Abstract
This study examined trajectories of cognitive change in psychometrically matched measures of episodic memory, semantic memory, and executive function in an ethnically, demographically, and cognitively diverse sample of older persons. Individual rates of change showed considerable heterogeneity in each domain. Baseline clinical diagnosis predicted differential change in semantic memory and executive function (dementia > mild cognitive impairment (MCI) > normal), but average decline in verbal episodic memory was similar across all three diagnostic groups. There was substantial overlap of distributions of cognitive change across baseline diagnostic groups for all three measures. Cognitive change was strongly related to change in clinical diagnosis. Rapid and similar change was present for all three cognitive measures in demented cases and in normals and cases with MCI who progressed clinically. In cognitively normal cases, verbal episodic memory change was greater than change in the other two domains. Global status, measured by the Clinical Dementia Rating scale, predicted change in semantic memory and executive function, while ApoE genotype predicted change in verbal episodic memory, and age had no effect on rates of change in any domain independent of global status and ApoE. Results show important limitations in using cross sectional diagnosis to predict prognosis, and suggest that research to identify robust predictors of cognitive change across the full spectrum from normal to dementia is needed for better early identification of diseases causing progressive decline.
Keywords: Cognitive change, diagnosis, dementia, mild cognitive impairment, normal cognition, aging
Trajectories of change in cognitive function in later adult life are heterogeneous. There are robust individual differences in cognitive functioning throughout adulthood and this heterogeneity is compounded by different courses of cognitive change that emerge as people age. Longitudinal studies of older individuals reveal widely different rates of cognitive decline, as well as stable function and even modest improvement in many cases (Albert, et al., 1995; Christensen, et al., 1999; Colsher & Wallace, 1991; Rubin, et al., 1998; Schaie, 1988; Wilson, Beckett, et al., 2002; Zelinski, Gilewski, & Schaie, 1993). Over time, differences in longitudinal trajectories translate into the increased variability of function within elderly populations (Christensen, et al., 1999) that is one of the basic observations of cross sectional studies of age effects on cognition. Understanding the sources of this heterogeneity so as to be able to predict change is a central issue in cognitive aging that has both scientific significance and clinical relevance.
Clinical diagnosis (e.g. normal cognitive function, mild cognitive impairment (MCI), dementia) has been the basic approach used to differentiate persons who have brain injury or disease from those who are pathology-free. The diagnosis of dementia presumably defines cases with a high probability of neuropathology; the diagnosis of normal cognitive function is associated with a low likelihood of pathology, and MCI with an intermediate likelihood. Likelihood of pathology can then reasonably be translated into prognostic statements about the cognitive course. The neuropathology underlying dementia in the elderly is most often Alzheimer's disease (AD), commonly in concert with varied other pathologies (Schneider, Arvanitakis, Bang, & Bennett, 2007; White, et al., 2005). Thus, dementia is usually progressive, and consequently, this diagnosis connotes cognitive decline that is far faster than in non-demented elders. The relative absence of pathology in normal cases, in contrast, leads to a prediction that the cognitive performance will be stable or perhaps decline very slowly. Mild cognitive impairment (MCI) is explicitly defined as falling between these two diagnoses and has been developed from the perspective of trying to capture early manifestations of AD with the goal of identifying “preclinical” cases or, alternatively, persons at substantially increased risk for future cognitive decline and dementia. It is now well recognized that MCI is etiologically heterogeneous (Bennett, et al., 2006; Saito & Murayama, 2007) and that the course of cognitive change in MCI is not always declining (Ganguli, Dodge, Shen, & DeKosky, 2004; Manly, et al., 2008; Palmer, Wang, Backman, Winblad, & Fratiglioni, 2002; Ritchie, Artero, & Touchon, 2001). Nonetheless, it is also clear that the diagnosis of MCI carries with it substantially increased risk of brain pathology and consequently cognitive decline (Bennett, et al., 2006; Manly, et al., 2008).
Exactly how much information diagnosis of cognitive syndrome conveys about the course of cognitive change is not well defined. It is clear that, on average, rates of cognitive decline increase from normal to MCI to dementia. However, group average rates of decline are determined both by the proportion of the group that declines, and how rapidly those who decline do so. Knowing the distribution of the full range of cognitive change paths, positive, negative, and neutral, allows one to better distinguish probability of decline and the rates of decline. Depending on the context, either or both parameters may be of interest.
Studies of cognitive change in dementia have often focused on rates of change (e.g. Barnes, et al., 2006; Scarmeas, Albert, Manly, & Stern, 2006; Stern, Albert, Tang, & Tsai, 1999; Wilson, Gilley, Bennett, Beckett, & Evans, 2000), while studies of MCI have generally focused on the probability of decline, frequently defined as “conversion” to the diagnosis of dementia (Mitchell & Shiri-Feshki, 2009). Studies of normal aging have tended to focus on rates (e.g. Ferrer, Salthouse, Stewart, & Schwartz, 2004; Wilson, Beckett, et al., 2002; Wilson, Li, Bienias, & Bennett, 2006) but the probability of decline has also been considered (Blasko, et al., 2008; Kryscio, Schmitt, Salazar, Mendiondo, & Markesbery, 2006). Because of these differing emphases in the literature it is difficult to determine exactly what prognostic information these syndromic diagnoses convey. The literature is further limited by the fact that many studies focus on a single group, frequently MCI, making it difficult to compare rates of change across the entire cognitive spectrum of older adults.
The purpose of this study was to examine cognitive change in a diverse sample of older persons; diverse with respect to race/ethnicity and associated demographic variables, diverse in cognitive function across the spectrum from normal to dementia, and presumably diverse with respect to presence and degree of age related diseases causing cognitive impairment. Longitudinal trajectories of change in cognition were evaluated using continuous measures of three clinically relevant cognitive domains, episodic memory, semantic memory, and executive function. These cognitive measures were developed using modern psychometric methods based on item response theory (Embretson & Reise, 2000; Hambleton & Swaminathan, 1985; Hambleton, Swaminathan, & Rogers, 1991) to have psychometric characteristics that are optimized for longitudinal research (Mungas, Reed, Crane, Haan, & González, 2004; Mungas, Reed, Haan, & Gonzalez, 2005; Mungas, Reed, Tomaszewski Farias, & DeCarli, 2005). We examined how rate of change in these measures differed in groups defined by baseline clinical diagnosis of normal cognition versus MCI versus dementia. Each participant in this study had longitudinal cognitive assessments with a clinical diagnosis at each assessment. The cognitive tests used to assess change were excluded from consideration in adjudicating clinical diagnosis, and this allowed us to examine how cognitive change corresponded to change in independently established diagnosis.
The design of this study allowed us both to characterize cognitive heterogeneity within and across diagnostic groups and to address the question of what an initial cross-sectional diagnosis tells us about cognitive change in older persons. We were interested in testing the basic hypothesis that diagnosis would predict rate of cognitive decline, with no decline in cognitively normal individuals, rapid decline in demented cases, and intermediate decline in MCI. We also expected that changes in clinical status would correspond to differences in cognitive trajectories.
Methods
Participants
This study included 369 participants in an ongoing longitudinal study of cognitive impairment in an educationally and ethnically diverse sample of older adults. These individuals were evaluated and followed within the research program of the University of California at Davis Alzheimer’s Disease Center (UCD ADC). All had at least two evaluations, but a rolling enrollment design was used and consequently the number of evaluations varied. Participants were recruited into the study through two routes: 1) memory clinic referrals and 2) community outreach. Approximately 68% of participants were recruited through community based recruitment protocols designed to enhance both the racial and ethnic diversity and the spectrum of cognitive dysfunction of the sample with an emphasis on normal cognition and MCI. Recruiters utilized various outreach methods such as soliciting in a community hospital lobby, a community survey, health fairs or word of mouth. The other 32% of the sample initially sought a clinical evaluation at the UCD ADC and subsequently were recruited for this study. These individuals predominantly had a clinical diagnosis of MCI. The overall sample included 107 African Americans, 88 Hispanics, 159 Caucasians, and 15 from other racial/ethnic groups.
Regardless of recruitment source, inclusion criteria were age greater than 60 and ability to speak English or Spanish. Exclusion criteria included unstable major medical illness, major primary psychiatric disorder (history of schizophrenia, bipolar disorder, or recurrent major depression), and substance abuse or dependence in the last five years. All participants signed informed consent, and all human subject involvement was overseen by institutional review boards at University of California at Davis, the Veterans Administration Northern California Health Care System and San Joaquin General Hospital in Stockton, California.
Clinical Evaluations
All participants received multidisciplinary diagnostic evaluations through the UCD ADC at baseline and at approximately annual intervals following the baseline evaluation. Baseline and follow-up evaluations followed the same protocol and included a detailed medical history and a physical and neurological exam. A physician fluent in Spanish examined subjects who spoke only Spanish. A family member or other informant with close contact with the participant was interviewed to obtain information about level of independent functioning. Information about change in the identified participant's cognitive and functional status prior to each evaluation was an important component of the clinical history, and was assessed by independent interviews with the participant and the informant. Clinical neuropsychological evaluation using standard neuropsychological tests was performed at baseline and at each follow-up. These clinical tests were distinct from the outcome measures used in analyses examining longitudinal trajectories. Routine dementia work-up laboratory tests were obtained at the baseline evaluation for all participants and when clinically indicated at the time of follow-up evaluations.
Diagnosis of cognitive syndrome (Normal, MCI, Dementia) and, for individuals with dementia, underlying etiology, was made according to standardized criteria and methods. Each case at baseline was initially diagnosed at a consensus conference by the clinical team evaluating the participant. Those appearing likely to be eligible for this study were then reviewed at a second, multidisciplinary UCD ADC-wide case adjudication conference. Follow-up cases were diagnosed at a case conference of the clinical team examining the participant, and in addition, were reviewed at the UCD ADC-wide case adjudication conference when the examining team identified a change in the diagnosis. Dementia was diagnosed using DSM-III R (American Psychiatric Association, 1987) criteria for dementia modified such that dementia could be diagnosed in the absence of memory impairment if there was significant impairment of two or more other cognitive domains. MCI was diagnosed according to standard clinical criteria and was further sub-typed according to current Alzheimer’s Disease Centers Uniform Data Set guidelines (J.C. Morris, et al., 2006). Normal cognitive function was diagnosed if there was no clinically significant cognitive impairment. All diagnoses were made blind to research neuropsychological testing. The Clinical Dementia Rating (CDR, Morris, 1993) was completed on the basis of a standardized interview with the identified participant and an informant; the sum of individual items or boxes (CDRSum) was used as a continuous measure of clinical status. The CDR was completed blind to other evaluation results including clinical and research neuropsychological test results, the physical and neurological exam, and the clinical diagnosis.
Research Neuropsychological Tests
The primary cognitive outcome measures in this study were from the Spanish and English Neuropsychological Assessment Scales (SENAS). These measures were administered at all evaluations. The SENAS has undergone extensive development as a battery of cognitive tests relevant to diseases of aging (Mungas, et al., 2004; Mungas, Reed, Haan, et al., 2005; Mungas, Reed, Marshall, & González, 2000; Mungas, Reed, Tomaszewski Farias, et al., 2005). Modern psychometric methods based on item response theory were used to create psychometrically matched measures across different scales and across English and Spanish versions. This study used a subset of SENAS tests to measure three cognitive domains: episodic memory, semantic memory, and executive function. Episodic Memory was a verbal episodic memory measure that was a composite score derived from a multi-trial word list learning test (Word List Learning 1, Mungas, et al., 2004). Semantic Memory was a composite of highly correlated verbal (Object Naming) and nonverbal (Picture Association) tasks. Executive Function was a composite measure constructed from component tasks of Category Fluency, Phonemic (letter) Fluency, and Working Memory (Digit Span Backward, Visual Span Backward, List Sorting). Measure development and psychometric characteristics have been reported in previous publications (Crane, et al., 2008; Mungas, et al., 2004; Mungas, Reed, Haan, et al., 2005).
There were three alternate forms of the word list learning task used for the Episodic Memory measure. These forms were alternated in the longitudinal evaluations to control for practice effects. Form 1 was administered for the baseline evaluation, Form 2 for the second assessment, and Form 3 for the third, and then the same sequence was repeated for subsequent evaluations. Forms were matched in terms of list structure, but the use of different forms in a longitudinal study raises questions about equivalence of forms, and consequently, form effects were evaluated in subsequent analyses.
Data Analysis
Descriptive analyses were performed to examine baseline characteristics of study participants. Analyses of variance were used to evaluate group differences in continuous measures, and the chi-square test was used for categorical variables. Mixed model random effects regression analyses were used to model rate of change of the cognitive outcome measures and to assess differences in rate of change across the clinical diagnoses. The longitudinal cognitive measures, Episodic Memory, Semantic Memory, and Executive Function were the primary outcomes of interest. Analyses were performed using SAS Proc Mixed and an unstructured covariance matrix for random effects was specified.
Mixed effects models for longitudinal data provide estimates of predicted baseline level and rate of change in the outcome, and also estimate how differences in baseline level and rate of change relate to variables of interest (fixed effects), such as diagnosis, that differ between subjects, and variables such as word list form that may vary from visit to visit within subjects. These models include random effects to account for a person's tendency to be above or below (random intercept) or to change more or less (random slope) than that predicted by their characteristics included in the model. They allow for different frequency of assessments and different lags between assessments across persons.
Baseline diagnosis and change in diagnosis were used in different analyses as independent variables to account for variability in cognitive baseline and change. Baseline diagnosis of Normal versus MCI versus Dementia was examined first, followed by a second analysis that included baseline diagnostic categories for MCI subtypes as well as Normal and Dementia. Change of diagnosis from the first to last evaluation was the independent variable in an additional set of analyses. This change variable included the following groups: Normal staying Normal (Norm-N), Normal progressing to MCI or Dementia (Norm-M), MCI reverting to Normal (MCI-N), MCI staying MCI (MCI-M), MCI converting to Dementia (MCI-D), and Dementia staying Demented (Dem-D). There were five cases with dementia at baseline that were not demented at the last evaluation, and these cases were excluded from the diagnosis change analyses because of the small size of this group. Pairwise comparisons of diagnostic group means were performed to evaluate patterns of significant differences in baseline level and rate of change. Bonferroni corrected p values were used to adjust for the number of possible pairwise comparisons.
The random effects models initially were run with diagnosis as the only independent variable. Subsequent models included demographic covariates: age at the baseline evaluation, gender, education, and ethnicity (African American, Hispanic, Caucasian; other minorities were excluded from these analyses since this was a small and heterogeneous group), and this was followed by models that included recruitment source (community versus clinic) along with these demographic covariates. Additional analyses examined the effects of age, ApoE, and baseline CDRSum and change in CDRSum on cognitive baseline and change. Change in CDRSum was a time varying independent variable and was calculated as the CDRSum at each evaluation minus CDRSum at the baseline evaluaiton. A final set of secondary analyses excluded cases that were lost to follow-up or died during the course of follow-up.
Mixed model regression analyses are sensitive to assumptions of linearity, normality, and constant variance. These assumptions were examined using graphical and statistical diagnostics. Residuals and random effects were examined to assure that they were normally distributed, and plots of residuals against predicted values and effects were examined to verify that non-linear trends in the data or non-constant variances were not present. Additional diagnostics included evaluation of variance components related to random effects and within subject error variance to address adequacy of statistical estimation procedures associated with the random effects modeling.
Form differences for Episodic Memory were evaluated by including a time-varying covariate that coded for the form administered at each evaluation. This was a fixed effect variable entered into the basic model along with time of the evaluation. This allows for estimating the independent contributions of time and form to within person variability in test scores; the form effect accounts for within-person variability in Episodic Memory that is related to the form administered but is independent of systematic trends across time. Practice is another potential factor that could impact all three cognitive scores (Ferrer, Salthouse, McArdle, Stewart, & Schwartz, 2005; Ferrer, et al., 2004; Wilson, et al., 2006). For Episodic Memory, we created a variable coding the number of times a given form was administered prior to the target evaluation and included this as an additional time varying fixed effect in a model with the time-varying form effect previously described to separate effects of practice from form differences. For all three cognitive outcome measures, we created a variable that coded previous versus no previous exposure to the test and included this as a fixed effect in models explaining baseline status and longitudinal change.
Results
Sample Characteristics
Table 1 shows demographic and follow-up characteristics of the whole sample and specific ethnic subgroups, and in addition, shows breakdown of baseline clinical diagnosis (Normal, MCI, Dementia). Mean education markedly differed across ethnic groups (F[3,365]=49.5, p<.001). Hispanics had about 9 years of education on average, compared with 13 to 14 years for the other groups. Standard deviations were also larger for Hispanics reflecting broader variability of education in Hispanics. Range of education was zero years to doctoral degrees in Hispanics, three years to doctoral in African Americans, and six years to doctoral in Caucasians and Other Minorities. Age differences were significant but less substantial (F[3,365]=3.2, p<.02); mean age was 1.5 to 2.9 years lower in Hispanics than the other groups. Group differences in gender were marginally significant (χ2[3]=8.0, p=.05); percentage of females were somewhat lower in the Caucasians (53%) than in the other groups (66–73%). About one half of the sample was cognitively normal at baseline evaluation, about one third had a diagnosis of MCI, and 15% were demented. Distribution of diagnoses differed across groups (χ2[3]=18.4, p=.005); Caucasians were more likely to have MCI and less likely to be normal, while African Americans were less likely to have dementia and were more likely than Hispanics to have MCI. Forty five of 56 cases (80.4%) with Dementia at baseline had an etiologic diagnosis of Alzheimer's disease, two had vascular dementia, four had mixed Alzheimer's and vascular disease, three had Lewy body dementia, one had frontotemporal dementia, and the etiology for one was undetermined.
Table 1.
Demographic and clinical characteristics and length of follow-up.
| African American (n=107) |
Hispanic (n=88) |
Caucasian (n=159) |
Other Minority (n=15) |
All (n=369) |
|
|---|---|---|---|---|---|
| Gender | |||||
| N (%) Female | 73 (68.2) | 58 (65.9) | 85 (53.5) | 11 (73.3) | 227 (61.5) |
| Education (years) | |||||
| Mean (S.D.) | 13.2 (3.1) | 8.6 (5.3) | 14.7 (3.3) | 12.8 (3.5) | 12.7 (4.5) |
| Age (years) | |||||
| Mean (S.D.) | 74.1 (7.4) | 72.6 (6.7) | 75.5 (6.8) | 74.6 (8.5) | 74.3 (7.1) |
| Clinical Diagnosis | |||||
| N (%) Normal | 63 (58.9) | 52 (59.0) | 65 (40.9) | 8 (53.3) | 188 (50.9) |
| N (%) MCI | 34 (31.8) | 18 (20.5) | 68 (42.8) | 5 (33.3) | 125 (33.9) |
| N (%) Dementia | 10 ( 9.3) | 18 (20.5) | 26 (16.3) | 2 (13.3) | 56 (15.2) |
| Follow-Up Time | |||||
| Mean (S.D.) | 2.5 (1.1) | 3.2 (1.3) | 3.0 (1.6) | 2.3 (1.0) | 2.9 (1.4) |
| Number of Evaluations | |||||
| N(%) 2 Evals | 43 (40.2) | 19 (19.3) | 48 (30.1) | 5 (33.3) | 113 (30.6) |
| N(%) 3 Evals | 35 (32.7) | 29 (33.0) | 34 (21.4) | 7 (46.7) | 105 (28.5) |
| N(%) 4 Evals | 21 (19.6) | 30 (34.1) | 39 (24.5) | 2 (13.3) | 92 (24.9) |
| N(%) ≥5 Evals | 8 ( 7.5) | 12 (13.6) | 38 (24.0) | 1 ( 6.7) | 59 (16.0) |
Number of evaluations and average follow-up duration are also presented in Table 1. Average follow-up duration for the 369 individuals was 2.9 years (s.d.=1.4, range = 0.6 – 7.7); 113 had two evaluations, 105 had three, 92 had four, 46 had five, and 13 had six or more. Amount of follow-up was similar across diagnostic groups (not shown) except that there was less follow-up in cases with a diagnosis of MCI at both evaluations (2.2 years on average) in comparison with the other groups (2.7 – 3.6 years). Three hundred ten of the 369 (84.0%) participants were actively followed up until the time of this study, 27 (7.3%) died during follow-up, and 32 (8.7%) were lost to follow-up.
Table 2 shows correspondence between baseline diagnosis and diagnosis at the last evaluation. Twenty three Normals at baseline (12%) progressed to MCI or Dementia, and these changes were distributed across all ethnicity/racial subgroups. For MCI, 45 of 125 (36%) converted to Dementia, 63 (50%) stayed MCI, and 17 (14%) reverted to Normal. MCI converters to Dementia were predominantly Caucasian, while stable MCI and MCI reverting to Normal were distributed across groups. Fifty one of 56 (91%) Dementia cases at baseline had Dementia at the last evaluation, but five (three Hispanics, two Caucasians) changed to a diagnosis of MCI.
Table 2.
Correspondence of baseline clinical diagnosis and diagnosis at the last evaluation.
| Diagnosis (Baseline - Final) |
African American (n=107) |
Hispanic (n=88) |
Caucasian (n=159) |
Other Minority (n=15) |
All (n=369) |
|---|---|---|---|---|---|
| Normal - Normal | 56 | 47 | 56 | 6 | 165 |
| Normal - MCI | 7 | 5 | 6 | 1 | 19 |
| Normal - Dementia | 0 | 0 | 3 | 1 | 4 |
| MCI - Normal | 6 | 6 | 5 | 0 | 17 |
| MCI - MCI | 25 | 9 | 25 | 4 | 63 |
| MCI - Dementia | 3 | 3 | 38 | 1 | 45 |
| Dementia - Normal | 0 | 0 | 0 | 0 | 0 |
| Dementia - MCI | 0 | 3 | 2 | 0 | 5 |
| Dementia - Dementia | 10 | 15 | 24 | 2 | 51 |
Episodic Memory Form Differences and Practice Effects
Form effects for Episodic Memory were evaluated in a mixed effects model that included time and form as independent fixed effects variables. Significant form differences were identified (Form 1: 0.24±0.05, Form 2: 0.10±0.05, Form 3: 0.00±0.06). When the variable coding amount of previous exposure to a form was included, form remained significant but amount of previous exposure was not independently related to test performance. Similarly, the variable coding previous exposure was not significantly related to test performance and did not alter the effect of form. These results show form differences, but fail to support practice effects either with respect to amount of exposure to a specific form or to previous exposure to this task regardless of form. Subsequent analyses involving Episodic Memory included form as a time varying covariate to control for form effects. Previous exposure was associated with better Semantic Memory performance (estimate=0.07±0.02, p<.007) but was not significantly related to Executive Function (p>.14). Previous exposure was included as a fixed effect in subsequent models for Semantic Memory.
Time Related Change
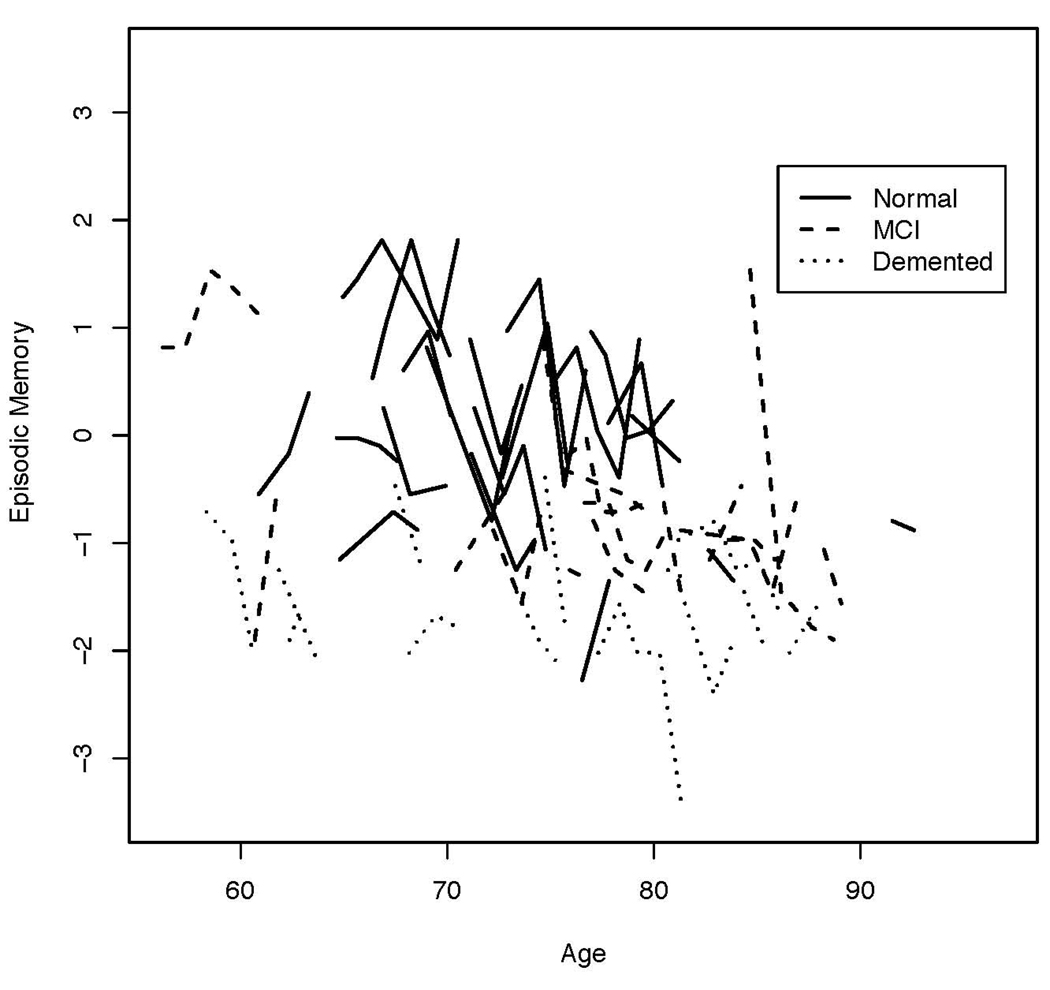
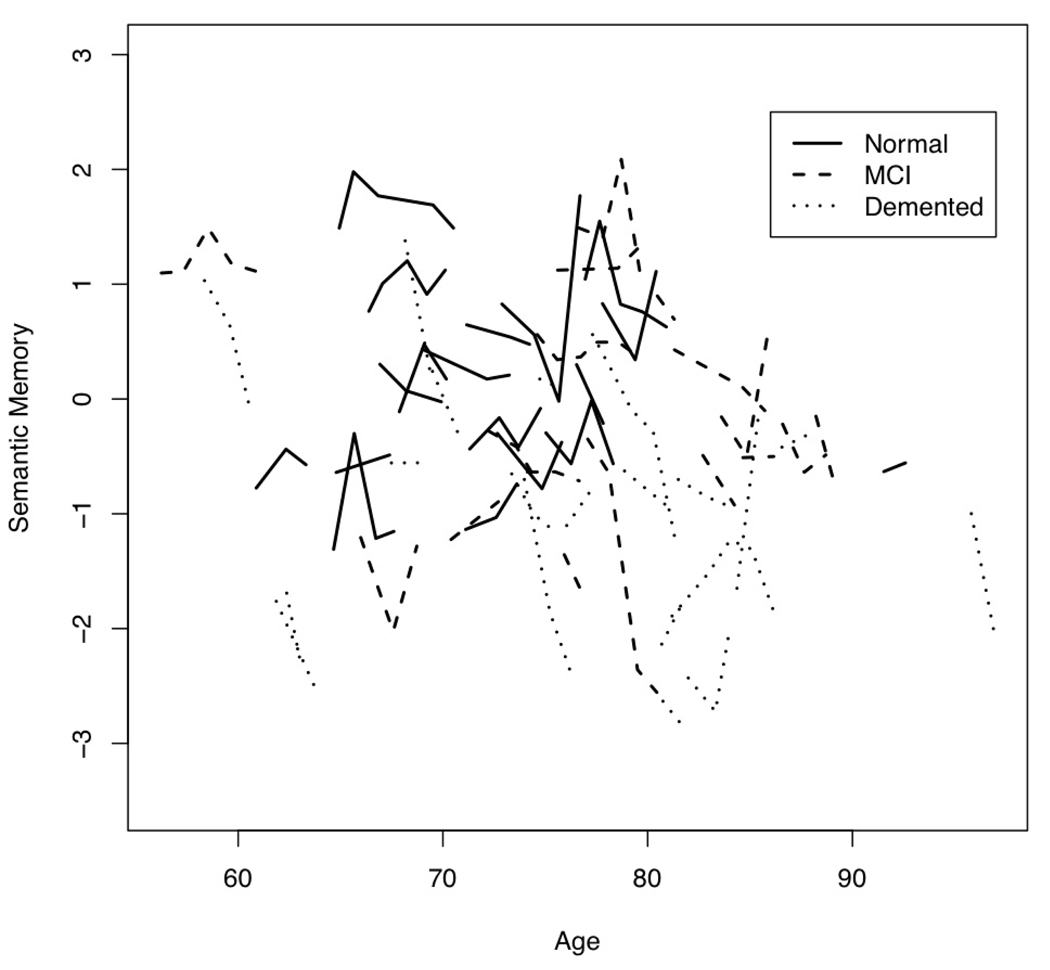
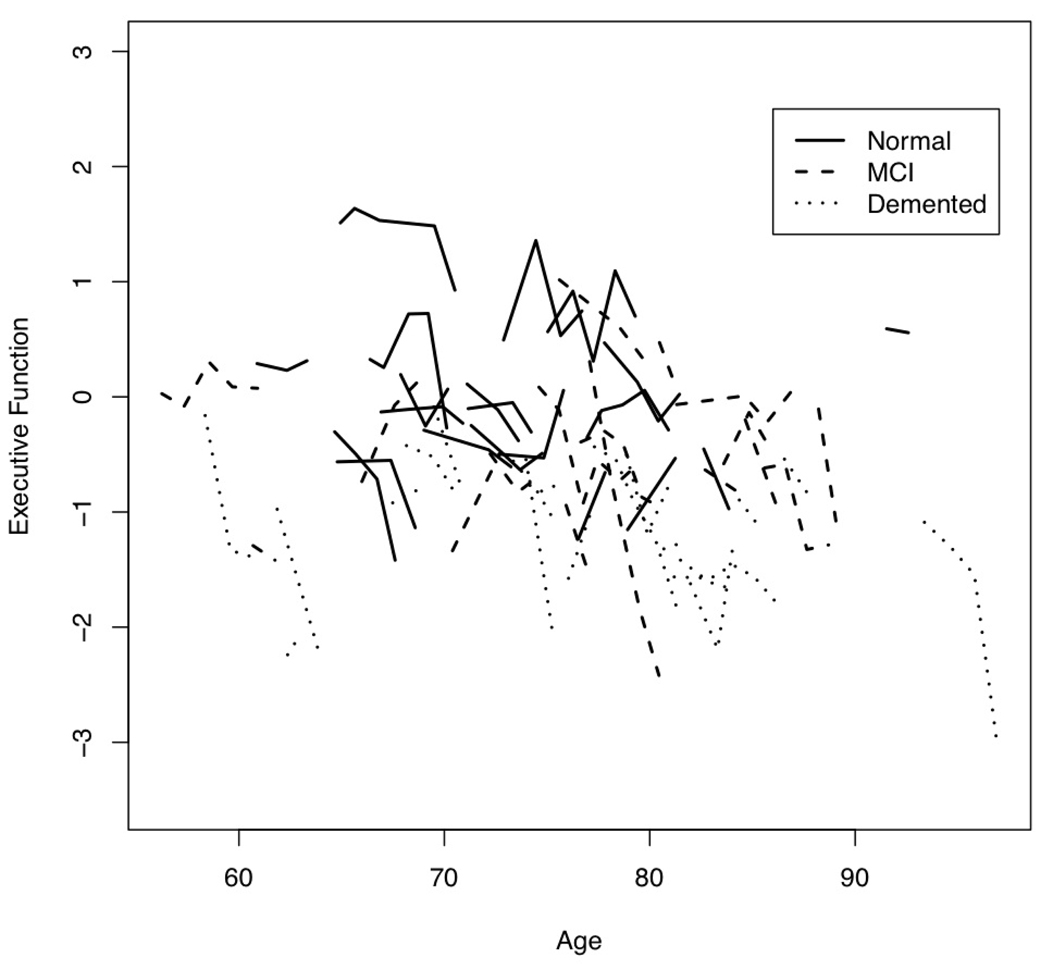
We first examined empirical trajectories for the three cognitive outcome variables. Figure 1 is a plot of Episodic Memory by age at evaluation for 20 randomly selected participants in each baseline diagnosis group. Figure 2 presents this same information for Semantic Memory, and Figure 3 for Executive Function.
Figure 1.
Plot of Episodic Memory by age at annual evaluations for 20 randomly selected participants from each baseline diagnosis group.
Figure 2.
Plot of Semantic Memory by age at annual evaluations for 20 randomly selected participants from each baseline diagnosis group.
Figure 3.
Plot of Executive Function by age at annual evaluations for 20 randomly selected participants from each baseline diagnosis group.
The next set of mixed effects models assessed overall rate of change by including time as the only predictor for Executive Function, adding Form to time for Episodic Memory, and adding previous exposure to time for Semantic Memory. Table 3 presents covariance parameters for the random effects for each of the three measures. Each cognitive measure showed significant decline over time in this aging cohort and there was significant between-person variation not only in baseline level but also in estimated rate of decline. Subsequent models examined predictors of this heterogeneity.
Table 3.
Random effect covariance parameter estimates for models with time as the only fixed effect parameter for Executive Function, time + form for Episodic Memory, and time + previous exposure for Semantic Memory.
| Cognitive Measure | Parameter | Estimate (S.E.) | p |
|---|---|---|---|
| Episodic Memory | Intercept Variance | 0.749 (0.067) | 0.001 |
| Slope Variance | 0.017 (0.004) | 0.001 | |
| Intercept-Slope Covariance | 0.006 (0.013) | ns | |
| Residual Variance | 0.199 (0.012) | 0.001 | |
| Semantic Memory | Intercept Variance | 0.683 (0.057) | 0.001 |
| Slope Variance | 0.007 (0.003) | 0.009 | |
| Intercept-Slope Covariance | 0.100 (0.100) | ns | |
| Residual Variance | 0.098 (0.007) | 0.001 | |
| Executive Function | Intercept Variance | 0.430 (0.036) | 0.001 |
| Slope Variance | 0.017 (0.003) | 0.001 | |
| Intercept-Slope Covariance | 0.005 (0.007) | ns | |
| Residual Variance | 0.078 (0.005) | 0.001 |
Baseline Diagnosis
The next stage of mixed model analyses examined how baseline diagnosis was associated with baseline scores and rates of change of the three cognitive outcome variables. Table 4 presents results of these analyses. There were very robust differences across groups in baseline scores for all three cognitive variables (p's<.001). Differences of group means for Episodic Memory were most substantial, spanning a range of about 1.7 s.d. in comparison with a range of 1.0 for Semantic Memory and 0.9 for Executive Function. Rates of change significantly differed across diagnostic groups for Semantic Memory and Executive Function (p’s<.001). For both of these variables, MCI and Dementia mean change significantly differed from that for Normals, but MCI – Dementia differences were not significant. Change for Normals for Semantic Memory did not differ from zero, while average change in Executive Function was slightly but significantly less than zero. Episodic Memory showed a different pattern of change in relation to diagnosis. All three groups, including Normals, showed significant decline, but rate of change did not significantly differ across the three diagnostic groups.
Table 4.
Cognitive baseline and change estimates by baseline clinical diagnosis.
| Model-Estimated Mean Baseline Level |
Model-Estimated Mean Annual Change |
||||
|---|---|---|---|---|---|
| Cognitive Measure | Diagnosis Group | Estimate | S.E. | Estimate | S.E. |
| Episodic Memory | Normal | 0.13bc | 0.05 | −0.05 | 0.02 |
| MCI | −0.86ab | 0.07 | −0.07 | 0.02 | |
| Dementia | −1.56ab | 0.10 | −0.12 | 0.04 | |
| Semantic Memory | Normal | 0.33bc | 0.06 | 0.00bc | 0.01 |
| MCI | 0.07ac | 0.07 | −0.11a | 0.02 | |
| Dementia | −0.66ab | 0.11 | −0.14a | 0.03 | |
| Executive Function | Normal | 0.07bc | 0.05 | −0.03bc | 0.01 |
| MCI | −0.27ac | 0.06 | −0.15a | 0.02 | |
| Dementia | −0.86ab | 0.08 | −0.15a | 0.03 | |
Group N's: Normal = 188, MCI = 125, Dementia = 56
Note. Results show model-estimated mean baseline level and annual change for each diagnostic group for each cognitive variable, presented in standard deviation units. Means that are significantly different from zero are bolded. Significant differences among pairwise comparisons of group means using a Bonferroni corrected p value of .017 are indicated by superscript letters.
(significantly different from Normal,
significantly different from MCI,
significantly different from Dementia)
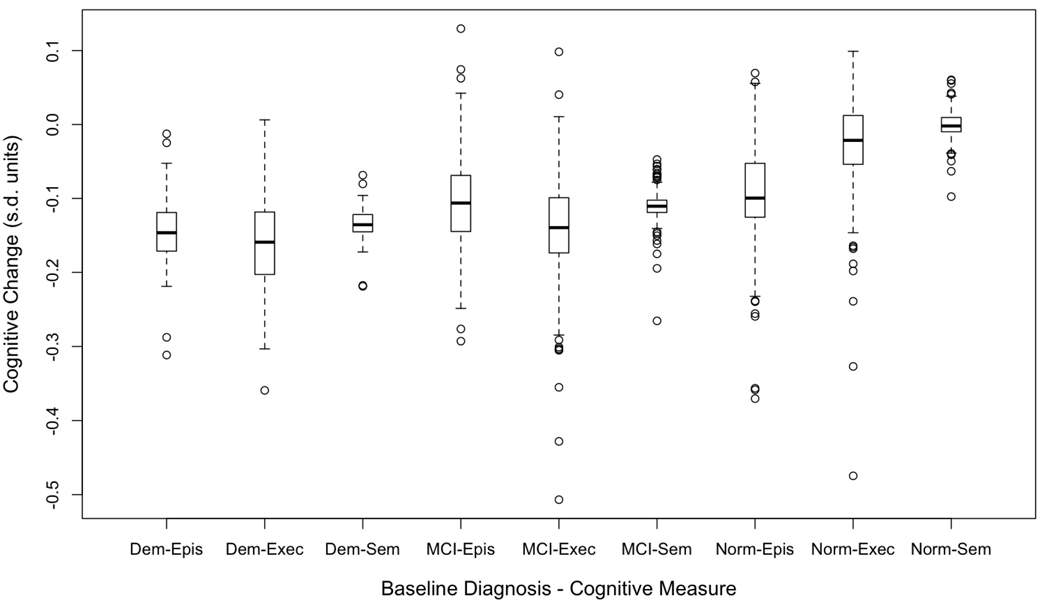
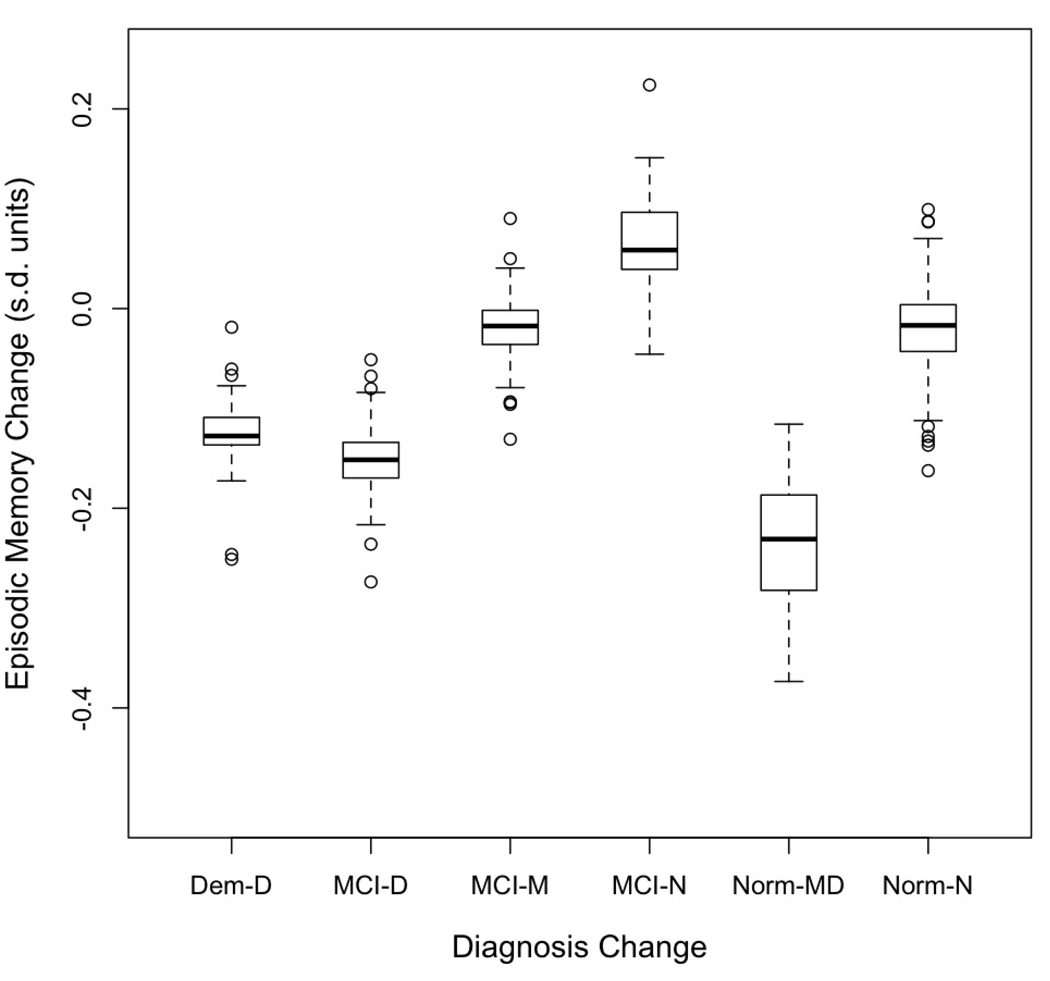
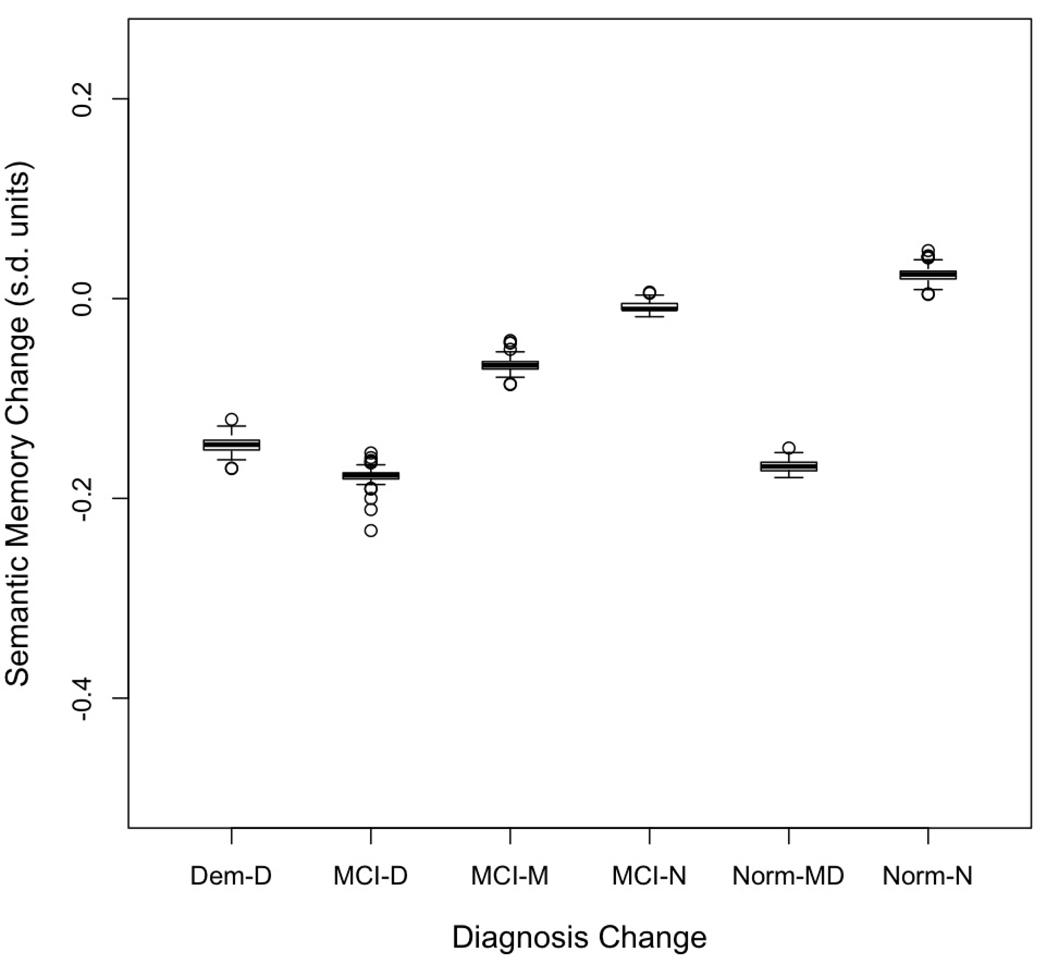
Figure 4 presents box plots of estimated person-specific change (sum of the predicted change and the person-specific random slope) in the three cognitive variables for the three diagnostic groups. Median annual change (center of the box) reflects the differences between the diagnostic groups summarized from Table 4, but variation (length of box and whiskers, presence of outliers) reveals additional patterns. First, variability of change in Semantic Memory was less than for Episodic Memory and Executive Function. Second, there was considerable overlap in rate of change in the three diagnostic groups with the possible exception that Normals showed less overlap with MCI and Dementia for Semantic Memory.
Figure 4.
Box plots showing distributions of annual rate of change in cognitive variables as measured by the sum of the predicted change for the diagnostic group and the person specific random deviation from this predicted value. The box demarcates the range from the 25th to the 75th percentile of the distribution, the line in the middle of the box is the 50th percentile, the lower horizontal line is at the 10th percentile, and the upper horizontal line is at the 90th percentile. Dem = Dementia, MCI = Mild Cognitive Impairment, Norm = Normal, Epis = Episodic Memory, Sem = Semantic Memory, Exec = Executive Function.
MCI Subtypes
The next analyses used an expanded baseline diagnosis variable that included MCI subtypes (Amnestic - n=57, Amnestic Plus - n=21, Non-Amnestic Single Domain - n=13, and Non-Amnestic Multiple Domain - n=24) along with Normal and Dementia. Differences in average rates of change of MCI subtypes were of primary interest. Table 5 shows average baseline and slope estimates for the MCI subtypes. The Amnestic Plus group showed the lowest average baseline scores and the highest average decline of the MCI subtypes. The Amnestic Plus subtype generally declined more rapidly than the other subtypes, showing significant and relatively large changes. Amnestic Plus was the only group to decline on Episodic Memory (pure Amnestic did not).
Table 5.
Cognitive baseline and change estimates by MCI subtypes from baseline clinical diagnosis.
| Model-Estimated Mean Baseline Level |
Model-Estimated Mean Annual Change |
||||
|---|---|---|---|---|---|
| Cognitive Measure | Diagnosis Group | Estimate | S.E. | Estimate | S.E. |
| Episodic Memory | Amnestic | −0.94 | 0.09 | −0.06 | 0.04 |
| Amnestic Plus | −1.21cd | 0.15 | −0.15 | 0.06 | |
| Non-Amnestic Single Domain |
−0.55b | 0.19 | 0.04a | 0.07 | |
| Non-Amnestic Multiple Domain |
−0.50b | 0.14 | −0.05 | 0.05 | |
| Semantic Memory | Amnestic | 0.33b | 0.11 | −0.10 | 0.03 |
| Amnestic Plus | −0.44a | 0.18 | −0.21 | 0.04 | |
| Non-Amnestic Single Domain |
0.26 | 0.22 | −0.05 | 0.05 | |
| Non-Amnestic Multiple Domain |
0.00 | 0.16 | −0.07 | 0.03 | |
| Executive Function | Amnestic | −0.03bd | 0.08 | −0.16 | 0.02 |
| Amnestic Plus | −0.62a | 0.13 | −0.25d | 0.04 | |
| Non-Amnestic Single Domain |
−0.20 | 0.16 | −0.09 | 0.05 | |
| Non-Amnestic Multiple Domain |
−0.44a | 0.12 | −0.06b | 0.04 | |
Group N's: Amnestic = 57, Amnestic Plus = 21, Non-Amnestic Single Domain = 13, Non-Amnestic Multiple Domain = 24, subtype not available = 10
Note. Results show model-estimated mean baseline level and annual change for each diagnostic group for each cognitive variable, presented in standard deviation units. Means that are significantly different from zero are bolded. Significant differences among pairwise comparisons of group means using a Bonferroni corrected p value of .008 are indicated by superscript letters.
(significantly different from Amnestic,
significantly different from Amnestic Plus,
significantly different from Non-Amnestic Single Domain,
significantly different from Non-Amnestic Multiple Domain)
Diagnosis Change
Change in diagnosis was the independent variable in the next set of random effects analyses. Table 6 presents mean estimated baseline scores and rates of change for diagnosis change groups, and indicates which estimates were statistically different from zero. This diagnosis variable was significantly related to the baseline and change effects for all three cognitive variables. Again, the change effects are of most interest. Figure 5 presents box plots showing medians and distributions of change scores for Episodic Memory, Figure 6 presents the same information for Semantic Memory, and Figure 7 for Executive Function. There was a consistent pattern of results across the three cognitive outcome variables. High and similar average rates of cognitive decline for all three cognitive variables were present for cases with Dementia, MCI progressing to Dementia, and Normal progressing to MCI or Dementia. Average decline for all three measures within these three groups ranged from 0.13 to 0.24 s.d. per year. Stable MCI had modest average change ranging from a decline of 0.02 to an increase of 0.09 s.d. per year. Change for MCI reverting to Normal was not significantly different from zero for any of the cognitive variables. Similarly, change for stable Normal was not different from zero.
Table 6.
Cognitive baseline and change estimates by change in clinical diagnosis from first to last evaluation.
| Model-Estimated Mean Baseline Level |
Model-Estimated Mean Annual Change |
||||
|---|---|---|---|---|---|
| Cognitive Measure | Diagnosis Group | Estimate | S.E. | Estimate | S.E. |
| Episodic Memory | Normal-Normal | 0.16cdef | 0.06 | −0.02be | 0.02 |
| Normal-MCI/Dem | −0.20def | 0.14 | −0.24acd | 0.04 | |
| MCI-Normal | −0.58af | 0.17 | 0.07bef | 0.05 | |
| MCI-MCI | −0.80abf | 0.09 | −0.02b | 0.04 | |
| MCI-Dementia | −1.07abf | 0.10 | −0.15ac | 0.03 | |
| Dementia-Dementia | −1.59abcde | 0.10 | −0.13c | 0.03 | |
| Semantic Memory | Normal-Normal | 0.31f | 0.06 | 0.02bdef | 0.01 |
| Normal-MCI/Dem | 0.40f | 0.17 | −0.17ac | 0.04 | |
| MCI-Normal | −0.07 | 0.19 | −0.01bef | 0.04 | |
| MCI-MCI | −0.04f | 0.10 | −0.07ae | 0.03 | |
| MCI-Dementia | 0.25f | 0.12 | −0.18acd | 0.02 | |
| Dementia-Dementia | −0.64abde | 0.12 | −0.15ac | 0.03 | |
| Executive Function | Normal-Normal | 0.07def | 0.05 | −0.00bdef | 0.01 |
| Normal-MCI/Dem | 0.05f | 0.13 | −0.20ac | 0.03 | |
| MCI-Normal | −0.15f | 0.15 | −0.01bef | 0.03 | |
| MCI-MCI | −0.34af | 0.08 | −0.09ae | 0.02 | |
| MCI-Dementia | −0.24af | 0.09 | −0.24acd | 0.02 | |
| Dementia-Dementia | −0.87abcde | 0.09 | −0.17ac | 0.02 | |
Group N's: Normal-Normal = 165, Normal-MCI/Dem = 23, MCI-Normal = 17, MCI-MCI = 63, MCI-Dementia = 45, Dementia-Dementia = 51
Note. Results show model-estimated mean baseline level and annual change for each diagnostic group for each cognitive variable, presented in standard deviation units. Means that are significantly different from zero are bolded. Significant differences among pairwise comparisons of group means using a Bonferroni corrected p value of .003 are indicated by superscript letters.
(significantly different from Normal-Normal,
significantly different from Normal-MCI/Dem,
significantly different from MCI-Normal,
significantly different from MCI-MCI
significantly different from MCI-Dementia,
significantly different from Dementia-Dementia)
Figure 5.
Box plots showing distributions of annual rate of change in Episodic Memory as measured by the sum of the predicted change for the diagnostic group and the person specific random deviation from this predicted value. The box demarcates the range from the 25th to the 75th percentile of the distribution, the line in the middle of the box is the 50th percentile, the lower horizontal line is at the 10th percentile, and the upper horizontal line is at the 90th percentile. Dem-D = Dementia at first and at last evaluation, MCI-D = MCI at first evaluation, Dementia at last, MCI-MCI = MCI at both evaluations, MCI-N = MCI at first and Normal at last, Norm-MD = Normal at first evaluation, MCI or Dementia at last, Norm-N = Normal at first and last evaluations.
Figure 6.
Box plots showing distributions of annual rate of change in Semantic Memory as measured by the sum of the predicted change for the diagnostic group and the person specific random deviation from this predicted value. The box demarcates the range from the 25th to the 75th percentile of the distribution, the line in the middle of the box is the 50th percentile, the lower horizontal line is at the 10th percentile, and the upper horizontal line is at the 90th percentile. Dem-D = Dementia at first and at last evaluation, MCI-D = MCI at first evaluation, Dementia at last, MCI-MCI = MCI at both evaluations, MCI-N = MCI at first and Normal at last, Norm-MD = Normal at first evaluation, MCI or Dementia at last, Norm-N = Normal at first and last evaluations.
Figure 7.
Box plots showing distributions of annual rate of change in Executive Function as measured by the sum of the predicted change for the diagnostic group and the person specific random deviation from this predicted value. The box demarcates the range from the 25th to the 75th percentile of the distribution, the line in the middle of the box is the 50th percentile, the lower horizontal line is at the 10th percentile, and the upper horizontal line is at the 90th percentile. Dem-D = Dementia at first and at last evaluation, MCI-D = MCI at first evaluation, Dementia at last, MCI-MCI = MCI at both evaluations, MCI-N = MCI at first and Normal at last, Norm-MD = Normal at first evaluation, MCI or Dementia at last, Norm-N = Normal at first and last evaluations.
Demographic Covariates and Diagnosis
Diagnosis was the only independent variable in the previously described models. Demographic covariates were omitted for simplicity of presentation, but subsequent models added gender, age at baseline evaluation, years of education, and ethnicity (African American, Hispanic, Caucasian) as independent variables to explain baseline and change in cognitive outcomes. The results were essentially unchanged by adding these covariates (not shown). Recruitment source (community versus clinic) also was added along with the other demographic covariates. Recruitment source was not independently related either to baseline status or rate of change for any measure, and did not change results in a meaningful way. An additional set of analyses excluded cases that had died or were lost to follow-up. Again, results were essentially the same.
Age, ApoE, CDR
Age, ApoE genotype, and the CDRSum were used as external explanatory variables in the next analyses. When entered alone, age at baseline was significantly, negatively associated with both baseline and change for all three cognitive variables. ApoE ε4 positive cases had lower baseline scores (−0.62±0.08 versus −0.36±0.07) and faster decline (−0.12±0.02 versus −0.04±0.02) for Episodic Memory. ApoE ε4 was related to decline of Executive Function (−0.14±0.02 versus −0.07±0.01), but was not associated with baseline status. Semantic Memory was not related to ApoE genotype. Baseline CDRSum was first entered alone and was negatively related to baseline (p's<.001) and change (p's<.001 for Semantic Memory and Executive Function, p=.002 for Episodic Memory) of all three cognitive variables. Change in CDRSum, when added as a time varying independent variable, was strongly related to all three variables (p's<.001).
Age, ApoE and baseline CDRSum were entered jointly as independent variables to examine independent predictive effects from the baseline evaluation. Age was independently related to baseline Episodic Memory and Executive Function (p's<.001), but was not related to change of any of the cognitive variables independent of ApoE and CDRSum. CDRSum was independently related to baseline for all three variables (p's<.001) and was related to change for Semantic Memory and Executive Function (p's<.001). ApoE was not related to baseline for any cognitive domain. ApoE was the only independent predictor of change in Episodic Memory (p<.001) and was not independently associated with change in the other two domains. CDRSum was the only independent predictor of change in Executive Function and Semantic Memory (p's<.001). Thus, change in Executive Function and Semantic Memory was predicted by baseline global status as measured by the CDRSum. While baseline Episodic Memory was strongly related to CDRSum, change in this measures was predicted by ApoE. Interestingly, age was not associated with cognitive change after accounting for ApoE and CDRSum effects.
Discussion
There were two primary findings from this study. First, there was evidence of considerable heterogeneity of cognitive trajectories in this very diverse sample. Second, baseline clinical diagnosis was limited in its ability to explain this heterogeneity. Of particular clinical relevance, MCI cases had variable cognitive trajectories such that some improved, some did not change, and some declined at a rapid rate. There also was important variability in those diagnosed as cognitively normal at baseline; the majority did not decline but a small subgroup declined at a particularly rapid rate. This latter group may represent an important target for early identification and treatment to prevent brain injury and cognitive decline.
Consistent with many previous studies of cognitive change in old age (Albert, et al., 1995; Christensen, et al., 1999; Colsher & Wallace, 1991; Rubin, et al., 1998; Schaie, 1988; Wilson, Beckett, et al., 2002; Zelinski, et al., 1993) we found extensive heterogeneity in individual trajectories of longitudinal change across all three cognitive domains, with rate of change for most participants ranging from decline of 0.3 standard deviations per year to improvement of 0.1 s.d. per year. Analyzing rates of change in relation to diagnosis revealed two principle findings. First, baseline diagnosis was informative regarding the probability of major decline, but otherwise held relatively little prognostic information. Of the cases normal at baseline, only 12% declined to MCI or dementia, whereas 36% of the baseline MCI cases became demented. Despite this, cognitive decline did not always differ according to diagnosis. For example, all three diagnostic groups declined on Episodic Memory at statistically significant rates that did not differ from each other, and while the MCI and dementia groups declined more rapidly than the Normal group on Semantic Memory and Executive Function, the two impaired groups did not decline at different rates. Equally important, the extent of overlap between the groups in the distribution of change rates on all the cognitive measures was considerable.
Second, we found that cognitive change for all three domains showed a closer correspondence to change in independently determined clinical diagnosis than to baseline diagnosis. Sorting cases so as to compare those that remained stable or improved diagnostically versus those that changed for the worse showed that rate of decline was substantial and similar in Normals converting to MCI or Dementia, MCI converting to Dementia, and those who had Dementia at the first and last evaluations. It would be expected that cognitive change should correspond to change in diagnosis since both presumably reflect the same underlying disease processes. These results nevertheless support the validity of both the cognitive outcome measures and the clinical diagnosis, especially since the cognitive outcome measures were excluded from consideration in establishing the diagnosis at baseline and follow-up evaluations. Change in diagnosis could result from random variation in cognitive test results from one assessment to the next within the limits of test reliability with no real change in underlying ability. Having cognitive outcome measures excluded from the diagnostic process minimizes a spurious source of correlation between clinical diagnosis and cognitive outcomes due to random unreliability influencing both variables in the same way. The strong relationship between change in the CDR sum of boxes and the cognitive outcome measures provided further independent validation of the ability of the cognitive outcome measures to detect clinically relevant change since the CDR was completed blind to all other results of the evaluations.
The finding that subsets of Normal, MCI, and Dementia cases who progressed clinically showed relatively rapid and similar rates of decline in multiple cognitive domains is particularly interesting. It is noteworthy that the rate of decline for the sub group of Normals that progressed clinically was equal to or exceeded that of the MCI and Dementia sub groups. The use of psychometrically matched measures facilitated investigation of differential effects across cognitive domains; these particular tests have high reliability over a wide ability range and these results are not artifacts of test floor or ceiling effects (Crane, et al., 2008; Mungas, et al., 2004; Mungas, Reed, Tomaszewski Farias, et al., 2005). Rather, the findings indicate that Normal and MCI cases that were deteriorating clinically were declining rapidly, at a rate similar to cases with Dementia, in all three domains that we tested. One hypothesis to explain the similar rates of decline in these three groups is that the observed cognitive change resulted from the same pathological process or processes.
Several findings suggest that verbal episodic memory is the cognitive domain most vulnerable to the early effects of pathology in this sample. Episodic Memory declined, on average, in the baseline Normal group at a rate that did not significantly differ from that of MCI and Dementia groups, was nearly double that of Executive Function, and about five times greater than for Semantic Memory. Group averages can obscure within group variability, and the average decline in the baseline Normals appeared to be due to the sub group that worsened clinically and declined at a particularly rapid rate; average change for stable Normals was not different from zero. In the Normals who worsened clinically, the rate of decline of Episodic Memory (−0.24±0.04) exceeded that of Executive Function (−0.20±0.03) and Semantic Memory (−0.17±0.04). In addition, differences between the diagnostic groups at baseline were greatest for Episodic Memory. In a cognitively diverse sample, baseline status provides a cross-sectional snapshot, taken at the time of the initial evaluation, of longitudinal trajectories. Consequently, these results suggest that there had been more decline in Episodic Memory preceding enrollment in this study than in other domains.
Various pathological changes could underlie this decline. Certainly, AD is a plausible etiology, and other studies have linked subtle verbal episodic memory impairment to AD neuropathology in cognitively normal individuals (Bennett, et al., 2006). However, several community based autopsy studies have now shown that the most common neuropathological substrate of dementia is a mix of AD and other pathologies, especially vascular (Schneider, et al., 2007; Schneider, Wilson, Bienias, Evans, & Bennett, 2004; White, et al., 2005). Episodic memory has been widely shown to be a sensitive indicator of varied abnormalities of brain structure and function. Episodic memory has been strongly linked to the hippocampus and a circuit of interconnected limbic structures. The hippocampus is vulnerable to injury from a variety of causes, including trauma, anoxia, infection, metabolic disorders, and most commonly in older persons, degenerative disease, especially AD. Results of this study are consistent with the hypothesis that the observed Episodic Memory decline is an indicator of increasing hippocampal damage that could result from AD as well as other pathologies. This hypothesis is supported by studies showing that hippocampal volume loss is associated with episodic memory decline (Kramer, et al., 2007; Mungas, Harvey, et al., 2005; Stoub, Rogalski, Leurgans, Bennett, & Detoledo-Morrell, 2008) and can be identified even in cognitively normal individuals (Csernansky, et al., 2005; Morra, et al., 2009; Xu, et al., 2008).
There has been much work focused on the concept and diagnosis of MCI, with a goal of identifying clinical characteristics that predict development of dementia and enabling treatment as early in the course as possible. While consistent with previous literature on MCI, particularly studies showing variable clinical outcomes in MCI cases from community samples (Ganguli, et al., 2004; Manly, et al., 2008; Palmer, et al., 2002; Ritchie, et al., 2001) and most rapid decline in MCI cases with multiple cognitive impairments (Arnaiz, et al., 2004; Artero, Petersen, Touchon, & Ritchie, 2006; Bozoki, Giordani, Heidebrink, Berent, & Foster, 2001; Guarch, Marcos, Salamero, & Blesa, 2004; Manly, et al., 2008; Tabert, et al., 2006), this study also makes a number of other points. The breadth of baseline cognitive functioning allowed us to identify a sub group of Normal individuals (12%) who progressed to MCI or Dementia at a rapid rate. This study did not sample in a way that permits estimation of population parameters and so our observation may over (or under) estimate rates of conversion in normal older adults. However, because the number of cognitively normal cases in the older population is large in relation to the number with MCI, confining attempts to detect persons at highest risk for rapid conversion to AD will miss large numbers of people who in fact deteriorate clinically over just a few years. This underscores the need to develop more sensitive and specific indicators of dementia risk.
Cognitive change for all three domains showed a closer correspondence to change in diagnosis than to baseline diagnosis. A central point is that less than optimal correspondence of cognitive trajectories with baseline diagnosis does not result from invalidity of the measures for assessing cognitive change because they do track as would be expected with change in diagnosis. From a clinical perspective, few would doubt that longitudinal follow-up provides better characterization of disease processes, but repeated evaluations are not always available. A clinical history of cognitive decline obtained in a single evaluation might provide information about longitudinal course and could potentially substitute for longitudinal evaluations. Unfortunately, results of this study do not support this idea. A clinical history of cognitive decline was routinely obtained as part of the clinical evaluations in this study, and the predictive utility of the baseline diagnosis was still limited. While clinical diagnosis based on a single evaluation may be useful for summarizing and efficiently communicating complex clinical results, the prognostic significance of diagnosis in diverse populations is less clear. That is because there is considerable variability of cognitive trajectories with substantial overlap across diagnostic groups.
Results related to ApoE and the CDR sum of boxes show how external variables can be evaluated as potential predictors of cognitive decline. The relationship of Episodic Memory with ApoE is consistent with previous research (Wilson, Schneider, et al., 2002) and supports the hypothesis that verbal episodic memory change is a good indicator of developing AD related brain injury across the full spectrum of impairment (Mungas, Harvey, et al., 2005). The obtained results fit expectations, and importantly, both of these variables were better predictors of subsequent change in Episodic Memory than was baseline diagnosis. The CDR essentially assesses real world function in several domains, and thus provides a global measure of impairment. These results are consistent with previous literature (Daly, et al., 2000) and show that greater functional impairment is associated with more rapid cognitive decline. MCI subtype analyses showed most rapid decline in Amnestic Plus MCI cases, and this further supports the hypothesis that rate of decline is more rapid in those who are more impaired. However, the rapidly declining Normals are a notable exception to this general rule. This is a group that is particularly important to identify and efforts to find predictors of decline in those without clinically significant cognitive impairment are especially important.
Semantic Memory showed the greatest stability across longitudinal measurements. This is not surprising because semantic memory is regarded as a crystallized ability that is acquired over a lifetime and is relatively resistant to factors causing decline of cognitive abilities. In contrast, verbal episodic memory and executive function are fluid abilities that are more susceptible to change. While Semantic Memory may not be the most sensitive indicator of cognitive decline, it did show sensitivity to change in the declining Normal subgroup, and results suggests that it might be a good measure of longitudinal change once the process of decline has been identified.
This study had a number of important methodological strengths. First, the sample was unusually diverse in many respects, notably in terms of demographic characteristics and range of baseline cognitive performance. This sample also included clinic patients as well as community recruits, which contributed to diversity of trajectories. Second, the cognitive outcome measures were constructed so that measurement properties were highly similar across measures of different domains as well as across groups of subjects, making it possible to evaluate domain and group differences independent of confounding psychometric characteristics and measurement bias. Third, the cognitive outcome measures used to define longitudinal trajectories were independent of clinical diagnosis. Finally, there was a relatively high follow-up rate of individuals enrolled in this study.
There also were important limitations of this study. First and foremost, the sample essentially was a sample of convenience, even though there was considerable and largely successful effort directed at community based recruitment to enroll individuals who were broadly representative of demographic characteristics and cognitive function in the target populations (Hinton, et al., In Press). Clinic patients were predominantly Caucasians and had higher levels of education, were predominantly MCI, and were more likely to progress from MCI to dementia than were community MCI cases (Tomaszewski Farias, Mungas, Reed, Harvey, & DeCarli, In Press ). A consequence of this complexity in the structure of the sample is that the ethnic groups in this study may systematically differ in clinical and disease characteristics that underlie trajectories of change, making it difficult to directly compare results across the different groups. The sample size also makes it difficult to directly compare predictors of cognitive decline across ethnic groups. These types of comparisons essentially involve interaction effects, and statistical power with the available sample is not adequate for interaction effects involving specific ethnic groups. Non-significant differences across groups, in particular, would be difficult to interpret since true lack of differences could not be distinguished from lack of adequate statistical power to detect differences. While this study did not directly compare findings across ethnic groups, it did evaluate relationships in an unusually diverse sample, which should facilitate generalizability to a broad older population. In addition, results were essentially unchanged after controlling for effects of recruitment source, ethnicity, and other demographic variables.
Another limitation is that amount of follow-up, especially for those more recently enrolled, was limited. Follow-up time influences stability of estimates of change, statistical power for identifying predictors of change, and ability to detect non-linear change. With respect to stability of estimated change, the random effects models used in this study take advantage of all available data to estimate model parameters including within person and between person error variances. Estimation of within person error variance is particularly important for estimation of within person trajectories, and typically a majority of cases must have three or more evaluations in order to obtain stable estimates of within person error. About 70% of the participants in this study had three or more assessments, and consequently, the available sample most likely is sufficient to reliably estimate within person change apart from random error. The main reason for shorter trajectories for some participants was rolling enrollment, thus short follow-up in some is unlikely to introduce bias in estimates of change due to informative drop-out or missingness. Empirically, all three cognitive outcome variables showed significant variability in linear change, and the sample size for this study was sufficient to identify with 80% power an external variable that explains less than 2% of the variance in cognitive change. This is a relatively small effect size for practical purposes, and thus statistical power is not a major limitation for this study. The relatively short overall follow-up time, however, does present limitations, notably that there is not sufficient follow-up to evaluate non-linear trends.
One of the most striking findings was the heterogeneity of rates of change within and across diagnostic groups. This study addressed the clinical question of what baseline diagnosis tells us about prognosis for subsequent cognitive decline. These results indicate that diagnosis alone provides a limited account of subsequent change. Brain injury and disease, the major drivers of cognitive decline, operate on the background of the maximal attained cognitive function. Variability in type, severity, extent, and localization of brain pathology and dysfunction is associated with variable change from the pre-existing baseline (Keller, 2006). Consequently, the cognitive status of an older person at any single time point is a complex function of the variables contributing to lifelong status, brain injury and disease, and other variables than can affect cognitive function including, for example, depression, health status, and exposure to substances and medications. Unraveling the multiple deleterious and protective factors that ultimately determine what is now unexplained variance in course of cognitive function in old age remains an enormous challenge. Results of this study suggest that investigations of these determinants will be most effective if samples include diversity of baseline cognitive function. Normal, MCI, and cases with dementia that are declining may be more similar etiologically than are cases within each of these diagnostic classification who decline at different rates, and these patterns will not be identifiable if the range of cognitive performance at baseline is arbitrarily restricted.
Acknowledgments
This work was supported in part by National Institute on Aging Grants AG10220, AG10129, and AG021028 and by the California Department of Public Health Alzheimer’s Disease Program Contracts 06-55311 and 06-55312.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pag
References
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychology and Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third Edition Revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- Arnaiz E, Almkvist O, Ivnik RJ, Tangalos EG, Wahlund LO, Winblad B, et al. Mild cognitive impairment: a cross-national comparison. Journal of Neurology Neurosurgery and Psychiatry. 2004;75:1275–1280. doi: 10.1136/jnnp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artero S, Petersen R, Touchon J, Ritchie K. Revised criteria for mild cognitive impairment: validation within a longitudinal population study. Dementia Geriatrics and Cognitive Disorders. 2006;22:465–470. doi: 10.1159/000096287. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Li Y, Gilley DW, Bennett DA, Evans DA. Change in cognitive function in Alzheimer's disease in African-American and white persons. Neuroepidemiology. 2006;26:16–22. doi: 10.1159/000089231. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Blasko I, Jellinger K, Kemmler G, Krampla W, Jungwirth S, Wichart I, et al. Conversion from cognitive health to mild cognitive impairment and Alzheimer's disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiology of Aging. 2008;29:1–11. doi: 10.1016/j.neurobiolaging.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Archives of Neurology. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, et al. An analysis of diversity in the cognitive performance of elderly community dwellers: individual differences in change scores as a function of age. Psychology and Aging. 1999;14:365–379. doi: 10.1037//0882-7974.14.3.365. [DOI] [PubMed] [Google Scholar]
- Colsher PL, Wallace RB. Longitudinal application of cognitive function measures in a defined population of community-dwelling elders. Annals of Epidemiology. 1991;1:215–230. doi: 10.1016/1047-2797(91)90001-s. [DOI] [PubMed] [Google Scholar]
- Crane PK, Narasimhalu K, Gibbons LE, Pedraza O, Mehta KM, Tang Y, et al. Composite scores for executive function items: demographic heterogeneity and relationships with quantitative magnetic resonance imaging. Journal of the International Neuropsychological Society. 2008;14:746–759. doi: 10.1017/S1355617708081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, et al. Preclinical detection of Alzheimer's disease: hippocampal shape and volume predict dementia onset in the elderly. Neuroimage. 2005;25:783–792. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Archives of Neurology. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- Embretson SE, Reise SP. Item response theory for psychologists. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Ferrer E, Salthouse TA, McArdle JJ, Stewart WF, Schwartz BS. Multivariate modeling of age and retest in longitudinal studies of cognitive abilities. Psychology and Aging. 2005;20:412–422. doi: 10.1037/0882-7974.20.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, Salthouse TA, Stewart WF, Schwartz BS. Modeling age and retest processes in longitudinal studies of cognitive abilities. Psychology and Aging. 2004;19:243–259. doi: 10.1037/0882-7974.19.2.243. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- Guarch J, Marcos T, Salamero M, Blesa R. Neuropsychological markers of dementia in patients with memory complaints. International Journal of Geriatric Psychiatry. 2004;19:352–358. doi: 10.1002/gps.1074. [DOI] [PubMed] [Google Scholar]
- Hambleton RK, Swaminathan H. Item response theory. Principles and applications. Boston: Kluwer-Nijhoff Publishing; 1985. [Google Scholar]
- Hambleton RK, Swaminathan H, Rogers HJ. Fundamentals of item response theory. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, et al. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer's Disease and Associated Disorders. doi: 10.1097/WAD.0b013e3181c1ee01. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN. Age-related neuropathology, cognitive decline, and Alzheimer's disease. Ageing Research Review. 2006;5:1–13. doi: 10.1016/j.arr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Reed BR, Wetzel M, Burnett M, Miller BL, et al. Longitudinal MRI and cognitive change in normal elderly. Journal of the International Neuropsychological Society. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66:828–832. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annals ofNeurology. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatrica Scandinavica. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, et al. Automated mapping of hippocampal atrophy in 1-year repeat MRI data from 490 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Neuroimage. 2009;45(1 Suppl):S3–S15. doi: 10.1016/j.neuroimage.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer's Disease and Associated Disorders. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): Further development and psychometric characteristics. Psychological Assessment. 2004;16:347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales: relationship to demographics, language, cognition, and independent function. Neuropsychology. 2005;19:466–475. doi: 10.1037/0894-4105.19.4.466. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Marshall SC, González HM. Development of psychometrically matched English and Spanish neuropsychological tests for older persons. Neuropsychology. 2000;14:209–223. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Tomaszewski Farias S, DeCarli C. Criterion-Referenced Validity of a Neuropsychological Test Battery: Equivalent Performance in Elderly Hispanics and Non-Hispanic Whites. Journal of the International Neuropsychological Society. 2005;11:620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K, Wang HX, Backman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. American Journal of Psychiatry. 2002;159:436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, Morris JC, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Archives of Neurology. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- Saito Y, Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007;27:578–584. doi: 10.1111/j.1440-1789.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Albert SM, Manly JJ, Stern Y. Education and rates of cognitive decline in incident Alzheimer's disease. Journal of Neurology Neurosurgery and Psychiatry. 2006;77:308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. Variability in cognitive function in the elderly: implications for societal participation. Basic Life Sciences. 1988;43:191–211. doi: 10.1007/978-1-4684-5460-4_20. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, Detoledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.003. doi:10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Archives of General Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- Tomaszewski Farias S, Mungas D, Reed B, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic versus community-based cohorts. Archives of Neurology. doi: 10.1001/archneurol.2009.106. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. Journal of Geriatric Psychiatry and Neurology. 2005;18:224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer's disease and their relation to age. Psychology and Aging. 2000;15:18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Li Y, Bienias JL, Bennett DA. Cognitive decline in old age: separating retest effects from the effects of growing older. Psychology and Aging. 2006;21:774–789. doi: 10.1037/0882-7974.21.4.774. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, et al. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Archives of Neurology. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- Xu Y, Valentino DJ, Scher AI, Dinov I, White LR, Thompson PM, et al. Age effects on hippocampal structural changes in old men: the HAAS. Neuroimage. 2008;40:1003–1015. doi: 10.1016/j.neuroimage.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ, Schaie KW. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychology and Aging. 1993;8:176–186. doi: 10.1037//0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]