Abstract
Epidermal growth factor (EGF) is a known mitogen for neural stem and progenitor cells (NS/NPCs) in the central nervous system (CNS). In vitro, EGF maintains NS/NPCs in the proliferative state, whereas in the normal rodent brain it promotes their proliferation and migration in the subventricular zone (SVZ). Additionally, EGF administration can augment neuronal replacement in the ischemic-injured adult striatum. Recently we found that the SVZ and the hippocampus display an injury-induced proliferative response following traumatic brain injury (TBI) that is linked to increased EGF expression. As adult neurogenesis is associated with cognitive function, we hypothesized that post-TBI administration of EGF could affect neurogenesis and cognitive recovery. Adult rats were intraventricularly infused with EGF or vehicle for 7 days following TBI. 5-Bromo-2-deoxyuridine (BrdU) was administered to label proliferating cells and the animals were sacrificed at 1 or 4 weeks post-injury. Using immunohistochemistry and stereology, we found that at 1 week post-injury, compared to vehicle-infused animals EGF-infused animals had significantly more BrdU-positive cells in the SVZ and hippocampus concomitant with enhanced EGF receptor expression. At 4 weeks post-injury, the number of BrdU-positive cells in the hippocampus was similar in both groups, suggesting that EGF does not support long-term survival of newly generated cells. Furthermore, we found that the EGF-induced proliferative population differentiated preferentially toward astroglial phenotype. Nevertheless, animals treated with EGF showed significant improvement in cognitive function, which was accompanied by reduced hippocampal neuronal cell loss. Collectively, the data from this study demonstrate that EGF exerts a neuroprotective rather than neurogenic effect in protecting the brain from injury.
Key words: epidermal growth factor, hippocampus, Morris water maze, neurogenesis, subventricular zone, traumatic brain injury
Introduction
It is now well established that neurogenesis in the adult brain persists in the regions of the subventricular zone (SVZ) and the dentate gyrus (DG) of the hippocampus. While the mechanism of adult neurogenesis is not fully understood, in the SVZ newly generated neurons migrate along the rostral migratory stream to the olfactory bulb to replace granule cells and periglomerular neurons (Lois and varez-Buylla, 1994) involved in fine-odor discrimination (Gheusi et al., 2000). In the DG, new neurons generated from the subgranular zone migrate to the granular layer and establish connections involved in hippocampal-dependent learning and memory function (Imayoshi et al., 2008;Trouche et al., 2009). Although neurogenesis persists throughout life, the number of new cells generated can be modulated by many neuropathological conditions such as TBI (Chirumamilla et al., 2002), stroke (Arvidsson et al., 2002; Jin et al., 2001), and epilepsy (Kuruba et al., 2009; Parent et al., 1997). For example, following TBI, heightened levels of cell proliferation have been observed in the SVZ and DG during the early post-injury time period (Chirumamilla et al., 2002; Dash et al., 2001; Sun et al., 2005). Furthermore, in the dentate gyrus newly-generated neurons integrate into the existing hippocampal neuronal circuitry, contributing to innate cognitive recovery post-TBI (Sun et al., 2007). These observations suggest that the adult brain has the innate potential for replenishing damaged neuronal populations through endogenous neurogenesis. Consequently, strategies to amplify this neurogenic response may have significant therapeutic potential for treating the injured brain.
Among the known factors that regulate proliferation and neuronal generation, growth factors have been widely accepted as the most important mediators. Studies have shown that a variety of growth factors are able to enhance endogenous neurogenesis in the normal brain (Aberg et al., 2000; Craig et al., 1996; Kuhn et al., 1997; Pencea et al., 2001). Likewise, growth factors can enhance neurogenesis and promote functional recovery following brain insults. For example, intraventricular infusion of basic fibroblast growth factor (bFGF) can significantly enhance post-TBI neurogenesis and improve cognitive functional recovery (Sun et al., 2009). Similarly, studies have shown that epidermal growth factor (EGF) infusion promotes cell proliferation, the generation and migration of striatal-specific neurons following ischemic injury (Ninomiya et al., 2006). However, the functional consequences of this EGF-induced proliferation are unclear. Nevertheless, as EGF can expand NS/NPCs in a similar fashion to bFGF (Gritti et al., 1999), and can dramatically enhance the migration of newly generated neurons to damaged brain areas (Teramoto et al., 2003), it is likely its actions have similar functional consequences as bFGF. Consequently, we sought to determine whether EGF can facilitate brain repair processes and functional recovery following TBI, and whether its actions are mediated through its neurogenic effect.
To explore the therapeutic potential of EGF for brain repair, this study chronicles the response of NS/NPCs in the injured brain to intraventricular administration of EGF, and correlates this with measurable functional recovery following TBI. Collectively, these studies seek to establish a link between the therapeutic manipulation of EGF-induced neurogenesis and cognitive recovery following brain injury.
Methods
Animals
A total of 47 male 3- to 4-month-old Sprague-Dawley rats (Harlan-Sprague Dawley, Inc., Indianapolis, IN) weighing approximately 300 g were used. The animals were housed in the animal facility with a 12-h light/dark cycle, and water and food provided ad libitum. All procedures were approved by our institutional animal care and use committee.
Surgical procedures
The animals were subjected to a moderate lateral fluid percussion injury following our previously published protocol (Sun et al., 2007; Sun et al., 2009). Briefly, adult rats were anesthetized in an acrylic glass chamber with 5% isoflurane, intubated and ventilated with 2% isoflurane in a gas mixture (30% oxygen and 70% nitrogen), and fixed on a stereotaxic frame. After a midline incision and skull exposure, a 4.9-mm craniotomy was trephinized on the left parietal bone halfway between the lambda and bregma sutures. A Luer-Lock syringe hub made from a 20-gauge needle was affixed to the craniotomy site with cyanoacrylate and further cemented with dental acrylic to the skull, at which point the anesthesia was switched off. Once the animal regained consciousness by showing toe and tail reflexes, the Luer-Lock fitting filled with saline was connected to a pre-calibrated fluid percussion device and a 2.2 ± 0.02 atm fluid pulse was administered. Sham animals went through the same surgical procedure without receiving the fluid pulse. After injury, the Luer-Lock fitting was removed, the animal was returned to the surgical table, and righting time was assessed. Fifteen minutes after the injury, the animal was re-anesthetized and an Alzet brain infusion cannula (Brain Infusion Kit II; DURECT, Cupertino, CA) was stereotactically implanted into the injured side posterior lateral ventricle under the guidance of an intracranial pressure monitoring system (coordinates: AP + 0.8 mm, lateral 1.4 mm, 3.5 mm beneath the pial surface). The infusion cannula was connected to an Alzet mini-osmotic pump (Model 1007D), which was placed subcutaneously on the back of the neck. Recombinant human EGF (rhEGF; Promega Corp., Madison, WI) was reconstituted in sterile artificial CSF (148 mM NaCl, 3 mM KCl, 1.4 mM CaCl2, 0.8 mM MgCl2, 1.5 mM Na2HPO4, and 0.2 mM NaH2PO4 [pH 7.4]) containing 100 μg/mL bovine serum albumin (BSA) at a concentration of 33 μg/mL. An Alzet mini-osmotic pump containing either rhEGF or vehicle (artificial CSF + 100 μg/mL BSA) was primed in a 37°C water bath for 2 h before use. The rhEGF solution was infused for 7 consecutive days at a flow rate of 0.5 μL/h (approximately 400 ng/d). The decision to use rhEGF and the infusion dosage was based on published studies that have shown the efficacy of EGF in inducing cell proliferation in both rats and mice (Kuhn et al., 1997; Craig et al., 1996). A total of 16 rats received rhEGF infusions, whereas 16 injured animals and 15 sham animals received a vehicle infusion. Beginning at 48 h after injury, all animals received daily single IP. injections of 5-bromo-2-deoxyuridine (BrdU; 50 mg/kg) for 5 consecutive days. A total of 18 animals (n = 6 in each group) were sacrificed at 7 days post-TBI to assess the extent of the cell proliferative response to TBI and EGF infusion. To determine the maturational fate and survival of cells generated following hrEGF infusion, 29 animals (n = 10 for both TBI groups, n = 9 for the sham group) were allowed to survive for 4 weeks. In this group of animals the infusion cannula and Alzet mini-osmotic pump were removed at 7 days post-injury. These long-term-survival animals were also assessed for cognitive recovery using the Morris water maze (MWM) test before sacrifice. To verify the efficiency of intraventricular infusion, after transcardiac perfusion, the infusion cannula was disconnected from the Alzet mini-osmotic pump and 20 μL of 2% Evan Blue dye was injected using a 26-gauge needle into the lateral ventricle via the infusion cannula in animals that were sacrificed at day 7. In all animals, after removal of the cannula and mini-osmotic pump, the fluid in the cannula and the remaining content in the Alzet mini-osmotic pump were examined.
Tissue preparation
At 1 or 4 weeks post-TBI, the animals were deeply anesthetized with an overdose of sodium pentobarbital, and transcardially perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. The brains were dissected and post-fixed in 4% paraformaldehyde for 48 h at 4°C, and then cut coronally into 60-μm sections with a vibratome throughout the rostro-caudal extent of the brain. The sections were collected in 24-well plates filled with PBS plus 0.01% sodium azide and stored at 4°C until use.
Immunohistochemistry
In order to assess the number of BrdU-labeled cells, every fourth section was processed for BrdU immunostaining. BrdU staining was performed following our previously published protocol (Sun et al., 2007). Briefly, the sections were washed with PBS, and DNA was denatured with 50% formamide for 60 min at 65°C, followed by a rinse in 2× saline-sodium citrate buffer, and then they were incubated with 2 N HCl for 30 min at 37°C. After denaturing, the sections were washed with PBS, and endogenous peroxidase was blocked using 3% H2O2. Following an overnight serum blocking with 5% normal horse serum in PBS, the sections were incubated with rat IgG pre-absorbed mouse anti-BrdU antibody (1:200; Dako North America, Inc., Carpenteria, CA) in PBST (PBS with 0.4% Triton) plus 5% normal horse serum at 4°C for 48 h with agitation. After rinsing with PBST, the sections were incubated with HRP-conjugated anti-mouse-IgG (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4°C and visualized with 5,5-diaminobenzidine. The sections were mounted on glass slides, lightly counterstained with 0.1% cresyl violet, and cover-slipped.
To determine the maturational fate of the newly generated cells, parallel sections were processed for immunofluorescence double labeling using antibodies against BrdU and markers for immature neurons (Turned On After Division, 64 kDa [TOAD-64] and Tuj-1), mature neurons (NeuN), astrocytes (vimentin and glial fibrillary acidic protein [GFAP]), and macrophages/activated microglia (ED1). The staining procedure was similar to the BrdU staining procedure described above. The primary antibodies used were rabbit anti-Tuj-1 (1:1000; Covance Inc., Princeton, NJ), rabbit anti-TOAD-64 (1:500; Chemicon International, Temecula, CA), mouse anti-NeuN (1:500; Chemicon), mouse anti-ED1 (1:500; Chemicon), mouse anti-vimentin (1:1000; Dako), rabbit anti-GFAP (1:1000; Dako), and mouse anti-BrdU (1:200; Dako) or rat anti-BrdU (1:200; Immunologicals Direct, Oxford, U.K.). The level of EGF receptor (EGFR) expression and the phenotypes of EGFR-expressing cells were also assessed in parallel sections using a rabbit monoclonal antibody against EGF receptor (EGFR, 1:500, Abcam, Cambridge, MA), which detects both phosphorylated and unphosphorylated EGFR, combined with mouse anti-Tuj-1 (1:500; Covance), and mouse anti-vimentin. The secondary antibodies used were Alexa Fluor 488 anti-rat IgG (mouse IgG pre-absorbed) with Alex Fluor 568 anti-mouse IgG (with rat IgG pre-absorbed), or Alex Fluor 568 anti-rabbit IgG (1:200; Molecular Probes, Eugene, OR). Briefly, for BrdU double-labeling, after DNA denaturing, endogenous peroxidase and serum blocking, the sections were incubated with primary antibodies for 72 h at 4°C with constant agitation. After washing, the sections were then incubated with secondary antibodies overnight at 4°C. For EGFR double-labeling, the staining procedure was similar to that described above, except DNA denaturing was omitted and a 10-min DAPI (1:1000; Vector Laboratories, Burlingame, CA) staining was added after incubation of the secondary antibodies. Finally, the sections were mounted on glass slides and cover-slipped with Vectashield (Vector Laboratories). To verify that immunofluorescence staining does not result from non-specific labeling or tissue autofluorescence, for each combination of double-labeling, a control section was included with the primary antibodies omitted.
Because BrdU was injected at 2–7 days post-injury, the number of BrdU-positive cells present at 4 weeks post-injury did not reflect cell proliferation at this time point. To assess whether EGF has a long-term effect on cell proliferation in the SVZ and hippocampus, brain sections from animals that had undergone MWM testing followed by sacrifice at 4 weeks post-injury were immunostained for Ki67, a proliferation marker. The immunostaining procedure for Ki67 was similar to the BrdU immunostaining protocol described above, with the omission of the denaturing steps with 50% formamide and 2 N HCl. Rabbit anti-Ki67 (1:500; Abcam) and biotin-conjugated goat anti-rabbit IgG (1:200; Jackson Laboratories, Bar Harbor, ME) were used, followed by an ABC kit and 5,5-diaminobenzidine substrate. To quantify the number of Ki67-positive cells, four animals in each experimental group with three sections per brain were examined, and every single Ki67-positive cell in the ipsilateral SVZ, subgranular zone (SGZ), and granular cell layer (GCL) was counted using a 60× oil-immersion objective. The number of Ki67-positive cells in each group was averaged and is presented as the number of cells per section.
Assessment of neuronal cell numbers in the hippocampus
To examine whether post-injury EGF treatment affects hippocampal neuronal survival, we used the stereological optical fractionator method to assess the number of pyramidal neurons in the ipsilateral CA3 and hilus hippocampal regions, where neurons are most susceptible to lateral fluid percussion injury. Fourth coronal parallel sections from animals sacrificed at 4 weeks following injury were mounted on microscope slides and processed for Giemsa histochemical staining. The number of neurons in the CA3 and hilus regions was quantified by a blinded observer.
Quantification of double-labeled cells
To quantify the percentage of newly-generated BrdU-labeled cells that had differentiated into specific phenotypes, immunofluorescence double-labeled sections were examined by confocal microscopy. In the hippocampus, the entire granule cell layer was assessed and every BrdU-labeled cell was examined to assess the co-labeling of BrdU with cell-type-specific markers. A minimum of 100 BrdU-positive cells from at least three sections per brain were examined for each marker. Each BrdU-positive cell was manually examined in its full “z” dimension, and only those cells for which the BrdU-positive nucleus was unambiguously associated with a given cell-type-specific marker were considered double-labeled. The percentage of double-labeled cells was calculated as the number of cells that were stained with both BrdU and a given cell-type-specific marker against the total number of BrdU-positive cells in the same section.
Stereological quantification
The optical fractionator method was used to estimate the total number of BrdU-positive cells in the SVZ and DG, as well the total number of neurons in the hippocampal CA3 and hilus. This design-based stereological method to estimate cell numbers has been widely used by our lab and others, and is described in detail elsewhere (Grady et al., 2003; Sun et al., 2009; Tran et al., 2006). Briefly, the region of interest was outlined using a 4× objective. A 60× oil immersion objective was used for cell counting. Within the region of interest, an optical dissector counting frame was used to count BrdU-positive cells or Giemsa-stained neuronal nuclei at predetermined regular x, y intervals. The area (a) of the counting frame was known relative to the stage-stepping intervals over the section, the sampling fraction (asf ) = a (frame)/a (x,y step). The dissector height (h) was known relative to the section thickness (t). With these parameters, the number of total cell counts (n) was estimated as n = ΣQ— · t/h · 1/asf · 1/ssf, where ssf was the section-sampling fraction (0.25 in this study), and ΣQ— was the number of cells counted.
To quantify the number of BrdU-positive cells in the SVZ and in the DG of the hippocampus, BrdU-stained sections were examined with an Olympus Image System CAST program (Olympus, Ballerup, Demark). Ten 60-μm-thick sections spaced 240 μm apart through the rostro-caudal extent of the SVZ (+1.7 mm to −0.8 mm of the bregma; Paxinos and Watson rat brain atlas coordinates), and 10 sections spanning the DG (−2.56 mm to −5 mm of the bregma) were examined by a blinded observer. Using a 60× oil immersion lens, BrdU-positive cells were counted within the counting frame, ignoring cells in the uppermost and lowermost focal planes, and focusing through the thickness of the section (optical dissector principle; Coggeshall and Lekan, 1996) to avoid oversampling errors. In the DG, BrdU-positive cells in the region of the SGZ and the granular region were counted together as the granule cell layer. For neuronal cell number assessment in the hippocampal CA3 and hilus, ten 60-μm-thick Giemsa-stained sections 240 μm apart through the rostro-caudal extent of the hippocampus (−2.56 mm to −5 mm of the bregma) were analyzed. Only neurons that exhibited clear nucleoli within a defined nuclear membrane were counted.
Morris water maze
To test whether post-TBI EGF infusion can improve cognitive recovery of injured animals, the rats were tested on hippocampal-dependent learning and memory tasks using MWM testing at 21–25 days post-injury by a blinded observer. MWM testing was performed following our previously published protocol (Sun et al., 2007). In MWM performance, goal latency and path length are equally sensitive measures (Hamm, 2001). We used goal latency as the primary dependent variable. Path length (to reach the goal in the MWM) and swim speed were also analyzed. Prior to MWM testing, a visual platform test was performed to confirm that the visual system of the animal was not impaired. Briefly, the animals were placed in the large circular tank containing opaque water and allowed to swim freely to find the hidden goal platform (1 cm below the water's surface) in order to escape from the water. Each animal was tested four times each day with a 5-min inter-trial interval. For each trial, the animal was randomly placed at one of four starting positions (N, E, S, or W). MWM performance was recorded using a computerized video tracking system (Columbus Instruments, Columbus, OH). The latency to find the platform, the total distance swam to reach the goal platform, and the swim speed was calculated for each trial. Upon finding the platform, the rat was left there for 30 sec before being removed from the maze and placed in a warm cage to dry. Animals that did not find the platform after 120 sec were placed on the platform for 30 sec and then removed from the maze.
Statistical analysis
The data generated were analyzed using SPSS software. For cell quantification a one-way analysis of variance (ANOVA) with post-hoc Fisher least significant difference (LSD) test or the Student's t-test with an applied Bonferroni correction for multiple groups was utilized, with p value <0.05 considered statistically significant. For MWM data analysis, the data were analyzed using a split-plot ANOVA (treatment × day) comparing effect of group on goal latency. A Fisher LSD test was performed to allow for pairwise group contrasts. Swim speed was also analyzed using a one-way ANOVA. Data are presented as mean ± SEM in all figures.
Results
Efficiency of intraventricular infusion
To verify that EGF or vehicle was indeed infused into the lateral ventricle, after perfusion, 20 μL of 2% Evan Blue dye was injected through the infusion cannula in animals that were sacrificed at 7 days post-injury. Evan Blue dye was found around the wall of ventricles in brain sections in those animals (data not shown). We also examined the fluid in the infusion cannula and the remaining content in the Alzet mini-pump in all animals after the removal of infusion pumps. No blockage of cannulas was found, and there was no or very little residual infusion material in the pump. This confirmed that the implantation of the cannula and infusion of EGF/vehicle into the lateral ventricle were effective.
Righting response
To confirm that injury severity was similar in all TBI animals, the post-injury righting response time in animals that received EGF infusion was compared to that of vehicle-treated animals. The righting time, which correlates with neuromotor deficits, is generally regarded as an indicator of injury severity (Hamm, 2001; Morehead et al., 1994). The mean (SEM) duration of suppression of the righting response after injury was 7.20 ± 0.30 min for the injured-vehicle group, and 6.8 ± 0.13 min for the injured-EGF group. A t-test on these data indicated that the groups did not significantly differ in the duration of the suppression of the righting response (t17 = 1.26, p = 0.22), suggesting that both groups received a similar severity of TBI.
Epidermal growth factor infusion enhances TBI-induced cell proliferation in the subventricular zone and the dentate gyrus of the hippocampus, but does not support long-term survival of newly-generated cells
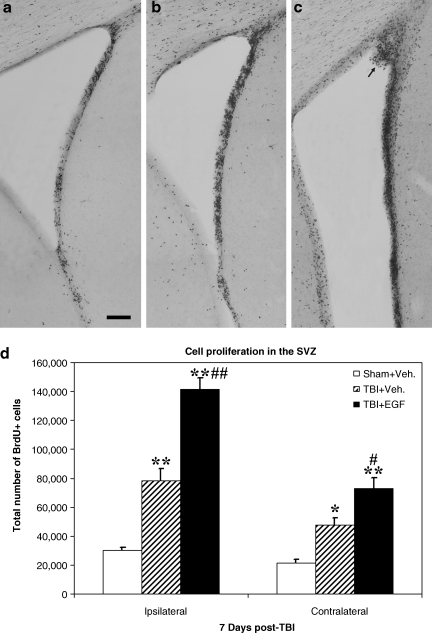
In the SVZ, many newborn cells as demonstrated by dense BrdU staining were observed in injured animals, both EGF- and vehicle-treated, compared to sham animals (Fig. 1a–c). Compared to vehicle infusion, injured animals receiving EGF displayed considerably more densely packed BrdU-positive cells in the SVZ, and in some instances polyp-like hyperplasias protruded into the ventricle (arrow in Fig. 1c). BrdU-positive cells were also observed in the SVZ-adjacent striatal and septal areas in EGF-treated animals. Unbiased stereological quantitative analysis of the total number of BrdU-positive cells revealed a significantly higher number of proliferating cells in injured animals with EGF or vehicle infusion in comparison to sham animals in both the ipsilateral and contralateral SVZ (p < 0.05 in the contralateral SVZ of the TBI-vehicle group, p < 0.01 for all other groups; Fig. 1d). Moreover, injured animals receiving EGF infusion had a significantly higher number of BrdU-positive cells in both sides of the SVZ, particularly in the ipsilateral side, compared to injured vehicle-treated animals (p < 0.01 in the ipsilateral SVZ, p < 0.05 in the contralateral SVZ; Fig. 1d).
FIG. 1.
Post-TBI intraventricular infusion of epidermal growth factor (EGF) for 7 days enhances cell proliferation in the subventricular zone (SVZ). Coronal sections of the ipsilateral SVZ taken from (a) a sham animal that received a 7-day infusion of vehicle only, (b) an injured animal that received a 7-day infusion of vehicle, and (c) an injured animal that received a 7-day infusion of EGF. Increased BrdU-labeling is observed in the injured-vehicle animal, and is further enhanced in the injured animal that received EGF compared to the sham animal. The arrow indicates a polyp-like hyperplasia in the SVZ of an EGF-infused animal (bar = 500 μm). (d) Quantitative analysis of the extent of cell proliferation in the SVZ. Compared to sham animals, injured animals with vehicle or EGF infusion have significantly enhanced cell proliferation in both the ipsilateral and contralateral SVZ (**p < 0.01, *p < 0.05). Compared to injured animals with vehicle infusion, injured animals with EGF infusion had significantly more BrdU-positive cells in both sides of the SVZ (#p < 0.01, ##p < 0.01; TBI, traumatic brain injury; BrdU, 5-bromo-2-deoxyuridine; Veh., vehicle).
In the DG of the hippocampus, clustered BrdU-positive cells were predominantly located in the SGZ and displayed enhanced levels in injured animals infused with either vehicle or EGF compared to sham animals (arrows in Fig. 2a–c). Stereological quantitative analysis showed that compared to sham animals, injured animals infused with EGF or vehicle had significantly higher total numbers of BrdU-positive cells in the granular layer (including the subgranular zone and the granular cell layer) in both hemispheres (p < 0.01; Fig. 2d) and the hilus region (p < 0.05 in the contralateral side of TBI EGF-infused animals, p < 0.01 in the other groups; Fig. 2e). Furthermore, injured animals that received EGF infusions had a significantly greater number of BrdU-positive cells in the ipsilateral granular layer compared to injured vehicle-treated animals (p < 0.05; Fig. 2d).
FIG. 2.
Post-TBI intraventricular infusion of epidermal growth factor (EGF) for 7 days enhances cell proliferation in the dentate gyrus (DG). Coronal sections of the ipsilateral DG taken from (a) a sham animal with vehicle infusion, (b) an injured animal with vehicle infusion, and (c) an injured animal with EGF infusion. Increased numbers of BrdU-positive cells, located predominantly in the subgranular zone (SGZ), were observed in the injured animals with vehicle or EGF infusions compared to sham animals (black dots indicated by the arrows; bar = 500 μm). (d) Quantitative analysis of the extent of cell proliferation in the granular zone. Compared to sham animals, injured animals with vehicle or EGF infusion had significantly higher numbers of BrdU-positive cells in the ipsilateral and contralateral granular zone (**p < 0.01). Compared to injured vehicle-infused animals, injured EGF-infused animals had significantly more BrdU + cells in the ipsilateral granular zone (#p < 0.05). (e) Quantitative analysis of cell proliferation in the hilus region. Compared to sham animals, the number of BrdU-positive proliferative cells in both the ipsilateral and contralateral hilus was significantly higher in the injured animals with either vehicle or EGF (*p < 0.05, **p < 0.01). Injured EGF-infused animals had slightly higher numbers of BrdU-positive cells in the ipsilateral hilus than the injured vehicle-infused animals, but the difference was not significant (TBI, traumatic brain injury; BrdU, 5-bromo-2-deoxyuridine; Veh., vehicle).
To examine whether EGF administration supported the survival of the newly-generated cells, the number of BrdU-positive cells was examined in animals that survived for 4 weeks after injury. Coronal brain sections from animals that underwent cognitive testing (see below) and were sacrificed at 4 weeks post-injury were processed for BrdU immunostaining. Because cells generated from the SVZ constantly migrate to the olfactory bulb, only a few BrdU-labeled cells were observed in this area at 4 weeks post-injury. The packed BrdU-positive hyperplasia polyps observed at 7 days post-injury had also disappeared by this time point (data not shown), likely due to migration along the rostral migratory stream. Because of the constant migration, the number of BrdU-positive cells in the SVZ at 4 weeks does not accurately represent the number of surviving cells. Therefore only the number of BrdU-positive cells in the DG at 4 weeks post-injury was quantified to assess the effect of EGF on post-injury cell survival. Specifically, the total number of BrdU-positive cells in the granular layer from animals sacrificed at 4 weeks post-injury was quantified using the stereological method described above, and compared to the total number of BrdU-positive cells in the same region at 7 days post-injury. The ratio between the two was taken to indicate the survival rate of newly-generated cells. In contrast to the number of BrdU-positive cells present at 7 days post-injury, there was a sharp decline in the number of BrdU-positive cells at 4 weeks post-injury, and this decline was more significant in the injured EGF-infused animals (Fig. 3). Specifically, 62% and 71% of BrdU-positive cells in the ipsilateral and contralateral DG, respectively, survived for 4 weeks post-injury in the sham group, but in injured vehicle-treated animals, 52% and 55% of newly-generated cells in the ipsilateral and contralateral DG, respectively, survived at 4 weeks. In injured EGF-infused animals 40% and 42% survival, respectively, was observed in the ipsilateral and contralateral DG. Because EGF was only infused for 7 days in this study, these data suggest that in the absence of EGF during the peak time of cell death, which was around 2 weeks after generation, EGF did not cause extended survival of the larger pool of newly-generated cells stimulated by EGF infusion. However, by 4 weeks post-TBI, the total numbers of surviving BrdU-positive cells in the granular zone in both injured animal groups were comparable, and were both significantly higher than in sham animals, in both the ipsilateral (p < 0.05; Fig. 3) and contralateral DG (p < 0.05p; Fig. 3).
FIG. 3.
Seven-day infusion of epidermal growth factor (EGF) does not support long-term survival of newly-generated cells in the dentate gyrus (DG) following injury. This graph shows the total number of BrdU-positive cells in the granular zone in sham, injured vehicle-infused, or injured EGF-infused animals at 4 weeks post-injury. At this time point post-injury, the numbers of BrdU-positive cells were still significantly higher in injured animals with vehicle or EGF infusion in both the ipsilateral and contralateral sides (*p < 0.05). However, the number of BrdU-positive cells in injured EGF-infused animals was similar to that found in injured vehicle-treated animals in both the ipsilateral and contralateral sides (BrdU, 5-bromo-2-deoxyuridine; Veh., vehicle).
Epidermal growth factor infusion enhances EGFR expression in the subventricular zone and the dentate gyrus of the hippocampus
Previous studies suggest that EGF receptor (EGFR) signaling is required for cell proliferation in the SVZ (Enwere et al., 2004). To assess whether EGF-enhanced cell proliferation following injury is modulated through EGFR, we studied EGFR expression using immunohistochemistry with an antibody that detects both phosphorylated and unphosphorylated EGF receptors. At 7 days post-injury, in both sham and injured vehicle-infused animals, EGFR expression was observed predominantly in the SVZ, with the injured vehicle-infused animals showing slightly increased staining intensity (Fig. 4a and b). Some scattered EGFR-positive cells were observed in the striatum area adjacent to the ipsilateral SVZ in the injured animals. In injured EGF-infused animals, drastically enhanced expression of EGFR was found in both the ipsilateral and contralateral SVZ (Fig. 4c). The polyp-like hyperplasia area in the SVZ was also strongly labeled with EGFR. Many chain-like EGFR-positive cells with neuronal morphologies were found in the adjacent striatum and septal nuclei areas of the ipsilateral SVZ. In the DG, low levels of EGFR immunoreactivity were observed in sham and injured-vehicle animals (Fig. 4d); whereas EGFR expression was enhanced in the EGF-treated animals, in both the granular layer and the hilus region (Fig. 4e). At 4 weeks post-injury, in EGF-infused animals, EGFR expression at the SVZ and the DG was decreased; however, EGFR-positive cells in the striatum adjacent to the ipsilateral SVZ were still prominent (data not shown). In sham and injured vehicle-infused animals, the staining pattern of EGFR at 4 weeks post-injury was similar to that seen at 1 week post-injury. No apparent EGFR staining was found in the CA3 region at either time point.
FIG. 4.
Epidermal growth factor (EGF) infusion enhances epidermal growth factor receptor (EGFR) expression in both the subventricular zone (SVZ) and the dentate gyrus (DG) of the hippocampus. (a–c) Fluorescence images show EGFR expression at 7 days post-injury in the ipsilateral SVZ in the sham (a), injured-vehicle infused (b), and injured EGF-infused animals (c). Note the robust EGFR expression in the EGF-treated animals in the SVZ, particularly in the hyperplasic polyp region. (d) This image shows very weak EGFR staining in the DG in an injured vehicle-treated animal, compared to (e) strong EGFR expression in the ipsilateral DG in an EGF-treated animal at 7 days post-TBI (bar = 200 μm). (f–k) Representative confocal microscopic images of the immunofluorescence double-labeling of EGFR with vimentin (f–h), and Tuj-1 (i–k), in the DG at 7 days post-TBI/EGF infusion. Note that the EGFR-positive cells were co-localized with vimentin (arrows in f–h), but not with Tuj-1 (arrows in i–k; scale bar = 30 μm; DAPI, 4,6-diamino-2-phenylindole). Color image is available online at www.liebertonline.com/neu.
EGFR immunofluorescence double-labeling of sections taken from the 7-day post-injury group showed that the majority of EGFR-positive cells had astroglial morphology and were co-labeled with vimentin (Fig. 4f–h), but not Tuj-1 (Fig. 4i–k), in both the DG and the SVZ. In the SVZ-adjacent striatum and septal nuclei areas, though many chain-like EGFR-positive cells were co-labeled with vimentin, a few EGFR-positive cells having neuronal morphology did not stain with vimentin or Tuj-1 (data not shown).
Seven-day infusion of EGF has no significant prolonged effect on cell proliferation
To examine whether 7-day EGF infusion had a prolonged effect on cell proliferation, the proliferation marker Ki67 was used to determine the degree of cell proliferation in the SVZ and the DG of the hippocampus in animals sacrificed at 4 weeks post-TBI. Ki67 immunoreactivity in the SVZ, which represented cell populations in the active cell cycle at the time of perfusion, exhibited a similar pattern of staining in all three groups (Fig. 5a). Some Ki67-positive cells were located in the striatal and septal areas close to the SVZ. In the DG, Ki67-positive cells were located predominantly in the SGZ (Fig. 5b). Quantitative analysis showed that the level of cell proliferation as represented by the numbers of Ki67-positive cells per section was not significantly different in sham, injured-vehicle, or injured EGF-infused animals, in both the SVZ and DG (Fig. 5d and e). These data suggest that neither TBI nor EGF treatment has any prolonged effect on cell proliferation in the SVZ or the hippocampus. It also indicates that the EGF-enhanced post-injury proliferative response of neural stem and progenitor cells in the SVZ and DG is transient.
FIG. 5.
Seven-day infusion of epidermal growth factor (EGF) does not have a prolonged effect on cell proliferation. (a–b) Representative Ki67 staining in the ipsilateral subventricular zone (SVZ) (a) and dentate gyrus (DG) (b), from an injured animal that received EGF infusion and was sacrificed at 4 weeks post-injury. Arrows indicate Ki67-positive cells (bar = 300 μm). (c) Enlarged image of the boxed region in (b). (d) This graph shows the number of Ki67-positive cells in the ipsilateral SVZ at 4 weeks post-injury. Injured animals that received EGF had slightly higher numbers of Ki67-positive cells than injured-vehicle infused or sham animals, but this difference was not statistically significant. (e) In the DG, the numbers of Ki67-positive cells in injured EGF-infused animals was no different from those of injured-vehicle or sham animals (Veh., vehicle; TBI, traumatic brain injury).
Epidermal growth factor promotes the differentiation of newly-generated cells toward an astroglial fate
Previously we reported that the majority of the cells that were generated in the DG following injury and had survived for an extended period of time ultimately matured into granular neurons (Sun et al., 2007). To examine whether a post-injury intraventricular infusion of EGF affects the differentiational fate of the newly-generated cells, immunofluorescence double-labeling for BrdU and the immature cell type markers TOAD-64, Tuj-1, and vimentin, as well as ED1, a marker for infiltrating macrophages and activated microglia, was performed on brain sections of EGF- and vehicle-treated injured animals or sham animals allowed to survive for 7 days post-injury. Hippocampal sections taken from animals that survived for 4 weeks post-TBI were processed for double-labeling of BrdU and the mature cell type markers NeuN, GFAP, or ED1.
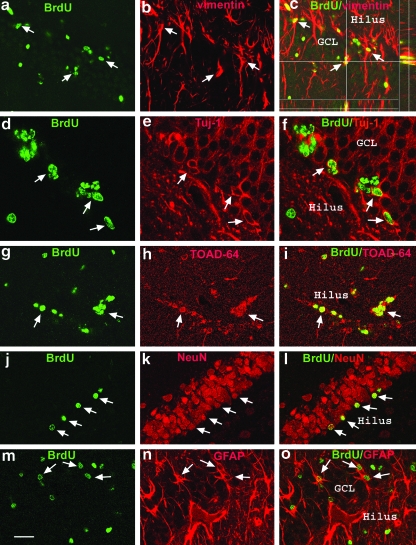
At 7 days post injury, the majority of BrdU-positive cells in the SVZ were co-labeled with TOAD-64, Tuj-1, or vimentin (Fig. 6). Vimentin, a marker for immature astrocytes, was expressed at high levels throughout the entire SVZ, as well as in the ependymal cells lining the ventricle. Many BrdU-positive cells were co-labeled with vimentin within the SVZ (Fig. 6a–c). The polyp-like hyperplasia also labeled positive for vimentin. The ependymal cells on the surface of the polyp were BrdU-positive. The polyp-like hyperplasia did not stain with the immature neuronal markers TOAD 64 or Tuj-1. However, many clustered BrdU-positive cells predominantly located at the rostral corner of the SVZ, as well as along the rostral migratory stream, were co-labeled with TOAD-64 (Fig. 6d–f) or Tuj-1 (Fig. 6h–j). TOAD-64 is a neural-specific protein that is transiently expressed in early post-mitotic neurons, whereas Tuj-1, which labels neuronal β-III tubulin, is not specific for post-mitotic neurons (Minturn et al., 1995). No BrdU/ED1-positive cells were found in the SVZ. In the DG of the hippocampus at 7 days post-injury, BrdU-positive cells were predominantly located in the SGZ, and many cells were co-localized with vimentin, TOAD-64, or Tuj-1 (Fig. 7a–i). BrdU/vimentin double-labeling was particularly evident in EGF-infused animals (Fig. 7 a–c). In injured animals both EGF- and vehicle-infused, a few BrdU/ED1-positive cells were found around the tip of the ventral blade of the GCL close to ventricle, indicating infiltrating macrophages from the ventricular system. These cells are round and have a larger BrdU-staining pattern, distinctly different from cells located at the SGZ. Because many newly-generated cells die during the 2–3 weeks after generation (Dayer et al., 2003), some of the cells that expressed these early differentiation markers likely did not survive to become mature cells that contribute to brain function; therefore, a quantitative analysis of these differentiation data was not performed at this time point.
FIG. 6.
Differentiation of newly-generated cells in the subventricular zone (SVZ) at 7 days post-injury. Representative confocal photomicrographs of the SVZ show newly-generated cells double-labeled with BrdU and vimentin, TOAD-64, or Tuj-1. (a–c) These images show BrdU/vimentin double-labeled cells with BrdU-stained nuclei (green) surrounded by vimentin-stained (red) cytoplasm and processes (arrows). The vimentin-stained hyperplasic polyp extends from the SVZ into the lateral ventricle (LV). Some BrdU-positive cells on the surface of the polyp are vimentin-positive ependymal cells. (d–f ) These images show many clustered BrdU-positive cells (green) co-labeled with TOAD-64 staining (red); arrows indicate the co-localization. BrdU and TOAD-64 double-labeled cells are predominantly accumulated at the rostral corner of the SVZ, as well as along the rostral migratory stream. (h–j) Many clustered BrdU-positive cells (green) in the SVZ that are also co-labeled with Tuj-1 (red) are indicated by arrows (bar = 80 μm; BrdU, 5-bromo-2-deoxyuridine; TOAD-64, Turned On After Division, 64 kDa). Color image is available online at www.liebertonline.com/neu.
FIG. 7.
Neuronal and glial differentiation of newly-generated cells in the dentate gyrus (DG). Representative confocal microscopic images of the DG demonstrate double-labeling of BrdU-positivity with immature neuronal and glial markers at 7 days post-injury (a–i), and with mature cell markers at 4 weeks post-injury (j–o). At 7 days post-injury, (a–c) arrows indicate BrdU-positive cells (green, a) in the DG are co-stained with the immature astrocyte marker vimentin (red, b) in the granular cell layer (GCL) throughout the z axis (c), (d–f ) Arrows indicate co-localization of BrdU (green, d) with Tuj-1 (red, e) in newly-generated cells in the inner layer of the GCL, (g–i) Arrows indicate BrdU-labeled cells (green, g) co-labeled with TOAD-64 (red, h). At 4 weeks post-injury, (j–l) the arrows indicate that many BrdU-positive cells (green, j) in the GCL have become NeuN-positive mature neurons (red, k and l), (m–o) Some BrdU-positive cells (green, m) in the GCL have become GFAP-positive mature astocytes (red, n and o; scale bar = 40 μm in d–f, and 80 μm in a–c and g–o; GFAP, glial fibrillary acidic protein; BrdU, 5-bromo-2-deoxyuridine;). Color image is available online at www.liebertonline.com/neu.
Because the majority of cells from the SVZ had migrated to the olfactory bulb by 4 weeks post-injury, only the maturational phenotypes of newly-generated cells in the DG were analyzed via double-labeling for BrdU, with NeuN, GFAP, or ED1. The percentage of newly-generated cells in the ipsilateral granular layer (SGZ + GCL) that had differentiated into mature neurons as demonstrated by double-labeling with BrdU and NeuN (Fig. 7j–l) was 72.7 ± 2.7% for sham animals, 66.7 ± 2.6% for injured vehicle-infused animals, and 47.1 ± 10.4% for injured EGF-infused animals. The percentage of cells that had differentiated into astrocytes as shown by BrdU/GFAP double-labeling (Fig. 7m–o) was 25.8 ± 7.7% for sham animals, 19 ± 3.8% for injured vehicle-infused animals, and 38.4 ± 3% for injured EGF-infused animals. Thus the percentage of BrdU/GFAP cells in the injured EGF-infused animals was significantly higher than that of sham or injured vehicle-infused animals (p < 0.05; Fig. 8). In the contralateral granular layer, similar percentages of BrdU/NeuN and BrdU/GFAP double-labeled cells were found as in the ipsilateral DG (Fig. 8). These data suggest that EGF preferentially drives endogenous NS/NPCs toward an astrocytic fate in the DG. In the granular layer, no BrdU/ED1 double-labeled cells were found.
FIG. 8.
Epidermal growth factor (EGF) infusion promotes newly-generated cells toward astroglial differentiation in the dentate gyrus (DG) at 4 weeks post-injury. Quantitative analysis shows that the percentage of BrdU/NeuN double-labeled cells was similar in sham and injured vehicle-infused animals in both the ipsilateral and contralateral DG. Injured animals infused with EGF had a lower percentage of BrdU/NeuN double-labeling than the other two groups; however, this difference was not statistically significant in either the ipsilateral or contralateral DG due to the variability observed in EGF-infused animals. Nevertheless, injured EGF-infused animals showed a significantly higher percentage of BrdU/GFAP double-labeled cells in the ipsilateral DG than the sham and injured vehicle groups (*p < 0.05). The percentages of BrdU/GFAP-labeled cells in sham and injured vehicle-infused animals was comparable (GFAP, glial fibrillary acidic protein; BrdU, 5-bromo-2-deoxyuridine; NeuN, mature neurons; Veh., vehicle; Ip., ipsilateral; Ct., contralateral).
Post-injury infusion of epidermal growth factor diminishes the cognitive deficits associated with TBI
Moderate lateral fluid percussion injury induces cognitive deficits that can be measured by Morris water maze testing. We previously showed that these deficits last for more than 1 month post-injury (Sun et al., 2007). In this study, we wanted to test whether EGF infusion could ameliorate the cognitive deficits associated with TBI. Specifically, MWM testing was performed in sham animals, as well as TBI animals that received intraventricular infusions of either EGF or vehicle for 7 days post-injury. The MWM tests were performed daily on days 21–25 post-TBI. The mean latency (in seconds) to reach the goal platform for each group is presented in Figure 9. Injured animals that received EGF infusions had a significantly shorter latency to reach the platform than injured vehicle-infused animals (p < 0.001), suggesting that EGF-infused animals displayed better performance or quicker learning in the MWM. The results of a split-plot ANOVA (group × day) revealed a significant group effect (F2,25 = 24.58; p < 0.001), and day effect (F4,100 = 64.69; p < 0.001), with no significant day × group interaction (F8,100 = 0.83; p = 0.57). To examine specific group differences, Fisher LSD tests were performed. This analysis found that the injured-vehicle group had significantly longer goal latencies than the injured-EGF and sham groups (p < 0.001). In addition, the injured-EGF group did not differ in goal latency from the sham group (p = 0.41). No differences were observed in swim speed between groups, indicating that motor impairments did not contribute to the different latencies. Taken together, these data demonstrate that injured animals with EGF infusion displayed a significant improvement in cognitive function.
FIG. 9.
Post-TBI epidermal growth factor (EGF) infusion improves recovery of cognitive function. This graph compares the MWM performance of sham and injured animals infused with vehicle or EGF. Compared to injured vehicle-infused animals, injured animals infused with EGF showed a significant improvement in cognitive recovery (*p < 0.001) through days 22–25 following injury. This cognitive recovery, as characterized by shorter goal latency in the water maze performance, reached similar levels to those observed in sham animals (MWM, Morris water maze; TBI, traumatic brain injury; Veh., vehicle).
Post-TBI infusion of epidermal growth factor preserved the total number of neurons in the hippocampal CA3 and hilus
Since EGF has both mitogenic and neurotrophic functions, we attempted to identify which of these properties was likely to participate in the cognitive recovery observed following EGF infusion in injured animals. Though EGF infusion significantly enhanced cell proliferation in the DG, it did not support the long-term survival of the larger pool of newly-generated cells, nor did it promote neuronal differentiation. Therefore, we speculated that the neurotrophic effect of EGF may play a role in improving cognitive function following injury. As hippocampal neurons in the CA3 and hilus regions are particularly vulnerable to secondary insults following TBI, and their loss contributes to cognitive deficits as assessed by MWM, we sought to determine whether post-injury administration of EGF protects these neuronal populations from injury-induced damage. Using unbiased stereological methods, the total number of neurons in the ipsilateral CA3 and hilus regions was quantified at 4 weeks post-injury in animals that underwent MWM testing. In both the CA3 and hilus regions, injured animals infused with either EGF or vehicle had more significant neuronal cell loss than sham animals (p < 0.01; Fig. 10). However, injured animals treated with EGF had significantly less neuronal cell loss in both regions than vehicle-infused animals (p < 0.05; Fig. 10). These data suggest that a 7-day intraventricular infusion of EGF immediately following TBI has a significant neural protective effect on the CA3 and hilus neurons in the injured hippocampus.
FIG. 10.
Epidermal growth factor (EGF) has a neuroprotective effect on neurons in the CA3 and hilus region following injury. At 4 weeks after fluid percussion injury, injured animals infused with vehicle or EGF had more significant neuronal cell loss in the ipsilateral CA3 region (a) and hilus region (b) than sham animals (**p < 0.01; n = 4 in each group). However, compared to injured vehicle-infused animals, injured animals receiving EGF had significantly less neuronal cell loss as evidenced by higher neuronal counts in both the CA3 and hilus regions (#p < 0.05; Veh., vehicle).
Discussion
The current study demonstrates that traumatic brain injury induces cell proliferation in the subventricular zone and the dentate gyrus of the hippocampus, which can be further augmented with an intraventricular infusion of EGF. However, at 4 weeks post-injury, the number of newly-generated cells in the hippocampus was similar in both the injured-vehicle-infused and injured-EGF-infused groups, suggesting that EGF does not support the long-term survival of these newly-generated cells. Furthermore, we found that the EGF-induced proliferative population differentiated preferentially toward an astrocytic phenotype. Nevertheless, injured animals treated with EGF showed significant improvements in cognitive function. This improvement in functional recovery following EGF infusion was associated with the reduced neuronal cell loss in the hippocampal CA3 and hilus regions of these animals. Collectively, the results of this study demonstrate that EGF exerted a neuroprotective effect rather than a neurogenic effect in promoting recovery following traumatic brain injury.
We have previously found that TBI-induced cell proliferation in the SVZ and the DG of the hippocampus can be enhanced with an intraventricular infusion of bFGF, and that this growth factor–mediated neurogenic response contributes to the cognitive recovery in injured animals (Sun et al., 2009). In the current study, we have demonstrated that EGF, while sharing many similarities with bFGF in regulating neural stem cell activities in vitro, displays distinctly different effects from bFGF when administered in vivo following injury. Similarly to bFGF, EGF increased injury-enhanced cell proliferation in the SVZ and the DG. However, unlike bFGF, EGF does not support the long-term survival of these cells in the hippocampus. Moreover, EGF promotes a higher proportion of the newly-generated cell population towards astroglia differentiation than bFGF. Furthermore, EGF infusion significantly reduces injury-induced neuronal cell loss of hippocampal neurons in the CA3 and hilus regions.
As expected, TBI in the form of fluid percussion injury induces cell proliferation in the SVZ and the DG. Intraventricular infusion of EGF further enhances this injury-induced cell proliferation in the SVZ, a finding in agreement with results of previously published studies showing that infusion of EGF into normal or ischemic injured rat brain results in a dramatic increase in the proliferation of SVZ cells (Craig et al., 1996; Kuhn et al., 1997; Ninomiya et al., 2006; Teramoto et al., 2003). Unlike the ischemically-injured brain, in which a 7-day infusion of EGF resulted in significant levels of proliferating cells migrating to the ischemically-injured striatum (Teramoto et al., 2003), in the current study only a few BrdU-positive cells were found in the SVZ-adjacent striatum and septum. This result is similar to what was reported when EGF was infused into the normal brain (Craig et al., 1996; Kuhn et al., 1997). This is likely due to the fact that the injured brain region in our model was located at the temporal lobe, which is some distance from the SVZ. Previously, Kuhn and associates (1997) reported that EGF infusion solely enhanced cell proliferation in the SVZ, without affecting cell proliferation in the normal DG. In the current study, we observed that EGF further enhances cell proliferation in the DG in injured animals. This is also supported by an organotypic hippocampal slice culture study, in which the authors demonstrated that following transection of CA2 Schaffer collaterals, EGF increased trauma-induced cell proliferation in the DG, but not neurogenesis (Laskowski et al., 2005). This disparity in the effects of EGF on cell proliferation in the DG could be due to differences between injured versus normal animals or the length of EGF administration, as well as differing sampling or quantification methods.
The effect of EGF on cell proliferation is thought to be modulated through EGF receptors. Prominent expression of EGF-receptor mRNA was detected throughout the SVZ, particularly in the dorsolateral aspect from birth to adulthood, and the numbers of labeled cells also diminished during development (Seroogy et al., 1995), emphasizing the important role of EGF and its family members in regulating cell genesis. In the postnatal and adult rat brain, EGFR is expressed in regions undergoing active neurogenesis, including the SVZ, the granular layer of the DG, and the cerebellar granular layer (Cameron et al., 1998; Tucker et al., 1993). EGFR knockout mice demonstrate defects in cortical neurogenesis and retinal histogenesis (Close et al., 2006). Similarly, diminished cell proliferation in the SVZ was observed for another EGFR ligand, TGF-α knockout mice, further implying a role of EGFR in cell proliferation (Wong, 2003). Following a focal ischemic injury, EGFR expression was significantly increased in the SVZ, which was accompanied by enhanced cell proliferation (Ninomiya et al., 2006b), whereas in the aged brain, reduced EGFR signaling is associated with diminished cell proliferation in the SVZ (Enwere et al., 2004). In the current study, we found enhanced EGFR expression in both the SVZ and the DG at 1 week post-injury, and its expression was decreased at 4 weeks. This EGFR expression pattern was similar to the levels of EGF-enhanced cell proliferation, and vimentin-positive cells were the dominant cell type expressing EGFR. Collectively, these studies point to the importance of EGF and its receptor in influencing cell proliferation, both during normal development and following brain injury.
In the current study, we also found that EGF infusion affects the maturational phenotype of newly-generated cells by increasing the generation of glial cells, and concomitantly decreasing neuronal differentiation. This effect of EGF of driving NC/NPCs toward an astrocytic phenotype following injury is in agreement with previous reports on the effects of EGF administration on cell differentiation in the normal rat brain (Craig et al., 1996; Kuhn et al., 1997). However, the mechanism by which EGF induces glial differentiation remains unclear. Doetsch and colleagues (2002) reported that in the SVZ, an intraventricular infusion of EGF at a higher level converts rapidly-dividing neuronal precursor cells in the SVZ into multipotent stem cells. These cells display elevated levels of tenascin, an astrocyte-associated extracellular matrix molecule, thus promoting the generation of GFAP-positive astrocytes, and decreasing neuronal production (Doetsch et al., 2002). Other studies have indicated that EGFR-mediated signaling contributes to the cells becoming of a glial phenotype. For example, previous studies in the retina have demonstrated that the level and density of EGF-receptor expression by progenitor cells affect the cell's ultimate fate. Specifically, a low level of EGFR expression in retina progenitor cells enhanced cell proliferation, whereas higher levels reduced proliferation and induced the generation of Müller glial cells (Lillien and Wancio, 1998). Adding EGFRs to E18 or P0 retinal progenitor cells resulted in the premature differentiation and overrepresentation of Müller glial cells when in the presence of high levels of the EGFR ligand TGF-α. Conversely, in the absence of the exogenous ligand and receptor, very few cells expressed a Müller glial phenotype (Lillien, 1995; Lillien and Wancio, 1998). Moreover, in the embryonic brain, the premature expression of high levels of the EGFR in progenitor cells in the ventricular zone through exogenous viral transduction resulted in the development of cortical astrocytes (Burrows et al., 1997). In vitro studies have also demonstrated that the action of EGF on cells is dose-dependent. In our study, as well as other in vivo studies, EGF at a dose of 400 ng/d was infused for 7 days or longer, which is a relatively high dose that likely drives cells towards glial differentiation. Taken together, these studies suggest that the EGF-induced cell fate is dose-dependent, with higher doses leading to an astroglial fate for NS/NPCs. Nevertheless, as it has been shown that SGZ astrocytes are neural stem cells (Seri et al., 2001), and EGF can convert neural precursor cells in the SVZ into multipotent stem cells (Doetsch et al., 2002b), the observation of EGF infusion inducing astroglial differentiation in the SGZ could be due to an effect of EGF maintaining NS/NPCs in an early stem cell state, rather than a maturational stage.
In our study, EGF infusion significantly improved cognitive functional recovery in injured animals. As our previous study demonstrated that bFGF infusion into TBI rats led to enhanced cell proliferation and a concomitant improvement of cognitive function, we hypothesized that EGF-induced neurogenesis in TBI rats would also be linked to cognitive improvements. However, in the current study, although EGF stimulated cell proliferation in both the SVZ and DG, survival of the newly-generated cells, as well as generation of new neurons, were not observed in EGF-treated animals. Therefore the effect of EGF in improving cognitive function may be due to other functions of EGF, rather than its neurogenic role. In fact, by examining the number of neurons in the hippocampus, we found that injured animals that received EGF treatment had significantly higher numbers of healthy neurons in the hippocampal CA3 and hilus regions than those seen in injured animals that received a vehicle infusion. This suggests that EGF plays a neuroprotective role in the hippocampal neurons following injury. This is not surprising, considering previous the results of in vitro and in vivo studies demonstrating the neuroprotective effect of EGF on CNS neurons. For example, in vitro studies have shown that EGF can protect cultured cerebellar and dopaminergic neurons from glutamate-induced neuronal death (Abe and Saito, 1992; Casper and Blum, 1995). Moreover, in primary hippocampal cell cultures, EGF can extend the survival of cultured neurons, facilitate neurite outgrowth, and prevent free radical–induced neuronal damage (Peng et al., 1998). In an in vivo gerbil ischemia model, an intraventricular infusion of EGF prevented CA1 neuronal death, decreased numbers of TUNEL-positive apoptotic cells in the ischemic CA1 region, and preserved synapses in the hippocampal CA1 region (Peng et al., 1998). Furthermore, studies have also found that EGF promotes the generation of long-term potentiation (LTP) in the DG. In hippocampal slices, EGF enhances short-term potentiation of evoked potentials in Schaffer/commissural-CA1 pyramidal cell synpases in a concentration-dependent manner, resulting in the generation of LTP (Abe et al., 1992). In vivo, an intraventricular infusion of EGF can promote LTP generation, which is associated with cognitive function, in the perforant path–dentate granule cell synapses of anesthetized rats, and in fimbria-fornix-lesioned rats (Abe et al., 1992; Ishiyama et al., 1991). Collectively, these studies and our current findings indicate that the neurotrophic effects of EGF on the injured brain are likely contributing to the improved functional recovery seen in the injured animals in this study.
The results of the current study demonstrate that an intraventricular infusion of EGF immediately following brain injury significantly enhances cell proliferation in the neurogenic SVZ and DG, concomitant with increased EGF-receptor expression in these regions. However, EGF does not support the long-term survival of newly-generated cells, nor does it enhance the generation of new neurons. Rather, EGF prevents injury-induced hippocampal neuronal cell loss. Thus the beneficial effect of EGF on the cognitive recovery of injured animals is likely due to its neurotrophic effect. Regardless of its mode of action, the therapeutic potential of EGF should be explored further.
Acknowledgments
This study was funded by the National Institutes of Health Grant no. NS055086 (to D.S.). The photomicrography for this study was performed at the Virginia Commonwealth University Department of Anatomy and Neurobiology Microscopy Facility, which is supported in part by funding from NIH-NINDS center core grant 5P30NS047463.
Author Disclosure Statement
No competing financial interests exist.
References
- Abe K. Ishiyama J. Saito H. Effects of epidermal growth factor and basic fibroblast growth factor on generation of long-term potentiation in the dentate gyrus of fimbria-fornix-lesioned rats. Brain Res. 1992;593:335–338. doi: 10.1016/0006-8993(92)91332-9. [DOI] [PubMed] [Google Scholar]
- Abe K. Saito H. Protective effect of epidermal growth factor on glutamate neurotoxicity in cultured cerebellar neurons. Neurosci. Res. 1992;14:117123. doi: 10.1016/0168-0102(92)90087-s. [DOI] [PubMed] [Google Scholar]
- Aberg M.A. Aberg N.D. Hedbacker H. Oscarsson J. Eriksson P.S. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A. Collin T. Kirik D. Kokaia Z. Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Burrows R.C. Wancio D. Levitt P. Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–267. doi: 10.1016/s0896-6273(00)80937-x. [DOI] [PubMed] [Google Scholar]
- Cameron H.A. Hazel T.G. McKay R.D. Regulation of neurogenesis by growth factors and neurotransmitters. J. Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Casper D. Blum M. Epidermal growth factor and basic fibroblast growth factor protect dopaminergic neurons from glutamate toxicity in culture. J. Neurochem. 1995;65:10161026. doi: 10.1046/j.1471-4159.1995.65031016.x. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S. Sun D. Bullock M.R. Colello R.J. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Close J.L. Liu J. Gumuscu B. Reh T.A. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006;54:94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- Coggeshall R.E. Lekan H.A. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J. Comp. Neurol. 1996;364:615. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Craig C.G. Tropepe V. Morshead C.M. Reynolds B.A. Weiss S. van der K.D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J. Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P.K. Mach S.A. Moore A.N. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dayer A.G. Ford A.A. Cleaver K.M. Yassaee M. Cameron H.A. Short-term and long-term survival of new neurons in the rat dentate gyrus. J. Comp. Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Doetsch F. Petreanu L. Caille I. Garcia-Verdugo J.M. varez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Enwere E. Shingo T. Gregg C. Fujikawa H. Ohta S. Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G. Cremer H. McLean H. Chazal G. Vincent J.D. Lledo P.M. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady M.S. Charleston J.S. Maris D. Witgen B.M. Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. J. Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Gritti A. Frolichsthal-Schoeller P. Galli R. Parati E.A. Cova L. Pagano S.F. Bjornson C.R. Vescovi A.L. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J. Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm R.J. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J. Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Imayoshi I. Sakamoto M. Ohtsuka T. Takao K. Miyakawa T. Yamaguchi M. Mori K. Ikeda T. Itohara S. Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Ishiyama J. Saito H. Abe K. Epidermal growth factor and basic fibroblast growth factor promote the generation of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci. Res. 1991;12:403–411. doi: 10.1016/0168-0102(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Jin K. Minami M. Lan J.Q. Mao X.O. Batteur S. Simon R.P. Greenberg D.A. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H.G. Winkler J. Kempermann G. Thal L.J. Gage F.H. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruba R. Hattiangady B. Shetty A.K. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav. 2009;14(Suppl. 1):65–73. doi: 10.1016/j.yebeh.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski A. Schmidt W. Dinkel K. Martinez-Sanchez M. Reymann K.G. bFGF and EGF modulate trauma-induced proliferation and neurogenesis in juvenile organotypic hippocampal slice cultures. Brain Res. 2005;1037:78–89. doi: 10.1016/j.brainres.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lillien L. Changes in retinal cell fate induced by overexpression of EGF receptor. Nature. 1995;377:158–162. doi: 10.1038/377158a0. [DOI] [PubMed] [Google Scholar]
- Lillien L. Wancio D. Changes in epidermal growth factor receptor expression and competence to generate glia regulate timing and choice of differentiation in the retina. Mol. Cell Neurosci. 1998;10:296–308. doi: 10.1006/mcne.1997.0659. [DOI] [PubMed] [Google Scholar]
- Lois C. varez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Minturn J.E. Geschwind D.H. Fryer H.J. Hockfield S. Early postmitotic neurons transiently express TOAD-64., a neural specific protein. J. Comp. Neurol. 1995;355:369–379. doi: 10.1002/cne.903550304. [DOI] [PubMed] [Google Scholar]
- Morehead M. Bartus R.T. Dean R.L. Miotke J.A. Murphy S. Sall J. Goldman H. Histopathologic consequences of moderate concussion in an animal model: correlations with duration of unconsciousness. J. Neurotrauma. 1994;11:657–667. doi: 10.1089/neu.1994.11.657. [DOI] [PubMed] [Google Scholar]
- Ninomiya M. Yamashita T. Araki N. Okano H. Sawamoto K. Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci. Lett. 2006;403:63–67. doi: 10.1016/j.neulet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Parent J.M. Yu T.W. Leibowitz R.T. Geschwind D.H. Sloviter R.S. Lowenstein D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea V. Bingaman K.D. Wiegand S.J. Luskin M.B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. Wen T.C. Tanaka J. Maeda N. Matsuda S. Desaki J. Sudo S. Zhang B. Sakanaka M. Epidermal growth factor protects neuronal cells in vivo and in vitro against transient forebrain ischemia- and free radical-induced injuries. J. Cereb. Blood Flow Metab. 1998;18:349–360. doi: 10.1097/00004647-199804000-00002. [DOI] [PubMed] [Google Scholar]
- Seri B. Garcia-Verdugo J.M. McEwen B.S. varez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroogy K.B. Gall C.M. Lee D.C. Kornblum H.I. Proliferative zones of postnatal rat brain express epidermal growth factor receptor mRNA. Brain Res. 1995;670:157–164. doi: 10.1016/0006-8993(94)01300-7. [DOI] [PubMed] [Google Scholar]
- Sun D. Bullock M.R. McGinn M.J. Zhou Z. Altememi N. Hagood S. Hamm R. Colello R.J. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. Colello R.J. Daugherty W.P. Kwon T.H. McGinn M.J. Harvey H.B. Bullock M.R. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J. Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- Sun D. McGinn M.J. Zhou Z. Harvey H.B. Bullock M.R. Colello R.J. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Teramoto T. Qiu J. Plumier J.C. Moskowitz M.A. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J. Clin. Invest. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L.D. Lifshitz J. Witgen B.M. Schwarzbach E. Cohen A.S. Grady M.S. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J. Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- Trouche S. Bontempi B. Roullet P. Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M.S. Khan I. Fuchs-Young R. Price S. Steininger T.L. Greene G. Wainer B.H. Rosner M.R. Localization of immunoreactive epidermal growth factor receptor in neonatal and adult rat hippocampus. Brain Res. 1993;631:65–71. doi: 10.1016/0006-8993(93)91187-w. [DOI] [PubMed] [Google Scholar]
- Wong R.W. Transgenic and knock-out mice for deciphering the roles of EGFR ligands. Cell Mol. Life Sci. 2003;60:113–118. doi: 10.1007/s000180300007. [DOI] [PMC free article] [PubMed] [Google Scholar]