Abstract
The purpose of this study was to evaluate the potential of new carboxylated multi-block copolymer of lactic acid and ethylene glycol (EL14) for nanoparticle (NP) formation and their ability to deliver high molecular weight hydrophobic drug—cyclosporine A (CsA). CsA-loaded EL14 NPs were compared with traditional poly(lactide-co-glycolide) (PLGA) NPs, both prepared by emulsion–diffusion–evaporation process. On the one hand, the increase in drug payload from 10 to 30 per cent for EL14 NPs showed no difference in particle size, however the entrapment efficiency tends to decrease from 50 to 43 per cent; on the other hand, the more hydrophobic PLGA showed an increasing trend in entrapment efficiency from 20 to 62 per cent with increasing particle size. Over 90 per cent of CsA was released in vitro from both the nanoparticulates; however, the release was much slower in the case of more hydrophobic PLGA. On in vivo evaluation in rats, the NPs made of EL14 showed a higher Cmax, a faster Tmax and enhanced tissue levels to that of PLGA that are crucial for CsA's activity and toxicity; however, the overall bioavailability of the nanoparticulates was similar and higher than Neoral. Together these data demonstrate the feasibility of NPs made of low molecular weight, hydrophilic polymer EL14 for efficient delivery of CsA.
Keywords: drug delivery, nanoparticles, pharmacokinetics, polymer
1. Introduction
The introduction of cyclosporine A (CsA) transformed the field of organ transplantation as it dramatically reduced the frequency of acute graft rejection (Cecka & Terasaki 1991). In addition to graft prevention, CsA found several applications in the treatment of psoriasis, Crohn's disease and rheumatoid arthritis. In spite of its wide applications, CsA is associated with adverse effects of which nephrotoxicity is the most severe (Faulds et al. 1993; Rezzani 2004). CsA also causes cardiovascular (Textor et al. 1994) and neurological complications (Polson et al. 1985) along with other non-nephrotoxic side effects, which include hypertrichosis, tremors and gingival hyperplasia (David-Neto et al. 2000). CsA being a critical dose drug with narrow therapeutic window, over- or under-exposure of the drug greatly affects the outcome of the therapy. Over-exposure to CsA results in toxicity while under-exposure causes graft rejection.
CsA is a highly lipophilic neutral cyclic peptide with a molecular formula of C62H111N11O12. CsA exhibits low oral bioavailability owing to its poor biopharmaceutical properties like high molecular weight (1202.64 Da), low solubility and permeability, and high presystemic metabolism (Faulds et al. 1993; Italia et al. 2006). Several formulations have been developed to overcome the poor biopharmaceutical properties of CsA. Introduction of the micro-emulsion, Neoral, has resulted in significant advancements as it established more dependable and consistent absorption profile (Mueller et al. 1994). There still remains a small degree of inter- and intra-patient variability in the absorption of the drug, however less than Sandimmune (Kahan et al. 1995), which can affect the therapeutic outcome of CsA treatment (Lindholm & Kahan 1993). Several polymeric nanoparticulate approaches have been investigated for CsA delivery, which includes pH-sensitive nanoparticles (NPs) of methacrylic acid–methacrylate copolymer (Dai et al. 2004), poly(isobutyl 2-cyanoacrylate) and polycaprolactone nanoparticulate formulations (Varela et al. 2001) and poly(lactic acid–b–poly(ethylene glycol)) (PLA–PEG) NPs (Gref et al. 2001). The developed formulations did improve overall bioavailability however, the efficacy and toxicity still remains a concern. In a recent report by our group, CsA-loaded poly(lactide-co-glycolide) (PLGA) NPs (Cmax, 448.5 ng ml−1) were found to be less nephrotoxic than commercial formulation Neoral, however the Cmax achieved was significantly lower than that of Neoral (Cmax, 1448.4 ng ml−1; Italia et al. 2007) that may fall well below therapeutic levels (Lindholm & Kahan 1993). Besides therapeutic efficacy, Cmax is found to be correlated with the occurrence of side effects. Hence, administration of CsA requires a delivery system that decreases the frequency and the severity of side effects, however still maintaining blood concentrations within the therapeutic levels.
Several reports have established the role of polymer matrix in determining the release profile of drugs (Gref et al. 2001; Mittal et al. 2007). PLGA has been exploited utmost for particulate delivery, because of its desired properties of biocompatibility, degradability and ease of fabrication (Anderson & Shive 1997; Bala et al. 2004; Mohamed & Van der Walle 2008). However, in the present case for CsA delivery, the regular approximately 40 kDa PLGA appears to fail in delivering therapeutic levels. Therefore, in an attempt to increase the Cmax, which is thought to play a crucial role in preventing graft rejection (Grant et al. 1999), we synthesized a more hydrophilic multi-block copolymer of lactic acid and ethylene glycol containing periodic side-chain carboxyl groups. The synthesized polymer should release CsA faster owing to decreased hydrophobicity and quicker hydrolytic degradation assisted by periodic side-chain carboxyl groups (Tracy et al. 1999) to achieve the desired CsA concentration.
2. Material and methods
2.1. Materials
CsA (Fluorochem Ltd. Derbyshire, UK), PLGA (Resomer RG 50∶50, MW 35 000–40 000, Boehringer Ingelheim, Ingelheim, Germany), N, N-didodecyl-N, N-dimethylammonium bromide (DMAB) (Aldrich) and HPLC grade ethanol (Fluka) were used as received. Labrasol was a gift sample from Gattefosse (France). All other chemicals used were of analytical grade.
2.2. Preparation of carboxylated chain-extended (ABA)n polymer
The polymer EL14 was prepared as described previously (Ankola et al. 2009). In brief, a solution of l-lactide, dried polyethylene glycol (PEG, MW 400) and 0.1 per cent (w/w) stannous octoate were heated at reflux in dry toluene for 24 h under dry nitrogen. Chain extension of lactide–ethylene glycol–lactide triblock copolymers was performed by introduction of triethylamine and pyromellitic dianhydride (1 : 2 : 1 copolymer∶amine∶dianhydride molar ratio) into the flask containing the pre-polymer solution under dry nitrogen atmosphere. The mixture was heated at 110°C for 1 h under stirring and then toluene was removed under vacuum. The dried polymer was dissolved in dichloromethane, washed with cold 0.5 N HCl and water, then precipitated from dichloromethane solution into diethyl ether. The precipitate was collected by filtration and dried under vacuum. The polymer was further purified by precipitation from acetone in 5 per cent NaHCO3, washing with water and dried under vacuum. The synthesized polymer had a MW of 16 300 and its comprehensive characterization was reported earlier (Ankola et al. 2009).
2.3. Preparation of NPs
Emulsion–diffusion–evaporation technique was used to prepare the NPs (Italia et al. 2007; Ankola et al. 2010). In brief, the method used was as follows: 50 mg of EL14 or PLGA were dissolved in 2.5 ml of organic solvent (ethyl acetate for PLGA and 4 : 1 ethyl acetate: dichloromethane for EL14) at room temperature and stirred for 2 h. The organic phase was then emulsified into an aqueous phase containing DMAB (0.25% w/v) as stabilizer. The resulting o/w emulsion was sonicated (Soniprep 150, MSE) at 10 µm amplitude for 1 min. Water was added to the above emulsion with constant stirring which resulted in nanoprecipitation. CsA-loaded NPs were prepared by dissolving the drug in minimum volume of organic phase and adding it to the polymeric solutions and proceeding as described above. The effect of drug loading on particle characteristics was studied at 10, 20 and 30 wt% of polymer load.
2.4. Size and zeta potential measurements
The mean particle size of the prepared NP suspensions was measured by dynamic light scattering (Nano ZS, Malvern, UK) taking the average of five measurements, which represented the hydrodynamic diameter of the particles. Mean size was derived from Cumulants analysis of the measured correlation curve, wherein a single particle size is assumed and a single exponential fit is applied to the autocorrelation function. In addition to the mean particle size, the instrument reports the Particle Dispersion Index (PDI) between 0 (monodispersed particles) and 1 (polydispersed particles). The zeta potential was estimated on the basis of electrophoretic mobility under an electric field as an average of 30 measurements. The frequency shift of an incident laser beam caused by these moving particles is converted to the zeta potential by the application of the Smoluchowski equation. The temperature was kept constant at 25°C during size and zeta potential measurements.
2.5. Entrapment efficiency
The percentage of CsA incorporated during NP preparation was determined by centrifuging the drug-loaded NPs at 14 000 rcf for 30 min and separating the supernatant. The pellet obtained was washed twice with water and CsA was extracted from the pellet using acetonitrile, followed by estimation of CsA in triplicate by HPLC. Entrapment efficiency was calculated as the percentage ratio of drug incorporated into the NPs to that initially added for loading. Estimation of CsA was carried out using Hypersil GOLD (15 cm × 4.6 mm, 5 µm) (C18) column and Hypersil 10 × 4 mm, 5 µm drop in guards connected by UNIGAUARD (Thermo Scientific). Elution of CsA was achieved using isocratic mobile phase (ethanol∶water) at 210 nm. The HPLC system consisted of ChromQuest acquisition software, Auto-sampler connected with LC Pump and PDA detector (Thermo Finnigan Surveyor System). The analytical method was validated according to the guidelines of the international conference on harmonization (ICH) of technical requirements for registration of pharmaceuticals for human use. Parameters validated included precision (repeatability (intra-day) and intermediate precision (inter-day)) and accuracy. Both the intra- and inter-day relative standard deviations of QC standards were less than 5 per cent over the selected range.
2.6. In vitro drug-release studies
Release of CsA from the nanoparticulates was observed using the dialysis membrane method. CsA-loaded NPs of PLGA and EL14 were centrifuged and redispersed in 1 ml of release medium (5% Labrasol solution in pH 7.4 phosphate buffer) and placed in the dialysis bags (Sigma) with a molecular mass cut-off of 12 000 Da. The bags were suspended in glass vials containing 5 ml of release medium. All the vials were kept in a shaker water-bath maintained at 37°C and 50 r.p.m. The release medium was completely replaced with fresh buffer at every sampling time interval. The sampling points were 6 h followed by sampling at every 24 h for the first week and every 48 h at later stages of release. The CsA content in the release medium was quantified using a validated HPLC method as described above.
2.7. Pharmacokinetic and tissue distribution studies
All animal experiments were carried out under a Project Licence issued under the U.K. Home Office Animals (Scientific Procedures) Act 1986. For the pharmacokinetic and tissue distribution studies, male SD rats weighing 200–250 g were used. Neoral and CsA nanoparticulate formulations of PLGA and EL14 were administered to rats by oral gavage tube at a dose of 15 mg kg−1 body weight. Blood samples were collected from animals by venipuncture of peripheral blood vessel at 0.5, 1, 2, 6, 12, 24, 36, 48 and 72 h in microcentrifuge tubes containing ethylenediaminetetraacetic acid sodium salt as anticoagulant. Blood samples were analysed for CsA concentration by cyclosporine-specific EIA KIT (Immunotech, Czech Republic). At the end of 72 h, animals were sacrificed and all major organs isolated. CsA concentration in all the organs was determined. In brief, the organs were isolated and weighed, to which water was added at 1 : 4 ratio and then homogenized to produce a fine homogenate. Two hundred microlitres of homogenate was transferred into a microcentrifuge tube to which 800 µl of acetonitrile was added to extract CsA from the tissues. The tubes were vortexed for 5 min and centrifuged. The samples were concentrated by collecting 800 µl of supernatant and evaporating it to dryness under vacuum. The obtained pellet was analysed for CsA content by cyclosporine-specific EIA KIT (Immunotech).
3. Results and discussion
3.1. Preparation of NPs
A careful consideration of polymer and drug properties is needed to optimize the formulation of NP-entrapped drug as both the surfactant and the solvent nature can significantly influence the particle characteristics that include particle size and drug encapsulation (Sahana et al. 2008). The method previously optimized for PLGA-CsA NPs (Italia et al. 2007; Ankola et al. 2010) was adapted with appropriate minor modifications for preparation of EL14 NPs. For PLGA NPs ethyl acetate was the oil phase, however for EL14, ethyl acetate : dichloromethane (4 : 1) was used for the preparation, as small amount of dichloromethane was required for complete polymer dissolution.
The EL14 and PLGA NPs were prepared by loading different concentrations of CsA (10–30% w/w of polymer weight). A slight increase in particle size from 107 to 119 nm was observed by increasing the drug payload from 10 to 30 per cent (table 1), whereas a significant increase in the entrapment efficiency from 20 to 62 per cent was observed for PLGA. On the other hand, EL14 NPs did not show any size increase, whereas CsA entrapment decreased. At 10 per cent loading, EL14 NPs were able to entrap higher amounts of CsA when compared with PLGA. These high entrapments at 10 per cent payload may be owing to several factors including the ionic interactions of CsA with the carboxyl groups present in the side chain of EL14. On the other hand, the commercial PLGA has only terminal carboxyl end groups therefore, the role of ionic interaction may be negligible when compared with EL14 that contains about 12–16 free carboxyl groups in polymer side chain (Ankola et al. 2009). The better orientation of CsA within the EL14 matrix than in PLGA could be another possibility.
Table 1.
Characteristics of CsA-encapsulated nanoparticles. EE, entrapment efficiency; ZP, zeta potential. All values are represented as mean ± s.d. (n = 3). Zeta potential was measured at pH 4.1 ± 0.5 pH. PDI of particles was in the range of 0.08–0.16.
| loading (%) | PLGA |
EL14 |
||||||
|---|---|---|---|---|---|---|---|---|
| size (nm) | EE (%) | CsA entrapped per 50 mg of PLGA (mg) | ZP (mV) | size (nm) | EE (%) | CsA entrapped per 50 mg of EL14 (mg) | ZP (mV) | |
| 10 | 107 ± 7 | 20 ± 2 | 1.0 ± 0.1 | 78 ± 6 | 136 ± 7 | 51 ± 11 | 2.5 ± 0.6 | 51 ± 6 |
| 20 | 108 ± 7 | 46 ± 4 | 4.6 ± 0.4 | 73 ± 12 | 139 ± 7 | 47 ± 3 | 4.7 ± 0.3 | 54 ± 9 |
| 30 | 119 ± 5 | 62 ± 4 | 9.3 ± 0.6 | 70 ± 7 | 138 ± 4 | 43 ± 3 | 6.4 ± 0.5 | 53 ± 7 |
By increasing the load to 30 per cent w/w of polymer, the efficiency of CsA entrapment decreased for EL14 NPs, whereas an increase was observed for PLGA NPs. The observed decrease for EL14 could be due to several factors, such as the limitation of the matrix itself owing to its lower molecular weight (16 300) compared with that of PLGA (approx. 40 000). Another reason could be a faster saturation of EL14, more hydrophilic matrix to hold highly hydrophobic CsA molecules in comparison with more hydrophobic PLGA. The entrapment of CsA was also found to depend upon the concentration of DMAB, which can be lowered to increase the CsA entrapment (Italia et al. 2007) as low entrapment would result in higher polymer administration at desired CsA dose. The NPs prepared using both the polymers were of low PDI (0.08–0.16). The observed zeta potential for both nanoparticulate formulations was around 75 mV for PLGA and 50 mV for EL14 NPs at pH 4.1 ± 0.5. The low PDI and high zeta potential indicates the high stability of both the nanoparticulate formulation. In the literature, besides PLGA several other polymers have also been explored for preparing CsA-loaded NPs. Block copolymers of lactic acid and ethylene glycol were used to prepare CsA-loaded NPs (Gref et al. 2001). Hyaluronic acid-coated poly(ɛ-caprolactone) particles have been explored for the delivery of CsA to the cornea (Yenice et al. 2008). In addition to polymers for specific molecules, several polymers for broader drug delivery and biomedical applications have also been synthesized. Several functional polyesters with pendant hydroxyl groups or tailored side chains have been developed (Cerbai et al. 2008). Amphiphilic diblock and triblock block copolymers consisting of alternating poly(ethylene glycol) and poly(ɛ-caprolactone) segments were synthesized (Signori et al. 2005). EL14 also represents a new class of polymer that can effectively entrap CsA, in the form of nanoparticulates in the current circumstances. As 20 per cent loading resulted in NPs with similar entrapment of CsA in PLGA and EL14 NPs, it was used for in vitro and in vivo evaluation.
3.2. In vitro drug-release studies
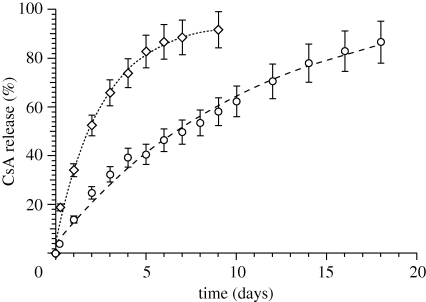
The in vitro release of drugs from polymeric NPs is mediated by diffusion-cum-degradation process and the release profiles for 20 per cent drug-loaded particles recorded were shown in figure 1. A 53 per cent release was recorded at 48 h for EL14 NPs and corresponding to that PLGA NPs recorded 25 per cent. After 48 h, EL14 NPs released approximately 7 per cent of drug daily and about 90 per cent by day 9, whereas PLGA NPs released 86 per cent of drug by day 18 with approximately 4 per cent daily release. Several factors could govern the faster release of CsA from EL14 NPs. The hydrophilic nature and low molecular weight of EL14 could have contributed to faster release of CsA from EL14 NPs. Other reasons may also include large amounts of surface-bound drug on EL14 NPs and faster degradation of EL14 polymer matrix.
Figure 1.
In vitro release pattern of CsA from PLGA (open circles) and EL14 (open diamonds) nanoparticles. All values represented as mean ± s.d. (n = 3).
The release profile of CsA from both the nanoparticulate formulations were modelled for different release mechanisms, mainly zero order (Najib & Suleiman 1985), first order (Desai et al. 1966), Higuchi's square root plot (Higuchi 1963), Hixson–Crowell cube root plot (Hixson & Crowell 1931) and Peppas–Korsmeyer power plot (Korsmeyer et al. 1983; table 2). CsA release from EL14 and PLGA NPs could have followed either Higuchi's model (diffusive) or first-order release based on higher correlation coefficient (R) and lower sum of squared errors (χ2). No conclusive differences between Higuchi's model (diffusive) or first-order release were found particularly if experimental errors (5–10%) were taken into account. However, if data for less than 85 per cent release are considered, the release of CsA from EL14 NPs fits exceptionally well with the Higuchi model. To further confirm, fitting to Peppas–Korsemeyer gave diffusional coefficient (n = 0.47 for EL14 NPs (n < 0.5, Fickian diffusion) indicating a diffusive release mechanism. The somewhat larger n = 0.685 was observed for PLGA NPs indicating a small deviation from the diffusive model, suggesting that diffusion is accompanied by erosion (n > 0.5, non-Fickian diffusion). The difference between the two polymers can be attributed to: hydration of hydrophilic EL14 NPs that facilitates drug diffusion; limited diffusion inside rather hydrophobic PLGA NPs and hence a diffusion–erosion mechanism. The faster but controlled release from EL14 NPs would help in achieving higher CsA blood levels in comparison to PLGA but at a controlled Cmax, desired for optimal CsA therapy.
Table 2.
In vitro release data fit to models.
| kinetic model | equation | parametersa | PLGA NPs | EL14 NPsb |
|---|---|---|---|---|
| zero order | Qt = k0t | R | 0.972 | 0.914 |
| χ2 | 1590 | 1516 | ||
| k0 | 5.78 | 9.72 | ||
| Higuchi | Qt = kHt1/2 | R | 0.997 | 0.988 (0.999) |
| χ2 | 155 | 260 (11.9) | ||
| kH | 21.5 | 34.5 (37.0) | ||
| first order | lnQt = lnQ = −k1t | R | 0.994 | 0.990 |
| χ2 | 105.1 | 62.1 | ||
| k1 | 0.0912 | 0.388 | ||
| Hixon–Crowell |
 ; ; 
|
R | 0.800 | 0.733 |
| χ2 | 44 565 | 43 164 | ||
| kHC | 0.165 | 0.337 | ||
| Peppas–Korsmeyer | Qt/Q0 = kPKtn; lnQt = lnQ0kPK + nlnt | R | 0.994 | 0.993 |
| χ2 | 187.8 | 200.8 | ||
| kPK | 0.170 | 0.366 | ||
| n | 0.685 | 0.470 |
aQt is the amount of drug released at time t; Q0 is the initial amount of the drug in the formulation; n is the diffusional coefficient; k0, k1, kH, kHC and kPK are the release rate constants for zero-order, first-order, Higuchi, Hixson–Crowell and Peppas–Korsmeyer rate equations, respectively. n is the diffusional coefficient in Peppas–Korsmeyer rate equations; R is the correlation coefficient and χ2 is the sum of squared errors.
bValues in parentheses refers to release values lower than 85 per cent.
3.3. Pharmacokinetic and tissue distribution study
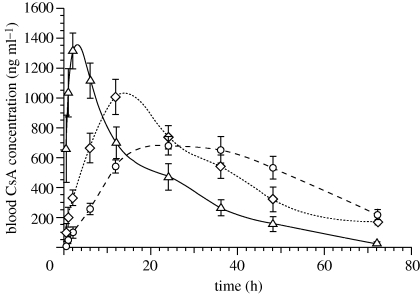
Pharmacokinetics and tissue distribution studies are noteworthy in the assessment of a delivery system, as they provide valuable information about the safety and efficacy of the formulation and also help in estimating clinical doses. Comparison of nanoparticulate formulation with the currently marketed formulation of CsA would provide additional information on therapeutic implications of the new formulations. Considering the above, the pharmacokinetic profile of EL14 and PLGA NPs of CsA were compared with currently marketed CsA formulation Neoral. The blood CsA levels with time for Neoral, EL14 and PLGA NPs on oral administration at 15 mg kg−1 are shown in figure 2 and the derived pharmacokinetics parameters in table 3.
Figure 2.
Comparative in vivo plasma concentration versus time profiles of CsA administered orally as Neoral (open triangles, 15 mg kg−1), CsA NPs of PLGA (open circles, 20% DL) and EL14 at 15 mg kg−1 (open diamonds, 20% DL). All values reported are mean ± s.e.m. (n = 3). DL, drug loading.
Table 3.
Pharmacokinetic parameters. AUC, area under curve.
| groups | AUC0–72 | Cmax(ng ml−1) | Tmax (h) | relative bioavailabilitya | relative bioavailabilityb |
|---|---|---|---|---|---|
| Neoral | 29 046 ± 1437 | 1328 ± 120 | 2 | 100 | n.a. |
| PLGA | 34 854 ± 2157 | 682 ± 66 | 24 | 120 | 100 |
| EL14 | 36 616 ± 2843 | 1006 ± 119 | 12 | 126 | 105 |
aIn relative to Neoral.
bIn relative to PLGA (all data are represented as mean ± s.e.m., n = 3).
Entrapment of CsA into EL14 and PLGA NPs resulted in the sustained release of the drug in comparison with the Neoral which is a micro-emulsion-based formulation. The Tmax was 2 h for Neoral and 12 and 24 h for EL14 and PLGA NPs, respectively. The Cmax of the formulations varied widely, depending upon the ability of the formulation to sustain the CsA release. Cmax was 1328, 1006 and 682 ng ml−1 for Neoral, EL14 NPs and PLGA NPs, respectively. Neoral resulted in a quick absorption profile, while PLGA NPs sustained the release of CsA longer than EL14 NPs. By 72 h after administration, EL14 and PLGA NPs had similar blood levels. The relative bioavailability of EL14 and PLGA NPs were 126 and 120 per cent, respectively, in comparison with Neoral and 105 for EL14 NPs in comparison with PLGA NPs.
In literature several studies have demonstrated the correlation of CsA concentrations to the incidence of side effects. The toxicities are found to depend on Cmax and the area under curve (AUC) of the drug. In a recent study in paediatrics with stable renal transplant it was found that hypertrichosis was related to AUC, whereas the tremors were more related to Cmax of CsA (David-Neto et al. 2000). Similar facts have also been established in acute nephrotoxicity caused by CsA, where decrease in glomerular filtration rate was closely related to the Cmax and not AUC (Perico et al. 1992). It was further suggested that hypertrichosis and tremor can be prevented by maintaining the AUC0–4 ≤ 4200 ng ml−1 h−1 and Cmax ≤ 900 ng ml−1, respectively (David-Neto et al. 2000). In circumstances to the above, EL14 NPs could be a preferred formulation for CsA delivery as observed Cmax was in the desired range, unlike Neoral that exceeded the limit significantly by approximately 400 ng ml−1.
After 72 h of oral administration, the animals were sacrificed and tissue concentration determined to have an insight on CsA biodistribution (table 4). CsA concentrations in all organs, except brain were lower for Neoral than for PLGA and EL14 NPs. For brain, Neoral had the highest CsA concentration followed in the order by EL14 and PLGA NPs, which may be owing to the fact that free CsA penetrated blood–brain barrier more easily than that entrapped in polymeric particles. When both the nanoparticulate formulations were compared with each other, EL14 NPs produced higher CsA tissue levels at lower blood CsA concentrations than PLGA NPs. This may be owing to the ability of EL14 NPs to distribute more in tissues as observed by higher tissue distribution coefficient (Kp, ratio of CsA's tissue concentration to blood concentration) as depicted in table 5. From the observed kinetic and tissue profile, it was clear that the nature of polymer matrix played a key role in determining the performance of given delivery system. EL14 in comparison with PLGA generated a kinetic profile, which is much closer to Neoral. The current commercial formulation of CsA experiences erratic bioavailability owing to its extensive metabolism in the gut, making it difficult to predict trough levels. The better bioavailability of CsA in the form of EL14 NPs could be because of the protective role of PEG present in the polymer chain, which has been found to have protective effects on proteins and peptides in gastric fluids (Tobio et al. 2000). The decreased gut-wall metabolism would allow better therapeutic monitoring of CsA.
Table 4.
Tissue concentrations of various CsA formulations at 72 h. All data are represented as mean ± s.e.m. (n = 3).
| tissue | µg g−1 of tissue or µg ml−1 of blood |
||
|---|---|---|---|
| Neoral | PLGA | EL14 | |
| blood | 0.04 ± 0.01 | 0.22 ± 0.04 | 0.17 ± 0.02 |
| brain | 3.4 ± 0.7 | 1.9 ± 0.2 | 2.7 ± 1.1 |
| heart | 5.0 ± 0.8 | 6.4 ± 1.5 | 6.8 ± 0.8 |
| lung | 5.3 ± 0.7 | 8.9 ± 3.6 | 10.7 ± 0.6 |
| kidney | 9.9 ± 1.6 | 14.0 ± 1.3 | 15.3 ± 1.5 |
| spleen | 6.9 ± 1.8 | 9.3 ± 2.1 | 10.8 ± 1.2 |
| liver | 10.7 ± 1.3 | 11.7 ± 0.8 | 16.4 ± 1.1 |
| intestine | 6.8 ± 0.8 | 7.5 ± 1.5 | 7.7 ± 1.2 |
Table 5.
Tissue distribution coefficient.
| tissue |
Kp |
|
|---|---|---|
| PLGA | EL14 | |
| blood | 1 | 1 |
| brain | 9 | 16 |
| heart | 29 | 41 |
| lung | 40 | 64 |
| kidney | 64 | 91 |
| spleen | 42 | 64 |
| liver | 53 | 98 |
| intestine | 34 | 46 |
4. Conclusion
Considering the critical dose drug type nature of CsA, which includes low and variable absorption, formulation-dependent bioavailability and individualization of dosing, nanoparticulate approach would be an ideal strategy to improve its performance and the current study indicates the potential of EL14 NPs for CsA delivery. EL14 NPs were successfully able to deliver CsA via oral route and at a controlled rate, withstanding drastic GI environment and metabolizing enzymes and complex elimination–excretion process of the body. These NPs when compared with PLGA NPs, released CsA faster; resulting in a higher Cmax but similar bioavailability.
Acknowledgements
Thanks are due to Prof. R. M. Wadsworth for constructive criticism on the manuscript. The start up funds from University of Strathclyde to M.N.V.R.K. is gratefully acknowledged.
Footnotes
One contribution to a Theme Supplement ‘Scaling the heights—challenges in medical materials: an issue in honour of William Bonfield, Part I. Particles and drug delivery’.
References
- Anderson J. M., Shive M. S. 1997. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Del. Rev. 28, 5–24. ( 10.1016/S0169-409X(97)00048-3) [DOI] [PubMed] [Google Scholar]
- Ankola D. D., Kumar M. N. V. R., Chiellini F., Solaro R. 2009. Multiblock copolymers of lactic acid and ethylene glycol containing periodic side-chain carboxyl groups: synthesis, characterization and nanoparticle preparation. Macromolecules 42, 7388–7395. ( 10.1021/ma9012253) [DOI] [Google Scholar]
- Ankola D. D., Durbin E. W., Buxton G. A., Schäfer J., Bakowsky U., Kumar M. N. V. R. 2010. Preparation, characterization and in silico modeling of biodegradable nanoparticles containing Cyclosporine A and Coenzyme Q10. Nanotechnology 21, 065104 ( 10.1088/0957-4484/21/6/065104) [DOI] [PubMed] [Google Scholar]
- Bala I., Hariharan S., Kumar M. N. V. R. 2004. PLGA nanoparticles in drug delivery: the state of the art. Crit. Rev. Ther. Drug Carrier Syst. 21, 387–422. ( 10.1615/CritRevTherDrugCarrierSyst.v21.i5.20) [DOI] [PubMed] [Google Scholar]
- Cecka J. M., Terasaki P. I. 1991. The UNOS Scientific Renal Transplant Registry. Clin. Transpl. 1–11. [PubMed] [Google Scholar]
- Cerbai B., Solaro R., Chiellini E. 2008. Synthesis and characterization of functional polyesters tailored for biomedical applications. J. Polym. Sci. A: Polym. Chem. 46, 2459–2476. ( 10.1002/pola.22579) [DOI] [Google Scholar]
- Dai J., Nagai T., Wang X., Zhang T., Meng M., Zhang Q. 2004. pH-sensitive nanoparticles for improving the oral bioavailability of cyclosporine A. Int. J. Pharm. 280, 229–240. ( 10.1016/j.ijpharm.2004.05.006) [DOI] [PubMed] [Google Scholar]
- David-Neto E., Lemos F. B. C., Furusawa E. A., Schwartzman B. S., Cavalcante J. S., Yagyu E. M., Romano P., Ianhez L. E. 2000. Impact of cyclosporin A pharmacokinetics on the presence of side effects in pediatric renal transplantation. J. Am. Soc. Nephrol. 11, 343–349. [DOI] [PubMed] [Google Scholar]
- Desai S. J., Singh P., Simonelli A. P., Higuchi W. I. 1966. Investigation of factors influencing release of solid drug dispersed in wax matrices. III. Quantitative studies involving polyethylene plastic matrix. J. Pharm. Sci. 55, 1230–1234. ( 10.1002/jps.2600551113) [DOI] [PubMed] [Google Scholar]
- Faulds D., Goa K. L., Benfield P. 1993. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 45, 953–1040. ( 10.2165/00003495-199345060-00007) [DOI] [PubMed] [Google Scholar]
- Grant D., Kneteman N., Tchervenkov J., Roy A., Murphy G., Tan A., Hendricks L., Guilbault N., Levy G. 1999. Peak cyclosporine levels (Cmax) correlate with freedom from liver graft rejection: results of a prospective, randomized comparison of Neoral and Sandimmune for liver transplantation (NOF-8)1,2. Transplantation 67, 1133–1137. ( 10.1097/00007890-199904270-00008) [DOI] [PubMed] [Google Scholar]
- Gref R., Quellec P., Sanchez A., Calvo P., Dellacherie E., Alonso M. J. 2001. Development and characterization of CyA-loaded poly(lactic acid)-poly(ethylene glycol)PEG micro- and nanoparticles. Comparison with conventional PLA particulate carriers. Eur. J. Biopharm. Pharm. 51, 111–118. ( 10.1016/S0939-6411(00)00143-0) [DOI] [PubMed] [Google Scholar]
- Higuchi T. 1963. Mechanism of sustained action medication, theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 52, 1145–1149. [DOI] [PubMed] [Google Scholar]
- Hixson A. W., Crowell J. H. 1931. Dependence of reaction velocity upon surface and agitation: I–Theoretical consideration. Ind. Eng. Chem. 23, 923–931. ( 10.1021/ie50260a018) [DOI] [Google Scholar]
- Italia J. L., Bhardwaj V., Kumar M. N. V. R. 2006. Disease, destination, dose and delivery aspects of cyclosporine: the state of the art. Drug Discov. Today 11, 846–854. ( 10.1016/j.drudis.2006.07.015) [DOI] [PubMed] [Google Scholar]
- Italia J. L., Bhatt D. K., Bhardwaj V., Tikoo K., Kumar M. N. V. R. 2007. PLGA nanoparticles for oral delivery of cyclosporine: nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral. J. Control. Rel. 119, 197–206. ( 10.1016/j.jconrel.2007.02.004) [DOI] [PubMed] [Google Scholar]
- Kahan B. D., Dunn J., Fitts C., Van Buren D., Wombolt D., Pollak R., Carson R., Alexander J. W., Choc M. 1995. Reduced inter- and intra-subject variability in cyclosporine pharmacokinetics in renal transplant recipients treated with a microemulsion formulation in conjunction with fasting, low-fat meals, or high-fat meals 1, 2. Transplantation 59, 505–511. [PubMed] [Google Scholar]
- Korsmeyer R. W., Gurny R., Doelker E. M., Buri P., Peppas N. A. 1983. Mechanism of solute release from porous hydrophilic polymers. Int. J. Pharm. 15, 25–35. ( 10.1016/0378-5173(83)90064-9) [DOI] [Google Scholar]
- Lindholm A., Kahan B. D. 1993. Influence of cyclosporine pharmacokinetics, trough concentrations, and AUC monitoring on outcome after kidney transplantation. Clin. Pharmacol. Ther. 54, 205–218. [DOI] [PubMed] [Google Scholar]
- Mittal G., Sahana D. K., Bhardwaj V., Kumar M. N. V. R. 2007. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Rel. 119, 77–85. ( 10.1016/j.jconrel.2007.01.016) [DOI] [PubMed] [Google Scholar]
- Mohamed F., Van der Walle C. F. 2008. Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J. Pharm. Sci. 97, 71–87. ( 10.1002/jps.21082) [DOI] [PubMed] [Google Scholar]
- Mueller E. A., Kovarik J. M., van Bree J. B., Tetzloff W., Grevel J., Kutz K. 1994. Improved dose linearity of cyclosporine pharmacokinetics from a microemulsion formulation. Pharm. Res. 11, 301–304. ( 10.1023/A:1018923912135) [DOI] [PubMed] [Google Scholar]
- Najib N., Suleiman M. 1985. The kinetics of drug release from ethyl cellulose solid dispersions. Drug Dev. Ind. Pharm. 11, 2169–2181. ( 10.3109/03639048509087779) [DOI] [Google Scholar]
- Perico N., Ruggenenti P., Gaspari F., Mosconi L., Benigni A., Amuchastegui C. S., Gasparini F., Remuzzi G. 1992. Daily renal hypoperfusion induced by cyclosporine in patients with renal transplantation. Transplantation 54, 56–60. [DOI] [PubMed] [Google Scholar]
- Polson R. J., Powel-Jackson P. R., Williams R. 1985. Convulsion associated with cyclosporine A in renal transplant recipients. Br. Med. J. 290, 1003–1010. ( 10.1136/bmj.290.6473.1003-a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezzani R. 2004. Cyclosporine A and adverse effects on organs: histochemical studies. Prog. Histochem. Cytochem. 39, 85–128. ( 10.1016/j.proghi.2004.04.001) [DOI] [PubMed] [Google Scholar]
- Sahana D. K., Mittal G., Bhardwaj V., Kumar M. N. V. R. 2008. PLGA nanoparticles for oral delivery of hydrophobic drugs: influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J. Pharm. Sci. 97, 1530–1542. ( 10.1002/jps.21158) [DOI] [PubMed] [Google Scholar]
- Signori F., Chiellini F., Solaro R. 2005. New self-assembling biocompatible–biodegradable amphiphilic. Polymer 46, 9642–9652. ( 10.1016/j.polymer.2005.07.071) [DOI] [Google Scholar]
- Textor S. C., Canzanello V. J., Taler S. J. 1994. Cyclosporine-induced hypertension after transplantation. Mayo Clin. Proc. 69, 1182–1193. [DOI] [PubMed] [Google Scholar]
- Tobio M., Sanchez A., Vila A., Soriano I., Evora C., Vila-Jato J. L., Alonso M. J. 2000. The role of PEG on the stability in digestive fluids and in vivo fate of PEG-PLA nanoparticles following oral administration. Colloids Surf. B: Biointerf. 18, 315–323. ( 10.1016/S0927-7765(99)00157-5) [DOI] [PubMed] [Google Scholar]
- Tracy M., Ward K., Firouzabadian L., Wang Y., Dong N., Qian R., Zhang Y. 1999. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials 20, 1057–1062. ( 10.1016/S0142-9612(99)00002-2) [DOI] [PubMed] [Google Scholar]
- Varela M. C., Guzman M., Molpeceres J., del Rosario Aberturas M., Rodriguez-Puyol D., Rodriguez-Puyol M. 2001. Cyclosporine-loaded polycaprolactone nanoparticles: immunosuppression and nephrotoxicity in rats. Eur. J. Pharm. Sci. 12, 471–478. [DOI] [PubMed] [Google Scholar]
- Yenice I., Mocan M. C., Palaska E., Bochot A., Bilensoy E., Vural I., Irkeç M., Hincal A. A. 2008. Hyaluronic acid coated poly-3-caprolactone nanospheres deliver high concentrations of cyclosporine A into the cornea. Exp. Eye Res. 87, 162–167. ( 10.1016/j.exer.2008.04.002) [DOI] [PubMed] [Google Scholar]