FLIPL and c-FLIPS) can also be recruited to the Fas DISC (259); they generally exert an anti-apoptotic function by antagonizing caspase 8 activation, although c-FLIPL has also been shown to promote Fas-induced caspase 8 activation through the formation of caspase 8:cFLIPL heterodimers (36, 189). High local concentrations of caspase 8 led to its activation by self-processing, which, in turn, start a signaling cascade resulting in the activation of effector downstream caspases, such as caspases 3, 6, and 7 (227, 237). However, the pathway to activate effector caspases differs in distinct cell types (228) (Fig. 5). In the so-called type I cells, such as lymphocytes and thymocytes, active caspase 8 proceeds to directly cleave and activate downstream effector caspases; mitochondria are not required for caspase activation in these cells but are engaged later in an amplification loop to maximize the death signal. Indeed, these cells undergo apoptosis even when the anti-apoptotic proteins Bcl-2 and Bcl-xL are overexpressed (229). Conversely, type II cells, such as hepatocytes, depend on caspase 8 cleavage of Bid and consequent mitochondria dysfunction to activate the effector caspases; consistently, Bid knockout hepatocytes are resistant to Fas-mediated apoptosis whereas thymocytes are sensitive (294). As expected, Fas-mediated apoptosis can be blocked by overexpression of Bcl-2 or Bcl-xL in type II cells (229). This selective response to Fas stimulation was initially attributed to the levels of activated caspase 8 generated at the DISC, with large amounts of caspase 8 being able to bypass the mitochondria and directly activate the effector caspases, and small amounts being sufficient to cleave Bid and engage the mitochondria, but not to activate the effector caspases (228). However, recent studies suggest that XIAP (X-chromosome linked inhibitor of apoptosis protein) may be the critical discriminator between type I and type II apoptosis signaling (127) (Fig. 5). Indeed, XIAP is stabilized by association with active caspase 3 in wild-type mouse hepatocytes during Fas-mediated apoptosis, preventing further progression of the apoptotic cascade, and cell death can only be achieved after mitochondrial dysfunction and release of the endogenous XIAP inhibitor SMAC/DIABLO (second mitochondria-derived activator of caspases/direct IAP-binding protein with low pI), which overcomes this blockage. However, when the mitochondrial pathway is inhibited, such as in Bid-deficient hepatocytes, Fas-mediated activation of effector caspases and apoptosis can be induced by depletion of XIAP, suggesting that effector caspases are indeed activated directly by caspase 8, but are inhibited by binding to XIAP. Loss of XIAP, therefore, turns hepatocytes into type I cells (127). Indeed, this pathway is so critical for hepatocyte apoptosis that mutants of cytochrome c (KA allele) which do not bind APAF-1 (resulting in failure to activate caspase 9) do not inhibit Fas-mediated liver injury (102). Finally, it should be noted that the dependence of hepatocytes on Bid for Fas-mediated cell death is a plastic phenomenon; in vitro cultured murine hepatocytes may resemble type I cells, and high concentrations of FasL may also result in type I signaling in vivo (232, 274).
3. Fas in liver pathobiology
Dysregulation of Fas apoptosis has been associated with several liver diseases (81). Toxic bile acids accumulating within the hepatocyte in cholestatic disease are known to induce hepatocyte apoptosis in a Fas-mediated, FasL-independent manner (70). Elevated intracellular concentrations of bile acids can induce Fas translocation from the cytosol and Golgi complex to the plasma membrane, where the increased surface density facilitates its oligomerization and initiation of the apoptotic signal (245) (Fig. 8). Consistently, Fas-deficient lpr mice are more resistant to liver injury after bile duct ligation (192).
Liver injury in viral hepatitis, both from HBV and HCV, is characterized by increased apoptosis; however, apoptosis is not caused by the virus, which by itself has a moderate cytopathic effect on the host cell, but rather by the host immune response to viral antigens. Although Fas-mediated apoptosis is not the only death pathway activated in viral hepatitis, it certainly plays a crucial role in the elimination of infected cells by CTL, as demonstrated by areas of FasL-positive infiltrating mononuclear cells in the liver of HBV- and HCV-infected patients (80, 111, 194). Consistently, Fas expression is increased in the liver of patients with chronic hepatitis B and C, although it is not clear whether this is regulated by viral proteins or by the inflammatory cytokines (80, 111, 174, 194). Fas-mediated apoptosis is also enhanced in the liver of patients with alcoholic hepatitis, where expression of both Fas and FasL is elevated compared with healthy livers, rendering the hepatocytes more sensitive to Fas-mediated apoptosis both by CTL and by autocrine and/or paracrine mechanisms (199). Finally, Fas expression and hepatocyte apoptosis are elevated in the liver of NASH patients, and sensitivity to Fas is increased in steatotic livers compared with normal livers, suggesting Fas-mediated cell death is an important feature of NASH (71, 72). Whereas increased Fas apoptosis is associated with liver diseases, the opposite is also true. Downregulation of Fas or inactivating mutations in the Fas death domain are, indeed, very frequent in human tumors, including hepatocellular carcinoma, and are associated with increased resistance to Fas-mediated apoptosis and poor prognosis (105, 120, 247, 291). Interestingly, although congenital or somatic mutations in the Fas death domain are fairly common, tumors very rarely display loss of heterozygosity, suggesting that maintaining one wild-type receptor may confer an oncogenic advantage (209). This advantage could be explained by the evidence that Fas-induced apoptosis requires two functional Fas alleles to ensure efficient DISC formation, whereas, in contrast, only one functional allele is sufficient to activate NF-κB and promote cell survival (158).
4. TRAIL signaling
DR4 (TRAIL-R1) and DR5 (TRAIL-R2) trigger apoptosis upon engagement by their natural ligand TRAIL (206, 207). Although both DR4 and DR5 are expressed in the liver at comparable levels (88), TRAIL binding affinity seems to be higher for DR5 at physiological temperatures (258), and, consistently, DR5 generally plays a more important role in TRAIL-mediated apoptosis (133). In addition, TRAIL also binds two “decoy receptors,” TRAIL-R3 (DcR1 or TRID or LIT) and TRAIL-R4 (DcR2/TRUNDD), so called because of their inability to induce cell death, due to either lack of the death domain (TRAIL-R3) or presence of a truncated, dysfunctional one (TRAIL-R4) (58, 59). Binding of TRAIL to these receptors can sometimes antagonize the apoptotic signal through DR4 and DR5. TRAIL is synthesized as a type II transmembrane protein and is expressed mainly by cells of the immune system, especially NK cells, NKT cells, and macrophages (88), and it is biologically active as a homotrimer (23). TRAIL-induced apoptosis is fundamental for maintaining T-cell homeostasis, as well as for the elimination of tumor and virally transformed cells (122, 244). Studies using TRAIL-deficient mice have, indeed, confirmed its function in antitumor immune surveillance (48). Despite the high expression of TRAIL and its receptors, normal hepatocytes are resistant to TRAIL-mediated cell death (90). On the other hand, liver cancer cells (as well as other tumors) often undergo apoptosis after TRAIL treatment, which prompted clinical trials for the use of TRAIL in cancer therapy (6). The reason for this different effect on transformed and nontransformed cells has not yet been completely clarified.
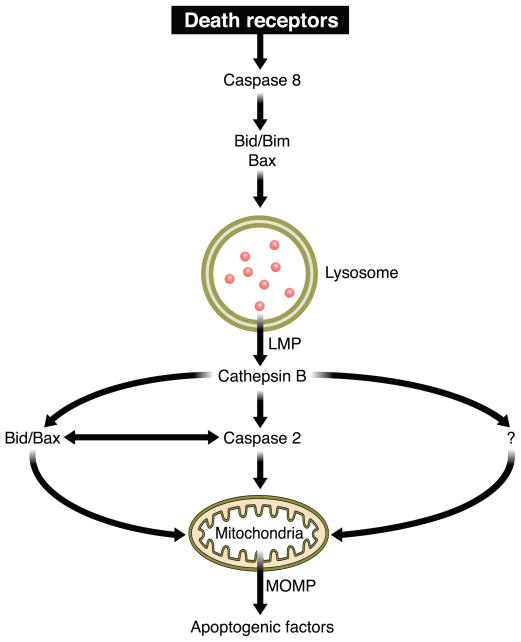
Binding of TRAIL to DR4 or DR5 is assumed to trigger similar signaling cascades, although some differences are likely to exist. Similar to Fas, the interaction between the ligand and its death receptors leads to the assembly of a multicomponent DISC at the cell membrane. The adaptor FADD is recruited to the receptor and functions as a docking protein to facilitate the recruitment of the initiator pro-caspase 8 and 10 to the receptor (145). The same distinction between type I and type II cells generally applies to TRAIL signaling as well (204), although it was originally referred to the Fas signaling pathway. Ligand-engaged DR4 and DR5 are rapidly endocytosed via both clathrin-dependent and clathrin-independent pathways in type I cells, but unlike Fas signaling, internalization of receptors is not required for transmission of its apoptotic signal (139). On the contrary, DR5 internalization is essential for TRAIL-induced apoptosis in hepatocytes and cholangiocytes (both examples of type II cells), suggesting that type I and type II cells have opposite requirements for receptor internalization during TRAIL-induced cell death (3). After TRAIL treatment of liver cells, DR5 is internalized and colocalizes with lysosomes, where it is believed to contribute to lysosomal membrane permeabilization (LMP) and lysosomal protease release into the cytosol. As hepatocytes and cholangiocytes heavily rely on LMP to mediate TRAIL killing, trafficking of receptor-bound DISC-containing vesicles to the lysosomes may explain the need for receptor internalization to initiate the apoptotic pathway in these cells. The release of lysosomal enzymes into the cytosol promotes mitochondrial dysfunction in liver cells, although the mechanisms involved are not fully understood (94). Prior studies, however, suggest that release of the lysosomal enzyme cathepsin B into the cytosol promotes activation of caspase 2 in hepatocytes, which, in turn, may facilitate efficient mitochondrial cytochrome c release and apoptosis (93). At the same time, mitochondrial dysfunction can also be induced by caspase 8-mediated cleavage of Bid and subsequent Bax/Bak activation, similarly to what was observed after Fas stimulation in type II cells.
5. TRAIL in liver pathobiology
TRAIL-mediated apoptosis is involved in the pathogenesis of viral hepatitis (196). Although hepatocytes are normally resistant to TRAIL-induced apoptosis, underlying pathological conditions, such as steatosis and viral infection, can sensitize them to TRAIL (196, 270). Cell infection by different viruses, such as reovirus, herpesvirus, HBV, and human immunodeficiency virus (HIV), has been shown to upregulate TRAIL and induce autocrine apoptosis (46, 173, 196, 235). Livers of patients with steatosis or HCV infection also display enhanced sensitivity to TRAIL-mediated apoptosis associated with increased expression of TRAIL receptors and upregulation of pro-apoptotic Bcl-2 proteins (270). Moreover, HCV core protein has been described to sensitize human hepatocellular carcinoma cells to TRAIL-mediated apoptosis via a mechanism dependent on sequential activation of caspase 8, Bid cleavage, and induction of mitochondria apoptosis signaling pathway (45).
In addition to promoting apoptosis of steatotic hepatocytes, TRAIL may also promote hepatocyte steatosis itself. For example, TRAIL expression in a murine model of HCV infection not only induces apoptosis of infected hepatocytes, but also leads to steatosis, a characteristic feature of liver diseases such as chronic hepatitis C, alcoholic liver disease, and nonalcoholic steatohepatitis (197). Moreover, TRAIL expression is also induced after alcohol consumption in mice and is associated with hepatic steatosis, but does not cause hepatocyte apoptosis, suggesting that TRAIL-mediated apoptosis and steatosis may be independently modulated after viral infection and alcohol intake (197). Several studies in vitro and in vivo have shown that TRAIL-induced apoptosis through DR5 also regulates cholestatic liver injury (106, 108, 109, 250) (Fig. 8). In particular, DR5 expression is increased in the liver of bile duct-ligated mice and in liver cells exposed to toxic bile acids (106, 108, 250). Cholestatic liver injury is also reduced in TRAIL-deficient mice following bile duct ligation (129). Importantly, TRAIL expression and apoptosis are significantly enhanced in cholangiocytes of human primary sclerosing cholangitis and primary biliary cirrhosis patients (250). These studies have also reported that treatment with an agonistic anti-DR5 monoclonal antibody triggers cholangiocyte apoptosis, and subsequently induced cholangitis and cholestatic liver injury (250). Notably, anti-DR5 monoclonal antibody-induced cholangitis exhibited the typical histological appearance of human primary sclerosing cholangitis. Thus TRAIL/DR5-mediated apoptosis may substantially contribute to chronic cholestatic disease, particularly primary sclerosing cholangitis.
6. TNF-α signaling
The ligand TNF-α is a pleiotropic cytokine mainly produced by macrophages, monocytes, and T cells in response to infection and inflammatory conditions and, occasionally, by other cell types, including hepatocytes. TNF-α exists in two biologically active forms, a membrane-bound form (mTNF-α) and a soluble form (sTNF-α), which bind to their cognate receptors TNF-R1 and TNF-R2 (p75/CD120b). However, since TNR-R2 lacks a functional death domain, only TNF-R1 is involved in transduction of the apoptotic signal (273).
In contrast to Fas and TRAIL receptors, signaling pathways from the TNF receptors are mainly pro-survival, although TNF-mediated apoptotic signals can be unmasked in certain pathological conditions. The complexity of the signaling triggered by TNF-α is demonstrated by the diverse cellular responses to this cytokine, ranging from regulation of inflammation and immunity, to proliferation, differentiation, and apoptosis of many different target cells (272). For the purpose of this article, we will focus only on the apoptotic signaling. The reader is referred elsewhere for more comprehensive reviews on TNF signaling (96, 272).
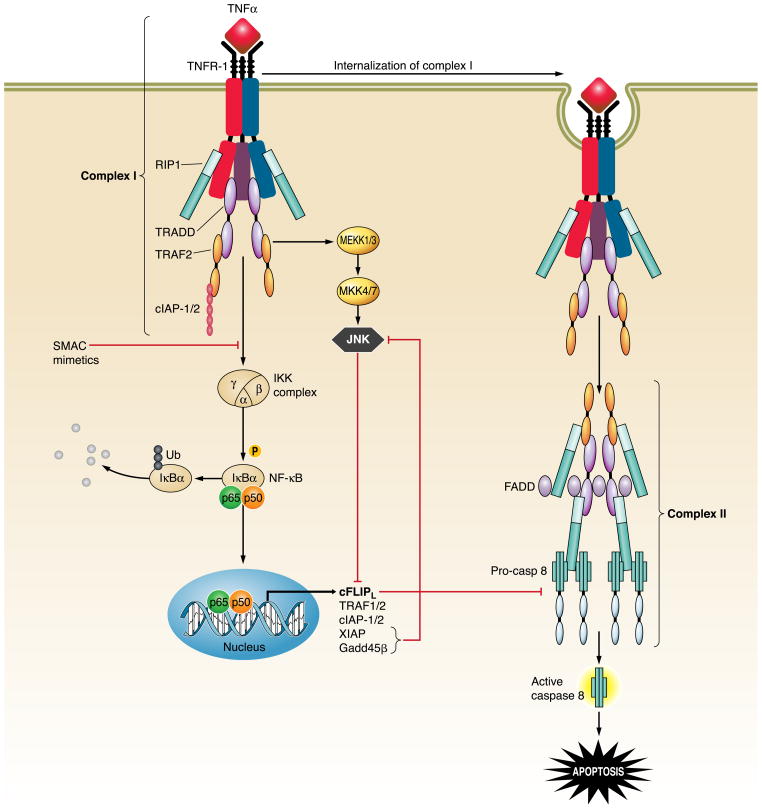
Engagement of TNF-R1 rapidly leads to the assembly of an initial plasma membrane-bound complex (complex I) comprising the receptor, TNF-R1-associated protein with death domain (TRADD), RIP1, TNF-receptor associated factor 2 (TRAF2), cIAP-1, and, possibly, other molecules (190) (Fig. 9). This complex I induces activation of the inhibitor of κB kinase (IKK) complex (including IKKα, IKKβ, and IKKγ/NEMO), which phosphorylates and promotes degradation of the NF-κB regulatory subunit IκBα, resulting in activation of NF-κB. NF-κB, in turn, regulates the transcription of anti-apoptotic target genes, such as cFLIPL, cIAP-1, cIAP-2, XIAP, TRAF1, TRAF2, A20, A1, and Bcl-xL that antagonize cell death (275). In addition to NF-κB activation, complex I also signals activation of different mitogen-activated protein kinase (MAPK) cascades, such as p38 MAPK, extracellular regulated kinase (ERK), and c-Jun NH2-terminal kinase (JNK) (19) (Fig. 9). Transient activation of JNK promotes proliferation, whereas prolonged JNK activation contributes to cell death (43), at least, in part, by promoting phosphorylation and proteasomal degradation of cFLIPL (37). NF-κB activation normally regulates the strength and duration of TNF-α-induced JNK activation and prevents sustained activation via transcription of target genes such as xiap and gadd45β (56, 254) (Fig. 9). Indeed, IKKβ-deficient mouse embryos display massive hepatocyte apoptosis at E14.5, whereas livers from IKKβ/JNK1 double knockout mice show no apoptotic damage at the same embryonic stage (37). Moreover, JNK activation plays a role in the development of TNF-mediated hepatitis, as JNK-deficient mice, as well as mice treated with a specific JNK inhibitor, are resistant to different models of fulminant hepatitis depending on TNF-R1 activation (54, 175). Hepatocyte-specific impairment of NF-κB activation also increases diethylnitrosamine-induced hepatocyte apoptosis, compensatory proliferation, and hepatocarcinogenesis via TNF-α-mediated JNK1 activation (226).
FIG. 9.
TNF-R1 signaling pathways. Engagement of TNF-R1 by TNF-α induces recruitment of TRADD, RIP1, TRAF2, and c-IAP1/2 to the receptor to form the membrane-bound complex I. Two distinct pathways originate from the association of RIP1 and TRAF-2 to the receptor. The first one promotes survival by activation of the catalytic IKK complex, leading to phosphorylation of the NF-κB inhibitory protein IκBα and its degradation via the ubiquitin-proteasome pathway, allowing NF-κB to translocate to the nucleus and initiate transcription of anti-apoptotic target genes, such as cFLIP, cIAP-1, cIAP-2, TRAF1, TRAF2, XIAP, and Gadd45β. The second one leads to activation of JNK via the consequential activation of MEKK1/3 and MKK4/7. Activation of JNK is usually transient due to prompt inhibition by NF-κB-regulated proteins, such as XIAP and Gadd45β; however, sustained activation of JNK due to defects in NF-κB transcriptional activity results in c-FLIP degradation, enhanced caspase 8 activation, and apoptosis. Following the transient formation of complex I, the receptor is internalized by endocytosis and RIP1, TRAF2 and TRADD dissociated from TNF-R1, allowing the recruitment of FADD and caspase 8. The newly formed cytosolic complex (named complex II) promotes the activation of caspase 8, initiating the apoptotic cascade. This apoptotic pathway is inhibited by NF-κB-mediated expression of cFLIPL, which binds to caspase 8 and prevents its proteolytic activation.
The receptor-containing complex I is subsequently internalized, and TRADD, TRAF2, and RIP1 dissociate from the complex and associate with FADD and caspase 8 and 10 to form a cytosolic complex named complex II, which promotes caspase activation and apoptosis (190) (Fig. 9). Under normal circumstances, NF-κB-induced cFLIPL associates with complex II, inhibiting the pro-apoptotic activity of caspase 8 (190). Therefore, TNF-induced apoptosis can only occur when the formation of complex I is prevented or disrupted, or when the cyto-protective activity of NF-κB is inhibited. Indeed, a critical role for NF-κB in preventing TNF-α-mediated apoptosis was established in the liver during studies employing mice lacking the RelA/p65 component of NF-κB, or IKKβ (12, 160); embryonic lethality occurs in these mice due to massive hepatocyte apoptosis with liver degeneration. This phenotype was rescued by crossing these mice with TNF-R1 knockout mice (222). Thus NF-κB activation is essential to prevent hepatocyte apoptosis by TNF-α.
TNF-α-mediated NF-κB activation can also be prevented by the pharmacological use of SMAC mimetics, small molecules designed to resemble the NH2 terminus of SMAC that inhibit XIAP, cIAP-1, and cIAP-2 (210, 268). These small molecules cause cIAP-1 and cIAP-2 autoubiquitination and proteasomal degradation (268); as a consequence, RIP1 is no longer ubiquitinated by cIAP-1 and cIAP-2 (17) and fails to phosphorylate NEMO and, thereby, to activate the IKK complex (Fig. 9). RIP1 is then released from complex I and associates with FADD and caspase 8, facilitating caspase 8 activation (210, 277). In liver cells, as previously described in Fas and TRAIL apoptosis, TNF-α-mediated caspase 8 activation results in cleavage of Bid and MOMP with release of pro-apoptogenic factors in the cytosol (301, 302). In addition to Bid, recent studies suggested another BH3-only protein, Bim, may play a role in TNF-α-mediated liver injury (132). TNF-α-induced mitochondria dysfunction is also mediated by the lysosomal cathepsin B in hepatocytes (94). Indeed, both mitochondria permeabilization and apoptosis are abrogated in cathepsin B-deficient hepatocytes in vitro and in vivo after TNF-α treatment (94, 98). Several experimental evidences suggest that other caspase-independent apoptosis-like pathways may also be activated by TNF-α in the liver (126, 148).
7. TNF-α in liver pathobiology
TNF-α-induced hepatocyte apoptosis is implicated in a wide range of liver diseases including viral hepatitis, alcoholic hepatitis, ischemia/reperfusion liver injury, and fulminant hepatic failure (85). In chronic and acute liver injury, hepatocyte apoptosis results largely from overactivation of the immune system and generation of inflammatory cell products. TNF-α is produced by the liver in response to hepatotoxins, such as carbon tetrachloride, galactosamine, and alcohol (51, 294). During chronic alcohol consumption, the number of liver NKT cells increases, promoting extensive hepatocyte apoptosis through both the Fas pathway and the TNF-R1 pathway (191). Expression of TNF-R1, however, is not enhanced in patients with alcoholic hepatitis, despite evidence of increased hepatocyte apoptosis (199). During ischemia/reperfusion (IR) injury, activated Kupffer cells release large amounts of TNF-α and chemokines to recruit inflammatory cells. Although TNF-α is involved in IR injury, as demonstrated by reduced IR liver damage in TNF-R1 knockout mice (224), the injury is likely not due to a direct toxic effect of TNF-α on the cells, but is rather the result of the recruitment and activation of inflammatory cells (47). Finally, TNF-α, together with other cytokines, is secreted by infiltrating cytotoxic T lymphocytes during HBV infection, which can promote apoptosis of viral-infected cells (187). Indeed, in a model of HBV transgenic hepatoma cell line, stimulation with TNF-α induces apoptosis (292). The pro-apoptotic effect of TNF-α in infected hepatocytes appears to be mediated by HBV protein X (HBVx) via activation of MAPK 1 (MKK1) and n-Myc (248).
B. Toll-like Receptors
1. Definition
Toll-like receptors, named for their similarity to the Toll gene product in Drosophila, are key pattern recognition receptor proteins in the innate immune system (18, 103, 212). They form a series of cell membrane and cytoplasmic receptors, which together provide a mechanism for recognition and response to fungal, bacterial, and viral products. Ten TLRs have been identified in humans and 13 in mice. TLRs and some of their ligands, including their primary ligand, are listed in Table 2. In addition to microbial recognition, TLRs also recognize endogenous DAMPs. Activation of the innate immune response is vital for host defense as well as priming of the adaptive immune system. However, excessive, continuous, or autoimmune activation of the innate immune system is a feature of many diseases. This sterile inflammatory response is also activated by DAMPs.
TABLE 2.
Toll-like receptors and their ligands
| Receptor | Ligand |
|---|---|
| TLR1 | Triacyl lipopeptides |
| TLR2 | Triacyl lipopeptides, peptidoglycans, lipoteichoic acid |
| TLR3 | Viral double-stranded RNA, poly I:C |
| TLR4 | Lipopolysaccharide, heparan, hyaluronate |
| TLR5 | Bacterial flagellin |
| TLR6 | Diacyl lipopeptides |
| TLR7 | Single-stranded RNA, short double-stranded RNA |
| TLR8 | Single-stranded RNA |
| TLR9 | Unmethylated CpG oligodeoxynucletides |
| TLR10 | Unknown |
| TLR11 | Profilin |
| TLR12 | Unknown |
| TLR13 | Unknown |
2. Toll-like receptors signaling
While understanding of TLR signaling is by no means complete, TLRs are known to be type I transmembrane proteins, single-spanning, with an extracellular NH2 terminus and an intracellular COOH terminus. TLRs form homodimers and, in some instances, heterodimers (TLR2 with TLR1 or TLR6) upon ligation. TLR signals emanate either from the cell surface (TLR1, TLR2, TLR4, TLR5, TLR6) or from the endolysosomal compartment (TLR3, TLR7, TLR9) (Fig. 7) (9). Upon ligation, they undergo a conformational change and recruit cytoplasmic adaptor proteins via a Toll/IL-1 receptor (TIR) domain. The proximal adaptor proteins that mediate TLR signaling are myeloid differentiation primary response gene 88 (MyD88), MyD88 adaptor-like protein (MAL, also known as TIR-domain-containing adaptor protein; TIRAP), TIR domain-containing adaptor protein inducing interferon-β (TRIF), and TRIF-related adaptor molecule (TRAM). TIRAP and MyD88 mediate signals from most of the TLRs, except TLR3. TRIF mediates signals from TLR3, and with the adaptor TRAM, also from TLR4. Downstream of MyD88, via other adaptor proteins, regulatory kinases are activated, leading to activation of NF-κB and MAPK pathways. Downstream of TRIF, type I interferons are induced. TLR4 is the only TLR that can activate both pathways, the TIRAP-MyD88 pathway from the cell membrane, followed by the TRAM-TRIF pathway from the early endosome (128). TLR7, -8, and -9 engagement leads to IFN-α production downstream of MyD88.
3. Toll-like receptors in liver pathobiology
Most of the known TLRs are widely expressed on many cell types in the liver in addition to cells of the innate immune system, including hepatocytes, cholangiocytes, and stellate cells, and play a vital role in hepatic inflammation. TLR9 mediates hepatic stellate cell activation in response to apoptotic hepatocyte DNA (278). In acetaminophen-induced acute liver injury, activation of inflammatory pathways occurs in a TLR9-dependent manner (117) (Fig. 6). Furthermore, DAMPs are released from damaged hepatocytes in this model, presumably activating TLR9, although this has not been directly demonstrated (184). Liver inflammation upon intraportal injection of apoptotic hepatocyte DNA also occurs in a TLR9-dependent manner (117). These studies highlight the interconnectedness of hepatocyte cell death and the sterile inflammatory response in the liver. In an alcoholic hepatitis mouse model, Kupffer cell TLR4 activation mediates hepatic steatosis, inflammation, and injury (265). Chronic alcohol feeding upregulates many TLRs in the liver, and the use of TLR agonists augments liver injury (99). HCV infection is associated with reduced TLR7 expression in human liver samples, and viral proteins modulate TLR7 expression at a transcriptional level, thus favoring immune evasion (38). HCV-mediated TLR2 activation also favors immune subversion by dampening the TLR3 response (260). HBV replication is inhibited by activation of most TLRs, except TLR2 in a transgenic mouse model (119). In primary biliary cirrhosis, monocytes from patients demonstrate an exaggerated response to TLR ligands, suggesting a role for innate immunity in autoreactivity (182). Hepatic stellate cells are activated by the TLR4 agonist LPS, and mice lacking TLR4 demonstrate reduction in hepatic fibrosis following bile duct ligation (205, 236).
C. Lysosomal Pathway
1. Lysosomal pathway of apoptosis
For many years, mitochondria and nuclei have been thought to be the only key organelles in the initiation and execution of apoptosis. Although their role is still indisputable, many studies during the past decade have demonstrated also the importance of other organelles, such as the lysosomal/endosomal system (97) and the endoplasmic reticulum (see below), in the process. The lysosomal/endosomal compartment comprises single membrane-bound, cytosolic organelles responsible for degradation and recycling of cellular components. Over 50 different hydrolases are present in the lysosomal lumen, which work optimally at the lysosomal acidic pH, and carry out the digestion of all the major cellular macromolecules (7). The largest group of these hydrolases are the cathepsin proteases, which include the cysteine cathepsins (B, C, F, H, K, L, O, S, V, W, and X), the aspartic proteases cathepsin D and E, and the serine protease cathepsin G (262). In the early stages of apoptosis following exposure to different stimuli, lysosomal proteases from the cathepsin family frequently translocate from the lysosomal lumen to the cytosol and nucleus. This release is associated with LMP and, contrary to what occurs during necrosis, is a moderate phenomenon that likely affects only a sub-population of susceptible lysosomes rather than all lysosomes (283). The mechanisms underlying LMP are still largely unknown, but several evidences suggest that the release of lysosomal enzymes occurs via non-specific mechanisms, such as increased membrane permeabilization or pore formation, rather than via specific transporters across the membrane. Genetic and pharmacological inhibition of two of the most highly expressed cathepsins, cathepsin B and D, has been proven effective in blocking apoptosis in several different models, including p53-dependent and -independent cell death as well as oxidative stress-, IFN-γ-, TNF-α-, Fas-L, and TRAIL-induced cell death, suggesting that these enzymes actively participate in the pathway and their release is not just the result of a late degenerative process (20, 61, 78, 94, 125, 219, 238, 284). In some models, apoptosis appears to be entirely cathepsin dependent (27, 34, 78), whereas in others both caspases and cathepsins are required for apoptosis initiation and execution (70, 94). Lysosomal permeabilization is often followed by mitochondrial dysfunction and release of pro-apoptogenic factors (20, 26, 27, 94, 300). Therefore, in many cases, cathepsins seem to function upstream of the mitochondria by provoking MOMP, possibly via direct activation of Bid or Bax (20, 104, 246). This sequence of subcellular alterations (lysosomal permeabilization → mitochondrial permeabilization→ caspase activation→ cell death) is known as the lysosomal pathway of apoptosis (Fig. 10).
FIG. 10.
The lysosomal pathway of apoptosis in hepatocytes. Death receptor engagement by their cognate ligands results in formation of a DISC promoting activation of caspase 8. Caspase 8 cleaves and activates Bid, which, in hepatocytes, facilitates lysosomal membrane permeabilization (LMP), likely by activating Bax. In cholangiocytes, another BH3-only protein, Bim, but not Bid, cooperates with Bax to induce LMP following phosphorylation/activation by JNK. LMP results in release of lysosomal components into the cytosol. Although not the only lysosomal protease released, cathepsin B plays a pivotal role in lysosomal-mediated cytotoxicity in liver cells by promoting mitochondrial dysfunction. The mechanisms by which cathepsin B causes MOMP are still largely unknown; however, cleavage and activation of Bid/Bax and caspase 2 have been described in different experimental models.
2. Lysosomal pathway of apoptosis in liver pathobiology
The contribution of the lysosomal pathway of apoptosis appears to be particularly significant in liver cell death in pathological conditions. LMP with release of lysosomal content has been shown in several liver diseases in association with increased apoptosis. In particular, cathepsin B seems to play a central role in the induction and progression of liver damage of different etiologies, as inhibition of cathepsin B significantly reduces hepatocyte apoptosis and liver injury in animal models of cholestasis (32), TNF-α-mediated acute liver failure (98), NASH (74, 75), and cold ischemia/warm reperfusion injury in steatotic livers (10). Cathepsin B is one of the most abundant lysosomal cysteine cathepsins and retains most of its enzymatic activity and stability even at cytosolic pH, which might explain its prominent role in the apoptotic process (261). In many of these models, apoptosis is triggered after the engagement of a death receptor, suggesting lysosomes are an important component of the death receptor-dependent apoptotic cascade in the liver (Fig. 10). In particular, Fas and DR5 are activated by bile acids, the bile component mainly responsible for tissue damage in cholestatic livers (70, 107, 108), whereas Fas, DR5, and the TNF/TNF-R system mediate cytotoxicity in fatty livers (74, 177). How engagement of death receptors signals LMP is still under investigation; however, recent studies have shown that internalization of the TRAIL/DR5 complex is required for lysosomal permeabilization in liver cancer cells (3). The subsequent trafficking of the complex to the lysosomes would bring the apoptotic machine in close proximity to the lysosomal membrane, allowing membrane permeabilization to occur, perhaps via formation of pores with mechanisms similar to those proposed for the outer mitochondrial membrane. Indeed, TRAIL-induced LMP in cholangiocarcinoma cells requires JNK-mediated activation of Bim and Bax (284). Likewise, TNF-α-mediated LMP is strongly reduced in Bid-deficient mouse hepatocytes (93, 282). Finally, hepatocyte treatment with toxic free fatty acids (an in vitro model of liver steatosis) results in Bax translocation to the lysosomes, reduced expression of the Bax antagonist Bcl-xL, and LMP, which can be prevented by pharmacological or genetic inhibition of Bax (75).
D. Endoplasmic Reticulum Stress
1. Endoplasmic reticulum stress signaling
Endoplasmic reticulum (ER) stress, defined in the context of the accumulation of misfolded proteins in the lumen of the ER, activates a series of ER-to-nucleus signals, collectively termed the unfolded protein response (UPR) (221, 231) (Fig. 11). Perturbations in ER homeostasis, such as calcium depletion, inhibition of glycosylation, alterations in the redox state, and lipid loading of the ER, also activate the UPR. Accumulation of misfolded ER proteins is sensed by three resident transmembrane proteins, inositol requiring protein 1α (IRE1α), ds-RNA-activated protein kinase (PKR) like ER kinase (PERK), and activating transcription factor 6 alpha (ATF6α) (Fig. 11). Release from inhibitory binding with the chaperone BiP activates all three sensors. PERK phosphorylates the eukaryotic initiation factor 2 α-subunit (eIF2α), leading to a global reduction in translation due to inhibition of the initiation of translation. Activating transcription factor 4 (ATF4) is preferentially translated following eIF2α phosphorylation, activating ER chaperone genes, ER associated protein degradation (ERAD) pathway genes, amino acid metabolism genes, as well as the transcription factor C/EBP homologous protein (CHOP). IRE1α has kinase as well as endoribonuclease activity; it is activated by trans-autophosphorylation, leading to the unconventional splicing of the transcription factor X-box binding protein-1 (XBP1) mRNA and formation of transcriptionally active spliced XBP1 (XBP1s). Spliced XBP1 activates chaperone UPR target genes, including chaperones and ERAD pathway genes. ATF6α is cleaved in the Golgi to form a transcriptionally active fragment that traffics to the nucleus to activate UPR target genes. All three arms of the UPR are aimed at adaptation and restoration of ER homeostasis; however, sustained ER stress can lead to apoptosis.
FIG. 11.
Unfolded protein response and cell death. Endoplasmic reticulum (ER) stress, such as the accumulation of misfolded proteins in the ER lumen, activates the unfolded protein response (UPR), aimed at restoring ER homeostasis through translational and transcriptional pathways. The UPR sensors IRE1α, PERK, and ATF6α activate these pathways. Activation of IRE1α leads to the unconventional splicing of the transcription factor XBP1 to the transcriptionally active spliced XBP1 (XBP1s), which activates UPR target genes aimed at restoration of ER homeostasis. Via the adaptor protein TRAF2, IRE1α can activate JNK. ATF6α is cleaved in the Golgi to an active transcription factor which induces expression of UPR target genes, also aimed at restoration of homeostasis. Phosphorylation of eIF2α by PERK results in attenuation of translation and selective translation of ATF4 and its target CHOP. Failure of restoration of ER homeostasis results in cell death. Some possible mechanisms include CHOP-induced expression of the proapoptotic protein Bim, and DR5, perturbations in ER calcium, and JNK-induced cell death. The anti-apoptotic protein Bax inhibitor 1 (BI-1) is a negative regulator of IRE1α, as well as negative regulator of ER-stress induced cell death.
The molecular mechanisms of ER stress-induced apoptosis are not fully understood; however, potentially apoptotic pathways are activated as part of the UPR. Deletion of the transcription factor CHOP offers protection from ER stress-induced apoptosis (303). Growth arrest and DNA damage-inducible protein (Gadd34) transcription occurs downstream of CHOP. Gadd34 binds protein phosphatase-1 and enhances eIF2α dephosphorylation, leading to restoration of translation. Thus the entry of nascent proteins into stressed ER could be further disruptive (183). CHOP can also enhance expression of the TRAIL receptor DR5 and the proapoptotic Bcl-2 family protein Bim (215, 289). Overexpression of CHOP in cell lines sensitizes to ER stress-induced apoptosis with reduced cellular glutathione and decreased expression of the anti-apoptotic protein Bcl-2 (188). IRE1α can recruit the adaptor protein TRAF2 activating JNK and NF-κB, both of which can mediate apoptosis (116, 267). Alterations in ER calcium homeostasis and activation of caspase 12 are other mechanisms for ER stress-induced apoptosis (198, 201, 216).
2. ER stress in liver pathobiology
ER stress is a feature of liver disease. An example of ER stress-associated liver injury is that mediated by FFA in the metabolic syndrome (Fig. 12). Saturated FFA induce an ER stress response (276, 280, 281), which activates PERK and IRE1α. PERK activation results in CHOP expression that not only induces expression of DR5 and Fas (177), but also of BH3-only protein Bim (178). In addition, IRE1α promotes sustained JNK activation resulting in PUMA expression (35). All of these cascades likely contribute to the lipoapoptosis observed in human NASH.
FIG. 12.
Molecular mechanisms involved in hepatocyte lipoapoptosis. Saturated FFAs accumulate in the ER eliciting an ER stress response mediated by phosphorylation and activation of IRE1α and PERK. Phosphorylated PERK promotes induction of the transcription factor CHOP, which upregulates the expression of the pro-apoptotic BH3-only protein Bim. Phosphorylated IRE1α activates JNK, which, in turn, phosphorylates the transcription factor c-Jun. Activation of c-Jun results in transcriptional upregulation of the pro-apoptotic BH3-only protein PUMA. Bim and PUMA activate the pro-apoptotic executioner protein Bax, resulting in MOMP, activation of effector caspases, and apoptosis. In addition, CHOP upregulates the expression of death receptors, such as DR5, increasing the susceptibility of steatotic hepatocytes to circulating death ligands (e.g., TRAIL).
In obese patients, UPR markers are detected in the liver and correlate with weight loss (92). Hepatitis C viral replicons can also activate the UPR, and HCV core protein can induce ER calcium depletion and apoptosis (14, 255). Intrahepatic cholestasis in mice is associated with activation of the UPR (22). In the bile duct-ligation model of cholestatic liver injury, CHOP-deficient mice demonstrate attenuation of hepatocyte cell death, liver injury, and fibrosis (253). In alcohol-induced liver injury in mice, the absence of CHOP abrogates hepatocyte apoptosis, without affecting hepatocyte steatosis (123). Furthermore, mice with genetic absence of any of the UPR sensors develop microvesicular hepatic steatosis upon acute ER stress (225).
V. THERAPEUTIC STRATEGIES FOR LIVER INJURY
The optimal therapeutic strategy for liver diseases is to directly inhibit the inciting mechanism for the liver injury. This is quite feasible for viral hepatitis where direct and indirect antiviral therapies are employed to treat human disease. However, the pathophysiological mechanisms for many cholestatic liver diseases (e.g., primary sclerosing cholangitis, primary biliary cirrhosis), metabolic diseases such as NASH, acute alcohol-mediated hepatitis, etc. remain to be identified. In addition, many patients with viral hepatitis fail antiviral therapy. Thus there is a role for the development of hepatoprotective agents. Such agents, by inhibiting mechanisms of liver injury, would retard fibrotic progression of the liver disease potentially preventing cirrhosis, portal hypertension, and even hepatocellular cancer. To date, the only example of a hepatoprotective agent used to treat a liver disease is the widespread use of ursodeoxycholate as primary therapy for primary biliary cirrhosis (154); indeed, ursodeoxycholate may be considered the first anti-apoptotic agent employed to treat liver diseases (220). We will limit this discussion to strategies that have proven viable in the pharmaceutical industry, namely, the potential use of small molecule protease and kinase inhibitors (Table 3). Potentially, antibodies could be used to target death ligands and receptors, but they target very specific molecules and likely would not be useful in complex pathophysiological conditions where often multiple pathways are operating simultaneously.
TABLE 3.
Anti-apoptotic therapeutic strategies for liver diseases
| Drug Type | Liver Diseases | Clinical Trial | Reference Nos. |
|---|---|---|---|
| Ursodeoxycholate | PBC | Phase III | 146, 162 |
| PSC | Phase III | 49, 165 | |
| NASH | Phase II | 8, 164 | |
| Cholestasis of pregnancy | Phase II | 86, 87 | |
| Chronic HCV | Phase I/II | 13, 203 | |
| Caspase inhibitors | Chronic HCV | Phase II | 211 |
| I/R injury | Phase II | 11 | |
| Cathepsin B inhibitors | I/R injury | Preclinical | 10 |
| Fibrosis | Preclinical | 32 | |
| MMP inhibitors | HCV | Phase II | |
| JNK inhibitors | Fibrosis | Preclinical | 138 |
| Necrostatins | Necrotic damage (?) | No studies in liver |
PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; NASH, nonalcoholic steatohepatitis; HCV, hepatitis C virus; I/R, ischemia/reperfusion.
A. Protease Inhibitors
1. Caspase inhibitors
As apoptosis plays a key role in many liver diseases and is largely caspase mediated, pharmacological caspase inhibitors are attractive hepatoprotective drugs for the treatment of human liver diseases. Indeed, pan-caspase inhibitors attenuate hepatic injury and fibrosis in preclinical models of cholestasis and nonalcoholic liver disease (30, 288). In a short-term trial, a pan-caspase inhibitor reduces serum transaminases in patients with HCV without affecting viral replication (186, 211). A pan-caspase inhibitor was shown to reduce liver preservation injury during rodent and human organ transplantation (11, 114). Thus these agents clearly warrant further consideration for the treatment of human liver diseases.
Although there is a theoretical concern that apoptosis inhibition by caspase inhibitors would promote carcinogenesis, this is unlikely. As mentioned above, there are two canonical pathways of apoptosis, a death receptor-mediated pathway and a mitochondrial pathway. The death receptor pathway is dependent on caspase 8 (132), while the mitochondrial pathway is mediated by caspase 9. In the liver, the genetic absence of caspase 8 attenuates hepatitis in experimental models mediated by death ligands (132, 297). In contrast, caspase 9 deletion delays, but does not prevent, cell death (65). Thus a caspase inhibitor would only be expected to inhibit death receptor-mediated apoptosis. As knockout murine models of various death receptors do not develop spontaneous cancers, caspase inhibitors should be safe with regard to cancer risk.
2. Cathepsin B inhibitors
Cathepsin B is a lysosomal protease that has been implicated in lysosomal disruption and apoptosis (94, 95, 282, 284). Preclinical studies showed that genetic and pharmacological inhibition of cathepsin B reduce TNF-α-mediated acute liver injury and improve survival in a mouse model (98). Likewise, cathepsin B inactivation attenuates liver injury, inflammation, and fibrogenesis after bile duct ligation, a model of cholestatic liver injury (32). Because the cathepsin B knockout animal does not have a spontaneous phenotype, a cathepsin B inhibitor should not have target toxicity in humans. Cathepsin B inhibitors, for the treatment of cholestatic liver diseases and, potentially, even for NASH, are an attractive strategy (74).
3. Matrix metalloprotease inhibitors
Matrix metalloprotease (MMP) inhibition is a potential strategy to minimize liver injury and reduce hepatic fibrogenesis. TNF-α converting enzyme (TACE) is a MMP required for the release of cell-bound TNF-α into the circulation. TNF-α also promotes the induction and activation of other MMP which further enhances liver injury. Indeed, inhibition of MMP activity blocks lethal hepatitis and apoptosis in murine models of TNF-α-associated acute liver injury (286). Also, a broad-spectrum MMP inhibitor reduces hepatocyte apoptosis and fibrosis in the bile duct-ligated mouse (130). A MMP inhibitor is currently in early phase trials for the treatment of HCV-associated liver disease.
B. Kinase Inhibitors
1. JNK inhibitors
Sustained JNK activation has been implicated in multiple forms of hepatocyte cell death, including drug-induced liver injury, FFA-mediated liver cytotoxicity, ER stress-associated disease states, I/R injury, and TNF-α-induced liver damage (50, 101, 178, 179, 230, 233, 234, 264). In addition, JNK inhibition is also antifibrotic in the liver (138). Although JNK inhibitors are widely employed in cell system studies, their use in vivo is limited. Also, multiple isoforms of JNK are encoded by two genes, JNK1 and -2, in the liver, and inhibition of one, the other, or both, may be necessary for different forms of injury. For example, in a model of NASH, JNK1 promotes liver injury while JNK2 is protective (230). Thus JNK isoform-specific inhibitors may be necessary to treat human liver diseases.
2. Necrostatins
RIP1 and -3 kinases have been implicated in cell death, especially that by death receptors (57). Recently, a group of small molecules termed necrostatins were found to inhibit cell death by allosterically inhibiting RIP kinases (60). Necrostatin-1 is a highly selective inhibitor of RIP1. Given the emerging role of RIP1 in various forms of necrotic cell injury, these observations suggest small molecular compounds can be developed to inhibit RIP kinases and prevent hepatic necrosis.
VI. CONCLUSIONS
This review focuses on current concepts of hepatocyte cytotoxicity, a common occurrence in clinical practice. There is much yet to be learned about liver injury, especially the complex interplay between the immune system and the hepatocyte in these pathophysiological states. Also, the mechanisms by which cell death promotes hepatic fibrosis are likely more multi-faceted and nuanced than current concepts suggest. Rational therapeutic hepatoprotective strategies must be predicated on a thorough understanding of how liver injury occurs. As this information becomes available, clinical trials with appropriate end points can be developed to hopefully establish therapeutic approaches to minimize hepatic injury and its sequelae. Hopefully, this review will serve as a guide in this quest.
Acknowledgments
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) RO1 Grants DK-41876 and DK-63947 (to G. J. Gores), NIDDK T32 Training Grant DK-07198-34 (to H. Malhi), and the Mayo Foundation.
References
- 1.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, Rehermann B. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alpha-dependent manner. Gastroenterology. 2010;138:325–335. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akazawa Y, Mott JL, Bronk SF, Werneburg NW, Kahraman A, Guicciardi ME, Meng XW, Kohno S, Shah VH, Kaufmann SH, McNiven MA, Gores GJ. Death receptor 5 internalization is required for lysosomal permeabilization by TRAIL in malignant liver cell lines. Gastroenterology. 2009;136:2365–2376. doi: 10.1053/j.gastro.2009.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009;50:1721–1734. doi: 10.1194/jlr.R900011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, Poniachik J. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci. 2004;106:635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–1990. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bainton DF. The discovery of lysosomes. J Cell Biol. 1981;91:66s–76s. doi: 10.1083/jcb.91.3.66s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balmer ML, Siegrist K, Zimmermann A, Dufour JF. Effects of ursodeoxycholic acid in combination with vitamin E on adipokines and apoptosis in patients with nonalcoholic steatohepatitis. Liver Int. 2009;29:1184–1188. doi: 10.1111/j.1478-3231.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 9.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baskin-Bey ES, Canbay A, Bronk SF, Werneburg N, Guicciardi ME, Nyberg SL, Gores GJ. Cathepsin B inactivation attenuates hepatocyte apoptosis and liver damage in steatotic livers after cold ischemia-warm reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2005;288:G396–G402. doi: 10.1152/ajpgi.00316.2004. [DOI] [PubMed] [Google Scholar]
- 11.Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, Burgart L, Garrity-Park M, van Vilsteren FG, Oliver LK, Rosen CB, Gores GJ. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7:218–225. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 12.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 13.Bellentani S, Podda M, Tiribelli C, Callea F, Marazzi M, Sodde M, Merlini R, Batezzati PM, Crosignani A, Zuin M. Ursodiol in the long-term treatment of chronic hepatitis: a double-blind multicenter clinical trial. J Hepatol. 1993;19:459–464. doi: 10.1016/s0168-8278(05)80558-6. [DOI] [PubMed] [Google Scholar]
- 14.Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, Buscail L, Bartenschlager R, Ichas F, Rizzuto R, Paterlini-Brechot P. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24:4921–4933. doi: 10.1038/sj.onc.1208673. [DOI] [PubMed] [Google Scholar]
- 15.Berke G. The CTL’s kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 16.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 17.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyaert R, Van Loo G, Heyninck K, Vandenabeele P. Signaling to gene activation and cell death by tumor necrosis factor receptors and Fas. Int Rev Cytol. 2002;214:225–272. doi: 10.1016/s0074-7696(02)14007-1. [DOI] [PubMed] [Google Scholar]
- 20.Bidere N, Lorenzo HK, Carmona S, Laforge M, Harper F, Dumont C, Senik A. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003;278:31401–31411. doi: 10.1074/jbc.M301911200. [DOI] [PubMed] [Google Scholar]
- 21.Blommaart EF, Luiken JJ, Meijer AJ. Autophagic proteolysis: control and specificity. Histochem J. 1997;29:365–385. doi: 10.1023/a:1026486801018. [DOI] [PubMed] [Google Scholar]
- 22.Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008;14:828–836. doi: 10.1038/nm.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodmer JL, Meier P, Tschopp J, Schneider P. Cysteine 230 is essential for the structure and activity of the cytotoxic ligand TRAIL. J Biol Chem. 2000;275:20632–20637. doi: 10.1074/jbc.M909721199. [DOI] [PubMed] [Google Scholar]
- 24.Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ, Kamath PS, Shah VH. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bortolami M, Kotsafti A, Cardin R, Farinati F. Fas/FasL system, IL-1beta expression and apoptosis in chronic HBV and HCV liver disease. J Viral Hepat. 2008;15:515–522. doi: 10.1111/j.1365-2893.2008.00974.x. [DOI] [PubMed] [Google Scholar]
- 26.Boya P, Andreau K, Poncet D, Zamzani N, Perfettini JL, Metivier D, Ojcius DM, Jaattela M, Kroemer G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med. 2003;197:1323–1334. doi: 10.1084/jem.20021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boya P, Gonzales-Polo RA, Poncet D, Andreau K, Vieira HLA, Roumier T, Perfettini JL, Kroemer G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22:3927–3936. doi: 10.1038/sj.onc.1206622. [DOI] [PubMed] [Google Scholar]
- 28.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Metivier D, Meley D, Souquere S, Yoshimori T, Pierron G, Codogno P, Kroemer G. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Branch AD, Seeff LB. Afterword: HCV in the decades ahead. Semin Liver Dis. 2000;20:233–237. [PubMed] [Google Scholar]
- 30.Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191–1196. doi: 10.1124/jpet.103.060129. [DOI] [PubMed] [Google Scholar]
- 31.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 32.Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M, Gores GJ. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112:152–159. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83:655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 34.Caruso JA, Mathieu PA, Joiakim A, Zhang H, Reiners JJ., Jr Aryl hydrocarbon receptor modulation of tumor necrosis factor-alpha-induced apoptosis and lysosomal disruption in a hepatoma model that is caspase-8-independent. J Biol Chem. 2006;281:10954–10967. doi: 10.1074/jbc.M508383200. [DOI] [PubMed] [Google Scholar]
- 35.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, Gores GJ. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–3714. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Chang S, Kodys K, Szabo G. Impaired expression and function of toll-like receptor 7 in hepatitis C virus infection in human hepatoma cells. Hepatology. 2010;51:35–42. doi: 10.1002/hep.23256. [DOI] [PubMed] [Google Scholar]
- 39.Charlotte F, L’Hermine A, Martin N, Geleyn Y, Nollet M, Gaulard P, Zafrani ES. Immunohistochemical detection of bcl-2 protein in normal and pathological human liver. Am J Pathol. 1994;144:460–465. [PMC free article] [PubMed] [Google Scholar]
- 40.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen JJ, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–1717. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 43.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 44.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou AH, Tsai HF, Wu YY, Hu CY, Hwang LH, Hsu PI, Hsu PN. Hepatitis C virus core protein modulates TRAIL-mediated apoptosis by enhancing Bid cleavage and activation of mitochondria apoptosis signaling pathway. J Immunol. 2005;174:2160–2166. doi: 10.4049/jimmunol.174.4.2160. [DOI] [PubMed] [Google Scholar]
- 46.Clarke P, Meintzer SM, Gibson S, Widmann C, Garrington TP, Johnson GL, Tyler KL. Reovirus-induced apoptosis is mediated by TRAIL. J Virol. 2000;74:8135–8139. doi: 10.1128/jvi.74.17.8135-8139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. The role of cytokine networks in the local liver injury following hepatic ischemia/reperfusion in the rat. Hepatology. 1996;23:506–514. doi: 10.1002/hep.510230315. [DOI] [PubMed] [Google Scholar]
- 48.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 49.Cullen SN, Rust C, Fleming K, Edwards C, Beuers U, Chapman RW. High dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis is safe and effective. J Hepatol. 2008;48:792–800. doi: 10.1016/j.jhep.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 50.Czaja MJ. The future of GI and liver research: editorial perspectives. III. JNK/AP-1 regulation of hepatocyte death. Am J Physiol Gastrointest Liver Physiol. 2003;284:G875–G879. doi: 10.1152/ajpgi.00549.2002. [DOI] [PubMed] [Google Scholar]
- 51.Czaja MJ, Flanders KC, Biempica L, Klein C, Zern MA, Weiner FR. Expression of tumor necrosis factor-alpha and transforming growth factor-beta 1 in acute liver injury. Growth Factors. 1989;1:219–226. doi: 10.3109/08977198908997998. [DOI] [PubMed] [Google Scholar]
- 52.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 53.Dart RC, Kuffner E. Alcohol and acetaminophen hepatotoxicity. Arch Intern Med. 2003;163:244–245. doi: 10.1001/archinte.163.2.244-c. [DOI] [PubMed] [Google Scholar]
- 54.Das M, Sabio G, Jiang F, Rincon M, Flavell RA, Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21:219–223. doi: 10.1054/clnu.2001.0529. [DOI] [PubMed] [Google Scholar]
- 56.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 57.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 59.Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deiss LP, Galinka H, Berissi H, Cohen O, Kimchi A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 1996;15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- 62.DeLeve LD. Hepatic microvasculature in liver injury. Semin Liver Dis. 2007;27:390–400. doi: 10.1055/s-2007-991515. [DOI] [PubMed] [Google Scholar]
- 63.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47:296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]
- 64.Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–G424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekert PG, Read SH, Silke J, Marsden VS, Kaufmann H, Hawkins CJ, Gerl R, Kumar S, Vaux DL. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J Cell Biol. 2004;165:835–842. doi: 10.1083/jcb.200312031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 67.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States. Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 69.Faubion WA, Gores GJ. Death receptors in liver biology and pathobiology. Hepatology. 1999;29:1–4. doi: 10.1002/hep.510290101. [DOI] [PubMed] [Google Scholar]
- 70.Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 72.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 73.Feldstein AE, Gores GJ. An apoptosis biomarker goes to the HCV clinic. Hepatology. 2004;40:1044–1046. doi: 10.1002/hep.20479. [DOI] [PubMed] [Google Scholar]
- 74.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 75.Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1339–G1346. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 77.Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, Orlandini A, Sacchelli L, Missale G, Ferrari C. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut. 2009;58:974–982. doi: 10.1136/gut.2008.163600. [DOI] [PubMed] [Google Scholar]
- 78.Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, Elling F, Leist M, Jaattela M. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol. 2001;153:999–1009. doi: 10.1083/jcb.153.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galle PR, Krammer PH. CD95-induced apoptosis in human liver disease. Semin Liver Dis. 1998;18:141–151. doi: 10.1055/s-2007-1007150. [DOI] [PubMed] [Google Scholar]
- 82.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 84.Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem. 2008;19:567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghavami S, Hashemi M, Kadkhoda K, Alavian SM, Bay GH, Los M. Apoptosis in liver diseases–detection and therapeutic applications. Med Sci Monit. 2005;11:RA337–RA345. [PubMed] [Google Scholar]
- 86.Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis of pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399–1405. doi: 10.1002/hep.20952. [DOI] [PubMed] [Google Scholar]
- 87.Glantz A, Reilly SJ, Benthin L, Lammert F, Mattsson LA, Marschall HU. Intrahepatic cholestasis of pregnancy: amelioration of pruritus by UDCA is associated with decreased progesterone disulphates in urine. Hepatology. 2008;47:544–551. doi: 10.1002/hep.21987. [DOI] [PubMed] [Google Scholar]
- 88.Golstein P. Cell death: TRAIL and its receptors. Curr Biol. 1997;7:R750–R753. doi: 10.1016/s0960-9822(06)90000-1. [DOI] [PubMed] [Google Scholar]
- 89.Gores GJ, Herman B, Lemasters JJ. Plasma membrane bleb formation and rupture: a common feature of hepatocellular injury. Hepatology. 1990;11:690–698. doi: 10.1002/hep.1840110425. [DOI] [PubMed] [Google Scholar]
- 90.Gores GJ, Kaufmann SH. Is TRAIL hepatotoxic? Hepatology. 2001;34:3–6. doi: 10.1053/jhep.2001.25173. [DOI] [PubMed] [Google Scholar]
- 91.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 92.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guicciardi ME, Bronk SF, Werneburg NW, Yin XM, Gores GJ. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology. 2005;129:269–284. doi: 10.1053/j.gastro.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 94.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis. 2002;34:387–392. doi: 10.1016/s1590-8658(02)80033-0. [DOI] [PubMed] [Google Scholar]
- 96.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 98.Guicciardi ME, Miyoshi H, Bronk SF, Gores GJ. Cathepsin B knockout mice are resistant to tumor necrosis factor-alpha-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am J Pathol. 2001;159:2045–2054. doi: 10.1016/s0002-9440(10)63056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 100.Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K, Hatakeyama S. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, Yeh WC, Ohashi P, Wang X, Mak TW. Specific ablation of the apoptotic functions of cytochrome c reveals a differential requirement for cytochrome c and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 103.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 104.Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, Wickel M, Schneider-Brachert W, Trauzold A, Hethke A, Schutze S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 105.Higaki K, Yano H, Kojiro M. Fas antigen expression and its relationship with apoptosis in human hepatocellular carcinoma and noncancerous tissues. Am J Pathol. 1996;149:429–437. [PMC free article] [PubMed] [Google Scholar]
- 106.Higuchi H, Bronk SF, Taniai M, Canbay A, Gores GJ. Cholestasis increases tumor necrosis factor-related apoptotis-inducing ligand (TRAIL)-R2/DR5 expression and sensitizes the liver to TRAIL-mediated cytotoxicity. J Pharmacol Exp Ther. 2002;303:461–467. doi: 10.1124/jpet.102.040030. [DOI] [PubMed] [Google Scholar]
- 107.Higuchi H, Gores GJ. Bile acid regulation of hepatic physiology. IV. Bile acids and death receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G734–G738. doi: 10.1152/ajpgi.00491.2002. [DOI] [PubMed] [Google Scholar]
- 108.Higuchi H, Grambihler A, Canbay A, Bronk SF, Gores GJ. Bile acids up-regulate death receptor 5/TRAIL-receptor 2 expression via a c-Jun N-terminal kinase-dependent pathway involving Sp1. J Biol Chem. 2004;279:51–60. doi: 10.1074/jbc.M309476200. [DOI] [PubMed] [Google Scholar]
- 109.Higuchi H, Yoon JH, Grambihler A, Werneburg N, Bronk SF, Gores GJ. Bile acids stimulate cFLIP phosphorylation enhancing TRAIL-mediated apoptosis. J Biol Chem. 2003;278:454–461. doi: 10.1074/jbc.M209387200. [DOI] [PubMed] [Google Scholar]
- 110.Hikita H, Takehara T, Shimizu S, Kodama T, Li W, Miyagi T, Hosui A, Ishida H, Ohkawa K, Kanto T, Hiramatsu N, Yin XM, Hennighausen L, Tatsumi T, Hayashi N. Mcl-1 and Bcl-xL cooperatively maintain integrity of hepatocytes in developing and adult murine liver. Hepatology. 2009;50:1217–1226. doi: 10.1002/hep.23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hiramatsu N, Hayashi N, Katayama K, Mochizuki K, Kawanishi Y, Kasahara A, Fusamoto H, Kamada T. Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology. 1994;19:1354–1359. [PubMed] [Google Scholar]
- 112.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci. 2009;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 114.Hoglen NC, Anselmo DM, Katori M, Kaldas M, Shen XD, Valentino KL, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Farmer DG. A caspase inhibitor, IDN-6556, ameliorates early hepatic injury in an ex vivo rat model of warm and cold ischemia. Liver Transpl. 2007;13:361–366. doi: 10.1002/lt.21016. [DOI] [PubMed] [Google Scholar]
- 115.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Inohara Chamaillard McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 119.Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ito Y, Takeda T, Umeshita K, Sakon M, Wakasa K, Matsuura N, Monden M. Fas antigen expression in hepatocellular carcinoma tissues. Oncol Rep. 1998;5:41–44. [PubMed] [Google Scholar]
- 121.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 122.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 123.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang JX, Mikami K, Venugopal S, Li Y, Torok NJ. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol. 2009;51:139–148. doi: 10.1016/j.jhep.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johansson AC, Steen H, Ollinger K, Roberg K. Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ. 2003;10:1253–1259. doi: 10.1038/sj.cdd.4401290. [DOI] [PubMed] [Google Scholar]
- 126.Jones BE, Lo CR, Liu H, Srinivasan A, Streetz K, Valentino KL, Czaja MJ. Hepatocytes sensitized to tumor necrosis factor-alpha cytotoxicity undergo apoptosis through caspase-dependent and caspase-independent pathways. J Biol Chem. 2000;275:705–712. doi: 10.1074/jbc.275.1.705. [DOI] [PubMed] [Google Scholar]
- 127.Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, Bouillet P, Thomas HE, Borner C, Silke J, Strasser A, Kaufmann T. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kahraman A, Barreyro FJ, Bronk SF, Werneburg NW, Mott JL, Akazawa Y, Masuoka HC, Howe CL, Gores GJ. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology. 2008;47:1317–1330. doi: 10.1002/hep.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kahraman A, Bronk SF, Cazanave S, Werneburg NW, Mott JL, Contreras PC, Gores GJ. Matrix metalloproteinase inhibitor, CTS-1027, attenuates liver injury and fibrosis in the bile duct-ligated mouse. Hepatol Res. 2009;39:805–813. doi: 10.1111/j.1872-034X.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 132.Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, Cretney E, Smyth MJ, Silke J, Hakem R, Bouillet P, Mak TW, Dixit VM, Strasser A. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, Pai R, Hymowitz SG, Ashkenazi A. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- 134.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kiener PA, Davis PM, Starling GC, Mehlin C, Klebanoff SJ, Ledbetter JA, Liles WC. Differential induction of apoptosis by Fas-Fas ligand interactions in human monocytes and macrophages. J Exp Med. 1997;185:1511–1516. doi: 10.1084/jem.185.8.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 138.Kluwe J, Pradere JP, Gwak GY, Mencin A, Minicis SD, Osterreicher CH, Colmenero J, Bataller R, Schwabe RF. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kohlhaas SL, Craxton A, Sun XM, Pinkoski MJ, Cohen GM. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem. 2007;282:12831–12841. doi: 10.1074/jbc.M700438200. [DOI] [PubMed] [Google Scholar]
- 140.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 142.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 144.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kuang AA, Diehl GE, Zhang J, Winoto A. FADD is required for DR4- and DR5-mediated apoptosis: lack of trail-induced apoptosis in FADD-deficient mouse embryonic fibroblasts. J Biol Chem. 2000;275:25065–25068. doi: 10.1074/jbc.C000284200. [DOI] [PubMed] [Google Scholar]
- 146.Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281–1287. doi: 10.1053/j.gastro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 147.Kumar S. Caspase 2 in apoptosis, the DNA damage response and tumour suppression: enigma no more? Nat Rev Cancer. 2009;9:897–903. doi: 10.1038/nrc2745. [DOI] [PubMed] [Google Scholar]
- 148.Kunstle G, Hentze H, Germann PG, Tiegs G, Meergans T, Wendel A. Concanavalin A hepatotoxicity in mice: tumor necrosis factor-mediated organ failure independent of caspase-3-like protease activation. Hepatology. 1999;30:1241–1251. doi: 10.1002/hep.510300517. [DOI] [PubMed] [Google Scholar]
- 149.Kurosawa H, Que FG, Roberts LR, Fesmier PJ, Gores GJ. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1587–G1593. doi: 10.1152/ajpgi.1997.272.6.G1587. [DOI] [PubMed] [Google Scholar]
- 150.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 151.Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27:173–193. doi: 10.1055/s-2007-979470. [DOI] [PubMed] [Google Scholar]
- 152.Lau AH, Szabo G, Thomson AW. Antigen-presenting cells under the influence of alcohol. Trends Immunol. 2009;30:13–22. doi: 10.1016/j.it.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 153.Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol. 2005;34(Suppl 1):S1–S3. doi: 10.1016/s1386-6532(05)00384-7. [DOI] [PubMed] [Google Scholar]
- 154.Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid “mechanisms of action and clinical use in hepatobiliary disorders. J Hepatol. 2001;35:134–146. doi: 10.1016/s0168-8278(01)00092-7. [DOI] [PubMed] [Google Scholar]
- 155.Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee CY, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128:1443–1455. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- 157.Lee KH, Feig C, Tchikov V, Schickel R, Hallas C, Schutze S, Peter ME, Chan AC. The role of receptor internalization in CD95 signaling. EMBO J. 2006;25:1009–1023. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Legembre P, Barnhart BC, Zheng L, Vijayan S, Straus SE, Puck J, Dale JK, Lenardo M, Peter ME. Induction of apoptosis and activation of NF-kappaB by CD95 require different signalling thresholds. EMBO Rep. 2004;5:1084–1089. doi: 10.1038/sj.embor.7400280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 160.Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, Kikuchi K, Ikehara S, Gershwin ME. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–2330. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 162.Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Harrison JM, Wiesner RH, Anderson ML, Lange SM. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–1290. doi: 10.1016/0016-5085(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 163.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 164.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 165.Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 169.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 170.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 171.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 173.Lum JJ, Pilon AA, Sanchez-Dardon J, Phenix BN, Kim JE, Mihowich J, Jamison K, Hawley-Foss N, Lynch DH, Badley AD. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l. J Virol. 2001;75:11128–11136. doi: 10.1128/JVI.75.22.11128-11136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Luo KX, Zhu YF, Zhang LX, He HT, Wang XS, Zhang L. In situ investigation of Fas/FasL expression in chronic hepatitis B infection and related liver diseases. J Viral Hepat. 1997;4:303–307. doi: 10.1046/j.1365-2893.1997.00053.x. [DOI] [PubMed] [Google Scholar]
- 175.Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- 176.Maher JJ. DAMPs ramp up drug toxicity. J Clin Invest. 2009;119:246–249. doi: 10.1172/JCI38178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 179.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 181.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, Coppel RL, Shimoda S, Ishibashi H, Gershwin ME. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802–808. doi: 10.1002/hep.20859. [DOI] [PubMed] [Google Scholar]
- 183.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 186.Masuoka HC, Guicciardi ME, Gores GJ. Caspase inhibitors for the treatment of hepatitis C. Clin Liver Dis. 2009;13:467–475. doi: 10.1016/j.cld.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74:2255–2264. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grutter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–45171. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 190.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 191.Minagawa M, Deng Q, Liu ZX, Tsukamoto H, Dennert G. Activated natural killer T cells induce liver injury by Fas and tumor necrosis factor-alpha during alcohol consumption. Gastroenterology. 2004;126:1387–1399. doi: 10.1053/j.gastro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 192.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 193.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mochizuki K, Hayashi N, Hiramatsu N, Katayama K, Kawanishi Y, Kasahara A, Fusamoto H, Kamada T. Fas antigen expression in liver tissues of patients with chronic hepatitis B. J Hepatol. 1996;24:1–7. doi: 10.1016/s0168-8278(96)80178-4. [DOI] [PubMed] [Google Scholar]
- 195.Mohd-Ismail NK, Deng L, Sukumaran SK, Yu VC, Hotta H, Tan YJ. The hepatitis C virus core protein contains a BH3 domain that regulates apoptosis through specific interaction with human Mcl-1. J Virol. 2009;83:9993–10006. doi: 10.1128/JVI.00509-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mundt B, Kuhnel F, Zender L, Paul Y, Tillmann H, Trautwein C, Manns MP, Kubicka S. Involvement of TRAIL and its receptors in viral hepatitis. FASEB J. 2003;17:94–96. doi: 10.1096/fj.02-0537fje. [DOI] [PubMed] [Google Scholar]
- 197.Mundt B, Wirth T, Zender L, Waltemathe M, Trautwein C, Manns MP, Kuhnel F, Kubicka S. Tumour necrosis factor related apoptosis inducing ligand (TRAIL) induces hepatic steatosis in viral hepatitis and after alcohol intake. Gut. 2005;54:1590–1596. doi: 10.1136/gut.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 199.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–253. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 200.Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46:2347–2352. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- 201.Oakes SA, Opferman JT, Pozzan T, Korsmeyer SJ, Scorrano L. Regulation of endoplasmic reticulum Ca2+ dynamics by pro-apoptotic BCL-2 family members. Biochem Pharmacol. 2003;66:1335–1340. doi: 10.1016/s0006-2952(03)00482-9. [DOI] [PubMed] [Google Scholar]
- 202.Olsson M, Vakifahmetoglu H, Abruzzo PM, Hogstrand K, Grandien A, Zhivotovsky B. DISC-mediated activation of caspase-2 in DNA damage-induced apoptosis. Oncogene. 2009;28:1949–1959. doi: 10.1038/onc.2009.36. [DOI] [PubMed] [Google Scholar]
- 203.Omata M, Yoshida H, Toyota J, Tomita E, Nishiguchi S, Hayashi N, Iino S, Makino I, Okita K, Toda G, Tanikawa K, Kumada H. A large-scale, multicentre, double-blind trial of ursodeoxycholic acid in patients with chronic hepatitis C. Gut. 2007;56:1747–1753. doi: 10.1136/gut.2007.120956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ozoren N, El-Deiry WS. Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia. 2002;4:551–557. doi: 10.1038/sj.neo.7900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 206.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 207.Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 208.Perlmutter DH. Autophagic disposal of the aggregation-prone protein that causes liver inflammation and carcinogenesis in alpha-1-antitrypsin deficiency. Cell Death Differ. 2009;16:39–45. doi: 10.1038/cdd.2008.103. [DOI] [PubMed] [Google Scholar]
- 209.Peter ME, Legembre P, Barnhart BC. Does CD95 have tumor promoting activities? Biochim Biophys Acta. 2005;1755:25–36. doi: 10.1016/j.bbcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 210.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pockros PJ, Schiff ER, Shiffman ML, McHutchison JG, Gish RG, Afdhal NH, Makhviladze M, Huyghe M, Hecht D, Olters-dorf T, Shapiro DA. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 212.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 213.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Kontgen F, Adams JM, Cory S. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 216.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 217.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 218.Ratziu V, Poynard T. Assessing the outcome of nonalcoholic steatohepatitis? It’s time to get serious. Hepatology. 2006;44:802–805. doi: 10.1002/hep.21391. [DOI] [PubMed] [Google Scholar]
- 219.Roberg K, Johansson U, Ollinger K. Lysosomal release of cathepsin D precedes relocation of cytochrome c and loss of mitochondrial transmembrane potential during apoptosis induced by oxidative stress. Free Radic Biol Med. 1999;27:1228–1237. doi: 10.1016/s0891-5849(99)00146-x. [DOI] [PubMed] [Google Scholar]
- 220.Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 222.Rosenfeld ME, Prichard L, Shiojiri N, Fausto N. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am J Pathol. 2000;156:997–1007. doi: 10.1016/S0002-9440(10)64967-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 224.Rudiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 225.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, Katze MG, Hussain MM, Song B, Swathirajan J, Wang J, Yau GD, Kaufman RJ. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci USA. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 230.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 231.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Schungel S, Buitrago-Molina LE, Nalapareddy P, Lebofsky M, Manns MP, Jaeschke H, Gross A, Vogel A. The strength of the Fas ligand signal determines whether hepatocytes act as type 1 or type 2 cells in murine livers. Hepatology. 2009;50:1558–1566. doi: 10.1002/hep.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Schwabe RF. Cell death in the liver-all roads lead to JNK. Gastroenterology. 2006;131:314–316. doi: 10.1053/j.gastro.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 234.Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 235.Secchiero P, Mirandola P, Zella D, Celeghini C, Gonelli A, Vitale M, Capitani S, Zauli G. Human herpesvirus 7 induces the functional up-regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) coupled to TRAIL-R1 down-modulation in CD4(+) T cells. Blood. 2001;98:2474–2481. doi: 10.1182/blood.v98.8.2474. [DOI] [PubMed] [Google Scholar]
- 236.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 237.Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 238.Shibata M, Kanamori S, Isahara K, Ohsawa Y, Konishi A, Kametaka S, Watanabe T, Ebisu S, Ishido K, Kominami E, Uchiyama Y. Participation of cathepsin B and D in apoptosis of PC12 cells following serum deprivation. Biochem Biophys Res Commun. 1998;251:199–203. doi: 10.1006/bbrc.1998.9422. [DOI] [PubMed] [Google Scholar]
- 239.Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, Lenardo MJ. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 240.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sirica AE, Nathanson MH, Gores GJ, Larusso NF. Pathobiology of biliary epithelia and cholangiocarcinoma: proceedings of the Henry M. and Lillian Stratton Basic Research Single-Topic Conference. Hepatology. 2008;48:2040–2046. doi: 10.1002/hep.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 244.Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature’s TRAIL–on a path to cancer immunotherapy. Immunity. 2003;18:1–6. doi: 10.1016/s1074-7613(02)00502-2. [DOI] [PubMed] [Google Scholar]
- 245.Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol. 2000;278:G992–G999. doi: 10.1152/ajpgi.2000.278.6.G992. [DOI] [PubMed] [Google Scholar]
- 246.Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson M, Bromme D, Krajewski S, Reed JC, Yin XM, Turk V, Salvesen GS. Lysosomal protease pathways to apoptosis. Cleavage of bid, not pro-caspases, is the most likely route. J Biol Chem. 2001;276:3149–3157. doi: 10.1074/jbc.M008944200. [DOI] [PubMed] [Google Scholar]
- 247.Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells–a mechanism of immune evasion? Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 248.Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc Natl Acad Sci USA. 1997;94:8744–8749. doi: 10.1073/pnas.94.16.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Takeda K, Kojima Y, Ikejima K, Harada K, Yamashina S, Okumura K, Aoyama T, Frese S, Ikeda H, Haynes NM, Cretney E, Yagita H, Sueyoshi N, Sato N, Nakanuma Y, Smyth MJ, Okumura K. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci USA. 2008;105:10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Takehara T, Takahashi H. Suppression of Bcl-xL deamidation in human hepatocellular carcinomas. Cancer Res. 2003;63:3054–3057. [PubMed] [Google Scholar]
- 252.Takehara T, Tatsumi T, Suzuki T, Rucker EB, 3rd, Hennighausen L, Jinushi M, Miyagi T, Kanazawa Y, Hayashi N. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 253.Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G498–G505. doi: 10.1152/ajpgi.00482.2007. [DOI] [PubMed] [Google Scholar]
- 254.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 255.Tardif KD, Mori K, Siddiqui A. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J Virol. 2002;76:7453–7459. doi: 10.1128/JVI.76.15.7453-7459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 257.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 258.Truneh A, Sharma S, Silverman C, Khandekar S, Reddy MP, Deen KC, McLaughlin MM, Srinivasula SM, Livi GP, Marshall LA, Alnemri ES, Williams WV, Doyle ML. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem. 2000;275:23319–23325. doi: 10.1074/jbc.M910438199. [DOI] [PubMed] [Google Scholar]
- 259.Tschopp J, Irmler M, Thome M. Inhibition of Fas death signals by FLIPs. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 260.Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology. 2010;138:305–314. doi: 10.1053/j.gastro.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 261.Turk B, Dolenc I, Zerovnik E, Turk D, Gubensek F, Turk V. Human cathepsin B is a metastable enzyme stabilized by specific ionic interactions associated with the active site. Biochemistry. 1994;33:14800–14806. doi: 10.1021/bi00253a019. [DOI] [PubMed] [Google Scholar]
- 262.Turk B, Stoka V, Rozman-Pungercar J, Cirman T, Droga-Mazovec G, Oresic K, Turk V. Apoptotic pathways: involvement of lysosomal proteases. Biol Chem. 2002;383:1035–1044. doi: 10.1515/BC.2002.112. [DOI] [PubMed] [Google Scholar]
- 263.Tzung SP, Fausto N, Hockenbery DM. Expression of Bcl-2 family during liver regeneration and identification of Bcl-x as a delayed early response gene. Am J Pathol. 1997;150:1985–1995. [PMC free article] [PubMed] [Google Scholar]
- 264.Uehara T, Bennett B, Sakata ST, Satoh Y, Bilter GK, Westwick JK, Brenner DA. JNK mediates hepatic ischemia reperfusion injury. J Hepatol. 2005;42:850–859. doi: 10.1016/j.jhep.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 265.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 266.Upton JP, Austgen K, Nishino M, Coakley KM, Hagen A, Han D, Papa FR, Oakes SA. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:3943–3951. doi: 10.1128/MCB.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 268.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 269.Vick B, Weber A, Urbanik T, Maass T, Teufel A, Krammer PH, Opferman JT, Schuchmann M, Galle PR, Schulze-Bergkamen H. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes. Hepatology. 2009;49:627–636. doi: 10.1002/hep.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Volkmann X, Fischer U, Bahr MJ, Ott M, Lehner F, Macfarlane M, Cohen GM, Manns MP, Schulze-Osthoff K, Bantel H. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46:1498–1508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 271.Von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R, Geerts A, Kolanus W, Knolle P, Diehl L. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology. 2009;49:1664–1672. doi: 10.1002/hep.22795. [DOI] [PubMed] [Google Scholar]
- 272.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 273.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 274.Walter D, Schmich K, Vogel S, Pick R, Kaufmann T, Hochmuth FC, Haber A, Neubert K, McNelly S, von Weizsacker F, Merfort I, Maurer U, Strasser A, Borner C. Switch from type II to I Fas/CD95 death signaling on in vitro culturing of primary hepatocytes. Hepatology. 2008;48:1942–1953. doi: 10.1002/hep.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 276.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 277.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 278.Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 279.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wei Y, Wang D, Gentile CL, Pagliassotti MJ. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem. 2009;331:31–40. doi: 10.1007/s11010-009-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 282.Werneburg N, Guicciardi ME, Yin XM, Gores GJ. TNF-alpha-mediated lysosomal permeabilization is FAN and caspase 8/Bid dependent. Am J Physiol Gastrointest Liver Physiol. 2004;287:G436–G443. doi: 10.1152/ajpgi.00019.2004. [DOI] [PubMed] [Google Scholar]
- 283.Werneburg NW, Guicciardi ME, Bronk SF, Gores GJ. Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am J Physiol Gastrointest Liver Physiol. 2002;283:G947–G956. doi: 10.1152/ajpgi.00151.2002. [DOI] [PubMed] [Google Scholar]
- 284.Werneburg NW, Guicciardi ME, Bronk SF, Kaufmann SH, Gores GJ. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. J Biol Chem. 2007;282:28960–28970. doi: 10.1074/jbc.M705671200. [DOI] [PubMed] [Google Scholar]
- 285.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 286.Wielockx B, Lannoy K, Shapiro SD, Itoh T, Itohara S, Vandekerckhove J, Libert C. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]
- 287.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 288.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, Omenetti A, Jung Y, Teaberry V, Choi SS, Guy CD, Pollard J, Charlton P, Diehl AM. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 289.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 290.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 291.Yano H, Fukuda K, Haramaki M, Momosaki S, Ogasawara S, Higaki K, Kojiro M. Expression of Fas and anti-Fas-mediated apoptosis in human hepatocellular carcinoma cell lines. J Hepatol. 1996;25:454–464. doi: 10.1016/s0168-8278(96)80204-2. [DOI] [PubMed] [Google Scholar]
- 292.Yared G, Hussain KB, Nathani MG, Moshier JA, Dosescu J, Mutchnick MG, Naylor PH. Cytokine-mediated apoptosis and inhibition of virus production and anchorage independent growth of viral transfected hepatoblastoma cells. Cytokine. 1998;10:586–595. doi: 10.1006/cyto.1998.0340. [DOI] [PubMed] [Google Scholar]
- 293.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 294.Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 295.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 296.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 297.Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B, Waltemathe M, Gosling T, Flemming P, Malek NP, Trautwein C, Manns MP, Kuhnel F, Kubicka S. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci USA. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 299.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 300.Zhao M, Antunes F, Eaton JW, Brunk UT. Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem. 2003;270:3778–3786. doi: 10.1046/j.1432-1033.2003.03765.x. [DOI] [PubMed] [Google Scholar]
- 301.Zhao Y, Ding WX, Qian T, Watkins S, Lemasters JJ, Yin XM. Bid activates multiple mitochondrial apoptotic mechanisms in primary hepatocytes after death receptor engagement. Gastroenterology. 2003;125:854–867. doi: 10.1016/s0016-5085(03)01066-7. [DOI] [PubMed] [Google Scholar]
- 302.Zhao Y, Li S, Childs EE, Kuharsky DK, Yin XM. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-alpha-induced liver injury. J Biol Chem. 2001;276:27432–27440. doi: 10.1074/jbc.M102465200. [DOI] [PubMed] [Google Scholar]
- 303.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]