Abstract
Background
During mate choice, individuals must classify potential mates according to species identity and relative attractiveness. In many species, females do so by evaluating variation in the signals produced by males. Male túngara frogs (Physalaemus pustulosus) can produce single note calls (whines) and multi-note calls (whine-chucks). While the whine alone is sufficient for species recognition, females greatly prefer the whine-chuck when given a choice.
Methodology/Principal Findings
To better understand how the brain responds to variation in male mating signals, we mapped neural activity patterns evoked by interspecific and intraspecific variation in mating calls in túngara frogs by measuring expression of egr-1. We predicted that egr-1 responses to conspecific calls would identify brain regions that are potentially important for species recognition and that at least some of those brain regions would vary in their egr-1 responses to mating calls that vary in attractiveness. We measured egr-1 in the auditory brainstem and its forebrain targets and found that conspecific whine-chucks elicited greater egr-1 expression than heterospecific whines in all but three regions. We found no evidence that preferred whine-chuck calls elicited greater egr-1 expression than conspecific whines in any of eleven brain regions examined, in contrast to predictions that mating preferences in túngara frogs emerge from greater responses in the auditory system.
Conclusions
Although selectivity for species-specific signals is apparent throughout the túngara frog brain, further studies are necessary to elucidate how neural activity patterns vary with the attractiveness of conspecific mating calls.
Introduction
Choosing a mate is one of the most important decisions that an animal makes. In many species, females make mate choice decisions based on communication signals produced by males. Males convey information about species identity in their signals and females pay attention to this information in order to avoid heterospecific matings, which often fail to produce viable offspring [1], [2]. Communication signals can also provide information that females use to discriminate among conspecifics, which can lead to variation in male mating success. Thus, the evolution of sender and receiver has been an important topic in speciation and sexual selection [3], [4]. Yet, a complete understanding of how selection acts on behavioral responses to mating signals requires understanding the mechanistic basis of signal processing.
One way to address questions about the mechanisms of sexual communication is to study how the brain responds to variation in male mating signals. In some systems, different signal features differentially predict mate choice in species recognition tasks and intraspecific discrimination, suggesting a hierarchical classification process. For example, in frogs and crickets, fine-scale temporal features (e.g., pulse rate) often influence species recognition whereas gross temporal features (e.g., call duration) often influence intraspecific discrimination [2], [5]. In songbirds, patterns of neural selectivity are consistent with a hierarchical classification process: selectivity to conspecific song is apparent in Field L of the forebrain [6], [7], a primary auditory area, whereas selectivity to preferred song types appears to emerge in secondary auditory forebrain areas such as the caudomedial mesopallium and the caudomedial nidopallium [8]–[11]. However, our understanding of the relationship between the neural mechanisms of species recognition and intraspecific discrimination is incomplete.
Túngara frogs (Physalaemus pustulosus) are an excellent model for investigating species recognition and intraspecific discrimination. Male túngara frogs and their close relatives produce advertisement calls, known as whines, that females use to locate and identify potential mates. Male túngara frogs can increase the attractiveness of their whines by adding a second note called a chuck. Although the whine is sufficient for species recognition [12], females prefer whines with chucks compared to whines [13]–[15]; however, the number of chucks does not influence the attractiveness of a male's call in most situations [16]. Based on behavioral choice tests, Wilczynski et al. [17] proposed a model by which the “call analysis system” of the túngara frog encodes acoustic signals. According to the model, species-specific spectral components of the whine trigger species recognition while the presence of chucks increases attractiveness by increasing the acoustic energy in the calls [17]. Thus, we predicted that conspecific calls, but not heterospecific calls, would evoke responses in brain regions involved in species recognition and attractive calls would elicit differential responses in at least some of those same brain regions, thereby serving as a potential mechanism to bias behavioral output towards attractive signals. Previous work in túngara frogs has identified parts of the auditory brainstem as potentially contributing to species recognition [18], [19]. In the present study, we extend those results by examining both the auditory brainstem and its forebrain targets, and by subsequently examining the effects of call attractiveness on those same brain regions. In addition, we focused on females because we were interested in acoustically induced neural activity patterns within the context of mate choice.
We first presented reproductively active female túngara frogs with conspecific whine-chucks, heterospecific whines of a congener, Physalaemus enesefae, or no sound and assessed neural responses in the auditory brainstem and its forebrain targets by measuring expression of the immediate early gene egr-1 (also known as zif268 and ZENK). All but three nuclei known to receive auditory projections demonstrated a greater egr-1 response to the conspecific calls than the heterospecific calls. We then exposed a second group of female túngara frogs to conspecific whines, conspecific whine-chucks, or no sound. We found that the whine-chucks did not elicit greater expression of egr-1 than the whines in any of eleven brain regions examined, suggesting that the magnitude of the responses in these brain regions do not explain intraspecific differences in attractiveness. Clearly, further studies that use alternative approaches will be required to identify brain regions that contribute to intraspecific discrimination in the túngara frog.
Methods
The governments of Costa Rica (INV-ACOSA-008-07; ATM-ACOSA-002-07) and Panama (SEX/A-133-07; SE/A-99-07) permitted tissue collection and export, and the University of North Carolina Institutional Animal Care and Use Committee (08-015) approved our experimental procedures.
Response patterns evoked by interspecific variation in mating calls
We captured female túngara frogs in a mating clasp with males on the Osa Peninsula, Costa Rica in July 2007 between 20:00 and 24:00 h. We released the males and brought the females to the laboratory at the Osa Biodiversity Center where we placed each in a rectangular mesh cage (18 cm×10 cm) inside one of 8 dark acoustic chambers (91 cm×20 cm×30 cm). Each chamber was equipped with a Tivoli Portable Audio Laboratory speaker (Tivoli Audio, Cambridge, MA) that was connected to an M-Audio Firewire 410 8-channel audio playback unit (M-Audio, Arcadia, CA) and a Macintosh computer. After an 11-hour acclimation period, we presented females with conspecific whine-chucks (n = 11), heterospecific P. enesefae whines (n = 11), or no sound (n = 8) for 30 minutes followed by 30 minutes of silence. We dispersed treatments across chambers and days. All females in the study remained gravid during the acclimation period and stimulus presentation. We rapidly decapitated females 1 hour after onset of stimuli, a time that corresponds to peak accumulation of acoustically induced egr-1 mRNA expression [20] and that occurs before habituation of the egr-1 response is apparent (R. Glaeser, L.A. Mangiamele, S.S. Burmeister, unpublished observation). After decapitation, we opened the skull in order to fix the brains for 10 min in 4% paraformaldehyde before removing them. We then rinsed the brains in phosphate buffered saline for 10 min before freezing them in liquid nitrogen in 2 ml tubes containing Tissue-Tek OCT embedding medium (Sakura, Finetek, Torrance, CA). We kept the brains on dry ice during transportation to University of North Carolina where we stored them at –80° C until further processing.
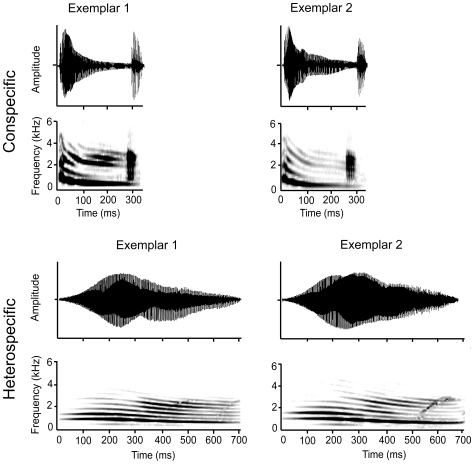
For our initial study on responses to conspecific calls, we chose to use the whine-chuck to represent conspecific calls because it contains both notes in the species's repertoire. We used two túngara frog whine-chucks, each with a single chuck (whine +1 chuck), that we recorded from two free-living males on the Osa Peninsula in 2005 using a digital recorder and a sampling rate of 44.1 kHz (Fig. 1). To represent heterospecific calls, we chose to use the calls of P. enesefae because, although they do not occur in Costa Rica, P. enesefae is the only congener that is sympatric with the túngara frog and the calls of P. ensefae are more similar to those of the túngara frog than more distantly related species that co-occur with the túngara frog in Costa Rica. We used two P. enesefae whine exemplars recorded by Dr. Zaida Tárano from two different males in Venezuela (Fig. 1). We presented each female with a single male call that was repeated every 2 seconds in order to reflect the average calling rate of P. pustulosus males and to be consistent with previous behavioral [e.g., 16] and egr-1 studies [e.g., 18]. We set the peak amplitude of the calls at 82 dB SPL (re 20 µPa) at a distance of approximately 5 cm from the speaker with a RadioShack (Fort Worth, Texas) sound pressure level meter.
Figure 1. Waveforms and spectrograms of the calls that we used to represent conspecific (Physalaemus pustulosus) and heterospecific (Physalaemus enesefae) mating calls in order to identify brain regions that contribute to species recognition.
Response patterns evoked by intraspecific variation in mating calls
We captured female túngara frogs in a mating clasp near Gamboa, Panama from 29 October to 25 November, 2007 between 20:00 and 22:45 h, transported them to the laboratory of the Smithsonian Tropical Research Institute, and placed each inside a circular mesh arena (8 cm diameter) inside one of 8 dark acoustic chambers (270 cm×190 cm×190 cm). After a 10-h acclimation period, we exposed females to túngara frog whines (n = 9), whine-chucks, each with three chucks (whine +3 chucks; n = 11), or no sound (n = 6) for 30 minutes followed by 30 minutes of silence before sacrifice. We dispersed treatments across chambers and days. We collected the females' brains according to the procedure described above, except that we did not rinse brains in phosphate buffered saline before freezing them.
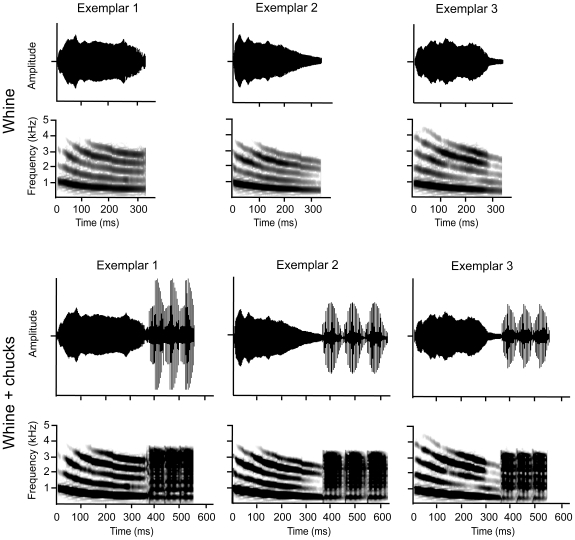
To create our stimuli, we started with three whine +1 chuck calls from Ryan and Rand [21], referred to as Oc, M, Sd in the original report, and modified them using Signal sound analysis software (Engineering Design, Berkeley, CA). To create our whine stimuli, we removed the chuck. To create our whine +3 chucks stimuli, we appended the original chuck onto the whine three times. Thus, each experimental group had the same three call exemplars that differed only in the presence of chucks (Fig. 2). Each female heard a single male call that was repeated every 2 seconds to approximate the average calling rate of túngara frog males. In addition, we modified our stimuli to account for the frequency response characteristics of our amplified speaker system by using Vibrotoolbox (Dr. Marcos Gridi-Papp, University of the Pacific) to create a transfer function of our speakers between 100 Hz and 6000 Hz and then filtering each acoustic stimulus by the inverse of this transfer function. We set playback amplitude at 82 dB (re 20 µPa) at approximately 25 cm from the speaker.
Figure 2. Waveforms and spectrograms of the túngara frog (Physalaemus pustulosus) calls that we used to represent the whine and whine-chuck in order to examine responses of brain regions to mating calls that vary in their attractiveness.
Radioactive in situ hybridization
We sectioned brains in the transverse plane at 16 µm in 3 series on a cryostat. To localize egr-1 mRNA, we used radioactive in situ hybridization following the procedure previously described in Burmeister et al. 2008 [20]. Briefly, we generated radioactively labeled (S-35) sense and antisense probes by reverse transcription of a 309-nucleotide subclone of P. pustulosus egr-1 (GenBank Accession No. AY562993) and hybridized the probes to the brain tissue at 65° C. We performed separate in situ hybridizations for brain tissue collected in each experiment. To visualize the bound riboprobe, we dipped slides in emulsion, allowed them to dry, and stored them in lightproof boxes at 4°C for 14 days before development and counterstaining with thionin. To confirm the specificity of our egr-1 riboprobe, we noted the absence of binding in brain tissue hybridized with sense strand riboprobe under identical hybridization conditions.
Quantification of egr-1 expression
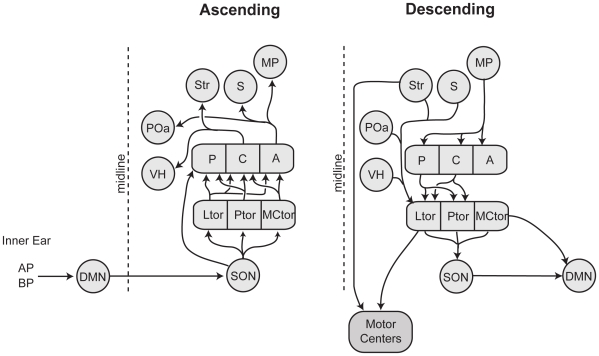
We measured egr-1 expression in the auditory brainstem and its forebrain targets (Fig. 3). We examined these regions because of their role in auditory processing [22] or their predicted involvement in female choice behavior in studies of other anurans [23]. The auditory brainstem includes the dorsal medullary nucleus (homolog of the mammalian cochlear nucleus), superior olivary nucleus, and midbrain torus semicircularis (homolog of the mammalian inferior colliculus). Within the torus semicircularis, we sampled from the principal, laminar, and magnocellular nuclei. Forebrain targets of the auditory system include the posterior, central, and anterior thalamic nuclei, the ventral hypothalamus, anterior preoptic nucleus, medial pallium, septum, and striatum. Within the medial pallium, we sampled from the dorsal part. Within the septum, we sampled from the ventrolateral septal nucleus. Within the striatum, we sampled from the ventral portion.
Figure 3. Diagram of the major ascending and descending connections in the frog auditory system.
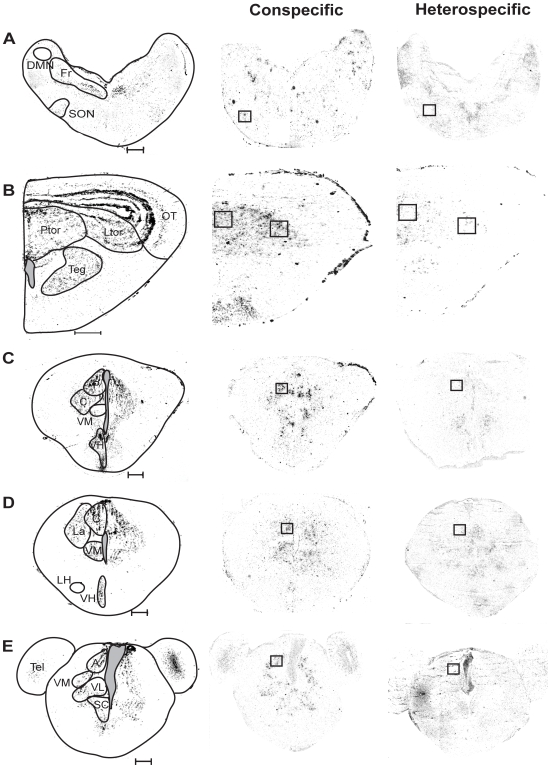
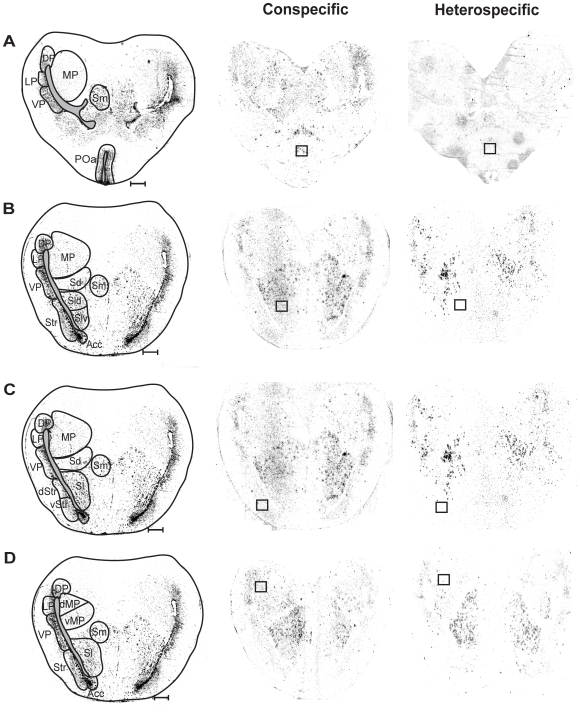
Abbreviations: A, anterior thalamus; AP, amphibian papilla; BP, basilar papilla; C, central thalamus; DMN, dorsal medullary nucleus; Ltor, laminar nucleus of the torus semicircularis; MCtor, magnocellular nucleus of the torus semicircularis; MP, medial pallium; P, posterior thalamus; POa, anterior preoptic nucleus; Ptor, principal nucleus of the torus semicircularis; S, septum; SON, superior olivary nucleus; Str, striatum; VH, ventral hypothalamus.
For each brain region, we quantified egr-1 mRNA expression from digital images taken at a magnification of 630× or 1000× from one hemisphere of the brain chosen at random. Due to variation in tissue quality, we were unable to collect data from all brain regions for every individual; overall, we collected data from an average of 10.6 brain regions per subject resulting in 18–30 subjects per brain region. In addition, the number of sections we sampled from for each brain region varied with the size of the region and quality of the sections, as follows: dorsal medullary nucleus, 2–7; superior olivary nucleus, 2–8; principal nucleus of the torus semicircularis, 3–6; laminar nucleus of the torus semicircularis, 3; magnocellular nucleus of the torus semicircularis, 2–5; posterior thalamus, 3–6; central thalamus, 3–6; anterior thalamus, 2–4; ventral hypothalamus, 3–11; preoptic area, 3–7; ventral striatum, 3–4; ventrolateral septal nucleus, 3–4; dorsal part of the medial pallium, 6. We quantified egr-1 expression as described in Burmeister et al. 2008 [20]. Briefly, we used ImageJ to count silver grains in the region of interest and in a nearby area of the slide that represented local background silver grain levels. We chose the sampling area for background silver grains by moving the microscope stage until the tissue was no longer visible. This provides an estimate of local background levels that may differ at different positions of the slide due to variation in emulsion thickness. We subtracted the number of background silver grains from the number of silver grains in the region of interest to calculate number of silver grains above background per image. For each image, we manually counted the number of cell bodies in the region of interest from separate photomicrographs. We expressed egr-1 expression as the number of silver grains above background per cell.
Statistical Analysis
When examining response patterns evoked by interspecific variation in mating calls, we conducted separate ANOVAs for each brain region in order to test for an effect of stimulus (no sound, heterospecific whine, conspecific whine +1 chuck) on silver grains per cell above background followed by t-tests between pairs of groups. Although brain regions are not independent of one another, we could not account for any covariation among brain regions with a multivariate analysis (e.g., repeated-measures ANOVA) because missing values for individual brain regions would result in the exclusion of most subjects. Because the no sound group does not have exemplars, we could not include exemplar as a factor in our analyses. Therefore, we tested for exemplar effects separately by conducting ANOVAs with stimulus, exemplar, and their interaction as factors, including only the groups receiving calls.
When examining response patterns evoked by intraspecific variation in mating calls, we used ANOVA to test for an effect of stimulus followed by t-tests to compare conspecific calls (whine and whine +3 chucks) to no sound and to compare the preferred, whine +3 chucks to whine for each brain region. In these analyses, we excluded the dorsal medullary nucleus and ventral hypothalamus because egr-1 expression was not modulated by conspecific calls in these regions in the first experiment (p>0.89 in both cases) or by variation in the attractiveness of the calls in the second experiment (data not shown). Again, we were unable to conduct a multivariate analysis to account for correlated variation across the brain because of missing values for individual brain regions.
Results
Response patterns evoked by interspecific variation in mating calls
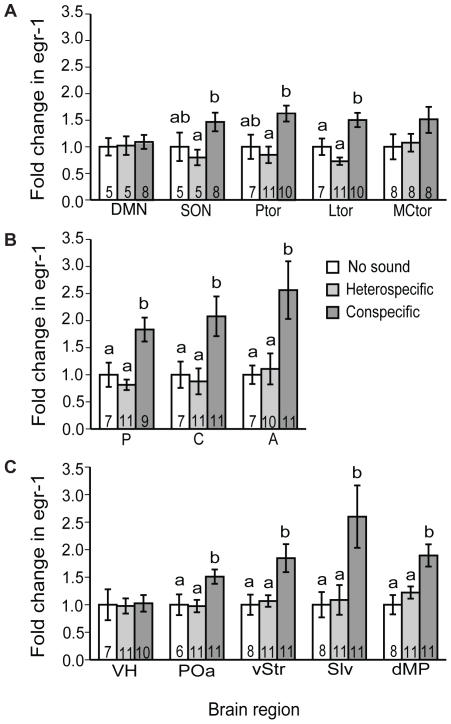
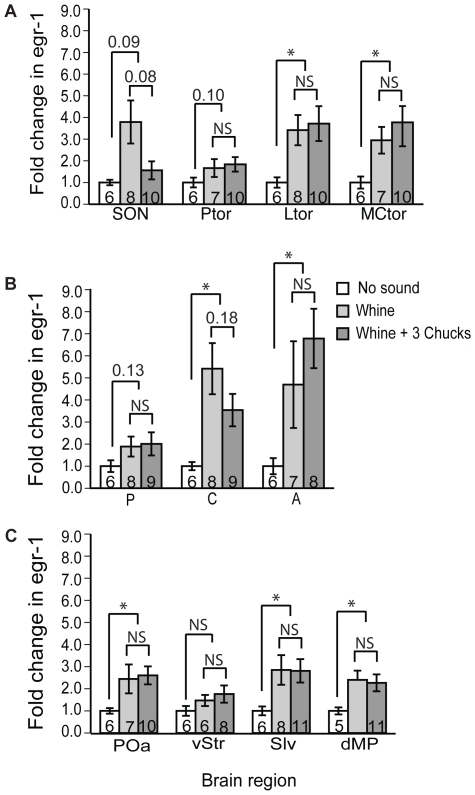
The conspecific, whine +1 chuck calls elicited robust expression of egr-1 in almost all nuclei of the auditory brainstem and its forebrain targets while heterospecific whines did not (Table 1; Fig. 4; Fig. 5; Fig. 6). One exception was the dorsal medullary nucleus, where sound had no effect on egr-1 expression, which was expressed at low but detectable levels. In the superior olivary nucleus, principal nucleus of the torus, and laminar nucleus of the torus, females exposed to conspecific whine-chucks had higher egr-1 expression than those exposed to heterospecific whines, although they did not always differ from females exposed to no sound, a pattern that reflects a slight decline in egr-1 expression in females hearing heterospecific whines compared to no sound (Fig. 4). In contrast, conspecific whine-chucks did not have a strong effect on egr-1 expression in the magnocellular nucleus of the torus. All but one of the auditory forebrain targets we sampled expressed higher levels of egr-1 expression in response to conspecific whine-chucks compared to heterospecific whines or no sound. In the ventral hypothalamus mating calls had no effect on egr-1 expression in spite of significant auditory input to this nucleus [24], [25], indicating that sound does not modulate egr-1 expression there. In no case did exemplar influence the egr-1 response to calls (exemplar × stimulus, all p>0.13), indicating that the increase in egr-1 expression in response to the whine-chucks we used may be generalizeable. In summary, conspecific calls elicited significant egr-1 responses from most parts of the auditory brainstem and its forebrain targets, including limbic and motor regions thought to be important in mate choice.
Table 1. Effects of acoustic stimuli on egr-1 expression when stimuli reflected interspecific (Experiment 1) and intraspecific (Experiment 2) variation in mating calls.
| Experiment 1 | Experiment 2 | |||||
| Region | df | F | p | df | F | p |
| DMN | 2, 15 | 0.11 | 0.90 | — | — | — |
| SON | 2, 15 | 3.23 | 0.07 | 2,21 | 4.09 | 0.03 |
| Ptor | 2, 25 | 6.23 | <0.01 | 2,20 | 1.46 | 0.25 |
| Ltor | 2, 25 | 13.34 | <0.01 | 2,22 | 3.87 | 0.04 |
| MCtor | 2, 23 | 1.66 | 0.21 | 2,19 | 4.40 | 0.03 |
| P | 2, 24 | 11.30 | <0.01 | 2,20 | 1.22 | 0.32 |
| C | 2, 26 | 6.27 | <0.01 | 2,20 | 5.82 | 0.01 |
| A | 2, 25 | 5.06 | 0.01 | 2,18 | 3.89 | 0.04 |
| VH | 2, 25 | 0.04 | 0.96 | — | — | — |
| POa | 2, 25 | 5.36 | 0.01 | 2,20 | 3.19 | 0.06 |
| vStr | 2, 27 | 6.02 | <0.01 | 2,21 | 1.0 | 0.38 |
| Slv | 2, 27 | 3.38 | 0.049 | 2,22 | 2.92 | 0.07 |
| dMP | 2, 27 | 7.71 | <0.01 | 2,22 | 2.46 | 0.11 |
Abbreviations: A, anterior thalamus; C, central thalamus; DMN, dorsal medullary nucleus; dMP, dosal part of the medial pallium; Ltor, laminar nucleus of the torus semicircularis; MCtor, magnocellular nucleus of the torus semicircularis; P, posterior thalamus; POa, anterior preoptic nucleus; Ptor, principal nucleus of the torus semicircularis; Slv, ventral part of the lateral septum; SON, superior olivary nucleus; VH, ventral hypothalamus; vStr, ventral striatum.
Figure 4. Effects of interspecific variation in mating calls on egr-1 mRNA expression in the auditory brainstem and its forebrain targets.
Data are shown as mean (± SE) fold change in silver grains per cell above background relative to the no sound group. Sample sizes are indicated for each group and letters above bars indicate groups that are statistically different (p<0.05). Abbreviations: A, anterior thalamus; C, central thalamus; DMN, dorsal medullary nucleus; dMP, dosal part of the medial pallium; Ltor, laminar nucleus of the torus semicircularis; MCtor, magnocellular nucleus of the torus semicircularis; P, posterior thalamus; POa, anterior preoptic nucleus; Ptor, principal nucleus of the torus semicircularis; Slv, ventrolateral septal nucleus; SON, superior olivary nucleus; VH, ventral hypothalamus; vStr, ventral striatum.
Figure 5. Brightfield images (left column) and inverted darkfield images of transverse sections showing egr-1 expression within sampling windows (boxes) in response to conspecific whine-chucks (middle column) and heterospecific whines (right column) in the auditory brainstem (A–B) and thalamus (C–E).
Scale bars represent 400 µm. Abbreviations: A, anterior thalamus; C, central thalamus; DMN, dorsal medullary nucleus; Fr, reticular formation; La, lateral thalamus; LH, lateral hypothalamus; Ltor, laminar nucleus of the torus semicircularis; OT, optic tectum; P, posterior thalamus; Ptor, principal nucleus of the torus semicircularis; SC, suprachiasmatic nucleus; SON, superior olivary nucleus; Teg, tegmentum; Tel, telencephalon; VH, ventral hypothalamus; VL, ventrolateral thalamus; VM, ventromedial thalamus.
Figure 6. Brightfield images (left column) and inverted darkfield images of transverse sections showing egr-1 expression within sampling windows (boxes) in response to conspecific whine-chucks (middle column) and heterospecific whines (right column) in the anterior preoptic nucleus (A), septum (B), striatum (C), and medial pallium (D).
Scale bars represent 400 µm. Abbreviations: Acc, nucleus accumbens; dMP, dorsal part of the medial pallium; DP, dorsal pallium; dStr, dorsal striatum; LP, lateral pallium; MP, medial pallium; POa, anterior preoptic nucleus; Sd, dorsal septal nucleus; Sl, lateral septal nucleus, Sld, dorsolateral septal nucleus; Slv, ventrolateral septal nucleus; Sm, medial septal nucleus; Str, striatum; vMP, ventral part of the medial pallium; VP, ventral pallium; vStr, ventral striatum.
Response patterns evoked by intraspecific variation in mating calls
Once again, conspecific calls increased egr-1 expression in most regions of the auditory brainstem and its forebrain targets (Table 1; Fig. 7). However, when we compared conspecific calls (whine and whine +3 chucks) to no sound (Fig. 7), we did not replicate the results from our first experiment (Fig. 4) in three brain regions. For the principal nucleus of the torus, posterior thalamus, and ventral striatum, conspecific calls failed to induce significant expression of egr-1, although, because the magnitude of the egr-1 response in these brain regions was similar in the two experiments, the difference in our results was probably due to differences in the between-subjects variance. In this experiment, however, the egr-1 response to conspecific calls in the magnocellular nucleus of the torus was more robust than in our first experiment (Fig. 4). We found no evidence for increased egr-1 responses in females hearing whine +3 chucks compared to those hearing whines in any brain region examined (Table 1; Fig. 7). In all brain regions, levels of egr-1 expression elicited by whines and whine +3 chucks were similar, with the possible exception of the superior olivary nucleus where the whine appeared to evoke greater egr-1 expression than the whine +3 chucks. A previous study in males also found no evidence for increased egr-1 responses to whine-chucks compared to whines in the torus semicirularis [18]. Thus, in spite of robust egr-1 responses to conspecific calls in most parts of the auditory brainstem and its targets, levels of egr-1 expression did not vary with the attractiveness of the call.
Figure 7. Effects of intraspecific variation in mating calls on egr-1 mRNA expression in the auditory brainstem and its forebrain targets.
Data are shown as mean (± SE) fold change in silver grains per cell above background relative to the no sound group. Sample sizes are indicated for each group. The bars above the columns indicate statistical comparisons between females hearing conspecific calls (whine or whine +3 chucks) to those hearing no sound and between females hearing the preferred, whine +3 chucks to those hearing whines; p values are indicated as follows: asterisks indicate p<0.05, actual p values are given for those tests where 0.05<p<0.2, and NS indicates p>0.2. Abbreviations: A, anterior thalamus; C, central thalamus; dMP, dosal part of the medial pallium; Ltor, laminar nucleus of the torus semicircularis; MCtor, magnocellular nucleus of the torus semicircularis; P, posterior thalamus; POa, anterior preoptic nucleus; Ptor, principal nucleus of the torus semicircularis; Slv, ventrolateral septal nucleus; SON, superior olivary nucleus; vStr, ventral striatum.
Discussion
When evaluating males as potential mates, females must classify them according to species identity and relative attractiveness. To better understand the neural systems underlying these classification tasks, we examined neural activity patterns in response to interspecific and intraspecific variation in mating calls in female túngara frogs by mapping expression of the activity dependent gene egr-1. First, we mapped responses to conspecific calls to identify brain regions that contribute to species recognition. Second, we examined responses of these brain regions to mating calls that vary in their attractiveness. We predicted that conspecific calls would differentially stimulate egr-1 expression in brain regions important for species recognition and that at least some of those brain regions would vary in their egr-1 responses to mating calls that vary in attractiveness. We found that conspecific whine-chuck calls evoked greater egr-1 expression than heterospecific whines in auditory, motor, and limbic regions of the brain. However, we found no evidence that preferred whine-chuck calls elicited greater egr-1 expression than conspecific whines in any brain region examined.
In order to identify neural activity patterns that are characteristic of species recognition, we compared egr-1 expression in response to conspecific calls to those elicited by the calls of P. enesefae, an allopatric congener. Although allopatric species have commonly been used in similar studies [e.g., 19,26,27], there are potential drawbacks. One drawback is that the subjects in all these studies will have had previous experience with the conspecific signals but not the heterospecific ones, resulting in a potential confound between familiarity and species identity (conspecific or heterospecific). Even for species that co-occur, individuals may be more familiar with conspecific than heterospecific signals if they aggregate when signaling, as in the case of many frogs. Nonetheless, at least in the case of túngara frogs, we think that the calls of P. enesefae are a useful representative of heterospecific signals because their calls are more similar to those of the túngara frog than other, more distantly related species that co-occur with the túngara frog in Costa Rica. Because phylogenetic relatedness is a good predictor of whether a female will recognize a heterospecific's call as an acceptable sexual signal [28], using a congener to represent heterospecifics is a reasonable approach in this case, even if we cannot rule out the potential contribution of familiarity.
Consistent with previous results [19], we found that differential egr-1 expression in response to conspecific calls over heterospecific calls emerged as early as the second synapse in the auditory hindbrain, before the emergence of feature detectors in the midbrain and thalamus. Similarly, the auditory midbrain and most of its forebrain targets responded differentially to conspecific mating calls. Surprisingly, the heterospecific P. enesefae whine was unable to elicit an egr-1 response, even though the ears of túngara frogs are sensitive to the spectral content of these calls [29] and behavioral studies confirm that túngara frogs perceive P. enesefae calls [30]. Thus, although P. enesefae calls are likely to elicit electrical activity in the túngara frog auditory system, they apparently do not activate the second messenger cascades required for induction of egr-1, suggesting that egr-1 responses are more selective than electrical responses in the auditory brainstem. The lack of egr-1 expression in response to heterospecific calls in túngara frogs is in apparent contrast with zebra finches, where canary song can induce ZENK (avian egr-1) expression in the auditory forebrain relative to no sound or tones [26].
We found that conspecific calls elicit differential egr-1 expression throughout the túngara frog brain, including all but three brain regions that receive significant auditory input. In addition to the auditory brainstem, conspecific calls differentially induced egr-1 in regions of the forebrain that have been implicated in phonotaxis, including the anterior preoptic nucleus, where lesions abolish phonotaxis, the septum, where lesions retard phonotaxis, and the striatum, where lesions abolish locomotion but not orientation [23]. Although the medial pallium, which is homologous to the hippocampus, is acoustically responsive [31], [32], its role in modulating behavior in frogs remains unclear [23]. In spite of the widespread nature of the egr-1 response, there is reason to think that it is specific to conspecific mating calls. While recordings of a conspecific mating chorus elicit robust responses from the auditory midbrain and some parts of the pallium [20], [31], they do not do so in regions processing other sensory modalities [31], indicating that conspecific mating calls do not elicit egr-1 responses through some general arousal system. However, we can say very little at this point about the response properties of the different brain regions, as each of them is probably responding to different acoustic traits of the conspecific calls, at least in the auditory brainstem. This point is particularly important in the context of identifying brain regions involved in species recognition since the stimuli we used go beyond those that are sufficient for species recognition in behavioral tests [17]. Whereas we used full-spectrum whine-chuck calls to represent conspecific mating calls, behavioral studies show that the fundamental frequency of the whine is sufficient for species recognition [12] and a sequence of descending tones can mimic the conspecific whine [17]. Future studies that use the minimum required acoustic elements for species recognition would help to elucidate which brain regions contribute to species recognition.
Although the spectral requirements for species recognition by the túngara frog are fairly specific, the requirements for call preferences are highly permissive. In the natural whine-chuck call, the chuck adds acoustic energy in the high frequency range (above 1500 Hz). However, one can emulate the whine-chuck preference with an artificial chuck that contains only the lower frequencies [33]. These types of behavioral studies inspired Wilczynski et al. [17] to propose that mating preferences in the túngara frog result from a simple summation of the acoustic energy in the call. We predicted, therefore, that the whine-chuck call would cause greater egr-1 expression than the whine in at least some of the brain regions that are responsive to conspecific calls in a manner similar to songbirds where preferred songs elicit greater expression of ZENK (avian egr-1) in the auditory forebrain [8], [10]. We examined eleven brain regions that were responsive to conspecific calls and none responded to the preferred whine-chuck call with higher expression of egr-1. Although not what we expected, our results are consistent with previous studies that also failed to find elevated immediate early gene expression in response to whine-chucks [18], [34]. Interpretation of negative results is always difficult. For example, it is possible that, by presenting stimuli for 30 minutes, we have induced similar levels of egr-1 expression with both the whines and whine-chucks due to a ceiling effect, rather than a true lack of difference in the calls' abilities to elicit egr-1 expression. Our interpretation of these results is further limited by the fact that changes in firing rates are not necessarily accompanied by changes in gene expression [35]. Because females show mating preferences for the whine-chuck call, there is no question that the whine-chuck elicits differential responses in the túngara frog brain. We are simply unable to detect these differences using activity dependent gene expression in the manner we have employed to date. At this point, however, we can conclude that our data are not consistent with predictions of the Wilczynski et al. [17] model that posits that mating preferences emerge from greater responses in the auditory system. One limitation of our approach to date is that each female heard only one type of call, whereas mating preferences are a consequence of comparisons among calls. The conspecific whine, by itself, is a very attractive stimulus that is only less attractive in the presence of whine-chucks.
In summary, we mapped neural activity patterns in response to interspecific and intraspecific variation in mating calls in order to better understand the neural mechanisms of mate choice. We found that conspecific calls evoked robust egr-1 expression in the auditory brainstem and many of its targets, but that preferred, whine-chuck mating calls failed to evoke greater egr-1 expression compared to whines, in contrast to our predictions based on studies showing that calls of greater acoustic energy evoke behavioral preferences [17]. Clearly we still have much to learn about the neural mechanisms of species recognition and mating preferences in túngara frogs. Because selection for species recognition can influence intraspecific discrimination [36]–[38], determining how the underlying processes are related will enable us to better understand how the evolution of one can influence the evolution of the other.
Acknowledgments
We thank Christina Lebonville, Mihnea (Mike) Mangalea, Sera Haith, Stuart Jeckel, Joan Winter, and Rachel Glaeser for their contributions to data collection. We also thank Dr. Michael J. Ryan, the Smithsonian Tropical Research Institute, Friends of the Osa, and the Organization for Tropical Studies for research support and assistance in obtaining permits.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by NSF IOB 0445682 to SSB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arnold ML. Natural Hybridization and Evolution; In: May RM, Harvey PH, editors. Oxford: Oxford University Press; 1997. 232 [Google Scholar]
- 2.Gerhardt HC, Huber F. Chicago: University of Chicago Press; 2002. Acoustic communication in insects and anurans.542 [Google Scholar]
- 3.Andersson M. Princeton: Princeton University Press; 1994. Sexual selection.624 [Google Scholar]
- 4.Endler JA. Signals, signal conditions and the direction of evolution. Am Nat. 1992;139:S125–S153. [Google Scholar]
- 5.Gerhardt HC. Female mate choice in treefrogs - static and dynamic acoustic criteria. Anim Behav. 1991;42:615–635. [Google Scholar]
- 6.Grace JA, Amin N, Singh NC, Theunissen FE. Selectivity for conspecific song in the zebra finch auditory forebrain. J Neurophysiol. 2003;89:472–487. doi: 10.1152/jn.00088.2002. [DOI] [PubMed] [Google Scholar]
- 7.Hauber ME, Cassey P, Woolley SMN, Theunissen FE. Neurophysiological response selectivity for conspecific songs over synthetic sounds in the auditory forebrain of non-singing female songbirds. J Comp Physiol A. 2007;193:765–774. doi: 10.1007/s00359-007-0231-0. [DOI] [PubMed] [Google Scholar]
- 8.Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64:275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- 9.Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc R Soc Lond B Biol Sci. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Rand AS, Ryan MJ, Wilczynski W. Signal redundancy and receiver permissiveness in acoustic mate recognition by the túngara frog, Physalaemus pustulosus. Am Zool. 1992;32:81–90. [Google Scholar]
- 13.Ryan M, Rand A. Mate recognition in túngara frogs: a review of some studies of brain, behavior, and evolution. Acta Zool Sin. 2003;49:713–772. [Google Scholar]
- 14.Ryan MJ. Female mate choice in a Neotropical frog. Science. 1980;209:523–525. doi: 10.1126/science.209.4455.523. [DOI] [PubMed] [Google Scholar]
- 15.Ryan MJ. Chicago: University of Chicago Press; 1985. The túngara frog, a study in sexual selection and communication.230 [Google Scholar]
- 16.Bernal X, Akre K, Baugh A, Rand A, Ryan M. Female and male behavioral response to advertisement calls of graded complexity in túngara frogs, Physalaemus pustulosus. Behav Ecol Sociobiol. 2009;63:1269–1279. [Google Scholar]
- 17.Wilczynski W, Rand AS, Ryan MJ. The processing of spectral cues by the call analysis system of the túngara frog, Physalaemus pustulosus. Anim Behav. 1995;49:911–929. [Google Scholar]
- 18.Hoke KL, Burmeister SS, Fernald RD, Rand AS, Ryan MJ, et al. Functional mapping of the auditory midbrain during mate call reception. J Neurosci. 2004;24:11264–11272. doi: 10.1523/JNEUROSCI.2079-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoke KL, Ryan MJ, Wilczynski W. Candidate neural locus for sex differences in reproductive decisions. Biol Lett. 2008;4:518–521. doi: 10.1098/rsbl.2008.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burmeister SS, Mangiamele LM, Lebonville CL. Acoustic modulation of immediate early gene expression in the auditory midbrain of female túngara frogs. Brain Res. 2008;1190:105–114. doi: 10.1016/j.brainres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Ryan MJ, Rand W, Hurd PL, Phelps SM, Rand AS. Generalization in response to mate recognition signals. Am Nat. 2003;161:380–394. doi: 10.1086/367588. [DOI] [PubMed] [Google Scholar]
- 22.Wilczynski W, Endepols H. Central auditory pathways in anuran amphibians: the anatomical basis of hearing and sound communication. In: Fay RR, Popper AN, editors. Hearing and sound communication in amphibians. New York: Springer; 2007. [Google Scholar]
- 23.Walkowiak W, Berlinger M, Schul J, Gerhardt HC. Significance of forebrain structures in acoustically guided behaviour in anurans. Eur J Morphol. 1999;37:177–181. doi: 10.1076/ejom.37.2.177.4740. [DOI] [PubMed] [Google Scholar]
- 24.Allison JD. Acoustic modulation of neural activity in the preoptic area and ventral hypothalamus of the green treefrog (Hyla cinerea). J Comp Physiol A. 1992;171:387–395. doi: 10.1007/BF00223968. [DOI] [PubMed] [Google Scholar]
- 25.Allison JD, Wilczynski W. Thalamic and midbrain auditory projections to the preoptic area and ventral hypothalamus in the green treefrog (Hyla cinerea). Brain Behav Evol. 1991;38:322–331. doi: 10.1159/000114398. [DOI] [PubMed] [Google Scholar]
- 26.Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terpstra NJ, Bolhuis JJ, Den Boer-Visser AM, Ten Cate C. Neuronal activation related to auditory perception in the brain of a non-songbird, the ring dove. J Comp Neurol. 2005;488:342–351. doi: 10.1002/cne.20592. [DOI] [PubMed] [Google Scholar]
- 28.Ryan MJ, Rand AS. Phylogenetic influence on mating call preferences in female túngara frogs, Physalaemus pustulosus. Anim Behav. 1999;57:945–956. doi: 10.1006/anbe.1998.1057. [DOI] [PubMed] [Google Scholar]
- 29.Wilczynski W, Rand AS, Ryan MJ. Evolution of calls and auditory tuning in the Physalaemus pustulosus species group. Brain Behav Evol. 2001;58:137–151. doi: 10.1159/000047268. [DOI] [PubMed] [Google Scholar]
- 30.Bernal XE, Rand AS, Ryan MJ. Sex differences in response to nonconspecific advertisement calls: receiver permissiveness in male and female túngara frogs. Anim Behav. 2007;73:955–964. [Google Scholar]
- 31.Mangiamele LM, Burmeister SS. Acoustically evoked immediate early gene expression in the pallium of female túngara frogs. Brain Behav Evol. 2008;72:239–250. doi: 10.1159/000171481. [DOI] [PubMed] [Google Scholar]
- 32.Mudry KM, Capranica RR. Evoked auditory activity within the telencephalon of the bullfrog (Rana catesbeiana). Brain Res. 1980;182:303–311. doi: 10.1016/0006-8993(80)91190-7. [DOI] [PubMed] [Google Scholar]
- 33.Ryan MJ, Rand AS. The sensory basis of sexual selection for complex calls in the túngara frog, Physalaemus pustulosus (Sexual selection for sensory exploitation). Evolution. 1990;44:305–314. doi: 10.1111/j.1558-5646.1990.tb05200.x. [DOI] [PubMed] [Google Scholar]
- 34.Mangiamele LM, Thomson CJ, Lebonville CL, Burmeister SS. Dev Neurobiol In press; 2010. Characterization of the plasticity-related gene, Arc, in the frog brain. [DOI] [PubMed] [Google Scholar]
- 35.Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- 36.Pfennig KS. The evolution of mate choice and the potential for conflict between species and mate-quality recognition. Proc R Soc Lond B Biol Sci. 1998;265:1743–1748. [Google Scholar]
- 37.Ryan MJ, Getz W. Signal decoding and receiver evolution - An analysis using an artificial neural network. Brain Behav Evol. 2000;56:45–62. doi: 10.1159/000006677. [DOI] [PubMed] [Google Scholar]
- 38.Safi K, Heinzle J, Reinhold K. Species recognition influences female mate preferences in the common European grasshopper (Chorthippus biguttulus Linnaeus, 1758). Ethology. 2006;112:1225–1230. [Google Scholar]