Abstract
Background
Excess mortality has declined among HIV infected patients but without evidence of a decline in patients with AIDS. We assessed temporal changes in excess mortality and elucidated risk factors for excess mortality in patients with AIDS diagnosed in the era of highly active antiretroviral therapy (HAART).
Methods
We included 1,188 patients of the Longitudinal Study of Ocular Complications in AIDS who were between 25-64 years old at enrollment and diagnosed with AIDS after 1995. We calculated excess mortality as the age-, year- and sex-adjusted difference in mortality rates between patients with AIDS and persons in the US general population, between 1999 and 2007, and used a relative survival model to identify risk factors for excess mortality.
Results
There were an average of 50 excess deaths (95% CI 44-57) per 1,000 person years between 1999 and 2007. Excess mortality almost halved with an annual decline of 8.0% per year (3.0-12.7 p=0.002) but remained high at 36 excess deaths per 1,000 person years in 2007. Viral load >400 vs. ≤400 copies/mL (risk ratio 3.4 [2.3-5.0]), CD4+ count <200 vs. ≥200 cells/μL (2.7 [1.9-3.9]) and cytomegalovirus retinitis (1.6 [1.2-2.1]) were the strongest risk factors for excess mortality.
Conclusions
Excess mortality among patients with AIDS was nearly halved in the HAART era and most strongly linked to stage of HIV disease. These results reflect the continuing improvements in AIDS management but also highlight that excess mortality remains about five times higher in patients with AIDS than in patients with HIV-infection but no AIDS.
Keywords: AIDS, mortality, Highly Active Antiretroviral Therapy, Cohort Studies
Introduction
A recent analysis from the CASCADE HIV cohorts (Concerted Action on Seroconversion to AIDS and Death in Europe) reported that excess mortality among HIV-infected persons has decreased dramatically since the introduction of highly active antiretroviral therapy (HAART).(1) Excess mortality, defined as deaths occurring in excess of the rate observed in the general population, declined from 41 per 1,000 person years in the pre-HAART era to only 6 per 1,000 person years in the years 2004 to 2006. The continued reduction in excess mortality paralleled the uptake of HAART as first line treatment.
Comparing mortality rates among HIV-infected patients to rates in the general population provides a natural reference point that indicates the impact of HIV infection upon survival at a population level and is an index of the effectiveness of antiretroviral treatment.(1-7) In addition, such comparisons may offer insights into factors that could explain the observed variability in excess mortality rates encountered among HIV-infected patients. Accounting for such variability can help formulate strategies on how to optimize HIV care.
Although HAART use has dramatically decreased death rates among persons with AIDS(6) it is unknown to what extent HAART-related decreases in excess mortality observed among HIV-infected persons exist among patients with AIDS. .. Also, although the recent analysis of the CASCADE cohorts was based on a large number of patients, covariates evaluated to assess the risks for excess mortality included age at seroconversion, sex and HIV exposure category only. Other, potentially more important correlates of mortality such as CD4+ T-cell count, cytomegalovirus (CMV) retinitis or quantitative plasma HIV RNA level (viral load) have not been explored. The Longitudinal Study of Ocular Complications in AIDS (LSOCA), funded by the National Eye Institute, is one of a few cohort studies limited to persons diagnosed with AIDS but without further exclusions.(8, 9) Our aims here are to assess the extent of decline in excess mortality over time and, to elucidate risk factors for excess mortality among patients participating in LSOCA who have been diagnosed with AIDS in the HAART era compared to persons in the general United States population.
Methods
LSOCA is a prospective observational study of patients with AIDS, who were at least 13 years of age at enrollment.(8, 9) Enrollees have AIDS diagnosed according to the 1993 Centers for Disease Control and Prevention case surveillance definition of AIDS. Patients with incident and prevalent AIDS are included. Since September 1998, recruitment has been performed at 19 clinical centers across the United States, located in urban areas with sizable HIV-infected populations. The study protocol was reviewed and approved by institutional review boards at each of the participating clinics and the coordinating center. The study is conducted in accordance with the principles of the Declaration of Helsinki. Adult patients have given written informed consent. For adolescents, a Consent Statement has been signed by parents or guardians and an Assent Statement signed by adolescents and their parents or guardians.
At enrollment, all participants provide a medical history, including information on AIDS-related illnesses and antiretroviral therapy, and undergo an ophthalmologic examination. Laboratory tests performed at enrollment and follow-up include hematology, lymphocyte subset analysis, and, in patients with a major ocular complication, serum chemistry. More detailed information about the study protocol, data forms and the study handbook is available on www.lsoca.com.
In this analysis, we included patients enrolled between January 1999 and December 2007who were diagnosed with AIDS during the HAART era (from January 1996 onwards). We did not consider the year 1998 in the analyses because recruitment had just started with too few patients enrolled (n=9) to provide a meaningful comparison to the death rates of the general population. We excluded the year 2008 from the analyses because of the yet incomplete reporting of final death data to the data coordination center. We also excluded patients younger than 25 (n=20) or older than 64 years of age (n=15) because a comparison of these age groups, that would need further stratification into calendar year and sex, against mortality rates of the general US population would be too imprecise. We decided against merging patients in these age groups with other age groups because mortality rates can change substantially from one 5-year category to the next (for example, 1,596 deaths per 100,000 persons for 60-64 year old males in 1999 versus 2479 in the 65-70 year old males).
Statistical analysis
The primary aim of the analysis was to assess declines in excess mortality in patients with AIDS enrolled in the LSOCA cohort. In an additional analysis, we assessed patient characteristics associated with excess mortality.
We defined excess mortality as the age-, year- and sex-adjusted difference in mortality rates between patients with AIDS and persons in the US general population. In a first step we calculated mortality rates for patients enrolled in LSOCA for each calendar year (1999-2007) and compared them to mortality rates of the US population adjusting for calendar year, age and sex. We retrieved the mortality rates of the US population from the Vital Statistics report of the US Center for Disease Control and Prevention. (http://www.cdc.gov/nchs/nvss.htm) For the years 2006 and 2007, for which mortality rates have not yet been released we used multiple imputation (“ice” command of STATA) to generate age- and sex-stratified mortality rates for 2006 and 2007. We based the multiple imputation on age-adjusted death rates from the Vital Statistics reports 1999 to 2005 (for men and women separately) and used weights to indicate that the death rate estimates are based on a large number of observations. We generated 100 data sets and took the medians as an estimate of the age- and sex-stratified mortality rates for 2006 and 2007.
Based on these mortality rates we compared, for each year of follow-up, the observed mortality rates (in the LSOCA cohort) with the mortality rates of the general US population, adjusting for age and sex. We considered matched individuals from the US population to be at risk until the corresponding LSOCA patient died or was censored (Ederer II method (10, 11)). In order to quantify the average extent of decline in mortality rates per year, we used Poisson regression analysis with the number of excess deaths as the dependent variable and the calendar year as independent variable and reported the average annual decline in %. In addition, we extracted the number of excess deaths, observation time and excess mortality per 1,000 person years from the publication on excess mortality in the CASCADE cohort in order to compare (declines in) excess mortality observed among AIDS (LSOCA cohort) and overall HIV-infected patients (CASCADE cohorts).
We stratified the analysis for moderate to high and for low CD4+ count (≥ or < 200 cells/μL) at enrollment, which reflects the long-term prognosis based on CD4+ counts. We conducted a number of sensitivity analyses to further assess the association of CD4+ count status (≥ or < 200 cells/μL) and change of excess mortality over time. First, we repeated the analysis excluding the years 1999 and 2000 because of the low number of enrollees in these years. Second, we considered CD4+ counts of individual patients for each year of observation following enrollment (no time lag between CD4+ measurement and year of observation) because CD4+ counts vary within individuals over time (with and without HAART). This analysis reflects the immediate association between CD4+ counts and mortality. Third, we also considered 2- and 4-year lags between CD4+ measurements and year of observation. Finally, we also planned to stratify the analysis for nadir CD4+ count before enrollment into LSOCA but the number of patients with nadir CD4+ count ≥200 cells/mm3 was too low to provide a meaningful analysis.
To assess the association of potential risk factors with excess mortality we used a Poisson regression model while offsetting the expected deaths, which adjusts for the mortality observed in the general population (background mortality).(11, 12) The model provides estimates of excess risk ratios, where the interpretation is similar to that of the risk ratio. The risk factors included age, sex, race (white, black, other), type of HIV exposure (men having sex with men, injection drug use, other), CD4+ cell count at enrollment into LSOCA (categorized into CD4+ of <200, 200-499 and ≥500 cells/μL), nadir CD4+ cell count (categorized into CD4+ of <100 and ≥100 cells/μL), plasma HIV RNA (viral load) level at enrollment (categorized into<400 and ≥400 copies/mL), CMV retinitis, time since AIDS diagnosis and calendar year of enrollment. To include all eligible patients in the analyses, we assumed that viral load was above 400 (copies/mL) in the 64 patients (5.4%) with missing data at study enrollment. We had complete data for the other variables. All analyses were conducted using STATA (STATA™ for Windows, version 10.1, Stata Corp; College Station, TX).
Results
As of 31 December 2008, there were 2,221 patients enrolled into LSOCA. We included 1,188 patients in the present analysis who were diagnosed with AIDS in the HAART era and enrolled into LSOCA between January 1999 and December 2007. We excluded patients with a diagnosis of AIDS before 1996 (n=863), enrollment in 1998 (n=9) or in 2008 (n=90), patients with a missing date of AIDS diagnosis (n=36), patients below 25 (n=20) or above 64 (n=15) years of age.
Patients were predominantly male (76.2%), of non-hispanic white (38.9%) or black ethnicity (41.4%), and diagnosed with AIDS more than a year before enrollment into LSOCA (61.6%, Table 1). There was a wide distribution of CD4+ cell counts and HIV RNA (viral load) levels and 19.6% of patients had CMV retinitis. Overall, 81.6% of patients received HAART and the proportions of patients who received HAART did not change over time; this was true for both patients who died (70.0%) or were censored (85.4%) between 1999 and 2008.
Table 1. Patient characteristics.
| Patient characteristics at enrollment | Overall (n=1188) | Vital status as of end of 2007 | |
|---|---|---|---|
| Alive (n=895) | Dead (n=293) | ||
| Age groups, n (%) | |||
| - 25-34 years | 226 (19.0) | 162 (18.1) | 64 (21.8) |
| - 35-44 years | 538 (45.3) | 404 (45.1) | 134 (45.7) |
| - 45-54 years | 337 (28.4) | 269 (30.1) | 68 (23.2) |
| - 55-64 years | 87 (7.3) | 60 (6.7) | 27 (9.2) |
| Sex, n (%) | |||
| - male | 905 (76.2) | 697 (77.9) | 208 (71.0) |
| - female | 283 (23.8) | 198 (22.1) | 85 (29.0) |
| Race/Ethnicity, n (%) | |||
| - White, non-Hispanic | 462 (38.9) | 357 (39.9) | 105 (35.8) |
| - Black, non-Hispanic | 492 (41.4) | 352 (49.3) | 140 (47.8) |
| - Hispanic | 195 (16.4) | 156 (17.4) | 39 (13.3) |
| - Other | 39 (3.3) | 30 (3.4) | 9 (3.0) |
| Time since AIDS diagnosis | |||
| - ≤ 1 year before enrollment into LSOCA (incident AIDS) | 456 (38.4) | 368 (41.1) | 88 (30.0) |
| - > 1 year before enrollment into LSOCA (prevalent AIDS) | 732 (61.6) | 527 (58.9) | 205 (70.0) |
| Reason for AIDS diagnosis# | |||
| - Clinical diagnosis | 429 (37.7) | 312 (36.6) | 117 (40.9) |
| - CD 4 count < 200 cells/μL | 710 (62.3) | 541 (63.4) | 169 (59.1) |
| HIV-exposure category, n (%) | |||
| - Men having sex with men | 495 (41.7) | 390 (43.6) | 105 (35.8) |
| - Injection drug use | 86 (7.2) | 60 (6.7) | 26 (8.9) |
| - Other | 607 (51.1) | 445 (49.7) | 162 (55.3) |
| CD 4 T-cell count (cells/μL) | |||
| - < 200 | 687 (57.8) | 455 (50.8) | 232 (79.2) |
| - 200-499 | 375 (31.6) | 330 (36.9) | 45 (15.4) |
| - ≥ 500 | 126 (10.6) | 110 (12.3) | 16 (5.5) |
| Nadir T-cell CD 4 count (cells/μL) | |||
| - < 100 | 905 (76.2) | 655 (73.2) | 250 (85.3) |
| - ≥ 100 | 283 (23.8) | 240 (26.8) | 43 (14.7) |
| HIV viral load at enrollment (copies/mL) | |||
| - < 400 | 501 (42.2) | 451 (50.4) | 50 (17.1) |
| Cytomegalovirus retinitis, n (%) | 233 (19.6) | 156 (17.5) | 77 (26.3) |
| HAART treatment, n (%) | 968 (81.6) | 763 (85.4) | 205 (70.0) |
Available for 1139 patients
Decline in excess mortality in AIDS patients from 1999 to 2007
There were 293 (24.7%) patients who died, with an average duration of follow-up of 4.67 years (SD 3.07). Over the entire study period, the mortality rate was 53.5 per 1,000 patient years (95% CI 47.7 to 60.0). The average excess mortality rate per year was 50.2 (95% CI 44.2 to 56.5) per 1000 person years. Excess mortality declined between 1999 and 2007 from approximately 70 excess deaths per 1,000 person years in 1999 through 2001 to approximately 40 excess deaths per 1,000 person years in 2006 and 2007 (Figure 1). Based on a Poisson regression model the average annual decline was 8.0% (95% CI 3.0 to 12.7 p=0.002), Excess mortality in the CASCADE HIV cohorts averaged 9.1 excess deaths per 1,000 person years between 1999 and 2005, the average annual decline in excess mortality in HIV patients enrolled in the CASCADE cohorts was 4.8% (95% CI 3.1 to 6.5, p<0.001).
Figure 1. Decline in excess mortality between 1999 and 2007.
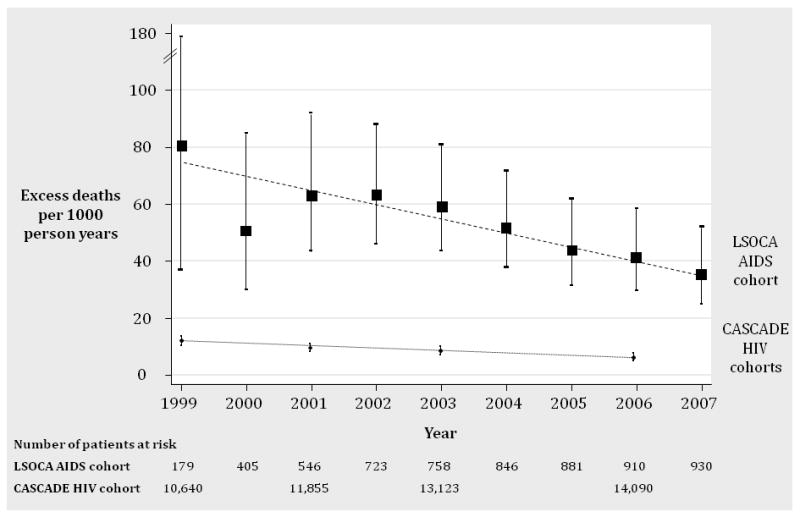
The figure shows the decline in excess mortality in the LSOCA AIDS cohort and in the CASCADE HIV cohorts between 1999 and 2007. The upper line in the graph shows the number of excess deaths per 1,000 person years (solid rectangles) in patients with AIDS enrolled in LSOCA and 95% confidence intervals. The dashed line represents the fitted line based on a Poisson regression model. The annual decline in excess mortality averaged 8.0% per year (95% CI 3.0 to 12.7, p=0.002). The lower line in the graph shows the number of excess deaths per 1,000 person years (solid circles) in HIV patients enrolled in the CASCADE cohorts and 95% confidence intervals. The dotted line represents the fitted line based on a Poisson regression model. The annual decline in excess mortality averaged 4.8% (95% CI 3.1 to 6.5, p<0.001).
For patients with low CD4+ cell count (<200 cells/ μL) the average excess mortality rate per year was 74.0 (95% CI 64.5-84.4) per 1,000 person years and in patients with moderate to high CD4+ counts (≥200 cells/ μL) it was 21.1 (95% CI 15.5 to 28.1) per 1,000 person years (Figure 2). Excess mortality declined substantially in patients with low CD4+ cell count between 1999 and 2007 (8.3% per year [95% CI 2.7 to 13.4], p=0.004) whereas there was no decline in patients with moderate to high CD4+ cell counts (1.4% [95% CI -10.7 to 15.3], p=0.83). The interaction between CD4+ count status and year was not statistically significant (p=0.16).
Figure 2. Decline in Excess Mortality Between 1999 and 2007, Stratified by CD4 Count at Enrollment.
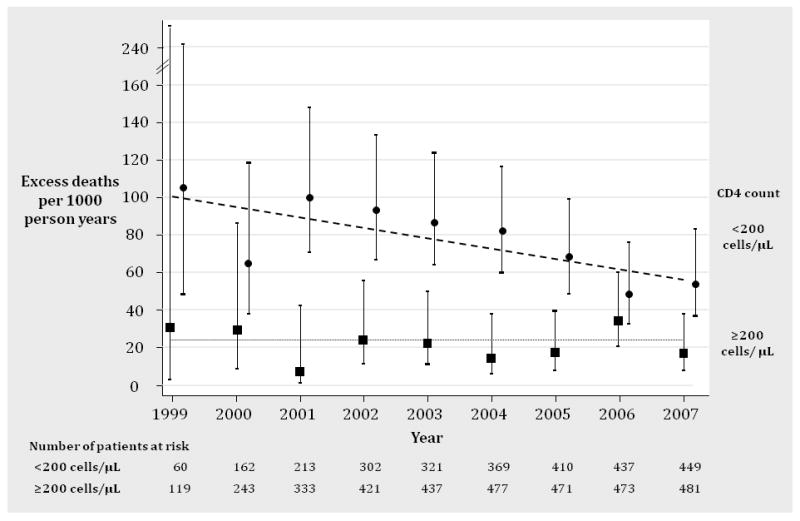
The figure shows the decline in excess mortality in the LSOCA AIDS cohort stratified for CD4+ count at study enrollment. The upper line in the graph shows the number of excess deaths per 1,000 person years (solid circles) in patients with low CD4+ counts and 95% confidence intervals. The dashed line represents the fitting line based on a Poisson regression model, which showed an average annual decline in excess mortality of 8.3% (95% CI 2.7 to 13.4, p=0.004). The lower line in the graph shows the number of excess deaths per 1,000 person years (solid rectangles) in patients with moderate to high CD4+ counts at study enrollment and 95% confidence intervals. The dotted line represents the fitting line based on a Poisson regression model. There was no change in excess mortality (1.4%, 95% CI -10.7 to 15.3, p=0.83). The interaction between CD4+ count status and year was not statistically significant (p=0.16).
In the sensitivity analysis here we only considered the years 2001 to 2007, excess mortality declined by 11.4% per year (8.3% [95% CI 4.8 to 17.5], p=0.001) in patients with low CD4+ cell count whereas there was no decline in patients with moderate to high CD4+ counts (6.1% [95% CI -9.0 to 23.8], p=0.45). The interaction between CD4+ count status and year was marginally significant (p=0.034). In additional sensitivity analyses where we considered CD4+ count for each year of observation (no time lag between CD4+ measurement and year of observation) and a 2- and 4-year lag we did not find statistically significant interactions between CD4+ count status and year (p=0.24 for no time lag, p=0.68 for 2-year time lag and p=0.18 for 4-year time lag) either that would indicate statistically significantly different declines in excess mortality between patients with low and moderate to CD4+ count over time.
Risk factors for excess mortality in AIDS patients
A high plasma HIV RNA (viral load,>400 copies/mL), and low CD4+ count (<200 cells/ μL) were the strongest independent factors (Table 2) in the relative survival model based on Poisson regression and independently associated with an approximately 3-fold increased risk for excess mortality (Table 2). Patients with CMV retinitis, no HAART, longer duration since AIDS diagnosis and early enrollment into LSOCA were also at statistically significantly higher risk for excess mortality. We did not find a statistically significant association between sex, age, race and HIV exposure category with excess mortality.
Table 2. Independent associations of patient characteristics with excess mortality.
| Patient characteristic | Comparison category | Reference category | Relative risk of excess mortality (95% CI) | p-value | |
|---|---|---|---|---|---|
| Sex | Female | Male | 1.25 (0.92-1.71) | 0.16 | |
| Age group at enrollment in years | 35-44 | 25-34 | 0.89 (0.65-1.23) | 0.49 | |
| 45-54 | 0.79 (0.54-1.15) | 0.22 | |||
| 55-64 | 1.43 (0.82-2.48) | 0.20 | |||
| Race | African-American | Other | 1.12 (0.86-1.47) | 0.41 | |
| Exposure | Injection drug use | Men having sex with men | 1.35 (0.82-2.24) | 0.24 | |
| Other exposure | 1.05 (0.77-1.44) | 0.77 | |||
| CD4+ at enrollment in cells/mL | <200 | ≥200 | 2.70 (1.87-3.89) | <0.0001 | |
| Nadir CD4+ in cells/μL * | <100 | ≥100 | 1.38 (0.92-2.07) | 0.12 | |
| HIV viral load at enrollment in copies/μL | ≥400 | <400 | 3.39 (2.28-5.03) | <0.0001 | |
| CMV retinitis at enrollment | Yes | No | 1.58 (1.19-2.10) | 0.002 | |
| Calendar year | Per year from 1999 to 2007 | 0.93 (0.87-0.99) | 0.025 | ||
| Time since AIDS diagnosis | Per year | 1.14 (1.08-1.21) | <0.0001 | ||
| HAART treatment | No HAART | HAART | 1.61 (1.23-2.12) | 0.001 | |
CD4+ categories 200-499 cells/μL and ≥500 cells/μL merged into ≥200 cells/ μL because they were similarly associated with excess deaths in the multiple Poisson regression model.
The number of excess deaths per 1,000 person years varied greatly depending upon the presence of the three strongest risk factors for increased excess mortality (plasma HIV RNA (viral load) >400 copies/mL, CD4+ cell count <200/μL and CMV retinitis, Table 3). For example, patients with none of these risks experienced 8 excess deaths per 1,000 person years (95% CI 5-16), versus 128 (95% CI 82-201) excess deaths per 1000 person years in patients with all three risk factors.
Table 3. Excess deaths per 1000 person years (95% CI) for different risk profiles at enrollment.
| Patients with CD4+ T-cells ≥200 (copies/μL) | ||
|---|---|---|
| HIV viral load | CMV retinitis | No CMV retinitis |
| >400 | 44 (25-80) | 26 (15-46) |
| ≤400 | 14 (8-26) | 8 (5-16) |
| Patients with CD4+ T-cells <200 (copies/μL) | ||
| HIV viral load | CMV retinitis | No CMV retinitis |
| >400 | 128 (82-201) | 98 (64-150) |
| ≤400 | 32 (18-55) | 23 (13-39) |
Adjusted for year, sex, age, race, exposure status, time since AIDS diagnosis, nadir CD4+ T-cell count and HAART treatment
Discussion
Among persons who initiated HAART after having been diagnosed with AIDS, excess mortality was nearly halved between 1999 and 2007 but remained high, with 36 excess deaths per 1000 person years in 2007. Excess mortality was thus, on average, more than five times higher than rates seen among HIV-infected patients in the CASCADE study.(1) The mortality rates observed in LSOCA closely reflect those of the entire US AIDS population. The Centers for Disease Control and Prevention (CDC) reported a mortality rate of 56.3 per 1,000 person years in 1999 with a decline to 30.0 per 1,000 person years in 2007 (www.cdc.gov/hiv/topics/surveillance/resources/reports). The extent of excess mortality was linked to the presence of identifiable mortality risk factors. Calendar year, time since AIDS diagnosis, higher plasma HIV RNA (viral load) levels, lower CD4+ cell counts and diagnosis with CMV retinitis were each independent risk factors for excess mortality. Depending on the presence of the latter three risks, the extent of excess mortality seen in AIDS patients ranged from 8 extra deaths per 1,000 patient years (twice as many as observed among all HIV-infected patients) to 128 extra deaths per 1,000 patient years, a level 16 times that observed among all HIV-infected patients.(1)
Previous studies have demonstrated that HIV-related mortality rates have markedly declined as a consequence of HAART introduction and prevalent use.(6, 7, 13) Results from CASCADE further demonstrated that mortality rate declines were progressive through the first decade of the HAART era despite stability in HAART use rates.(1) Potential reasons for continued declines in death rates include improvements in overall health care for HIV-infected persons, earlier identification of HIV infection (making earlier HAART initiation possible), trends toward earlier HAART use in general, more attention to patient adherence, as well as improvements in the efficacy and tolerability of specific antiretroviral therapies available. Our results suggest that, among patients with AIDS, the effects of improvements in HAART management might be even more pronounced. The decline in excess mortality that we observed is likely a consequence of progressive optimization of antiretroviral management (for example, timing of therapy initiation, specific drug combinations used) rather than an increased rate of HAART utilization overall; the proportion of patients receiving HAART did not change between 1999 and 2007.
The stratified analyses suggested a possible association of CD4+ count and excess mortality. Although this analysis was limited by the sample size and although the interaction between CD4+ count and year was not statistically significant in neither the main nor in two sensitivity analyses, it is remarkable that there was differential decline in excess mortality depending on CD4+ counts. A possible explanation that needs more evidence that provide by our study could be that further mortality reductions among patients with AIDS who had higher CD4+ counts becoming increasingly difficult, even with the use of more effective treatment, or is going to require substantially longer periods of follow-up.
The main limitation of our analysis was sample size. Our estimates of excess mortality suffer from a lack of precision as do the analyses stratified for CD4+ count. Another limitation of the present analyses is the focus on opportunistic infections of the eyes, which may be overrepresented among all opportunistic infections in LSOCA patients. We did not assess the (competing) risk of different opportunistic infections for excess mortality. There is, however, very limited evidence on the prevalence of opportunistic infections and their association with prognosis in AIDS patients from the USA. Furthermore, we did not explore the influence of additional factors upon mortality such as use of specific types of HAART because this was beyond the scope of this article. Finally, we focused on all-cause mortality as do most excess mortality analyses, which informs public health on changes in mortality at a population level and may serve as an index of the effectiveness of antiretroviral treatment. However, mortality among HAART treated patients clearly may not only be due to AIDS-related conditions, but also (and increasingly) is a consequence of co-morbid conditions (5, 18)
In conclusion, our study demonstrated that excess mortality among patients with AIDS was nearly halved in the HAART era and most strongly linked to stage of HIV disease. These results reflect the continuing improvements in AIDS management but also highlight that excess mortality remains about five times higher than in patients with HIV-infection but no AIDS.
Acknowledgments
We thank Dr. Alvaro Munoz (Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health) for his advice concerning the statistical analyses.
Financial support: LSOCA is supported by cooperative agreements from the National Eye Institute, Bethesda, Maryland, to Mount Sinai School of Medicine (grant no. U10 EY 08052), Johns Hopkins University Bloomberg School of Public Health (grant no. U10 EY 08057), and University of Wisconsin, Madison (grant no. U10 EY 08067). Additional support was provided by the National Center for Research Resources, Bethesda, Maryland, through General Clinical Research Center grants 5MO1 RR 00188 (Baylor College of Medicine), MO1 RR 00052 (Johns Hopkins University School of Medicine), M01 RR00096 (NYU School of Medicine), 5MO1 RR 05096 (Louisiana State University, Tulane, Charity Hospital), 5MO1 RR 00865 (University of California, Los Angeles), 5MO1 RR 05280 (University of Miami), 5M01 RR00046 (University of North Carolina, Chapel Hill), 5MO1 RR 00043 (University of Southern California), and 5MO1 RR 00047 (Weill Medical College of Cornell University). Support also is provided through cooperative agreements U01 AI 27674 (Louisiana State University, Tulane), U01 AI 27660 (University of California, Los Angeles), U01 AI 276670 (University of California, San Diego), U01 AI 27663 (University of California, San Francisco), U01 AI 25858 (University of North Carolina, Chapel Hill), U01 AI 25903 (Washington University at St. Louis), and U01 AI 32783 (University of Pennsylvania) from the National Institutes of Health.
Participating Clinical Centers
Baylor College of Medicine, Cullen Eye Institute, Houston, TX: Richard Alan Lewis, MD, MS (Director); John Michael Bourg; Victor Fainstein, MD; Zbigniew Krason, CRA; Joseph F. Morales, CRA; Silvia Orengo-Nania, MD; Tobias C. Samo, MD; Steven Spencer, BA, COMT; Mitchell P. Weikert, MD. Former Members: Richard C. Allen, MD; Pamela Frady, COMT; Ronald Gross, MD; Allison Schmidt, CRA; Laura Shawver, COT/CCRP; James Shigley, CRA; Benita Slight, COT; Rachel Sotuyo, COT; Stephen Travers, CRA.
Emory University Eye Center, Atlanta, GA: Sunil K. Srivastava, MD (Director); Allison Gibbs, BS; Deborah Gibbs, COMT; Debora Jordan, CRA; Bob Myles, CRA; Janna Rutter, CRA. Former Members: Antonio Capone, Jr. MD; David Furukuwa, PA; Baker Hubbard, MD; Daniel F. Martin, MD.
Indiana University, Indianapolis, IN: Former Members: Mitchell Goldman, MD (Director); Janice Brown; Thomas Ciulla, MD; Jean Craft, RN, CS; Ronald Danis, MD; Paul Fry; Hua Gao,MD; Samir Gupta, MD; Janet Hernandez, RN; Debra Poe; Linda Pratt, RN; James D. Richardson, MD; Tim Steffens, CRA; L. Joseph Wheat, MD; Beth Zwickl, RN, CS, MSN.
Johns Hopkins University School of Medicine, Baltimore, MD: J.P. Dunn, MD (Director); Diane M. Brown, RN; Dennis Cain; David Emmert; Mark Herring; Adam Jacobowitz, MD; Henry A. Leder, MD; Alison G. Livingston, RN, BSN; Yavette Morton; Kisten D. Nolan, RN, BSN, MPH; Richard D. Semba, MD, MPH; Priscilla Soto; Jennifer E. Thorne, MD, PhD. Former Members: Patricia Barditch-Crovo, MD; Marie-Lyne Bélair, MD; Stephen G. Bolton, CRNP; Joseph B. Brodine; Lisa M. Brune, RN, BSN; Anat Galor, MD; Douglas A. Jabs, MD, MBA; Meera Kapoor; Sanjay R. Kedhar, MD; John H. Kempen, MD, PhD; Stephen J. Kim, MD; Armando L. Oliver, MD; George B. Peters, III, MD; Ricardo Stevenson, MD; Michelle Tarver-Carr, MD, PhD; Susan Wittenberg, MD; Michelle Yue Wang, MD.
Louisiana State University Health Sciences Center, New Orleans, LA: Donald Bergsma, MD (Director); Rebecca Clark, MD; Robin Cooper, COMT; Jasmine Elison, MD; Butler Fuller, MD; Christine Jarrott, RN, ACRN; Lynn Otillio, COT; Maria Reinoso, MD; Christine Romero, COT, ROUB. Former Members: Bruce Barron, MD; Robin Bye, RN; Mandi Conway, MD; Larry Dillon, COT/CRA; Audrey Lombard, RN; Gholman Peyman, MD.
New Jersey Medical School, Newark, NJ: Former Members: Ronald Rescigno, MD (Director); Neelakshi Bhagat, MD; Rosa Paez-Boham, COMT; Marta Paez-Quinde.
New York Hospital - Cornell Medical Center, New York, NY: Murk-Hein Heinemann, MD (Director); Susana Coleman; Sara Daniel; Roberta Janis, RN, BSN; Aziz Khanifer, MD; Andrzej Kozbial; Diane Iglesias Rivera, COA; Kent Sepkowitz, MD. Former Members: Kenneth Boyd; Robinson V.P. Chan, MD; Cynthia Chiu, MD; Charles Cole, MD; Charles Doering, MD; Jasmine Elison, MD; Sangwoo Lee, MD; Fang Lu; Joseph Murphy; Sophia Pachydaki, MD; Christina Peroni, MD; Firas M. Rahhal, MD; Ashok Reddy, MD; Scott Warden, MD.
New York University Medical Center, New York, NY: Dorothy N. Friedberg, MD, PhD (Director); Adrienne Addessi, MA, RN; Douglas Dieterich, MD; Monica Lorenzo-Latkany, MD; Maria Pei, COA. Former Member: Alex McMeeking, MD.
Northwestern University, Chicago, IL: Alice T. Lyon, MD (Director); Lori Ackatz, RN, MPH; Manjot Gill, MD; Lori Kaminski, RN, MS; Rukshana Mirza, MD; Robert Murphy, MD; Frank Palella, MD; Carmen Ramirez; Zuzanna Rozenbajgier; Dawn Ryan; Evica Simjanoski; Former Members: Alexander Habib; Jill Koecher; Jeevan Mathura, MD; Annmarie Muñana, RN; Jonathan Shankle; David V. Weinberg, MD; James Yuhr.
Rush University, Chicago, IL: Former Members: Mathew W. MacCumber, MD, PhD (Director); Bruce Gaynes, OD, PharmD; Christina Giannoulis; Pamela Hulvey; Harold Kessler, MD; Heena S. Khan; Andrea Kopp; Pauline Merrill, MD; Frank Morini; Nada Smith; Allen Tenorio, MD; Denise Voskuil-Marre; Kisung Woo.
University of California, Irvine: Former Members: Baruch D. Kuppermann, MD, PhD (Director); Bogdan Alexandiescu, MD; Donald N. Forthal, MD; Jeff Grijalva, COT; Faisal Jehan, MD; Karen Lopez; Rosie Magallon, BA; Nader Moinfar, MD; Bret Trump; Melody Vega, COA; Randy Williams.
University of California, Los Angeles: Gary N. Holland, MD (Director); Robert D. Almanzor, COA; Margrit E. Carlson, MD; Jose T. Castellanos, COT; Jeffrey A. Craddock, COT; Serina Gonzales; Ann K. Johiro, MN, RN,BC, FNP-C, AACRN, AAHIVS; Partho S. Kalyani, MD; Michael A. Kapamajian, MD; David L. LeBeck; Kristin M. Lipka; Susan S. Ransome, MD. Former Members: Suzette A. Chafey, RN, NP; Alexander C. Charonis, MD; Peter J. Kappel, MD; Ardis A. Moe, MD; Germán Piñón; Angela Sanderson; Kayur H. Shah, MD; Robert Stalling, COA; Dennis Thayer, CRA; Jean D. Vaudaux, MD.
University of California, San Diego: William R. Freeman, MD (Director); Denise Cochran; Igor Kozak, MD; Megan Loughran; Luzandra Magana; Victoria Morrison, MD; Vivian Nguyen; Stephen Oster, MD. Former Members: Sunan Chaidhawanqual, MD; Lingyun Cheng, MD; Tom Clark; Mark Cleveland; Randall L. Gannon; Claudio Garcia, MD; Daniel Goldberg, MD; Joshua Hedaya, MD; Marietta Karavellas, MD; Tiara Kemper; Brian Kosobucki; Alona Mask; Nicole Reagan MD; Mi-Kyoung Song, MD; Francesca Torriani, MD; Dorothy Wong; Tekeena Young.
University of California, San Francisco: Jacque Duncan, MD (Director); Fermin Ballesteros, Jr.; Robert Bhisitkul, MD, PhD; Debra Brown; David Clay; Michael Deiner; Donald Eubank; Mark Jacobson, MD; Mary Lew, COT; Todd Margolis, MD, PhD. Former Members: Judith Aberg, MD; Jacqueline Hoffman; Alexander Irvine, MD; James Larson; Jody Lawrence, MD; Michael Narahara; Monique Trinidad.
University of North Carolina, Chapel Hill: Travis A. Meredith, MD (Director); Sandy Barnhart; Debra Cantrell; Seema Garg, MD, PhD; Elizabeth Hartnett, MD; Maurice B. Landers, MD; Sarah Moyer; David Wohl, MD;. Former Members: Stephanie Betran; Kelly DeBoer; David Eifrig, MD; John Foley, MD; Angela Jeffries; Jan Kylstra, MD; Barbara Longmire; Sharon Myers; Fatima N'Dure, COA; Kean T. Oh, MD; Jeremy Pantell; Susan Pedersen, RN; Cadmus Rich, MD; Cecilia A. Sotelo, RN; Charles van der Horst, MD; Samir Wadhvania.
University of Pennsylvania Medical Center, Philadelphia, PA: Charles W. Nichols, MD (Director); Mark Bardsley, BSN; Cheryl C. Devine; Jay Kostman, MD; Albert Maguire, MD; William Nyberg; Leslie Smith, RN. Former Members: Chris Helker, RN; RobRoy MacGregor, MD; Karen McGibney, RN; Keith Mickelberg, RN.
University of Southern California, Los Angeles, CA: Former Members: Jennifer I. Lim, MD (Director); Rizwan Bhatti, MD; John Canzano, MD; Thomas S. Chang, MD; Alexander Charonis, MD; Lawrence Chong, MD; Robert Equi, MD; Amani Fawzi, MD; Christina Flaxel, MD; Jesus Garcia; Todd Klesert, MD; Francoise Kramer, MD; Lori Levin, MPH; Tracy Nichols, COA, CRA; Christopher Pelzek, MD; Margaret Podilla, BS; Len Richine; Danny Romo, COA; Srinivas Sadda, MD; Richard Scartozzi, MD; Robert See, MD; Kevin Shiramizu, MD; Mark Thomas; A. Frances Walonker, CO, MPH; Alexander Walsh, MD; Ziquiang Wu, MD.
University of South Florida, Tampa, FL: Peter Reed Pavan, MD (Director); JoAnn Leto, COT; Brian Madow, MD; Richard Oehler, MD; Nandesh Patel, MD; Wyatt Saxon; Susan Sherouse, COT. Former Members: Andrew Burrows, MD; Steve Carlton; Burton Goldstein, MD; Sandra Gompf, MD; Bonnie Hernandez, COT; Mohan Iyer, MD; Patrick Kelty, MD; Amy Kramer, COT; Sharon Millard, RN, COT; Jeffrey Nadler, MD; Scott E. Paulter, MD; Jennifer Tordilla-Wadia, MD; Nancy Walker, COA.
University of Texas Medical Branch, Galveston, TX: Former Members: Garvin Davis, MD (Director); Robert Blem, MD; J. Mike Bourg, VA; John Horna, BS; Craig Kelso; Zbigniew Krason, BS; Helen K. Li, MD; Lan-Chi Nguyen, COMT; Rhonda Nolen, BS, CRC; Michelle Onarato, MD; David Paar, MD; Steven Rivas; Vicky Seitz, COT; Happy Spillar; Sami Uwaydat, MD.
Chairman's Office, Mount Sinai School of Medicine, New York, New York: Douglas A. Jabs, MD, MBA (Study Chairman); Yasmin Hilal, MHS; Melissa Nieves, BA; Karen Pascual, BBA; Jill Slutsky, MPA; Maria Stevens, CM. Former member: Judith C. Southall.
Coordinating Center, The Johns Hopkins University Bloomberg School and Public Health: Curtis L. Meinert, PhD (Director); Alka Ahuja, MS; Debra A. Amend-Libercci; Karen L. Collins; Betty J. Collison; Ryan Colvin; John Dodge; Michele Donithan, MHS; Cathleen Ewing; Kevin Frick, PhD; Janet T. Holbrook, MS, MPH, PhD; Milana R. Isaacson, BS; Rosetta M. Jackson; Hope Livingston; Lee McCaffrey, MA; Milo Puhan, MD, PhD; Girlie Reyes; Jacki Smith; Michael Smith; Elizabeth Sugar, PhD; Jennifer E. Thorne, MD, PhD; James A. Tonascia, PhD; Mark L. Van Natta, MHS; Annette Wagoner. Former members: Carley Benham; Gregory Foster; Judith Harle; Adele M. Kaplan Gilpin, JD, PhD; John H. Kempen, MD, PhD; Barbara K. Martin, PhD; Nancy Min, MPH, PhD; Laurel Murrow, MS; Maria J. Oziemkowska, MS, MPH; Wai Ping Ng, BS; Pamela E. Scott, MA; Erica Smothers; Emily West; Claudine Woo, MPH; Albert Wu, MD, MPH; Alice Zong.
Fundus Photograph Reading Center, University of Wisconsin: Ronald Danis, MD (Director); Charles Chandler; Sapna Gangaputra, MD, MPH; Gregory Guilfoil; Larry Hubbard, MAT; Jeffrey Joyce; Thomas Pauli; Nancy Robinson; Dennis Thayer; Jeong Won Pak; Grace Zhang. Former members: Michael Altaweel, MD; Jane Armstrong; Matthew D. Davis, MD; Sheri Glaeser; Katrina Hughes; Dolores Hurlburt; Linda Kastorff; Michael Neider, BA; Therese Traut; Marilyn Vanderhoof-Young; Hugh Wabers;.
National Eye Institute, Bethesda, MD: Natalie Kurinij, PhD.
Officers of the Study: Douglas A. Jabs, MD, MBA (Chair); Ronald Danis, MD; Natalie Kurinij, PhD; Curtis L. Meinert, PhD; Jennifer E. Thorne, MD, PhD. Former Members: Matthew D. Davis, MD; Janet T. Holbrook, MS, MPH, PhD.
Steering Committee: Douglas A. Jabs, MD, MBA (Chair); Ronald Danis, MD; James P. Dunn, MD; Gary N. Holland, MD; Milana R. Isaccson, BS; Mark Jacobson, MD; Natalie Kurinij, PhD; Richard Lewis, MD, MS; Kisten D. Nolan, RN, BSN, MPH; Curtis L. Meinert, PhD; William Nyberg; Frank Palella, MD; Jennifer E. Thorne, MD, PhD. Former Members: Adrienne Addessi, MA, RN; Lisa Brune, RN, BSN; Rebecca Clark, MD; Tom Clark, CRA; Janet Davis, MD; Matthew D. Davis, MD; William R. Freeman, MD; Dorothy Friedberg, MD; James Gilman; Janet T. Holbrook, MS, MPH, PhD; John Horna; Larry Hubbard, MAT; Mark Jacobson, MD; Daniel F. Martin, MD; Travis A. Meredith, MD; Annmarie Muñana, RN; Robert Murphy, MD; P. Reed Pavan, MD; Steven Spencer, BA, COMT; Tim Steffens, CRA; Dennis Thayer; Charles van der Horst, MD; Fran Wallach.
Policy and Data Monitoring Board: John P. Phair, MD (Chair); Brian P. Conway, MD; Barry R. Davis, MD, PhD; Douglas A. Jabs, MD, MBA; Natalie Kurinij, PhD; Curtis L. Meinert, PhD; David Musch, PhD; Robert B. Nussenblatt, MD; Jennifer E. Thorne, MD, PhD; Richard Whitley, MD. Former Members: B. William Brown, Jr., PhD; Matthew D. Davis, MD; James Grizzle, PhD; Argye Hillis, PhD; Janet T. Holbrook, MS, MPH, PhD; Harmon Smith, PhD; James A. Tonascia, PhD.
Visual Function Quality Assurance Committee: Steven Spencer, BA, COMT (Chair); Robert D. Almanzor; Deborah Gibbs, COMT; Milana Isaacson, BS; Mary Lew, COT; Richard Alan Lewis, MD, MS (Advisor);. Former Members: Ferman Ballesteros; Jeff Grijalva, COT; Karen Lopez; Laura G. Neisser, COT; Rosa Paez-Boham, COST.
Footnotes
Potential conflicts of interest: All authors: no conflicts.
References
- 1.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008 Jul 2;300(1):51–9. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Ewings FM, Bhaskaran K, McLean K, Hawkins D, Fisher M, Fidler S, et al. Survival following HIV infection of a cohort followed up from seroconversion in the UK. AIDS. 2008 Jan 2;22(1):89–95. doi: 10.1097/QAD.0b013e3282f3915e. [DOI] [PubMed] [Google Scholar]
- 3.Jaggy C, von Overbeck J, Ledergerber B, Schwarz C, Egger M, Rickenbach M, et al. Mortality in the Swiss HIV Cohort Study (SHCS) and the Swiss general population. Lancet. 2003 Sep 13;362(9387):877–8. doi: 10.1016/S0140-6736(03)14307-3. [DOI] [PubMed] [Google Scholar]
- 4.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007 Jan 16;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 5.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006 Sep;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 6.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 7.Smit C, Geskus R, Uitenbroek D, Mulder D, Van Den Hoek A, Coutinho RA, et al. Declining AIDS mortality in Amsterdam: contributions of declining HIV incidence and effective therapy. Epidemiology. 2004 Sep;15(5):536–42. doi: 10.1097/01.ede.0000135171.07103.f0. [DOI] [PubMed] [Google Scholar]
- 8.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007 Apr;114(4):780–6. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007 Apr;114(4):787–93. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 10.Brenner H, Hakulinen T. On crude and age-adjusted relative survival rates. J Clin Epidemiol. 2003 Dec;56(12):1185–91. doi: 10.1016/s0895-4356(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 11.Dickman PC. Modeling of the cure fraction in survival studies. STATA Journal. 2007;7(3):1–25. [Google Scholar]
- 12.Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. 2004 Jan 15;23(1):51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002 Jul 13;360(9327):119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 14.Late HIV testing - 34 states, 1996-2005. MMWR Morb Mortal Wkly Rep. 2009 Jun 26;58(24):661–5. [PubMed] [Google Scholar]
- 15.Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, et al. Survival benefit of initiating antiretroviral therapy in HIV-infected persons in different CD4+ cell strata. Ann Intern Med. 2003 Apr 15;138(8):620–6. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009 Apr 30;360(18):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HIV prevalence estimates--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008 Oct 3;57(39):1073–6. [PubMed] [Google Scholar]
- 18.Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d'Arminio Monforte A, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003 May 23;17(8):1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]


