Abstract
Background
Cardiopulmonary adverse events after transfusion include acute lung injury (TRALI) and circulatory overload (TACO), which are potentially lethal and incompletely understood.
Study Design and Methods
To determine whether the incidence of TRALI and TACO was affected by leukoreduction we conducted a retrospective, before and after study of acute transfusion reactions for the seven years prior to and after introduction of universal leukoreduction in 2000, involving 778,559 blood components.
Results
Substantial decreases occurred in the rates of TRALI (−83%; from 2.8 cases per 100,000 components pre- to 0.48 post-universal leukoreduction; p=0.01), TACO (−49%; 7.4 to 3.8 cases per 100,000; p=0.03) and febrile reactions (−35%; 11.4 to 7.4 cases per 10,000; p<0.0001). The incidence of allergic reactions remained unchanged (7.0 per 100,000 pre- and post-universal leukoreduction). These outcomes were primarily attributable to decreased TRALI/TACO associated with RBC and platelet transfusions (−64%) with notably smaller decreases associated with FFP or cryoprecipitate transfusions (−29%). The incidence of TRALI/TACO after 28,120 washed red cell and 69,325 platelet transfusions was zero.
Conclusion
These data suggest novel hypotheses for further testing in animal models, in prospective clinical trials, and via the new US Hemovigilance System : (1) Is TACO or TRALI mitigated by leukoreduction? (2) Is the mechanism of TACO more complex than excessive blood volume? (3) Does washing mitigate TRALI and TACO due to platelet and RBC transfusions?
Introduction
Transfusion related acute lung injury (TRALI) 1 and transfusion associated circulatory overload (TACO) 2 are infrequent complications that are amongst the most commonly reported fatal events after blood transfusions. 3,4 The mechanism of TRALI has long been thought to be anti-white cell antibody (usually the donor’s) reacting with antigen positive cells, leading to a capillary leak syndrome. However, recent research suggests that other mechanisms, involving white blood cell (e.g., platelet activating factor) 5 and platelet derived (e.g., CD40L) mediators are important. 6 TACO has been presumed to be due to the inability of the recipient’s cardiovascular system to accommodate the volume infused.
Universal leukoreduction has been associated, in one report, with a decreased incidence of adult respiratory distress syndrome in a cohort of critically ill patients. 7 The odds ratio for mortality in acute lung injury patients was 1.06 in recipients of leukoreduced red cells as compared with 1.14 in recipients of non-leukoreduced red cells in one recent cohort study. 8 In trauma patients in a randomized trial, no reduction in lung injury was associated with use of leukoreduced transfusions. 9
We initially hypothesized that leukoreduction might influence the overall incidence of TRALI, but not TACO. Pre-storage leukoreduction of blood components reduces the quantity of white cell and platelet derived mediators transfused. 10 We reasoned that universal leukoreduction, and subsequent removal of platelets (from transfused red cells), white blood cells (from both red cells and platelets) and their mediators, might decrease the risk of TRALI. We further hypothesized, based upon prior work of Silliman and colleagues, that saline washed red cells and platelets would be associated with a lower incidence of TRALI. 5
Recent findings support a role for white cell derived bioactive mediators such as IL-6 and TNF-α in heart failure,11 with which TACO shares some similarities. After initial review of the data, we further hypothesized that the incidence of TACO would also be influenced by leukoreduction of blood transfusions. We tested these hypotheses using retrospective analysis of transfusion reaction data.
Methods
Patients
This was designed as a before and after retrospective observational assessment of reported transfusion reactions, employing aggregate transfusion service data. The data consisted of reports of transfusion reactions jointly investigated by reporting clinical staff and transfusion service physicians at the time of the adverse event. The setting is a 750 bed university teaching hospital that functions as a community hospital, as well as a tertiary care institution. All reactions that were reported and considered definitely or possibly transfusion related by the transfusion service physician were included. Because these data are summary transfusion service data, a limitation of our analysis is that detailed clinical or laboratory data are not available on the patients involved.
Procedures
Annual reports of transfusion reactions evaluated by transfusion service physicians were divided by the total components transfused (RBC, platelets, FFP, cryoprecipitate) during seven years before (1993-1999) and after (2001-2007) the implementation of universal leukoreduction to derive an incidence rate before and after implementation of universal leukoreduction in 2000. Virtually all transfusions were derived from whole blood donations for all these components, including platelets. Leukoreduction for red cells was pre-storage by filtration of either red cell concentrates or whole blood prior to receipt at our transfusion service. Leukoreduction of platelets was performed during pooling immediately prior to release for transfusion. All units of FFP were FFP, not 24 hour or thawed plasma, and FFP units were not leukoreduced prior to storage. An unknown number of FFP units were manufactured from leukoreduced whole blood during the post-universal leukoreduction period. No effort was made by our suppliers to remove female donor sources from the FFP pool during the period reviewed. A number of local practices that might influence transfusion reaction rates are (1) for the entire period of 1993-2007 patients undergoing chemotherapy or stem cell transplant for hematologic malignancies received only leukoreduced, ABO identical transfusions and (2) these patients frequently by protocol received only washed, leukoreduced ABO identical platelets and red cells. 12
The same two senior attending physicians were involved in clinical practice throughout the 14 years of the study, and we used consistent criteria for assessment of transfusion reactions. No changes in criteria or diagnostic evaluation methods for TRALI, TACO, febrile or allergic reactions occurred during this period. In general, non-cardiogenic pulmonary edema, or worsening acute lung injury occurring during or shortly after transfusion, with compatible chest radiologic findings (bilateral infiltrates with no hemodynamic evidence of heart failure) was considered TRALI, particularly in the absence of other likely causes. No laboratory investigations of white cell antibodies in donor or recipient were considered in the diagnosis. In general, all pulmonary reported reactions not clearly explained by alternative diagnoses were classified as TRALI, without descriptors such as possible, unlikely, etc. New or worsening cardiogenic pulmonary edema, particularly accompanied by typical chest radiologic and physical findings and responding to diuretics, were classified as TACO if no other more likely cause was present. Febrile reactions consisted of new or worsening fever, and/or rigors, and/or chills in the absence of other obvious causes during or shortly after transfusion. Blood or other bacterial/fungal cultures were performed at the discretion of the attending clinical staff, but the results were not employed in the classification of reactions. Allergic reactions were acute, de novo reports of rash, urticaria, airway obstruction, etc. during or shortly after transfusion with no other more likely etiology.
Because the diagnostic database was created prior to the generation of the hypotheses of this study, it is unlikely that observer bias could play a role in the findings. No reactions or patients were excluded, and all transfusion reactions and transfused blood components during this 14 year period were included in the dataset. During this period, some indeterminate number of leukoreduced transfusions (<20% of total) were given during the period prior to universal leukoreduction being implemented, thus any differences observed likely would understate the effects of leukoreduction.
None of the plasma components were leukoreduced during the pre-universal leukoreduction period, but after introduction of universal leukoreduction, an unknown number of units of FFP and cryoprecipitate were manufactured after leukoreduction of donated whole blood. Thus, an inherent control for the study is that universal leukoreduction would be expected to have a more modest to no effect on TRALI or TACO due to FFP and cryoprecipitate. In addition, there is no basis in current knowledge for assuming that allergic reactions would be altered by universal leukoreduction, serving as an additional control on confounding and bias.
Statistical analysis
Statistical evaluation employed Chi square for 2 × 2 tables, without Yates correction, confirmed using Fisher’s exact test for n<10. P values are presented, as always in retrospective studies, as probabilistic assessments of the strength of associations, not as indications of causal inference. P values of <0.05 are considered significant. Odds ratios and 95% confidence intervals are presented in the tables, along with percent change in incidence of the reactions. 95% confidence intervals that did not overlap 1.0 are considered statistically significant.
Role of the funding source
There was no extramural funding source. Two authors (NB, RPP) received partial salary support from NIH grants during the period this work was conducted. Data retrieval and analysis were conducted as part of a clinical quality assurance project by the authors, who all are members of the transfusion service medical and technical staff. Because these data were collected as part of institutional quality assurance within a department, no human subjects approval was required.
Results
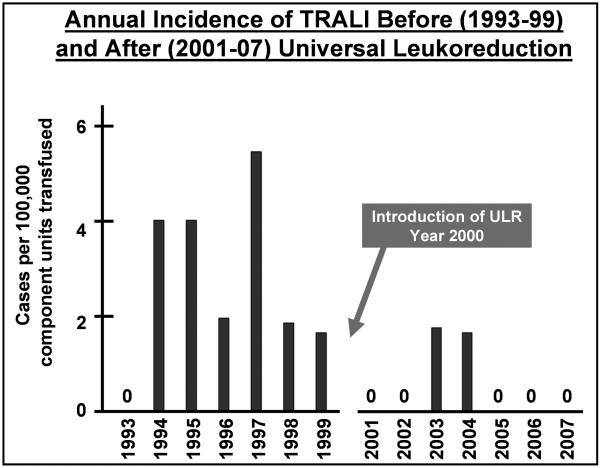
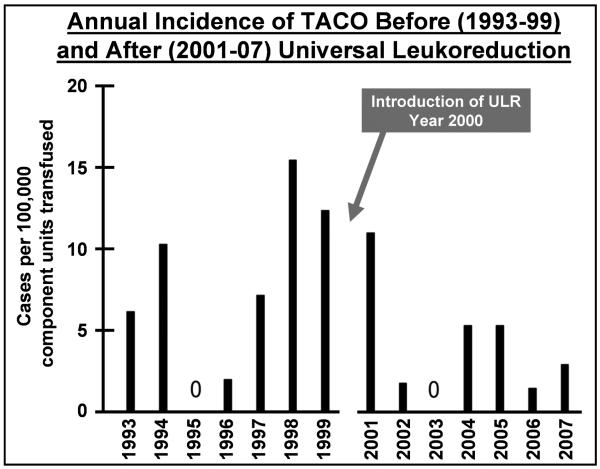
The annual incidence of TRALI and TACO incidence are displayed in Figures 1 and 2 respectively, demonstrating the decreased incidence associated with the introduction of universal leukoreduction of transfusions in mid-2000. In the 7 years after the implementation of universal leukoreduction, the incidence of TRALI and TACO reports decreased by 83% and 49% respectively (p= 0.002 for these cardiopulmonary complications combined) (Table 1). The total number of cases of TRALI/TACO associated with cellular transfusions of red cells decreased substantially (from 3 TRALI and 19 TACO cases before universal leukoreduction to 1 and 9 after), despite a significant increase in the number of transfusions. (Tables 2-4). The same was true of cases associated with platelet transfusion (3 TRALI and 5 TACO before universal leukoreduction decreasing to 0 and 1 after). In contrast, the number of cases of TRALI/TACO associated with FFP and cryoprecipitate remained unchanged at a total of 7 cases during the seven year periods before universal leukoreduction (4 TRALI and 2 TACO cases due to FFP; 0 and 1 due to cryoprecipitate) and after universal leukoreduction (1 TRALI and 6 TACO due to FFP; 0 cases of TRALI and 0 of TACO due to cryoprecipitate). After the introduction of universal leukoreduction, the magnitude of the decrease in incidence of TRALI/TACO associated with cellular components was more than two-fold of that due to plasma components.
Figure 1.
The annual incidence of TRALI before (1993-99) and after (2001-07) the introduction of universal leukoreduction (ULR) in the year 2000 is shown. No cases of TRALI were reported in five of the seven years post-introduction of ULR, as compared with only one of seven years pre-introduction of ULR in which cases of TRALI were not reported pre-introduction of ULR.
Figure 2.
The annual incidence of TACO before (1993-99) and after (2001-07) the introduction of universal leukoreduction (ULR) in the year 2000 is shown. The incidence rate was 5 or fewer cases per 100,000 components transfused in six of the seven years post-introduction of ULR, compared with only two of the seven years pre-introduction of ULR.
Table 1. Transfusion reaction incidence pre- and post-implementation of universal leukoreduction (ULR).
| Pre–universal leukoreduction (n; incidence) 1993-1999 |
Post–universal leukoreduction (n; incidence) 2001-2007 |
Odds Ratio Post-/Pre-ULR (95% confidence interval) |
Net Change In Incidence |
p value (incidence pre/post- ULR) |
|
|---|---|---|---|---|---|
| Transfused Components |
362,784 | 415,775 | ------ | ----- | ----- |
| TRALI cases |
10 (2.8*) | 2 (0.48*) | 0.17 (0.04-0.80) | −83% | 0.01 |
| TACO cases | 27 (7.4*) | 16 (3.8*) | 0.52 (0.28-0.96) | −49% | 0.03 |
| Allergic Reactions |
255 (7.0†) | 290 (7.0†) | 0.99 (0.84-1.17) | 0% | 0.96 |
| Fever/Rigor Reactions |
413 (11.4†) | 309 (7.4†) | 0.65 (0.56-0.76) | −35% | <0.0001 |
Rate per 100,000 components transfused
Rate per 10,000 components transfused
Table 2. TRALI incidence by blood component pre- and post-implementation of universal leukoreduction.
| Pre–universal leukoreduction TRALI (n; incidence) 1993-1999 |
Post–universal leukoreduction TRALI (n; incidence) 2001-2007 |
Odds Ratio Post-/Pre-ULR (95% confidence interval) |
Net Change In Incidence |
|
|---|---|---|---|---|
| Cryoprecipitate (Cryo) |
0 of 19,152 (0) | 0 of 18,870 (0) | Not Applicable | 0% |
| Fresh Frozen Plasma (FFP) |
4 of 56,201 (7.1)* |
1 of 87,744 (1.1)* |
0.16 (0.02 – 1.4) | −85% |
| Red Cells (RBC) |
3 of 147,789 (2.0)* |
1 of 173,244 (0.58*) |
0.28 (0.03 – 2.7) | −71% |
| Platelets | 3 of 139,642 (2.1*) |
0 of 135,917 (0*) |
0.15 (0.008 – 2.8) | −100% |
| Total FFP/Cryo | 4 of 75,353 (5.3)* |
1 of 106,614 (0.94)* |
0.18 (0.02 –1.6) | −82% |
| Total RBC/Platelets |
6 of 287,431 (2.1)* |
1 of 309,161 (0.32)* |
0.16 (0.02 –1.3) | −85% |
Rate per 100,000 components transfused
Table 4. Combined TRALI and TACO incidence by blood component pre- and post-implementation of universal leukoreduction.
| Pre–universal leukoreduction TRALI/TACO (n; incidence) 1993-1999 |
Post–universal leukoreduction TRALI/TACO (n; incidence) 2001-2007 |
Odds Ratio Post-/Pre-ULR (95% confidence interval) |
Net Change In Incidence |
|
|---|---|---|---|---|
| Cryoprecipitate (Cryo) |
1 of 19,152 (5.2) | 0 of 18,870 (0) | 0.33 (0.01 – 8.1) | −100% |
| Fresh Frozen Plasma (FFP) |
6 of 56,201 (11)* | 7 of 87,744 (8.0)* |
0.75 (0.25 – 2.2) | −27% |
| Red Cells (RBC) | 22 of 147,789 (15)* | 10 of 173,244 (5.8*) |
0.39 (0.18 – 0.82) | −61% |
| Platelets | 8 of 139,642 (5.7*) | 1 of 135,917 (0.7*) |
0.13 (0.02 –1.03) | −88% |
| Total FFP/Cryo | 7 of 75,353 (9.3)* | 7 of 106,614 (6.6)* |
0.71 (0.25 –2.0) | −29% |
| Total RBC/Platelets |
30 of 287,431 (10)* | 11 of 309,161 (3.6)* |
0.34 (0.17 – 0.68) | −64% |
Rate per 100,000 components transfused
The overall incidence of allergic reactions remained unchanged after introduction of universal leukoreduction. Fever/rigor reactions decreased 34% after universal leukoreduction implementation (p<0.0001), similar to previous reports.4 Finally, there were no reported cases of TRALI or TACO associated with transfusions of washed, leukoreduced RBC (n=28,120) and platelets (n=69,325) during these 14 years. This is significantly fewer cases than the number of TRALI and TACO cases associated with leukoreduced transfusions of RBC and platelets during 2001-2007 (11 of 309,161; p=0.049).
Discussion
Within the limitations of aggregate transfusion service data and the clinical skills of the reporting and evaluating physicians, we describe two unexpected and unexplained associations between the rates of adverse reactions to transfusions and the introduction of universal leukoreduction. These observations do not prove causality but rather provide novel, exciting and potentially important hypotheses for further testing in animal models, national hemovigilance data, and more exacting clinical research.
We observed a significant overall decrease in the absolute number of cases and incidence per component transfused of TRALI associated with introduction of universal leukoreduction in our hospital (Table 1/Figure 1). However, the total number of cases analyzed is quite small, and the results are, perhaps due to insufficient numbers, not statistically significant when individual blood component types are analyzed (Table 2). These non-significant trends toward declining reports of TRALI were observed for both plasma and cellular transfusions. This raises the hypothesis that leukoreduction of whole blood donations influences cases of TRALI mediated by white cells, platelets and their mediators in FFP, as well as in RBC and platelets. White cell and platelet derived mediators have been proposed and are increasingly accepted as potential contributing factors to TRALI. 4-6 Animal model and in vitro experimental data suggest that mediators such as bioactive lipids and soluble CD40L (CD154) that accumulate in stored red cells and platelets may interact with neutrophils and endothelial cells to mediate the capillary leak syndrome of TRALI. These mediators accumulate at significantly lower levels in some studies of leukoreduced red cells.
Most surprisingly and interestingly, the absolute number of cases and the incidence of TACO reported also decreased significantly after introduction of universal leukoreduction. (Table 1/Figure 1) This observation was limited, in terms of statistical significance for specific transfusion components, to transfusions of red cells, although a similar trend was seen for platelets. The number of cases and incidence of TACO due to FFP actually increased during this period, although this trend was not statistically significant. These results suggest a number of hypotheses to be tested in future animal and clinical investigations. Given the possibility that heart failure may be exacerbated by inflammatory mediators, 11 we speculate that TACO is sometimes not solely due to volume overload, but exacerbated by white cell and platelet mediators that are largely absent from leukoreduced RBC and platelet transfusions.
It is particularly surprising that reports of TRALI and TACO associated with platelet transfusions decreased post-implementation of universal leukoreduction, given that leukoreduction of platelets was performed at release rather than pre-storage. If this association is reproducible and causal, removal of platelet/white cell derived microparticles (or other mediators of capillary leak such as vascular endothelial growth factor-VEGF) through adherence to the leukoreduction filter is a mechanism worth further investigation.
Because of the small number of cases and the retrospective nature of this study, defining the reported associations as causal would be premature. These associations suggest that development of animal models, and investigations in larger numbers of patients employing more detailed and exacting clinical data would be important next steps. The new US Hemovigilance system being implemented by CDC and AABB should eventually be able to contribute larger numbers of reactions and more detailed clinical information to support or refute the hypothesis that leukoreduced transfusions are less likely to cause TRALI and TACO. The present findings supporting an association between leukoreduction and reduced TRALI/TACO are strengthened by the observation that, as has been previously reported by others, 13 the allergic reaction rate incidence in our center was not at all affected by universal leukoreduction. Our data are limited primarily by reliance on clinical judgment in practice, rather than scientifically rigorous definitions, and the absence of blinded assessments of the reactions reported. By the nature of this study we cannot provide laboratory measurements of potential transfused mediators that might have contributed to the clinical events reported.
Additional limitations of these data are their retrospective nature, and the possibility of undetected changes in transfusion reaction reporting by clinical staff. Changes in reporting seem unlikely to explain these findings given that the dramatic drop in TRALI/TACO incidence associated with RBC and platelet transfusions occurred during a period when allergic reaction incidence was unchanged and TACO incidence associated with FFP actually increased. Furthermore, throughout the entire 14 year period, voluntary reporting mechanisms were unchanged, and the same two attending physicians evaluated reported reactions. No changes in criteria for TRALI or TACO occurred as far as we can determine. No educational activities were undertaken to reduce transfusions in critically ill patients, and no decreases in red cell or platelet utilization occurred during the period reported. Underreporting is unlikely as our incidence of TRALI and TACO is very similar to that reported in UK nationwide public health data, 14 and our overall reaction rates are in the range of 0.5-1.0% reported by others. 13
These associations were unexpected. The hypothesis underlying this retrospective study was not generated until long after the reported events were clinically evaluated by the authors of this report, minimizing the possibility of observer bias. Nonetheless, undetected observer bias and confounding are always issues in public health or aggregate clinical data reporting, and no posthoc analysis can completely rule out the presence of bias or confounding. Prospective or blinded evaluation of data from other centers will be needed to confirm or refute the findings reported here, and will determine whether our findings can be generalized. Animal model studies will be needed to assess whether a credible mechanistic explanation exists for the reported associations.
In summary, we believe it plausible that leukoreduction favorably influences the incidence and/or severity of cardiopulmonary adverse effects after blood transfusion, particularly for cellular components such as red cells and platelets. Given the zero incidence of TRALI and TACO associated with tens of thousands of washed red cells and platelets, supernatant mediators in red cells and platelets may play a role in both TRALI and TACO. While selection of only male donors may reduce the incidence of TRALI due to FFP14-16, our data suggest leukoreduction is a potential strategy that might further reduce the incidence of TRALI and TACO. In addition, clinical trials of saline washing as a strategy for reducing the incidence of TRALI and TACO due to platelet and red cell transfusions are warranted based upon our preliminary findings.
These data represent the first report that leukoreduction is associated with a decrease in the incidence of TACO, an important and occasionally life threatening complication of transfusion, and provide additional evidence that leukoreduction is associated with a decrease in the incidence of TRALI. Finally, the data suggest the hypothesis that saline washing may mitigate the occurrence of TRALI and TACO due to cellular transfusions. Further investigation will be necessary to determine if these associations are indeed causal, and whether leukoreduction and/or washing has a clinically significant beneficial effect in reducing the incidence of TRALI and TACO.
Table 3. TACO incidence by blood component pre- and post-implementation of universal leukoreduction.
| Pre–universal leukoreduction TACO (n; incidence) 1993-1999 |
Post–universal leukoreduction TACO (n; incidence) 2001-2007 |
Odds Ratio Post-/Pre-ULR (95% confidence interval) |
Net Change In Incidence |
|
|---|---|---|---|---|
| Cryoprecipitate (Cryo) |
1 of 19,152 (5.2)* |
0 of 18,870 (0) | 0.33 (0.01 – 8.1) | −100% |
| Fresh Frozen Plasma (FFP) |
2 of 56,201 (3.6)* |
6 of 87,744 (6.8)* |
1.9 (0.39 - 9.5) | +89% |
| Red Cells (RBC) | 19 of 147,789 (12.8)* |
9 of 173,244 (5.2*) |
0.40 (0.18 – 0.89) | −59% |
| Platelets | 5 of 139,642 (3.6*) |
1 of 135,917 (0.74*) |
0.21 (0.02 –1.8) | −79% |
| Total Cryo/FFP | 3 of 75,353 (4.0)* |
6 of 106,614 (5.6)* |
1.4 (0.35 – 5.7) | +40% |
| Total RBC/Platelets |
24 of 287,431 (8.4)* |
10 of 309,161 (3.2)* |
0.39 (0.19 – 0.81) | −62% |
Rate per 100,000 components transfused
Acknowledgements
Supported in part by NIH grants ES01247, HL078603, HL095467, HL100051.
Footnotes
Disclosure of Conflicts of Interest
NB and JMH have received lecture honoraria, consulting fees and research support from Pall Biomedical and Fenwal, manufacturers of leukoreduction filters, as well as from Caridian, manufacturers of cell washing equipment and supplies. The other authors declare they have no potential conflicts of interest.
References
- 1.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–6. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 2.Gajic O, Gropper MA, Hubmayr RD. Pulmonary edema after transfusion: how to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit Care Med. 2006;34:S109–13. doi: 10.1097/01.CCM.0000214311.56231.23. [DOI] [PubMed] [Google Scholar]
- 3.Popovsky MA. Pulmonary consequences of transfusion: TRALI and TACO. Transfus Apher Sci. 2006;34:243–4. doi: 10.1016/j.transci.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Benson AB, Moss M, Silliman CC. Transfusion-related acute lung injury (TRALI): a clinical review with emphasis on the critically ill. Br J Haematol. 2009;147:431–43. doi: 10.1111/j.1365-2141.2009.07840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silliman CC, Moore EE, Johnson JL, et al. Transfusion of the injured patient: proceed with caution. Shock. 2004;21:291–9. doi: 10.1097/00024382-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plurad D, Belzberg H, Shulman I, et al. Leukoreduction is associated with a decreased incidence of late onset acute respiratory distress syndrome after injury. Am Surg. 2008;74:117–23. [PubMed] [Google Scholar]
- 8.Netzer G, Shah CV, Iwashyna TJ, et al. Association of RBC transfusion with mortality in patients with acute lung injury. Chest. 2007;132:1116–23. doi: 10.1378/chest.07-0145. [DOI] [PubMed] [Google Scholar]
- 9.Watkins TR, Rubenfeld GD, Martin TR, et al. Effects of leukoreduced blood on acute lung injury after trauma: a randomized controlled trial. Crit Care Med. 2008;36:1493–9. doi: 10.1097/CCM.0b013e318170a9ce. [DOI] [PubMed] [Google Scholar]
- 10.Seghatchian J. Platelet storage lesion: an update on the impact of various leukoreduction processes on the biological response modifiers. Transfus Apher Sci. 2006;34:125–30. doi: 10.1016/j.transci.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Celis R, Torre-Martinez G, Torre-Amione G. Evidence for activation of immune system in heart failure: is there a role for anti-inflammatory therapy? Curr Opin Cardiol. 2008;23:254–60. doi: 10.1097/HCO.0b013e3282fbfbc7. [DOI] [PubMed] [Google Scholar]
- 12.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia [ISRCTN76536440] BMC Blood Disord. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paglino JC, Pomper GJ, Fisch GS, et al. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 14.Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 15.Eder AF, Benjamin RJ. TRALI risk reduction: donor and component management strategies. J Clin Apher. 2009;24:122–9. doi: 10.1002/jca.20198. [DOI] [PubMed] [Google Scholar]
- 16.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49:1825–35. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]