Abstract
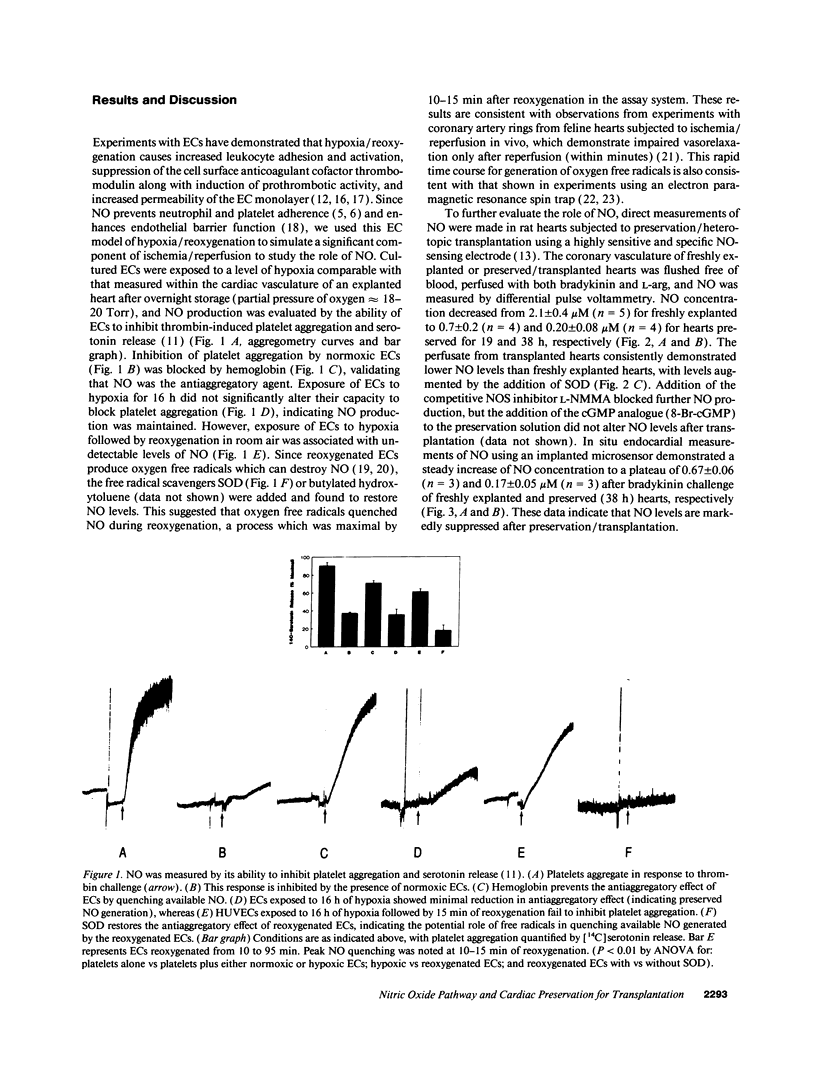
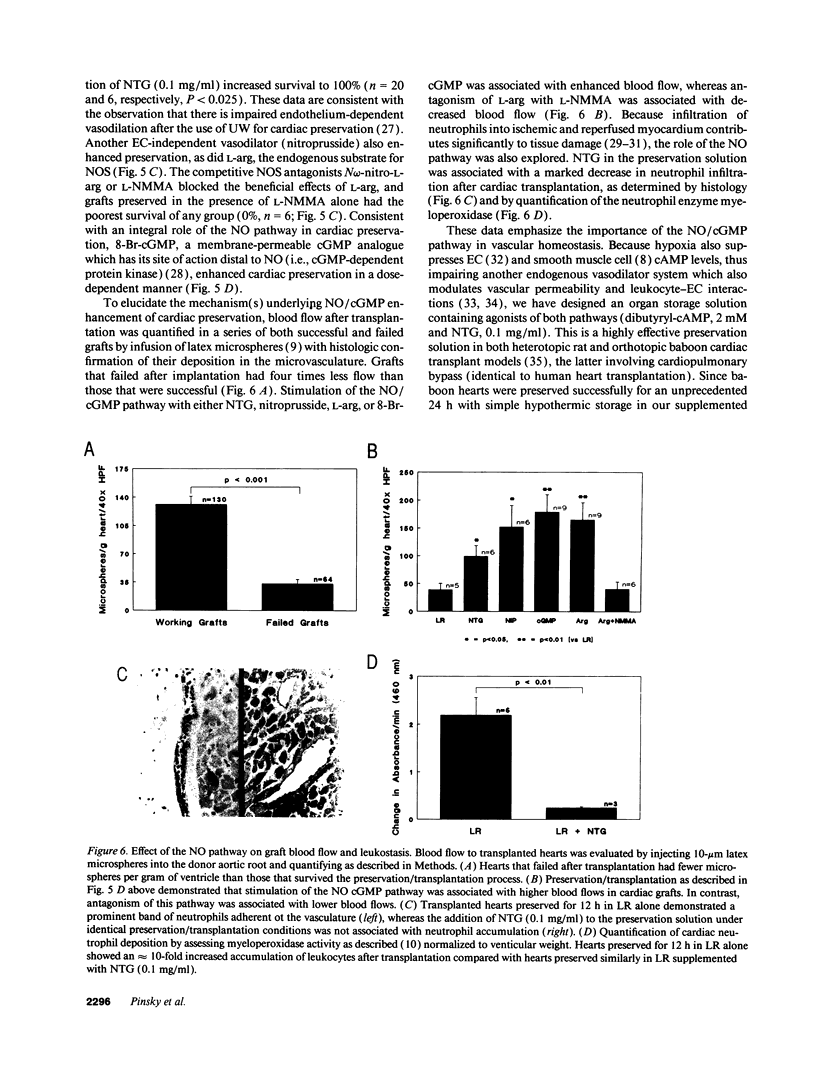
Nitric oxide (NO) is a novel biologic messenger with diverse effects but its role in organ transplantation remains poorly understood. Using a porphyrinic microsensor, the first direct measurements of coronary vascular and endocardial NO production were made. NO was measured directly in the effluent of preserved, heterotopically transplanted rat hearts stimulated with L-arginine and bradykinin; NO concentrations fell from 2.1 +/- 0.4 microM for freshly explanted hearts to 0.7 +/- 0.2 and 0.2 +/- 0.08 microM for hearts preserved for 19 and 38 h, respectively. NO levels were increased by SOD, suggesting a role for superoxide-mediated destruction of NO. Consistent with these data, addition of the NO donor nitroglycerin (NTG) to a balanced salt preservation solution enhanced graft survival in a time- and dose-dependent manner, with 92% of hearts supplemented with NTG surviving 12 h of preservation versus only 17% in its absence. NTG similarly enhanced preservation of hearts stored in University of Wisconsin solution, the clinical standard for preservation. Other stimulators of the NO pathway, including nitroprusside, L-arginine, or 8-bromoguanosine 3',5' monophosphate, also enhanced graft survival, whereas the competitive NO synthase antagonist NG-monomethyl-L-arginine was associated with poor preservation. Likely mechanisms whereby supplementation of the NO pathway enhanced preservation included increased blood flow to the reperfused graft and decreased graft leukostasis. NO was also measured in endothelial cells subjected to hypoxia/reoxygenation and detected based on its ability to inhibit thrombin-mediated platelet aggregation and serotonin release. NO became undetectable in endothelial cells exposed to hypoxia followed by reoxygenation and was restored to normoxic levels on addition of SOD. These studies suggest that the NO pathway fails during preservation/transplantation because of formation of oxygen free radicals during reperfusion, which quench available NO. Augmentation of NO/cGMP-dependent mechanisms enhances vascular function after ischemia and reperfusion and provides a new strategy for transplantation of vascular organs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbs C. F., Cregor M. D., Turek J. J., Badylak S. F. Endothelial superoxide production in the isolated rat heart during early reperfusion after ischemia. A histochemical study. Am J Pathol. 1991 Nov;139(5):1069–1080. [PMC free article] [PubMed] [Google Scholar]
- Belzer F. O., Southard J. H. Principles of solid-organ preservation by cold storage. Transplantation. 1988 Apr;45(4):673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Baehner R. L. Diminished polymorphonuclear leukocyte adherence. Function dependent on release of cyclic AMP by endothelial cells after stimulation of beta-receptors by epinephrine. J Clin Invest. 1980 Aug;66(2):268–274. doi: 10.1172/JCI109853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Roeder T. L., Shasby D. M. Insight into the nature and site of oxygen-centered free radical generation by endothelial cell monolayers using a novel spin trapping technique. Blood. 1992 Feb 1;79(3):699–707. [PubMed] [Google Scholar]
- Broekman M. J., Eiroa A. M., Marcus A. J. Inhibition of human platelet reactivity by endothelium-derived relaxing factor from human umbilical vein endothelial cells in suspension: blockade of aggregation and secretion by an aspirin-insensitive mechanism. Blood. 1991 Aug 15;78(4):1033–1040. [PubMed] [Google Scholar]
- Crawford M. H., Grover F. L., Kolb W. P., McMahan C. A., O'Rourke R. A., McManus L. M., Pinckard R. N. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988 Dec;78(6):1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Goldblum S. E., Wu K. M., Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol (1985) 1985 Dec;59(6):1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- Hale S. L., Alker K. J., Kloner R. A. Evaluation of nonradioactive, colored microspheres for measurement of regional myocardial blood flow in dogs. Circulation. 1988 Aug;78(2):428–434. doi: 10.1161/01.cir.78.2.428. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevanandam V., Barr M. L., Auteri J. S., Sanchez J. A., Ott G. Y., Schenkel F. A., Marboe C., Smith C. R., Rose E. A. University of Wisconsin solution for human donor heart preservation: initial clinical experience. Ann Thorac Surg. 1991 Dec;52(6):1213–1216. doi: 10.1016/0003-4975(91)90003-9. [DOI] [PubMed] [Google Scholar]
- Johnson G., 3rd, Tsao P. S., Mulloy D., Lefer A. M. Cardioprotective effects of acidified sodium nitrite in myocardial ischemia with reperfusion. J Pharmacol Exp Ther. 1990 Jan;252(1):35–41. [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefer A. M., Tsao P. S., Lefer D. J., Ma X. L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991 Apr;5(7):2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Tsao P. S., Lefer A. M. Antibody to CD-18 exerts endothelial and cardiac protective effects in myocardial ischemia and reperfusion. J Clin Invest. 1991 Oct;88(4):1237–1243. doi: 10.1172/JCI115427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinski T., Taha Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature. 1992 Aug 20;358(6388):676–678. doi: 10.1038/358676a0. [DOI] [PubMed] [Google Scholar]
- Minnear F. L., Johnson A., Malik A. B. Beta-adrenergic modulation of pulmonary transvascular fluid and protein exchange. J Appl Physiol (1985) 1986 Jan;60(1):266–274. doi: 10.1152/jappl.1986.60.1.266. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Ogawa S., Gerlach H., Esposito C., Pasagian-Macaulay A., Brett J., Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990 Apr;85(4):1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Koga S., Kuwabara K., Brett J., Morrow B., Morris S. A., Bilezikian J. P., Silverstein S. C., Stern D. Hypoxia-induced increased permeability of endothelial monolayers occurs through lowering of cellular cAMP levels. Am J Physiol. 1992 Mar;262(3 Pt 1):C546–C554. doi: 10.1152/ajpcell.1992.262.3.C546. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pinsky D., Oz M., Liao H., Morris S., Brett J., Sciacca R., Karakurum M., Van Lookeren Campagne M., Platt J., Nowygrod R. Restoration of the cAMP second messenger pathway enhances cardiac preservation for transplantation in a heterotopic rat model. J Clin Invest. 1993 Dec;92(6):2994–3002. doi: 10.1172/JCI116922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Shreeniwas R., Koga S., Karakurum M., Pinsky D., Kaiser E., Brett J., Wolitzky B. A., Norton C., Plocinski J., Benjamin W. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest. 1992 Dec;90(6):2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson D. K., Pasaoglu I., Berkoff H. A., Southard J. A., Hegge J. O. Improved heart preservation with UW preservation solution. J Heart Transplant. 1988 Nov-Dec;7(6):456–467. [PubMed] [Google Scholar]
- Walter U. Physiological role of cGMP and cGMP-dependent protein kinase in the cardiovascular system. Rev Physiol Biochem Pharmacol. 1989;113:41–88. doi: 10.1007/BFb0032675. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988 Jan 25;263(3):1353–1357. [PubMed] [Google Scholar]




