Abstract
Context
Studies suggest that many survivors of critical illness suffer long-term cognitive impairment, but have not included pre-morbid measures of cognitive functioning and have not evaluated risk for dementia associated with critical illness.
Objective
To determine whether decline in cognitive function was greater among older individuals who experienced acute care or critical illness hospitalizations relative to those not hospitalized, and to determine whether the risk for incident dementia differed by these exposures.
Design
Analysis of data from a prospective cohort study 1994-2007.
Subjects
We drew the study population of 2,929 individuals from an ongoing prospective cohort of non-demented persons 65 years old and older residing in the Seattle area and belonging to a consumer-governed HMO. We included participants in the parent study with two or more study visits, but individuals experiencing hospitalizations for a diagnosis of primary brain injury were censored at the time of such injury.
Outcome Measures
The outcomes were the score on the Cognitive Abilities Screening Instrument (CASI) at follow-up study visits and incident dementia diagnosed in study participants, adjusted for baseline cognitive scores, age, and other risk factors.
Results
There were 1,601 subjects without a hospitalization, 1,287 subjects with one or more non-critical illness hospitalization, and 41 subjects with one or more critical illness hospitalizations. Adjusted CASI scores averaged 1.00 point lower for visits following acute care illness hospitalization compared to follow-up visits not following any hospitalization (95% CI -1.33 to - 0.70; p<0.001) and 2.13 points lower on average for visits following critical illness hospitalization (95% CI -4.24 to -0.03; p<0.047). There were 146 cases of dementia among those not hospitalized, 228 cases of dementia among those with one or more non-critical illness hospitalization, and 5 cases of dementia among those with one or more critical illness hospitalizations. The adjusted hazard ratio for incident dementia was 1.4 following a non-critical illness hospitalization (95% CI 1.1 to 1.7; p=0.001) and 2.3 following a critical illness hospitalization (95% confidence interval 0.9 to 5.7; p=0.089).
Conclusions
Among a cohort of non-demented older adults, acute care hospitalization and critical illness hospitalization were associated with greater cognitive decline when compared to no hospitalization. Non-critical illness hospitalization was significantly associated with the development of dementia.
Introduction
The incidence of critical illness syndromes such as acute lung injury and severe sepsis and the use of critical care procedures such as mechanical ventilation are increasing in the United States, and are higher for older adults 1-4. These trends, coupled with an aging population and a declining mortality rate among the critically ill, are resulting in a growing number of patients who are survivors of critical illness.
These survivors often suffer significant long-term morbidity, such as reduced physical function, increased psychological symptoms, and reduced quality of life that appears to be a direct consequence of critical illness and critical care therapies.5-7 Since over half of ICU bed-days in the U.S. are accounted for by patients 65 years of age and older, a large proportion of these survivors are in this age group.8, 9 There is a growing body of literature describing an association between critical illness and long-term cognitive impairment.6, 10-15 Abnormalities in cognitive domains of executive function, attention, and memory appear to be the most common, and have been demonstrated as long as 6 years after hospital discharge. 10, 11, 16 However, none of the studies thus far has included objective measures of cognitive function before critical illness, and none have evaluated the risk of incident dementia among survivors of critical illness.
Understanding the association between critical illness and neurocognitive impairment has the potential to improve the care of critically ill patients in several ways. First, a better understanding of such an association may allow improved anticipatory guidance, as physicians will be able inform patients and patients' families about the potential for cognitive impairment as a complication of critical illness. Second, understanding such factors has the potential to improve the care of critically ill patients by generating research aimed at modifying those therapeutic practices that may contribute to chronic neurocognitive impairment or by guiding the creation of specific rehabilitation programs for these patients. We used an existing cohort study conducting serial cognitive testing on older adults combined with administrative data from hospitalizations to examine associations between hospitalizations for acute illness or critical illness and cognitive decline and dementia in older persons.
Methods
Study Sample
This study involves analysis of data from an ongoing prospective cohort study, The Adult Changes in Thought (ACT) study. ACT is a population-based longitudinal study of aging and dementia designed to determine the incidence of cognitive impairment and dementia as well as risk factors for these conditions. The details of this study have been described elsewhere.17-19 The ACT study population was created from a random sample of non-demented persons 65 years old and older residing in the Seattle-area, not residing in a nursing home at baseline, and belonging to the Group Health Cooperative (GHC), a consumer-governed health maintenance organization. Participants were interviewed with structured questionnaires to obtain data, including demographic characteristics, medical history, memory and general functioning, and potential epidemiologic risk factors. Participants also received the Cognitive Abilities Screening Instrument (CASI)20 as initial screening for cognitive function, and were retested at each study visit. Those individuals identified as having dementia based upon the evaluations performed at the initial study visit were not enrolled in the parent study cohort. The original cohort was enrolled between 1994 and 1996, with 2581 out of 5422 eligible individuals agreeing to participate.17 Between 2000 and 2002, an expansion cohort was recruited, with an additional 811 individuals. In recent years a continuous enrollment strategy has been utilized with the goal of keeping the number of alive and at-risk individuals in the cohort at approximately 2000. Each study participant was evaluated approximately once every two years. The ACT study has an excellent completeness of follow-up index21 of over 95%. 21 of over 95%. Participants with 2 or more study visits during which a valid cognitive screening score was obtained in the parent study were included in the present study.
Cognitive Performance Test
The CASI provides quantitative assessment of attention, concentration, orientation, short-term memory, long-term memory, language ability, visual construction, list-generating fluency, abstraction, and judgment.20 The CASI has a potential range of 0 to 100, with higher scores indicating better cognitive performance. A score of 86 corresponds to a Mini-Mental State Examination score of 25 to 26. 22 In prior analyses of data from the ACT study, we found that the CASI had curvilinear scaling properties, such that a given number of standard CASI points was associated with a variable amount of cognitive ability at different parts of the ability spectrum. In that same paper we performed some simulation studies and demonstrated that use of standard scores with curvilinear tests produced biased estimates of rates of change compared to using item response theory (IRT) scores23. In the current study, we were interested in the effects of hospitalization and critical illness on estimates of cognition over time, so we needed a dependent variable that had linear scaling properties. We therefore performed analyses using IRT scores. We used parameter estimates from our previously published analyses23 to generate IRT scores at each study visit for each study participant on the same metric. We used Samejima's graded response model24, 25 and expectation a posteriori scoring using Parscale26. The scale for IRT scores was defined such that 0 was the mean score of non-demented participants at the most recent study visit and the standard deviation was 1.
Exposure Definitions
The present study linked data from this ongoing prospective cohort study with claims data from hospitalizations of study participants that were submitted to the HMO to which all study participants belonged. We examined diagnosis and procedure codes from all hospitalizations. Hospitalizations were identified as including critical illness by the presence of any one of a list of critical illness diagnosis and procedure codes from the International Classification of Diseases, Ninth Revision (ICD-9) (Table 1a). Non critical-ill hospitalization and critical illness hospitalization were each coded with an indicator variable, and this variable changed after the relevant exposure such that visits before such a hospitalization were considered unexposed and visits afterwards were considered exposed. In this way, the exposures to acute care and critical illness hospitalizations were each considered in a time-dependant fashion.We censored individuals who received one or more diagnosis codes for primary brain injury during any hospitalization (Table 1b) at the time such a hospitalization occurred.
Table 1. Diagnosis and Procedure Codes used to Identify Critical Illness Hospitalization.
| Description | ICD-9 Diagnosis Code(s) |
|---|---|
| Shock without mention of trauma | 785.5 (and all 5 digit breakouts) |
| Severe sepsis | 995.92 |
| Traumatic shock | 958.4 |
| Postoperative shock | 998.0 |
| Acute respiratory failure | 518.81 |
| Other pulmonary insufficiency, not elsewhere classified | 518.82 |
| Acute on chronic respiratory failure | 518.84 |
| Hypotension | 458 |
| Respiratory arrest | 799.1 |
| Cardiac arrest | 427.5 |
| Description | ICD-9 Procedure Code |
| Cardiopulmonary resuscitation, not otherwise specified | 99.60 |
| Closed chest cardiac massage | 99.63 |
| Continuous mechanical ventilation for 96 consecutive hours or more | 96.72 |
| Table 1b: Diagnosis Codes Defining Primary Brain Injury | |
|---|---|
| Description | ICD-9 Diagnosis Codes |
| Ischemic stroke | 430, 431, 432.0, 432.1, 432.9, 433.01, 433.11, 433.21, 433.31, 433.91, 434.01, 434.11, 434.9 |
| Brain hemorrhage | 430, 431, 432.1, 432.0, 432.9 |
| Head Trauma | 851, 852, 853, 854 |
Incident Dementia
We screened participants every two years using the CASI to identify cases of incident dementia. Scores on the CASI that were less than 86 prompted a full standardized clinical examination.19 The results of rescreening by the CASI and clinical and neuropsychological examinations were reviewed at a consensus diagnosis conference that included at least the examining physician, a neuropsychologist, another study physician, and the study nurse. Persons who did not meet the criteria for dementia continued to be followed in the ACT cohort17, 18. Persons who met the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)27, criteria for dementia were considered to have incident dementia. We examined dementia subtype (categorized as vascular dementia using DSM-IV criteria27, Alzheimer's disease using NINCDS-ADRDA criteria28, or other) by exposure category using the χ2 statistic.
Statistical Analysis
For those individuals who were hospitalized, we compared the change in standard total CASI score for the interval that included hospitalization to the change in standard total CASI score for the interval that preceded hospitalization. To further assess the association of critical illness or non-critical illness hospitalizations with cognitive function, we developed multiple linear regression models using population-averaged generalized estimating equations (GEE).29 The use of GEE requires the specification of a “working” correlation matrix, and if model-based standard error estimates are relied upon, correct inferential statistics require that the specified working matrix correctly describe correlation within the data. An exchangeable correlation matrix was specified, and the robust standard error (“Huber-White sandwich”) estimator was used to allow for valid inference even if the working correlation matrix was incorrectly specified.30 The outcome variable for these models was CASI score at follow-up study visits. The variables of baseline CASI score, age at study visit, gender, years of education, time since baseline study visit, and the presence of self-reported coronary heart disease (CHD, including a history of congestive heart failure, myocardial infarction, angina, or coronary artery bypass grafting) and cerebrovascular disease (CVD, including a history of stroke, cerebral hemorrhage, or small strokes/transient ischemic attack) at the baseline visit were added to the model a priori based upon known associations between these predictors and the risk of cognitive decline. To evaluate the effect of critical illness and non-critical illness hospitalizations on the slope of CASI decline, we tested interactions between time and the critical illness and non-critical hospitalization indicators. An additional model added race/ethnicity (self-indicated by study participants), baseline presence of self-indicated renal disease, pulmonary disease (including COPD and asthma), malignancy (including solid tumors, leukemia, and lymphoma but excluding skin cancer), rheumatologic disease (including rheumatoid arthritis, lupus, or autoimmune disease), and smoking history (with a binary indicator of active cigarette smoking and the total pack-years smoked). Results were compared to our primary model as a sensitivity analysis. These models were repeated using CASI IRT as the outcome variable. The pre-specified (primary) analyses for CASI and CASI IRT were performed as complete-case analyses, in that observations with missing values for any of the covariates in the models were excluded.
We assessed for associations between critical illness and non-critical illness hospitalizations and incident dementia using Cox proportional hazards regression. The time of dementia diagnosis was considered to be the study visit at which the dementia evaluation was initiated (i.e. when the individual scored ≤ 86 on the CASI) for those individuals subsequently found to meet diagnostic criteria for dementia. This time-point was chosen because our hypothesis was that critical illness results in the development of chronic cognitive impairment over a very short time period. This hypothesis was derived from literature demonstrating that a substantial proportion of survivors of critical illness have profound cognitive dysfunction present at hospital discharge.31 The time axis of the regression models was age at study visit, left-truncated at age at study entry. Persons who left the study or died before developing dementia were censored at their last examinations, and persons who remained dementia-free at their most recent study visit were censored at the most recent follow-up date. Age at study entry was included in the model. The baseline cognition score, gender, years of education, and the self-reported presence of CVD and CHD at study entry were considered probable confounders and were included in the regression model a priori. An additional model added race/ethnicity, baseline presence of self-indicated renal disease, pulmonary disease, malignancy, and rheumatologic disease as well as smoking history. Results were compared to our primary model as a sensitivity analysis. The Schoenfeld residual test was used to evaluate for violations of the proportional hazards assumption. The variable indicating the presence of CHD at baseline was found to violate the assumption of proportionality, and therefore was included in models as a time-varying-covariate. The prespecified (primary) analyses for incident dementia was performed as a complete-case analyses, in that observations with missing values for any of the covariates in that model were excluded.
We performed a sensitivity analysis excluding individuals observed for less than 4 years to evaluate for potential bias introduced by the fact that those patients who were observed to have experienced hospitalizations during study participation had greater median time under study. We performed an additional sensitivity analysis to evaluate for potential bias introduced by censoring or death. Persons with a low CASI score at the time they were censored might have a higher probability of developing dementia, and random censoring for those persons might not be appropriate. Therefore, we examined the last CASI score for those who died or withdrew from the study. Those persons having a CASI score lower than 86 at his or her final study visit were assumed to have gone on to develop dementia 1 year after their last study visit. We repeated analyses with this assumption in place to determine whether the association between hospitalization and incident dementia was altered.
We estimate that the study had power of 0.98 to detect a mean difference in follow-up CASI score of 4.2 points (the difference between mean baseline score and a score of 89, indicating possible mild cognitive impairment) between those with critical illness and those without hospitalization. We estimate that this study had power of 0.64 to detect a significant difference in the survival curves for time to incident dementia between those with critical illness and those never hospitalized using the log-rank test.
Statistical analyses were performed using Stata 10 (College Station, TX). All reported p-values were 2-sided, and results were considered statistically significant at the p<0.05 level. Both the parent study and this specific study were approved by the Institutional Review Board of Group Health Cooperative. All study participants provided written consent to study participation at study enrollment.
Results
There were 1,601 study participants who experienced no hospitalizations during study participation. There were 1,287 study participants who were hospitalized but never critically ill during study participation, and they experienced 2,514 hospitalizations. There were 41 patients meeting criteria for critical illness, and they experienced 43 critical-illness hospitalizations. Thirty of the individuals experiencing a critical illness during study participation experienced a total of 95 non-critical hospitalizations, although only 14 of these non-critical hospitalizations in 9 of these individuals occurred before the first interval during which they experienced a critical illness hospitalization. There were 2,931 follow-up study visits occurring any time after a non-critical illness hospitalization, 76 follow-up study visits occurring after critical-illness hospitalization, and 8,675 follow-up study visits occurring after no hospitalizations. Participants spent a median of 4.02 years under study before any hospitalization. Those who experienced acute care hospitalization spent a median of 4.07 years under study after this hospitalization, and those who experienced a critical care hospitalization spent a median of 3.67 years under study after critical illness hospitalization. The median time between the most recent non-critical illness hospital discharge and the first study visit following this hospitalization was 307 days, with an interquartile range (IQR) of 150-501 days. The median time between the most recent hospital discharge during an interval that included critical illness and the next study visit following this hospitalization was 365 days, with an IQR of 196-412 days; 94.3% of the study visits after an interval that included hospitalization took place more than 45 days after the most recent hospital discharge. Individuals experiencing a critical illness hospitalization during study participation were more likely to be men, had slightly lower mean education but slightly higher baseline CASI and CASI IRT scores, and were more likely to indicate a history of CHD at study entry (Table 2). There were 28 individuals who had missing values for one or more of the covariates included in the primary models (0.95% of the sample) and who were excluded from analyses.
Table 2. Characteristics of study patients at baseline visit, by hospitalization status.
| No hospitalizations During study (n=1601) |
One or more non-critical hospitalizations (n=1287) |
One or more critical illness hospitalizations (n=41) |
|
|---|---|---|---|
| Women, n(%) | 969 (60.5) | 752 (58.4) | 18 (44) |
| Mean age in years, (standard deviation) | 74.6 (6.0) | 75.4 (6.2) | 75.4 (6.6) |
| Race | |||
| White, n(%) | 1415 (88.4) | 1191 (92.5) | 39 (95) |
| Black | 79 (4.9) | 50 (3.9) | 1 (2) |
| Asian | 71 (4.4) | 32 (2.5) | 1 (2) |
| Am. Indian/Alaskan Native | 2 (0.1) | 3 (0.2) | -- |
| Other, including multiple | 33 (2.1) | 11 (0.9) | -- |
| Missing value | 1 (0.1) | -- | -- |
| Mean Education in years*, (sd) | 14.3 (3.1) | 13.8 (2.9) | 13 (3.1) |
| Coronary Heart Disease | |||
| Yes, n(%) | 253 (15.8) | 294 (22.8) | 15 (37) |
| No | 1344 (84.0) | 986 (76.6) | 25 (61) |
| Missing value | 4 (0.3) | 7 (0.5) | 1 (2) |
| Cerebrovascular Disease | |||
| Yes | 133 (8.3) | 141 (11.0) | 4 (10) |
| No | 1459 (91.1) | 1142 (88.7) | 37 (90) |
| Missing value | 9 (0.6) | 4 (0.3) | -- |
| Pulmonary Disease | |||
| Yes | 257 (16.1) | 228 (17.7) | 8 (20) |
| No | 1338 (83.6) | 1053 (81.8) | 33 (80) |
| Missing value | 6 (0.4) | 6 (0.5) | -- |
| Diabetes | |||
| Yes | 125 (7.8) | 146 (11.3) | 6 (15) |
| No | 1475 (92.1) | 1140 (88.6) | 35 (85) |
| Missing value | 1 (0.1) | 1 (0.1) | -- |
| Kidney Disease | |||
| Yes | 85 (5.3) | 93 (7.2) | 4 (10) |
| No | 1516 (94.7) | 1192 (92.6) | 37 (90) |
| Missing | -- | 2 (0.2) | -- |
| Malignancy | |||
| Yes | 267 (16.7) | 244 (19.0) | 6 (15) |
| No | 1333 (83.3) | 1043 (81.0) | 35 (85) |
| Missing | 1 (0.1) | -- | -- |
| Mean CASI score at baseline (sd) | 93.2 (4.7) | 92.9 (4.7) | 93.9 (4.4) |
| Median CASI IRT at baseline (interquartile range) | 0.27 (-0.23, 0.79) | 0.23 (-0.25, 0.72) | 0.59 (0.06, 0.95) |
| Mean change in CASI score, baseline study visit to last study visit (sd) | -1.83 (6.48) | -3.81 (8.10) | -5.28 (10.34) |
| Median follow-up time in years, (iqr) | 4.1 (2.0, 9.9) | 7.9 (4.1, 9.9) | 8.0 (4.1, 9.9) |
| Median number of study visits (iqr) | 3 (2, 5) | 5 (3, 6) | 4 (3, 6) |
Education missing for1 individual in each category
For those individuals with a critical illness hospitalization, the mean change in CASI score was -2.44 for the interval that included a critical illness hospitalization, compared to -0.84 for the preceding interval. For those individuals with a non-critical hospitalization, the mean change in CASI score was -1.75 for the interval that included a non-critical hospitalization, compared to -0.78 for the preceding interval. After adjusting for age at study visit, sex, baseline CASI score, years of education, time since baseline visit, and the baseline comorbidities of CHD and CVD, CASI scores were found to be 1.00 point lower on average for visits following non-critical illness hospitalization compared to follow-up visits not following any hospitalization (95% confidence interval 1.32 to 0.68 points lower; p<0.001) and 2.13 points lower on average for visits following critical illness hospitalization compared to follow-up visits not following any hospitalization (95% confidence interval 4.24 to 0.02 points lower; p=0.047) (Table 3). We observed similar results when CASI IRT rather than standard totel CASI was used (Table 3). The estimated differences in follow-up CASI and CASI IRT scores for visits following non-critical and critical illness hospitalizations were similar in models adjusting for the more exhaustive list of possible confounders (results not shown). Since the interaction term between time since baseline visit and the indicator variable for critical illness was not significant (p=0.387 in the model using CASI, and p=0.255 in the model using CASI IRT), we did not have evidence that the rate of decline of CASI scores differed before and after critical illness, though we may have been underpowered to detect this.
Table 3. Difference in follow-up cognitive scores, by hospitalization status*.
| Visit Status | |||
|---|---|---|---|
| Not following hospitalization | Following non-critical illness hospitalization | Following critical illness hospitalization | |
| Difference in follow-up CASI (95% CI, p-value) |
Referent | -2.27 (-2.61 to -1.93, p<0.001) |
-2.92 (-5.00 to -0.86, p=0.006) |
| Adjustedτ difference in follow-up CASI (95% CI, p-value) |
Referent | -1.00 (-1.33 to -0.70, p<0.001) |
-2.13 (-4.24 to -0.03, p=0.047) |
| Difference in follow-up CASI IRT (95%CI, p-value) |
Referent | -0.28 (-0.32 to -0.24, p<0.001) |
-0.27 (-0.45 to -0.09, p=0.003) |
| Adjustedτ difference in follow-up CASI IRT (95% CI, p-value) |
Referent | -0.12 (-0.16 to -0.08, p<0.001) |
-0.19 (-0.38 to -0.01, p=0.042) |
Linear regression with GEE to account for repeated observations, specifying an exchangeable correlation matrix and robust variance estimates
Adjusted for age at study visit, sex, baseline cognitive score, years of education, time since baseline visit, and the baseline comorbidities CHD and CVD.
There were 146 cases of dementia among those never hospitalized during study participation, 228 cases of dementia among those experiencing one or more non-critical illness hospitalizations but no critical illness hospitalizations during study participation, and 5 cases of dementia among those experiencing one or more critical illness hospitalizations during study participation. Review of the subtype of dementia diagnosed shows that among those diagnosed with dementia in this study, a higher proportion of individuals in the never-hospitalized group were diagnosed with Alzheimer's disease than in the other two groups (76% in the never-hospitalized group versus 60% in the group with one or more non critical-illness hospitalizations and 40% in the critical illness hospitalization group, χ2 test for difference in this proportion across groups p=0.004; Table 4). Additionally, there were 403 individuals referred for dementia evaluation but found not to meet criteria for dementia (157 in the never-hospitalized group, 243 in the non-critical illness hospitalization group, and 4 in the critical illness group). These individuals remained in the study following the assessments for dementia. After adjusting for age at study entry, sex, baseline CASI IRT score, years of education, and the baseline comorbidities of CHD and CVD, the hazard ratio for incident dementia following a non-critical illness hospitalization was 1.4 (95% confidence interval 1.1 to 1.7; p=0.002) and 2.2 following a critical illness hospitalization (95% confidence interval 0.9 to 5.7; p=0.093) (Table 5). Results of an analysis including the more exhaustive list of comorbidities as possible confounders were not substantially different (data not shown). When analysis was restricted to those individuals observed on study for at least 4 years, the observed association between non-critical illness hospitalization and dementia was not substantially different (hazard ratio 1.5, 95% confidence interval 1.2 to 2.0, p=0.001), but the precision of the estimated association between critical illness hospitalization and incident dementia was substantially reduced (point estimate of the hazard ratio 2.0, 95% confidence interval 0.6 to 6.5, p=0.263) due to the dramatically reduced sample size. When those censored individuals whose last observed CASI score was less than 86 were assumed to have developed dementia 1 year after their last study visit, the association between non-critical illness hospitalization and incident dementia remained significant but was attenuated (hazard ratio 1.2, 95% confidence interval 1.0 to 1.4, p=0.044) and the estimated association between critical illness hospitalization and incident dementia was also slightly attenuated (hazard ratio 1.7, 95% confidence interval 0.8 to 3.6, p=0.156).
Table 4. Dementia subtype (DSM-IV) by hospitalization status.
| No hospitalizations during study (n=146) |
One or more non-critical hospitalizations (n=228) |
One or more critical illness hospitalizations (n=5) |
|
|---|---|---|---|
| Alzheimer's type, n (%) | 111 (76) | 138 (60) | 2 (40) |
| Vascular, n(%) | 10 (7) | 26 (11) | 1 (20) |
| Other*, n(%) | 25 (17) | 64 (28) | 2 (40) |
Other category includes ‘Dementias due to General Medical Conditions’, ‘Substance-Induced Persisting Dementia, ‘Dementia due to Multiple Etiologies’, ‘Other or unknown cause’
Table 5. Risk of incident dementia by hospitalization status*.
| Hospitalization Status | |||
|---|---|---|---|
| No hospitalizations during study (n=1601) |
One or more non-critical hospitalizations (n=1287) |
One or more critical illness hospitalizations (n=41) |
|
| Cases of incident dementia, n | 146 | 228 | 5 |
| Risk of incident dementia, hazard ratio (95% CI, p-value) | Referent | 1.5 (1.3 to 1.9, p<0.001) | 1.7 (0.7 to 4.0, p=0.272) |
| Adjusted risk of incident dementia, hazard ratio (95% CI, p-value) | Referent | 1.4 (1.1 to 1.7, p=0.001) | 2.3 (0.9 to 5.7, p=0.089) |
Cox proportional hazards regression, with age as the time axis, left-truncated at age at study entry
Hazard ratios after adjusting for age at study entry, sex, baseline CASI IRT score, years of education, and baseline comorbidities of CHD and CVD, with the latter included as a time varying covariate.
Discussion
We have found that non-critical and critical illness hospitalizations were each associated with greater decline in cognitive functioning scores in a cohort of older adults. It is possible that hospitalization is merely a marker for cognitive decline or dementia that is in a pre-clinical stage or has not yet been diagnosed, and thus it is cognitive decline that is causing more hospitalizations. However, this is the first study demonstrating the association between critical illness and cognitive decline that has been able to adjust for pre-morbid cognitive screening scores as well as comorbid illness, and is also unique for its utilization of a control group for comparison. These features provide some evidence suggesting that factors associated with acute illness, and to a greater degree with critical illness, may be causally related with cognitive decline. We also found a significant association between non-critical illness hospitalizations and incident dementia, while thethe association between critical illness hospitalization and incident dementia was not statistically significant. These findings further strengthen the existing evidence regarding an association between hospitalization and cognitive impairment, and underscore the clinical relevance of this association. Given the absence of a significant interaction between time and our exposure indicators, we did not find evidence that the rate of cognitive decline changed after either non-critical or critical illness hospitalization. This suggests that an acute or critical illness may cause an abrupt loss of cognitive function rather than steepening the slope of decline or simply being a marker of cognitive decline.
The mechanisms through which critical illness may contribute to neurocognitive impairment are likely multiple, with evidence suggesting hypoxemia31-33, delirium34-36, hypotension37, glucose dysregulation38, systemic inflammation39, and sedative and analgesic medications40, 41 all may potentially play a role. Older adults are at greatly increased risk of dementia, and preexisting risk factors for cognitive decline likely put patients in this age group at elevated risk of brain injury from the mechanisms listed above. Patients cared for in an intensive care unit (ICU) are more likely to experience these exposures, but the exposures are likely present to a lesser degree in many acutely ill patients hospitalized outside of an ICU and not meeting our criteria for critical illness. While previous studies of long-term cognitive function in survivors of acute lung injury have failed to show an association between severity of illness and cognitive impairment6, 42, those studies have only examined small groups of patients with uniformly high severity of illness. In clinical practice, severity of acute illness exists on a continuum. Thus, the graded association or “dose-effect” we observed in this study, with greater impairment in cognitive function among survivors of critical illness than in non-critically ill hospitalized patients (albeit with overlapping confidence intervals), is not only biologically plausible but provides some support for an the inference of causation.
Further research to clarify the role that specific aspects of critical illness or its treatment play in the development of cognitive impairment is necessary. Such information will be important in the search for factors that are modifiable, with the hope of reducing the prevalence of cognitive impairment among survivors of critical illness. Such information will also assist clinicians in providing patients and their loved ones better information about long-term outcomes as they make the difficult decisions that often surround critical illness. The ability to predict those at greatest risk of cognitive impairment might also bolster the feasibility of early cognitive rehabilitation programs for survivors of critical illness. Finally, a better understanding of the mechanisms linking critical illness and cognitive impairment may advance our understanding of the pathophysiology of dementia more generally.
Limitations
There are several important limitations of this study. First, since our definition of critical illness is derived only from administrative data, it relies upon ICD-9 diagnosis and procedure codes. This definition has not been validated, and there is a risk of misclassification of exposure. However, a gold standard definition of what constitutes critical illness does not exist and misclassification seems likely to bias the results toward the null hypothesis which would make our estimates of cognitive decline due to critical illness conservative. Second, the time interval between study visits was substantial. While the longitudinal nature of this study is a strength, the fact that study visits occurred every two years means that a hospitalization is only one of a number of possible significant events that could result in cognitive decline. Third, this study is also limited by the lack of information regarding the minimal clinically relevant decline in CASI score, which creates challenges in interpreting the impact of follow-up CASI scores that were 2.13 points lower, on average, following critical illness. However, patients experiencing a hospitalization were found to have a subsequent risk of incident dementia that was 40% higher than those not hospitalized. Individuals experiencing a critical illness hospitalization were estimated to have 2.2 times the risk of incident dementia than those not hospitalized, although this association was not statistically significant. An association between hospitalization and incident dementia would have clearer clinical significance than a decline in CASI scores. Fourth is the possibility that survivors of critical illness who continued to participate in the ACT study may not be representative of all older survivors of critical illness, and this may limit the generalizability of the study findings. However, since such patients continuing on in the study may be healthier and likely have better cognitive and physical functioning than survivors as a whole, this limitation likely means that our findings may be an underestimate of the cognitive impact of critical illness. Finally, the fact that there were only 41 individuals identified who experienced critical illness and were observed for one or more study visits after such a hospitalization limited the power of this study to detect a statistically significant association between critical illness and incident dementia. This lack of power also prevented exploration of specific critical illness syndromes and cognitive decline. Despite these limitations, this is the first study to include a control group and adjust for baseline cognitive function, thereby providing stronger support for the hypothesis that critical illness leads to significant decline in cognitive function in some patients.
Summary
In conclusion, we found associations between acute care and critical illness hospitalizations and greater cognitive decline in analyses that adjusted for baseline (pre-morbid) cognitive function. We also found an association between acute care hospitalization and the subsequent risk for incident dementia.The estimated association between critical illness and the subsequent risk for incident dementia was of greater magnitude but not statistically significant. Further studies are needed to better understand the factors associated with acute and critical illness that may contribute to cognitive impairment.
Supplementary Material
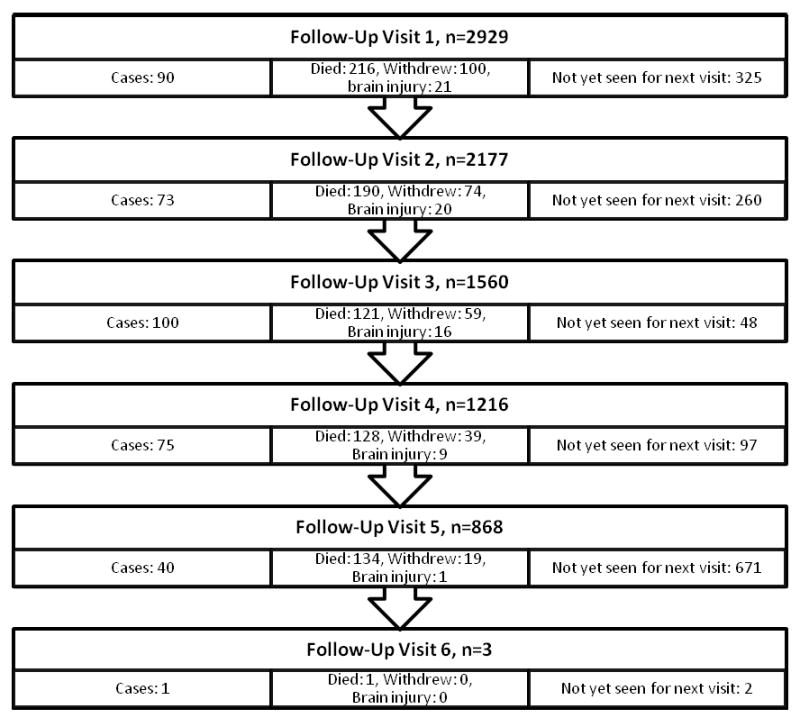
Figure 1. Flowchart of Follow-Up Visits.
Acknowledgments
Funding/Support: The Adult Changes in Though Study is funded by NIH National Institute on Aging Cooperative Grant (UO1 AG06781, PI: Eric B. Larson). Additional funding for this study was provided by John A. Hartford Foundation (PI: Catherine L. Hough) and a K24 Award from NHLBI (1 K24 HL068593, PI: J. Randall Curtis). Dr. Ehlenbach was supported by an NIH Respiratory Research Training Grant (5T32HL007287-30, PI: Leonard Hudson) and a Department of Veterans Affairs Health Services Research and Development Postdoctoral Fellowship.
Footnotes
Author Contributions: Dr. Ehlenbach had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ehlenbach, Hough, Carson, Curtis, Larson
Acquisition of data: Larson
Analysis and interpretation of data: Ehlenbach, Hough, Haneuse, Curtis, Larson
Drafting of the manuscript: Ehlenbach
Critical revision of the manuscript for important intellectual content: Ehlenbach, Hough, Crane, Haneuse, Carson, Curtis, Larson
Statistical analysis: Ehlenbach, Haneuse
Obtained funding: Larson, Hough, Curtis, Crane
Administrative, technical, or material support: Larson, Crane
Study supervision: Larson
Additional Contributions: We gratefully acknowledge the contributions of our colleague Steven L. Balch, BA, BS, MA, MBA, of the Group Health Research Institute, who assisted with data cleaning, management and analysis dataset creation. He did not receive compensation for these contributions.
Financial Disclosures: None reported.
Role of the Sponsors: The funding agencies played no role in the design and conduct of the study; the collection, analysis, or interpretation of the data; or the drafting of the manuscript.
References
- 1.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest. 2007 Feb;131(2):554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001 Jul;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Carson SS, Cox CE, Holmes GM, Howard A, Carey TS. The changing epidemiology of mechanical ventilation: a population-based study. J Intensive Care Med. 2006 May-Jun;21(3):173–182. doi: 10.1177/0885066605282784. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003 Feb 20;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005 Feb 15;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JC, Hart RP, Gordon SM, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003 Apr;31(4):1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J., Jr Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000 Dec 6;284(21):2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 9.Carson SS. The epidemiology of critical illness in the elderly. Crit Care Clin. 2003 Oct;19(4):605–617. v. doi: 10.1016/s0749-0704(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 10.Suchyta MR, Hopkins RO, White J, Jephson A, Morris AH. The incidence of cognitive dysfunction after ARDS. [abstract] Am J Respir Crit Care Med. 2004;169:A18. [Google Scholar]
- 11.Rothenhausler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001 Mar-Apr;23(2):90–96. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 12.Al-Saidi F, McAndrews MP, Cheung AM, et al. Neuropsychological sequelae in ARDS survivors [abstract] Am J Respir Crit Care Med. 2003;167:A737. [Google Scholar]
- 13.Hopkins RO, Herridge MS. Quality of life, emotional abnormalities, and cognitive dysfunction in survivors of acute lung injury/acute respiratory distress syndrome. Clin Chest Med. 2006 Dec;27(4):679–689. doi: 10.1016/j.ccm.2006.06.003. abstract x. [DOI] [PubMed] [Google Scholar]
- 14.Sukantarat KT, Burgess PW, Williamson RC, Brett SJ. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia. 2005 Sep;60(9):847–853. doi: 10.1111/j.1365-2044.2005.04148.x. [DOI] [PubMed] [Google Scholar]
- 15.Marquis K, Curtis J, Caldwell E, et al. Neuropsychological sequelae in survivors of ARDS compared with critically ill control patients [abstract] Am J Respir Crit Care Med. 2000;161:A383. [Google Scholar]
- 16.Hopkins RO, Jackson JC. Long-term neurocognitive function after critical illness. Chest. 2006 Sep;130(3):869–878. doi: 10.1378/chest.130.3.869. [DOI] [PubMed] [Google Scholar]
- 17.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002 Nov;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, van Belle G, Kukull WB, Larson EB. Predictors of functional change: a longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc. 2002 Sep;50(9):1525–1534. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- 19.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006 Jan 17;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994 Spring;6(1):45–58. doi: 10.1017/s1041610294001602. discussion 62. [DOI] [PubMed] [Google Scholar]
- 21.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002 Apr 13;359(9314):1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004 Dec;52(12):2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 23.Crane PK, Narasimhalu K, Gibbons LE, et al. Item response theory facilitated cocalibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol. 2008 Oct;61(10):1018–1027 e1019. doi: 10.1016/j.jclinepi.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samejima F. Estimation of latent ability using a response pattern of graded scores. Psychometrika Monograph. 1969:17. [Google Scholar]
- 25.Samejima F. Graded response model. NY: Springer; 1997. [Google Scholar]
- 26.PARSCALE for Windows [computer program] Version 4.1. Chicago: Scientific Software International; 2003. [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV. 4th. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association. Task Force on DSM-IV. [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–130. [PubMed] [Google Scholar]
- 30.Diggle PJ, Heagerty P, Liang K, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 2002. [Google Scholar]
- 31.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999 Jul;160(1):50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins RO, Gale SD, Johnson SC, et al. Severe anoxia with and without concomitant brain atrophy and neuropsychological impairments. J Int Neuropsychol Soc. 1995 Sep;1(5):501–509. doi: 10.1017/s135561770000059x. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain Cogn. 1995 Mar;27(2):180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- 34.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004 Jun;14(2):87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 35.Girard TD, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Ely EW. Duration of Delirium as a Predictor of Long-Term Cognitive Impairment in Survivors of Critical Illness. Am J Respir Crit Care Med. 2009 April 1;179(1_MeetingAbstracts):A5477. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolph JL, Marcantonio ER, Culley DJ, et al. Delirium is associated with early postoperative cognitive dysfunction. Anaesthesia. 2008 Sep;63(9):941–947. doi: 10.1111/j.1365-2044.2008.05523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopkins RO, Weaver LK, Chan KJ, Orme JF., Jr Quality of life, emotional, and cognitive function following acute respiratory distress syndrome. J Int Neuropsychol Soc. 2004 Nov;10(7):1005–1017. doi: 10.1017/s135561770410711x. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins RO, Jackson JC, Wallace C. Neurocognitive impairments in ICU patients with prolonged mechanical ventilation [abstract]. International Neuropsychological Society 33rd Annual Meeting Program and Abstracts; 2005. p. 60. [Google Scholar]
- 39.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 40.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007 Dec 12;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 41.Starr JM, Whalley LJ. Drug-induced dementia. Incidence, management and prevention. Drug Saf. 1994 Nov;11(5):310–317. doi: 10.2165/00002018-199411050-00003. [DOI] [PubMed] [Google Scholar]
- 42.Jackson JC, Gordon SM, Burger C, Ely EW, Thomason JW, Hopkins RO. Acute respiratory distress disorder and long-term cognitive impairment: a case study [abstract] Archives of Clinical Neuropsychology. 2003 October;18:687–807. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


