Abstract
Background
The increasing incidence of thyroid cancer may be an artifact of increased diagnostic scrutiny, permitting detection of smaller, sub-clinical thyroid cancers. Our objective was to examine trends in the incidence of well-differentiated thyroid cancers with large size and adverse pathologic features.
Methods
Detailed population-based analysis of incidence trends in well-differentiated thyroid carcinoma (1973–2006) in the Surveillance Epidemiology and End Results dataset, using weighted least-squares and joinpoint regression models.
Results
The incidence of WDTC in the United States has tripled since 1973 (p<0.0001). Incidence trends differ significantly between geographic regions and racial groups. Large WDTCs, including those >4cm or >6cm in size, have more than doubled in incidence (p<0.0001). Cancers with extrathyroidal extension and with cervical metastases have also more than doubled in incidence (p<0.0001).
Discussion
While the model of improving screening does explain increased diagnoses of small thyroid cancers, significant rises in the incidence of large cancers, and cancers with clinically significant pathologic adverse features, are harder to explain. Alternative hypotheses, including a true increase in cancer incidence, would seem to merit exploration.
Keywords: Incidence, screening, detection, ultrasound, papillary, differentiated thyroid cancer
INTRODUCTION
The incidence of well-differentiated thyroid cancer (WDTC) in the United States continues to increase, having more than doubled over the past three decades 1–4. It remains unclear whether there has been a true increase in the incidence of cancer, or whether this is merely an artifact of improved screening and detection of cancer.
A number of experts have argued that the rising incidence of thyroid cancer is attributable to improved screening practices – more widespread use of neck ultrasound, fine needle aspiration biopsy of small nodules, as well as more frequent “incidentalomas” found on non-thyroid ultrasound or cross-sectional imaging1,4. Several population-based analyses drawn from large cancer registries have demonstrated that the majority of the increase in thyroid cancer diagnosis is due to small papillary thyroid cancers (PTC) 1,2, with no contemporaneous change in thyroid cancer mortality. These data are consistent with the argument that advancing diagnostic technology has allowed us to detect a steadily higher proportion of a large subclinical reservoir of thyroid cancer which, in many cases, would likely have remained asymptomatic. Davies and Welch have argued that this represents “overdiagnosis”1.
However, the fact that most of the increase in incidence has come from small PTC is not wholly unexpected. At baseline, the majority of WDTC are small (<2cm) PTC, and we would therefore expect the majority of the increase in incidence to also come from such tumors. If excess cancers are solely coming from a putative reservoir of small, occult tumors, we would expect no change in the incidence of clinically significant thyroid cancers – those of large, palpable size, or with other adverse pathologic features.
Accordingly, the aim of this study was to characterize trends in well-differentiated thyroid cancer incidence over the past 30 years in greater detail, with attention to demographic and pathologic criteria. A large population-based cancer registry is an appropriate method of studying cancer incidence trends. If the model of improved screening were correct, we would expect little or no increase in the incidence of large thyroid tumors, or in the incidence of tumors with clinically significant adverse features such as metastases and extrathyroidal extension.
METHODS
Incidence rates are most accurately studied using population-based data. We performed a population-based study of incidence data representing 50,212 cases of thyroid cancer in the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database between 1973–2006. This database is considered the gold standard cancer registry, having collected clinical data since 1973, and now capturing 26% of the United States population. Quality control is an integral part of the SEER program5. Because SEER is a de-identified dataset, the NCI does not require institutional review board oversight.
In order to ensure consistency in the incidence data, analysis was limited to the original 9 SEER registries which provide continuous data from 1973. The dataset was accessed using SEER*Stat software, release 6.5.2 (2009; NCI Cancer Statistics Branch, Bethesda, MD). We identified cases originating within the thyroid gland, and used the corresponding ICD-O-3 (International Classification of Diseases for Oncology, 3rd edition6) histology codes for “papillary carcinoma of thyroid” and its variants (8050, 8052, 8130, 8260, 8340–44, 8450, 8452) and “follicular carcinoma” (8290, 8330–8332, 8335). Variants of papillary and follicular carcinoma were considered together as well-differentiated thyroid cancers (WDTC). Because of changes in World Health Organization criteria for follicular carcinoma7, which would be expected to shift diagnoses into the follicular variant of PTC category, analysis focused on WDTC as a whole, rather than on papillary and follicular carcinomas individually. Cases of medullary and anaplastic thyroid cancer were not included, as we and others have previously reported that incidence in these cancers has remained low and unchanged over the past 30 years1,8.
Incidence data was age-adjusted and further stratified by age, gender, race, geographic registry, tumor size, and presence of extrathyroidal extension and cervical metastases. Analysis of cervical metastases was limited to patients older than 45 years, because regional metastases are believed to most negatively influence outcome in this age group. The SEER Historic Stage category was not utilized as it is assigned by cancer registrars and does not represent primary data. Hispanic ethnicity has been recorded in the registry since 1992 and may be applied to patients of any race; these individuals were considered separately in the Hispanic category, and all analyses by race were limited to the 1992–2006 period.
Trends in incidence were analyzed with both weighted-least squares regression and log-linear joinpoint regression9 in Joinpoint v. 3.3.1 (2008; NCI Statistical Research and Applications Branch, Bethesda, MD), and the R statistical platform v. 2.9.1 (R Foundation for Statistical Computing, Vienna, Austria). The two-tailed level of significance was set at p<0.05.
RESULTS
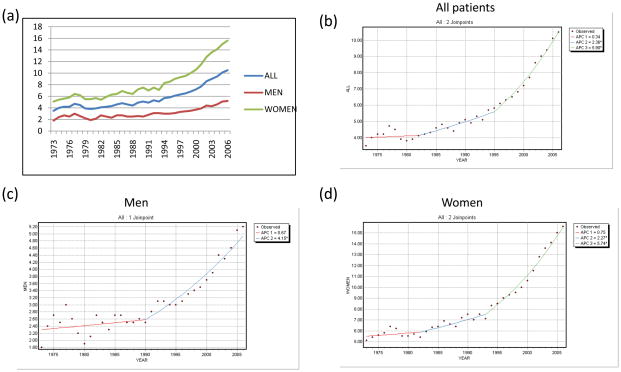
The incidence of WDTC in the United States tripled between 1973 and 2006, increasing from 3.5 per 100,000 (95%CI 3.2–3.8) to 10.5 per 100,000 (95%CI 10.1–10.9), at an annual percentage change (APC) of 3.5% per annum (p<0.0001). On joinpoint regression, three distinct slopes were apparent, representing a non-significant APC of 0.3% from 1973–1982 (p=0.66), followed by a 2.4% APC from 1982–1995 (p<0.0001), and a 5.9% APC from 1995–2006 (p<0.0001).
The APC in women was significantly higher than in men, such that the incidence of WDTC in women has more than tripled, from 5.1 per 100,000 (95%CI 4.6–5.6) in 1973 to 15.6 per 100,000 (95%CI 15–16.3) in 2006. Trends for the population as a whole, and for men and women, are detailed in Table 1 and Figure 1.
Table 1.
Incidence trends in WDTC, age-adjusted, and stratified by gender and race, from 1973–2006.
| Segment | Years | APC | 95% CI | p value |
|---|---|---|---|---|
| ALL WDTC | ||||
| 1 | 1973–1982 | 0.3 | −3.1–1.9 | 0.66 |
| 2 | 1982–1995 | 2.4 | 1.4–3.4 | <0.0001 |
| 3 | 1995–2006 | 5.9 | 4.7–7.1 | <0.0001 |
| MEN | ||||
| 1 | 1973–1990 | 0.7 | −0.4–1.7 | 0.20 |
| 2 | 1990–2006 | 4.1 | 3.0–5.3 | <0.0001 |
| WOMEN | ||||
| 1 | 1973–1982 | 0.7 | −0.5–2.0 | 0.24 |
| 2 | 1982–1993 | 2.3 | 1.2–3.4 | <0.0001 |
| 3 | 1993–2006 | 5.7 | 5.0–6.5 | <0.0001 |
| WHITE | ||||
| 1 | 1992–1998 | 4.3 | 3.3–5.3 | <0.0001 |
| 2 | 1998–2006 | 6.7 | 6.1–7.4 | <0.0001 |
| BLACK | ||||
| 1 | 1992–2006 | 4.9 | 3.7–6.0 | <0.0001 |
| HISPANIC | ||||
| 1 | 1992–2006 | 1.1 | −1.7–4.0 | 0.42 |
| ASIAN/PACIFIC ISLANDER | ||||
| 1 | 1992–2006 | 2.1 | 1.2–3.0 | <0.0001 |
“Segment” indicates joinpoint segments in the regression curve.
Numbers in bold are significant at p<0.05.
WDTC: well-differentiated thyroid cancer
APC: annual percentage change
Figure 1. Trends in the incidence of thyroid cancer in men and women.
(a) Incidence per 100,000 of WDTC in the entire population, and men and women
(b) Joinpoint regression of incidence trends in the entire population, (c) men and (d) women.
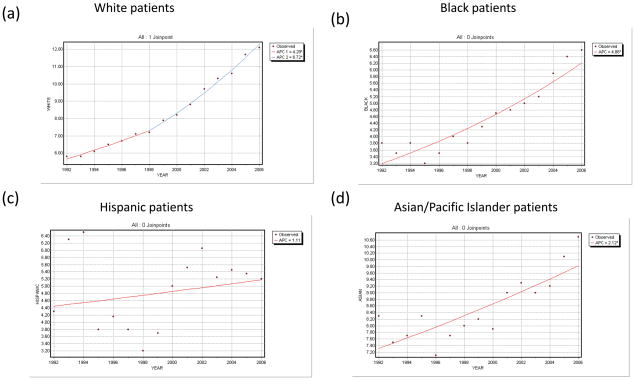
The incidence in white Americans has also more than tripled since 1973, and is currently increasing with APC of 6.7% (95%CI 6.1–7.4). Incidence has increased more slowly in black Americans (APC 4.9%, 95%CI 3.7–6.0), and yet more slowly among Asian individuals (APC 2.1%, 95%CI 1.2–3.0). Incidence has not statistically changed in the Hispanic population (APC 1.1%, 95%CI−1.7–4.0). These trends are detailed in Table 1 and Figure 2.
Figure 2. Trends in the incidence of WDTC by race.
(a) Joinpoint regression of incidence trend among (a) white, (b) black, (c) Hispanic, and (d) Asian/Pacific Islander individuals. Note that Hispanic ethnicity was not recorded prior to 1992, and that data is therefore limited to the 1992–2006 period.
Incidence has increased significantly in each SEER geographic registry, with a wide range between regions. Between 1973 and 2006, incidence in Hawaii increased 50.2% (APC 0.7%, p<0.0001), but more than quintupled in Connecticut, with an increase of 433.4% (APC 5.4%, p<0.0001).
When WDTC cases were stratified by papillary or follicular subtype, only papillary cancers demonstrated a statistically significant increase in incidence since 1973. PTC incidence has more than tripled, from 2.7 per 100,000 (95% CI 2.4–3.0) to 9.4 per 100,000 (95%CI 9.0–9.8), representing an APC of 3.9% (95%CI 3.4–4.3; p<0.0001). Follicular carcinoma has increased from 0.8 per 100,000 (95%CI 0.7–1.0) to 1.0 per 100,000 (95%CI 0.8–1.1), representing a non-significant APC of 0.3% (95%CI −0.1–0.7; p=0.10). Accordingly, nearly all (95.7%) of the increase in WDTC incidence is due to PTC. It should again be noted that the time interval includes changes in World Health Organization criteria for follicular carcinoma and follicular variant of PTC.
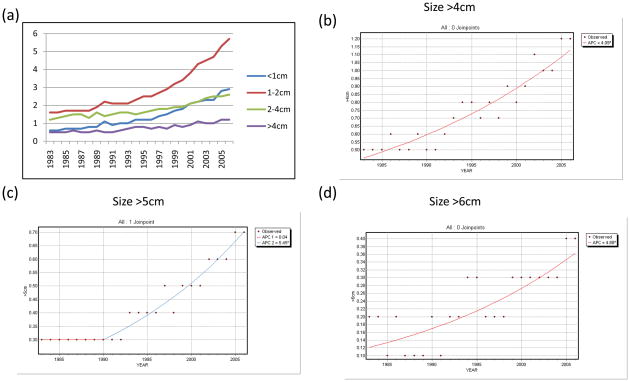
Trends in WDTC incidence was then stratified by primary tumor size, as shown in Figure 3. Size data has been collected since 1983. As expected, sub-centimeter WDTCs, which accounted for 22.3% of WDTCs during the study period, also accounted for 36.5% of the increased incidence during this time. Similarly, <2cm WDTCs accounted for 50.0% of all WDTCs during this period, and were responsible for 65.1% of the increase. Large WDTCs accounted for less of the increase in incidence. For example, >4cm tumors (making up 12.9% of WDTCs) accounted for only 11.1% of the increase in incidence since 1983.
Figure 3. Trends in the incidence of WDTC by primary tumor size.
(a) Incidence per 100,000 of WDTC stratified by size. (b) Joinpoint regression of incidence trends in cancers larger than 4cm, (c) larger than 5cm, and (d) larger than 6cm.
Nevertheless, across the board, among well-differentiated thyroid cancers of all sizes, incidence has significantly increased since 1983 (Table 2). While sub-centimeter tumors have nearly quintupled, larger tumors have also dramatically increased in incidence. The incidence of tumors of clinically palpable size (>2cm) has more than doubled, and larger thyroid cancers, those >4cm or even >6cm in size, have all more than doubled in incidence, changes which were highly significant (p<0.0001).
Table 2.
Percentage increase in incidence of WDTC, stratified by tumor size, from 1973–2006. Cancers in all size categories have increased significantly in incidence.
| Size (cm) | % Increase (1983–2006) | p value |
|---|---|---|
| 0–1 | 383 | p<0.0001 |
| 0–2 | 356 | p<0.0001 |
| 0–4 | 293 | p<0.0001 |
| 0–6 | 284 | p<0.0001 |
| 1–2 | 329 | p<0.0001 |
| 2–4 | 217 | p<0.0001 |
| >2 | 211 | p<0.0001 |
| >4 | 240 | p<0.0001 |
| >6 | 200 | p<0.0001 |
On joinpoint regression, these large cancers were seen to mainly have exhibited rising incidence in the period since 1990, with APCs of 4.9% for >4cm tumors, 5.5% for >5cm tumors, and 4.9% for >6cm tumors (all p<0.0001; Table 3, Figure 3)
Table 3.
Incidence trends in WDTC, age-adjusted, and stratified by tumor size, from 1973–2006.
| Segment | Years | APC | 95% CI | p value |
|---|---|---|---|---|
| SIZE <1cm | ||||
| 1 | 1983–2006 | 7.2 | 6.7–7.7 | <0.0001 |
| SIZE 1–2cm | ||||
| 1 | 1983–1996 | 3.7 | 3.1–4.4 | <0.0001 |
| 2 | 1996–2000 | 8.7 | 7.7–9.8 | <0.0001 |
| SIZE 2–4cm | ||||
| 1 | 1983–1995 | 1.7 | 0.8–2.7 | 0.001 |
| 2 | 1995–2006 | 4.6 | 3.5–5.7 | <0.0001 |
| SIZE >4cm | ||||
| 1 | 1983–2006 | 4.1 | 3.4–4.8 | <0.0001 |
| SIZE >5cm | ||||
| 1 | 1983–1990 | 0 | −2.7–2.8 | 0.98 |
| 2 | 1990–2006 | 5.5 | 4.6–6.3 | <0.0001 |
| SIZE >6cm | ||||
| 1 | 1983–2006 | 4.9 | 3.0–6.8 | <0.0001 |
Segments represent joinpoint segments in the regression curve.
Numbers in bold are significant at p<0.05.
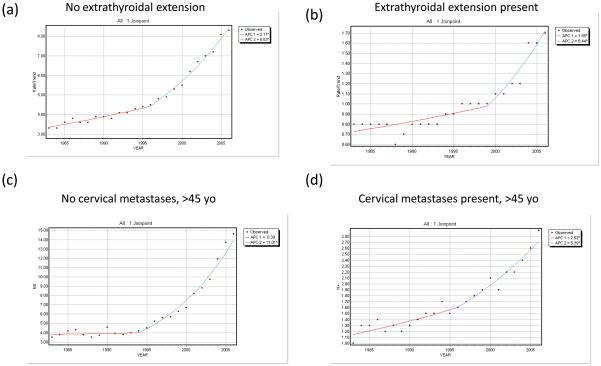
Data on extrathyroidal extension was also available from 1983–2006. The incidence of WDTCs with extrathyroidal extension more than doubled, increasing from 0.8 per 100,000 (95%CI 0.7–0.9) to 1.7 per 100,000 (95%CI 1.6–1.8). Both intrathyroidal and extrathyroidal cancers have demonstrated significant increase in incidence during this time period (p<0.0001; Figure 4a–4b).
Figure 4. Trends in the incidence of WDTC by adverse pathologic features.
(a) Joinpoint regression of incidence trends in (a) cancers without extrathyroidal extension, (b) cancers with extrathyroidal extension, (c) cancers without cervical metastases (in patients >45 years old), and (d) cancers with cervical metastases (in patients >45 years old).
Similarly, data on regional lymph node metastasis in patients older than 45 years of age was available from 1983–2006. The incidence of WDTCs presenting with cervical metastases in this age group nearly tripled, from 1.0 per 100,000 (95%CI 0.7–1.2) to 2.9 per 100,000 (95%CI 2.6–3.2), at an APC of 5.4% (p<0.0001; Figure 4c–4d). When limited to patients older than 65, a similar pattern was observed (data not shown).
DISCUSSION
To date, discussion of the dramatically rising incidence of thyroid carcinoma has centered around two possible explanations: either a true increase in the incidence of disease, or an artifact of improved screening and diagnostic activity.
Emerging evidence points toward diagnostic artifact as the main contributor. Rising incidence has been documented in several large studies in the United States and Western Europe, without a concomitant increase in mortality, which would be expected if there were truly increasing cancer incidence1–4,10–11. Because much of the increase has stemmed from small papillary thyroid cancers, the notion of “overdiagnosis” of non-lethal disease has been advanced. A large subclinical reservoir of small thyroid cancers is believed to exist, based on inferences from classical autopsy studies demonstrating occult thyroid cancers in 5–36%12–16 of cadavers. At the same time, there is evidence for significantly increasing utilization of ultrasound and fine needle aspiration biopsies, paralleling the rise in thyroid cancer incidence4,11.
However, the diagnostic artifact hypothesis remains speculative. In fact, Verkooijen et al reported a signifcant change in diagnostic practices over 30 years in Switzerland, without any appreciable change in WDTC incidence, or the incidence of subclinical and subcentimeter disease10. An alternative explanation would be that there is an increasing incidence of thyroid cancer, due to an unknown risk factor, and that current diagnostic practices have permitted early diagnosis while cancers are small, thereby keeping mortality low.
We have previously speculated that population-wide dietary, hormonal, or genetic risk factors may play some role in the changing incidence of thyroid cancer8. Iodine deficiency, one causative factor, has been eradicated in the United States, although iodine excess may be related to the development of PTC17. Increasing exposure to low-dose ionizing radiation from wider use of radiographic imaging has also been raised as a potential, although very low risk, cause of secondary malignancy18. With similar changes in ultrasound and FNA use having also occurred in Western Europe, the much higher incidence of cancer in the United States is difficult to explain. Furthermore, the wide range in thyroid cancer incidence within the United States, in different geographic regions, and in different racial groups, also seems difficult to ascribe entirely to differences in diagnostic scrutiny8.
Therefore, more detailed consideration of incidence trends may be helpful in understanding the cause of rising thyroid cancer incidence. The bulk of the rising incidence is attributable to small cancers; this is not surprising. Because most thyroid cancers are small, an across-the-board increase in incidence would mainly manifest among small tumors. Trends in the incidence of larger cancers and tumors with other adverse features have not been extensively studied.
We report significant increases in the incidence of well-differentiated thyroid cancers of palpable size (>2cm), as well as larger WDTCs (>4cm and >6cm), all of which were at least twice as common in 2006 as they were in 1983. If the rising incidence of thyroid cancer were entirely due to diagnostic practices, we would expect great increases in identification of small, sub-clinical disease, but we would not expect any change in our ability to detect large tumors which are already clinically apparent. Essentially all occult thyroid cancers in autopsy series have been <1cm, and most are smaller than 1mm12–16. The detection of large tumors does not require aggressive use of screening technologies. While some palpable cancers may have been missed in the past, it seems unlikely that most >6cm thyroid cancers were escaping identification as recently as the 1980s and 1990s.
Our size data are in partial contrast to the definitive population-based study of thyroid cancer incidence trends, by Davies and Welch1. These authors did not report increases in large thyroid cancers, or in cancers with adverse features. Methodological differences may have contributed to our divergent results. Davies and Welch reported relative risk differences for small cancers (a 2.9-fold increase), but only absolute risk differences for large cancers (0.15 per 100,000; representing a statistically significant relative change). Furthermore, we mainly observed increases in large cancers after the first decade of data (1983–1993), and our use of joinpoint regression techniques is more sensitive to interval changes in the trend line which would be diluted by simple linear regression. Our results are in agreement with a recent population-based study by Chen et al19, who also note an increasing incidence of thyroid cancers of all sizes in recent years, using a joinpoint model.
In addition, our results include significant increases in the incidence of thyroid cancers with extrathyroidal extension and neck metastases. Again, improved diagnostic identification of small, subclinical disease, does not explain the rising incidence of cancers with these adverse features, which are less likely to remain clinically occult. Extra-thyroidal extension, in particular, is a significant negative prognostic factor. While some cancers with minimal extra-thyroidal extension might remain asymptomatic, it seems likely that most of these high risk WDTCs would have come to clinical attention in the past – in an era predating widespread screening.
The finding of neck metastases is harder to interpret. It is possible that more cervical lymph nodes are being examined on ultrasound and subjected to needle biopsy, although this practice is relatively recent (incorporated into American Thyroid Association guidelines in 2006). At the same time, however, elective neck dissections are performed for clinically node-negative WDTC far less commonly than in the past20. Nevertheless, regional metastases in patients older than 45 do carry significantly negative prognosis21, and the model of detection artifact would require that half of these cases were escaping clinical detection a decade ago.
In addition, the wide range in incidence trends in different geographic regions of the United States, and in different racial/ethnic groups, is difficult to explain. Under the model of improved screening, these geographic and racial differences would require drastic differences in diagnostic practices in different parts of the country, and among different racial groups. If screening practices were entirely responsible, observed results would require that mainly white Americans have been subjected to increased screening, less so black Americans, and not Hispanic and Asian Americans. While this is possible, we are not aware of any literature supporting such a phenomenon, which seems no more likely than an environmental, dietary or genetic cause.
Racially divergent results have been reported in a cohort study of thyroid cancer incidence in Northern California: among 200,000 patients treated over 30 years in the same health care network, there was a wide range in the incidence of thyroid cancer. With white patients as the referent group, black patients had a low relative risk (0.55) and Asians, a high relative risk (2.86), of developing thyroid cancer – in a set of patients all likely to receive similar access to health care and similar thyroid screening22.
Accordingly, our results suggest that the “improved detection” hypothesis is not sufficient to explain the tripling of thyroid cancer incidence in the United States since 1973. Improved detection has undoubtedly occurred and may explain much of the increase in small well-differentiated cancers. However, this hypothesis cannot explain significant increases in large cancers, as well as those with other significant adverse features.
Other than the explanation of truly increasing WDTC incidence, there is another hypothesis which has not been extensively considered: the model of increased pathologist scrutiny. Under this hypothesis, the number of large thyroid cancers undergoing surgery would not have changed, but pathologists may be examining more sections within thyroid specimens. In large multinodular goiters, for example, this might lead to detection of more incidental PTCs, or microscopic extrathyroidal extension which was not apparent at surgery. This hypothesis would reflect increased work in sectioning and histologic sampling by pathologists, not necessarily advances in technology.
Unfortunately, there is currently no evidence to support this hypothesis, apart from an innovative study by Grodski et al which reviewed 2,260 retrosternal goiters undergoing surgery, and reported that the incidence of WDTC in the specimens doubled over four decades. At the same time, the number of blocks sampled per thyroid increased from 2.5 to 9.1, leading the authors to attribute the excess cancers to improved detection by pathologists. However, the entire increase in cancer was papillary microcarcinomas – there was no change over time in the incidence of >1cm cancers23. In any era, most >6cm thyroid nodules would be submitted to surgery, and pathologists would be unlikely to miss a cancer diagnosis.
Overall, while there is evidence consistent with presumably improved detection of occult thyroid cancer, improved detection cannot entirely explain the findings we have reported. We believe it is premature to completely ascribe the dramatically rising incidence of thyroid cancer to one cause, because such a model does not parsimoniously account for increases in the incidence of large thyroid cancers, cancers with adverse pathologic features, and widely divergent trends in different geographic regions and racial/ethnic groups. Indeed, it may not be possible to explain these complex findings with one solitary model. There are two alternate hypotheses: increased pathologist scrutiny; or a true increase in the incidence of thyroid cancer, due to unknown hormonal, dietary, genetic or environmental risk factors. Further investigation in these areas seems prudent.
SUMMARY
Although the dramatically rising incidence of well-differentiated thyroid cancer has been attributed to improved detection due to screening practices, this model does not sufficiently explain significant increases in the incidence of large well-differentiated thyroid cancers and tumors with adverse pathologic characteristics.
Footnotes
Presented in part at the 2007 Clinical Congress of the American College of Surgeons, October 7, 2007, New Orleans, LA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson NC, Button J, Solorzano CC. Thyroid cancer: is the incidence still increasing? Ann Surg Oncol. 2004;11:1093–1097. doi: 10.1245/ASO.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 3.Haselkorn T, Bernstein L, Preston-Martin S, Cozen W, Mack WJ. Descriptive epidemiology of thyroid cancer in Los Angeles County, 1972–1995. Cancer Causes Control. 2000;11:163–170. doi: 10.1023/a:1008932123830. [DOI] [PubMed] [Google Scholar]
- 4.Burgess JR, Tucker P. Incidence trends for papillary thyroid carcinoma and their correlation with thyroid surgery and thyroid fine-needle aspirate cytology. Thyroid. 2006;16:47–53. doi: 10.1089/thy.2006.16.47. [DOI] [PubMed] [Google Scholar]
- 5.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the Surveillance, Epidemiology and End Results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Percy C, Fritz A, Jack A, Shanmugurathan S, Sobin L, Parkin DM, Whelan S. International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 7.Hedinger C, Williams ED, Sobin LH. Histological typing of thyroid tumors. 2. Berlin: Springer; 1988. [Google Scholar]
- 8.Morris LG, Sikora AG, Myssiorek D, DeLacure MD. The basis of racial differences in the incidence of thyroid cancer. Ann Surg Oncol. 2008;15:1169–1176. doi: 10.1245/s10434-008-9812-6. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Verkooijen HM, Fioretta G, Pache JC, Franceschi S, Raymond L, Schubert H, Bouchardy C. Diagnostic changes as a reason for an increase in papillary thyroid cancer incidence in Geneva, Switzerland. Cancer Causes Control. 2003;14:13–17. doi: 10.1023/a:1022593923603. [DOI] [PubMed] [Google Scholar]
- 11.Leenhardt L, Bernier MO, Boin-Pineau MH, et al. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur J Endocrinol. 2004;150:133–139. doi: 10.1530/eje.0.1500133. [DOI] [PubMed] [Google Scholar]
- 12.VanderLaan W. The occurrence of carcinoma of the thyroid gland in autopsy material. N Engl J Med. 1947;237:221–222. doi: 10.1056/NEJM194708142370703. [DOI] [PubMed] [Google Scholar]
- 13.Bondeson L, Ljungberg O. Occult thyroid cancer at autopsy in Malmo, Sweden. Cancer. 1981;47:319–323. doi: 10.1002/1097-0142(19810115)47:2<319::aid-cncr2820470218>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Heitz P, Moser H, Staub JJ. Thyroid cancer: a study of 573 thyroid tumors and 161 autopsy cases observed over a thirty year period. Cancer. 1976;37:2329–2337. doi: 10.1002/1097-0142(197605)37:5<2329::aid-cncr2820370523>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Solares CA, Penalonzo MA, Xu M, Orellana E. Occult papillary thyroid carcinoma in postmortem species: prevalence at autopsy. Am J Otolaryngol. 2005;26:87–90. doi: 10.1016/j.amjoto.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid: a “normal” finding in Finland. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Belfiore A, LaRosa GL, LaPorta GA, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine uptake, sex, age and multinodularity. Am J Med. 1992;93:363–9. doi: 10.1016/0002-9343(92)90164-7. [DOI] [PubMed] [Google Scholar]
- 18.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–78. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 19.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 20.Heller KS. Do all cancers need to be treated? The role of thyroglobulin in the management of thyroid cancer. Arch Otolaryngol Head Neck Surg. 2007;133:639–43. doi: 10.1001/archotol.133.7.639. [DOI] [PubMed] [Google Scholar]
- 21.Hughes CJ, Shaha AR, Shah JP, Loree TR. Impact of lymph node metastases in differentiated carcinoma of the thyroid: a matched-pair analysis. Head Neck. 1996;18:127–132. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<127::AID-HED3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Iribarren C, Haselkorn T, Tekawa IS, Friedman GD. Cohort study of thyroid cancer in a San Francisco Bay Area population. Int J Cancer. 2001;93:745–750. doi: 10.1002/ijc.1377. [DOI] [PubMed] [Google Scholar]
- 23.Grodski S, Brown T, Sidhu S, et al. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008;144:1038–1043. doi: 10.1016/j.surg.2008.08.023. [DOI] [PubMed] [Google Scholar]