Abstract
Background
Human minor histocompatibility antigens (mHA) and clinically relevant immune responses to them have not been well defined in organ transplantation. We hypothesized that women with male kidney transplants would develop antibodies against H-Y, the mHA encoded on the Y-chromosome, in association with graft rejection.
Methods
We tested sera from 118 consecutive transplant recipients with kidney biopsies. Antibodies that specifically recognized the recombinant H-Y antigens RPS4Y1 or DDX3Y were detected by IgG enzyme-linked immunosorbent assay and western blotting. Immunogenic epitopes were further identified using overlapping H-Y antigen peptides for both the H-Y proteins.
Results
In the 26 female recipients of male kidneys, H-Y antibody development posttransplant (1) was more frequent (46%) than in other gender combinations (P<0.001), (2) showed strong correlation with acute rejection (P=0.00048), (3) correlated with plasma cell infiltrates in biopsied kidneys (P=0.04), and (4) did not correlate with C4d deposition or donor-specific anti-human leukocyte antigen (HLA) antibodies. Of the two H-Y antigens, RPS4Y1 was more frequently recognized (P=0.005).
Conclusion
This first demonstration of a strong association between H-Y antibody development and acute rejection in kidney transplant recipients shows that in solid organ allografts, humoral immune responses against well defined mHA have clear clinical correlates, can be easily monitored, and warrant study for possible effects on long-term graft function.
Keywords: Acute rejection, Alloantibodies, Kidney transplantation, H-Y antigens
Immunity to donor antigens belonging to the human major histocompatibility (human leukocyte antigen—HLA) complex (MHC) has long been recognized as important in the outcome of solid organ transplant. Clinical assays for detecting anti-HLA antibodies pretransplant have nearly eliminated the risk of hyperacute rejection and, recently, the clinical significance of posttransplant humoral immunity to HLA antigens has also been appreciated (1, 2). For example, posttransplant development of donor-specific HLA antibodies (DSA) can be detected in patients with acute rejection (AR) or chronic allograft nephropathy, and is predictive of subsequent graft failure (2–6). Additional evidence associating humoral immunity with graft damage in kidney allograft rejections include C4d deposition, B-cell (CD20+) infiltration, and transcription profiling consistent with B-cell activity (7–11).
Immunity to HLA antigens, however, does not explain all graft rejection because even HLA identical organ grafts can suffer AR and chronic allograft nephropathy, and ultimately fail. Evidence for the role of immunity to non-HLA (i.e., minor histocompatibility) antigens, is the higher rates of graft rejection associated with increased parity, prior blood transfusions, second transplants (12), MHC class I related chain A sensitization (13), and high panel reactive antibody (PRA) levels before HLA-identical related donor transplants (14).
Several minor histocompatibility antigens (mHA) have been shown to be polymorphic proteins capable of eliciting a coordinated T and B-cell response including generation of alloantibodies (allo-ab) (15). Unfortunately, the alloimmune responses to mHA often lack clear identification of the molecular targets or have weak clinical correlations. One model system for studying human allogeneic mHA immune responses is that of gender-mismatched transplantation (16–18). To characterize allo-ab responses to mHAs in kidney transplantation, we used the gender mismatch model system and focused on two genes, RPS4Y1 and DDX3Y, located on the Y chromosome. Each of these H-Y genes has an X chromosome homolog that is, respectively, 91% and 93% identical at the amino acid level, ubiquitously expressed, and escapes X inactivation (19–21). Males develop tolerance to their H-Y self-antigens, but female T and B cells are capable of recognizing peptides derived from H-Y proteins, a phenomenon observed in male recipients with female hematopoietic stem cell transplants (HCT) (22). We therefore hypothesized that female recipients of male kidneys (M→F) will develop allo-ab against H-Y antigens of the donor. This study shows that high titer antibodies do develop against H-Y antigens and their development strongly associates with renal allograft AR, thus providing the first evidence for clinically relevant allo-ab to well-defined human mHA in solid organ transplantation.
MATERIALS AND METHODS
Patient Characteristics
We studied 118 consecutive patients who underwent posttransplant renal biopsy, 112 (including all 26 of the M→F patients) for graft dysfunction and 6 per protocol, at Stanford University from 2003 to 2006. Among pretransplant characteristics (Table 1), only PRA showed significant differences. As expected, female recipients showed more HLA allosensitization (P=0.005), but without significant differences for M→F versus F→F or M→M versus F→M. An additional 15 patients with stable kidney function were obtained from the same transplant time-frame as age and gender-matched controls for the 26 M→F patients. Pretransplant sera and sera obtained at time of biopsy were available from all patients. These patients had been transplanted at Stanford Medical Center between 1995 and 2005 and were enrolled in an Institutional Review Board approved research protocol. Initial immunosuppressive agents varied but consisted primarily of a calcineurin inhibitor, steroids, and mycophenolate mofetil. Most patients with deceased donor transplants received induction with antithymocyte globulin but none had intravenous immunoglobulin.
TABLE 1.
Pretransplant characteristics of 118 patients with allograft dysfunction who underwent biopsy
| Recipient |
Female |
Male |
P | ||
|---|---|---|---|---|---|
| Donor | Male | Female | Female | Male | |
| Number | 26 | 22 | 31 | 39 | |
| Age, median (range) | 43 (26–76) | 42 (17–71) | 49 (24–70) | 44 (21–75) | NS |
| Deceased donor | 20 | 16 | 17 | 27 | NS |
| Second transplant | 2 | 1 | 3 | 6 | NS |
| Parity | 21 | 16 | NA | NA | NS |
| Delayed graft function | 6 | 2 | 6 | 9 | NS |
| HLA MM (mean±SD) | 2.8±2.4 | 3.7±1.9 | 4.1±1.6 | 3.9±1.8 | NS |
| Peak PRA >10% | 17 (65%) | 10 (45%) | 11 (35%) | 9 (27%) | (1) 0.005 for M (n=70) vs. F (n=48) recipients; (2) NS for M→F vs. F→F or for F→M vs. M→M |
| Race | |||||
| White | 12 | 7 | 11 | 10 | NS |
| Black | 3 | 1 | 2 | 3 | |
| Other | 11 | 14 | 18 | 26 | |
Detection of Anti-Human Leukocyte Antigen IgG Antibodies (Panel Reactive Antibodies or Donor Specific Antibodies) in Human Serum
Anti-class I and class II HLA IgG antibodies in patient sera were detected using the LABScreen Single Antigen kit (One Lambda, Canoga Park, CA) per manufacturer’s instructions.
Detection of H-Y and H-X Antibodies by Enzyme-Linked Immunosorbent Assay
Using H-Y antigen expression methods previously published (22), two H-Y genes (RPS4Y1 [Entrez gene ID=6192] and DDX3Y [Entrez gene ID=8653]) and their corresponding 91% to 93% identical X homologs (RPS4X [Entrez gene ID=6191] and DDX3X [Entrez gene ID=1654]) were reverse transcribed from male peripheral blood mononuclear cells, polymerase chain reaction amplified with primers derived from GenBank sequences, and complementary DNA sequences were confirmed. Each H-Y and H-X clone was expressed with a C-terminal V5 epitope tag and six histidine residues in Escherichia coli and purified by histidine affinity chromatography. Human immunodeficiency virus p24 was expressed and purified in a similar fashion to serve as a negative control for background nonspecific binding. Sera (1:50) were tested by H-Y enzyme-linked immunosorbent assay (ELISA) methods previously described (18).
Detection of Antibodies to H-Y and H-X Proteins Using Western Blotting
Purified H-Y and H-X proteins (2 μg/lane) were separated by sodium-dodecyl sulfate-polyacrylamide gel electrophoresis and were electrophoretically transferred onto Hybond C+ membranes (Amersham Biosciences, NJ). Proteins were detected with anti-V5 (Invitrogen, CA), or patient plasma (1:500). After washing, the membranes were incubated with secondary antibody goat anti-human IgG conjugated to horse radish peroxidase (Jackson Immuno Research Laboratories, West Grove, PA), and visualized by enhanced chemiluminescence (Amersham Biosciences).
Detection of H-Y Peptide Antibodies by Enzyme-Linked Immunosorbent Assay
Antibodies were tested by IgG ELISA against 93 individual overlapping DDX3Y and 23 RPS4Y1 peptides (New England Peptides, Fitchburg, MA). As previously described (22), peptides were generated using the amino acid sequence of the full length protein. RPS4Y1 peptides along with DDX3Y peptides are listed in supplemental Figure 1, available for viewing online only. The same IgG ELISA was performed with sera from a reference panel of 30 healthy men and the threshold for seropositivity was determined as mean +3 SD.
Histology and Immunohistochemistry
Initial diagnoses of hematoxylin-eosin and periodic acid-Schiff stained kidney biopsies were confirmed using the revised Banff criteria (3). Presence of plasma cells greater than 5% of cellular infiltrate was scored positive. Formalin fixed, paraffin embedded tissue was stained with polyclonal antiserum to C4d (Biomedica Gruppe, Austria), CD20 (DAKO Carpinteria, CA), CD138 (Serotec, Raleigh, NC), and CD38 (Novacastra, UK). C4d staining was considered positive if present in more than 10% of the peritubular capillaries or in any glomerular endothelial cells (23, 24). CD20 stain was positive if more than 275 stained cells were present per high power field. CD138 and CD38 stained for plasma cells and plasmablasts and the biopsies were semiquantitatively scored on a scale of 0 to 3, with normal less than 2. The CD38 scoring was validated by counting the positive cells in the biopsy core using 20× objective and the average number of positive cells/20× high power field was calculated. Compared with CD138, CD38 stain also highlights a subset of activated T and B lymphocytes but not the tubular epithelium that makes the interpretation of CD138 difficult.
Statistical Method
Significant differences between different gender donor-recipient groups were analyzed using Fisher’s exact test in univariate analysis. All P values were two-tailed. One-way analysis of variance with F statistics was used to measure differences between HLA mismatches, parity, and PRA measurements and Kruskal-Wallis rank-sum method was used to measure differences between age, race, and time of biopsy within the different gender combination subsets of patients. Correlations between antibody recognition of peptides versus cognate recombinant antigens were tested using Kappa Statistics. Multivariate analysis was performed by linear regression.
RESULTS
H-Y Antibody Responses Develop Primarily in Women With Male Kidney Transplants
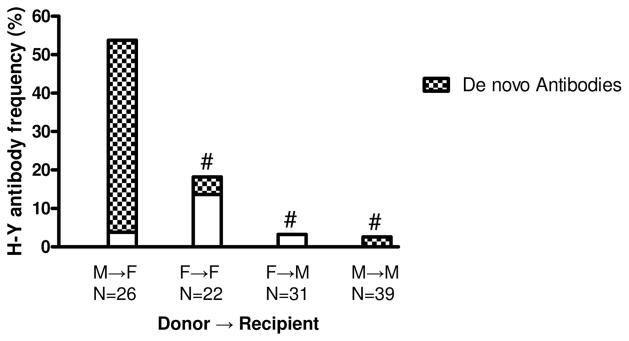
Posttransplant sera collected at the time of biopsy from 118 consecutive patients were tested by ELISA for IgG antibodies against the two H-Y antigens RPS4Y1 and DDX3Y. Figure 1 shows total antibody responses (stacked bars) by donor→recipient gender groups at the time of biopsy. As anticipated, H-Y antibody frequency was highest, 54%, in M→F patients (four made RPS4Y1 only, three made DDX3Y only, and seven made both). This was significantly more than the 3% in F→M (one patient made DDX3Y only; P=0.00001), the 3% in M→M (one patient made DDX3Y only; P=0.000001) or the 18% in F→F patients (three made RPS4Y1 only and one made DDX3Y only; P=0.01).
FIGURE 1.
H-Y antibodies are more frequently detected in M→F patients. Frequency of patients with RPS4Y1 and/or DDX3Y Ab at the time of biopsy. Heights of the stacked bars represent the frequency in each transplant group of H-Y antibodies detected by IgG ELISA at the time of biopsy. De novo antibodies against DDX3Y or RPS4Y1 at the time of biopsy are shown by the hatched areas. Both total (P<0.01) and de novo (P<0.001) H-Y antibody in M→F vs. each other group were highly significant (Fisher’s exact test, #P<0.001). Other group comparisons did not show statistical significance.
As H-Y antibodies present at the time of rejection could have formed pretransplant or developed posttransplant, we separately analyzed de novo (i.e., developed posttransplant) antibodies (Fig. 1—hatched bars). In the group most at risk, the 26 M→F patients, only seven had preformed antibody. In contrast, de novo antibodies to H-Y were relatively common; 10 of 11 anti-RPS4Y1 were de novo, as were 6 of 10 anti-DDX3Y. Comparison of de novo antibody in the different gender combinations was also informative. For M→F patients, 46% developed de novo responses to one or both of the H-Y antigens tested (six RPS4Y1 only, two DDX3Y only, and four both). This is significantly higher than in any of the other gender combinations; that is, versus 3% for F→F (P=0.001), versus 3% for M→M (P<0.00002), and versus 0% for F→M (P<0.00002).
Antibodies Are Specific for H-Y and Not H-X in M→F Patients
To confirm this first demonstration of H-Y antibodies in solid organ transplant recipients, we tested all 26 M→F patients by western blot. There was complete concordance between ELISA and western blots for both, RPS4Y1 and DDX3Y (Supplemental Fig. 2, available for viewing online only), demonstrating antibody responses were specific, for example, reacting with RPS4Y1 but not its 93% identical X homolog, RPS4X.
Twenty-three overlapping peptides were synthesized to cover the entire 260 amino acid RPS4Y1 and 93 peptides for 660 amino acid DDX3Y protein (22) (Supplemental Fig. 1, available for viewing online only). M→F and M→M patient sera were tested for IgG antibody by ELISA for each individual peptide. The presence of antibodies to full-length recombinant RPS4Y1 or DDX3Y protein correlated with the presence of antibody to detecting at least one constituent peptide (Supplemental Table 1, available for viewing online only) thus validating this approach. Of the 11 patients with antibodies against RPS4Y1 protein, eight (73%) were also positive for at least one RPS4Y1 peptide (range 1–4 peptides). Conversely, the 15 patients lacking antibody against RPS4Y1 protein were also negative for peptide recognition (κ=0.755). As controls, all 39 M→M kidney transplant patients were also screened for antibodies to RPS4Y1 peptides by IgG ELISA. Only one of the 39 M→M patients recognized a peptide but did not recognize the protein (κ=0.655). Similar concordance between protein and peptide recognition was found with DDX3Y (κ=0.76 for 26 M→F patients and κ=1.0 for M→M patients; Supplemental Table 1, available for viewing online only).
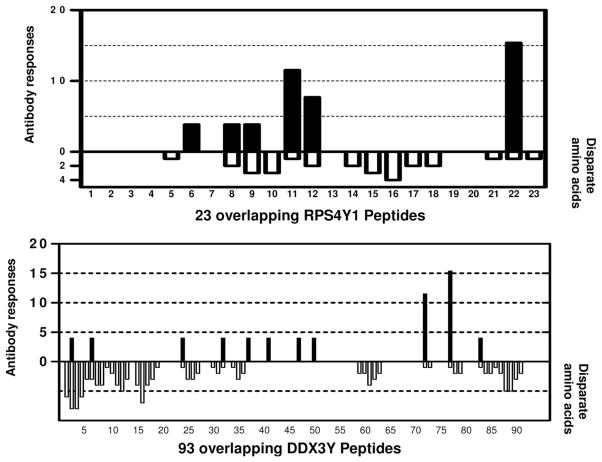
Figure 2 presents the frequency with which the 26 M→F patients recognized each individual RPS4Y1 peptide and DDX3Y peptides by IgG ELISA. The number of disparate amino acids between RPS4Y1 and RPS4X homologous peptide sequences is shown below the x-axis, demonstrating that the antibodies preferentially recognized regions that were disparate between the X and Y homologs. RPS4Y1 peptide 22 and DDX3Y peptide 77 were the most frequently recognized peptides. Of the six RPS4Y1 peptides recognized by antibody, five had disparate amino acids from their RPS4X homologs. We synthesized and tested the X-homologs of all these five peptides, and except for a single patient’s antibody reacting to RPS4X peptide 11, all responses were Y-homolog-specific (data not shown). One patient (with AR) developed an antibody response against a nondisparate RPS4Y1 peptide (peptide 6) and two patients (non-AR) developed antibody responses against four nondisparate regions of DDX3Y (one patient recognized nondisparate peptides 38 and 50 plus disparate peptide 83, and one patient recognized nondisparate peptides 41 and 47 plus disparate peptide 2). Because nondisparate regions of Y encoded protein were targeted in these three patients, we hypothesized that sera of these patients would also recognize the X-encoded relevant full length proteins. Two of these three patients’ sera did react against the relevant RPS4X or DDX3X protein, thus further supporting concordance of peptide specificity with full length protein recognition.
FIGURE 2.
Frequency of antibody responses against RPS4Y1 and DDX3Y peptides in 26 M→F patients. Sera from 26 M→F patients were tested by IgG ELISA, for antibodies against 23 overlapping RPS4Y1 peptides and 93 overlapping DDX3Y peptides. The solid bars indicate the frequency of the antibody responses to each peptide and the open bars report the number of amino acids disparate for X vs. Y homologs by comparing RPS4YX/DDX3X sequence with each RPS4Y1/DDX3Y peptide tested.
Although anti-H-Y antibody would be expected only in female patients, we did observe the rare occurrence of de novo anti-H-Y activity in our IgG ELISA assay for one male patient (Fig. 1). For this single M→M patient, serum reacted against the nondisparate peptides RPS4Y1 peptides 4 and 7. Interestingly, nonsynonymous single nucleotide polymorphisms (nsSNP) have been detected in the corresponding RPS4X regions (blocked residues in Supplemental Fig. 1A, available for viewing online only), and it is possible that disparity in nongender associated nsSNP could induce antibodies. However, these SNP allele frequencies are currently unknown, and the absence of donor DNA precluded genotyping to address this issue. Overall, our peptide epitope mapping studies showed only rare gender cross-reactivity, with most of the anti-H-Y antibodies targeting only disparate male-specific epitopes.
Pretransplant Characteristics and De Novo H-Y Antibody
Relevant preclinical features of the 26 M→F patients are listed in Table 2. By univariate analysis, only donor age was significantly associated with de novo antibody development however significance was lost in the posttransplant multivariate analysis. Importantly, H-Y antibodies developed in patients receiving mismatched or matched HLA grafts and were not dependent on HLA presensitization (PRA >10%) or previous pregnancies. Antibodies specific to RPS4Y1 or DDX3Y were not associated with donor HLA—A, B, DRB1, or recipient DRB1 antigens.
TABLE 2.
Univariate analysis of de novo H-Y antibody development and pretransplant characteristics in 26 M→F patients with graft dysfunction
| Patient characteristics (n=26 M→F) | H-Y antibody detected |
P | |
|---|---|---|---|
| Yes (n=12) | No (n=14) | ||
| Age of donor: median (range) | 31 (13–49) | 47 (24–57) | 0.02 |
| Age of recipient: median (range) | 44 (33–67) | 41 (26–76) | NS |
| Donor | |||
| Deceased | 11 | 9 | NS |
| Living | 1 | 5 | |
| HLA-mismatches: mean ± SD (number with 0 mismatches) | 3.0±2.4 (5) | 2.7±2.3 (5) | NS |
| Sensitized (peak PRA >10%) | 9 | 8 | NS |
| Race | |||
| White | 5 | 5 | NS |
| African American | 1 | 4 | |
| Others | 6 | 5 | |
| Diabetes | 3 | 1 | NS |
| Parity | 12 | 9 | NS |
Posttransplant Characteristics and De Novo H-Y Antibody: The Association With Acute Rejection in M→F Transplants
Preformed H-Y antibodies were present in seven of the 26 M→F patients. None of the 26 experienced hyperacute or accelerated ARs and only three of the seven patients suffered AR. Thus, the numbers were too small to reveal statistically significant differences.
All 26 patients were biopsied because of graft dysfunction. Fourteen patients (54%) had histologically proven AR; by Banff there were two borderline, three IA, six IB, one IIA, and two IIB. Twelve patients did not have AR on biopsy (eight drug toxicity, two recurrent disease, one acute tubular necrosis, one BK nephropathy). Of note, 11 of the 14 patients with AR developed de novo H-Y antibody versus only 1 of 12 without AR (P=0.00048, Table 3). Between the two H-Y antigens tested, RPS4Y1 was most frequently associated with AR (by univariate analysis), developing in 9 of 14 patients with AR, versus only 1 of 12 without AR (P=0.005).
TABLE 3.
De novo H-Y antibody development and histopathologic correlations in M→F patients with graft dysfunction
| Patient characteristics (n=26 M→F) | H-Y antibody detected |
P | |
|---|---|---|---|
| Yes (n=12) | No (n=14) | ||
| Biopsy assessment | |||
| AR | 11 (92%) | 3 (21%) | 0.00048 |
| Non-AR | 1 (8%) | 11 (79%) | |
| C4d positive | 1 (8%) | 0 (0%) | NS |
| CD20+B cells | 6 (50%) | 3 (21%) | NS |
| CD138+/CD38+/plasma cells | 8 (67%) | 3 (21%) | 0.04 |
| Clinical assessment | |||
| Delayed graft function | 5 (42%) | 3 (21%) | NS |
| DSA positive | 2 (17%) | 5 (36%) | NS |
| Graft failure | 1 (8%) | 2 (14%) | NS |
| Time of biopsy (d), median (range) | 724 (28–3386) | 435 (33–3077) | NS |
A control group of 15 additional M→F patients with stable allograft function, matched for age and time posttransplant, were also tested. Only two of the 15 controls showed de novo antibody development, a rate similar to that of non-AR patients. As shown in Figure 3, there was a strong association between de novo anti-H-Y antibodies and AR.
FIGURE 3.
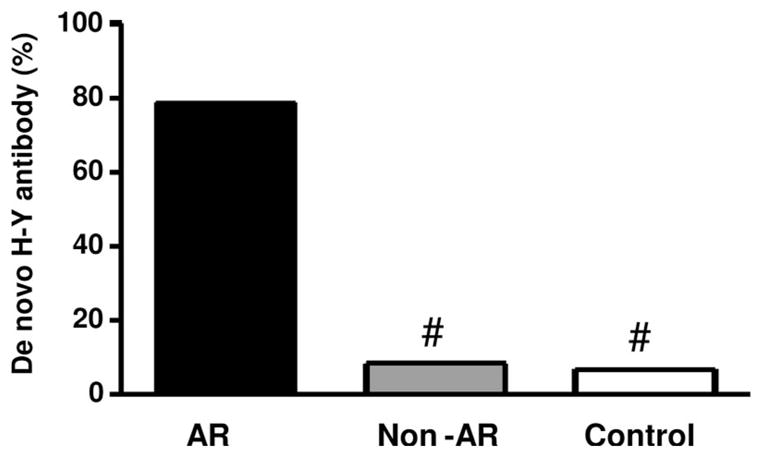
De novo H-Y antibodies associate with AR in M→F patients. Sera from 26 M→F patients with allograft dysfunction and who underwent biopsy were tested for de novo anti-H-Y by IgG ELISA. Fourteen patients had biopsy confirmed AR and 12 did not (non-AR). Sera from 15 patients with stable graft function were also tested as a control group. De novo antibody detection was significantly greater in patients with AR compared with patients with non-AR or stable graft function (Fisher’s exact test, # P<0.001).
De novo H-Y antibody development did not correlate with other indicators of humoral immunity such as C4d deposition, CD20 staining or with donor-specific anti-HLA antibodies (Table 3). Of 12 patients with de novo H-Y antibody responses, eight stained specifically for CD138, CD38 and had plasma cell infiltrates in their biopsies. In contrast, of the14 patients without de novo H-Y antibody formation, only three had any plasma cells (P=0.04). (A kidney biopsy from a female recipient with a 0 HLA mismatched male allograft is shown in Supplemental Figure 3 (available for viewing online only) demonstrating prominent plasma cell infiltrates with extensive tubular staining with both CD138 and CD38.) Plasma cell infiltration showed an association with H-Y antibody development and included three of the five patients transplanted with 0 HLA mismatches.
Those characteristics (donor age, AR, and plasma cell infiltration) that in univariate testing showed statistically significant associations with de novo antibody development were subjected to multivariate analysis. In this three variable model, the AR association remained highly significant (P=0.005) and the plasma cell infiltration association just missed the standard cutoff for significance (P=0.07), whereas the association with donor age failed to reach significance (P=0.3).
DISCUSSION
Alloimmunity studies in human solid organ allograft rejection have focused on both classical and nonclassical antigens in the MHC (HLA), with substantial progress in molecular definition of targets, clinically relevant associations, and understanding of both cellular and humoral immune mechanisms (2, 13).
In contrast, alloimmunity involving clinically relevant mHAs has not been well defined. Although non-HLA alloimmune responses have been suggested (14), they often lack clear identification of the molecular targets or have weak clinical correlations. The present cross-sectional analysis is the first study identifying the H-Y antigens, RPS4Y1 and DDX3Y, as likely clinically relevant mHAs which, in sex-mismatched kidney transplants, showed not only strong association between H-Y alloantibody development and AR, but also had substantial molecular characterization of the targeted antigens.
H-Y antigens were identified as mHA after observing that male to female mouse skin grafts were more often rejected than other gender combinations (16). Thus far, human mHA molecular identification has been pursued primarily in HCT where gender-mismatch is a significant risk factor associated with graft rejection or graft versus host disease (17, 25–28), and previous studies have demonstrated H-Y specific cellular and humoral immunity (18, 25). Human H-Y antigens have multiple T cell epitopes, with different HLA restrictions derived from Y-chromosome encoded proteins RPS4Y1, DDX3Y, UTY, and SMCY inducing robust immune responses with clinical significance (15, 25, 26, 29, 30). In contrast to alleles of autosomal encoded genes which have 2 to 3 coding SNP on average, the genes for H-Y antigens encode 1 to 245 polymorphisms in comparison with their X homologs. This high degree of polymorphism likely accounts for H-Y antigen immunogenicity and specificity. This is consistent with what we observe in our study where almost half of at risk patients (i.e., 46% of M→F) developed de novo allo-ab that specifically recognized H-Y antigens but not their X homologs.
To assess clinical relevance of antibodies to H-Y antigens for this study, the two best characterized outcome measures were graft loss or AR (30). We selected AR over graft loss because the smaller number of the latter would have been inadequate for statistical analysis. In this retrospective study of kidney transplant recipients, all subjects (except the 15 controls with stable graft function) underwent allograft biopsies, a step essential to establishing accurate diagnosis of AR. The availability of serum samples both pretransplant and at the time of biopsy allowed study of the relationship between AR and H-Y antibodies that were preformed versus those that were developed in response to the allogeneic stimulus of the transplant. Preformed H-Y antibodies were detected in 7 of 26 (26.9%) of our M→F patients but were too infrequent to significantly predict subsequent AR of male kidney.
In contrast, de novo antibodies to H-Y antigens developed in 46% of M→F after transplantation and strongly associated with AR in both univariate (P=0.00048) and multivariate (P=0.005) analysis. Our study is insufficient to explain the difference in clinical relevance for preformed versus de novo antibodies to H-Y antigens, but further on-going larger prospective studies of immunoglobulin isotypes, titers, and peptide epitope specificities may help elucidate these differences. H-Y antigens RPS4Y1 and DDX3Y are intracellular cytosolic and nuclear antigens, and therefore preexisting H-Y antibodies may be unable to cause hyperacute rejection by binding cell surface antigens as do anti-HLA or ABO antibodies. We hypothesize that the initial H-Y antigen recognition may result from graft destruction because of other causes, however, once H-Y antigen is indirectly presented to the host’s T and B cells, strong allogeneic antibodies could accelerate this alloimmune response through antibody dependent antigen presentation.
Even though this is a retrospective cross-sectional study, with respect to kinetics, antibody formation was observed with rejection episodes that occurred as early as 4 weeks and as late as 9 years posttransplant. This contrasts to our previous findings in HCT where H-Y antibodies developed late (i.e., no earlier than 4 months posttransplant) (18), presumably due to the time necessary for donor hematopoietic cells to establish immune reconstitution in their new host. With respect to antibody response profile, among at risk kidney transplant patients, antibody to RPS4Y1 developed fairly frequently (42% of the M→F). In contrast, our previous studies of HCT patients showed antibodies to RPS4Y1 developed only rarely (18) despite RPS4Y1 being ubiquitously expressed as an essential component of the ribosome. One explanation for the different profiles is that high expression of RPS4Y1 may elicit robust serologic response after single solid organ transplantation into a host with a mature immune system, but in HCT may instead induce tolerance when encountering the newly transplanted, donor lymphocytes that are not yet fully matured. Antibody studies were also carried out in our patients using two additional H-Y proteins, ZFY and UTY, however de novo allo-ab to those two H-Y antigens were observed too infrequently to allow meaningful statistical testing for significance (data not shown).
Identification of the immunogenic epitopes on the H-Y target molecules was investigated using overlapping peptides from RPS4Y1 and from DDX3Y as antigen targets. By antibody reactivity, there was a strong correlation between recognition of peptide and recognition of the cognate recombinant protein. Further, antibody allospecificity for Y versus X peptide homologs was clearly shown, with most responses directed against the disparate regions of the Y as compared with its X protein homolog. Consistent with gender allospecificity, none of the male patients had antibodies to RPS4Y1 however anomalous antibodies to DDX3Y was seen in one F→M patient pretransplant, one M→M de novo, and one F→F de novo. The fact that a very small number of the male recipients and one F→F recipient showed any anti-H-Y at all, along with the reactivity against nondisparate peptides for a small number of sera raise the question of auto versus allo H-Y antibodies. At present, we cannot exclude the possibility of some, albeit infrequent, autoantibody, particularly to DDX3Y where 4/11 peptides recognized by patient antibodies had no sequence difference between their X and Y homologs. An alternative hypothesis would invoke the existence of nongender-linked polymorphisms among H-Y or H-X genes. Studies are ongoing to test this hypothesis, that is, that M→M or F→F kidney transplant patients may have H-Y or H-X genes encoding nongender-linked nsSNPs encoding amino acid differences between the donor kidney and the recipient, and thus capable of eliciting alloantibody response to foreign H-Y or H-X proteins. For example, in addition to the 21 amino acids that differ in comparison with RPS4Y1, RPS4X has 17 nongender-linked nsSNPs (allele frequencies unknown; Supplemental Fig. 1A, available for viewing online only), disparity for which could induce antibody; and H-Y genes would be expected to show a similar degree of polymorphism.
Histological correlations of H-Y antibody production were also sought. Up-regulation of cells effecting humoral immunity has been reported in association with renal allograft rejection (8, 11), and this is often attributed to anti-HLA activity. In particular, plasma cell infiltrates in AR are associated with poor clinical outcomes in renal transplantation (31–33). Among our patients, an association between plasma cell infiltration and de novo H-Y antibody formation attained borderline statistical significance and we believe this deserves close examination in subsequent studies. In our current study, however, it is unlikely that the frequent plasma cell up-regulation we observed was entirely because of anti-HLA antibodies because donor specific anti-HLA antibodies (DSA) were detected in seven of the 26 (27%) M→F patients, but only two with H-Y antibody formation (16%) had DSA antibody. Further, five M→F patients had 0 HLA mismatch allografts and developed AR. All developed de novo antibodies to H-Y, three had plasma cell infiltrates, and one was an HLA genotypic match for whom nonclassical MHC (MHC class I related chain A) antibody can be excluded (13). Taken together this suggests plasma cell infiltrates in AR may target non-HLA molecules such as H-Y antigens. Thus far, however, our attempts to stain kidney biopsies of M→F with AR in situ using select FITC-conjugated RPS4Y1 and DDX3Y peptides have failed to demonstrate specific Borplasma cell staining(data not shown). An additional histological marker that has proven useful in the study of the humoral component of allograft rejection, at least for HLA incompatibilities, is immunostaining for deposited complement proteins. Specifically, HLA antibody mediated rejection after renal transplantation results in C4d deposition in 4% to 37% of biopsies (34, 35). In our study, C4d deposition was detected in only 1 of 14 (7%) M→F patients with AR, suggesting both that HLA antibody was not involved in the observed rejections or that anti-H-Y antibodies did not appear to fix significant amounts of complement.
This study demonstrates that posttransplant humoral allograft immunity can target mHAs encoded on the Y chromosome in female recipients of male kidney allografts. Ongoing longitudinal and prospective studies (36), and newer analysis of large registry databases (Dr. Alan Leichtman, personal communication, 2007), with strong multivariate analysis, are expected to provide further insight and support for the relevance of gender disparity in renal transplant outcomes. The association of humoral immunity to H-Y antigens and AR should be useful as a paradigm in the search for other relevant mHAs in solid organ clinical transplantation and may serve as a clinically useful biomarker of alloimmunity in gender-mismatch transplantation.
Supplementary Material
Acknowledgments
The authors thank the staff of the Stanford Histocompatibility Laboratory for their technical assistance.
Footnotes
The first two authors contributed equally to this study.
References
- 1.Terasaki PI, McClelland JD. Microdroplet assay of human serum cytotoxins. Nature. 1964;204:998. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 2.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3:665. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 3.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 4.Ishii Y, Sawada T, Kubota K, et al. Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int. 2005;67:321. doi: 10.1111/j.1523-1755.2005.00085.x. [DOI] [PubMed] [Google Scholar]
- 5.Hourmant M, Cesbron-Gautier A, Terasaki PI, et al. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16:2804. doi: 10.1681/ASN.2004121130. [DOI] [PubMed] [Google Scholar]
- 6.Andresdottir MB, Haasnoot GW, Doxiadis II, et al. Exclusive characteristics of graft survival and risk factors in recipients with immunoglobulin A nephropathy: A retrospective analysis of registry data. Transplantation. 2005;80:1012. doi: 10.1097/01.tp.0000179150.84803.56. [DOI] [PubMed] [Google Scholar]
- 7.Crespo M, Sole M, Arostegui JL, et al. Diagnostic value of C4d in renal allograft biopsies in different clinical settings: Absence of C4d in grafts from non-heart-beating donors. Transplant Proc. 2005;37:3688. doi: 10.1016/j.transproceed.2005.09.110. [DOI] [PubMed] [Google Scholar]
- 8.Hippen BE, DeMattos A, Cook WJ, et al. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant. 2005;5:2248. doi: 10.1111/j.1600-6143.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- 9.Herman J, Lerut E, van Damme-Lombaerts R, et al. Capillary deposition of complement C4d and C3d in pediatric renal allograft biopsies. Transplantation. 2005;79:1435. doi: 10.1097/01.tp.0000158420.26623.0f. [DOI] [PubMed] [Google Scholar]
- 10.Koo DD, Roberts IS, Quiroga I, et al. C4d deposition in early renal allograft protocol biopsies. Transplantation. 2004;78:398. doi: 10.1097/01.tp.0000128328.68106.54. [DOI] [PubMed] [Google Scholar]
- 11.Sarwal M, Chua MS, Kambham N, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 12.Opelz G. Success rate and impact of HLA matching on kidney graft survival in highly immunized recipients. Collaborative transplant study. Transpl Int. 1992;5(suppl 1):S601. doi: 10.1007/978-3-642-77423-2_176. [DOI] [PubMed] [Google Scholar]
- 13.Zou Y, Stastny P, Susal C, et al. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 14.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 15.Zorn E, Miklos DB, Floyd BH, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199:1133. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichwald EJ, Silmser CR. Skin graft success is limited by sex. Transplant Bull. 1955;2:148. [PubMed] [Google Scholar]
- 17.Goulmy E, Termijtelen A, Bradley BA, et al. Alloimmunity to human H-Y. Lancet. 1976;2:1206. doi: 10.1016/s0140-6736(76)91727-x. [DOI] [PubMed] [Google Scholar]
- 18.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenfield A, Carrel L, Pennisi D, et al. The UTX gene escapes X inactivation in mice and humans. Hum Mol Genet. 1998;7:737. doi: 10.1093/hmg/7.4.737. [DOI] [PubMed] [Google Scholar]
- 20.Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science. 1997;278:675. doi: 10.1126/science.278.5338.675. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet. 2002;11:1409. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- 22.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troxell ML, Weintraub LA, Higgins JP, et al. Comparison of C4d immunostaining methods in renal allograft biopsies. Clin J Am Soc Nephrol. 2006;1:583. doi: 10.2215/CJN.00900805. [DOI] [PubMed] [Google Scholar]
- 24.Sijpkens YW, Joosten SA, Wong MC, et al. Immunologic risk factors and glomerular C4d deposits in chronic transplant glomerulopathy. Kidney Int. 2004;65:2409. doi: 10.1111/j.1523-1755.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 25.Goulmy E, Termijtelen A, Bradley BA, et al. Y-antigen killing by T cells of women is restricted by HLA. Nature. 1977;266:544. doi: 10.1038/266544a0. [DOI] [PubMed] [Google Scholar]
- 26.Voogt PJ, Fibbe WE, Marijt WA, et al. Rejection of bone-marrow graft by recipient-derived cytotoxic T lymphocytes against minor histocompatibility antigens. Lancet. 1990;335:131. doi: 10.1016/0140-6736(90)90003-n. [DOI] [PubMed] [Google Scholar]
- 27.Spierings E, Vermeulen CJ, Vogt MH, et al. Identification of HLA class II-restricted H-Y-specific T-helper epitope evoking CD4+ T-helper cells in H-Y-mismatched transplantation. Lancet. 2003;362:610. doi: 10.1016/S0140-6736(03)14191-8. [DOI] [PubMed] [Google Scholar]
- 28.Randolph SS, Gooley TA, Warren EH, et al. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004;103:347. doi: 10.1182/blood-2003-07-2603. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Meadows LR, den Haan JM, et al. Human H-Y: A male-specific histocompatibility antigen derived from the SMCY protein. Science. 1995;269:1588. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- 30.Matas AJ, Gillingham KJ, Humar A, et al. Immunologic and nonimmunologic factors: Different risks for cadaver and living donor transplantation. Transplantation. 2000;69:54. doi: 10.1097/00007890-200001150-00011. [DOI] [PubMed] [Google Scholar]
- 31.Meehan SM, Domer P, Josephson M, et al. The clinical and pathologic implications of plasmacytic infiltrates in percutaneous renal allograft biopsies. Hum Pathol. 2001;32:205. doi: 10.1053/hupa.2001.21574. [DOI] [PubMed] [Google Scholar]
- 32.Charney DA, Nadasdy T, Lo AW, et al. Plasma cell-rich acute renal allograft rejection. Transplantation. 1999;68:791. doi: 10.1097/00007890-199909270-00011. [DOI] [PubMed] [Google Scholar]
- 33.Desvaux D, Le Gouvello S, Pastural M, et al. Acute renal allograft rejections with major interstitial oedema and plasma cell-rich infiltrates: High gamma-interferon expression and poor clinical outcome. Nephrol Dial Transplant. 2004;19:933. doi: 10.1093/ndt/gfh027. [DOI] [PubMed] [Google Scholar]
- 34.Herzenberg AM, Gill JS, Djurdjev O, et al. C4d deposition in acute rejection: An independent long-term prognostic factor. J Am Soc Nephrol. 2002;13:234. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]
- 35.Mengel M, Bogers J, Bosmans JL, et al. Incidence of C4d stain in protocol biopsies from renal allografts: Results from a multicenter trial. Am J Transplant. 2005;5:1050. doi: 10.1111/j.1600-6143.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 36.Tan JC, Wadia P, Grumet FC, et al. FOCIS. San Francisco, CA: 2006. Antibodies against H-Y minor histocompatibility antigens are associated with acute rejection in female recipients of male kidney transplants [abstract] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.