Abstract
Background
Apolipoprotein E ε4 (APOEε4) allele carrier status has been well established as a risk factor for developing Alzheimer’s disease. However, the specific influence of APOEε4 allele status on cognitive and functional rates of decline in MCI is poorly understood. We examine the prospective association of APOEε4 allele status on measures of cognitive and functional decline in subjects with amnestic Mild Cognitive Impairment (aMCI).
Methods
516 aMCI participants aged 55 to 90 who received placebo or Vitamin E from the Alzheimer’s Disease Cooperative Study’s MCI treatment trial were evaluated. During the 36 month study period, neurocognitive and functional measures were collected. These measures were assessed over time for change and association with APOEε4 status. Generalized Estimating Equations were performed to model each outcome measure over the study period.
Results
APOEε4 status had a significant impact on cognitive and functional decline on multiple measures; those who were APOEε4 positive had significantly more rapid decline in performance on all cognitive and functional measures except Number Cancellation and Maze tracing (p<0.05). The greatest decline was seen in global measures of cognition and function including the Clinical Diagnostic Rating scale, followed by the MMSE, Global Deterioration scale, and the ADAS-cog.
Conclusions
These findings demonstrate that APOEε4 genotype is predictive of increased general rates of decline with global measures of cognition and function most affected. With accelerated declines in common clinical trial primary efficacy measures, APOEε4 status needs to be accounted for in treatment trials of mild cognitive impairment.
Keywords: All Cognitive Disorders/Dementia, MCI (mild cognitive impairment), Alzheimer's disease, Risk factors in epidemiology, All genetics
1. Introduction
Mild cognitive impairment (MCI) has been accepted as a transitional state between normal aging and dementia. MCI can be delineated into two subtypes: amnestic MCI (aMCI) which includes memory impairment and non-amnestic MCI which includes non-memory cognitive impairment in domains such as attention, calculation, and visuspatial function (1). The aMCI subtype is of particular interest because those with this subtype are likely to progress to Alzheimer’s disease (AD) (1, 2); individuals with aMCI progress to AD at a rate of 10–15% per year compared to 1–2% per year among normal aging population (3, 4). Recognition of aMCI thus facilitates prediction of progression and perhaps initiation of treatment.
Several risk factors predict development of AD over one’s lifetime; however predictors associated with rates of decline in aMCI are still not well understood. The Apolipoprotein E e4 allele (APOEε4) is the best known genetic risk factor for late onset AD (5). Non-demented carriers of APOEε4 may experience accelerated cognitive decline compared to non-carriers and are at an increased risk of progressing from MCI to AD when controlling for other risk factors (6–9). APOEε4 is associated with increased overall rates of progression to AD, and may influence response to donepezil treatment, yet there is arguably insufficient data to support acetylcholinesterase inhibitor (ACHEI) use in this population (8). For this reason, there is still much irresolution amongst clinicians whether to test for APOEε4 status in aMCI patients (8). Research investigating rates of decline of various cognitive and functional scales by APOEε4 status among those with MCI has so far proven to be inconclusive. Several longitudinal studies have reported that APOEε4 is associated with cognitive decline among those without dementia (6, 10–15), and have shown APOEε4 to be predictive of the progression from aMCI to AD (8, 16). In contrast, a few studies have reported no association between APOEε4 and cognitive decline (17–19) or found it not to be predictive, by itself, in the progression of aMCI to AD (7, 20). Our current longitudinal cohort, derived from a randomized placebo-controlled treatment trial, allows us to better establish and define APOEε4-associated effects over time on specific cognitive and functional measures.
This analysis explored whether people with aMCI had differential decline over time in cognitive or functional measures associated with APOEε4 status. This study represents an unplanned post-hoc analysis of a cohort of participants from the Alzheimer’s Disease Cooperative Study’s (ADCS) MCI treatment trial (Clinicaltrials.gov identifier: NCT00000173) (8).
2. Methods
2.1 Participants
Cognitive and functional scores were obtained from participants in the ADCS randomized clinical drug trial of donepezil, vitamin E, or placebo investigating progression from aMCI to AD over 36 months, conducted between March 1999 and January 2004 (8, 21). A total of 2,264 participants were recruited from 69 ADCS sites from the United States and Canada. Initially 790 aMCI participants were randomized and 769 had baseline evaluations in the primary treatment trial. To be included, participants needed to be between 55 and 90 years old, meet the criteria for amnestic MCI of the degenerative nature (22), not meet criteria for dementia according to National Institute of Neurological and Communicative Disease and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (23), have impaired memory confirmed by an informant, a Clinical Dementia Rating (CDR) of 0.5, and a score of 24 to 30 on the Mini-Mental State Examination (MMSE). Participants were excluded from the study if they had a history of cerebral vascular disease resulting in a Hachinski score >4, depression measured as >12 on the Hamilton Depression Rating Scale, had any medical diseases that could potentially interfere with the study or were taking vitamins or supplements. Furthermore, all participants included in the study provided a blood sample for APOE genotyping. Details of study design were previously presented (8, 24).
Previous analysis of this aMCI cohort revealed no differential overall treatment effect of donepezil or vitamin E on progression to AD at 36 months. However, the donepezil arm appeared to have improved survival without AD at 12, 24 and 36 months in the APOEε4 positive subgroup (24). Therefore, to evaluate APOEε4-associated cognitive and functional decline, avoiding differential treatment effects, this analysis only evaluated those subjects randomized to placebo or vitamin E. This resulted in a cohort of 516 participants (257 in Vitamin E arm and 259 in Placebo arm) representing a sample of ‘untreated’ MCI participants.
2.2 Outcome measures
The primary outcome of the ADCS MCI trial was time to the development of possible or probable AD according to NINCDS-ADRDA (23). When participants had a clinical diagnosis of AD, all cognitive and functional data were first sent to the ADCS Coordinating Center then sent to a review committee for an agreement of the diagnosis. The outcome of interest in this current analysis was to assess whether baseline APOEε4 status results in a differential rate of change as measured by cognitive and functional scores. Clinical variables included MMSE (25), Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-cog) (26), Delayed Word List Recall (of ADAS-cog word list) (27) the New York University (NYU) Paragraph Recall Test (immediate and delayed) (28), the Symbol Digit Modalities Test (29), Category Fluency Test (30), a number cancellation test (31), Boston Naming Test (32), Digits Backward Test (33), clock drawing, and a maze tracing task (31). Furthermore, all participants were assessed regarding overall dementia severity and functional status; these variables included the Clinical Dementia Rating scale Sum of Boxes (CDR-SOB) (34), ADCS MCI- Activities of Daily Living (ADL) scale (35), and the Global Deterioration Scale (GDS) (36).
Participants underwent a screening and baseline assessment with 3 years of follow-up for all outcome measures which were collected every 3 months for the first 6 months of the trial (with the exception of MMSE, CDR-SOB, ADL and GDS which did not receive a month three assessment) and then every 6 months until 36 months or diagnosis of AD.
2.3 Conduct of study
This study was conducted according to Good Clinical Practice guidelines, the Declaration of Helsinki, and U.S. Code of Federal Regulations title 21 Part 50-Protection of Human Subjects and Part 56-Institutional Review Boards. Written informed consent was obtained from all participants and study partners before the study commenced.
2.4 Statistics
Analyses were performed on participants who received either the placebo (n=259) or Vitamin E (n=257). APOEε4 status was defined as negative (APOEε4−), no E4 alleles present, or positive (APOEε4+), at least one e4 allele present. Age, sex, and education were chosen a priori as potential confounders. Univariate analyses of the cognitive and functional scores were completed at each visit by APOEε4 status. Specifically, comparisons across APOEε4 status were conducted with Wilcoxon Rank-Sum test for continuous variables and Chi-Square tests for categorical variables. Correlation matrices of repeated scores at each follow-up visit using Pearson’s correlation were generated overall and by APOEε4 status for each outcome measure, as appropriate..
The Generalized Estimating Equations (GEE) approach for continuous or count data, as appropriate, was used to model each cognitive measure over the study period to assess differences in these outcomes of interest. The independent variables included in each model were APOEε4 status, time, and time by APOEε4 status interaction. Baseline ADAS-Cog11 (or CDR-SOB scores for ADAS-Cog outcomes) total score was included in each model to adjust for baseline severity. Time was treated as continuous, coded as months from baseline assessment; compound symmetry was assumed as the correlation structure unless the observed correlation matrix suggested otherwise. For each analysis, the potential confounders of baseline age, sex and years of education were assessed for balance by APOEε4 status and association with the outcome. If any of these variables were observed to be confounders, they were included in the model as a covariate. Additionally, a sensitivity analysis was performed on the primary outcome of interest, the ADASCog, using a mixed-effects regression model
Furthermore, in an attempt to compare rates of decline across outcome measures and graphically represent the results, each outcome was standardized by converting each subject’s raw score at each scheduled visit into a Z-score based on the baseline APOEε4 group specific mean and standard deviation, to represent unit-less group specific change from baseline. Then GEE analysis was repeated using the standardized scores to allow comparisons between rates of decline of each measure to illustrate which measures were most effected by APOEε4 status.
Since analyses were exploratory, no adjustments for multiple comparisons were made. P-value <0.05 was considered statistically significant. All analyses were conducted using the statistical software R (R Foundation for Statistical Computing, Vienna, Austria), version 2.6.2.
3. Results
Of the 516 participants who received either the placebo or vitamin E, 239 (46.3%) were APOEε4 negative and 277 (53.7%) were APOEε4 positive, with 18% of these participants having two e4 alleles present. 136 (52.5%) of the participants in the placebo arm and 141 (54.9%) in the vitamin E were APOEε4 positive (p = 0.65). There were no significant group differences at baseline in age (p = 0.85), education (p = 0.95) or sex (p = 0.38). APOEε4 carriers were more impaired at baseline on ADAS-Cog 11, ADAS-Cog 13, Delayed Word List Recall, MMSE, CDR-SOB, GDS, Clock Drawing, Category Fluency, NYU Delayed Paragraph Recall Immediate, NYU Delayed Paragraph Recall Delayed, Number Cancellation Target Hits, and Symbol Digit Modalities (all p-values <0.05). Baseline characteristics categorized by APOEε4 status are shown in Table 1.
Table 1.
Baseline characteristics
| APOEε4 non-carriers | APOEε4 carriers | P-value | |
|---|---|---|---|
| Participants (no.) | 239 (46.3%) | 277(53.7%) | |
| Age | 72.74±8.10 | 72.92±6.77 | 0.848 |
| Sex (%male) | 55.65% | 51.62% | 0.377 |
| Education (years) | 14.65±3.14 | 14.71±3.17 | 0.953 |
| Cognitive Measures | |||
| ADAS-cog-11 | 10.06±3.92 | 12.28±4.41 | <0.001 |
| ADAS-cog-13 | 15.87±5.50 | 19.30±6.0 | <0.001 |
| Delayed Word List Recall errors | 5.61±2.15 | 6.83±2.08 | <0.001 |
| MMSE | 27.54±1.84 | 27.05±1.83 | 0.003 |
| Digit Backwards | 6.33±2.08 | 6.18±1.92 | 0.366 |
| Boston Naming Test | 6.92±2.41 | 6.78±2.56 | 0.665 |
| Clock Drawing | 4.36±0.89 | 4.17±1.04 | 0.045 |
| Category Fluency | 16.63±5.28 | 15.22±4.99 | 0.003 |
| NYU Paragraph Recall Immediate |
4.66±2.41 | 3.65±2.12 | <0.001 |
| NYU Delayed Paragraph Recall Delayed |
4.44±2.97 | 2.94±2.65 | <0.001 |
| Number Cancellation Target Hits | 22.74±7.09 | 21.60±5.94 | 0.044 |
| Number Cancellation Target Errors | 0.11±0.59 | 0.20±1.2 | 0.371 |
| Symbol Digit Modalities | 32.61±11.51 | 30.83±10.25 | 0.035 |
| Maze Tracing* | - | - | 0.195 |
| Global Measures | |||
| CDR-SOB | 1.70±0.75 | 1.93±0.79 | <0.001 |
| GDS | 2.57±0.58 | 2.78±0.58 | <0.001 |
| Functional Measures | |||
| ADL | 45.70±5.25 | 45.72±4.71 | 0.59 |
P-value from Fisher’s exact test
ADAS-cog= Alzheimer’s Disease Assessment Scale-Cognitive Subscale; MMSE= Mini Mental State Exam; CDR-SOB=Clinical Dementia Rating Scale Sum of Boxes; GDS= Global Deterioration Scale; ADL= Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale.
Each cognitive score was compared at each completed visit by APOEε4 status. APOEε4 carriers were more impaired on the ADAS-Cog 11 compared to APOEε4 non-carriers at all visits (all p < 0.001). Similarly, APOEε4 carriers scored worse on ADAS-Cog 13, Delayed Word List Recall, NYU Delayed Paragraph Recall Delayed, NYU Delayed Paragraph Recall Immediate, MMSE, Category Fluency, Number Cancellation Target Hits and Symbol Digit Modalities compared to APOEε4 non-carriers at all visits (all p < 0.05). The Boston Naming and Clock Drawing baseline scores through month 6 were similar by APOEε4 status; however APOEε4 carriers began to score significantly lower at month 12 (all p< 0.05). The Digit Backward baseline scores through month 12 were similar by APOEε4 status (excluding month 6, at which time APOEε4 carriers scored significantly worse); however APOEε4 carriers began to score significantly worse compared to APOEε4 non-carriers from 18–36 months (all p< 0.05). The Maze Tracing baseline scores through month 18 were similar by APOEε4 status; however APOEε4 carriers began to score significantly worse compared to their APOEε4 non-carriers in subsequent visits (all p< 0.05). APOEε4 carriers did not differ from their APOEε4 non-carriers on the Number Cancellation Target Errors at any time point, except at Month 30 (p= 0.03). APOEε4 carriers were more impaired compared to APOEε4 non-carriers on the CDR-SOB, and the GDS at all visits (p < 0.001). Activities of daily living scores were similar by APOEε4 status through month 6; however APOEε4 carriers began to score significantly lower in subsequent visits (all p< 0.05).
The correlation matrices between observed raw scores over time for each cognitive, global and functional score appeared similar by APOEε4 status and supported the use of compound symmetry for the GEE Modeling. Age, education and sex did not meet our pre-specified definition of a confounder; therefore the final GEE model of each of the outcome measures remained unadjusted for these variables. Annualized rates of decline in raw cognitive, global and functional scores are shown in Table 2, with p-values representing the significance level of change over time associated with APOEε4 status from the GEE models. GEE models demonstrated that APOEε4 carriers had significantly increased rates of decline, with and without controlling for differences in baseline global cognitive status, on all outcome measures except Number Cancellation Target Errors (p-value 0.33) and Maze tracing (p-value 0.21). This included a statistically significant decline over time, with the APOEε4 carriers declining faster than non-carriers, on the CDR-SOB (p<0.001), MMSE (p<0.001), GDS (p<0.001), ADAS Cog11 (p<0.001), ADAS-Cog13 (p<0.001), ADL (p<0.001), Delayed Word List Recall (p<0.001), Digit Backwards (p<0.001), Boston Naming Test (p<0.001), Clock Drawing (p<0.004), NYU Paragraph Delayed Recall (p<0.001), NYU Paragraph Immediate Recall (p=0.012), Category Fluency (p<0.001), Symbol Digit Modality (p<0.001) and on the Number Cancellation Target Hits (p<0.001). Lastly, the estimate of APOEε4 by time interaction from the mixed-effects regression model evaluating sensitivity was 0.118 (SE 0.009) which was similar to the ones obtained by the GEE model 0.117 (RSE 0.017). Final GEE models of cognitive, global and functional outcome measures by APOEε4 status are shown in Table 2.
Table 2.
Annual rates of change in raw scores for continuous outcome measures
| APOEε4 non-carriers | APOEε4 carriers | P-value * | |
|---|---|---|---|
| Cognitive Measures | |||
| ADAS-cog-11 | 0.48 | 1.89 | <0.001 |
| ADAS-cog-13 | 0.58 | 2.37 | <0.001 |
| Delayed Word List Recall | |||
| errors | 0.07 | 0.37 | <0.001 |
| MMSE | −0.21 | −1.09 | <0.001 |
| Digit Backwards | 0.10 | −0.12 | <0.001 |
| Boston Naming Test | 0.08 | −0.02 | <0.001 |
| Clock Drawing† | −0.01 | −0.04 | 0.004 |
| Category Fluency | −0.08 | −0.81 | <0.001 |
| NYU Delayed | |||
| Paragraph Recall | |||
| Immediate | −0.05 | −0.20 | 0.012 |
| NYU Delayed | |||
| Paragraph Recall | |||
| Delayed | −0.13 | −0.36 | 0.001 |
| Number Cancellation | |||
| Target Hits | −0.47 | −1.09 | <0.001 |
| Number Cancellation | |||
| Target Errors † | 0.08 | 0.30 | 0.327 |
| Symbol Digit Modalities | |||
| 0.01 | −1.34 | <0.001 | |
| Global Measures | |||
| CDR-SOB | 0.18 | 0.73 | <0.001 |
| GDS | 0.05 | 0.27 | <0.001 |
| Functional Measures | |||
| ADL | −0.84 | −2.62 | <0.001 |
P-value relates to the significant level of the interaction term of APOEε4 status and time in each GEE model; unadjusted for multiple comparisons
GEE with outcome as Poisson.
For maze tracing task (ordinal), annual rate of decline is not appropriate to report. Neither group, APOEε4 non-carriers or APOEε4 carriers, showed a significant increase in number of errors (0,1,2) over time. P-value for interaction term= 0.21
ADAS-cog= Alzheimer’s Disease Assessment Scale-Cognitive Subscale; MMSE= Mini Mental State Exam; CDR-SOB= Clinical Dementia Rating Scale Sum of Boxes; GDS= Global Deterioration Scale; ADL= Alzheimer’s Disease Cooperative Study Activities of Daily Living Scale.
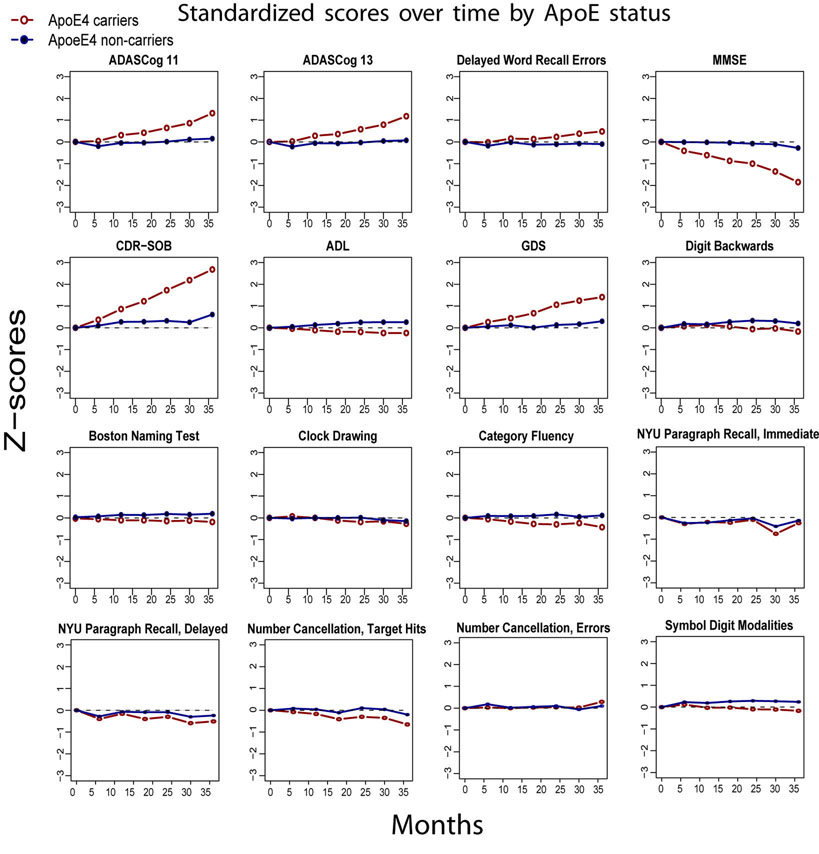
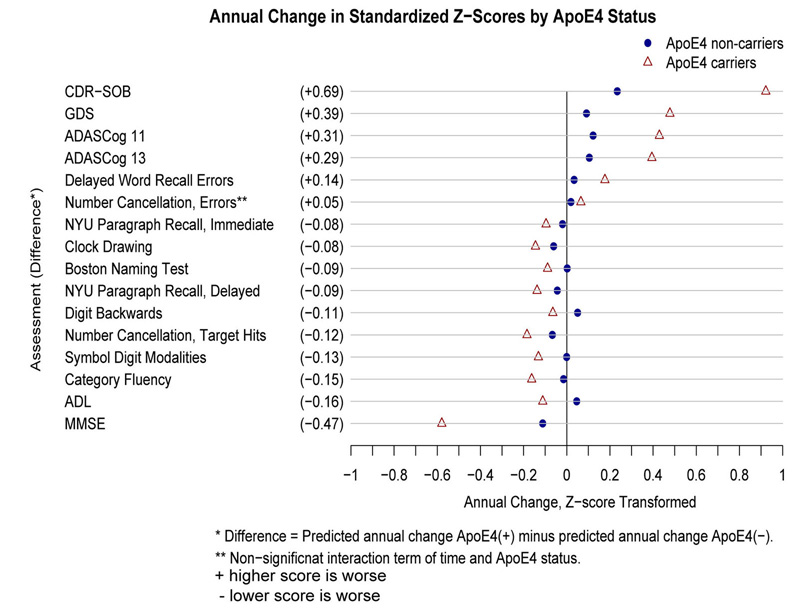
Transformation of the raw score outcome measures to standardized Z-scores demonstrated relative differences in rates of decline between outcome measures. Plots of standardized outcome measures over 36 months illustrating comparative trajectories of change over time for APOEε4 carriers versus non-carriers are shown in Figure 1. Figure 2 shows the rank ordering of relative rates of decline between measures, with the CDR-SOB, MMSE, GDS and ADAS-Cog showing the largest differences in standardized rates of decline between APOEε4 status groups, compared to measures of specific cognitive domains and activities of daily living.
Figure 1.
Thirty-six month plots of outcome measure scores converted to standardized Z-scores, illustrating differences in slopes of change between APOEε4 carriers and non-carries. Relative influences of the APOEε4 gene on each outcome measure are demonstrated.
Figure 2.
Plot demonstrating Z-score differences in standardized mean rates of change between APOEε4 carriers and non-carriers. It can be seen that APOEε4 carriers had more rapid rates of decline on most measures, with global cognitive and dementia status scores demonstrating the widest separation in APOEε4 groups. Note that scores marked with “+” indicate that higher scores are worse, and those with “−” indicate that lower scores are worse for specific measures.
4. Discussion
This study has observed that individuals with amnestic mild cognitive impairment who have one or more copies of the APOEε4 allele experience a more rapid rate of global cognitive decline and deterioration than those without the APOEε4 gene. This is detectible in measures of most cognitive domains, but is most apparent when looking at composite measures of global cognitive function or dementia severity such as the CDR, ADAS-cog, MMSE or Global Deterioration scale. Not only do APOEε4 carriers have an earlier age of dementia onset (37), they also appear to have a more rapid clinical decline once a diagnosis of amnestic MCI is established. This has potential implications in clinical management as well as clinical trial design. For instance, if a treatment trial is not stratified or balanced by APOEε4 status between treatment arms, it could bias trial results. Alternatively, enriching early treatment trial cohorts with APOEε4 subjects may improve treatment effect sizes and enable shorter trials with smaller cohorts for this target population; on the other hand, such a strategy would reduce the generalizability of the results. In the primary analysis of the ADCS donepezil and vitamin E treatment trial, there was no treatment effect in the full cohort at 36 months, yet the APOEε4 subset saw a significant treatment effect out to 36 months, with a one third reduction in conversions to AD in those subjects on donepezil (8). This APOEε4 influence likely drove the treatment effects seen in the full cohort at 12 months.
The prevalence of the APOE4 genotype in our aMCI cohort, 53.7%, is similar to prevalence rates reported in other population based aMCI studies (38). Previous studies have demonstrated that the apolipoprotein E ε4 gene is associated with increased risk of progressing from MCI to AD (39, 40), although not without controversy (20, 40, 41). It has also been found to be related to worse memory scores in MCI (42) and with memory decline in cognitively normal individuals (43, 44). This association with worsened early cognitive status and decline is accompanied by evidence of increased AD pathology associated with APOEε4 in cognitively normal individuals (45), MCI (46) and AD (47). These findings support a hypothesis that increased rates of cognitive decline, as presented here, may be associated with increased AD pathology burden in MCI subjects who carry the APOEε4 allele. Overall, we now know that the APOEε4 cohort from this study had more AD converters in 36 months (39), had more brain atrophy (48) and may have responded better to donepezil treatment (8). And this study demonstrates that specific global and neurocognitive rates of decline are accelerated in MCI based on APOEε4 allele presence.
The CDR-SOB, a measure of both cognition and function, stood out with the largest increase in standardized rates of change compared to non- APOEε4 carriers (Figure 2). The GDS, a seven point clinician assessment of disease severity, the MMSE and the ADAS-Cog were also strongly associated with APOEε4 status. Differences in rates of decline in activities of daily living and measures of individual cognitive domains appeared to be substantially less influenced. The ADAS-Cog is a composite score from multiple neurocognitive domain measures, and previous analyses demonstrated that it is strongly predictive of progression to AD within 36 months. And individual measures of episodic memory such as the delayed word list and paragraph recall were also quite strong predictors of future AD (39). This makes intuitive sense considering that episodic memory impairment is well described as one of the earliest abnormalities in MCI and AD (49, 50). We were unable to directly compare standardized z-score rates of decline due to statistical limitations. For this reason the relative lesser affect of APOEε4 on specific measures compared to global cognitive and functional measures are subjective numeric comparisons and thus difficult to interpret.
This study was limited by the fact that it assessed a highly selected cohort established for the purpose of a clinical treatment trial. As such, individuals had higher levels of educations and likely suffered fewer co-morbidities than the general population. This type of cohort selection bias may appreciably affect the applicability of our results to general community samples, even though participants were from 69 various regions in the United States and Canada. It is also important to note that this cohort was limited to individuals who met more severe criteria for amnestic MCI, chosen specifically for prominent memory impairment. Therefore our results would not necessarily apply to individuals with milder disease or non-amnestic MCI. In addition, this data represents a post hoc exploratory analysis in a study not powered to assess effects of APOEε4 allele status on clinical progression. For these reasons, despite a strong relationship between accelerated disease progression and APOEε4 status, our data does not provide an indication for testing of APOEε4 status in patients with aMCI in clinical practice. Without preventative treatments available for individuals with aMCI, knowing APOEε4 status, as of yet, provides no clear clinical benefit. And decisions regarding testing should include appropriate genetic counseling and be decided on a case-by-case basis by clinicians, patients, and their families.
In summary this study has observed the influence of APOEε4 on common cognitive and functional measures often used as outcome measures in clinical treatment trials. It emphasizes the strengths of global cognitive and functional measures compared to tests of individual cognitive domains for assessing rates of change in this population. This cohort does represent a typical aMCI population likely to be enrolled in treatment trials for secondary prevention of Alzheimer’s type dementia in early cognitive disease. Therefore this study highlights the need to account for APOEε4 status in early treatment trials, with the potential for tailoring treatments to individuals at higher risk for rapid decline. This will improve specificity of treatment development and ultimately lay the foundation for primary prevention trials in high risk preclinical populations.
Acknowledgements
Supported by a grant from the National Institute on Aging (UO1 AG10483), The Institute for the Study of Aging, Pfizer Inc., and Eisai Inc.
Abbreviations
- APOEε4
Apolipoprotein E ε4
- aMCI
Amnestic Mild Cognitive Impairment
- AD
Alzheimer’s disease
- MCI
Mild cognitive impairment
- ACHEI
Acetylcholinesterase inhibitor
- ADCS
Alzheimer’s Disease Cooperative Study
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disease and Stroke/Alzheimer’s Disease and Related Disorders Association
- ADAS-cog
Alzheimer’s Disease Assessment Scale-Cognitive subscale
- CDR-SOB
Clinical Dementia Rating scale Sum of Boxes
- ADL
ADCS MCI- Activities of Daily Living
- GDS
Global Deterioration Scale
- GEE
Generalized Estimating Equations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: For all authors there are no disclosures to be made of any financial support from, or equity positions in, manufacturers of drugs or products mentioned in the manuscript. These authors and the Alzheimer’s Disease Cooperative study had full publication rights without influence or restrictions from the Institute for the Study of Aging, Pfizer Inc., or Eisai Inc. All authors have agreed to conditions noted on the Author Disclosure Form. All of the authors have been instrumental in the interpretation and presentation of data in this paper. This manuscript has not been simultaneously submitted for publication to any other journal. Thank you for your consideration.
All author financial disclosures for the past two years regardless of relation to this study:
Danielle C. Whitehair: none
Abdullah Sherzai: Dr. Sherzai is a speaker for Novartis (Exelon patch)
Jennifer Emond: none
Rema Raman: none
Paul S. Aisen: Dr. Aisen consults with pharmaceutical companies regarding AD drug development, including Elan, Wyeth, Eisai, Neurochem, Schering-Plough, Bristol Myers Squibb, Lilly, Neurophage, Merck, Roche, Amgen, Genentech, Abbott, Pfizer, Novartis and Medivation.
Ronald C. Petersen: Dr. Petersen consults with pharmaceutical companies regarding AD drug development, including Elan, GE Healthcare and Wyeth. He also is a speaker for Eisai, Janssen, Novartis and Pfizer.
Adam S. Fleisher: none
References
- 1.Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectrums. 2008;13:45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- 2.Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. Journal Of Neurology, Neurosurgery, And Psychiatry. 2006;77:429–438. doi: 10.1136/jnnp.2005.072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauthier S. Cholinesterase inhibitors in late-stage Alzheimer's disease. Lancet Neurol. 2006;5:468–469. doi: 10.1016/S1474-4422(06)70454-9. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 6.Caselli RJ, Reiman EM, Osborne D, et al. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62:1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- 7.Devanand DP, Pelton GH, Zamora D, et al. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch Neurol. 2005;62:975–980. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 9.Tierney MC, Szalai JP, Snow WG, et al. A prospective study of the clinical utility of ApoE genotype in the prediction of outcome in patients with memory impairment. Neurology. 1996;46:149–154. doi: 10.1212/wnl.46.1.149. [DOI] [PubMed] [Google Scholar]
- 10.Deary IJ, Whiteman MC, Pattie A, et al. Apolipoprotein e gene variability and cognitive functions at age 79: a follow-up of the Scottish mental survey of 1932. Psychology And Aging. 2004;19:367–371. doi: 10.1037/0882-7974.19.2.367. [DOI] [PubMed] [Google Scholar]
- 11.Hofer SM, Christensen H, Mackinnon AJ, et al. Change in cognitive functioning associated with apoE genotype in a community sample of older adults. Psychology And Aging. 2002;17:194–208. [PubMed] [Google Scholar]
- 12.Packard CJ, Westendorp RGJ, Stott DJ, et al. Association between apolipoprotein E4 and cognitive decline in elderly adults. Journal Of The American Geriatrics Society. 2007;55:1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith JD. Apolipoproteins and aging: emerging mechanisms. Ageing Research Reviews. 2002;1:345–365. doi: 10.1016/s1568-1637(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 14.Swan GE, Lessov-Schlaggar CN, Carmelli D, Schellenberg GD, La Rue A. Apolipoprotein E epsilon4 and change in cognitive functioning in community-dwelling older adults. Journal Of Geriatric Psychiatry And Neurology. 2005;18:196–201. doi: 10.1177/0891988705281864. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Schneider JA, Barnes LL, et al. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Archives Of Neurology. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Mild cognitive impairment in different functional domains and incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:1479–1484. doi: 10.1136/jnnp.2004.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunce D, Fratiglioni L, Small BJ, Winblad B, Bäckman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004;63:816–821. doi: 10.1212/01.wnl.0000137041.86153.42. [DOI] [PubMed] [Google Scholar]
- 18.Pendleton N, Payton A, van den Boogerd EH, et al. Apolipoprotein E genotype does not predict decline in intelligence in healthy older adults. Neuroscience Letters. 2002;324:74–76. doi: 10.1016/s0304-3940(02)00135-0. [DOI] [PubMed] [Google Scholar]
- 19.Yip AG, Brayne C, Easton D, Rubinsztein DC. Apolipoprotein E4 is only a weak predictor of dementia and cognitive decline in the general population. Journal Of Medical Genetics. 2002;39:639–643. doi: 10.1136/jmg.39.9.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66:828–832. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 21.Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives Of Neurology. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 22.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68:1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal Of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. The American Journal Of Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 27.Mohs RC. The Alzheimer's Disease Assessment Scale. International Psychogeriatrics / IPA. 1996;8:195–203. doi: 10.1017/s1041610296002578. [DOI] [PubMed] [Google Scholar]
- 28.Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. Journal Of Geriatric Psychiatry And Neurology. 1999;12:168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- 29.Smith A. Symbol Digit Modalities Test Manual-Revised. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 30.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives Of Neurology. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 31.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Disease And Associated Disorders. 1997;11 Suppl 2:S13–S21. [PubMed] [Google Scholar]
- 32.Kaplan EF, Goodglass I, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febige; 1983. [Google Scholar]
- 33.Wechsler D. Manual for the Wechsler Adult Intelligence Scale (rev. ed.) New York: The Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1981. [Google Scholar]
- 34.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:3. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 35.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Disease And Associated Disorders. 1997;11 Suppl 2:S33–S39. [PubMed] [Google Scholar]
- 36.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. The American Journal Of Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 37.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 38.Lautenschlager NT, Riemenschneider M, Drzezga A, Kurz AF. Primary degenerative mild cognitive impairment: study population, clinical, brain imaging and biochemical findings. Dementia and Geriatric Cognitive Disorers. 2001;12:379–386. doi: 10.1159/000051284. [DOI] [PubMed] [Google Scholar]
- 39.Fleisher AS, Sowell BB, Taylor C, Gamst AC, Petersen RC, Thal LJ. Clinical predictors of progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2007;68:1588–1595. doi: 10.1212/01.wnl.0000258542.58725.4c. [DOI] [PubMed] [Google Scholar]
- 40.Devanand DP, Pelton GH, Zamora D, et al. Predictive Utility of Apolipoprotein E Genotype for Alzheimer Disease in Outpatients With Mild Cognitive Impairment. Arch Neurol. 2005;62:975–980. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- 41.Visser PJ, Verhey FRJ, Ponds RWHM, Cruts m, van Broeckhoven CL, Jolles J. Course of objective memory impairment in non-demented subjects attending a memory clinic and predictors of outcome. Int J of Geriatr Psychiatry. 2000;15:363–372. doi: 10.1002/(sici)1099-1166(200004)15:4<363::aid-gps129>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Ramakers IH, Visser PJ, Aalten P, et al. The association between APOE genotype and memory dysfunction in subjects with mild cognitive impairment is related to age and Alzheimer pathology. Dement Geriatr Cogn Disord. 2008;26:101–108. doi: 10.1159/000144072. Epub 2008 Jul 2011. [DOI] [PubMed] [Google Scholar]
- 43.Caselli RJ, Graff-Radford NR, Reiman EM, et al. Preclinical memory decline in cognitively normal apolipoprotein E-epsilon4 homozygotes. Neurology. 1999;53:201–207. doi: 10.1212/wnl.53.1.201. [DOI] [PubMed] [Google Scholar]
- 44.Caselli RJ, Reiman EM, Locke DE, et al. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol. 2007;64:1306–1311. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- 45.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-{beta} burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc.Natl.Acad.Sci.U.S.A. 2009 doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ewers M, Zhong Z, Burger K, et al. Increased CSF-BACE 1 activity is associated with ApoE-epsilon 4 genotype in subjects with mild cognitive impairment and Alzheimer's disease. Brain. 2008;131:1252–1258. doi: 10.1093/brain/awn034. Epub 2008 Mar 1211. [DOI] [PubMed] [Google Scholar]
- 47.Drzezga A, Grimmer T, Henriksen G, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;1:1. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 48.Fleisher AS, Sun S, Taylor C, et al. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology. 2008;70:191–199. doi: 10.1212/01.wnl.0000287091.57376.65. [DOI] [PubMed] [Google Scholar]
- 49.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Mild cognitive impairment in different functional domains and incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:1479–1484. doi: 10.1136/jnnp.2004.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]