Abstract
BACKGROUND
Slug is a transcription factor of the Snail/Slug zinc-finger family and is implicated in metastasis of tumors, but its role in cell proliferation of prostate cancers is unclear.
METHODS
Expression level of Slug and other genes was examined by Western blot, RT-PCR, and QPCR analyses. The forced expression of Slug was mediated by retroviruses and adenoviruses. Slug was down-regulated by shRNA. Cell growth was measured by the MTT assay and the quick cell proliferation assay.
RESULTS
Here, we demonstrated that Slug expression is elevated in mouse prostate tumors, and human prostate cancer cell lines LNCaP, PC-3, and 22RV1. Forced expression of Slug inhibited proliferation of prostate cancer cells PC-3 and DU-145. Conversely, reduced expression of Slug by shRNA promoted growth of PC-3 cancer cells. Consistent with these data, we found that forced expression of Slug in prostate cancer cells led to G1 cell cycle arrest. Furthermore, ectopic expression of Slug decreased cyclin D1 expression in both PC-3 and DU-145 cells, and knockdown of Slug by shRNA upregulated cyclin D1 expression in these cancer cells. In addition, we demonstrated that ectopic expression of cyclin D1 relieved Slug-mediated inhibition of proliferation of prostate cancer cells.
CONCLUSIONS
We provide the first compelling evidence that Slug is a negative regulator of proliferation of prostate cancer cells. Our findings in this study are distinct from the previously reported role of Slug as a promoter for tumor metastasis, and suggest that Slug is a prognostic marker and potential therapeutic target.
Keywords: Slug, prostate cancer, cell growth
Introduction
Prostate cancer is a leading cause of illness and death among men in the United States and Western Europe, and is the fourth most common male malignancy worldwide (1). Incidence and mortality rates vary tremendously among countries, similar to the variations seen among distinct ethnic groups in the United States. Prostate cancer is predominantly a disease of elderly men, with more than 75% of new prostate cancers being diagnosed in men older than 65 years. Recently, the standard therapies include androgen ablation that initially causes tumor regression. However, cancer cells eventually recur and develop into hormone refractory prostate cancer (2,3). New strategies developed to treat patients with advanced prostate cancer require a thorough understanding of the molecular mechanisms that regulate prostate cancer development and progression.
The androgen receptor (AR) is the key determinant in the development and progression of prostate cancer. AR is expressed in almost all prostate cancer regardless of their hormone sensitivity (4). Activation of AR stimulates proliferation of prostate cancer cells (5). In contrast, inactivation of AR prevents prostate cancer cells from replicating DNA and entering the S phase, and induces cell death (6,7). Although AR plays a central role in regulation of the proliferation of prostate cancer, other factors such as Pten, Sox9, and GSK3β are also critical for tumorigenesis of prostate cancers (8–10). Key transcription factors that regulate growth of prostate cancers remain to be identified.
Slug is a zinc-finger transcription factor of the Slug/Snail superfamily. All members of this family share similar structure and the same binding motif (AGAGGTG), and are involved in the ingression of the early mesodermal cells at gastrulation, and in the delamination of the neural crest from the neural tube during development (11,12). We previously demonstrated that Slug protects mice from γ-radiation-induced death in a cell autonomous manner by inhibiting the mitochondria-dependent apoptotic pathway in myeloid progenitor cells, thereby allowing early hematopoietic progenitors to evade lethal effects of genotoxic agents (13). In addition to its antiapoptotic function, Slug and Snail play important roles in the epithelial-mesenchymal transition of mesodermal cells. Epithelial-mesenchymal transition is a critical process during morphogenesis of multi-cellular organisms (14,15). Epithelial-mesenchymal transition is not only a normal developmental process but is also essential for tumor invasion and metastasis (16–20).
Mounting evidence indicates that elevated expression Slug or Snail promotes cancer cells to become motile and invasive, by down-regulating epithelial markers (cytokeratin-18, Muc-1, desmoplakin, E-cadherin) and up-regulating mesenchymal markers (fibronectin, vimentin, Rho GTPases) (14,15,19,21). Elevated expression of Slug and Snail is associated with (i) postoperative relapse, (ii) shorter patient survival, (iii) reduced E-cadherin expression, and (iv) lymph node metastasis of a variety of epithelial tumors, such as breast cancer, lung cancer, esophageal squamous cell carcinoma, gastric cancer, colorectal cancer, pancreatic cancer, ovarian carcinoma, and hepatocellular carcinoma (22–29).
Snail/Slug family members also regulate cell-cycle progression and survival. Snail1 regulates components of the early to late G1 transition and G1/S checkpoint, including the repression of cyclin D2 transcription and the increase in p21 (30). However, it remains to be determined whether or not Slug alters cell cycle and proliferation of prostate cancer cells.
In this study, we determined that Slug expression is significantly up-regulated in prostate tumor tissues from the transgenic adenocarcinoma mouse prostate (TRAMP) model (31) and human prostate cancer cell lines (LNCaP, PC-3, 22RV1). Ectopic expression of Slug in PC-3 and DU-145 cancer cells inhibited growth of these cancer cells and resulted in G1 cell cycle arrest. By gain-of-function and loss-of-function approaches, we determined that Slug inhibited proliferation of prostate cancer cells via downregulation of cyclin D1 expression. Together, our data strongly suggest that Slug may represent a prognostic marker and a potential therapeutic target of this highly aggressive tumor.
Materials and Methods
Cell culture
PC-3, 22RV1, LNCaP, and DU-145 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). These cells were maintained in culture medium, according to the manufacturer’s instructions.
Plasmids
The pMIGw-cyclin D1-HA and the pMIGw-Cylin D1-HA T286A were constructed by subcloning a 1,151-bp XhoI-BamHI fragment from pcDNA cyclin D1 HA and pcDNA cyclin D1 HA T286A (32) respectively, into pMIGw vector. pLKO.1-Slug shRNA1 (target sequence- 5’ CAGCTGTAAATACTGTGACAA3’) and pLKO.1-Slug shRNA2 (target sequence- 5’ GCCAAATCATTTCAACTGAAA’) were obtained as a set (RHS4533-MN_003068) from Open Biosystem (Huntsville, AL). pLKO.1-Slug shRNA3 (target sequence- 5’CAGACCCAT TCTGAT GTAAAG, Addgene plasmid #10905) (33) or pLKO.1-control shRNA (containing non-target scramble shRNA, Addgene plasmid #1864) (34) were purchased from Addgene (Cambridge, MA).
Generation of chicken anti-Slug antibody
The chicken anti-Slug antibody was custom made using a short peptide (LQ[S/P]KLSDPHAIEAEK) and purified by Aves Labs (Tigard, OR).
SDS-PAGE and Western blot analysis
Mouse prostate cancer tissues were dissected from TRAMP mice. Normal mouse prostate tissue was cut into pieces and washed three times in 1×PBS (phosphate-buffered saline, pH 7.4). Tumor tissue and cells were lysed in the RIPA buffer (50 mM Tris-Hcl at PH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM EDTA, 1 mM EGTA, 1 mM beta glycerophosphate, 1mM sodium orthovanadate), supplemented with 1 ml protease inhibiter cocktail (Sigma, St. Louis, MO). Protein samples were analyzed by Western blot analysis using a ECL kit (Pierce, Rockford, IL) with antibodies against following antigens: Slug, cyclin D1 (ANASPEC, Fremont, CA), HA-tag (Bethyl Laboratories, Montgomery, TX), α-tublin (Santa Cruz Inc, Santa Cruz, CA) and β-actin (Bethyl Laboratories, Montgomery, TX).
Viral production and infection
293T cells were seeded at 1 × 105 cells per 35 mm dish. The next day, a mixture of plasmid DNA was transfected separately into 293T cells using FuGENE 6 transfection reagent (Roche, Indianapolis, IN). For retrovirus production, pEco (packaging plasmid) was mixed with pMIGR1-based retroviral vectors for expression of Slug, cyclin D1-HA, cylin D1-HA T286A, or their control. For generation of lentiviruses, the packaging plasmids (pCMV-VSVG and psPAX2) were co-transfected with pLKO.1-Slug shRNA or pLKO.1-control shRNA (containing non-target shRNA) into 293T cells. For viral infection, PC-3 or DU-145 cells were seeded at 50% confluence in 12-well plates. The next day, virus-containing supernatants from 293T cultures were mixed with polybrene (Sigma, St. Louis, MO) at final concentration of 8 µg/ml, and added to the cells in each well. The plate was centrifuged at 2000 rpm for one hour at 35°C, and subsequently was returned to the cell culture incubator. PC-3 cells infected with pLKO.1 lentiviruses were selected with puromycin (1 ug/ml), starting at 48 hours after infection.
RNA isolation, reverse transcription, RT- PCR, and QPCR analysis
RNA of the cultured cells was prepared using the Trizol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was carried out using random primers of the SuperScript III First-Strand Synthesis SuperMix kit (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocols. Primers used for the RT-PCR and QPCR analysis were synthesized by Integrated DNA Technologies (Coralville, IA). QPCR was performed on a Bio-Rad MyiQ system using the HotstarTaq DNA polymerase kit (QIAGEN, Valencia, CA), according to the manufacturer’s protocols. Data were analyzed by using the comparative CT method (35). Quantities of gene-specific mRNA expression were determined by the CT method: CT refers to the “threshold cycle,” and is determined for each experiment using MyiQ software. Amplification of GAPDH was performed for each reverse-transcribed sample as an endogenous quantification standard. The fold-difference in gene expression was determined by 2− ΔΔCT. ΔΔCT is equal to (ΔCT of experimental conditions − ΔCT of control conditions). ΔCT is equal to (gene-specific CT − GAPDH CT). The primers are as following: Snail1, 5’-AATACTGCAACAAGGAATACCTCAGCCTGG-3’ and 5’-GGACAGGAGAAGGGCTTCTCGCCAGTGTG-3’; Snail2/Slug, 5’-CTTCCTGGTCAAGAAGCA-3’ and 5’-GGGAAATAATCACTGTATGTGTG-3’; Snail3, 5’ GCTGGAGACGCAGAGAGAAATCAATGGTGC-3’ and 5’-CCAGAGCTTCCTCGATCCGTGGCAGGAGG-3’; Scratch1, 5’-CGCGCCACTGCACGATAAAGGGTACCTC-3’ and 5’-GTCGCCGTTGATGTAGCCGCCCGCAGC-3’; Scratch2, 5’-GCCTCCCGGGGACAACGGGTACGC-3’ and 5’-GCGTCCATGGAGTAGCTGTCGGTCACTG-3’; cyclin D1, 5’-TCGCCACCTGGATGCTGGAGGTCTG-3’ and 5’-CACCAGGAGCAGCTCCATTTGCAGCAG-3’; GAPDH, 5’-ATTGACCTCAACTACATGGTTTACATG-3’ and 5’-TTGGAGGGATCTCGCTCCTGGAAG-3’.
Analysis of cell cycle by flow cytometry
The cells were harvested from the dishes, fixed in 70% ethanol (Sigma) on ice for 30 minutes. The fixed cells were resuspended in 100 µl 1×PBS supplemented with 0.05 mg/ml propidium iodide, 0.1 mg/ml RNase A, and 0.05% Triton X−100, followed by incubation for 40 minutes at 37°C. The treated cells were washed two times and resuspended in 500 µl PBS. The cells were analyzed for their DNA content on a BD FACSCalibur. Only signals from the single cells were included in the analysis (10,000 cells/assay).
MTT assay
The cells were seeded at 1000 to 5000 cells per well (triplicates) in 48-well tissue culture plates. 100 µl MTT (5 mg/ml in culture medium) was added to each well. The cells were incubated for four hours at 37°C to allow the MTT to be metabolized. The cells were washed twice in 1×PBS, followed by the addition of 100 µl DMSO to dissolve formazan crystals. Absorbance of the dye solutions was measured at 492 nm on a multi-well spectrophotometer (Power Wave×340, Bio-Tek).
Quick cell proliferation assay
The cells were seeded at 1000–5000 cells per well (triplicates) in 48-well tissue culture plates. For measuring cell proliferation, 100 µl growth medium supplemented with 10 µl water soluble tetrazolium (WST, BioVision, Mountain View, CA) was added to the cells in each well. The cells were incubated for 2.5 hours at 37°C to allow the WST to be metabolized. Absorbance of the dye solutions was measured at 492 nm on a multi-well spectrophotometer (Power Wave×340, Bio-Tek).
Statistical Analysis
QPCR data and cell growth data were analyzed by the Student's t-test (one-tailed). P < 0.05 was used to define statistically significant differences.
RESULTS
Slug protein expression is elevated in prostate gland tumors
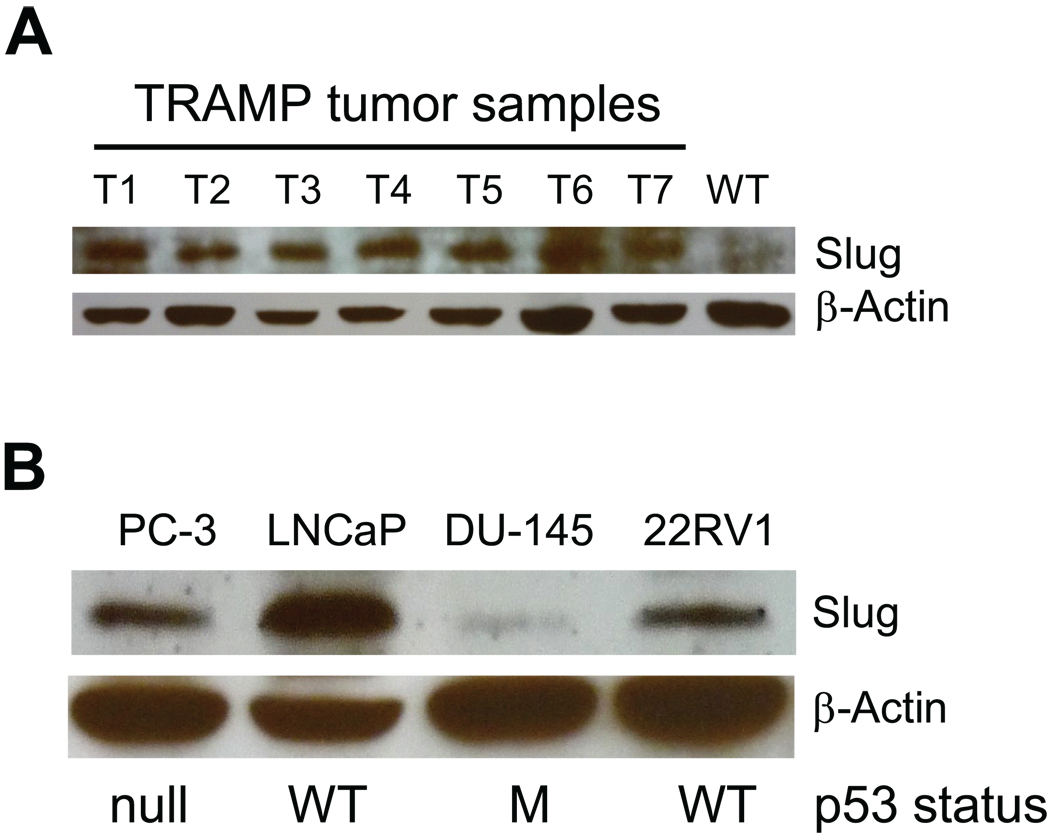
Slug is implicated in tumor metastasis of multiple tumors, including mammary gland tumors, ovarian cancer, and lung adenocarcinoma. To facilitate analysis of Slug protein expression, we validated specificity of the chicken anti-Slug antibody (Supplementary Fig. S1). To determine whether Slug plays a role in regulation of prostate tumor cells, we examined Slug expression by Western blot analysis in prostate tumors from mice harboring the transgenic adenocarcinoma of the mouse prostate (TRAMP) (31). Our data indicated that Slug protein was highly expressed in tumor samples but not in normal prostate tissue (Fig. 1A). In addition, we studied Slug expression in human prostate cancer cell lines and found high Slug protein levels in PC-3, LNCaP, and 22RV1 cell lines. In contrast, Slug expression was minimal in the DU-145 cell line. Together, these data suggest that Slug may play a role in regulation of prostate cancer. In contrast to a recent report (36), Slug protein expression level was not inversely correlated with p53 status.
Fig. 1. Examination of Slug protein expression in prostate cancer.
A: Western blot analysis of Slug protein in prostate tumors from the TRAMP mouse model. Prostate tumors were dissected from 21-week-old TRAMP mice (carrying large T-antigen), and all proteins extracted from these tumor samples were immunoblotted using anti-Slug antibody. Normal prostates (NP) were isolated from 21-week-old wild-type mice. β-actin was the loading control.
B: Western blot analysis of Slug protein in human prostate cancer cell lines. Proteins were extracted from human cancer cell lines (PC-3, LNCaP, DU-145, and 22RV1) and analyzed by Western blot using anti-Slug antibody. β-actin was the loading control. p53 status in these cell lines: null, p53−/−; WT, wild-type p53; M, mutated p53.
Slug is a member of the Snail/Slug superfamily selectively expressed in human prostate cancer cell lines
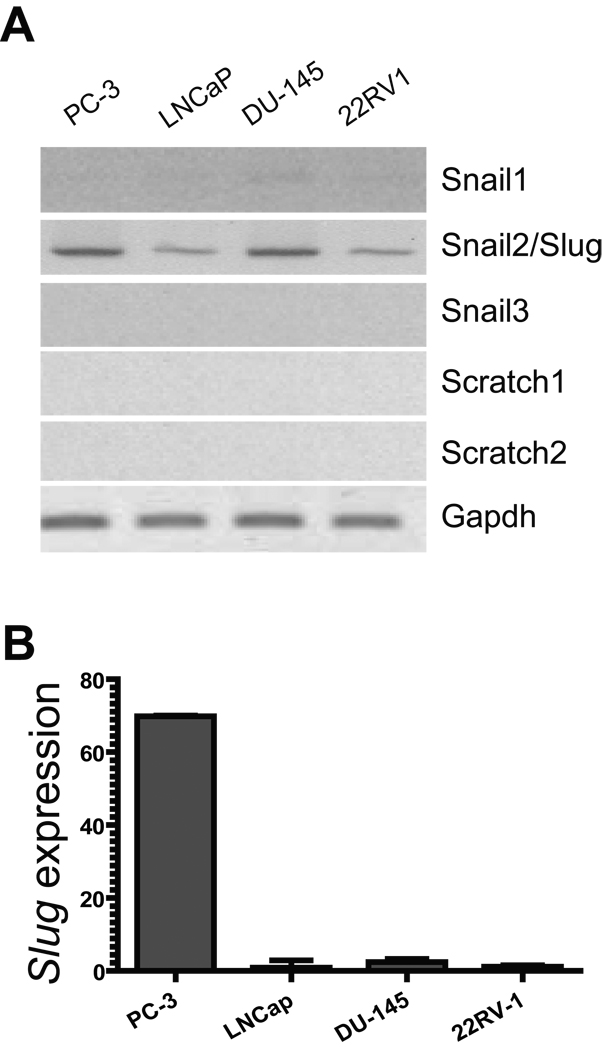
Slug belongs to Slug/Snail superfamily of the zinc finger transcription factors, which has five members. It is possible that each of these members is differentially expressed in prostate tumors, thus has distinct role in regulation of tumor growth. By RT-PCR (Fig. 2A) and QPCR (Fig. 2B), we analyzed RNA transcripts of the Slug/Snail superfamily members, including Snail1, Snail2/ Slug, Snail3, Scratch1, and Scratch2 in the four human prostate cancer cell lines (PC-3, LNCaP, DU-145, and 22RV1). Among the five Slug/Snail superfamily members, only Slug transcripts were prominent in prostate cancer cell lines PC-3 and DU-145, and became evident in the other two cell lines (LNCap and 22RV1) when the number of PCR cycle increased from 27 to 35 (Fig. 2). Other members of this family were expressed at a minimum level in these cell lines. Interestingly, although Slug protein is expressed in LNCaP, PC-3, and 22RV1 cell lines (Fig. 1B), its transcripts were only highly expressed in PC-3 cells. Collectively, our data suggest that Slug, as a Slug/Snail family member, was selectively expressed in human prostate cancer cell lines; its expression may be subject to regulation at the transcriptional or post-translational level. Slug may play a role in regulation of cell growth of human prostate cancer cells.
Fig. 2. RNA transcript levels of Slug/Snail family members in human prostate cancer cell lines.
A: RT-PCR analysis of RNA transcripts of Slug/Snail family members. RNA was extracted from PC-3, LNCaP, DU-145, and 22RV1 prostate cancer cell lines and subjected to cDNA synthesis. mRNA transcript level of each Slug/Snail family member in these cell lines was analyzed by PCR using primers specific for Snail1, Snail2/Slug, Snail3, Scratch1, and Scratch2. The number of PCR cycle is 35 for Snail2/Slug and 27 for the other members. Gapdh was included as a loading control. Only Snail2/Slug was highly expressed in human prostate cancer cell lines (PC-3 and DU-145).
B: QPCR analysis of Slug transcript in human prostate cancer cell lines. RNA was extracted from PC-3, LNCaP, DU-145, and 22RV1 prostate cancer cell lines, and. Slug transcript level was analyzed by QPCR. Slug transcript level is greater in PC-3 versus LNCaP, DU-145, and 22RV1 lines.
Forced expression of Slug suppresses cell growth of prostate cancer cell lines and resulted in G1-phase cell cycle arrest
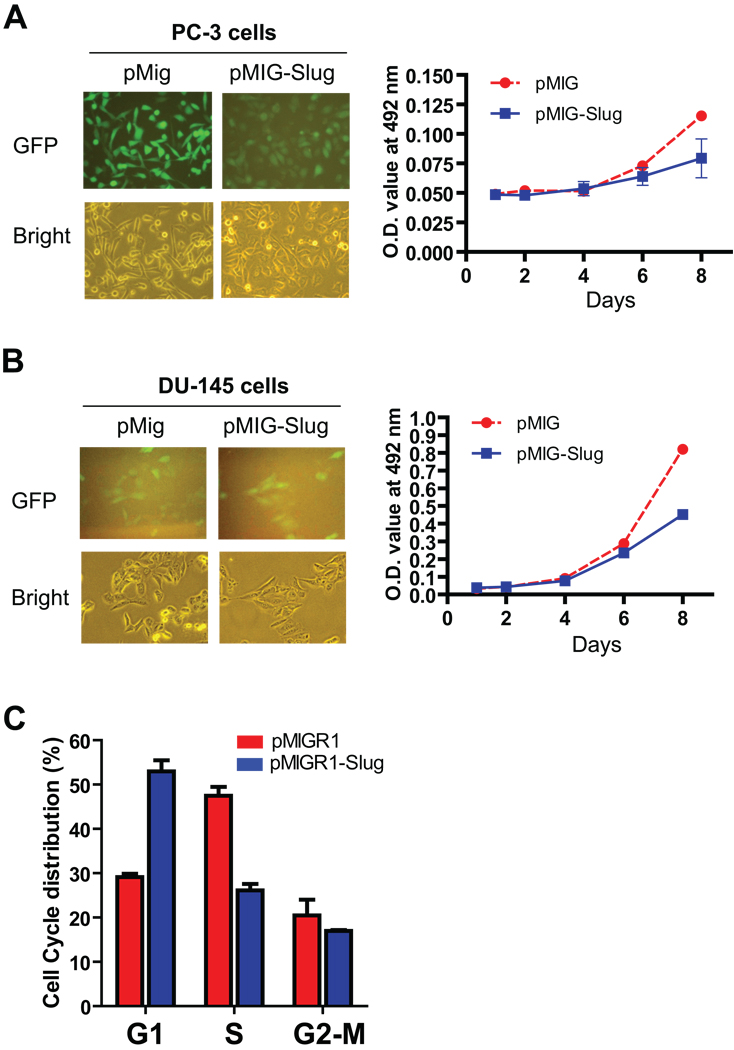
To test whether Slug can regulate cell growth of prostate cancer cell lines, we infected human PC-3 cells with control retroviruses or retroviruses expressing Slug (Fig. 3A, left panel), and measured cell growth of these two stable cell lines by MTT assay. We found that forced expression of Slug significantly inhibited PC-3 cell growth (Fig. 3A, right panel). In addition, we generated two DU-145 cell lines stably harboring vector or Slug expression vector via retroviral infection (Fig. 3B, left panel). Similar to the result using PC-3 cells, elevated level of Slug suppressed DU-145 cell growth, as determined by the quick cell proliferation assay (Fig. 3B, right panel). Together, these data indicated that growth inhibition of prostate cancer cells by forced expression of Slug is not cell type-specific.
Fig. 3. Cell growth curve of PC-3 and DU-145 prostate cancer cells stably expressing Slug.
A: Cell growth curve of PC-3 cells stably harboring pMIGR1-Slug or pMIGR1. PC-3 cells were infected with retroviruses generated from pMIGR1-Slug vector or pMIGR1 (left panel). Slug and GFP genes were driven by a common MMCV promoter. The PC-3 cells stably expressing Slug or vector were seeded in 48-well tissue culture plates (1000 cells per well), and their growth was measured by MTT assay at the indicated time points. Data shown are the average values from triplicate measurements. p < 0.05 at days 6 and 8.
B: Cell growth curve of DU-145 cells stably expressing pMIGR1-Slug or pMIGR1. DU-145 cells were infected with retroviruses generated from pMIGR1-Slug vector or pMIGR1 (left panel). The DU-145 cells stably expressing Slug or vector were seeded in 48-well tissue culture plates (500 cells per well), and their growth was measured by the quick cell proliferation assay at the indicated time points. Data shown are the average values from triplicate measurements. p < 0.05 at days 6 and 8.
C: Cell cycle analysis of PC-3 stable cell lines. PC-3 cells carrying pMIGR1 vector or expressing Slug (pMIGR1-Slug) were fixed in 70% ethanol followed by propidium iodide staining and cell cycle analysis. The percentages of cells in each phase are shown. The percentage of cells in G1-phase was significantly higher in PC-3 cells expressing Slug than in the cells carrying vector (pMIGR1). **p < 0.01
To study the mechanisms by which Slug inhibits proliferation of prostate cell lines, we analyzed cell cycle profile of PC-3 cells carrying vector (pMIGR1) or expressing Slug (pMIGR1-Slug). In agreement with its inhibitory function, forced expression of Slug resulted in G0/G1 cell cycle arrest, leading to decreased percentage of S-phase cells (Fig. 3C). These data imply that Slug may regulate some cell cycle regulators in G1-phase.
Knockdown of Slug expression promotes prostate cancer cell growth
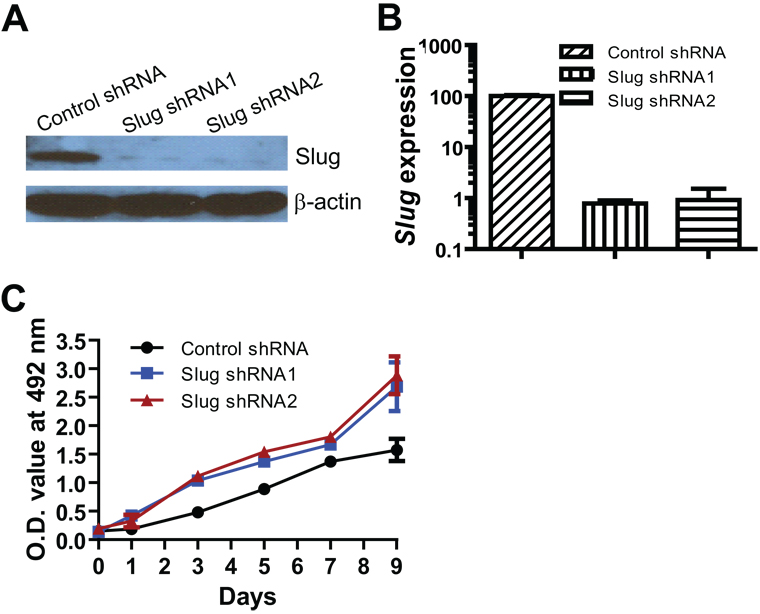
In addition to gain-of-function studies (Fig. 3), we used a loss-of-function approach to assess the effects of Slug on cell growth of human prostate cancer cells. We first established three PC-3 stable cell lines by infection of lentiviruses expressing control shRNA or small hairpin RNA (shRNA) targeting the human Slug gene, followed by selection with puromycin. As we expected, Slug protein expression was significantly reduced by two Slug shRNAs (Fig. 4A). QPCR analysis further confirmed that Slug RNA level was decreased more than 10-fold in two PC-3 cell lines stably expressing Slug-specific shRNAs, compared with PC-3 cells expressing control shRNA (non-target shRNA) (Fig. 4B).
Fig. 4. Knockdown of Slug expression and cell growth curve of PC-3 cells stably expressing Slug shRNAs.
A: Western blot analysis of Slug expression in PC-3 cell lines harboring control shRNA or Slug shRNAs. PC-3 cells were infected with lentiviruses expressing control shRNA, Slug shRNA1, or Slug shRNA2, and selected with puromycin (1 µg/ ml). Proteins were extracted from the stable lines and analyzed by Western blot with anti-Slug antibody and anti-β-actin antibody.
B: QPCR analysis of Slug RNA transcript in the stable lines harboring control shRNA or Slug shRNAs.
C: Cell growth curve of PC-3 cells stably expressing Slug shRNAs or control shRNA. The PC-3 stable cell lines were seeded in 48-well tissue culture plates (5000 cells per well) in the cell culture medium (2% FBS). Their growth was measured by the quick cell proliferation assay at the indicated time points. Data shown are the average values from triplicate measurements. p < 0.05 at days 3, 5, 7, and 9.
To assess the effect of Slug suppression on PC-3 prostate cancer cell growth, we measured cell growth of these two PC-3 sub-lines by the quick cell proliferation assay. As shown in Figure 4C, PC-3 cells with reduced Slug expression started to grow faster, starting at day 4 after plating. The cell growth rate of the PC-3 cell line harboring Slug shRNA was almost double, compared with the control cell line at 9 days after plating. In addition, we obtained a lentiviral Slug shRNA3 that effectively downregulates human Slug in melanocytes (33). Consistently, Slug shRNA3 relieved Slug-mediated growth inhibition of prostate cancer cells (Supplementary Fig. S2). Together with the results shown in Figure 3, this data indicated that Slug is a potent negative regulator of cell growth for prostate cancers.
Slug regulates cyclin D1 expression in prostate cancer cells
To study underlying molecular mechanisms by which Slug negatively regulates cell growth of prostate cancer cell lines, we analyzed several cell cycle regulators by Western blot and QPCR analyses. We found that cyclin D1 protein was reduced 2-fold in PC-3 cells infected with retroviruses expressing Slug, compared with cells infected with control viruses (Fig. 5A).
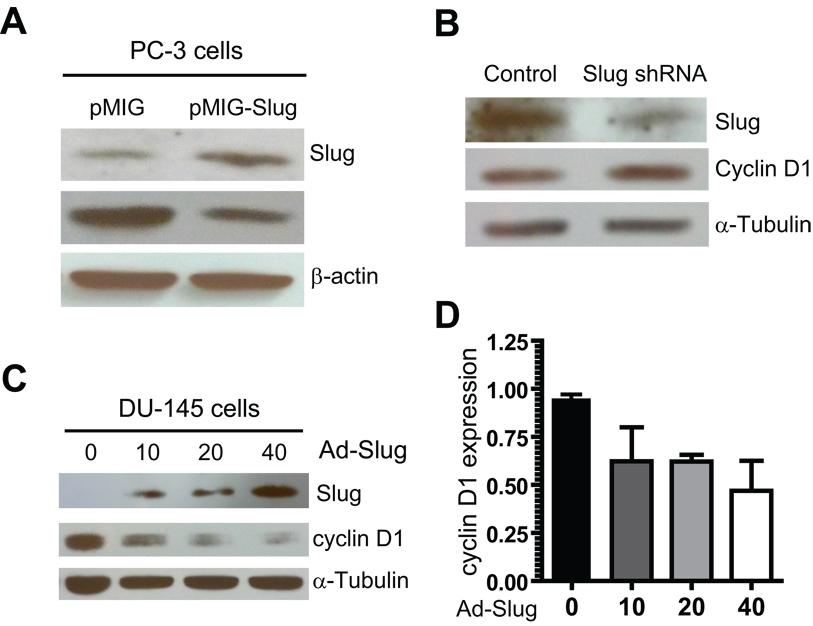
Fig. 5. Slug regulates cyclin D1 expression in PC-3 and DU-145 cell line.
A: Western blot analysis of cyclin D1 protein expression in PC-3 cell lines stably expressing Slug. All protein was extracted from the PC-3 cell lines stably carrying pMIGR1-Slug or pMIGR1 (vector), and examined by Western blot analysis using anti-Slug, anti-cyclin D1, and anti-β-actin antibodies.
B: Western blot analysis of cyclin D1 protein expression in PC-3 cells expressing Slug shRNA. All protein was extracted from PC-3 cells stably expressing Slug shRNA3 or control shRNA (Fig. S2), and immunoblotted with anti-cyclin D1 and anti-α-tubulin (loading control) antibodies.
C: Western blot analysis of cyclin D1 protein expression in PC-3 cells after infection with adenovirus-mediated delivery of Slug. PC-3 cells were infected with an increasing dose of adenoviruses expressing Slug (Ad-Slug). Four days after infection, all protein was extracted from infected cells and analyzed by Western blot analysis using anti-Slug, anti-cyclin D1, and anti-α-tubulin antibodies (loading control).
D: QPCR analysis of cyclin D1 RNA transcripts. PC-3 cells were infected with an increasing dose of Ad-Slug, and all RNA was extracted from these cells four days after infection. The cyclin D1 transcript level was analyzed by QPCR.
Mounting evidence indicates that the cyclin D1 gene is amplified or overexpressed in several types of human cancer, including cancers of the breast, esophagus, head, and neck. Recently, it was reported that the expression of cyclin D1 is increased in several prostate cancer cell lines and in a subset of primary prostate cancer. Thus, cyclin D1 is a potential target of Slug in prostate cancer cells. To further test this possibility, we used shRNA to knockdown protein expression of endogenous Slug in PC-3 prostate cancer cells. We found that reduced protein expression of Slug led to an elevated cyclin D1 expression (Fig. 5B). Furthermore, we infected DU-145 prostate cells with increasing doses of adenoviruses expressing Slug, and then analyzed cyclin D1 expression by Western blot analysis four days after viral infection. Our data showed that expression of cyclin D1 is dramatically down-regulated by forced expression of Slug (Fig. 5C).
To assess whether elevated expression of Slug down-modulated cyclin D1 expression at the transcription level, we analyzed cyclin D1 expression by QPCR analysis. We found that cyclin D1 RNA level was reduced 2-fold by adenovirus-mediated overexpression of Slug in DU-145 cells (Fig. 5D). In summary, Slug negatively regulated cyclin D1 expression at both protein and RNA level, suggesting that cyclin D1 is an in vivo target of Slug in prostate cells. However, it remains to be determined whether or not Slug directly regulates cyclin D1.
Forced expression of cyclin D1 relieves Slug-mediated cell growth inhibition of prostate cancer cells
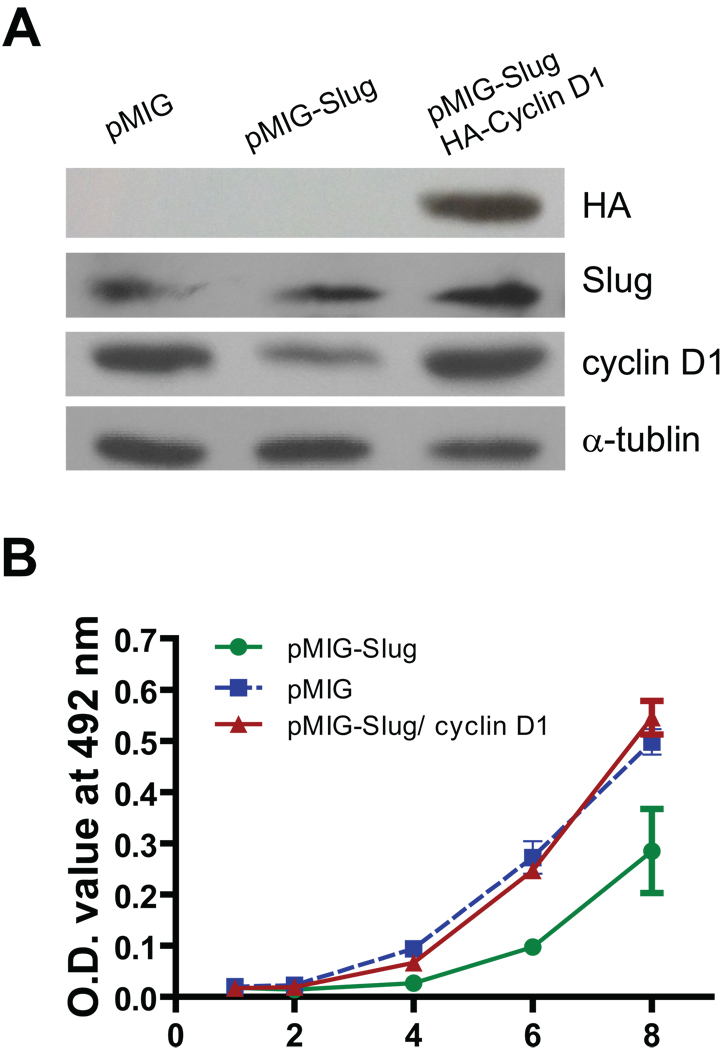
Our data indicated that increased expression of Slug inhibits cell growth of prostate cancer cells (Fig. 3), and that reduced expression of Slug accelerates cell growth of these cancer cells (Fig. 4). In addition, Slug negatively regulated cyclin D1 expression in prostate cancer cells (Fig. 5). Because cyclin D1 is a major cell cycle regulator for both normal and varied tumor cells, we tested whether forced expression of cyclin D1 relieves Slug-mediated cell growth inhibition of prostate cancer cells. To this end, we generated three stable PC-3 cell lines: (i) cells carrying vector only, (ii) cells expressing Slug, and (iii) cells expressing both Slug and cyclin D1. The expression of cyclin D1 was decreased in the PC-3 cells harboring pMIGR1-Slug, and elevated in the PC-3 cells expressing both Slug and cyclin D1 (Fig. 6A). We measured cell growth of these three cancer cell lines for up to eight days. As we expected, cell growth of PC-3 cancer cells stably expressing Slug was significantly decreased, starting from day 4 up to day 8 after plating (Fig. 6B). Interestingly, forced expression of cyclin D1 completely mitigated Slug-mediated cell growth inhibition of PC-3 prostate cancer cells. These data, together with Figure 5, demonstrated that cyclin D1 is a functional target of Slug in the regulation of cell growth of prostate cancer cells.
Fig. 6. Forced expression of cyclin D1 reverses Slug-mediated cell growth inhibition.
A: Western blot analysis of PC-3 stable cell lines. PC-3 cells were infected with retroviruses prepared from pMIGR1 (vector), pMIGR1-Slug, or pMIGR1-Slug plus pMIGR1-HA-cyclin D1. Protein was extracted from cells and immunoblotted with anti-HA or anti-α-tubulin antibodies.
B: Cell growth curve of PC-3 stable cell lines. PC-3 cell lines carrying pMIGR1 (vector), pMIGR1-Slug, or pMIGR1-Slug plus pMIGR1-HA-cyclin D1 were seeded in 48-well plates (1000 cells per well). Growth of these three cell lines was measured by the quick cell proliferation assay at the indicated time points. Data shown are the average values from triplicate measurements. p < 0.05 at days 4, 6, and 8.
DISCUSSION
It has been extensively documented that Slug is involved in triggering epithelial-mesenchymal transition during embryonic development and tumor metastasis, thereby implicating its direct function in cadherin regulation (11). However, the role of Slug in regulation of cancer cell growth remains to be determined. In this study, we studied the regulative function of Slug in prostate cancer cell models by showing its remarkable inhibitory effect on the growth of PC-3 and DU-145 cells. Western blot analysis showed that Slug was expressed in both primary prostate tumors from TRAMP mice (a murine prostate cancer model) carrying SV40 T-antigen (37) and human prostate cancer cell lines. These data suggest that Slug is a common regulator of prostate cancers.
Interestingly, Slug expression is minimal in the normal murine prostate, compared with primary prostate cancer tumors, implying that Slug plays an important role in regulation of prostate cancer but not normal prostate cells. A recent study suggests that wild-type p53 suppresses cancer invasion by inducing Slug degradation, whereas mutant p53 stabilizes Slug protein (36). Thus, we also showed p53 status in these cancer cell lines, and attempted to establish an inverse relationship between p53 and Slug. Our data indicated that Slug protein level is highest in LNCaP cancer cells carrying wild-type p53 and is lowest in p53-mutated DU-145 cancer cells. Taken together, these data indicate that Slug expression in prostate cancer cells is not regulated by p53.
Slug belongs to the Slug/Snail family that includes five members (Snail1, Snail2/Slug, Snail3, Scratch1, and Scratch2) in human cells. Although this family was implicated in various cancers, Slug appears to be more highly expressed in human prostate cancer cell lines when compared with the other four members, suggesting that Slug may play a unique role in the tumorigenesis of prostate cancer cells.
By comparing Western blot and the PCR analyses, we noticed that Slug expression is the highest in LNCaP cells and lowest in DU-145 at protein level; in contrast, Slug RNA level is significantly lower in LNCaP than in DU-145 cells. These data suggest that Slug stability is regulated in prostate cancer cells by a post-translational mechanism. Indeed, SP Wang et al. and AE Vernon et al. showed that Slug protein stability is regulated by the p53-MDM2 complex in non-small-cell lung cancers (36) and by the F-box protein Ppa during neural crest development (38). We anticipate that Slug degradation is not mediated by the p53-MDM2 in prostate cancer cells, because Slug protein level does not inversely correlate with p53 status in these cells.
By using both gain-of-function and loss-of-function approaches, we confirmed that Slug is a potent negative regulator of cell growth of prostate cancer cells. In this study, we used three Slug specific shRNAs to assure that knockdown is specific for Slug gene and not due to off-target effects (39). To study underlying molecular mechanisms by which Slug regulates the growth of prostate cancer cells, we found that cyclin D1, a key regulator of cell cycle (G1-phase) for both normal and tumor cells, is negatively regulated by Slug in prostate cancer cells. Consistent with this finding, forced expression of Slug resulted in cell cycle G1-phase arrest. This mechanism is unlikely cell type-specific, because forced expression of Slug down-regulated cyclin D1 expression in both PC-3 and DU-145 cells. Although cyclin D1 RNA level was reduced in DU-145 cells after infection with an adenovirus expressing Slug, we cannot exclude the possibility that elevated expression of Slug accelerates cyclin D1 degradation through indirect or direct protein-protein interaction. Because Slug protein is labile in cells and is regulated by the 26S proteasome (36), we will explore whether Slug facilitate cyclin D1 degradation through proteasome degradation pathways in the future. Nevertheless, Slug-mediated inhibition of cyclin D1 expression supports the finding that Slug suppressed cell proliferation of prostate cells. It has been well established that Slug promotes tumor metastasis by down-regulating epithelial related genes (14,15,19,21). Thus, it is important to study whether Slug promotes tumor metastases at the expense of tumor cell proliferation.
In addition, we alleviated Slug-mediated cell growth inhibition of prostate cancer cells by overexpression of cyclin D1. Our data showed that the rescue of cell growth in Slug-expressing stable PC-3 cells by cyclin D1 is not due to decreasing Slug expression. Thus, cyclin D1 is a functional target of Slug in prostate cancer cells. In this study, we used a cell culture model to demonstrate that Slug is a potent negative regulator of prostate cell growth via down-regulating cyclin D1. However, it remains to be tested whether forced expression of Slug inhibits tumorigenesis of prostate cancer cells in vivo, such as in a xenograft tumor model in nude mice. Furthermore, because genetically defined mouse prostate cancer models such as TRAMP mouse and Pten−/− mice are readily available, we should investigate the role of endogenous Slug in the initiation, promotion, and progression of prostate tumors. These future studies will add substantively to the knowledge of the underlying molecular mechanisms by which Slug regulates tumorigenesis of prostate cancer.
CONCLUSIONS
We were the first to determine that Slug was up-regulated in mouse prostate tumors and human prostate cancer lines. Forced expression of SLUG inhibited proliferation of prostate cancer cells through downregulation of cyclin D1 expression. Our data might suggest that SLUG may promote migration of tumor cells at the expense of their proliferation.
Supplementary Material
ACKNOWLEDGMENTS
Financial Support:
This study was supported by Maine Medical Center institutional support. J.L. was supported by a Scholarship (2008610038) from China Scholarship Council. W.S.W. was supported by an NIH K01 award from the National Institute of Diabetes and Digestive and Kidney Disease (K01DK078180).
We thank our colleagues at MMCRI for their critical review. We also thank the Cell Separation and Analysis Core at MMCRI for the cell analysis (as supported by NIH grant P20 RR018789).
Footnotes
Disclosure of Potential Conflicts of Interest: There are no potential conflicts of interest.
REFERENCES
- 1.McDavid K, Lee J, Fulton JP, Tonita J, Thompson TD. Prostate cancer incidence and mortality rates and trends in the United States and Canada. Public Health Rep. 2004;119(2):174–186. doi: 10.1177/003335490411900211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer. 1993;72(12 Suppl):3888–3895. doi: 10.1002/1097-0142(19931215)72:12+<3888::aid-cncr2820721726>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49(1):8–31. doi: 10.3322/canjclin.49.1.8. 31. [DOI] [PubMed] [Google Scholar]
- 4.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 5.Culig Z, Hobisch A, Bartsch G, Klocker H. Androgen receptor--an update of mechanisms of action in prostate cancer. Urol Res. 2000;28(4):211–219. doi: 10.1007/s002400000111. [DOI] [PubMed] [Google Scholar]
- 6.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62(4):1008–1013. [PubMed] [Google Scholar]
- 7.Cifuentes E, Croxen R, Menon M, Barrack ER, Reddy GP. Synchronized prostate cancer cells for studying androgen regulated events in cell cycle progression from G1 into S phase. J Cell Physiol. 2003;195(3):337–345. doi: 10.1002/jcp.10317. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4(3):209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, McKnight NC, Zhang T, Lu ML, Balk SP, Yuan X. SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res. 2007;67(2):528–536. doi: 10.1158/0008-5472.CAN-06-1672. [DOI] [PubMed] [Google Scholar]
- 10.Mulholland DJ, Dedhar S, Wu H, Nelson CC. PTEN and GSK3beta: key regulators of progression to androgen-independent prostate cancer. Oncogene. 2006;25(3):329–337. doi: 10.1038/sj.onc.1209020. [DOI] [PubMed] [Google Scholar]
- 11.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3(3):155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 12.Alberga A, Boulay JL, Kempe E, Dennefeld C, Haenlin M. The snail gene required for mesoderm formation in Drosophila is expressed dynamically in derivatives of all three germ layers. Development. 1991;111(4):983–992. doi: 10.1242/dev.111.4.983. [DOI] [PubMed] [Google Scholar]
- 13.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123(4):641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 15.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 16.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154(1):8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 17.Hemavathy K, Ashraf SI, Ip YT. Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene. 2000;257(1):1–12. doi: 10.1016/s0378-1119(00)00371-1. [DOI] [PubMed] [Google Scholar]
- 18.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 19.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Oda H, Tsukita S, Takeichi M. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev Biol. 1998;203(2):435–450. doi: 10.1006/dbio.1998.9047. [DOI] [PubMed] [Google Scholar]
- 22.Come C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C, Savagner P. Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res. 2006;12(18):5395–5402. doi: 10.1158/1078-0432.CCR-06-0478. [DOI] [PubMed] [Google Scholar]
- 23.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8(3):197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Tamura S, Shiozaki H, Miyata M, Kadowaki T, Inoue M, Matsui S, Iwazawa T, Takayama T, Takeichi M, Monden M. Decreased E-cadherin expression is associated with haematogenous recurrence and poor prognosis in patients with squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83(11):1608–1614. doi: 10.1002/bjs.1800831138. [DOI] [PubMed] [Google Scholar]
- 25.Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211(5):507–515. doi: 10.1002/path.2138. [DOI] [PubMed] [Google Scholar]
- 26.Ghadimi BM, Behrens J, Hoffmann I, Haensch W, Birchmeier W, Schlag PM. Immunohistological analysis of E-cadherin, alpha-, beta- and gamma-catenin expression in colorectal cancer: implications for cell adhesion and signaling. Eur J Cancer. 1999;35(1):60–65. doi: 10.1016/s0959-8049(98)00344-x. [DOI] [PubMed] [Google Scholar]
- 27.Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with transforming growth factor-beta1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129(5):1375–1383. doi: 10.1053/j.gastro.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 28.Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13(16):4769–4776. doi: 10.1158/1078-0432.CCR-06-2926. [DOI] [PubMed] [Google Scholar]
- 29.Elloul S, Silins I, Trope CG, Benshushan A, Davidson B, Reich R. Expression of E-cadherin transcriptional regulators in ovarian carcinoma. Virchows Arch. 2006;449(5):520–528. doi: 10.1007/s00428-006-0274-6. [DOI] [PubMed] [Google Scholar]
- 30.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18(10):1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garabedian EM, Humphrey PA, Gordon JI. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc Natl Acad Sci U S A. 1998;95(26):15382–15387. doi: 10.1073/pnas.95.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman RM, Mobascher A, Mangold U, Koike C, Diah S, Schmidt M, Finley D, Zetter BR. Antizyme targets cyclin D1 for degradation. A novel mechanism for cell growth repression. J Biol Chem. 2004;279(40):41504–41511. doi: 10.1074/jbc.M407349200. [DOI] [PubMed] [Google Scholar]
- 33.Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, Naber SP, Weinberg RA. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37(10):1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 35.Tsai CN, Tsai CL, Tse KP, Chang HY, Chang YS. The Epstein-Barr virus oncogene product, latent membrane protein 1, induces the downregulation of E-cadherin gene expression via activation of DNA methyltransferases. Proc Natl Acad Sci U S A. 2002;99(15):10084–10089. doi: 10.1073/pnas.152059399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, Li KC, Hong TM, Yang PC. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat Cell Biol. 2009;11(6):694–704. doi: 10.1038/ncb1875. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vernon AE, LaBonne C. Slug stability is dynamically regulated during neural crest development by the F-box protein Ppa. Development. 2006;133(17):3359–3370. doi: 10.1242/dev.02504. [DOI] [PubMed] [Google Scholar]
- 39.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21(6):635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.