Abstract
BACKGROUND
Symptoms and functional status are major concerns for heart transplant (HT) patients.
OBJECTIVE
To examine gender and age differences in symptom distress and functional disability at 1 year after HT surgery.
METHODS
The sample (N = 237) consisted of 44 females and 193 males, who were divided into younger (n = 66) and older (n = 171) groups with the breakpoint at age 50. Data from chart review and 2 questionnaires (HT Symptom Checklist and Sickness Impact Profile) were analyzed with chi square, t-tests, ANOVA, and MANOVA.
RESULTS
Women reported worse symptom distress (overall, plus cardiovascular, gastro-intestinal, dermatologic symptoms) and more functional disability (overall, plus disability in ambulation, mobility, self-care, home management). Older patients reported more disability in ambulation and work. Gender by age interactions showed that older men reported worse genito-urinary symptoms and younger women reported worse dermatologic symptoms.
CONCLUSION
There were more gender than age differences in symptoms and disability.
Keywords: symptoms, disability, gender and age differences, heart transplant patients
Symptom distress is a major issue for heart transplant (HT) recipients1–4 and has been identified as a predictor of functional status and work status.5–8 Transplant patients must take immunosuppressant drugs for the rest of their lives to prevent organ rejection, and these drugs have many side effects including: infections, diabetes, hypertension, renal dysfunction, cancer, osteoporosis, gastro-intestinal (GI) problems, tremors, and weakness. Post-transplant acute rejection and chronic rejection (cardiac allograft vasculopathy) can cause additional symptom distress. Gender and age differences in symptom distress after heart transplantation have been suggested by a few studies,1–3 but more research is needed to provide additional information for patient care.
A goal of heart transplantation is to reduce disability from heart failure and improve functioning. However, even though functional status improved from before to after surgery,5,9,10 many patients still experienced multiple problems with activities of daily living 1 year later5 and 5 years later,6 with female gender and older age predicting worse disability on short-term5 and long-term follow-up.6,7 Also, more symptom distress and disability negatively impacted post-transplant quality of life11–14 and patient satisfaction with the transplant.15
Hayes and Redberg16 call for gender-specific reporting of research findings because they found that only 25% of over 600 cardiovascular clinical trials cited gender results, which is disturbing since heart disease is the leading cause of death in women. Rogers and Ballantyne17 also found that only 7% of 400 clinical studies reported gender analyses. Thus, there is insufficient data on gender differences in treatment outcomes that can provide direction for clinical practice.16
Age too is an important factor to examine in HT patients because the age limit for heart transplants has long been a point of discussion because of the shortage of donor organs, and there is ongoing concern for how well older patients do after HT surgery.18,19 Moreover, studies have produced conflicting results on the influence of older age on functional outcomes after heart transplantation.7,10,20–24 When HT studies used age as a dichotomous variable to examine outcomes, various age breakpoints ranging from 50 to 70 have been employed. The current research used a breakpoint of age 50, which was the mean age of HT recipients reported by the Registry of the International Society for Heart and Lung Transplantation.25
OBJECTIVES
Research objectives were: (1) identify gender and age differences in total symptom distress scores plus 6 types of symptom distress at 1 year after HT surgery; (2) identify gender and age differences in total functional disability scores plus disability in 12 areas of functioning; and (3) determine if there are any interactions between gender and age on symptom distress and functional disability.
METHODS
This research was part of a 10-year, 2-site study funded by the National Institutes of Health that investigated multiple quality of life outcomes, plus gender and age differences in outcomes, in adult HT patients at standardized intervals during the wait for a new heart and up to 5 years after surgery. Criteria for study enrollment were: on the HT waiting list at Loyola University of Chicago Medical Center or University of Alabama at Birmingham Medical Center, at least 18 years of age, scheduled for only a heart transplant, and willing to complete the study booklet at the required times (see Grady et al11 for a description of the questionnaires in the booklet). Prior to study participation, patients reviewed the booklet and signed a consent form. The research was approved by the Institutional Review Board at each site.
Sample
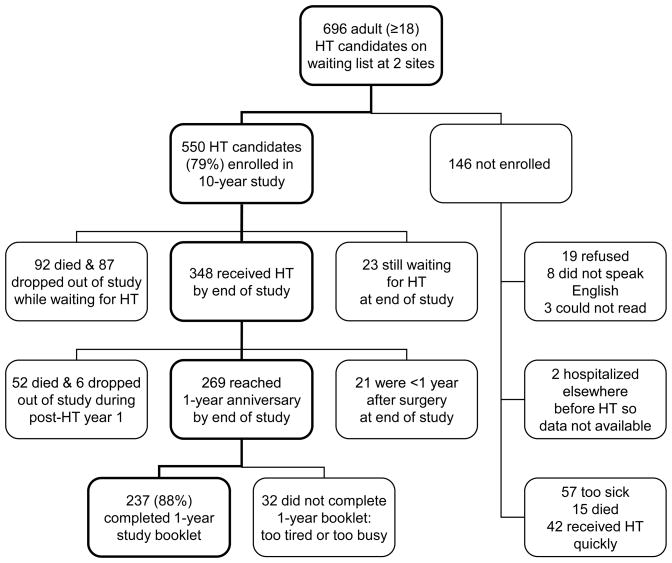
The sample consisted of 237 HT patients who reached the 1-year anniversary after surgery by the end of the study and completed the study booklet at that time. The 1-year anniversary was used for this assessment because it is considered a clinically significant milepost. Figure 1 shows how the sample size was derived for this time point. The sample was divided into gender and age (<50, ≥50) groups for analysis.
Figure 1.
Flow chart showing how the sample size was derived for analysis of 237 heart transplant (HT) patients at 1 year after surgery.
Data
The data for this analysis were collected from chart review and from patient-completed questionnaires on symptoms and functional status. Clinical data were retrieved by nurses experienced in cardiac care and included medical and surgical history, hospital admissions, medications, complications, and laboratory results (see Jalowiec et al26 for information on collection and reliability verification of chart data).
Instruments
Symptoms
Mere presence of a symptom does not indicate how much the symptom bothers the patient, so this assessment focused on the amount of distress caused by a symptom not on symptom frequency. Therefore, symptom distress was defined as how much a symptom bothered a patient and was measured with the Heart Transplant Symptom Checklist,27 which listed 92 symptoms related to heart disease, immunosuppressant side effects, and post-HT complications. Six types of symptoms were included: cardiopulmonary, GI, genito-urinary (GU), neuromuscular, dermatologic, and psychological. The patient rated how much each symptom bothered him/her, from “not bothered at all” (0) to “very bothered” (3). Percentage scores were used for the total scale and the 6 types of symptoms, with higher scores indicating more symptom distress. For this sample, Cronbach alpha reliability for the total symptom score was 0.96 and alphas for the 6 subscales ranged from 0.55 (GU) to 0.93 (psychological).
Functioning
Functional status was defined as the ability to perform activities of daily living and was measured with the Sickness Impact Profile (SIP),28 which assessed the amount of disability in 12 areas of functioning: ambulation, mobility, self-care, eating, sleeping, home management, social interaction, recreation, communication, alertness, emotional behavior, and work. A modified version of the SIP was used with 110 of the original 136 items. The patient indicated which activities he/she had problems performing by checking “yes” or “no” for each item.
The SIP assesses the degree of disability (not just the presence) because: (1) the items are stated in varying degrees of severity (e.g., “I climb the stairs more slowly than I used to” vs “I cannot climb the stairs at all anymore”), and (2) weights denoting the severity of disability were assigned to each activity by a panel used by the SIP developers. Those weights were the scores used for each activity that the patient checked “yes” as being a problem; if “no” was checked, the item score was zero. Raw SIP scores were converted to percentage scores for total disability and the 12 types of disability, with higher scores indicating more disability.
Even though the SIP is a self-assessment of functional status, SIP scores in previous studies correlated well with objective exercise tests.29–31 In the current study, data were collected on treadmill tests but only 13 patients (5%) completed treadmill tests at the 1-year anniversary so that objective data could not be used to compare to the subjective SIP scores. However, left ventricular ejection fraction (LVEF) is an objective measure of functional status in cardiac patients and showed a significant negative correlation with SIP scores in this sample, thus supporting validity of the SIP. For this sample, Cronbach alpha reliability for the total SIP score was 0.94 and alphas for the 12 subscales ranged from 0.53 (sleeping) to 0.80 (alertness).
Statistical analysis
Demographic and clinical differences were examined with chi square for categorical variables and t-tests for continuous variables. Correlations assessed the relationships between clinical variables and symptoms and disability. Statistical normality tests and graphical plots indicated that all symptom and disability percentage scores had non-normal distributions so square root transformations were used in the analysis. However, for ease of interpretation of the results on easily understood 0–100% scales, untransformed percentage scores are shown on the tables instead of square root scores.
ANOVA (analysis of variance) and MANOVA (multivariate analysis of variance) in SPSS V13 were used to analyze data for the 3 research objectives. Two-factor (gender and age group) ANOVA was run on total symptom and disability scores to determine the main effects of gender and age and any interaction effects. Because the subscales on an instrument are multiple and correlated, MANOVA was used to test the subscales. Two-factor MANOVA was run on the 6 symptom subscales together and then on the 12 disability subscales together to determine the main and interaction effects of gender and age. If the multivariate F test was significant for the entire group of subscales, then the univariate F tests were examined to identify which individual subscales were significant. Significance was set at p ≤ 0.05. If an interaction was significant, the Tukey post-hoc test on ANOVA was used to determine the significance of pair-wise comparisons between younger females, older females, younger males, and older males.
RESULTS
Demographic characteristics
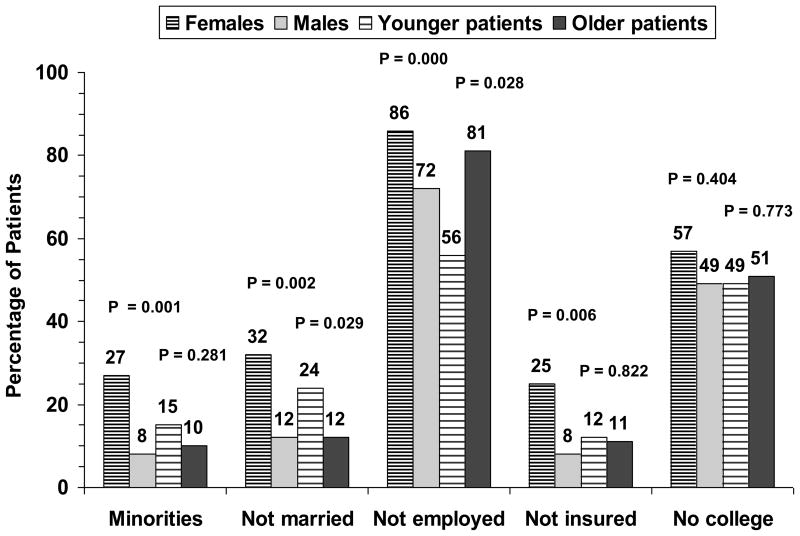
There were 44 females (19%) and 193 males (81%) in the sample of 237, with ages ranging from 24 to 71 years (mean = 54 ± 9). There was no significant age difference between females and males (both means = 54). Younger patients (<50 years of age) comprised 28% of the sample (n = 66); older patients (≥50) made up 72% (n = 171). There was no significant gender difference between younger and older patients (20% younger females, 18% older females). Significantly more females were minorities, not married, not employed, and not insured (Figure 2). Also, more younger patients were not married and more older patients were not employed (Figure 2). College education vs no college was not significant by gender or age group (Figure 2).
Figure 2.
Demographic differences by chi square between 44 females vs 193 males and 66 younger vs 171 older heart transplant patients at 1 year after surgery.
Clinical characteristics
Fifty-six percent of the patients had ischemic heart disease recorded as the reason for transplant. Nine patients (4%) received their second heart transplant. Most patients were on a triple immunosuppressant regimen of cyclosporine, prednisone, and azathioprine at 1 year after surgery. Less frequent immunosuppressants were mycophenolate mofetil, tacrolimus, methotrexate, and cyclophosphamide.
Other clinical characteristics are summarized on Table I by gender and age group. The only significant differences in clinical variables were in: (1) serum creatinine: higher in men and older patients; (2) cardiac index: lower in older patients; (3) azathioprine dose: higher in men; and (4) the number of complications recorded on the chart during the previous 3 months: more in older patients.
Table I.
Clinical characteristics of 237 heart transplant patients at 1 year after surgery by gender and age group
| Variable | Females (n = 44) | Males (n = 193) | P | Youngera (n = 66) | Oldera (n = 171) | P |
|---|---|---|---|---|---|---|
| Treated acute rejections in past 3 months (#) | 0.3 ± 0.5 | 0.2 ± 0.5 | 0.203 | 0.1 ± 0.3 | 0.2 ± 0.5 | 0.559 |
| IV-treated infections in past 3 months (#) | 0.1 ± 0.3 | 0.1 ± 0.4 | 0.599 | 0.1 ± 0.2 | 0.1 ± 0.5 | 0.352 |
| Complications in past 3 months (#) | 3.3 ± 2.1 | 3.2 ± 1.8 | 0.845 | 2.8 ± 1.4 | 3.4 ± 1.9 | 0.015 |
| Days hospitalized in past 3 months (#) | 5.4 ± 8.7 | 2.6 ± 7.1 | 0.057 | 2.2 ± 5.6 | 3.5 ± 8.1 | 0.203 |
| Cyclosporine dose (mg) | 314 ± 136 | 359 ± 146 | 0.064 | 373 ± 129 | 342 ± 151 | 0.149 |
| Prednisone dose (mg) | 11 ± 6 | 13 ± 4 | 0.471 | 13 ± 4 | 12 ± 5 | 0.179 |
| Azathioprine dose (mg) | 91 ± 58 | 124 ± 53 | 0.001 | 122 ± 58 | 115 ± 54 | 0.464 |
| LVEF (%) | 54.8 ± 8.2 | 55.1 ± 7.1 | 0.788 | 55.6 ± 6.1 | 54.8 ± 7.7 | 0.445 |
| Cardiac index (L/min/m2) | 2.8 ± 0.6 | 2.8 ± 0.5 | 0.638 | 2.9 ± 0.5 | 2.7 ± 0.6 | 0.008 |
| Serum creatinine (mg/dL) | 1.5 ± 0.5 | 1.7 ± 0.5 | 0.001 | 1.6 ± 0.4 | 1.7 ± 0.4 | 0.026 |
| Cholesterol (mg/dL) | 236 ± 61 | 233 ± 49 | 0.722 | 229 ± 52 | 235 ± 51 | 0.431 |
| Body mass index (kg/m2) | 27.2 ± 4.9 | 28.2 ± 4.5 | 0.185 | 28.9 ± 5.6 | 27.7 ± 4.1 | 0.101 |
| CAV | 1 (2.3) | 8 (4.1) | 1.000 | 1 (1.5) | 8 (4.7) | 0.451 |
| Diabetes | 10 (22.7) | 59 (30.6) | 0.360 | 15 (22.7) | 54 (31.6) | 0.204 |
| Hypertension | 38 (86.4) | 181 (93.8) | 0.113 | 64 (97.0) | 155 (90.6) | 0.168 |
| Renal dysfunction | 3 (6.8) | 23 (11.9) | 0.430 | 5 (7.6) | 21 (12.3) | 0.361 |
| Lymphoma | 2 (4.5) | 3 (1.6) | 0.234 | 1 (1.5) | 4 (2.3) | 1.000 |
Abbreviations: LVEF = left ventricular ejection fraction; CAV = cardiac allograft vasculopathy.
Note: Continuous variables are expressed as mean ± SD and were tested with t-test; categorical variables are expressed as N (%) and were tested with chi square.
Younger patients = <50 years; older patients = ≥50.
There were no significant gender or age group differences in the following clinical variables (Table I): the number of treated acute rejections or IV-treated infections during the previous 3 months, the number of days hospitalized during the previous 3 months, LVEF at 1 year, cholesterol or body mass index, cyclosporine or prednisone dose, or the percentages of patients with cardiac allograft vasculopathy, diabetes, renal dysfunction, hypertension, or lymphoma.
Clinical correlates of symptoms and disability
Clinical correlates of symptom distress were LVEF at 1 year (P = 0.006), the number of complications recorded on the chart in the previous 3 months (P = 0.005), and having a repeat transplant (P = 0.030). Clinical correlates of functional disability were LVEF (P = 0.000), the number of complications recorded on the chart (P = 0.003), and the number of days hospitalized during the previous 3 months (P = 0.005).
Symptom distress
On the total symptom distress score, female patients reported significantly worse overall symptom distress (Table II) but age group (Table II) and the interaction between gender and age group (Table III) were not significant. On the group of 6 symptom distress subscales, the multivariate effect of gender was significant, with significant univariate differences on 3 subscales: cardiopulmonary, GI, and dermatologic; females had the higher mean score on all 3 subscales (Table II). The multivariate effect of age group on the symptom subscales was significant but none of the univariate F tests were significant (Table II).
Table II.
Gender and age group differences in symptom distress
| Symptom distress score | Females (n = 44) Mean ± SD | Males (n = 193) Mean ± SD | Fa | P | Youngerb (n = 66) Mean ± SD | Olderb (n = 171) Mean ± SD | Fa | P |
|---|---|---|---|---|---|---|---|---|
| Total score | 16.9 ± 10.3 | 13.7 ± 11.1 | 7.18 | 0.008 | 14.0 ± 10.7 | 14.4 ± 11.2 | 0.91 | 0.342 |
| Cardiopulmonary | 9.7 ± 12.3 | 6.0 ± 8.3 | 4.95 | 0.027 | 6.5 ± 8.9 | 6.8 ± 9.4 | 0.32 | 0.574 |
| Gastro-intestinal | 22.9 ± 13.4 | 17.1 ± 14.1 | 9.98 | 0.002 | 19.5 ± 15.1 | 17.6 ± 13.8 | 1.77 | 0.185 |
| Genito-urinary | 10.7 ± 13.0 | 13.0 ± 13.4 | 0.16 | 0.690 | 11.3 ± 13.6 | 13.1 ± 13.2 | 1.27 | 0.262 |
| Neuromuscular | 16.4 ± 11.7 | 14.1 ± 13.5 | 3.21 | 0.074 | 12.5 ± 12.5 | 15.3 ± 13.4 | 0.34 | 0.562 |
| Dermatologic | 21.1 ± 13.5 | 14.0 ± 11.7 | 16.41 | 0.000 | 15.9 ± 11.8 | 15.0 ± 12.6 | 2.81 | 0.095 |
| Psychological | 16.2 ± 17.4 | 15.3 ± 19.0 | 0.61 | 0.437 | 17.0 ± 19.3 | 14.8 ± 18.5 | 1.72 | 0.191 |
Note: Scores are from the Heart Transplant Symptom Checklist and can range from 0–100%; higher scores = more symptom distress.
Univariate F from 2-factor ANOVA for total score and 2-factor MANOVA for subscales; MANOVA multivariate F for entire group of subscales for gender = 4.92, P = 0.000 and for age group = 2.59, P = 0.019.
Younger patients = <50 years; older patients = ≥50.
Table III.
Interactions between gender and age group on symptom distress
| Symptom distress score | Youngera females (n = 13) Mean ± SD | Oldera females (n = 31) Mean ± SD | Youngera males (n = 53) Mean ± SD | Oldera males (n = 140) Mean ± SD | Fb | P |
|---|---|---|---|---|---|---|
| Total score | 20.6 ± 9.2 | 15.4 ± 10.5 | 12.4 ± 10.5 | 14.1 ± 11.4 | 3.31 | 0.070 |
| Cardiopulmonary | 10.9 ± 11.9 | 9.2 ± 12.6 | 5.4 ± 7.7 | 6.3 ± 8.5 | 1.12 | 0.291 |
| Gastro-intestinal | 28.9 ± 15.3 | 20.4 ± 11.8 | 17.2 ± 14.3 | 17.0 ± 14.1 | 1.87 | 0.173 |
| Genito-urinary | 12.4 ± 17.7 | 7.1 ± 8.4c | 9.3 ± 11.8d | 14.4 ± 13.7c,d | 11.73 | 0.001 |
| Neuromuscular | 17.4 ± 11.9 | 15.9 ± 11.8 | 11.3 ± 12.5 | 15.2 ± 13.7 | 1.34 | 0.248 |
| Dermatologic | 27.1 ± 9.9e,f | 18.5 ± 14.2 | 13.1 ± 10.5e | 14.3 ± 12.1f | 5.25 | 0.023 |
| Psychological | 19.6 ± 15.2 | 14.7 ± 18.3 | 16.4 ± 20.3 | 14.8 ± 18.6 | 1.02 | 0.314 |
Note: Scores are from the Heart Transplant Symptom Checklist and can range from 0–100%; higher scores = more symptom distress.
Younger patients = <50 years; older patients = ≥50.
Univariate F from 2-factor ANOVA for total score and 2-factor MANOVA for subscales; MANOVA multivariate F for entire group of subscales = 2.33, P = 0.034.
Groups with same superscript are significantly different by Tukey post-hoc comparisons.
However, there was a significant multivariate interaction between gender and age group on the symptom distress subscales, with significant univariate differences on 2 subscales: GU and dermatologic (Table III). On the GU subscale, older males had worse symptom distress than younger males (Tukey post-hoc P = 0.046) and older females (P = 0.028). On the dermatologic subscale, younger females had worse scores than younger males (P = 0.001) and older males (P = 0.002).
Functional disability
On the total functional disability score, female patients reported significantly more overall disability (Table IV) but age group (Table IV) and the interaction between gender and age group (Table V) were not significant. On the group of 12 disability subscales, the multivariate effect of gender was significant, with significant univariate differences on 4 subscales: ambulation, mobility, self-care, and home management; females had the higher mean score on all 4 subscales (Table IV).
Table IV.
Gender and age group differences in functional disability
| Functional disability score | Females (n = 44) Mean ± SD | Males (n = 193) Mean ± SD | Fa | P | Youngerb (n = 66) Mean ± SD | Olderb (n = 171) Mean ± SD | Fa | P |
|---|---|---|---|---|---|---|---|---|
| Total score | 16.6 ± 11.1 | 12.7 ± 9.7 | 6.80 | 0.010 | 11.1 ± 9.9 | 14.3 ± 9.9 | 2.38 | 0.124 |
| Ambulation | 15.8 ± 16.1 | 8.9 ± 12.0 | 11.00 | 0.001 | 6.5 ± 10.7 | 11.6 ± 13.7 | 3.84 | 0.050 |
| Mobility | 9.7 ± 13.2 | 4.0 ± 8.0 | 12.86 | 0.000 | 4.2 ± 8.6 | 5.4 ± 9.8 | 0.71 | 0.402 |
| Self-care | 7.0 ± 11.1 | 3.5 ± 6.9 | 5.46 | 0.020 | 2.4 ± 4.8 | 4.8 ± 8.8 | 3.64 | 0.058 |
| Eating | 9.6 ± 9.1 | 8.2 ± 8.3 | 0.49 | 0.484 | 8.2 ± 5.6 | 8.6 ± 9.3 | 0.58 | 0.447 |
| Sleeping | 7.7 ± 10.1 | 8.5 ± 15.4 | 0.95 | 0.331 | 7.3 ± 11.6 | 8.7 ± 15.6 | 0.51 | 0.478 |
| Home management | 21.8 ± 21.4 | 15.6 ± 20.3 | 6.74 | 0.010 | 10.1 ± 13.2 | 19.3 ± 22.3 | 3.08 | 0.081 |
| Social interaction | 18.0 ± 21.7 | 15.6 ± 16.8 | 0.28 | 0.600 | 16.4 ± 20.9 | 15.9 ± 16.4 | 0.01 | 0.934 |
| Recreation | 23.9 ± 22.4 | 18.9 ± 20.1 | 1.78 | 0.184 | 16.5 ± 19.6 | 21.1 ± 20.9 | 0.85 | 0.358 |
| Communication | 7.0 ± 12.8 | 6.8 ± 15.0 | 0.48 | 0.491 | 7.3 ± 14.5 | 6.6 ± 14.5 | 0.36 | 0.551 |
| Alertness | 16.9 ± 25.5 | 17.7 ± 28.6 | 1.85 | 0.175 | 15.0 ± 26.8 | 18.6 ± 28.5 | 0.82 | 0.367 |
| Emotional behavior | 12.8 ± 19.9 | 9.4 ± 13.1 | 1.37 | 0.243 | 12.0 ± 17.0 | 9.3 ± 13.5 | 2.29 | 0.131 |
| Work | 87.8 ± 31.4 | 75.9 ± 39.4 | 2.16 | 0.143 | 62.8 ± 43.6 | 84.0 ± 34.4 | 10.33 | 0.001 |
Note: Scores are from the Sickness Impact Profile and can range from 0–100%; higher scores = more disability.
Univariate F from 2-factor ANOVA for total score and 2-factor MANOVA for subscales; MANOVA multivariate F for entire group of subscales for gender = 2.19, P = 0.013 and for age group = 1.97, P = 0.028.
Younger patients = <50 years; older patients = ≥50.
Table V.
Interactions between gender and age group on functional disability
| Functional disability score | Youngera females (n = 13) Mean ± SD | Oldera females (n = 31) Mean ± SD | Youngera males (n = 53) Mean ± SD | Oldera males (n = 140) Mean ± SD | Fb | P |
|---|---|---|---|---|---|---|
| Total score | 16.0 ± 11.1 | 16.8 ± 11.3 | 9.9 ± 9.4 | 13.7 ± 9.6 | 0.82 | 0.365 |
| Ambulation | 13.7 ± 14.7 | 16.7 ± 16.8 | 4.7 ± 8.8 | 10.5 ± 12.7 | 0.60 | 0.438 |
| Mobility | 8.5 ± 11.3 | 10.3 ± 14.0 | 3.1 ± 7.5 | 4.3 ± 8.2 | 0.01 | 0.913 |
| Self-care | 4.5 ± 7.5 | 8.0 ± 12.2 | 1.8 ± 3.8 | 4.1 ± 7.7 | 0.15 | 0.695 |
| Eating | 7.8 ± 4.4 | 10.4 ± 10.5 | 8.3 ± 5.9 | 8.2 ± 9.0 | 0.96 | 0.329 |
| Sleeping | 11.2 ± 11.7 | 6.2 ± 9.2 | 6.3 ± 11.5 | 13.7 ± 9.6 | 2.87 | 0.092 |
| Home management | 16.9 ± 11.2 | 23.8 ± 24.3 | 8.4 ± 13.2 | 18.3 ± 21.8 | 1.24 | 0.274 |
| Social interaction | 20.8 ± 26.3 | 16.9 ± 19.8 | 15.4 ± 19.5 | 15.7 ± 15.7 | 0.37 | 0.542 |
| Recreation | 23.1 ± 22.2 | 24.2 ± 22.9 | 14.9 ± 18.7 | 20.5 ± 20.5 | 0.39 | 0.535 |
| Communication | 9.2 ± 15.4 | 6.1 ± 11.7 | 6.9 ± 15.1 | 6.8 ± 15.0 | 0.42 | 0.519 |
| Alertness | 10.8 ± 16.9 | 17.6 ± 28.0 | 10.9 ± 22.7 | 20.3 ± 30.2 | 3.22 | 0.075 |
| Emotional behavior | 19.9 ± 23.7 | 9.8 ± 17.6 | 10.0 ± 14.6 | 9.2 ± 12.5 | 2.17 | 0.142 |
| Work | 71.2 ± 45.4 | 94.7 ± 20.4 | 60.8 ± 43.3 | 81.6 ± 36.4 | 0.26 | 0.610 |
Note: Scores are from the Sickness Impact Profile and can range from 0–100%; higher scores = more disability.
Younger patients = <50 years; older patients = ≥50.
Univariate F from 2-factor ANOVA for total score and 2-factor MANOVA for subscales; MANOVA multivariate F for entire group of subscales = 1.49, P = 0.128.
The multivariate effect of age group on the disability subscales was also significant, with significant univariate differences on 2 subscales: ambulation and work; older patients had the higher mean score on both subscales (Table IV). However, there was no significant multivariate or univariate interaction between gender and age group on the disability subscales (Table V).
Summary of gender and age differences
Significant gender differences were found on total symptom distress, on 3 of 6 types of symptom distress (cardiopulmonary, GI, dermatologic), on total functional disability, and on 4 of 12 types of disability (ambulation, mobility, self-care, home management). Significant age differences were observed only in ambulation and work functioning. Significant gender by age interactions were found on GU and dermatologic symptom distress.
DISCUSSION
Gender differences
The main conclusion to be drawn from these results is that there were more differences in symptom distress and functional disability related to female gender than related to age or the interaction between gender and age. At 1 year after HT surgery, women reported significantly worse overall symptom distress and also worse distress due to cardiopulmonary, GI, and dermatologic symptoms. In addition, women reported significantly more overall functional disability and also more disability in ambulation, mobility, self-care, and home management.
A recent review of 18 studies by Kugler et al2 concluded that female gender was consistently related to higher levels of symptom distress in patients with solid organ transplants (including heart). Moons’ study3 also found more symptom distress in women after HT surgery, whereas studies by Grady1 (using the same symptom tool as this study) and by Lough4 found more frequent symptoms in HT women but no significant gender difference in symptom distress. In addition, Grady and colleagues6,7 reported that women had more physical disability at 5 to 10 years after HT surgery (also using the SIP), which was influenced by the level of symptom distress. However, De Santo et al32 found that gender did not affect functional recovery after heart transplantation.
To try to explain the greater symptom distress and disability in women, variables that had been tested for gender differences were reviewed. No significant gender differences were observed in the following variables: age, LVEF, cardiac index, cholesterol, body mass index, prednisone or cyclosporine dose, number of rejections or infections, or percentages of patients with cardiac allograft vasculopathy, diabetes, renal dysfunction, hypertension, or lymphoma.
However, women spent more days in the hospital during the previous 3 months (see Table 1), although this was only marginally significant at .057. Further examination of post-transplant complications also showed that there were no significant differences between females and males in 11 categories of complications (such as cardiovascular, pulmonary, renal, endocrine), so the reasons for the increased hospitalization in women are not clear. Therefore, it is hard to determine from the clinical data analyzed for this report what was causing greater symptom distress and functional disability in the women in this sample.
However, significantly more women were minorities, not married, not employed, and not insured. This could indicate the lack of readily available personal and financial resources to help them manage their illness and symptoms, their own care, and their home, thereby resulting in a greater impact on disability in the female HT recipients. Bohachick’s study33 did find that worse support resources correlated with worse functional outcomes after HT surgery.
Age differences
Age analysis of symptom scores showed no significant differences in symptom distress by age group, either overall or by type of symptom. This finding provides new information since only 1 study was found on age differences in symptom distress after heart transplantation: Grady et al1 reported more overall distress in younger patients at 5 to 10 years after HT surgery (using the same symptom tool).
Age analysis of SIP total functional scores also showed no significant difference in overall disability. However, older HT patients (≥50) reported significantly more disability in 2 categories on the SIP: ambulation and work. The work finding cannot be attributed solely to patients being too old to work because only 7% of the older group (12/171) were beyond the traditional retirement age of 65, whereas 81% (139/171) were not employed, so illness problems (not age) seemed to account for the majority of work-related disability.
The older patients in this sample had a lower cardiac index, higher serum creatinine, and had more complications recorded on their charts in the previous 3 months, which could help to explain their greater disability in some areas. Further examination of complications showed that older HT patients had more complications of 3 types: hematologic, neurologic, and oncologic.
Previous research has resulted in equivocal findings on the influence of older age on functional ability after heart transplantation. For example, Fisher’s study10 found no age differences in SIP functional scores for up to 5 years after HT surgery and neither did Rosenblum’s study20 for up to 10 years afterward (also using the SIP). In contrast, studies by Martinelli,21 Politi,22 Rickenbacher,23 and Heroux24 reported worse functional outcomes in older HT patients on both short-term and long-term follow-up. In addition, Grady et al7 identified older age as a predictor of physical disability at 5 to 10 years post-HT (using the SIP).
Impact of age breakpoint on age findings
More symptom distress and functional disability were expected in the older age group based on previous disability research and the fact that the older patients in this sample had more complications recorded on their charts, leading one to assume that they would also have more symptoms and disability. Because so few age differences were found in this study using the age 50 breakpoint, follow-up post-hoc analysis was conducted to determine if the reason was too young a breakpoint, so symptom and disability analyses were rerun using an age break of 60. However, for both symptom distress and functional disability, age 60 produced the same results as age 50. Therefore, using a breakpoint of age 50 was not the reason for finding so few age differences in the results.
The good news from the lack of many significant differences between the older and younger patients in this research is that this finding helps support heart transplantation of older patients (the oldest patient in this report was 70 at the time of transplant; 25% were ≥60). It shows that symptom and functional outcomes in older HT patients can approach the outcomes observed in younger patients.
Interactions between gender and age
Significant interactions were found between gender and age group on only 2 variables: GU and dermatologic symptom distress. Older males had higher GU scores and younger females had higher dermatologic scores. Benign prostatic hypertrophy, which is common in older men, might be partly to blame for the GU symptoms. In addition, the GU subscale included several items on sexual function, which could also explain why older men scored worse on GU symptom distress. Moons et al3 and Lough et al4 found that sexual impotence was the most distressing symptom reported by the men in their HT sample, and other studies also noted that sexual concerns are common for men after HT surgery.34,35
The dermatologic subscale with the significant interaction included such items as change in facial features (such as “moon face” from prednisone), change in body features (such as “buffalo hump” from prednisone), and excessive hairiness (hirsutism from prednisone and cyclosporine). Since many symptoms on the dermatologic subscale are cosmetic, it is not surprising that younger women had much higher symptom distress scores on this subscale than the other 3 subgroups.
Summary and conclusion
At 1 year after HT surgery, women reported worse symptom distress (overall, plus cardiovascular, GI, and dermatologic symptoms) and also more functional disability (overall, plus disability in ambulation, mobility, self-care, and home management). Older patients reported more disability in ambulation and work. Older men reported worse GU symptoms and younger women reported worse dermatologic symptoms. Thus, there were more gender than age differences in symptom distress and functional disability at 1 year after heart transplantation.
Limitations
Study limitations include: (1) the number of females was small (44) so the study needs to be replicated with a larger sample (however, the proportion of females was similar to HT registry data25); and (2) the sample consisted of patients who survived the transplant to the 1-year anniversary and completed the study booklet at that time, so the results probably underestimate the amount of symptom distress and functional disability in the overall HT population.
Clinical application
The gender and age differences observed in symptom distress and functional disability in HT recipients emphasize the importance of identifying these differences when assessing patients. The goal is to tailor the treatment and care regimens to fit the different symptom and disability experiences of patients after HT surgery and also to facilitate access to appropriate resources to better meet their needs.
Study findings suggest clinical implications for managing individual patients following heart transplantation. For example, women may require more rigorous management of cardiopulmonary, GI, and dermatologic symptoms because the study showed that these symptoms are particularly distressing for women. Also, younger women who are distressed by the cosmetic changes caused by the immunosuppressants may benefit from psychological counseling to help them cope better. Older men should be evaluated for distressing problems with urination and sexual dysfunction, and medications ordered if appropriate. In addition, marital counseling may promote better understanding and more constructive handling of illness-related and medication-related sexual problems.
Women and older patients may benefit from physical therapy or cardiac rehabilitation to improve ambulation and mobility. Women with difficulties in self-care and home management should receive occupational therapy to improve their ability to take care of themselves and function better at home. Social services also can identify home care support resources as needed, especially for older patients or for women with children or elderly parents to care for while at the same time trying to take care of their own illness needs. Patients who feel well enough to work but are not employed may benefit from job counseling and identification of barriers to returning to work after surgery. All such interventions will enhance the quality of life of HT recipients over the long term.
Acknowledgments
Funding: National Institutes of Health (NINR: #NR01693, #NR01693/S, #5R01-NR01693; NHLBI: #HL49336), Sandoz Pharmaceuticals Corporation, Earl Bane Estate, American Association of Critical-Care Nurses, Sigma Theta Tau, Loyola University Research Committee, Loyola University School of Nursing, Loyola University Medical Center. Study PI: Dr Anne Jalowiec.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grady KL, Wang E, Higgins R, et al. Symptom frequency and distress from 5 to 10 years after heart transplantation. J Heart Lung Transplant. 2009;28:759–68. doi: 10.1016/j.healun.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kugler C, Geyer S, Gottlieb J, et al. Symptom experience after solid organ transplantation. J Psychosom Res. 2009;66:101–10. doi: 10.1016/j.jpsychores.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Moons P, De Geest S, Abraham I, Van Cleemput J, Vanhaecke J. Symptom experience associated with maintenance immunosuppression after heart transplantation: patients’ appraisal of side effects. Heart Lung. 1998;27:315–25. doi: 10.1016/s0147-9563(98)90052-8. [DOI] [PubMed] [Google Scholar]
- 4.Lough ME, Lindsey AM, Shinn JA, Stotts NA. Impact of symptom frequency and symptom distress on self-reported quality of life in heart transplant recipients. Heart Lung. 1987;16:193–200. [PubMed] [Google Scholar]
- 5.Jalowiec A, Grady KL, White-Williams C. Functional status one year after heart transplant. J Cardiopulm Rehabil Prev. 2007;27:24–32. doi: 10.1097/01.hcr.0000265029.25392.6e. [DOI] [PubMed] [Google Scholar]
- 6.Grady KL, Naftel DC, Kirklin JK, et al. Predictors of physical functional disability at 5 to 6 years after heart transplantation. J Heart Lung Transplant. 2005;24:2279–85. doi: 10.1016/j.healun.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Grady KL, Naftel DC, Young JB, et al. Patterns and predictors of physical functional disability at 5 to 10 years after heart transplantation. J Heart Lung Transplant. 2007;26:1182–91. doi: 10.1016/j.healun.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White-Williams C, Jalowiec A, Grady K. Who returns to work after heart transplantation? J Heart Lung Transplant. 2005;24:2255–61. doi: 10.1016/j.healun.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Grady KL, Jalowiec A, White-Williams C. Improvement in quality of life in patients with heart failure who undergo heart transplantation. J Heart Lung Transplant. 1996;15:749–57. [PubMed] [Google Scholar]
- 10.Fisher DC, Lake KD, Reutzel TJ, Emery RW. Changes in health-related quality of life and depression in heart transplant recipients. J Heart Lung Transplant. 1995;14:373–81. [PubMed] [Google Scholar]
- 11.Grady KL, Jalowiec A, White-Williams C. Predictors of quality of life in patients at one year after heart transplantation. J Heart Lung Transplant. 1999;18:202–10. doi: 10.1016/s1053-2498(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 12.Grady KL, Naftel DC, Kobashigawa J, et al. Patterns and predictors of quality of life in patients at 5 to 10 years after heart transplantation. J Heart Lung Transplant. 2007;26:535–43. doi: 10.1016/j.healun.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelista LS, Moser D, Dracup K, Doering L, Kobashigawa J. Functional status and perceived control influence quality of life in female heart transplant recipients. J Heart Lung Transplant. 2004;23:360–7. doi: 10.1016/S1053-2498(03)00196-7. [DOI] [PubMed] [Google Scholar]
- 14.Butler J, McCoin NS, Feurer ID, et al. Modeling the effects of functional performance and post-transplant comorbidities on health-related quality of life after heart transplantation. J Heart Lung Transplant. 2003;22:1149–56. doi: 10.1016/s1053-2498(02)01188-9. [DOI] [PubMed] [Google Scholar]
- 15.Jalowiec A, Grady KL, White-Williams C. Satisfaction with heart transplantation. Prog Cardiovasc Nurs. 2006;22:134–9. doi: 10.1111/j.0889-7204.2006.05565.x. [DOI] [PubMed] [Google Scholar]
- 16.Hayes SN, Redberg RF. Dispelling the myths: calling for sex-specific reporting of trial results. Mayo Clin Proc. 2008;83:523–5. doi: 10.4065/83.5.523. [DOI] [PubMed] [Google Scholar]
- 17.Rogers WA, Ballantyne AJ. Exclusion of women from clinical research: myth or reality? Mayo Clin Proc. 2008;83:536–42. doi: 10.4065/83.5.536. [DOI] [PubMed] [Google Scholar]
- 18.Weiss ES, Nwakanma LU, Patel ND, Yuh DD. Outcomes in patients older than 60 years of age undergoing orthotopic heart transplantation: an analysis of the UNOS database. J Heart Lung Transplant. 2008;27:184–91. doi: 10.1016/j.healun.2007.11.566. [DOI] [PubMed] [Google Scholar]
- 19.Tjang YS, van der Heijden GJ, Tenderich G, Korfer R, Grobbee DE. Impact of recipient’s age on heart transplantation outcome. Ann Thorac Surg. 2008;85:2051–5. doi: 10.1016/j.athoracsur.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Rosenblum DS, Rosen ML, Pine ZM, Rosen SH, Borg-Stein J. Health status and quality of life following cardiac transplantation. Arch Phys Med Rehabil. 1993;74:490–3. doi: 10.1016/0003-9993(93)90111-m. [DOI] [PubMed] [Google Scholar]
- 21.Martinelli V, Fusar-Poli P, Emanuele E, et al. Getting old with a new heart: impact of age on depression and quality of life in long-term heart transplant recipients. J Heart Lung Transplant. 2007;26:544–8. doi: 10.1016/j.healun.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Politi P, Piccinelli M, Poli PP, et al. Ten years of “extended” life: quality of life among heart transplantation survivors. Transplantation. 2004;78:257–63. doi: 10.1097/01.tp.0000133537.87951.f2. [DOI] [PubMed] [Google Scholar]
- 23.Rickenbacher PR, Lewis NP, Valantine HA, et al. Heart transplantation in patients over 54 years of age: mortality, morbidity, and quality of life. Eur Heart J. 1997;18:870–8. [PubMed] [Google Scholar]
- 24.Heroux AL, Costanzo-Nordin MR, O’Sullivan JE, et al. Heart transplantation as a treatment option for end-stage heart disease in patients older then 65 years of age. J Heart Lung Transplant. 1993;12:573–8. [PubMed] [Google Scholar]
- 25.Taylor DO, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report –2008. J Heart Lung Transplant. 2008;27:943–56. doi: 10.1016/j.healun.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Jalowiec A, Grady KL, White-Williams C. Predictors of rehospitalization time during the first year after heart transplant. Heart Lung. 2008;37:344–55. doi: 10.1016/j.hrtlng.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalowiec A, Grady KL, White-Williams C, et al. Symptom distress three months after heart transplantation. J Heart Lung Transplant. 1997;16:604–14. [PubMed] [Google Scholar]
- 28.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981;8:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Leyenson V, Furukawa S, Kuzma AM, et al. Correlation of changes in quality of life after lung volume reduction surgery with changes in lung function, exercise, and gas exchange. Chest. 2000;118:728–35. doi: 10.1378/chest.118.3.728. [DOI] [PubMed] [Google Scholar]
- 30.Noonan VK, Dean E, Dallimore M. The relationship between self-reports and objective measures of disability in patients with late sequelae of poliomyelitis: a validation study. Arch Phys Med Rehabil. 2000;81:1422–7. doi: 10.1053/apmr.2000.9172. [DOI] [PubMed] [Google Scholar]
- 31.Salsich GB, Mueller MJ. Relationships between measures of function, strength, and walking speed in patients with diabetes and transmetatarsal amputation. Clin Rehabil. 1997;11:60–7. doi: 10.1177/026921559701100109. [DOI] [PubMed] [Google Scholar]
- 32.De Santo LS, Marra C, De Feo M, et al. The impact of gender on heart transplantation outcomes: a single center experience. Ital Heart J. 2002;3:419–23. [PubMed] [Google Scholar]
- 33.Bohachick P, Taylor MV, Sereika S, Reeder S, Anton BB. Social support, personal control, and psychosocial recovery following heart transplantation. Clin Nurs Res. 2002;11:34–51. doi: 10.1177/105477380201100104. [DOI] [PubMed] [Google Scholar]
- 34.Tabler JB, Frierson RL. Sexual concerns after heart transplantation. J Heart Transplant. 1990;9:397–403. [PubMed] [Google Scholar]
- 35.Mulligan T, Sheehan H, Hanrahan J. Sexual function after heart transplantation. J Heart Lung Transplant. 1991;10:125–8. [PubMed] [Google Scholar]