Abstract
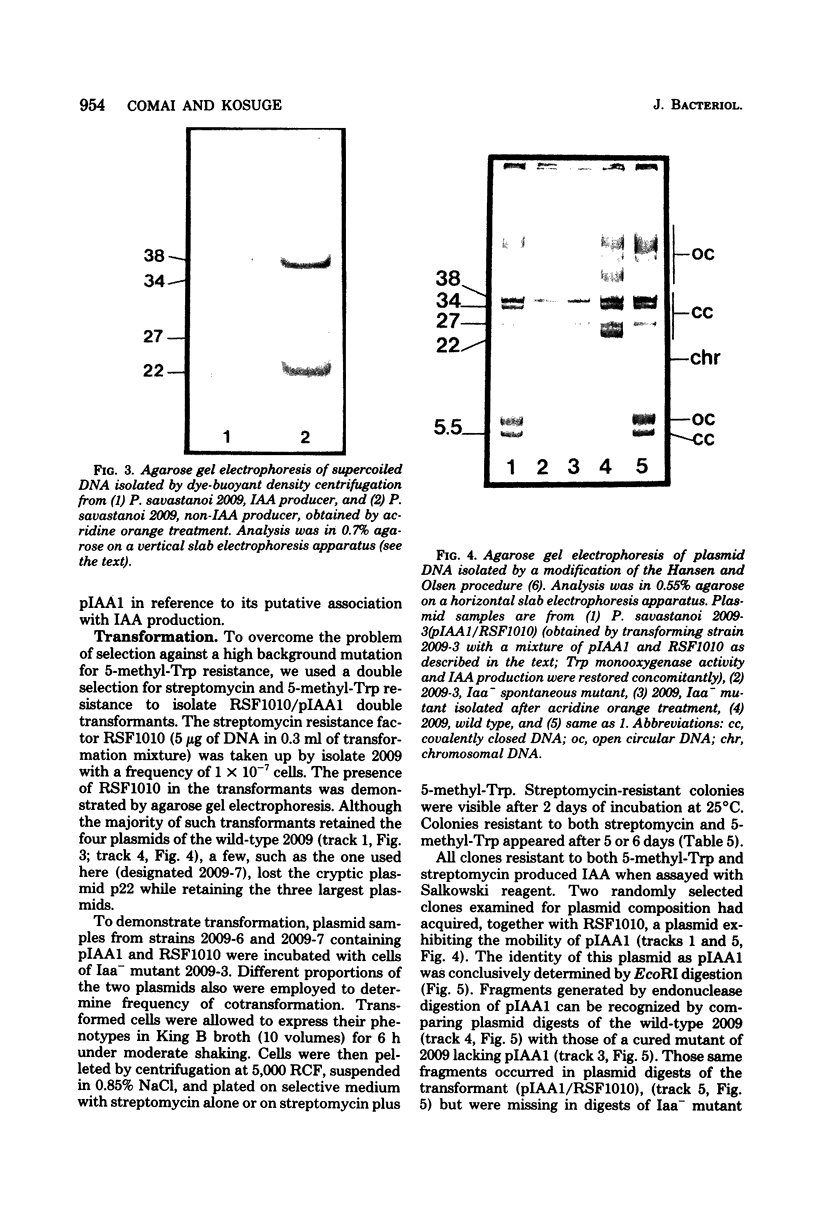
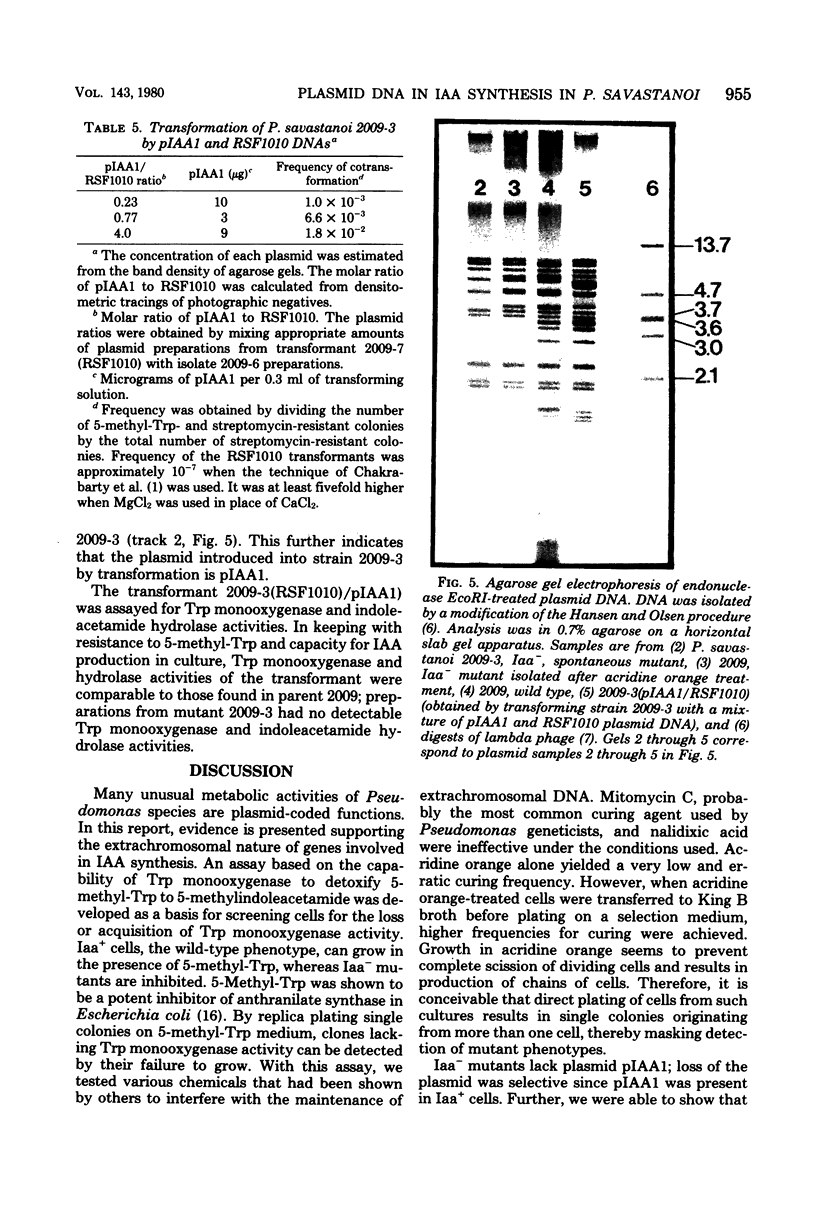
Olive (or oleander) knot is a plant disease incited by Pseudomonas savastanoi. Disease symptoms consist of tumorous outgrowths induced in the plant by bacterial production of indole-3-acetic acid (IAA). Synthesis of IAA occurs by the following reactions: L-tryptophan leads to indoleacetamide leads to indoleacetic acid, catalyzed by tryptophan 2-monooxygenase and indoleacetamide hydrolase, respectively. Whereas the enzymology of IAA synthesis is well characterized, nothing is known about the genetics of the system. We devised a positive selection for the presence of tryptophan 2-monooxygenase based on its capacity to use as a substrate the toxic tryptophan analogue 5-methyltryptophan. Efficient curing of the bacterium of tryptophan 2-monoxygenase, indoleacetamide hydrolase, and IAA production was obtained by acridine orange treatment. Further, loss of capacity to produce IAA by curing was correlated with loss of a plasmid of 34 X 10(6) molecular weight. This plasmid, here called pIAA1, when reintroduced into Iaa- mutants by transformation, restored tryptophan 2-monooxygenase and indoleacetamide hydrolase activities and production of IAA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chakrabarty A. M., Mylroie J. R., Friello D. A., Vacca J. G. Transformation of Pseudomonas putida and Escherichia coli with plasmid-linked drug-resistance factor DNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3647–3651. doi: 10.1073/pnas.72.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kosuge T., Heskett M. G., Wilson E. E. Microbial synthesis and degradation of indole-3-acetic acid. I. The conversion of L-tryptophan to indole-3-acetamide by an enzyme system from Pseudomonas savastanoi. J Biol Chem. 1966 Aug 25;241(16):3738–3744. [PubMed] [Google Scholar]
- Kretschmer F. J., Chang A. C., Cohen S. N. Indirect selection of bacterial plasmids lacking identifiable phenotypic properties. J Bacteriol. 1975 Oct;124(1):225–231. doi: 10.1128/jb.124.1.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. T., Kado C. I. Indoleacetic acid production: a plasmid function of Agrobacterium tumefaciens C58. Biochem Biophys Res Commun. 1979 Sep 12;90(1):171–178. doi: 10.1016/0006-291x(79)91605-x. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- Meyer R., Figurski D., Helinski D. R. Physical and genetic studies with restriction endonucleases on the broad host-range plasmid RK2. Mol Gen Genet. 1977 Apr 29;152(3):129–135. doi: 10.1007/BF00268809. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis L. The genetics of dissimilarity pathways in Pseudomonas. Annu Rev Microbiol. 1975;29:505–524. doi: 10.1146/annurev.mi.29.100175.002445. [DOI] [PubMed] [Google Scholar]