Abstract
Normal aging has been shown to impact performance during human eyeblink classical conditioning as older adults showed lower conditioning levels compared to younger adults. Previous findings showed younger adults can acquire both delay and trace conditioning concurrently, but it is not known whether older adults can learn under the same conditions. Present results indicated older adults failed to produce a significantly greater number of conditioned responses during acquisition, but their ability to time eyeblink responses prior to the unconditioned stimulus was preserved. The decline in eyeblink conditioning that typically accompanies aging has been extended to concurrent presentations of delay and trace conditioning trials.
Classical eyeblink conditioning is a model system for characterizing age-related changes in human learning and memory. In this fundamental form of learning a neutral conditioned stimulus (CS) is paired with a biologically salient, unconditioned stimulus (US). After repeated pairings, the CS alone reliably elicits a conditioned response (CR), indicating that a CS-US association has been formed. In the delay conditioning procedure the CS and US coterminate while in trace conditioning, a silent “trace interval” elapses between the CS offset and US onset.
Age-related effects on behavioral performance and neural activity during eyeblink conditioning have been investigated (Solomon, Beal, & Pendlebury, 1988; Woodruff-Pak, 1988). Numerous human studies have consistently demonstrated that older adults showed fewer conditioned responses than younger adults (Bellebaum & Daum, 2004; Finkbiner & Woodruff-Pak, 1991; Knuttinen, Power, Preston, & Disterhoft, 2001; Solomon, Pomerleau, Bennett, James, & Morse, 1989; Woodruff-Pak & Thompson, 1988). These age-related learning deficits are evident in a number of different conditioning paradigms. Single cue delay (Solomon et al., 1989; Woodruff-Pak & Thompson, 1988), single cue trace (Finkbiner & Woodruff-Pak, 1991), differential trace (Knuttinen et al., 2001), and conditional discrimination (Bellebaum & Daum, 2004) studies reported that younger adults produced higher conditioning levels than older adults.
In addition to its correlation with conditioning performance, normal aging is also associated with functional and morphological changes within two brain regions that are often targeted during investigations of memory and cognition, including eyeblink conditioning: the hippocampus and cerebellum (Rosenzweig & Barnes, 2003; Solomon et al., 1988; Wilson, Gallagher, Eichenbaum, & Tanila, 2006; Woodruff-Pak, 1988). In the hippocampus, fewer synaptic connections, weakened synaptic plasticity, and a volumetric reduction have been found (Barnes, Rao, & Houston, 2000; Geinisman, de Toledo-Morrell, Morrell, Persina, & Rossi, 1992; Golomb et al., 1993; Raz et al., 2004). Similarly in the cerebellum, Purkinje cell loss, fewer cerebellar cortical synapses, and a reduction in parallel fiber conduction velocity have been found in older animals (Andersen, Gundersen, Pakkenberg, 2003; Glick & Bondareff, 1979; Rogers, Zornetzer, & Bloom, 1981). These age-related changes in the brain are often linked to impairments in cognitive functioning. For example, magnetic resonance imaging (MRI) data showed that cerebellar volume strongly correlated with the production of conditioned eyeblink responses (Woodruff-Pak, Goldenberg, Downey-Lamb, Boyko, & Lemieux, 2000) and the degree of hippocampal atrophy predicted performance on a variety of memory tests (Golomb et al., 1996; Petersen et al., 2000). Further, the hippocampus has been shown to be critical for trace learning (Moyer, Deyo, & Disterhoft, 1990; Solomon, Vander Schaaf, Thompson, & Weisz, 1986) and significant hippocampal activity has also been reported during delay learning (Berger, Alger, & Thompson, 1976). Functional MRI (fMRI) results from a recent eyeblink conditioning study (Cheng, Disterhoft, Power, Ellis, & Desmond, 2008) showed trace trials elicited significantly greater human hippocampal responding than delay trials, suggesting that this region is critically relied upon during the associative processes mediating trace conditioning. One possible reason for the conditioning deficits typically seen in older adults may be that both the cerebellum and hippocampus experience significant changes across the lifespan.
The present study is a behavioral investigation of whether older adults can demonstrate both delay and trace conditioning within the same acquisition period. Younger adults have shown significant learning during a conditioning design in which both delay and trace trials are presented within the same acquisition session (Cheng et al., 2008; Knight, Cheng, Smith, Stein, & Helmstetter, 2004) while older adults have only been tested in their capacity to learn either delay or trace conditioning separately (Finkbiner & Woodruff-Pak, 1991; Solomon et al., 1989; Knuttinen et al.. 2001; Woodruff-Pak & Thompson, 1988). Presenting two different conditioning trial types during acquisition introduces a modest level of complexity not present in single-cue experiments. Behavioral CRs from these previous studies (Cheng et al., 2008; Knight et al., 2004) suggest that delay and trace conditioning was possible using this within-subjects design in younger adults, but given the increased design complexity and the effects of aging on the hippocampus and cerebellum, older adults may have difficulty demonstrating significant learning under such conditions.
Method
Participants
Seventeen healthy, right handed volunteers were compensated $20/hr for their participation. Participants were recruited from television advertisements and university flyers. Nine were older adults (64.1 ± 3.8 years; 6 females, 3 males) and eight were younger adults (22.3 ± 2.2 years; 5 females, 3 males). All procedures were approved by the Institutional Review Boards for human subject research at the Johns Hopkins University School of Medicine.
Apparatus
Stimulus presentation and behavioral data acquisition were controlled and analyzed using a laptop computer running custom software developed under LabView version 7.1. Standard headphones were used to present auditory stimuli. Charlie Chaplin’s The Gold Rush was presented on a monitor located approximately two feet from the participant. Standard safety goggles were modified to accommodate both airpuff delivery and an infrared sensor for recording eyeblinks. A fiber-optic probe measured the reflectance of infrared light from the left eye (Miller, Weiss, Disterhoft, & Wyrwicz, 2005) and airpuff delivery was controlled by a solenoid valve.
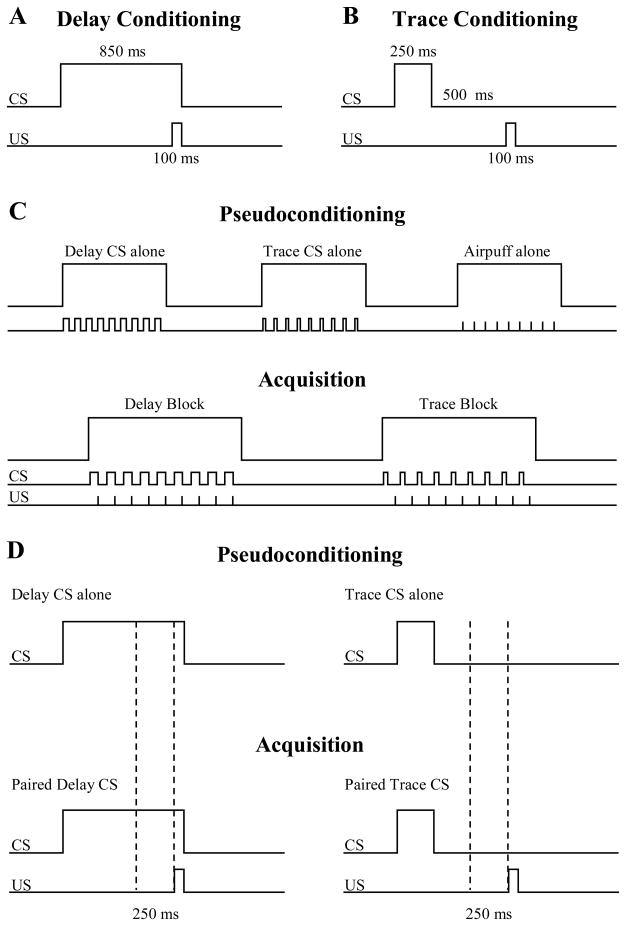
Delay and trace CSs were counterbalanced between a binaural 1200 Hz tone and white noise (90 dB). The delay stimulus lasted 850 ms and coterminated with a 100 ms corneal airpuff (4 psi) to the left eye (Figure 1a). The trace stimulus lasted 250 ms and was followed by a 500 ms trace period before airpuff presentation (Figure 1b). Trials were grouped into blocks: 9 trials/block, 2 sec/trial, 3 sec ITI (CS onset to CS onset), such that each block lasted 27 sec. Pseudoconditioning consisted of alternating four delay alone, four trace alone, and four airpuff alone blocks. Acquisition consisted of alternating 16 delay and 16 trace blocks (e.g. delay-trace-delay, etc; Figure 1c). Pseudoconditioning was seamlessly followed by acquisition blocks and presentation order was counterbalanced amongst subjects.
Figure 1.
The temporal relationship between the CS and US in both delay and trace conditioning. Delay CSs lasted 850 ms and coterminated with a 100 ms US presentation (a). Trace CSs lasted 250 ms and were followed by a 500 ms trace interval before a 100 ms US presentation (b). During pseudoconditioning, participants received four blocks each of delay CS alone, trace CS alone and airpuff alone while in acquisition, participants received 16 delay and 16 trace blocks in an alternating fashion (e). Dotted lines represent the 250 ms time window used to analyze data during pseudoconditioning (top) and acquisition (bottom) (d).
Procedure
Audiometric testing (Solomon et al., 1989) was administered to participants prior to conditioning. Participants were instructed to pay attention to the silent movie while distracting sounds and airpuffs were presented. They were informed that this study investigated the effects that distracting sounds and airpuffs have on their ability to remember details about the movie. Following conditioning, a movie quiz and post-experimental questionnaire assessed awareness of the CS-US contingencies (Clark & Squire, 1998). General cognitive abilities were assessed with the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975), a simple reaction time task, the Alzheimer’s Disease Assessment Scale (recall and recognition) (Rosen, Mohs, & Davis, 1984), subtests of the Wechsler Adult Intelligence Scale-III (WAIS-III; Matrix Reasoning, Digit Span, Digit Symbol, Vocabulary; Wechsler, 1997), and the National Adult Reading Test (Nelson, 1982). Scores from the WAIS-III were age adjusted for each participant’s age.
Data Analysis
A response was considered a CR if the response amplitude (reflectance as measured in voltage; Miller et al., 2005) within a 250 ms pre-US time window exceeded four times the standard deviation of the mean of the baseline period (250 ms pre- CS). This pre-US time window (Figure 1d) was selected to minimize the inclusion of voluntary and alpha (non-associative, orienting responses to the CS) responses as CRs (Gormezano, 1966). Performance was expressed as % CR (number of CRs divided by number of trials). Latency of Peak Response within this same pre-US time window was also calculated as an additional measure of conditioning. This particular index was intended to focus only on temporal properties of responses and did not account for amplitude (as % CR did). Consequently, peak responses did not necessarily meet the definition of conditioned responses. Data were evenly parsed into blocks of 36 trials to assess time-dependent changes as a function of learning. The decision to use the current threshold (4 sd) was made so that comparisons could be made to previous behavioral results from our laboratory (Cheng et al., 2008).
Results
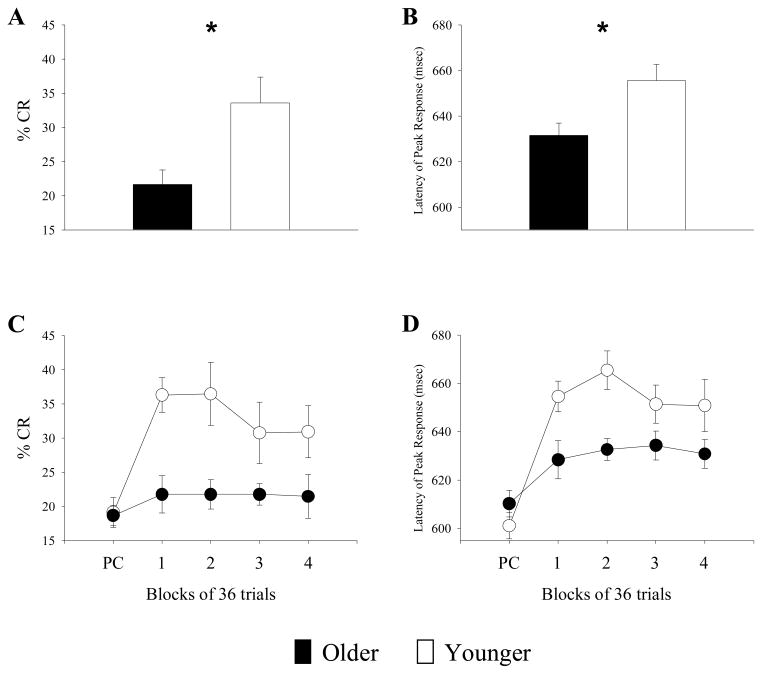
A Group (older, younger) x Block (pseudoconditioning, Blocks 1–4) × CS Type (delay, trace) mixed factor analysis of variance (ANOVA) with repeated measures was performed. A main effect of Group was observed on both % CR, F(1, 15) = 7.71, p<.05, and response latencies, F(1, 15) = 5.61, p<.05. Post-hoc tests of the acquisition phase indicated that younger adults produced significantly greater % CR, t(15) = 2.87, p<.05, and also timed their peak responses to occur closer to US presentation, t(15) = 2.73, p<.05, than older adults (Figures 2a and 2b). A main effect of Block was also significant for both measures of conditioning. Relative to pseudoconditioning, greater % CR, F(4, 60) = 9.29, p<.05, and longer peak responses latencies, F(4, 60) = 22.50, p<.05, were found for all participants during acquisition. A significant Group x Block interaction was also found for both % CR, F(4, 60) = 4.51, p<.05, and latency, F(4, 60) = 4.86, p<.05. However, all other interactions involving CS Type (Group × CS, Block × CS, and Group × Block × CS) were not statistically significant (p’s > .05). Furthermore, since there was also no main effect of CS Type (p’s > .05), we collapsed both delay and trace conditioning trials in Figures 2c and 2d. These findings suggest that younger adults learned better than older adults on two measures of conditioning: % CR and Latency of Peak Response.
Figure 2.
Top: Aging effects on two measures of conditioning: % CR and Latency of Peak Response. Younger adults showed a significantly greater number of conditioned responses during acquisition (mean ± standard error, younger: 33.59 ± 3.74, older: 21.68 ± 2.09) (a) and also timed their maximal eyeblink response closer to US presentation (younger: 655.55 ± 7.14, older: 631.34 ± 5.34) (b). These behavioral findings suggest that younger adults condition better than older adults during the concurrent presentation of both delay and trace trials. Bottom: The impact of age on learning rates using % CR and Latency of Peak Response. Since no main effect or interaction involving CS Type was detected, delay and trace trials were collapsed. Older adults failed to show acquisition in the number of their conditioned responses (black circles in 2c) but were able to shift the time of their maximal eyeblink response significantly closer to US onset time relative to pseudoconditioning (black circles in 2d). Younger adults showed learning-related changes on both measures of conditioning (white circles in 2c & 2d). Although there was a significant decline in % CR that accompanied aging, the ability to time eyeblink responses appeared to be preserved in older adults. (Error bars represent ± standard error of the mean; PC = pseudoconditioning)
Because a significant Group x Block interaction was detected, further statistical analyses were performed in an effort to better understand learning rates produced by each age group. These results showed that younger adults successfully learned the CS-US association on both indices of conditioning by showing greater % CR, F(1, 7) = 22.97, p<.05, and longer peak response latencies, F(1, 7) = 14.28, p<.05, during acquisition relative to pseudoconditioning (white circles in Figs. 2c and 2d). Post hoc tests on both variables showed each time point in acquisition (1–4) differed significantly from pseudoconditioning in younger adults (t’s > 2.71). However, older adults showed learning-related changes only in their peak response latencies, F(1, 8) = 7.45, p<.05, but not % CR, F(1, 8) = 0.96, p > .05 (black circles in Figs. 2c and 2d). Post hoc tests on latency measures showed time points 2–4 differed significantly from pseudoconditioning in older adults (t’s > 2.62). These findings suggest that % CR may be more sensitive to aging effects.
Furthermore, we addressed the possibility that any differences reported between the younger and older adults were due to nonassociative factors such as spontaneous blink rates, unconditioned response (UCR) amplitudes, or general cognitive abilities. We measured eyeblink variability during baseline and no significant differences between our two groups were found, t(15) = 0.26, p > .05. UCR amplitude measures were also similar between the younger and older adults, t(15) = 0.60, p > .05. Group differences in cognitive tests were not statistically significant (p’s > .05). Both age groups correctly answered the same number of movie and post-experimental awareness questions, and furthermore, correlations between % CR, Latency of Peak Response, cognitive test results, and subject’s awareness scores were not statistically significant (p’s > .05).
Discussion
Previous work from our laboratory showed that younger adults (20–30 yrs old) were capable of demonstrating both delay and trace eyeblink conditioning within the same acquisition session (Cheng et al. 2008). The present behavioral investigation was performed to determine if older adults (60–70 yrs old) could show similar learning. Evidence of learning in the older adults was subtle in comparison to their younger counterparts. Younger adults learned the CS-US relationship on two measures of conditioning (white circles in Figs 2c and 2d) while older adults failed to produce any significant differences in % CR during acquisition (black circles in Figure 2c), but a closer examination of their peak response latencies showed a temporal shift to more closely approximate US presentation (black circles in Figure 2d), suggesting that learning in older adults was not eliminated entirely.
Age-related effects were present in % CRs as younger adults produced more conditioned responses than older adults during the acquisition period (Figure 2a). Age effects were also evident in peak response latency measures, as maximal eyelid closure for younger adults occurred significantly closer to the time of US presentation than older adults (Figure 2b). Collectively, these age-related findings are consistent with previous reports of aging in eyeblink conditioning (Bellebaum & Daum, 2004; Finkbiner & Woodruff-Pak, 1991; Knuttinen et al., 2001; Solomon et al., 1989; Woodruff-Pak & Thompson, 1988).
Although significant learning-related changes were measured, the level of conditioning in the current study was less robust than what has been typically reported (Disterhoft et al., 2003). Factors such as presenting pseudoconditioning trials before conditioning trials and a very conservative CR time window (250 ms pre US) likely contributed to the level of conditioning observed in the present study. Since it is possible that the criteria used for CRs were too stringent, a re-analysis of the % CR data was performed using a more liberal threshold (> 2 sd). The older adults still failed to show learning-related changes over acquisition while younger adults continued to show significant conditioning. These findings, along with the UCR analysis, suggest that the conditioning performance of both groups was not due to age-related differences in eyeblink amplitude. Furthermore, the performance of our younger adults in the current study is consistent with the levels of conditioning reported from our previous study (Cheng et al., 2008). Finally, it should also be noted that asymptotic performance was achieved during the first few acquisition trials and this finding is in agreement with other single-cue conditioning papers (Manns, Clark, & Squire, 2000; 2001).
Conditioning performance has been shown to be affected by manipulating levels of cognitive demand (Bellebaum & Daum, 2004; Carillo, Gabrieli, & Disterhoft, 2000). Bellebaum and Daum (2004) tested younger and older adults using an eyeblink conditional discrimination procedure, and notably, only tones preceded by a specific visual stimulus were paired with the airpuff. Younger, but not older adults showed conditioning using this paradigm. Attenuated conditioning as a result of increased cognitive demands is not unique to older adults. Carillo et al. (2000) studied younger adults and reported that levels of attention did not affect performance during simple, single-cue conditioning but did adversely impact conditioning for the more complex differential conditioning design. The current design may not be as cognitively demanding as a conditional discrimination procedure but presenting delay and trace trials within the same acquisition period does introduce a modest level of complexity not present in single-cue designs. It is possible that additional CSs may distract or interfere with a subject’s ability to normally condition to a single CS. Additionally, delay and trace conditioning rely on distinct neural circuits (Cheng et al., 2008; Clark & Squire, 1998; Knight et al., 2004) and engaging these circuits in an on-off manner could have affected normal acquisition. Presentation of both conditioning trial types within the same acquisition session in the current study may have contributed to a complexity level that was particularly susceptible to aging effects.
Although some studies show normal acquisition by older adults in the delay paradigm (Knuttinen et al., 2001; Smith, Clark, Manns, & Squire 2005), other laboratories have reported age-related impairments (Solomon et al., 1989; Woodruff-Pak & Thompson, 1988). One possible reason for the older adult’s lower % CR rate in both delay and trace conditioning in the present study may be due to age-related cerebellar cortical atrophy (Andersen et al., 2003; Dimitrova et al., 2008; Glick & Bondareff, 1979; Rogers et al., 1981; Woodruff-Pak et al., 2000). Fortier, Disterhoft, & McGlinchey-Berroth (2000) found that a patient with cerebellar cortical degeneration was able to perform simple single-cue conditioning paradigms but had difficulty with more complex designs. This patient showed conditioning deficits when presented with two delay conditioned stimuli each paired with two different ISIs (350 ms vs 750 ms) but showed normal acquisition when presented with one delay CS. The present findings suggest that the attenuated level of conditioning as a result of more complex conditioning designs may be exacerbated by age.
Interestingly, a closer examination of the behavioral data revealed that in contrast to % CR measures, older adults showed evidence of learning in their response latency measurements. Figure 2d (black circles) showed that the time of maximal eyeblink response shifted closer to US onset time relative to pseudoconditioning, suggesting the ability to appropriately time responses may be preserved in older adults. Lesion studies have shown that eyeblink response timing has been linked to both the hippocampus and cerebellum (Gerwig et al., 2005; McGlinchey-Berroth, Brawn, & Disterhoft, 1999). McGinchey-Berroth et al. (1999) tested medial temporal lobe amnesics and showed that these patient’s CRs occurred closer to CS onset than controls, indicating that the hippocampus plays an important role in the timing of adaptive responses to the US. Gerwig et al. (2005) showed that cerebellar lesions in lobule HV extending into anterior HVI correlated with short CR onset latencies. Furthermore, they report a 20 ms difference between cerebellar patients and controls in CR timing. This timing difference is similar to the latency shift (from pseudoconditioning to acquisition) for older adults in the present study. Furthermore, the dissociation between the % CR and latency learning curves in older adults supports the idea that the production and timing of eyeblink CRs are separate processes and may be mediated by unique neural circuits. Additional research is needed to determine if these structures and their role in eyeblink classical conditioning are differentially susceptible to normal aging. For example, a parametric study using different age groups could determine if/when older adults fail to show conditioning on both % CR and latency measures. In the current study, it is possible that changes in the hippocampus and cerebellum related to aging may be responsible for the group differences in peak response latencies. Such changes may have been sufficient to produce differences in response timing between the two age groups during acquisition (Figure 2b), but not to the degree that prevents learning-related behavioral changes in older adults (Figure 2d).
In summary, age-related decline in conditioning has been extended to concurrent delay and trace learning. Compared to pseudoconditioning, older adults failed to show significantly more conditioned responses to either trial types during acquisition. However, their ability to time eyeblink responses was preserved, although not to the same extent as younger adults. Animal studies suggest that hippocampal and cerebellar changes underlie this age-related deficit in eyeblink conditioning. However, it should be noted that the current behavioral study cannot directly address the role of these structures during eyeblink conditioning. Future neuroimaging studies in humans will be needed to further investigate the most relevant neural correlates of this interesting behavioral aging effect.
Acknowledgments
This work was supported by the National Institute on Aging Grant (R01 AG021501) awarded to John E. Desmond
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/pag
Contributor Information
Dominic T. Cheng, Johns Hopkins University School of Medicine
Monica L. Faulkner, Johns Hopkins University School of Medicine
John F. Disterhoft, Northwestern University Feinberg School of Medicine
John E. Desmond, Johns Hopkins University School of Medicine
References
- Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: A stereological study. Journal of Comparative Neurology. 2003;466:356–365. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Houston FP. LTP induction threshold change in old rats at the perforant path-granule cell synapse. Neurobiology of Aging. 2000;21:613–620. doi: 10.1016/s0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Daum I. Effects of age and awareness on eyeblink conditional discrimination learning. Behavioral Neuroscience. 2004;118:1157–1165. doi: 10.1037/0735-7044.118.6.1157. [DOI] [PubMed] [Google Scholar]
- Berger TW, Alger B, Thompson RF. Neural substrate of classical conditioning in the hippocampus. Science. 1976;192:483–485. doi: 10.1126/science.1257783. [DOI] [PubMed] [Google Scholar]
- Carillo MC, Gabrieli JDE, Disterhoft JF. Selective effects of division of attention on discrimination conditioning. Psychobiology. 2000;28:293–302. [Google Scholar]
- Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. Proceedings of the National Academy of Sciences. 2008;105:8108–8113. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: The role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Carillo MC, Fortier CB, Gabrieli JDE, Knuttinen M-G, McGlinchey-Berroth R, Preston A, Weiss C. Impact of temporal lobe amnesia, aging, and awareness on human eyeblink conditioning. In: Squire LR, Schachter D, editors. Neuropsychology of Memory. Guilford; New York: 2003. pp. 97–113. [Google Scholar]
- Dimitrova A, Gerwig M, Brol B, Gizewski ER, Forsting M, Beck A, Aurich V, Kolb FP, Timmann D. Correlation of cerebellar volume with eyeblink conditioning in healthy subjects and in patients with cerebellar cortical degeneration. Brain Research. 2008;1198:73–84. doi: 10.1016/j.brainres.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Finkbiner RG, Woodruff-Pak DS. Classical eyeblink conditioning in adulthood: Effects of age and interstimulus interval on acquisition in the trace paradigm. Psychology and Aging. 1991;6:109–117. doi: 10.1037//0882-7974.6.1.109. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Disterhoft JF, McGlinchey-Berroth R. Cerebellar cortical degeneration disrupts discrimination learning but not delay or trace classical eyeblink conditioning. Neuropsychology. 2000;4:537–550. doi: 10.1037//0894-4105.14.4.537. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, Thilmann AF, Forsting M, Diener HC, Timmann D. Timing of conditioned eyeblink responses is impaired in cerebellar patients. Journal of Neuroscience. 2005;25:3919–3931. doi: 10.1523/JNEUROSCI.0266-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick R, Bondareff W. Loss of synapses in the cerebellar cortex of the senescent rat. Journal of Gerontology. 1979;34:818–822. doi: 10.1093/geronj/34.6.818. [DOI] [PubMed] [Google Scholar]
- Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH. Hippocampal atrophy in normal aging. An association with recent memory impairment. Archives of Neurology. 1993;50:967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Mittleman M, Cohen J, George AE. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- Gormezano I. Classical Conditioning. In: Sidowski JB, editor. Experimental Methods and Instrumentation in Psychology. McGraw-Hill; New York: 1966. pp. 385–420. [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. Journal of Neuroscience. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuttinen M-G, Power JM, Preston AR, Disterhoft JF. Awareness in classical differential eyeblink conditioning in young and aging humans. Behavioral Neuroscience. 2001;115:747–757. doi: 10.1037//0735-7044.115.4.747. [DOI] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Awareness predicts the magnitude of single-cue trace eyeblink conditioning. Hippocampus. 2000;10:181–186. doi: 10.1002/(SICI)1098-1063(2000)10:2<181::AID-HIPO7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Single-cue delay eyeblink conditioning is unrelated to awareness. Cognitive, Affective, & Behavioral Neuroscience. 2001;1:192–198. doi: 10.3758/cabn.1.2.192. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Brawn C, Disterhoft JF. Temporal discrimination learning in severe amnesic patients reveals an alteration in the timing of eyeblink conditioned responses. Behavioral Neuroscience. 1999;113:10–18. doi: 10.1037//0735-7044.113.1.10. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Li L, Weiss C, Disterhoft JF, Wyrwicz AM. A fiber optic-based system for behavioral eyeblink measurement in a MRI environment. Journal of Neuroscience Methods. 2005;141:83–87. doi: 10.1016/j.jneumeth.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test (NART) Test Manual. Berkshire, England: NFER-NELSON Publishing Company, Ltd; 1982. [Google Scholar]
- Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiology of Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Zornetzer SF, Bloom FE. Senescent pathology of cerebellum: Purkinje neurons and their parallel fiber afferents. Neurobiology of Aging. 1981;2:15–25. doi: 10.1016/0197-4580(81)90054-3. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Progress in Neurobiology. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Smith CN, Clark RE, Manns JR, Squire LR. Acquisition of differential delay eyeblink classical conditioning is independent of awareness. Behavioral Neuroscience. 2005;119:78–86. doi: 10.1037/0735-7044.119.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Beal MF, Pendlebury W. Age-related disruption of classical conditioning: A model systems approach to memory disorders. Neurobiology of Aging. 1988;9:535–546. doi: 10.1016/s0197-4580(88)80110-6. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Pomerleau D, Bennett L, James J, Morse DL. Acquisition of the classically conditioned eyeblink response in humans over the lifespan. Psychology and Aging. 1989;4:34–41. doi: 10.1037//0882-7974.4.1.34. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: Prior memories hinder new hippocampal encoding. Trends in Neuroscience. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Aging and classical conditioning: Parallel studies in rabbits and humans. Neurobiology of Aging. 1988;9:511–522. doi: 10.1016/s0197-4580(88)80108-8. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Goldenberg G, Downey-Lamb MM, Boyko OB, Lemieux SK. Cerebellar volume in humans related to magnitude of classical conditioning. Neuroreport. 2000;11:609–615. doi: 10.1097/00001756-200002280-00035. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18–83 years. Psychology and Aging. 1988;3:219–229. doi: 10.1037//0882-7974.3.3.219. [DOI] [PubMed] [Google Scholar]