Abstract
Rationale
In 2007 the American Thoracic Society (ATS) recommended guidelines for acceptability and repeatability for assessing spirometry in preschool children. The authors aim to determine the feasibility of spirometry among children in this age group performing spirometry for the first time in a busy clinical practice.
Methods
First-time spirometry for children age 4 to 5 years old was selected from the Children’s Hospital Boston Pulmonary Function Test (PFT) database. Maneuvers were deemed acceptable if (1) the flow-volume loop showed rapid rise and smooth descent; (2) the back extrapolated volume (Vbe), the volume leaked by a subject prior to the forced maneuver, was ≤80 ml and 12.5% of forced vital capacity (FVC); and (3) cessation of expiratory flow was at a point ≤10% of peak expiratory flow rate (PEFR). Repeatability was determined by another acceptable maneuver with forced expiratory volume in t seconds (FEVt) and FVC within 10% or 0.1 L of the best acceptable maneuver. Post hoc analysis compared spirometry values for those with asthma and cystic fibrosis to normative values.
Results
Two hundred and forty-eight preschool children performed spirometry for the first time between August 26, 2006, and August 25, 2008. At least one technically acceptable maneuver was found in 82.3% (n = 204) of the tests performed. Overall, 54% of children were able to perform acceptable and repeatable spirometry based on the ATS criteria. Children with asthma or cystic fibrosis did not have spirometry values that differed significantly from healthy controls. However, up to 29% of the overall cohort displayed at least one abnormal spirometry value.
Conclusions
Many preschool-aged children are able to perform technically acceptable and repeatable spirometry under normal conditions in a busy clinical setting. Spirometry may be a useful screen for abnormal lung function in this age group.
Keywords: asthma, child, cystic fibrosis preschool, spirometry
Introduction
Spirometry has been the primary mode of evaluating lung function for many pediatric and adult respiratory diseases (1, 2); however, it has historically been limited to children older than 6 years old. Performing spirometry requires concentration, cooperation with a spirometry technician, and control of breathing that has long been felt to be inadequate in preschool-aged children.
Over the past two decades, several authors have reported success in coaching preschool-aged children to perform useful spirometry (3–7). In 2007, the American Thoracic Society (ATS) and European Respiratory Society (ERS) published, for the first time, a statement on the use of pulmonary function testing (PFT) in preschool-aged children (8) that specifically addressed issues related to quality control for spirometry in this age group. It offers specific suggestions for criteria for acceptability and repeatability distinct from older children and adults in that it offers more lenient start and end of test criteria, and consider that small children may not be able to exhale for any significant length of time. Furthermore, the shape of the flow-volume curve is carefully scrutinized because children of this age group frequently display convexity of the curve. Additionally, repeatability is defined as a minimum of only one other effort with forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) within 10% or 100 ml of the best effort, as opposed three efforts within 5% or 200 ml in older children and adults.
Although several studies have shown that these young children are able to perform successful spirometry maneuvers under the rigorous conditions of a prospective clinical research study, there is little information available regarding real-time clinical use of spirometry in this population. Given the success of prospective studies, we hypothesize that the majority of preschool-aged children referred for testing in the clinical setting would be able to achieve acceptable and repeatable spirometry. We aimed to determine the feasibility of spirometry in the preschool-aged population by reviewing the spirometry performed by children aged 4 through 5 years old in our busy, clinic-based PFT laboratory under normal clinic conditions. We further aimed to explore factors that may influence the success of producing acceptable and repeatable spirometry based on the 2007 ATS/ERS criteria.
Materials and Methods
The Children’s Hospital Boston Pulmonary Function Test database (Morgan Scientific, Haverhill, MA) contains over 64,000 individual pulmonary function tests from 1986 to the present. The database was queried with the following parameters: Age at testing from 4 through 5 years old, date of test between August 26, 2006, and August 25, 2008, and measured forced vital capacity (FVC) >0.00 L, in order to limit results to performed spirometry. The database was limited to a 2-year period surrounding the release of the ATS/ERS statement on pulmonary function testing in preschool children in order to obtain a temporally relevant sampling. As such, the techniques used for obtaining spirometry in our sample were current, but not influenced by the ATS/ERS statement. In order to eliminate the learning effect of performing spirometry over multiple visits, the results were further limited to subjects without prior spirometry measures. Primary diagnosis was collected from the order form filled out by the referring physician for each patient. For the purposes of this study, several of the diagnoses were considered in aggregate because they were clinically similar, such as asthma and reactive airways disease (RAD), and cough and chronic cough. Primary diagnoses that were clinically distinct from the others and had a frequency of two cases or less were grouped as “other.”
Spirometry was performed with rolling-seal volume sensing spirometers for children without cystic fibrosis and with flow sensing spirometers (Morgan Scientific) for children with cystic fibrosis as indicated by our infection control protocol. Spirometers were calibrated daily per ATS recommendations (1). Standard spirometry instruction was given prior to efforts and each effort was coached by an experienced staff PFT technician in a dedicated pediatric PFT laboratory housed in the pulmonary clinic. Spirometry was performed in the seated position with a nose clip. Volume-incentive computer games were available and used at the discretion of the PFT technician.
Each effort in each spirometry test session was evaluated by a single investigator (J.M.G.) to determine acceptability and repeatability in reference to the 2007 ATS/ERS statement in pulmonary function testing in preschool children criteria (8). Efforts were interpreted de novo by the investigator from the raw spirometry data without knowledge of how it was clinically interpreted. Up to eight efforts per subject were evaluated. Postbronchodilator efforts were not evaluated when prebronchodilator efforts were available. Flow-volume curves were visually inspected to determine rapid rise to peak flow and smooth descending limb. Premature cessation of flow was visually assessed and manually measured if inspection was inconclusive. Back extrapolated volume (Vbe), the volume of air leaked by a subject prior to the forced expiratory maneuver, was calculated and reported by the spirometry software (Morgan Scientific).
A spirometry effort was deemed to be acceptable if it fulfilled the 2007 ATS statement on pulmonary function testing in preschool-aged children: (1) appropriate start and end of test and (2) demonstrated a rapid rise to peak flow and smooth descending limb free from significant artifact, such as glottic closure or leak. Per these criteria, appropriate start of test was defined as Vbe ≤ 80 ml and 12.5% of forced vital capacity (FVC) and a flow volume curve was deemed to have premature termination if cessation of flow occurred at greater than 10% of peak flow. Repeatability was defined as a second acceptable effort, based on the criteria above, with a forced expiratory volume in 0.5 or 1 second (FEVt) and FVC within 0.1 L or 10% of the best acceptable effort.
A post hoc analysis was performed to determine if spirometry from these subject differed from normal predicted values for healthy children of similar age. Using the prediction model proposed by Eigen et al. (4), percent predicted results for FVC, FEV1, and forced expiratory flow between 25% and 75% of FVC (FEF25–75%) were calculated for each subject. The FEV1/FVC ratio was calculated and expressed as a percent. Mean percent predicted values were further categorized by primary diagnosis for asthma and cystic fibrosis, the two most common chronic respiratory diseases in this cohort, to determine if there was deviation from normal. Symptomatic and potentially episodic diagnoses, such as cough and pneumonia, were excluded from individual analysis. Bronchopulmonary dysplasia was excluded from individual grouping due to small sample size achieving successful spirometry.
This study was approved by the Children’s Hospital Boston institutional review board.
Statistical Analysis
Descriptive statistics determined the prevalence of subjects achieving acceptable and repeatable spirometry, stratified by age. Statistical analysis was performed with SPSS version 17.0.2 (SPSS, Chicago, IL).
Univariate analysis was used to determine any significant associations between demographic variables, such as age, race, and gender, or primary clinical diagnosis and outcome of achievement of acceptable and repeatable efforts. In the absence of a disease-free group, subjects who had each primary diagnosis were compared with those without the diagnosis. Odds ratios were calculated to determine effect size and chi-square test determined statistical significance. Race was analyzed by logistic regression with White as the reference group because it had the largest sample size, lending greatest power to find an association.
A random sample of 15 spirometry efforts was independently reviewed by a second reviewer (T.R.M.) to determine internal consistency. An interrater reliability coefficient was calculated.
Percent predicted values for FVC, FEV1, and FEF25–75% were calculated for the best spirometric value for each subject completing both acceptable and repeatable spirometry by dividing the actual results by those predicted in a similar cohort described by Eigen et al. (4). Mean percent predicted values and interquartile range was reported for the group as a whole and then stratified by primary diagnosis of asthma and cystic fibrosis. The distribution of values for each parameter was displayed.
Results
Between August 2006 and August 2008, 298 children aged 4 through 5 years old performed spirometry at the Pulmonary Function Test Laboratory at Children’s Hospital Boston. Two hundred and forty-eight of them performed spirometry for the first time. Across age groups, 82.2% of the children were able to produce at least one acceptable maneuver. Of those, 66.2% were able to produce at least one other similar maneuver and be considered to have repeatable spirometry meeting the 2007 ATS/ERS criteria. Overall 54.4% of the children tested were able to perform both acceptable and repeatable spirometry.
Table 1 illustrates the demographics of the study subjects. Slightly more than half of the subjects were male. The majority of children were White, followed by small numbers of Latino and Black. Nearly three quarters of the subjects were 5 years old at the time of testing and one quarter 4 years old. The most commonly reported primary diagnosis was asthma/reactive airway disease (RAD) representing nearly half of the subjects, followed by cough/chronic cough. Twenty-one subjects (8.5%) had a diagnosis of cystic fibrosis.
Table 1.
Characteristics of the study subjects.
| Frequency (N = 248) | Percent of total | |
|---|---|---|
| Primary diagnosis | ||
| Asthma/RAD | 111 | 44.8 |
| Cough/chronic cough | 47 | 19.0 |
| Cystic fibrosis | 21 | 8.5 |
| Pneumonia | 17 | 6.9 |
| BPD | 6 | 2.4 |
| Other | 46 | 18.5 |
| Age at testing | ||
| 5 years old | 185 | 74.6 |
| 4 years old | 63 | 25.4 |
| Race | ||
| White | 206 | 83.1 |
| Latino | 20 | 7.9 |
| Black | 12 | 4.8 |
| Asian | 6 | 2.4 |
| Indian | 2 | 0.8 |
| Other | 2 | 0.8 |
| Gender | ||
| Male | 140 | 56.5 |
| Female | 108 | 43.5 |
Note. RAD = reactive airways disease; BPD = bronchopulmonary dysplasia.
Table 2 summarizes the proportion of subjects able to perform acceptable and repeatable spirometry by age group. Eighty-one percent of 5-year-old children and 84% of 4-year-old children were able to produce at least one acceptable effort by the 2007 ATS/ERS criteria. The 5-year-old children had the greatest success at repeating a similar acceptable maneuver (56%), followed by 50% of 4-year-old children. In aggregate, the overall success rate for producing acceptable, repeatable spirometry in preschool-aged children naïive to spirometry was 54%. There was substantial concordance between reviewers (agreement 87%, kappa 0.66, p = .01) (9).
Table 2.
Prevalence of successful spirometry stratified by age.
| Age at testing | Acceptability ≥ 1 acceptable effort No. (%) | Repeatability ≥ 1 repeatable effort No. (%) |
|---|---|---|
| 4 years old | 53 (84.1) | 32 (50.7) |
| 5 years old | 151 (81.6) | 103 (55.7) |
| Total | 204 (82.3) | 135 (54.4) |
Analyses of the association of each variable with successful completion of a single acceptable spirometry maneuver (Table 3) found no significant association for age, race, gender, or primary diagnosis.
Table 3.
Univariate analysis of predictors of completing successful spirometry.
| Variable | 95% confidence interval |
|||
|---|---|---|---|---|
| Odds ratio | Lower | Upper | p value | |
| Gender (male) | 0.81 | 0.49 | 1.34 | .41 |
| Age (5 vs. 4 years old) | 1.22 | 0.69 | 2.16 | .50 |
| Race | ||||
| White (reference) | ||||
| Black | 1.18 | 0.36 | 3.8 | .79 |
| Asian | 0.84 | 0.17 | 4.26 | .83 |
| Latino | 1.56 | 0.60 | 4.07 | .36 |
| Diagnosis | ||||
| BPD | 0.83 | 0.165 | 4.21 | .82 |
| Asthma | 0.75 | 0.45 | 1.24 | .26 |
| Cystic fibrosis | 0.60 | 0.24 | 1.48 | .27 |
| Pneumonia | 2.11 | 0.72 | 6.17 | .17 |
| Cough | 1.16 | 0.61 | 2.21 | .65 |
Note. BPD = bronchopulmonary dysplasia.
Table 4 summarizes the mean percent predicted values with interquartile range for each spirometry parameter in subjects fulfilling both acceptability and repeatability criteria when compared to standardized values. Asthma and cystic fibrosis were individually evaluated. The mean values for FEV1, FEF25–75%, and FEV1/FVC were similar to the normative data predicted for age and height from a cohort of healthy children (4). However, mean percent predicted for FVC tended to be lower than predicted. This was also true for subjects with primary diagnosis of asthma.
Table 4.
Mean percent predicted spirometry values stratified by primary diagnosis.
| Mean percent predicted [interquartile range] |
||||
|---|---|---|---|---|
| FVC | FEV1 | FEF25–75% | FEV1/FVCa | |
| Asthma (n = 56) | 89 [77–103] | 96 [86–110] | 96 [71–116] | 102 [98–107] |
| Cystic fibrosis (n = 9) | 94 [84–100] | 98 [85–108] | 99 [76–116] | 99 [95–106] |
| Total (n = 135) | 89 [77–101] | 95b [84–107] | 96c [73–114] | 100 [96–106] |
Note. FVC = forced vital capacity; FEV1 = forced expiratory volume in one second; FEF25–75% = forced expiratory flow between 25% and 75% of FVC.
FEV1/FVC was calculated as a ratio and transformed to a percentage.
133 children produced an FEV1.
104 children produced a value for FEF25–75%.
Figure 1 illustrates the distribution of each spirometry parameter performed by children able to successfully complete acceptable and repeatable spirometry. Notably, 29% of values for FVC, 17% of values for FEV1, and 28% of values for FEF25–75% were below 80% predicted. Fourteen percent of FEV1/FVC ratios were less than 85.
Figure 1.
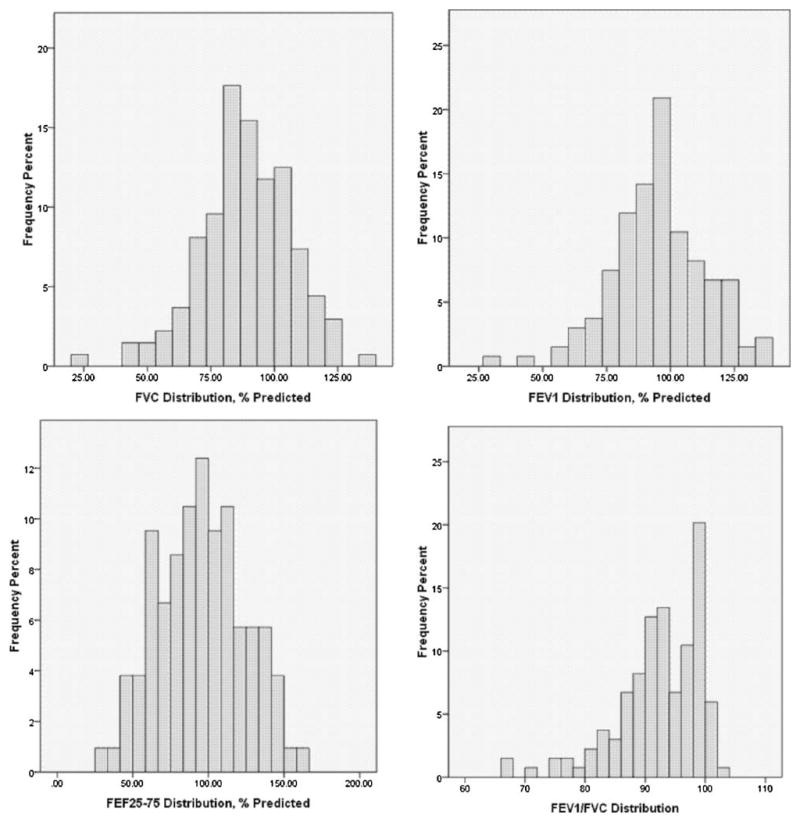
Distribution of individual variables among children able to successfully complete spirometry.
Discussion
Pulmonary function testing in the preschool-aged population has gained significant support as several carefully designed and implemented clinical studies have shown children in this age group to be able to successfully perform the test (3–8, 10–16). This is the first study to evaluate the use of spirometry in preschool children in the context of a busy, clinic-based, pulmonary function testing laboratory under normal clinical conditions. Our findings show that on the first occasion of performing spirometry, more than half of preschool-aged children are able to produce acceptable and repeatable spirometry based on the recommendations put forth in the 2007 ATS/ERS statement on the use of pulmonary function testing (PFT) in preschool-aged children. We found that over 80% of children were able to produce at least one acceptable spirometry effort, with 54% able to repeat similar efforts.
The success rate in our study is lower than many prospective studies assessing spirometry in similarly aged children, which have found acceptable and repeatable results in 65% to 92% of subjects (4, 6, 7, 10–13, 15, 17). The direct comparison is limited in that the referenced studies had varied criteria for determining acceptability and reproducibility, as there was no international guiding standard. This study adds to the important work done by Loeb et al. (5) who prospectively studied a cohort of children of all ages in light of the 2007 ATS/ERS criteria. For the 33 4- to 5-year-old children included in that study, they reported a success rate to produce acceptable and repeatable spirometry of roughly 42%. The current study greatly expands the power to determine a success rate in preschool-aged children because it draws from nearly 250 4- to 5-year-old subjects. Our overall success rate of 54% for these children is greater than that found by Loeb and colleagues and may reflect differences in sample size favoring the current study to more closely approximate the true population success rate. However, differences in the study cohorts, spirometry coaching, and subjective interpretations of spirometry by reviewers may have also played a role. Crenesse et al. (18) found similar success rates (55%) in a cohort of 355 3- to 5-year-old children who were also studied in a retrospectively designed study when using similar criteria for repeatability. In that study repeatability was defined as change less than 0.1 L or 5%, as opposed to 0.1 L or 10% in the current ATS criteria, and the acceptability criteria were directly adapted from adult guidelines (18, 19). In a prospective clinical trial, the PEAK study also reported a success rate of 56% in young children, though the criteria for acceptability and repeatability were not well described (20).
This study offers the unique perspective of spirometry performed in a busy clinic-based PFT laboratory during routine clinical conditions. These conditions differ from those offered in prospective clinical trials, which enjoy the benefit of a carefully recruited population and dedicated research staff, and they likely reflect a more accurate depiction of what a clinician may encounter. Consistent with this notion is that many prospective studies had much higher success rates, which may reflect the special conditions of the research laboratory that do not exist in routine clinical practice. Furthermore, this is the largest study to analyze spirometry in the context of the 2007 ATS/ERS statement on pulmonary function testing in preschool children.
We present the age-specific acceptability and repeatability data (Table 2) in an effort to fully illustrate the stratification of the results by age group and the effect on the success rate as a whole. Our clinical practice is such that 4-year-old children are generally referred for spirometry after a practitioner has seen the child and deemed him or her to have an adequate level of maturity and cooperation to be successful with the test. On the other hand, most 5-year-old children are routinely referred for pulmonary function testing, especially if they are known or suspected of having a chronic respiratory disease. Even if the practitioner is doubtful any useful information will be gained, spirometry referral is still made in order to introduce the test to the child and to provide some practice. As this is a retrospective study specifically looking at the real-life clinical feasibility of performing spirometry on preschool-aged children, it was impossible to avoid this degree of selection bias. However, the data collected on 5-year-old children likely reflects the true ability of children in this age group, as it is a normal age for beginning spirometry in our practice and the success rates reported here are similar to some prospective studies (10, 18). We feel that the data for the 4-year-old children is still instructive. Although the success rate for the younger children may be inflated, the sample size of 53 4-year-old children is large enough to show that it is not a rare event to find 4-year-old children who are able to perform spirometry, even if screening for the most cooperative ones. As previous studies have reported, consistent use of computer incentive programs may better elicit informative spirometry (3, 13). Furthermore, we only looked at efforts for children naïve to performing spirometry in order to consistently compare subjects. We anticipate that the success rate of performing spirometry will be greatly increased after children practice spirometry over several visits, thus the numbers we report here are likely to be a conservative estimate of the clinical utility over time.
In this study, none of the selected variables were significant predictors of successful completion of spirometry. We looked specifically at gender, race, age, and several of the most common diagnoses in this age group. In large part, this corroborates previous findings that gender, race, and primary diagnosis do not predict successful spirometry (3, 5). It has been suggested that children with neuromuscular disease are less likely to perform successful spirometry (5); however, we were unable to test this hypothesis because there was only one child in our cohort with neuromuscular disease.
Although the focus of this study was to determine feasibility of preschool-aged children to perform adequate spirometry in the clinical setting, we further attempted to determine the clinical utility of these measurements. When preschool children with asthma and cystic fibrosis were compared with the predicted spirometry values for age and height (4), most parameters of forced expiratory volume were close to 100% predicted. FVC was found to be lower than predicted, particularly for the asthmatic group; however, the distribution of values was large. It may not be surprising that this group did not have significant differences than the normative values, as children with asthma frequently have normal lung function when well. Although in older children spirometry is important in the diagnosis and management of asthma (2), it is less clear in the preschool age group (8–11). However, it is important to note that 18% of children with asthma had an FEV1 less than 80% predicted and 31% had an FEF25–75%, an important measure of small- to medium-sized airway disease, less than 80% predicted. When all children able to complete at least two acceptable and repeatable maneuvers were included, there was even greater variability and a larger proportion of children with abnormal lung function was identified. For these children, spirometry may offer important clinical information that may influence the management of the child’s respiratory disease. This type of airflow obstruction has been reported elsewhere for cohorts of children with symptomatic asthma (13) and cystic fibrosis (15, 21), corroborating spirometry’s usefulness as a diagnostic tool.
This post hoc analysis is limited by the lack of bronchial hyperresponsiveness or bronchodilator reversibility data for children diagnosed with asthma, which may have led to misclassification in some cases. Although there are some data on the feasibility of bronchoprovocation testing in preschool-aged children (22), this was not routinely done in our clinical practice during the study period. In this study, we rely on physician diagnosis as indicated by the clinician at the time of ordering the spirometry test.
In conclusion, we demonstrate that a majority of preschool-aged children naïve to spirometry are able to perform acceptable and repeatable spirometry in a busy PFT laboratory under normal clinical circumstances; however, the clinical success rate may be lower than described by previous prospective clinical trials. These findings are an important clinical correlate to the mounting evidence that spirometry is an appropriate test in this age group. Further studies are needed to address the clinical utility of spirometry in the diagnosis and management of chronic respiratory diseases, such as asthma and cystic fibrosis, in preschool-aged children.
Acknowledgments
Dr. Gaffin is supported by NIH KL2 RR025757-0 1, Harvard Clinical and Translational Science Center (KL1), and the American Thoracic Society Fellow Career Development Award, which was made possible by an educational grant from GlaxoSmithKline; Dr. Phipatanakul is funded by NIH/NIAID R-01 grant (AI-073964) and NIH/NHLBI AsthmaNet 1U10HL098102.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 2.Nair SJ, Daigle KL, DeCuir P, Lapin CD, Schramm CM. The influence of pulmonary function testing on the management of asthma in children. J Pediatr. 2005;147:797–801. doi: 10.1016/j.jpeds.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Aurora P, Stocks J, Oliver C, Saunders C, Castle R, Chaziparasidis G, Bush A. Quality control for spirometry in preschool children with and without lung disease. Am J Respir Crit Care Med. 2004;169:1152–1159. doi: 10.1164/rccm.200310-1453OC. [DOI] [PubMed] [Google Scholar]
- 4.Eigen H, Bieler H, Grant D, Christoph K, Terrill D, Heilman DK, Ambrosius WT, Tepper RS. Spirometric pulmonary function in healthy preschool children. Am J Respir Crit Care Med. 2001;163(3 Pt 1):619–623. doi: 10.1164/ajrccm.163.3.2002054. [DOI] [PubMed] [Google Scholar]
- 5.Loeb JS, Blower WC, Feldstein JF, Koch BA, Munlin AL, Hardie WD. Acceptability and repeatability of spirometry in children using updated ATS/ERS criteria. Pediatr Pulmonol. 2008;43:1020–1024. doi: 10.1002/ppul.20908. [DOI] [PubMed] [Google Scholar]
- 6.Neve V, Edme JL, Devos P, Deschildre A, Thumerelle C, Santos C, Methlin CM, Matran M, Matran R. Spirometry in 3–5-year-old children with asthma. Pediatr Pulmonol. 2006;41:735–743. doi: 10.1002/ppul.20389. [DOI] [PubMed] [Google Scholar]
- 7.Nystad W, Samuelsen SO, Nafstad P, Edvardsen E, Stensrud T, Jaakkola JJ. Feasibility of measuring lung function in preschool children. Thorax. 2002;57:1021–1027. doi: 10.1136/thorax.57.12.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 9.McGinn T, Wyer PC, Newman TB, Keitz S, Leipzig R, For GG. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic) CMAJ. 2004;171:1369–1373. doi: 10.1503/cmaj.1031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner SW, Craig LC, Harbour PJ, Forbes SH, McNeill G, Seaton A, Devereux G, Helms PJ. Spirometry in 5-year-olds—validation of current guidelines and the relation with asthma. Pediatr Pulmonol. 2007;42:1144–1151. doi: 10.1002/ppul.20709. [DOI] [PubMed] [Google Scholar]
- 11.Piccioni P, Borraccino A, Forneris MP, Migliore E, Carena C, Bignamini E, Fassio S, Cordola G, Arossa W, Bugiani M. Reference values of Forced Expiratory Volumes and pulmonary flows in 3–6 year children: a cross-sectional study. Respir Res. 2007;8:14. doi: 10.1186/1465-9921-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pesant C, Santschi M, Praud JP, Geoffroy M, Niyonsenga T, Vlachos-Mayer H. Spirometric pulmonary function in 3- to 5-year-old children. Pediatr Pulmonol. 2007;42:263–271. doi: 10.1002/ppul.20564. [DOI] [PubMed] [Google Scholar]
- 13.Vilozni D, Barak A, Efrati O, Augarten A, Springer C, Yahav Y, Bentur L. The role of computer games in measuring spirometry in healthy and “asthmatic” preschool children. Chest. 2005;128:1146–1155. doi: 10.1378/chest.128.3.1146. [DOI] [PubMed] [Google Scholar]
- 14.Kozlowska W, Aurora P, Stocks J. The use of computer-animation programs during spirometry in preschool children. Eur Respir J. 2004;23:494–495. doi: 10.1183/09031936.04.00126904. author reply 495. [DOI] [PubMed] [Google Scholar]
- 15.Marostica PJ, Weist AD, Eigen H, Angelicchio C, Christoph K, Savage J, Grant D, Tepper RS. Spirometry in 3- to 6-year-old children with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:67–71. doi: 10.1164/rccm.200111-056OC. [DOI] [PubMed] [Google Scholar]
- 16.Kanengiser S, Dozor AJ. Forced expiratory maneuvers in children aged 3 to 5 years. Pediatr Pulmonol. 1994;18:144–149. doi: 10.1002/ppul.1950180305. [DOI] [PubMed] [Google Scholar]
- 17.Arets HG, Brackel HJ, Van Der Ent CK. Forced expiratory manoeuvres in children: do they meet ATS and ERS criteria for spirometry? Eur Respir J. 2001;18:655–660. doi: 10.1183/09031936.01.00204301. [DOI] [PubMed] [Google Scholar]
- 18.Crenesse D, Berlioz M, Bourrier T, Albertini M. Spirometry in children aged 3 to 5 years: reliability of forced expiratory maneuvers. Pediatr Pulmonol. 2001;32:56–61. doi: 10.1002/ppul.1089. [DOI] [PubMed] [Google Scholar]
- 19.Kanner RE, Schenker MB, Munoz A, Speizer FE. Spirometry in children. Methodology for obtaining optimal results for clinical and epidemiologic studies. Am Rev Respir Dis. 1983;127:720–724. doi: 10.1164/arrd.1983.127.6.720. [DOI] [PubMed] [Google Scholar]
- 20.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 21.Vilozni D, Bentur L, Efrati O, Minuskin T, Barak A, Szeinberg A, et al. Spirometry in early childhood in cystic fibrosis patients. Chest. 2007;131:356–361. doi: 10.1378/chest.06-1351. [DOI] [PubMed] [Google Scholar]
- 22.Vilozni D, Livnat G, Dabbah H, Elias N, Hakim F, Bentur L. The potential use of spirometry during methacholine challenge test in young children with respiratory symptoms. Pediatr Pulmonol. 2009;44:720–727. doi: 10.1002/ppul.20978. [DOI] [PubMed] [Google Scholar]


