Abstract
Background
Self-care management in heart failure (HF) involves decision-making to evaluate, and actions to ameliorate symptoms when they occur. The purpose of this study was to compare the risk of all-cause mortality, hospitalization or emergency room admission among HF patients who practice above average self-care management, those who practice below average self-care management, and those who are symptom-free.
Methods
A secondary analysis was completed of data collected on 195 HF patients. A Cox proportional hazards model was used to examine the association between self-care management and event risk.
Results
The sample was in older adulthood (61.3±11 years), predominantly male (64.6%), with an ejection fraction of 34.7%±15.3%; 60.1% had New York Heart Association class III or IV HF. During an average follow-up period of 364±288 days, there were 4 deaths, 82 hospitalizations, and 5 emergency room visits as first events. Controlling for fifteen common confounders, those who engaged in above average self-care management (HR, 0.44; 95% CI, 0.22-0.88; P<.05) and those who were symptom-free (HR, 0.48; 95% CI, 0.24-0.97; P<.05) had a lower risk of an event during follow-up, than those engaged in below average self-care management.
Conclusion
Symptomatic HF patients who practice above average self-care management have an event-free survival benefit similar to that of symptom-free HF patients.
Introduction
Heart failure (HF) is a common endpoint of highly prevalent cardiovascular disorders.1 Both the one-year mortality rate2 and composite risk of death or re-hospitalization within one year after discharge from a HF hospitalization3 remain substantial despite advances in the medical care of patients with HF. It has been suggested that more than 50% of hospitalizations for HF,4 and ostensibly a proportion of HF deaths are preventable. Moreover, strategies for managing HF that have additional benefit over optimal medical care, such as enhancing self-care, have been presented as being critical to improving health outcomes in this population.5, 6
HF self-care is a naturalistic decision-making process involving patient behaviors that help maintain physiologic homeostasis and prevent exacerbations.7 These daily routine behaviors, referred to as self-care maintenance or adherence behaviors, reflect the degree to which a patient follows healthcare providers' recommendations.8 Importantly, HF self-care also involves active patient decision-making to evaluate and actions that effectively ameliorate HF symptoms when they occur.9 These behaviors, referred to as self-care management behaviors, are triggered by symptoms, which not all HF patients experience routinely. Although it is assumed that effective HF self-care management helps optimize health outcomes, relatively little is known about the effectiveness of self-care management behaviors in this population.
Accordingly, the purpose of this analysis was to describe the significance of HF self-care management in estimating risk of all-cause mortality, hospitalization or emergency room admission. First, we examined and described group differences in bio-behavioral characteristics among persons with HF who are symptom-free, persons who are engaged in below average HF self-care management, and persons who are engaged in above average HF self-care management. Second, under the hypothesis that self-care management would help explain event-risk, we described risk of all-cause mortality, hospitalization or emergency room admission among persons who were symptom-free, those engaged in below average HF self-care management, and those engaged in above average HF self-care management.
Methods
To test our hypothesis, we completed a secondary analysis of merged data collected on 195 HF patients as part of three parent studies10, 11 conducted by a team of HF investigators from 2004-2007. Measures of HF self-care and clinical event data were available on all 195 subjects included in this analysis. All parent study protocols were reviewed and approved by an appropriate institutional review board at each participating center, and written informed consent was obtained from all study participants. Patients were recruited from academic medical centers in East South Central, and South Atlantic regions of the United States.
Measurement
Patient Characteristics
Baseline demographic, clinical and self-care data included in this secondary analysis were collected at enrollment during each of the parent studies. Patient characteristics of age, gender, and ethnicity were collected using patient interview and medical record review. Height and weight were measured using a stadiometer and beam scale respectively. Comorbid conditions were assessed with the widely used 17-item Charlson Comorbidity Index.12 A list of 17 comorbid diseases was evaluated with the possible score ranging from 0 to 30. All participants had a score of at least 1 because all had HF; scores of 1 or 2 indicate low risk, scores of 3 or 4 indicate medium risk, and scores of 5 or more indicate high risk. Depressive symptoms were assessed using Beck Depression Inventory II (BDI).13 Higher values of the BDI indicate a greater level of depressive symptoms. BDI scores ranging from 0-13, 14-19, 20-28, and 29-63 indicate minimal, mild, moderate and severe depressive symptoms respectively.
Illness and Treatment Characteristics
HF etiology, left ventricular ejection fraction (LVEF), and prescribed HF medications were assessed through a review of the medical record. NYHA functional classification was assessed by clinicians and identified through medical record review. Subjective functional capacity was assessed using the Duke Activity Status Index (DASI),14 a 12-item self-reported scale that assesses the level of difficulty experienced in completing physical tasks. Higher scores (range 0 to 58.2) on the DASI indicate greater functional capacity. Health-related quality of life was measured using the Minnesota Living with Heart Failure Questionnaire (MLHFQ).15 Higher scores (range 0 to 105) on the MLHFQ indicate worse HF health-related quality of life.
Measures of Self-Care Maintenance and Confidence
Self-care maintenance was measured using the Medical Outcomes Study specific adherence scale (MOS) score. The MOS consists of nine self-rated questions that capture adherence to routine daily practices of exercise, taking HF medications as prescribed, maintaining alcohol, tobacco and dietary restrictions, as well as daily weight measurement and symptom monitoring. Higher scores on the MOS indicate better adherence to prescribed therapy. Self-care maintenance was also measured at enrollment with the Self-Care of Heart Failure Index (SCHFI).7 The SCHFI contains 15 items measured on a four-point Likert scale, which form three scales: a) self-care maintenance, b) self-care management, and c) self-care confidence. Items measuring self-care maintenance capture adherence to routine daily practices of weight maintenance and measurement, symptom monitoring, dietary restriction, and physical activity. The self-care confidence subscale captures patients' perceived ability to engage in self-care behaviors (keep free from HF symptoms, follow treatment advice, evaluate the importance of HF symptoms etc.). Scores on each of the SCHFI scales range from 0-100, with higher scores indicating better self-care.
Self-Care Management
Self-care management, the focus of this research, also was assessed at enrollment using the SCHFI. Self-care management items (Table 1) capture HF symptom evaluation, likelihood of self-initiated symptom management strategies, and evaluation of treatment effectiveness. Scores on the SCHFI self-care management scale also range from 0-100, with higher scores indicating better self-care management. Self-care management items of the SCHFI are only completed if the person with HF has experienced symptoms in the preceding three months. Thus, patients who were symptom-free in the three months preceding assessment did not fill out this section of the SCHFI and do not have a SCHFI management scale score.
Table 1. Self-Care of Heart Failure Index Management Subscale Questions.
Original Self-Care of Heart Failure Index self-care management items.
| In the past 3 months, have you had trouble breathing or ankle swelling? | Yes or No | |||||
|---|---|---|---|---|---|---|
| Have not had these | I did not recognize it | Not Quickly | Somewhat Quickly | Quickly | Very Quickly | |
| How quickly did you recognize it as a symptom of heart failure? | N/A | 0 | 1 | 2 | 3 | 4 |
| If you have trouble breathing or ankle swelling, how likely are you to try one of these remedies? | Not Likely | Somewhat Likely | Likely | Very Likely | ||
| Reduce the salt in your diet | 1 | 2 | 3 | 4 | ||
| Reduce your fluid intake | 1 | 2 | 3 | 4 | ||
| Take an extra water pill | 1 | 2 | 3 | 4 | ||
| Call your doctor or nurse for guidance | 1 | 2 | 3 | 4 | ||
| Think of a remedy you tried the last time you had trouble breathing or ankle swelling; | Not Applicable | Not Sure | Somewhat Sure | Sure | Very Sure | |
| How sure were you that the remedy helped or did not help? | N/A | 1 | 2 | 3 | 4 | |
Statistical Analysis
Self-care management scores were transformed into the following categories: patients who had HF symptoms but reported self-care management below the sample arithmetic mean 63.63 (below average self-care management), patients who had symptoms but reported self-care management above the sample arithmetic mean (above average self-care management), and patients who did not have to engage in HF self-care management as they were symptom-free (symptom-free).
Comparisons between persons with HF who were symptom-free, and persons who engaged in HF self-care management were made using Student's t tests, Mann-Whitney U tests, Fisher's exact tests, or Pearson χ2 analysis where appropriate. Comparisons among persons with HF who were symptom-free, persons who engaged in below average HF self-care management, and persons who engaged in above average HF self-care management were made using analysis of variance, Kruskal-Wallis tests, or Pearson χ2 analysis where appropriate. Results are expressed in mean ± standard deviation (SD) or proportions where appropriate. Statistical significance was predetermined at P < .05.
Hierarchical Cox proportional hazards modeling was used in the analysis of risk of first event, including all-cause mortality, hospitalization or emergency room admission as a cumulative endpoint. Patient characteristics (age, gender, BMI calculated as (weight (lb)/[height (in)]2×703), BDI score, and Charlson Comorbidity Index score) were included in the first block of the model. HF characteristics (HF etiology, LVEF, NYHA class, prescription of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), prescription of a β-adrenergic receptor blocker, MLHFQ score, and DASI score) were included in the second block. Indices of self-care maintenance and confidence (MOS score, and SCHFI maintenance and confidence scale scores) were included in the third block of the model. To assess the additive influence self-care management, all three categories (below average self-care management, above average self-care management, and symptom-free) were added in the final block of the model. The influence of each group of variables was assessed by evaluating the statistical significance of the block and overall model χ2. Hazard Ratios (HR) with 95% Confidence Intervals (CI) were calculated to determine the influence of individual variables in explaining event-risk. Risk of near-extreme multicolinearity was predetermined as a linear correlation of independent variable regression coefficients greater than 0.8.16 Assuming alpha of 0.05 and preserving power of 0.80, data on 195 patients allowed for the detection of minimal Cox proportional hazard ratios of 1.500 or 0.665. All analyses were performed using SPSS version 15.0 (Chicago, IL).
Results
Comparing data among the three parent studies on patients characteristics, HF characteristics, and indices of self-care maintenance, management and confidence, the one significant difference was the proportion of women enrolled (χ2=8.426, P=.015). As such, gender was included in the event-risk model. The sample (Table 2) was predominantly male (67.6%), in older adulthood (61±11 years), and the vast majority self-identified as being Caucasian (79%). Average BMI was 32.2±7.7 kg/m2, and the sample had minimal depressive symptoms (BDI=11.1±7.7). The typical participant had a moderate level of comorbidity. The majority of subjects had ischemic HF (56.1%), most were in NYHA function class II or III HF, were prescribed ACE inhibitors or ARBs (85%) and β-adrenergic blockers, and had a moderately reduced LVEF. Most subjects were engaged in HF self-care management, but about one-third were symptom-free in the three months preceding enrollment.
Table 2. Sociodemographic and Clinical Characteristics of the Sample.
“*”P<.05 between symptom free patients and those engaged in self-care management based on Fisher's exact test, Mann-Whitney U test, Pearson χ2 test, or Student's t test where appropriate. “†”P<.05 among symptom free, below average self-care management, and above average self-care management groups based on Pearson χ2 test, Kruskal-Wallis test, or analysis of variance where appropriate. Abbreviations: ACE = angiotensin converting enzyme, ARB = angiotensin receptor blocker, MLHFQ = Minnesota Living with Heart Failure Questionnaire, MOS = Medical Outcome Study, SC = Self-Care, S-CHFI = Self-Care of Heart Failure Index, SD = standard deviation.
| Category of Self-Care Management | ||||
|---|---|---|---|---|
| Total n=195 |
Symptom Free n=65 |
Below Average n=70 |
Above Average n=60 |
|
| Variables | Mean±SD or % | Mean±SD or % | Mean±SD or % | Mean±SD or % |
| Age | 61.31 ±11.05 | 63.3±10.4 | 60.6±10.7 | 60.0±11.8 |
| Gender (% female) | 35.4 | 24.6* | 34.3 | 48.3† |
| Ethnicity | ||||
| Caucasian | 79 | 83.1 | 80 | 75 |
| African American | 18.9 | 15.4 | 18.6 | 23.3 |
| Other | 2.1 | 1.5 | 1.4 | 1.7 |
| Body Mass Index | 32.15±7.66 | 30.3±7.8* | 32.4±7.2 | 33.9±8.5† |
| Beck Depression Inventory II | 11.13±8.84 | 7.60±7.3* | 12.7±8.4 | 13.2±9.8† |
| Charlson Comorbidity Index Score | 3.75±1.60 | 3.54±1.5 | 3.89±1.7 | 3.80±1.6 |
| Heart Failure Etiology | ||||
| Ischemic | 56.1 | 61.5 | 52.2 | 54.4 |
| Idiopathic | 17.4 | 12.3 | 20.9 | 19.3 |
| Other (known) | 26.4 | 26.2 | 26.9 | 26.3 |
| Left Ventricular Ejection Fraction | 34.73±15.34 | 35.1 ±14.8 | 34.7±15.3 | 34.4±16.2 |
| New York Heart Association Class | ||||
| Class I | 4.8 | 9.5* | 4.5 | 0† |
| Class II | 34.9 | 54* | 32.8 | 16.1† |
| Class III | 47.3 | 31.7* | 44.8 | 67.9† |
| Class IV | 12.9 | 4.8* | 17.9 | 16.1† |
| Heart Failure Medications | ||||
| ACE inhibitor or ARB | 85.0 | 80 | 70.6 | 73.3 |
| β-adrenergic blocker | 85.0 | 86.2 | 79.4 | 90 |
| MLHFQ Score | 41.10±25.55 | 25.5±20.4* | 46.5±24.6 | 51.7±23.9† |
| Duke Activity Status Index score | 14.56±13.44 | 21.0±15.3* | 13.0±11.9 | 9.3±9.9† |
| MOS Specific Adherence Score | 25.30±07.25 | 25.7±7.7 | 23.6±6.6 | 27.3±7.0† |
| SCHFI Maintenance Scale Score | 63.63±17.07 | 66.2±17.6 | 57.3±16.5 | 68.3±15.0† |
| SCHFI Confidence Scale Score | 67.69±15.31 | 71.8±14.3* | 62.0±14.2 | 70.0±15.8† |
| SCHFI Management Scale Score | 47.6±12.1 | 81.2±10.1 | ||
Bio-behavioral Differences by Category of Self-Care Management
Persons engaged in above average self-care management were more likely to be female (P<.05), have a greater BMI (P<.05), have more depressive symptoms (P<.001), higher NYHA functional class (P<.001), poorer quality of life (P<.001), and worse subjective functional capacity (P<.001), compared to those who were symptom-free and persons engaged in below average self-care management (Table 2).
Self-Care Management and Event-Risk
Over the average follow-up period of 364±288 days, there were 4 deaths (2 HF deaths, 2 other deaths), 82 hospitalizations (15 for HF, 23 for other cardiac issues, 44 for non-cardiac reasons), and 5 emergency room visits (2 for HF and 3 for other cardiac issues) as first clinical events. The slight majority of subjects (n=104) completing enrollment in the parent studies were event-free. In the Cox proportional hazards model there was no detectable risk of near-extreme multicolinearity.
Sociodemographic and clinical patient characteristics (age, gender, BMI, BDI score, and Charlson Comorbidity Index score) explained risk of an event during follow-up (χ2=23.31, P<.001). HF-specific characteristics (HF etiology, LVEF, NYHA functional class, prescription of an ACE inhibitor or ARB and/or β-adrenergic receptor blocker, MLHFQ total score, and DASI score) added significantly to the model (Block χ2=18.84, P<.05; Model χ2=43.97, P<.001). Measures of self-care maintenance and confidence (MOS adherence score, and SCHFI maintenance and confidence subscale scores) did not add to the model (Block χ2=6.07, P=.11; Model χ2=50.05, P<.001). But, the three categories of self-care management (below average self-care management, above average self-care management, and symptom-free) added significantly to the model (Block χ2=6.72, P<.05; Model χ2=55.69, P<.0001).
Individual factors associated significantly with risk of all-cause mortality, hospitalization or emergency room admission in the full model (Table 3) were age (HR, 0.967; 95% CI, 0.941-0.995) BDI score (HR, 1.058; 95% CI, 1.019-1.099), prescription of a β-adrenergic blocker (HR, 0.346; 95% CI, 0.187-0.641), and DASI score (HR, 0.960; 95% CI, 0.930-0.991). In addition, all categories of self-care management were significantly associated with event risk during follow-up. Relative to persons reporting below average self-care management scores, those with above average self-care management scores (HR, 0.441; 95% CI, 0.222-0.877; P < .05) and those who were symptom-free (HR, 0.481; 95% CI, 0.238-0.971; P < .05) were less likely to have an event during follow-up (Figure 1).
Table 3. Self-Care Management and Event Risk: Cox Proportional Hazard Model.
Results shown controlling for the influence of gender, body mass index, comorbid conditions, HF etiology, left ventricular ejection fraction, New York Heart Association functional class, prescription of an ACE inhibitor or ARB, Minnesota Living with Heart Failure Questionnaire score, Medical Outcomes Study Specific Adherence Score, and SCHFI maintenance and confidence subscale scores. “*” P<.05, “†” relative to persons with SCHFI Self-Care Management scores below the sample mean. Abbreviations: ACE = angiotensin converting enzyme, ARB = angiotensin receptor blocker, CI = confidence interval, HR = Hazard Ratio, SCHFI = Self-Care of Heart Failure Index.
| Variable | Adjusted HR | 95%CI of HR |
|---|---|---|
| Age in years | 0.967* | (0.941-0.995) |
| Beck Depression Inventory II score | 1.074* | (1.038-1.111) |
| Prescribed Beta adrenergic blocker | 0.346* | (0.187-0.641) |
| Duke Activity Status Index Score | 0.960* | (0.930-0.991) |
| Symptom-Free † | 0.481* | (0.238-0.971) |
| Above Average Self-Care Management Score † | 0.441* | (0.222-0.877) |
Figure 1. Event-Free Survival: Cox Proportional Hazard Model.
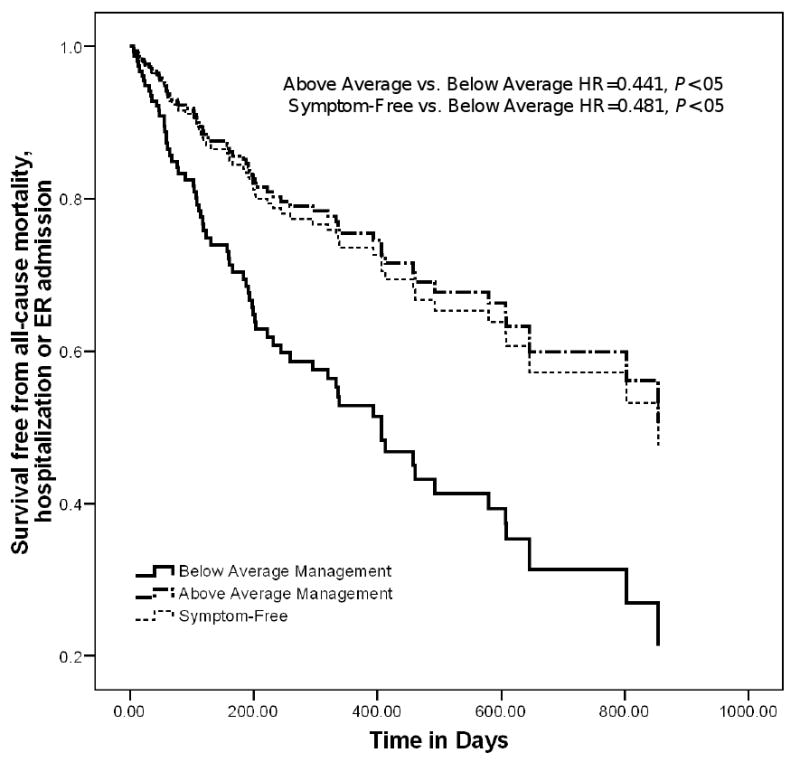
Cox proportional hazards model results show the difference in composite event-risk for patients who were symptom-free, those who practiced above average self-care management, and those who practiced below average self-care management. Results shown controlling for age, gender, body mass index, Beck Depression Inventory II score, comorbid conditions, left ventricular ejection fraction, heart failure etiology, New York Heart Association functional class, prescription of ACE inhibitors or ARBs, and/or β-adrenergic blockers, Duke Activity Status Index total score, Minnesota Living with Heart Failure Questionnaire total score, Medical Outcomes Study specific adherence score, and Self-Care of Heart Failure Index maintenance and confidence subscale scores.
Abbreviations: ACE = angiotensin converting enzyme, ARB = angiotensin receptor antagonist, ER = emergency room, HR = adjusted hazard ratio.
Discussion
In this study, we found that persons more engaged in HF self-care management had a 56% reduction (95% CI 12% to 78%) in the risk of all-cause mortality, hospitalization or emergency room admission than persons who were less engaged, after taking into consideration factors shown by others to be associated with these events. Second, persons with HF who were more engaged in self-care management had a an event risk nearly equivalent to those who were symptom-free, despite being a group that had more depressive symptoms, more severe NYHA functional class, lower functional capacity, poorer quality-of-life, and less confidence in their abilities to engage in self-care. Moreover, in this study the effect of HF self-care management on event-risk was close to the effect of having been prescribed a β-adrenergic receptor blocker.
Self-Care Management and Event-Free Survival
To the best of our knowledge, this is the first evidence that subjective self-care management (evaluation and self-treatment of HF symptoms) is associated with objective outcomes in persons with HF. Approximately 90% of persons admitted with HF have signs of clinical congestion,17 the early detection and treatment of which have been proposed as influencing outcomes in persons with HF.18 Until now, however, there has been no empirical data to support the hypothesis that effective HF self-care management (recognizing and treating clinical congestion) improves all-cause event-free survival. In fact, self-care management is rarely considered an important factor in models that determine risk of health outcomes in this population. This evidence of the clinical importance of the decision-making inherent in self-care management, however, suggests that patients who quickly recognize and act to ameliorate HF symptoms can greatly improve their outcomes. Moreover, we have provided preliminary evidence that patients who are better at self-care management may reduce their risk of events that may or may not be related to HF.
HF patients who were symptomatic but more engaged in self-care management practices had a risk of untoward events similar to that of symptom-free patients, even after adjusting for patient and clinical factors that clinicians typically judge to be important, including treatment adherence. Patient decision-making and actions of HF self-care management are quite different than routine practices of treatment adherence. Although both our indices of adherence were highest in persons who were more engaged in self-care management, measures of adherence were not associated with event-free survival either individually or in combination after adjustment for covariates. That is not to say that adherence to prescribed therapy is unimportant. Rather, we have identified modifiable self-care behaviors that have an additional event-free survival benefit over HF treatment and treatment adherence.
Two systematic reviews provided preliminary evidence of a relationship between self-care and risk of hospitalization in patients with HF. As part of a systematic review, McAlister and colleagues19 reported that enrollment in interventional programs designed to enhance HF patient self-care significantly reduced re-hospitalization for HF (RR 0.66) and all-cause re-hospitalization (RR 0.73). Similarly, Jovicic and colleagues20 reported a significant decrease in HF readmission (OR 0.44) and all-cause readmission (OR 0.59) in a separate systematic review of self-care interventions. The interventions included in these reviews never confirmed that self-care actually improved, however. Further, most interventions focused only on self-care maintenance behaviors. Thus, despite the appeal of general inferences that can be drawn from these reviews, they did not provided definitive evidence for the direct relationship between self-care and health outcomes or for the importance of self-care management, which were demonstrated in this study.
Several factors other than self-care were associated with risk of an event during follow-up in this study. In our analysis, increasing age was associated with a small-but-significant decrease in event-risk. This result differs from that previously reported by Rich21 Huynh22 and Goldberg23 and their respective colleagues, all of whom report an increased risk of mortality and/or hospitalization with age in the HF population. In the Huynh22 and Goldberg23 studies, the mean age was at least 15 years older than the mean age of our sample, while Rich and colleagues21 categorized age to estimate risk of mortality and hospitalization. These differences alone may explain why we did not detect the same influence of age. Further, including such factors as depression, activity and functional limitation, quality of life, and measures of self-care in our multivariate model may have accounted for the influence of age found in prior studies.
In our study, incremental (every 1-point) increases in depressive symptoms added a 6% increase in all-cause event-risk, consistent with the findings of other investigators. For example, Junger and colleagues24 identified that depression increased the risk of mortality by 8% (HR, 1.08; CI 1.01-1.15), although this effect was time-dependent, and Jiang and colleagues25 reported a 2% increase in risk of mortality per every 1-point increase in BDI score. Prescription of a β-adrenergic blocker reduced event risk in our analysis by 66%, confirming results from large clinical trials,26-28 despite comparative differences in sample age, LVEF and overall mortality rates. Better functional capacity was associated with a small-but-significant decrease in event risk, consistent with results from Shaw and colleagues.29 NYHA functional class was not a significant predictor of event risk, perhaps because we used all-cause outcome measures not just those related to HF.
Comment on Bio-behavioral Differences
We found significant differences in bio-behavioral characteristics among groups of self-care management. These results were largely confirmatory, adding to a growing body of evidence that HF symptoms are related to gender,30 BMI,31 quality of life,32 depression,33 and functional capacity.34 While these and several other univariate differences in bio-behavioral characteristics warrant further investigation, they are important to this study primarily for one reason. That is, the influence of bio-behavioral factors, including self-care maintenance and confidence, must be controlled in order to determine the relative importance of self-care management as a predictor of event-free survival.
Clinical Implications
Formally, there are many ways to measure self-care behaviors of patients with HF. In day to day clinical practice, however, HF clinicians most commonly judge engagement in self-care based on observation and patient self-report. In this paper, we have provided evidence that HF patients who are more engaged in self-care management and those who are symptom-free are less than half as likely to have an event during follow-up, than patients who are less engaged in self-care management as assessed by self-report. These findings are important to clinical practice for two reasons. First, these data provide the preliminary base of evidence to support the teaching and fostering self-care practices in this patient population. Particularly, teaching HF patients the actions to take when symptoms occur, and evaluating patients' skills in this regard during follow-up are important additions to routine HF patient education. Second, these results serve as evidence to impress upon patients the vital role they play in determining their own outcomes.
Strengths and Limitations
This was a secondary analysis of observational study data. As such, certain characteristics including patient age and functional parameters may not resemble efficacy study or other HF populations. Thus, inferences to a broad range of HF patients may be limited. Although we attempted to control for confounding factors, other, unidentified factors could have influenced the relationship between self-care management and event risk that were not included in the analysis. In previous studies, age,22 gender,35 BMI,36 depressive symptoms,25 comorbid conditions,37 LVEF,38 NYHA functional class,39 ischemic etiology,40 treatment with ACE inhibitors41 and β-blockers,26 quality of life,42 and activity limitations29 were identified as significant determinants of mortality or hospitalization in persons with HF. Thus, a strength of our study was that we controlled the influence of these factors. In addition to these important factors, we controlled for self-care maintenance and confidence, both which varied among categories of self-care management. Although the use of Cox proportional hazards modeling allows for the control of differing patient characteristics, we did not account for potential time-dependent covariates that may be influential. Finally, only significant associations and not causal mechanisms between self-care management and event risk during follow-up can be described.
Conclusion
In summary, measures of HF self-care management had independent predictive value in the determination of all-cause event-free survival after controlling for important clinical and psychosocial factors including treatment adherence. Patients more engaged in self-care management had less than half the risk of all-cause mortality, hospitalization or emergency room admission than patients less engaged in self-care management. Further, those who were more engaged in self-care management had an all-cause event risk nearly equivalent to patients who were symptom-free, despite being a group that has a markedly worse bio-behavioral risk profile. Future research that measures health outcomes in persons with HF should include self-care management as a predictive factor. The relationship between self-care management and HF-specific events in larger populations also warrants future research. Further, interventions to enhance self-care management, not just self-care maintenance or adherence, should be developed to improve outcomes in this population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher S. Lee, Lecturer, University of Pennsylvania School of Nursing.
Debra K. Moser, Professor, University of Kentucky College of Nursing.
Terry A. Lennie, Associate Professor, University of Kentucky College of Nursing.
Barbara Riegel, Professor, University of Pennsylvania School of Nursing, Philadelphia, PA.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Cowie MR, Fox KF, Wood DA, Metcalfe C, Thompson SG, Coats AJ, et al. Hospitalization of patients with heart failure: a population-based study. Eur Heart J. 2002;23(11):877–85. doi: 10.1053/euhj.2001.2973. [DOI] [PubMed] [Google Scholar]
- 4.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80(5):437–41. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havranek EP, Masoudi FA, Rumsfeld JS, Steiner JF. A broader paradigm for understanding and treating heart failure. J Card Fail. 2003;9(2):147–52. doi: 10.1054/jcaf.2003.21. [DOI] [PubMed] [Google Scholar]
- 6.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120(12):1141–63. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 7.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10(4):350–60. doi: 10.1016/j.cardfail.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Cleemput I, Kesteloot K. Economic implications of non-compliance in health care. Lancet. 2002;359(9324):2129–30. doi: 10.1016/S0140-6736(02)09114-6. [DOI] [PubMed] [Google Scholar]
- 9.Riegel B, Carlson B, Glaser D. Development and testing of a clinical tool measuring self-management of heart failure. Heart Lung. 2000;29(1):4–15. doi: 10.1016/s0147-9563(00)90033-5. [DOI] [PubMed] [Google Scholar]
- 10.Heo S, Lennie TA, Moser DK, Riegel B, Chung M. Gender differences in and factors related to self-care behaviors: A cross-sectional, correlational study of patients with heart failure. International Journal of Nursing Studies. 2008;45(12):1807–1815. doi: 10.1016/j.ijnurstu.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moser DK, Chung ML, Riegel B, Rayens MK, Lennie TA. Nonadherence is a mediator of the link between depressive symptoms, and rehospitalization or mortality in patients with heart failure. Circulation. 2006;144(suppl):II–518. [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35(8):785–91. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 14.cHlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64(10):651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 15.Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71(12):1106–7. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 16.Allison PD. Multiple Regression: A Primer. Thousand Oaks, CA: Pine Forge Press; 1999. [Google Scholar]
- 17.De Luca L, Fonarow GC, Adams KF, Jr, Mebazaa A, Tavazzi L, Swedberg K, et al. Acute heart failure syndromes: clinical scenarios and pathophysiologic targets for therapy. Heart Fail Rev. 2007;12(2):97–104. doi: 10.1007/s10741-007-9011-8. [DOI] [PubMed] [Google Scholar]
- 18.De Luca L, Abraham WT, Fonarow GC, Gheorghiade M. Congestion in acute heart failure syndromes: importance of early recognition and treatment. Rev Cardiovasc Med. 2006;7(2):69–74. [PubMed] [Google Scholar]
- 19.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44(4):810–9. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 20.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich MW, McSherry F, Williford WO, Yusuf S. Effect of age on mortality, hospitalizations and response to digoxin in patients with heart failure: the DIG study. J Am Coll Cardiol. 2001;38(3):806–13. doi: 10.1016/s0735-1097(01)01442-5. [DOI] [PubMed] [Google Scholar]
- 22.Huynh BC, Rovner A, Rich MW. Long-term survival in elderly patients hospitalized for heart failure: 14-year follow-up from a prospective randomized trial. Arch Intern Med. 2006;166(17):1892–8. doi: 10.1001/archinte.166.17.1892. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg RJ, Ciampa J, Lessard D, Meyer TE, Spencer FA. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med. 2007;167(5):490–6. doi: 10.1001/archinte.167.5.490. [DOI] [PubMed] [Google Scholar]
- 24.Junger J, Schellberg D, Muller-Tasch T, Raupp G, Zugck C, Haunstetter A, et al. Depression increasingly predicts mortality in the course of congestive heart failure. Eur J Heart Fail. 2005;7(2):261–7. doi: 10.1016/j.ejheart.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, et al. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154(1):102–8. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 27.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Carvedilol use at discharge in patients hospitalized for heart failure is associated with improved survival: an analysis from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2007;153(1):82e1–11. doi: 10.1016/j.ahj.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Gattis WA, O'Connor CM, Gallup DS, Hasselblad V, Gheorghiade M. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43(9):1534–41. doi: 10.1016/j.jacc.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Shaw LJ, Olson MB, Kip K, Kelsey SF, Johnson BD, Mark DB, et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol. 2006;47(3 Suppl):S36–43. doi: 10.1016/j.jacc.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 30.Heo S, Moser DK, Widener J. Gender differences in the effects of physical and emotional symptoms on health-related quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2007;6(2):146–52. doi: 10.1016/j.ejcnurse.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153(1):74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients' perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12(2):87–92. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Bekelman DB, Havranek EP, Becker DM, Kutner JS, Peterson PN, Wittstein IS, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643–8. doi: 10.1016/j.cardfail.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;12(6):439–45. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Alla F, Al-Hindi AY, Lee CR, Schwartz TA, Patterson JH, Adams KF., Jr Relation of sex to morbidity and mortality in patients with heart failure and reduced or preserved left ventricular ejection fraction. Am Heart J. 2007;153(6):1074–80. doi: 10.1016/j.ahj.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Burger AJ, Han Y, Aronson D. The relationship between body mass index and mortality in patients with acute decompensated heart failure. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 37.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27(1):65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 38.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112(24):3738–44. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed A. A propensity matched study of New York Heart Association class and natural history end points in heart failure. Am J Cardiol. 2007;99(4):549–53. doi: 10.1016/j.amjcard.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41(6):997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 41.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327(10):685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 42.Tate CW, 3rd, Robertson AD, Zolty R, Shakar SF, Lindenfeld J, Wolfel EE, et al. Quality of life and prognosis in heart failure: results of the Beta-Blocker Evaluation of Survival Trial (BEST) J Card Fail. 2007;13(9):732–7. doi: 10.1016/j.cardfail.2007.07.001. [DOI] [PubMed] [Google Scholar]


