Abstract
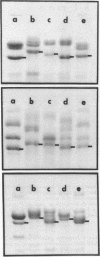
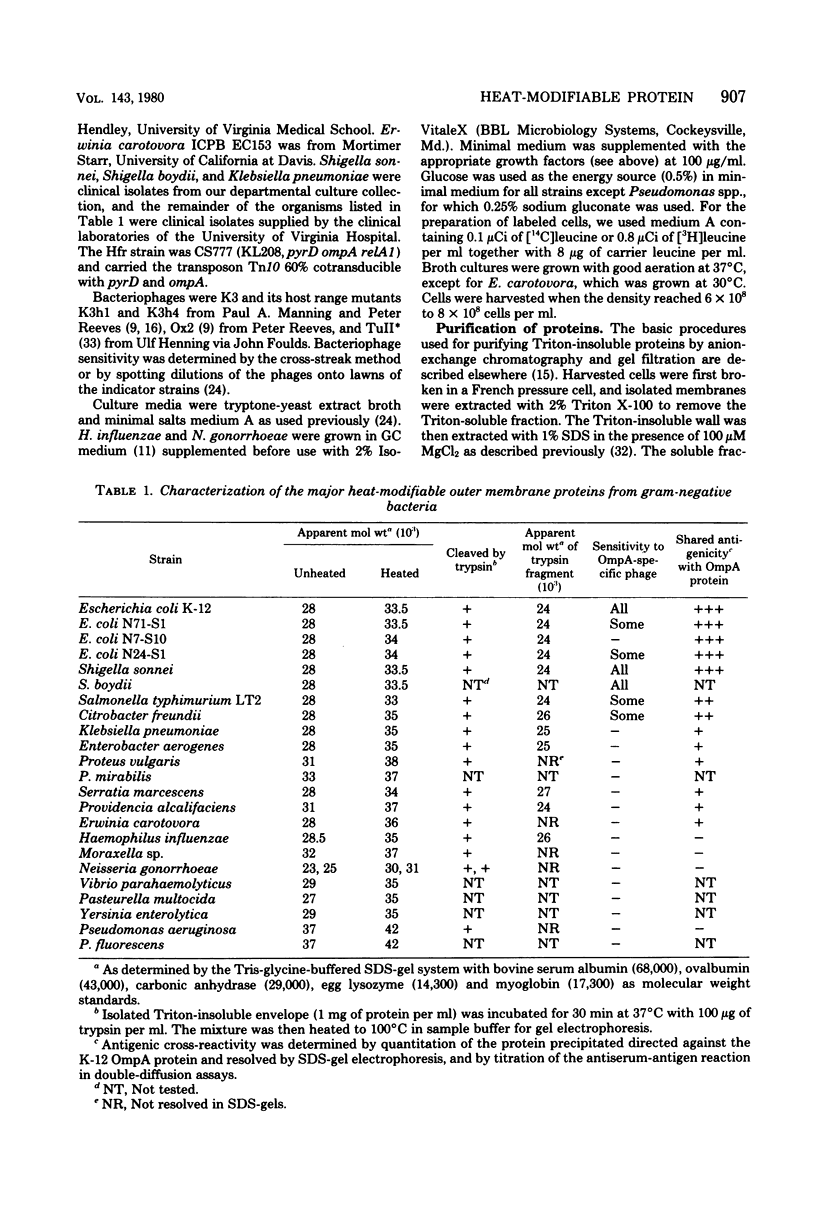
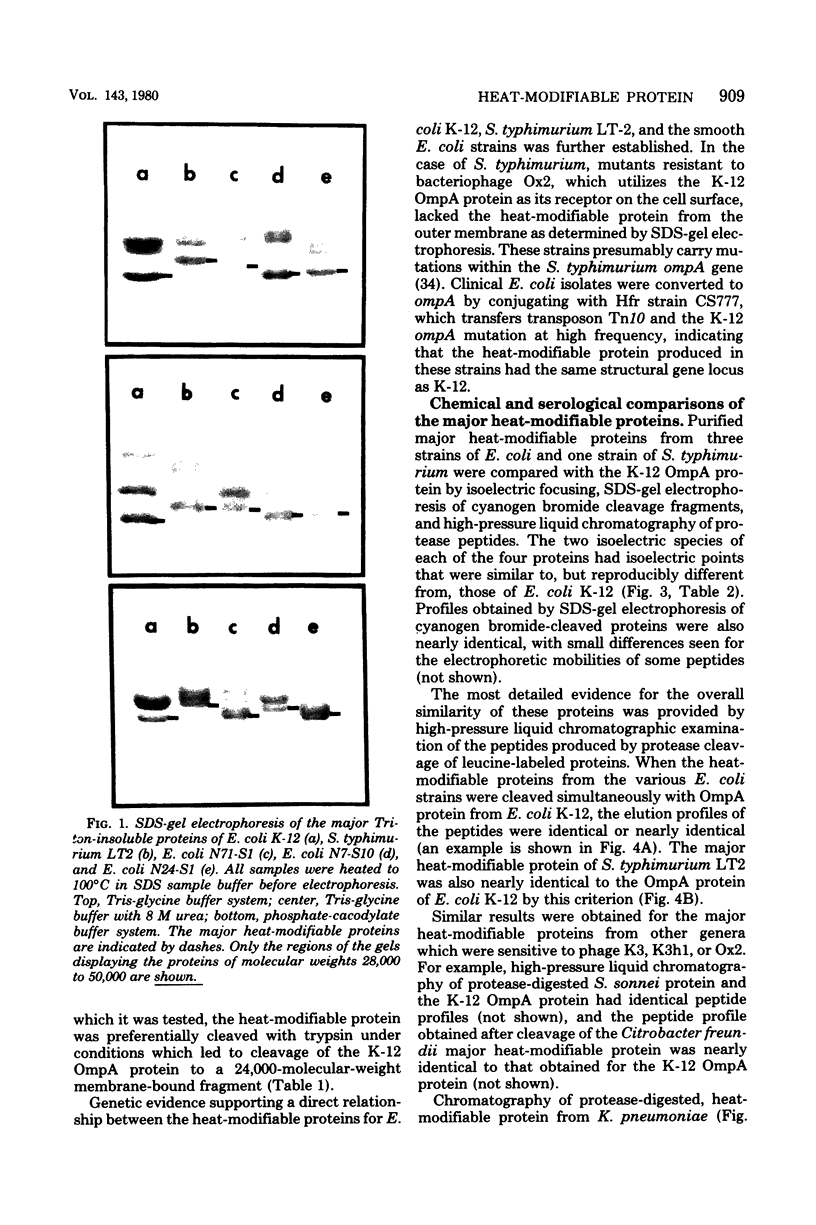
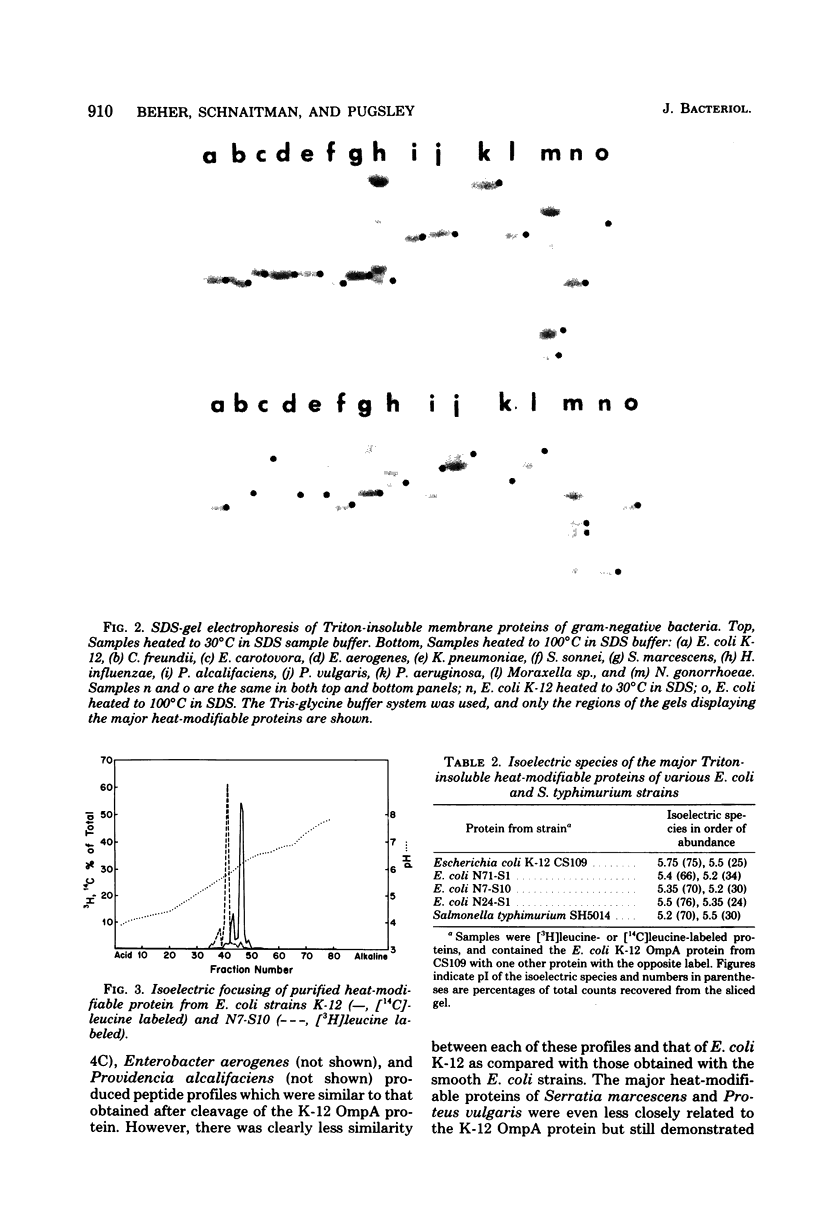
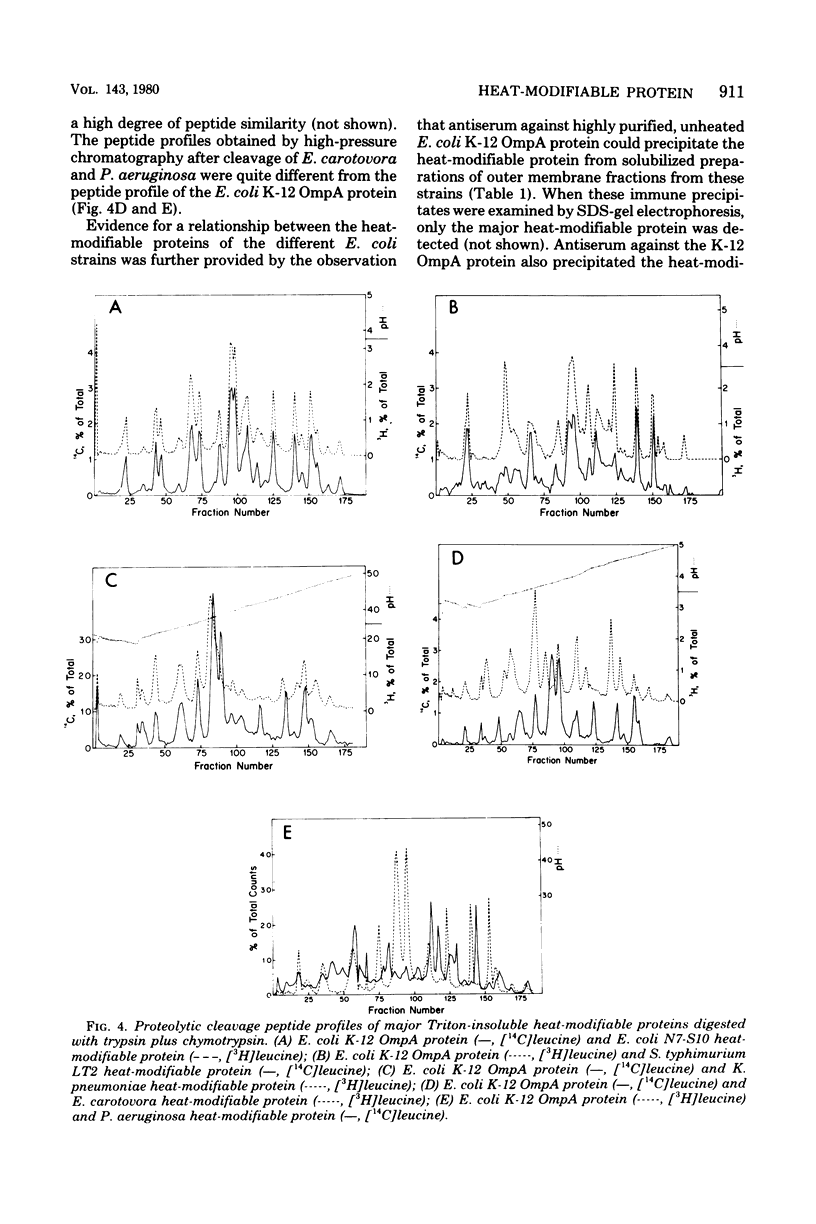
The outer membranes of several strains of Escherichia coli, other enteric bacteria, and a variety of nonenteric gram-negative bacteria all contain a major heat-modifiable protein similar to the OmpA protein of E. coli K-12. The heat-modifiable proteins from these bacteria resemble the K-12 protein in molecular weight, in preferential release from the outer membrane by sodium dodecyl sulfate in the presence of Mg2+, and in characteristic cleavage by proteases to yield a smaller fragment which remains membrane bound. Antiserum directed against the K-12 protein precipitated the heat-modifiable protein from all strains of Enterobacteriaceae, and chemical comparison by isoelectric focusing, cyanogen bromide cleavage profiles, and proteolytic peptide analysis indicated that the proteins from the various enteric bacteria were nearly identical in primary structure. The heat-modifiable proteins from bacteria phylogenically distant from E. coli shared many of the properties of the E. coli protein but were chemically distinct. Thus, it appears that the structure (and, presumably, the function) of the heat-modifiable protein of gram-negative bacteria is strongly conserved during evolution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Enzyme evolution in the Enterobacteriaceae. J Bacteriol. 1972 Jun;110(3):793–802. doi: 10.1128/jb.110.3.793-802.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Krämer C., Henning U. Diploidy for a structural gene specifying a major protein of the outer cell envelope membrane from Escherichia coli K-12. J Bacteriol. 1976 Dec;128(3):834–841. doi: 10.1128/jb.128.3.834-841.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Mocca L. F. Heat-modifiable outer membrane proteins of Neisseria meningitidis and their organization within the membrane. J Bacteriol. 1978 Dec;136(3):1127–1134. doi: 10.1128/jb.136.3.1127-1134.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface properties of Neisseria gonorrhoeae: topographical distribution of the outer membrane protein antigens. J Gen Microbiol. 1978 Oct;108(2):213–219. doi: 10.1099/00221287-108-2-213. [DOI] [PubMed] [Google Scholar]
- Hendley J. W., Powell K. R., Rodewald R., Holzgrefe H. H., Lyles R. Demonstration of a capsule on Neisseria gonorrhoeae. N Engl J Med. 1977 Mar 17;296(11):608–611. doi: 10.1056/NEJM197703172961105. [DOI] [PubMed] [Google Scholar]
- Henning U., Sonntag I., Hindennach I. Mutants (ompA) affecting a major outer membrane protein of Escherichia coli K12. Eur J Biochem. 1978 Dec;92(2):491–498. doi: 10.1111/j.1432-1033.1978.tb12771.x. [DOI] [PubMed] [Google Scholar]
- Hori H. Molecular evolution of 5S RNA. Mol Gen Genet. 1976 May 7;145(2):119–123. doi: 10.1007/BF00269583. [DOI] [PubMed] [Google Scholar]
- Lee D. R., Schnaitman C. A. Comparison of outer membrane porin proteins produced by Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Jun;142(3):1019–1022. doi: 10.1128/jb.142.3.1019-1022.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. R., Schnaitman C. A., Pugsley A. P. Chemical heterogeneity of major outer membrane pore proteins of Escherichia coli. J Bacteriol. 1979 Jun;138(3):861–870. doi: 10.1128/jb.138.3.861-870.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Pugsley A. P., Reeves P. Defective growth functions in mutants of Escherichia coli K12 lacking a major outer membrane protein. J Mol Biol. 1977 Oct 25;116(2):285–300. doi: 10.1016/0022-2836(77)90217-0. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Puspurs A., Reeves P. Outer membrane of Escherichia coli K-12: isolation of mutants with altered protein 3A by using host range mutants of bacteriophage K3. J Bacteriol. 1976 Sep;127(3):1080–1084. doi: 10.1128/jb.127.3.1080-1084.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Isolation of characterization of a major outer membrane protein of Pseudomonas aeruginosa. Evidence for the occurrence of a lipoprotein. J Biochem. 1979 Jan;85(1):115–122. doi: 10.1093/oxfordjournals.jbchem.a132300. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Pirtle R. M., Inouye M. Homology of the gene coding for outer membrane lipoprotein within various Gram-negative bacteria. J Bacteriol. 1979 Jan;137(1):595–604. doi: 10.1128/jb.137.1.595-604.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Outer membrane proteins of Escherichia coli. VII. Evidence that bacteriophage-directed protein 2 functions as a pore. J Bacteriol. 1978 Mar;133(3):1181–1189. doi: 10.1128/jb.133.3.1181-1189.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Proteolytic digestion and labelling studies of the organization of the proteins in the outer membrane of Escherichia coli. Can J Biochem. 1977 Oct;55(10):1082–1090. doi: 10.1139/o77-160. [DOI] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Purification and characterization of heat-modifiable protein from the outer membrane of Escherichia coli. FEBS Lett. 1974 May 1;41(2):195–198. doi: 10.1016/0014-5793(74)81210-x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schweizer M., Henning U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J Bacteriol. 1977 Mar;129(3):1651–1652. doi: 10.1128/jb.129.3.1651-1652.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga H., Tokunaga M., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 1. Chemical analysis. Eur J Biochem. 1979 Apr;95(3):433–439. doi: 10.1111/j.1432-1033.1979.tb12982.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]