Abstract
Traumatic brain injury (TBI) survivors often suffer from a wide range of post-traumatic deficits, including impairments in behavioral, cognitive, and motor function. Regulation of glutamate signaling is vital for proper neuronal excitation in the central nervous system. Without proper regulation, increases in extracellular glutamate can contribute to the pathophysiology and neurological dysfunction seen in TBI. In the present studies, enzyme-based microelectrode arrays (MEAs) that selectively measure extracellular glutamate at 2 Hz enabled the examination of tonic glutamate levels and potassium chloride (KCl)-evoked glutamate release in the prefrontal cortex, dentate gyrus, and striatum of adult male rats 2 days after mild or moderate midline fluid percussion brain injury. Moderate brain injury significantly increased tonic extracellular glutamate levels by 256% in the dentate gyrus and 178% in the dorsal striatum. In the dorsal striatum, mild brain injury significantly increased tonic glutamate levels by 200%. Tonic glutamate levels were significantly correlated with injury severity in the dentate gyrus and striatum. The amplitudes of KCl-evoked glutamate release were increased significantly only in the striatum after moderate injury, with a 249% increase seen in the dorsal striatum. Thus, with the MEAs, we measured discrete regional changes in both tonic and KCl-evoked glutamate signaling, which were dependent on injury severity. Future studies may reveal the specific mechanisms responsible for glutamate dysregulation in the post-traumatic period, and may provide novel therapeutic means to improve outcomes after TBI.
Key words: amperometry, hippocampus, microdialysis, synaptic release
Introduction
Approximately 1.5 million Americans suffer from traumatic brain injury (TBI) each year, with mild TBI accounting for as many as 75% of all injuries (Centers for Disease Control and Prevention, 2003). Survivors of TBI often suffer from a wide variety of neurological deficits that impair quality of life (Baddeley, 1992; Capruso and Levin, 1992; Levine et al., 2002; McAllister, 1992). Glutamate signaling in the central nervous system (CNS) plays a pivotal role in the acute pathophysiology of TBI. Primary mechanical forces of the initial injury can compromise the blood–brain barrier and cellular membranes, allowing increased glutamate release into the extracellular space (Bullock et al., 1998; Farkas et al., 2006; Schmidt and Grady, 1993). The redistribution of ions after injury can further depolarize neurons and augment the release of glutamate (Katayama et al., 1990). Excessive glutamate receptor activation can promote cellular damage through secondary injury cascades by disrupting ionic homeostasis in the cell, activating calcium-dependent proteases and phospholipases, uncoupling mitochondrial ATP synthesis, promoting oxidative stress, producing reactive oxygen species, depleting glutathione levels, and increasing the energy demand of the cell (Faden et al., 1989; Hall et al., 1994; McIntosh et al., 1996; Yi and Hazell, 2006). Further, clinical reports have reported increases in extracellular glutamate up to 4 days after injury that correlated with increased intracranial pressure and unfavorable outcomes (Bullock et al., 1995).These prior studies emphasize the consequences of CNS glutamate dysregulation, and the need to examine changes in glutamate signaling that occur in the post-traumatic period after TBI that may be responsible for aberrant neuronal signaling and contribute to neurological deficits.
Glutamate is released constantly into the extracellular space via stimulated and spontaneous exocytosis of synaptic vesicles, and by carrier-mediated exchange with other amino acids (Jabaudon et al., 1999; Jensen et al., 2000). Because there is no enzyme in the extracellular fluid to degrade glutamate, the synaptic concentration of glutamate is lowered by diffusion away from the synapse and through glutamate uptake. Since diffusion of glutamate is limited by the extrasynaptic anatomy, glutamate uptake is the primary mechanism to maintain the extracellular concentration of glutamate. In the CNS, almost all of the glutamate clearance is achieved by the sodium-dependent excitatory amino acid transporters (EAATs) GLAST and GLT-1, predominantly located on glia (Danbolt, 2001).
Previous studies have reported several-fold increases in the extracellular concentration of glutamate initiated by a TBI, which subside within 2 h (Faden et al., 1989; Katayama et al., 1990; Matsushita et al., 2000; Nilsson et al., 1990). These studies employed microdialysis, a common method for neurochemical sampling in vivo. However, microdialysis suffers from spatial and temporal limitations that restrict the ability to sample dynamic fluctuations of glutamate near the synapse (Hillered et al., 2005; Obrenovitch et al., 2000). The large sampling area of the microdialysis membrane (1–4 mm in length), and extensive damage from implantation of the microdialysis probe, limit the ability to detect neuronal release (Borland et al., 2005; Jaquins-Gerstl and Michael, 2009). Further, the low temporal resolution of microdialysis (1–20 min) is inadequate to sample the fast dynamics of glutamate release and clearance that occurs on the order of milliseconds to seconds (Diamond, 2005).
Interestingly, experimental TBI has yet to produce pathological extracellular concentrations of glutamate, even immediately post-injury (Katayama et al., 1990; Matsushita et al., 2000), which raises doubt about glutamate excitotoxicity after TBI (Carbonell and Grady, 1999; Obrenovitch, 1999). In this study we used midline fluid percussion injury (FPI), a model of a diffuse closed-head injury that affects both hemispheres equally and shares pathological features of human head injuries (Dixon et al., 1987; McIntosh et al., 1987), to examine changes in glutamate signaling 2 days after mild or moderate diffuse brain injury. To study injury-induced alterations in glutamate neurotransmission, we employed a novel technique, in vivo amperometry coupled to enzyme-based microelectrode arrays (MEAs), with high temporal resolution (2 Hz), a low limit of detection (<1 μM), and high spatial resolution, to selectively measure extracellular glutamate close to synapses (Burmeister and Gerhardt, 2001; Burmeister et al., 2002). The improved spatial and temporal resolution of the MEAs allowed us to examine injury-induced alterations in tonic glutamate levels and potassium chloride (KCl)-evoked glutamate release in the prefrontal cortex, dentate gyrus of the hippocampus, and striatum, brain regions known to play a role in the behavioral, cognitive, and motor deficits suffered by TBI survivors (Baddeley, 1992; Capruso and Levin, 1992; Levine et al., 2002; McAllister, 1992).
Methods
Animals
Male Sprague-Dawley rats (weighing 350–400 g; Harlan Laboratories Inc., Indianapolis, IN) were used for all experiments. The animals were housed in a 12-h light/dark cycle with food and water ad libitum according to the Association for Assessment and Accreditation of Laboratory Animal Care International. The animals were acclimated to their environment for at least 1 week before any experiments. After surgery, the animals were monitored daily for postoperative health. The animal care techniques used were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Midline fluid percussion injury
The animals were subjected to midline FPI as previously described (Lifshitz, 2008). The rats were anesthetized with 4% isoflurane in 100% oxygen for 5 min and placed in a stereotaxic frame in a sterile surgery hood with continuously delivered 2% isoflurane. The head was shaved and a 70% alcohol and povidone-iodine solution was applied. The eyes were treated with artificial tears and body temperature was maintained with a delta-phase heating pad (Braintree Scientific, Braintree, MA). A midline incision was made and the fascia was removed from the skull. A 4.7-mm trephine was used for the craniotomy, centered on the sagittal suture between the bregma and the lambda without disrupting the underlying dura or superior sagittal sinus. Anchoring screws were secured in pilot holes remote from the craniotomy. An injury hub was constructed from a Luer-Loc hub, and the outer diameter of the hub was beveled to match the craniotomy. The injury hub was fitted into the craniotomy and cyanoacrylate gel was applied to seal the skull-hub interface. To secure the injury hub to the anchoring screws, methyl-methacrylate (Hygenic Corp., Akron, OH) was applied. The hub was filled with sterile saline and the wound was sutured to prevent tearing. The animals were allowed to recover for 30–60 min until ambulatory, and then re-anesthetized with 5% isoflurane for 5 min. The incision was opened and the injury hub was inspected. The female Luer-Loc injury hub was connected to the male Luer-Loc hub of the injury device (Custom Design and Fabrication, Virginia Commonwealth University, Richmond, VA). As reflexive responses returned, a pendulum hammer was dropped onto the fluid-filled piston to induce either a mild or moderate injury. Sham-injured animals underwent the same procedure without induction of injury. The fluid pressure pulse for mild injury was 1.1 ± 0.01 atm, and was 2.0 ± 0.02 atm for moderate injury. The injury hub was removed and any bleeding was controlled with Gelfoam (Pharmacia, Kalamazoo, MI). The incision was closed with staples. Righting reflex recovery times were recorded for the injury group, from the time of impact to the time when the animals spontaneously righted. Mildly-injured animals had righting reflex times of 144 ± 34 sec (n = 10), and moderate injured animals had times of 606 ± 85 sec (n = 11). Sham-injured animals had righting reflex times of less than 15 sec (n = 10). No animals died from complications caused by the injury.
Enzyme-based microelectrode arrays
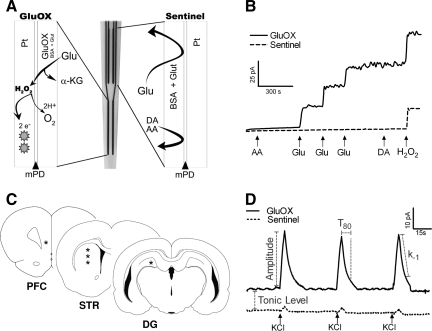
Ceramic-based MEAs, consisting of four platinum recording sites (15 × 333 μm) arranged in dual pairs, were prepared and selected for in vivo recordings as previously described (Burmeister et al., 2002; Day et al., 2006). Glutamate-oxidase (GluOX) covered one pair of recording sites to allow for the enzymatic conversion of glutamate to α-ketoglutarate and the generation of the reporter molecule hydrogen peroxide (H2O2). An inactive protein matrix covered the other pair of recording sites (sentinel sites). The two different coatings allowed the use of a self-referencing technique, in which the background current of the sentinel sites can be subtracted from the current of the GluOX sites, thereby producing a more selective glutamate measure (Burmeister and Gerhardt, 2001; Burmeister et al., 2002). A size exclusion layer of 1,3-phenylenediamine (mPD) restricts the passage of large molecules that can be oxidized, such as ascorbic acid (AA) and dopamine (DA). Small molecules such as H2O2 diffuse through the exclusion layer, reaching the platinum recording sites (Fig. 1A).
FIG. 1.
(A) Schematic diagram showing the configuration and coatings applied to the microelectrode arrays (MEAs). The lower pair of platinum electrode sites was prepared with a mixture of glutamate-oxidase (GluOX), bovine serum albumin (BSA), and glutaraldehyde (Glut), as illustrated at left. The upper pair of platinum sites was prepared only with BSA and Glut, as illustrated at right. A layer of m-phenylenediamine (mPD) was electroplated onto the recording sites, providing a size-exclusion layer to block major interferents, such as ascorbic acid (AA) and dopamine (DA). (B) MEAs in vitro calibration measuring the change in current on a GluOX site (solid line) and a sentinel site (dashed line) with addition of multiple analytes ( ↑ ). The addition of interferents such as AA and DA produced no change in current on the GluOX or sentinel sites. Three glutamate (Glu) additions showed a stepwise increase of current on the GluOX site, with no response on the sentinel site. The addition of hydrogen peroxide (H2O2) produces an increase in current on both the GluOX and sentinel sites. (C) Illustrations of coronal sections of the rat brain (Paxinos and Watson, 1998) highlight the regions of interest for glutamate recording (*), in the prefrontal cortex (PFC, AP: 3.2 mm), striatum (STR, AP: 1.0 mm), and dentate gyrus (DG, AP: −4.3 mm). (D) Representative in vivo recording from a GluOX site and a sentinel site. Local application of potassium chloride (KCl) ( ↑ ) produced reproducible glutamate release, highlighting the fast kinetics of glutamate release and uptake. Tonic glutamate was calculated in each brain region after the microelectrode had reached a stable baseline. KCl-evoked glutamate release was analyzed using the following parameters: amplitude, T80, and k−1, for each signal. The parameters are shown separately for clarity.
A 10-μL solution of 1% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO), 0.125% glutaraldehyde (Glut) (Sigma-Aldrich), and 1% GluOX (Seikagaku America, East Falmouth, MA) was prepared. Using a dissecting microscope, a microsyringe was used to manually apply a small drop (∼0.1 μL) of the GluOX solution onto the bottom pair of platinum recording sites. Three enzyme coats were applied to the platinum recording sites, with a 1-min drying period between each coat. The same procedure was used to coat the top pair of platinum recording sites (sentinel sites), with a solution containing 1% BSA and 0.125% glutaraldehyde. After coating, the MEAs were cured for at least 48 h in a low-humidity environment. The coated MEAs were connected to the FAST-16 mkII system (Fast Analytical Sensor Technology Mark II; Quanteon, L.L.C., Nicholasville, KY), and the tips of the MEAs were placed in a 5-mM mPD solution (Acros Organics, Morris Plains, NJ). Electroplating software was used to apply a potential as a triangular wave with an offset of –0.5 V, peak-to-peak amplitude equal to 0.25 V, at a frequency of 0.05 Hz, for a period of 20 min, to electroplate the mPD onto all platinum recording sites. The MEAs were cured for an additional 24 h in a low-humidity environment before use.
Microelectrode array calibration
Calibrations were conducted to test the capability of the MEAs to measure glutamate and to generate a standard curve for the conversion of current to glutamate concentration. Constant potential amperometry was performed with the MEAs using the FAST-16 mkII system. A potential of +0.7 V versus an Ag/AgCl reference was applied to oxidize the reporter molecule, H2O2, which is a two-electron oxidation reaction that occurs at the platinum recording sites of the MEAs (Fig. 1A). The resulting current was amplified and digitized by the recording system. The platinum recording sites of the MEAs were placed in a continuously stirred 40-mL solution of 0.05 M phosphate-buffered saline (PBS) maintained at 37°C with a recirculating water bath (Gaymar Industries Inc., Orchard Park, NY). The MEAs were exposed to final concentrations of 250 μM ascorbic acid (AA), 20, 40, and 60 μM glutamate, 2 μM dopamine (DA), and 8.8 μM H2O2 (Fig. 1B). The parameters tested were limit of detection (LOD), selectivity for glutamate over AA, slope of the electrode (sensitivity), and linearity of the glutamate response (R2). The average LOD was 0.8 ± 0.1 μM, selectivity was 167 ± 19:1 (glutamate:ascorbic acid), and slope was 3.4 ± 0.3 pA/μM (n = 51 GluOX recording sites).
In a separate study, we examined MEA performance after in vivo recordings. The post-implantation calibrations demonstrated that the parameters were no different (pre- versus post-implantation; n = 24 GluOX recording sites, by paired t-test): limit of detection (1.1 ± 0.1 μM versus 1.5 ± 0.4 μM; p = 0.37), selectivity (93 ± 21 versus 83 ± 26; p = 0.77), and slope (2.1 ± 0.2 pA/μM versus 2.2 ± 0.1 pA/μM; p = 0.68).
Oxygen is a required co-factor of GluOx for the production of the reporter molecule, hydrogen peroxide. Therefore we examined if low oxygen concentrations would affect MEA performance. Glutamate responses were minimally affected by oxygen concentration, and the performance even at 0 μM oxygen was within 80% of the MEA response obtained in air-saturated buffer (200 μM; Supplementary Figure 1; see online supplementary material at http://www.liebertonline.com).
Microelectrode array/micropipette assembly
For local application of solutions in the rat brain, glass micropipettes (1 mm outside diameter and 0.58 mm inside diameter [ID]; A-M Systems, Inc., Everett, WA) were pulled (Kopf Instruments, Tujunga, CA), and the tips of the micropipettes were bumped to create a tip with an ID of 10–15 μm. The micropipettes were placed centrally among all four platinum recording sites and mounted 50–100 μm above the MEAs. The micropipettes were filled with sterile filtered (0.20 μm) isotonic KCl solution (70 mM KCl, 79 mM NaCl, and 2.5 mM CaCl2, or 120 mM KCl, 29 mM NaCl, and 2.5 mM CaCl2; pH 7.4), at concentrations previously used to elicit reproducible KCl-evoked glutamate release in the brain regions of interest (Burmeister et al., 2002; Day et al., 2006; Stephens et al., 2009). The micropipette was attached to a Picospritzer III (Parker-Hannifin, Cleveland, OH), with settings adjusted to consistently deliver volumes between 50 and 100 nL. Pressure was applied from 2–20 p.s.i. for 1 sec. Volume displacement was monitored with the use of a stereomicroscope fitted with a reticule (Friedemann and Gerhardt, 1992).
In vivo anesthetized recording
Two days after midline FPI, the rats were anesthetized with urethane (1.25 g/kg IP; Sigma-Aldrich), and prepared for in vivo electrochemical recordings as previously described (Burmeister et al., 2002; Day et al., 2006; Pomerleau et al., 2003). Briefly, the animals were placed in a stereotaxic frame and body temperature was maintained at 37°C with a water pad connected to a recirculating water bath. A craniotomy was performed to provide access to the prefrontal cortex (AP: + 3.2 mm, ML: + 0.8 mm, DV: −4.5 mm), dentate gyrus (AP: −4.3 mm, ML: + 2.1 mm, DV: −4.2), and striatum (AP: + 1.0 mm, ML: + 2.5 mm, DV: −4.0, −4.5, and −5.0 mm; Paxinos and Watson, 1998; Fig, 1C). An Ag/AgCl reference wire was implanted into the lateral parietal cortex in the opposite hemisphere from the recording areas. The MEA was lowered into the brain using a microdrive (MO-10; Narishige International, East Meadow, NY). All MEA recordings were performed at a frequency of 2 Hz using constant potential amperometry. After the MEA reached a stable baseline (10–15 min), tonic glutamate levels (μM) were calculated by averaging extracellular glutamate levels over 10 sec. Local application of KCl produced glutamate signals that were reproducible every 30 sec. Each signal was analyzed using the following parameters: amplitude (μM), T80 (sec) the time for the signal to decay by 80% from the peak amplitude, and k−1 (sec−1), the slope of the linear regression of the natural log transformation of the decay over time (Fig. 1D; Thomas et al., 2009). Approximately eight reproducible signals were evoked at each location, and then averaged into a representative signal for comparisons between injury groups. After recording from all locations, an MEA with an attached micropipette was used to locally apply green waterproof drawing ink (Higgins; Eberhard Faber Inc., Lewisburg, TN), which was used to confirm MEA placement during brain sectioning.
Statistical analysis
Amperometric data were analyzed using custom Microsoft Excel™-based software to determine the tonic levels and KCl-evoked signal parameters. To determine tonic levels of glutamate in the different brain regions, the background current from the sentinel site was subtracted from the current of the GluOX site. Then the resulting current (pA) was divided by the slope (μM/pA) obtained during the calibration to determine the tonic glutamate concentration in a given brain region. Data from the dentate gyrus and prefrontal cortex were analyzed by a one-way analysis of variance (ANOVA), followed by a Bonferonni post-hoc test. For the striatum, data were analyzed using a repeated-measures two-way ANOVA, followed by a Bonferonni post-hoc test (injury severity versus depth). Data are presented as mean ± SEM, and statistical significance was defined as p < 0.05.
Results
Prefrontal cortex: Tonic and evoked glutamate release
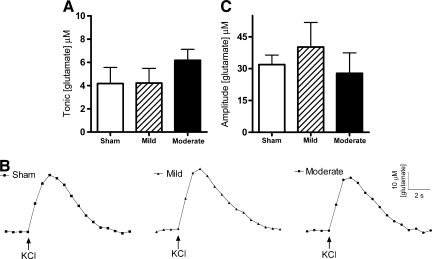
Tonic glutamate levels were not significantly changed in the prefrontal cortex 2 days after mild or moderate diffuse brain injury [ANOVA: F (2,18) = 0.8, p = 0.43; Fig. 2A]. Tonic levels averaged 4.1 ± 1.4 μM (sham), 4.2 ± 1.3 μM (mild), and 6.2 ± 2.5 μM (moderate). Local application of ∼50 nL of 120 mM KCl produced reproducible glutamate release in the prefrontal cortex. Representative recordings demonstrate similar evoked-glutamate signals from local application of KCl between the sham and injury groups (Fig. 2B). The amplitudes of KCl-evoked glutamate release in the prefrontal cortex were not significantly changed after diffuse brain injury [ANOVA: F (2,15) = 0.49, p = 0.62; Fig. 2C]. The amplitudes of KCl-evoked glutamate release in the sham, mild, and moderate brain-injured rats were 5–10 times larger than the corresponding tonic levels of glutamate. Amplitudes averaged 31.9 ± 4.5 μM, 40.2 ± 11.5 μM, and 27.8 ± 9.6 μM in the sham, mild, and moderate brain-injured groups, respectively. Clearance parameters (T80 and k−1) of the KCl-evoked glutamate signal back to tonic levels were equivalent among all groups (Table 1). Thus, mild or moderate midline FPI did not produce significant changes in the tonic glutamate concentration, the amplitude of KCl-evoked glutamate release, or glutamate clearance parameters in the prefrontal cortex.
FIG. 2.
Tonic and potassium chloride (KCl)-evoked release of glutamate in the prefrontal cortex of the urethane-anesthetized rat. (A) Tonic glutamate levels were not significantly different after mild or moderate midline fluid percussion injury (FPI) (n = 7). (B) Baseline-matched representative recordings exhibit no significant change in KCl-evoked glutamate release after midline FPI. Local application of KCl ( ↑ ) produced a robust increase in extracellular glutamate that rapidly returned to tonic levels. (C) The average amplitudes of KCl-evoked glutamate release were not significantly different after mild or moderate midline FPI (n = 6).
Table 1.
Clearance Parameters of KCl-Evoked Glutamate Release
| |
T80 (sec) |
K−1 (sec−1) |
||||
|---|---|---|---|---|---|---|
| Sham | Mild FPI | Moderate FPI | Sham | Mild FPI | Moderate FPI | |
| Prefrontal cortex | 7.0 ± 0.6 | 8.7 ± 1.5 | 7.4 ± 1.6 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.15 ± 0.01 |
| Dentate gyrus | 5.5 ± 0.8 | 7.1 ± 1.3 | 5.6 ± 0.6 | 0.17 ± 0.03 | 0.17 ± 0.03 | 0.16 ± 0.05 |
| Striatum (−4.0 mm) | 5.4 ± 0.4 | 6.0 ± 0.6 | 6.9 ± 0.8 | 0.14 ± 0.02 | 0.14 ± 0.03 | 0.13 ± 0.02 |
| Striatum (−4.5 mm) | 4.3 ± 0.3 | 5.3 ± 1.2 | 5.1 ± 0.8 | 0.21 ± 0.04 | 0.19 ± 0.05 | 0.25 ± 0.02 |
| Striatum (−5.0 mm) | 4.4 ± 0.6 | 5.9 ± 1.5 | 5.9 ± 1.1 | 0.27 ± 0.06 | 0.18 ± 0.04 | 0.21 ± 0.03 |
Mean ± SEM; prefrontal cortex n = 6; dentate gyrus n = 7; striatum n = 8.
KCl, potassium chloride; FPI, fluid percussion injury; SEM, standard error of the mean.
Dentate gyrus: Tonic and evoked glutamate release
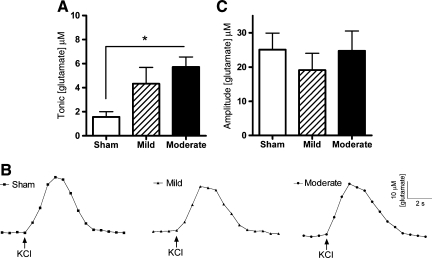
Tonic glutamate levels in the hippocampal dentate gyrus were significantly elevated after brain injury [ANOVA: F (2,21) = 4.9, p = 0.018]. Tonic levels averaged 1.6 ± 0.4 μM (sham), 4.3 ± 1.4 μM (mild), and 5.7 ± 2.3 μM (moderate). Moderate brain-injured animals exhibited a significant 256% increase in tonic glutamate levels compared to sham controls (Fig. 3A). Tonic glutamate levels in mild brain-injured animals were not significantly different from sham or moderate brain-injured animals. Local application of ∼75 nL of 70 mM KCl produced reproducible glutamate release in the dentate gyrus. Representative recordings demonstrate similar evoked-glutamate signals from local application of KCl for the sham and brain-injured groups (Fig. 3B). The amplitudes of evoked-glutamate release in the dentate gyrus were not significantly different between groups [ANOVA: F (2,18) = 0.41, p = 0.67; Fig. 3C]. The amplitude of KCl-evoked glutamate release in the sham-, mild-, and moderate injured rats were 4–15 times larger than the tonic glutamate levels, as amplitudes averaged 25.1 ± 4.9 μM, 19.1 ± 4.9 μM, and 24.7 ± 5.9 μM, respectively. Clearance parameters (T80 and k−1) of the KCl-evoked glutamate signal back to tonic levels were equivalent among all groups (Table 1). Thus, tonic levels of extracellular glutamate were significantly increased after moderate midline FPI, with no significant changes in the amplitude of KCl-evoked glutamate release or glutamate clearance parameters in the dentate gyrus after mild or moderate midline FPI.
FIG. 3.
Tonic and potassium chloride (KCl)-evoked release of glutamate in the dentate gyrus in the hippocampus of the urethane-anesthetized rat. (A) Tonic glutamate levels were significantly increased after moderate midline fluid percussion injury (FPI) in the dentate gyrus (*p < 0.05, n = 8). (B) Baseline-matched representative recordings exhibit no significant change in KCl-evoked glutamate release after midline FPI. (C) The average amplitudes of KCl-evoked glutamate release were not significantly different after mild or moderate injury (n = 7).
Striatum: Tonic and evoked glutamate release
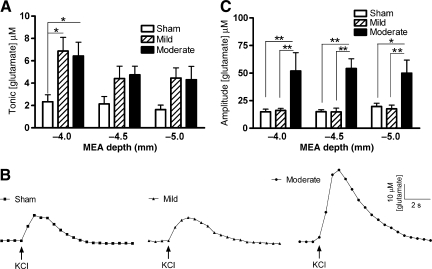
With the high spatial resolution of the platinum recording sites, glutamate responses were sampled from multiple depths of the large heterogeneous structure of the rat striatum (DV: −4.0, −4.5, and −5.0 mm). Tonic glutamate levels were significantly affected by brain injury severity [ANOVA: F (2,42) = 9.26, p = 0.0005], and by recording depth in the striatum [ANOVA: F (2,42) = 4.87, p = 0.0183]. At −4.0 mm in the striatum, tonic levels averaged 2.3 ± 0.6 μM (sham), 6.9 ± 1.2 μM (mild), and 6.4 ± 1.2 μM (moderate). Mild and moderate brain injury produced a significant increase in tonic glutamate levels at a depth of −4.0 mm compared to sham control animals, with a significant 200% increase after mild brain injury, and a significant 178% increase after moderate brain injury (Fig. 4A). The injury-induced increases in tonic glutamate levels after mild and moderate brain injury compared to sham animals at recording depths of −4.5 and −5.0 mm did not reach statistical significance.
FIG. 4.
Tonic and potassium chloride (KCl)-evoked release of glutamate in the striatum of the urethane-anesthetized rat. (A) Tonic glutamate levels were significantly increased after mild or moderate injury in the striatum at a depth of −4.0 mm, with no significant changes at −4.5 mm or −5.0 mm (*p < 0.05, n = 8). (B) Baseline-matched representative recordings of KCl-evoked glutamate release showed that there was a significant increase in the amplitude of glutamate release after moderate midline fluid percussion injury (FPI). Local application of KCl ( ↑ ) produced a robust increase in extracellular glutamate that rapidly returned to tonic levels. (C) The average amplitudes of KCl-evoked glutamate release in the rat striatum at −4.0, −4.5, and −5.0 mm were significantly increased after moderate midline FPI with local application of 100 nL of 70 mM KCl, compared to sham or mild injury animals (*p < 0.05, **p < 0.01, n = 8; MEAs, microelectrode arrays).
Local application of ∼100 nL of 70-mM KCl produced reproducible glutamate release in all depths of the striatum. Representative recordings of KCl-evoked glutamate release in the dorsal striatum (−4.0 mm) demonstrate the significantly elevated amplitude of glutamate release after moderate brain injury (Fig. 4B). At −4.0 mm in the striatum, the amplitude of KCl-evoked glutamate release was 10–25 times larger than the tonic glutamate concentration, as amplitudes averaged 14.9 ± 2.4, 16.1 ± 1.9, and 52.0 ± 16.6 μM in the sham, mild, and moderate brain-injured animals, respectively. The amplitude of evoked-glutamate release was significantly affected by brain injury severity [ANOVA: F (2,42) = 24.96, p < 0.0001], but not by recording depth of the MEA [ANOVA: F (2,42) = 0.02, p = 0.97]. Amplitudes of the KCl-evoked glutamate release after moderate brain injury were significantly elevated, by 249%, 302%, 153%, at −4.0, −4.5, and −5.0 mm, respectively (Fig. 4C). Clearance parameters (T80 and k−1) of the KCl-evoked glutamate signal back to tonic levels were equivalent at all depths of the striatum between all groups (Table 1). Thus the striatum exhibits significantly elevated tonic glutamate levels after mild and moderate midline FPI, whereas the amplitude of KCl-evoked glutamate release was elevated significantly only after moderate brain injury.
Tonic glutamate levels correlate with injury force
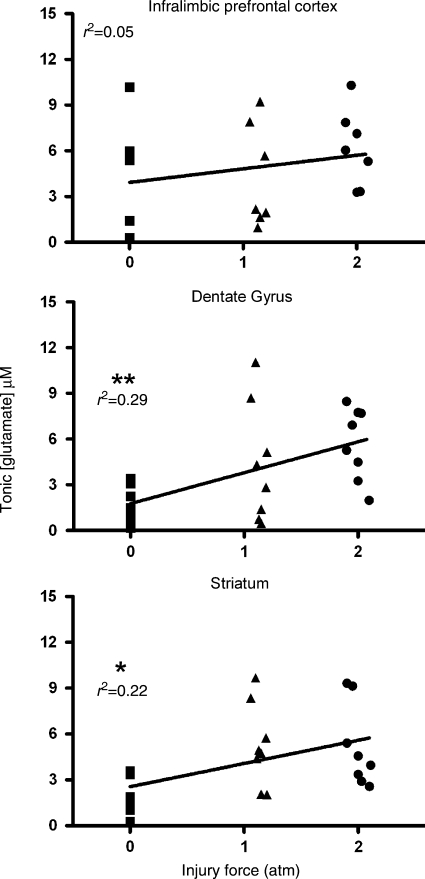
To examine if injury force (atm) is predictive of tonic glutamate levels, linear correlations were calculated for the three brain regions. The correlation was not significant in the prefrontal cortex (Pearson r2 = 0.05; p = 0.30). However, tonic glutamate levels were significantly correlated with injury force in the dentate gyrus (Pearson r2 = 0.29; p = 0.006), and in the striatum, when the three recording depths were averaged into a single value (Pearson r2 = 0.22; p = 0.019; Fig. 5). Thus injury force was predictive of elevated tonic glutamate levels in the dentate gyrus and the striatum at 2 days after diffuse brain injury.
FIG. 5.
Correlations of tonic glutamate levels with injury force in the rat prefrontal cortex, dentate gyrus, and striatum. Tonic glutamate levels were not significantly correlated with injury force in the prefrontal cortex (n = 7), but were significantly correlated in the dentate gyrus (**p < 0.01; n = 8), and in the striatum (*p < 0.05; n = 8; atm, atmosphere).
Discussion
We examined the extent of glutamate dysregulation 2 days after diffuse brain injury, which may contribute to aberrant neuronal signaling and the propagation of secondary injury cascades. The present study was a first attempt to use enzyme-based MEAs to examine regional changes in glutamate signaling after TBI. First, the prefrontal cortex exhibited no significant alterations in glutamate signaling 2 days after diffuse injury. Second, tonic glutamate levels in the dentate gyrus and striatum were significantly increased after midline FPI. Third, moderate midline FPI produced significant increases in the amplitudes of evoked-glutamate release at multiple depths of the striatum. Finally, tonic glutamate levels in the dentate gyrus and striatum were significantly correlated with the mechanical forces of injury.
MEAs have improved upon some limitations inherent to microdialysis for sampling glutamate in vivo. The high spatial resolution of the platinum recording sites allowed glutamate measurements from discrete regions, which is vital in studying local glutamate regulation. Also, the 2-Hz sampling rate of the MEAs records the fast dynamics of glutamate signaling that may be susceptible to TBI pathophysiology. The improved spatial resolution and limited damage to the surrounding tissue (Rutherford et al., 2007) allow MEAs to sample glutamate spillover from nearby synapses that are tetrodotoxin (TTX)-sensitive (Day et al., 2006; Hascup et al., 2008). In contrast, microdialysate samples of glutamate are largely TTX-independent and poorly correlated with the concentration of glutamate near synapses (Timmerman and Westerink, 1997; Obrenovitch, 1999). Moreover, the small recording sites of the MEAs require little tissue oxygen utilization, and the electrochemical oxidation of peroxide produces oxygen as supplementary substrate for the GluOX enzyme. However, in anoxic tissue MEAs performance may be compromised.
Tonic glutamate levels in the dentate gyrus and striatum are positively correlated with injury severity. However, the concentrations do not appear to reach excitotoxic levels, as even a continuous infusion of 1.8 M glutamate at 0.5 μL/h was insufficient to produce a neuropathological lesion (Mangano and Schwarcz, 1983). The present study was carried out under urethane anesthesia, which can decrease tonic glutamate levels by 60–80% (Rutherford et al., 2007). Therefore, the values reported here may represent only a fraction of the tonic glutamate concentration in the awake animal. Still, the increased tonic glutamate levels may contribute to the development or maintenance of diffuse brain injury pathophysiology. The observed tonic glutamate levels may explain elevated neuronal calcium levels, and altered calcium regulation, which result in the calpain-mediated spectrin proteolysis reported after midline FPI (Sun et al., 2008; McGinn et al., 2009). With regard to secondary injuries, increased tonic glutamate levels after the initial injury may produce increased susceptibility to excitotoxic damage, or provide neuroprotection via preconditioning. Future work should examine the effect of increased tonic glutamate levels on a secondary insult, and identify alternate sources of glutamate that contribute to the increased tonic glutamate levels.
Tonic glutamate levels appear to be independent of evoked-glutamate release, as we report significant increases in tonic glutamate levels in the presence and absence of alterations in KCl-evoked glutamate release, depending on brain region. Evoked-glutamate release, unlike the graded response in tonic glutamate levels, exhibited an all-or-none response with injury severity. Local application of isotonic KCl depolarized local axons and pre-synaptic terminals, evoking neurotransmitter release. The evoked increase in extracellular glutamate was transient, as glutamate uptake rapidly restores the glutamate concentration back to tonic levels. Although transient, elevated evoked-glutamate release may prolong secondary injury cascades, by excessively activating ionotropic receptors, increasing calcium influx, and activating proteases (McIntosh et al., 1996). Elevated evoked-glutamate release could arise from several different mechanisms. First, the pre-synaptic terminal could release more glutamate vesicles or vesicles with higher glutamate concentrations. Lateral FPI produces transient changes in the synaptic machinery, specifically complexin I and II, up to 1 week after injury, which could alter neurotransmitter packaging and synaptic release (Yi and Hazell, 2006). Furthermore, free radicals can increase KCl-evoked glutamate release (Pellegrini-Giampietro et al., 1990). Second, reduced glutamate transporter function would increase the extracellular glutamate concentration. Controlled cortical impact decreases the expression of GLT-1 and GLAST, the EAATs that mediate most of the glutamate uptake in the rat brain (Rothstein et al., 1996; Rao et al., 1998). Third, synaptic reorganization may alter synaptic structure and function. Future work could explore clearance of locally applied glutamate into the extracellular space to evaluate EAATs surface expression (Nickell et al., 2007).
Midline FPI produces a diffuse, multi-focal pathology with significant neurological impairments after mild and moderate injury, without overt cavitation (Hamm, 2001; Kelley et al., 2007; McIntosh et al., 1987; Schmidt and Grady, 1993). In addition, physiological disturbance may contribute to observed post-traumatic deficits (Delahunty et al., 1995; Lyeth et al., 1990). The spectrum of deficits includes behavioral, cognitive, and motor impairments, ascribed to the prefrontal cortex, hippocampus, and striatum (Baddeley, 1992; Capruso and Levin, 1992; Khan et al., 2003; Levine et al., 2002; McAllister, 1992).
The medial prefrontal cortex receives glutamatergic projections from the mediodorsal nucleus of the thalamus, hippocampus, and amygdala (Steketee, 2003). Post-traumatic impairments in working memory have been linked to damage in the medial prefrontal cortex (Baddeley, 1992; Delatour and Gisquet-Verrier, 2000; Levine et al., 2002). Here, we report no significant changes in tonic glutamate levels or evoked glutamate release in the medial prefrontal cortex, likely due to the injury type or the selected time point. Few reports have examined the prefrontal cortex after midline FPI, reporting blood–brain barrier disruption within 24 h post-injury (McIntosh et al., 1987; Schmidt and Grady, 1993). After controlled cortical impact, impairments in working memory have been attributed to excessive GABA-meditated inhibition weeks post-injury (Kobori and Dash, 2006; Kobori et al., 2006). Thus diffuse brain injury in the medial prefrontal cortex retains glutamate regulation at 2 days post-injury.
The dentate gyrus of the hippocampus receives glutamate projections from the entorhinal cortex and projects to area CA3 (Andersen, 2007). Survivors of TBI suffer from impairments in learning and memory attributed to hippocampal damage (Tate and Bigler, 2000). Midline FPI produces significant deficits in Morris water maze memory tests 11–15 days after injury (Hamm et al., 1993; Liu et al., 1994). In the absence of significant neuronal cell death, physiological changes in the hippocampus may contribute to functional deficits (Delahunty et al., 1995; Grady et al., 2003; Lyeth et al., 1990). The significantly elevated tonic glutamate levels seen after moderate brain injury may provide a potential substrate for the increased hippocampal excitability reported 2 days after midline FPI (Reeves et al., 1995), and the ensuing cognitive deficits.
The striatum receives glutamatergic projections from the thalamus and virtually all regions of the neocortex (Fonnum et al., 1981). Survivors of TBI suffer motor and cognitive impairments (Khan et al., 2003), as reported with glutamate and dopamine dysregulation in the striatum (Canales et al., 2002). Injury-induced increases in tonic and evoked-glutamate release in the striatum at 2 days post-injury may underlie the motor deficits seen within 5 days post-injury, and the memory deficits seen within 11–15 days post-injury (Hamm, 2001; Liu et al., 1994). Two weeks after controlled cortical impact, dopamine neurotransmission in the striatum exhibited decreased electrically-induced dopamine overflow, decreased levels of the dopamine transporters, and decreased dopamine uptake from the extracellular space (Wagner et al., 2005). Activation of ionotropic glutamate receptors reduces evoked-dopamine release in the striatum (Wu et al., 2000). The glutamate-dopamine interactions underlying motor and cognitive function warrant further investigation to explain the neurological deficits observed in experimental and clinical TBI.
In conclusion, we used MEAs in combination with midline FPI to examine how diffuse brain injury alters glutamate signaling, without the influence of tissue destruction seen in focal injury models. Our results demonstrate that elevated glutamate signaling contributes to the pathophysiology of diffuse brain injury, establishing a basis for further functional deficits. The specific mechanisms responsible for the elevations in tonic glutamate levels and evoked-glutamate release in vivo should be explored over a more complete time course to identify novel therapeutic targets to improve outcomes after TBI.
Supplementary Material
Acknowledgments
The authors thank Amanda M. Lisembee and Kelley D. Hall for technical assistance with the FPI, Laura C. Crawford and Emily D. Cottrell for assistance with in vivo recordings and MEA placement, and Michelle L. Stephens for providing the coordinates for the hippocampus. Support was provided by U.S. Public Health Service grants DA017186, AG00242, NS3978, and AG013494, National Science Fund grant (ERC) EEC-0310723, and the University of Kentucky College of Medicine, and Kentucky Spinal Cord and Head Injury Research Trust Grant 7-11.
Author Disclosure Statement
Greg A. Gerhardt, Ph.D. is the principal owner of Quanteon LLC.
References
- Andersen P. The Hippocampus Book. Oxford University Press; New York: 2007. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Borland L.M. Shi G. Yang H. Michael A.C. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J. Neurosci. Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bullock R. Zauner A. Myseros J.S. Marmarou A. Woodward J.J. Young H.F. Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann. N.Y. Acad. Sci. 1995;765:290–297. doi: 10.1111/j.1749-6632.1995.tb16586.x. [DOI] [PubMed] [Google Scholar]
- Bullock R. Zauner A. Woodward J.J. Myseros J. Choi S.C. Ward J.D. Marmarou A. Young H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- Burmeister J.J. Gerhardt G.A. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal. Chem. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Burmeister J.J. Pomerleau F. Palmer M. Day B.K. Huettl P. Gerhardt G.A. Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J. Neurosci. Methods. 2002;119:163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Canales J.J. Capper-Loup C. Hu D. Choe E.S. Upadhyay U. Graybiel A.M. Shifts in striatal responsivity evoked by chronic stimulation of dopamine and glutamate systems. Brain. 2002;125:2353–2363. doi: 10.1093/brain/awf239. [DOI] [PubMed] [Google Scholar]
- Capruso D.X. Levin H.S. Cognitive impairment following closed head injury. Neurol. Clin. 1992;10:879–893. [PubMed] [Google Scholar]
- Carbonell W.S. Grady M.S. Evidence disputing the importance of excitotoxicity in hippocampal neuron death after experimental traumatic brain injury. Ann. N.Y. Acad. Sci. 1999;890:287–298. doi: 10.1111/j.1749-6632.1999.tb08005.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. National Center for Injury Prevention and Control; Atlanta, GA: 2003. [Google Scholar]
- Danbolt N.C. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Day B.K. Pomerleau F. Burmeister J.J. Huettl P. Gerhardt G.A. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J. Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Delahunty T.M. Jiang J.Y. Gong Q.Z. Black R.T. Lyeth B.G. Differential consequences of lateral and central fluid percussion brain injury on receptor coupling in rat hippocampus. J. Neurotrauma. 1995;12:1045–1057. doi: 10.1089/neu.1995.12.1045. [DOI] [PubMed] [Google Scholar]
- Delatour B. Gisquet-Verrier P. Functional role of rat prelimbic-infralimbic cortices in spatial memory: evidence for their involvement in attention and behavioural flexibility. Behav. Brain Res. 2000;109:113–128. doi: 10.1016/s0166-4328(99)00168-0. [DOI] [PubMed] [Google Scholar]
- Diamond J.S. Deriving the glutamate clearance time course from transporter currents in CA1 hippocampal astrocytes: transmitter uptake gets faster during development. J. Neurosci. 2005;25:2906–2916. doi: 10.1523/JNEUROSCI.5125-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Faden A.I. Demediuk P. Panter S.S. Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Farkas O. Lifshitz J. Povlishock J.T. Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury? J. Neurosci. 2006;26:3130–3140. doi: 10.1523/JNEUROSCI.5119-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Storm-Mathisen J. Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience. 1981;6:863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Friedemann M.N. Gerhardt G.A. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol. Aging. 1992;13:325–332. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Grady M.S. Charleston J.S. Maris D. Witgen B.M. Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. J. Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Andrus P.K. Yonkers P.A. Smith S.L. Zhang J.R. Taylor B.M. Sun F.F. Generation and detection of hydroxyl radical following experimental head injury. Ann. N.Y. Acad. Sci. 1994;738:15–24. doi: 10.1111/j.1749-6632.1994.tb21785.x. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Lyeth B.G. Jenkins L.W. O'Dell D.M. Pike B.R. Selective cognitive impairment following traumatic brain injury in rats. Behav. Brain Res. 1993;59:169–173. doi: 10.1016/0166-4328(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Hamm R.J. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Hascup K.N. Hascup E.R. Pomerleau F. Huettl P. Gerhardt G.A. Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J. Pharmacol. Exp. Ther. 2008;324:725–731. doi: 10.1124/jpet.107.131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillered L. Vespa P.M. Hovda D.A. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J. Neurotrauma. 2005;22:3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- Jabaudon D. Shimamoto K. Yasuda-Kamatani Y. Scanziani M. Gahwiler B.H. Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquins-Gerstl A. Michael A.C. Comparison of the brain penetration injury associated with microdialysis and voltammetry. J. Neurosci. Methods. 2009;183:127–135. doi: 10.1016/j.jneumeth.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.B. Pickering D.S. Schousboe A. Depolarization-induced release of [(3)H]D-aspartate from GABAergic neurons caused by reversal of glutamate transporters. Int. J. Dev. Neurosci. 2000;18:309–315. doi: 10.1016/s0736-5748(99)00099-4. [DOI] [PubMed] [Google Scholar]
- Katayama Y. Becker D.P. Tamura T. Hovda D.A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- Kelley B.J. Lifshitz J. Povlishock J.T. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J. Neuropathol. Exp. Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Khan F. Baguley I.J. Cameron I.D. 4: Rehabilitation after traumatic brain injury. Med. J. Aust. 2003;178:290–295. doi: 10.5694/j.1326-5377.2003.tb05199.x. [DOI] [PubMed] [Google Scholar]
- Kobori N. Dash P.K. Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. J. Neurosci. 2006;26:4236–4246. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori N. Clifton G.L. Dash P.K. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J. Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- Levine B. Cabeza R. McIntosh A.R. Black S.E. Grady C.L. Stuss D.T. Functional reorganisation of memory after traumatic brain injury: a study with H(2)(15)0 positron emission tomography. J. Neurol. Neurosurg. Psychiatry. 2002;73:173–181. doi: 10.1136/jnnp.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshitz J. Fluid percussion injury. In: Chen J, editor; Xu Z., editor; Xu X.-M., editor; Zhang J., editor. Animal Models of Acute Neurological Injuries. The Humana Press; Totowa, NJ: 2008. [Google Scholar]
- Liu S. Lyeth B.G. Hamm R.J. Protective effect of galanin on behavioral deficits in experimental traumatic brain injury. J. Neurotrauma. 1994;11:73–82. doi: 10.1089/neu.1994.11.73. [DOI] [PubMed] [Google Scholar]
- Lyeth B.G. Jenkins L.W. Hamm R.J. Dixon C.E. Phillips L.L. Clifton G.L. Young H.F. Hayes R.L. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Mangano R.M. Schwarcz R. Chronic infusion of endogenous excitatory amino acids into rat striatum and hippocampus. Brain Res. Bull. 1983;10:47–51. doi: 10.1016/0361-9230(83)90073-4. [DOI] [PubMed] [Google Scholar]
- Matsushita Y. Shima K. Nawashiro H. Wada K. Tsuzuki N. Miyazawa T. Real time monitoring of glutamate following fluid percussion brain injury with hypoxia in the rat. Acta Neurochir. 2000;(Suppl. 76):207–212. doi: 10.1007/978-3-7091-6346-7_42. [DOI] [PubMed] [Google Scholar]
- McAllister T.W. Neuropsychiatric sequelae of head injuries. Psychiatr. Clin. North Am. 1992;15:395–413. [PubMed] [Google Scholar]
- McGinn M.J. Kelley B.J. Akinyi L. Oli M.W. Liu M.C. Hayes R.L. Wang K.K. Povlishock J.T. Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J. Neuropathol. Exp. Neurol. 2009;68:241–249. doi: 10.1097/NEN.0b013e3181996bfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T.K. Noble L. Andrews B. Faden A.I. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent. Nerv. Syst. Trauma. 1987;4:119–134. doi: 10.1089/cns.1987.4.119. [DOI] [PubMed] [Google Scholar]
- McIntosh T.K. Smith D.H. Meaney D.F. Kotapka M.J. Gennarelli T.A. Graham D.I. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab. Invest. 1996;74:315–342. [PubMed] [Google Scholar]
- Nickell J. Salvatore M.F. Pomerleau F. Apparsundaram S. Gerhardt G.A. Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol Aging. 2007;28:1737–1748. doi: 10.1016/j.neurobiolaging.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Nilsson P. Hillered L. Ponten U. Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- Obrenovitch T.P. High extracellular glutamate and neuronal death in neurological disorders. Cause, contribution or consequence? Ann. N.Y. Acad. Sci. 1999;890:273–286. doi: 10.1111/j.1749-6632.1999.tb08004.x. [DOI] [PubMed] [Google Scholar]
- Obrenovitch T.P. Urenjak J. Zilkha E. Jay T.M. Excitotoxicity in neurological disorders—the glutamate paradox. Int. J. Dev. Neurosci. 2000;18:281–287. doi: 10.1016/s0736-5748(99)00096-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Pellegrini-Giampietro D.E. Cherici G. Alesiani M. Carla V. Moroni F. Excitatory amino acid release and free radical formation may cooperate in the genesis of ischemia-induced neuronal damage. J. Neurosci. 1990;10:1035–1041. doi: 10.1523/JNEUROSCI.10-03-01035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau F. Day B.K. Huettl P. Burmeister J.J. Gerhardt G.A. Real time in vivo measures of L-glutamate in the rat central nervous system using ceramic-based multisite microelectrode arrays. Ann. N.Y. Acad. Sci. 2003;1003:454–457. doi: 10.1196/annals.1300.051. [DOI] [PubMed] [Google Scholar]
- Rao V.L. Baskaya M.K. Dogan A. Rothstein J.D. Dempsey R.J. Traumatic brain injury down-regulates glial glutamate transporter (GLT-1 and GLAST) proteins in rat brain. J. Neurochem. 1998;70:2020–2027. doi: 10.1046/j.1471-4159.1998.70052020.x. [DOI] [PubMed] [Google Scholar]
- Reeves T.M. Lyeth B.G. Povlishock J.T. Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp. Brain Res. 1995;106:248–256. doi: 10.1007/BF00241120. [DOI] [PubMed] [Google Scholar]
- Rothstein J.D. Dykes-Hoberg M. Pardo C.A. Bristol L.A. Jin L. Kuncl R.W. Kanai Y. Hediger M.A. Wang Y. Schielke J.P. Welty D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rutherford E.C. Pomerleau F. Huettl P. Stromberg I. Gerhardt G.A. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J. Neurochem. 2007;102:712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R.H. Grady M.S. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J. Neurotrauma. 1993;10:415–430. doi: 10.1089/neu.1993.10.415. [DOI] [PubMed] [Google Scholar]
- Steketee J.D. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res. Brain Res. Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Stephens M.L. Quintero J.E. Pomerleau F. Huettl P. Gerhardt G.A. Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D.A. Deshpande L.S. Sombati S. Baranova A. Wilson M.S. Hamm R.J. DeLorenzo R.J. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2 + homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur. J. Neurosci. 2008;27:1659–1672. doi: 10.1111/j.1460-9568.2008.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate D.F. Bigler E.D. Fornix and hippocampal atrophy in traumatic brain injury. Learn. Mem. 2000;7:442–446. doi: 10.1101/lm.33000. [DOI] [PubMed] [Google Scholar]
- Thomas T.C. Grandy D.K. Gerhardt G.A. Glaser P.E. Decreased dopamine D4 receptor expression increases extracellular glutamate and alters its regulation in mouse striatum. Neuropsychopharmacology. 2009;34:436–445. doi: 10.1038/npp.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman W. Westerink B.H. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wagner A.K. Sokoloski J.E. Ren D. Chen X. Khan A.S. Zafonte R.D. Michael A.C. Dixon C.E. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem. 2005;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- Wu Y. Pearl S.M. Zigmond M.J. Michael A.C. Inhibitory glutamatergic of evoked dopamine release in striatum. Neuroscience. 2000;96:65–72. doi: 10.1016/s0306-4522(99)00539-4. [DOI] [PubMed] [Google Scholar]
- Yi J.H. Hazell A.S. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem. Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.