Abstract
Neisseria gonorrhoeae can engage human complement receptor 3 (CR3) directly or through surface-bound iC3b. Factor H (fH) that binds to bacteria facilitates conversion of C3b to iC3b. fH also binds directly to CR3 on professional phagocytes. Certain non-professional phagocytes such as primary cervical epithelial cells also express CR3. We hypothesized that fH could bridge bacteria to CR3 and facilitate gonococcal association with host cells. Specificity of the fH-CR3 interaction was confirmed using human CR3-transfected CHO (CHO/CR3) cells. Using recombinant proteins that comprised contiguous fH domains (fH contains 20 short consensus repeat (SCR) domains) fused to murine Fc, we observed strong binding through SCRs 18–20, while weaker binding occurred through SCRs 6–10. Both regions also bound to unsialylated porin (Por) B.1A-expressing N. gonorrhoeae. Accordingly, fH-related protein 1 (CFHR1) (three of its five SCRs are highly homologous to fH SCRs 18–20) bound to CHO-CR3 and to unsialylated PorB.1A gonococci. An alternatively spliced variant of fH called fH-like protein-1 (FHL-1) (contains fH SCRs 1–7) bound to gonococci but minimally to CHO/CR3. A fH SCR 6–20 construct enhanced binding of unsialylated PorB.1A gonococci to CHO/CR3. However, a construct that contained only the apparently relevant SCRs (6–7 and 18–20) bound to CHO/CR3 and to gonococci separately, but did not enhance bacteria-CR3 interactions, suggesting that the intervening SCRs (8–17) may impart a configurational and spatial requirement for fH to bridge gonococci to CR3. These results indicate adherence between fH-coated gonococci and CR3 and may provide a means for gonococci to gain sanctuary into non-professional phagocytes.
Keywords: complement, complement receptor 3, Factor H, Neisseria gonorrhoeae
Introduction
Neisseria gonorrhoeae is the causative agent of the sexually transmitted infection gonorrhea. As many as 80% of women infected with N. gonorrhoeae may be asymptomatic or have minimal signs and symptoms. However, in 15–20 % of untreated women, gonococcal infection ascends into the upper reproductive tract and causes pelvic inflammatory disease (PID) that encompasses a range of pathologic conditions including endometritis, pelvic peritonitis, tubal abscess and salpingitis. The chronic sequelae associated with PID, i.e. pelvic pain, tubal damage, ectopic pregnancy and infertility are also recognized as important public health problems. In vitro studies have established that N. gonorrhoeae use different mechanisms to infect male and female genital tract epithelia. In vitro, asialoglycoprotein receptor-mediated endocytosis of gonococci is the main mechanism of entry into male urethral epithelial cells (1), whereas infection in primary cervical epithelial cells induces ruffling of cells via complement receptor 3 (CR3) that leads to a macropinocytic mechanism of entry (2, 3). CR3 is present in cells and tissues derived from the endo- and ectocervix, the endometrium and fallopian tubes although it is absent in tissues from the male urethra (2). The engagement of the CR3 receptor serves as a primary mechanism that enables gonococci to invade cervical epithelial cells (2).
CR3 is a heterodimeric β2 integrin consisting of a unique α subunit (CD11b) and a noncovalently associated β subunit, (CD18). CR3 is involved in immunity and inflammation and also mediates cell-cell, cell-extracellular matrix and, cell-pathogen interactions (4, 5). The hemolytically inactive form of complement component C3b, iC3b, binds to the I-domain of CR3, which is located within the N-terminal region of the α subunit, CD11b. Edwards et al (6) have shown previously that the gonococcal outer membrane proteins, porin and pilin, also play significant roles in adherence to CR3 present on cervical epithelial cells. Adherence largely occurs in a cooperative manner with iC3b and facilitates invasion of bacteria into these cells.
Factor H (fH) is a complement regulatory protein of the alternative pathway and is present in human plasma at concentrations of 116–562 µg/ml (7). fH is a ~150 kD single polypeptide glycoprotein composed of 20 domains comprising approximately 60 amino acids each (8, 9) called short consensus repeats (SCRs) (or complement control protein [CCP] modules), which are arranged in a consecutive fashion. fH acts a cofactor in the factor I-mediated cleavage of C3b to hemolytically inactive iC3b. In addition, fH irreversibly dissociates factor Bb from alternative pathway C3 and C5 convertases, which serves to limit C3 deposition and subsequent C5 cleavage (10). The main site of fH synthesis is the liver (11, 12); however, extrahepatic production of fH may provide sufficient amounts locally to aid in the protection of host cells from complement activation directly at sites of infection and inflammation (13–15). The fH protein family also includes fH-like protein 1 (FHL-1) and six additional human fH-related (FHR) proteins (CFHR1, -2, -3, -4A, -4B, and -5; these proteins have also been referred to as FHR-1 through FHR-5) (16, 17). These proteins are immunologically and structurally related and are composed exclusively of SCRs. CFHR1 has been shown recently to inhibit C5 convertase activity and interferes with C5b deposition and C5b-9 formation (18) and is present in the human plasma at a concentration of 70–100 µg/ml (18). FHL-1 consists of the 7 N-terminal SCRs of fH plus four unique amino acids at the C-terminus (19) and is present in the human plasma at a concentration of 10–50 µg/ml (20). Akin to fH, FHL-1 also possesses complement inhibiting activity. In addition, FHL-1 can facilitate cell adhesion (21).
Gonococci isolated from cervical secretions of infected women are coated with complement components (22). Complement present in cervical secretions (6, 18–21) may be the result of “spill over” into the female genitourinary tract from the systemic circulation or from de novo synthesis by cervical epithelial cells (6, 23). One of these components, fH, binds to gonococci in significant quantity (22). In addition to its function as a complement inhibitor both in solution and on cell surfaces, fH is also an adhesion ligand for neutrophils and platelets and may also participate in immune adherence in other host tissues (24–26).
N. gonorrhoeae can scavenge 5′-cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA) from the host to sialylate the lacto-N-neotetraose (LNT) structure of their lipooligosaccharide (LOS) molecules. We have shown previously that sialylation of gonococcal LOS enhances binding of fH to bacteria (27, 28), via porin (Por) molecules (10, 28). However, certain strains of N. gonorrhoeae can bind to fH independently of LOS sialylation. The Por molecule plays an important role in enabling gonococci (both sialylated and unsialylated) to bind to fH (10, 29). In N. gonorrhoeae, Por is an allelic protein and exists as two forms called PorB.1A and PorB.1B (30). In the unsialylated state, PorB.1A gonococcal strains bind more fH than PorB.1B strains (29). PorB.1A strains usually cause disseminated disease whereas PorB.1B strains more frequently are the causative agent of gonococcal PID and less symptomatic local disease in women (30, 31). Two regions in fH, one spanning SCRs 18–20 and the other in SCR 6 are involved in binding to gonococci (32).
In this study, we examined the role of fH and related molecules, CFHR1 and FHL-1 that bind to gonococci in facilitating the interaction between bacteria and CR3 on eukaryotic cells. We reasoned that because CR3 is expressed by cervical epithelial cells and alternative complement components are present in the cervical mucosa, the interaction between fH and CR3 could contribute to binding of gonococci to cervical epithelia. We also characterized the specific regions of fH that play a role in facilitating gonococcal adherence to CR3. We used CR3 transfected CHO cells to capitalize on an existing eukaryotic non-professional phagocyte model that isolates events surrounding interactions with CR3. Better understanding of the mechanism(s) of engagement between gonococci and host tissues is important because effective intervention strategies that may interrupt the organism’s ability to secure a foothold and cause infection would be important in disease prevention.
Materials and Methods
Cell lines
Chinese Hamster Ovary (CHO) cells stably transfected with CR3 (CHO/CR3) (33) and control CHO cells transfected with the ‘empty’ vector that contained the selection marker neomycin phosphotransferase (CHO/NEO) (34) were the gift of Dr. Douglas T. Golenbock (University of Massachusetts Medical School, Worcester, MA). Cell lines were maintained at 37°C in 5% CO2 in Ham’s Nutrient mixture F-12 medium (Gibco BRL Life Technologies, Gaithersburg, MD) supplemented with 10% heat inactivated fetal bovine serum and the antibiotic, geneticin (500 µg/ml). Media was changed to antibiotic-free Ham’s Nutrient mixture F12 medium, 12 hours before experiments were performed.
Sera and complement reagents
Normal human serum (NHS) specimens from 10 healthy volunteers with no history of gonococcal infection were pooled and stored at −70°C until used as a source of active complement. Complement was inactivated by heating NHS to 56°C for 30 min. Purified human fH, iC3b and C3b were purchased from Complement Technology, Inc. (Tyler, TX).
Gonococcal strains
N. gonorrhoeae 252, described previously (29), is a (stable) serum-resistant PorB.1A strain that binds fH (32) in the presence or absence of sialylation. Strain UU1 (PorB.1A) was isolated from an individual with disseminated gonococcal infection (DGI; (35) and also binds fH, but relatively weakly compared to 252. Strain F62 (PorB.1B) (32) binds barely detectable levels of fH in the unsialylated state. All strains were transformed with plasmid pEG2 (a gift from Dr. Myron Christodoulides (36)) to express green fluorescent protein (GFP) and maintained on GC agar media supplemented with 1% Isovitalex equivalent (37) containing ampicillin (5 µg/ml). For use in experiments, gonococci were harvested from overnight cultures and inoculated into GC broth (37) supplemented with the equivalent of 1% Isovitalex and grown to mid-log phase. When required, sialylation of gonococcal lipooligosaccharide (LOS) was achieved by adding CMP-NANA to the growth media (1 µg/ml). Bacteria were washed and resuspended in Hanks’ Balanced Salt Solution containing 0.15mM CaCl2 and 1mM MgCl2 (HBSS++) for use in binding and cell association assays.
Antibodies and immunochemicals
Expression of CR3 on CHO/CR3 was confirmed using anti-human PE-CD11b (Caltag [Carlsbad, CA]) and anti-human PE-Cy5-CD18 (BD Biosciences Pharmingen [Carlsbad, CA]) by FACS™ analysis. Biotin-labeled goat anti-mouse IgG primary antibody followed by Streptavidin-labeled AlexaFluor A647 (both from Molecular Probes [Carlsbad, CA]) were used in FACS experiments (below) to detect fH/Fc fragments bound to CHO cells and to gonococci. Specificity of fH binding to CHO/CR3 was determined by inhibition experiments using C3b or iC3b (each at a concentration of 220 nM), or monoclonal antibodies (mAbs) against anti-CD11b and anti-CD18 (Sigma [St. Louis, MO]), each at a concentration of 294 and 526 nM.
To measure binding of human fH, FHL-1, CFHR1, SCR 6, 7, 18–20 or SCR 6–20 to CHO/CR3 cells or to gonococci in FACS experiments, we used polyclonal antibody against fH that was made by immunizing goats with purified human fH (Bethyl Laboratories, Inc., Montgomery, TX) as primary antibody and anti-goat IgG conjugated to AlexaFluor A647 (Molecular Probes [Carlsbad, CA]) was used as the secondary antibody. mAb against human fH that is specific for an epitope within SCRs 18–20 (Quidel Corporation (Cat. No. A229) was used in capture ELISA to estimate the concentration of recombinant fH constructs SCR 6, 7, 18–20 and SCR 6–20 (see below).
Recombinant complement (C) proteins
We constructed five fH/murine Fc fusion proteins that contained contiguous fH SCR domains (SCR1–5, 6–10, 11–15, 16–20 and 18–20) fused in frame at their C-terminal ends to the N-terminus of Fc fragment of murine IgG2a (fH/Fc fusion proteins) as previously described (38). This allowed us to use the Fc region as a detection site (tag) for symmetric detection of each fusion protein using anti-mouse IgG. Deletion mutants in the SCR 16–20 domain were also constructed where SCR 16, 17, 18, 19 and 20 were each individually removed. To construct deletion mutants, pBluescript that contained cloned human factor H SCR16–20 was used as a template to construct the gene encoding the desired recombinant protein. Overlapping PCR was used to delete either SCR 17, 18 or 19 (primers listed in Table I). To delete SCR 16, the forward primer was designed at the 5’ of SCR 17 and to delete SCR 20 the reverse primer was designed at the 3’end of SCR 19 (Table 1). Each fH fragment was cloned into the Asc1 and Not1 site of pcDNA3 and the sequence was confirmed by automated DNA sequencing. CHO cells were transfected with the resulting construct using lipofectin (Invitrogen Life Technologies). Proteins were expressed, purified and quantified as described previously (38).
Table I.
Primers used for fH/Fc deletion mutant constructions
| Primer name* | Primer sequence |
|---|---|
| SCR 16–20Δ16 F AscI | GGCGCGCCAAAAACAGATTGTCTCAGTTTACC |
| SCR 16–20Δ16 R NotI | GCGGCCGCTCTTTTAGCACAAGTTGGATACT |
| SCR 16–20Δ17 R | CAGGAGGTGTCTATGCATGATGGAGGGTGAGAC |
| SCR 16–20Δ17 F | CCATCATGCATAGACACCTCCTGTGTGAATCCG |
| SCR 16–20Δ18 R | TCCTGTAGAATCTCTGCATGTTGGCCTTCCTG |
| SCR 16–20Δ18 F | CCAACATGCAGAGATTCTACAGGAAAATGTGGGC |
| SCR 16–20Δ19 R | TACACACGGATGTTTGCATTGAGGTGGTTCCGTC |
| SCR 16–20Δ19 F | CCTCAATGCAAACATCCGTGTGTAATATCCCGA |
| SCR 16–20Δ20 F AscI | GGCGCGCCCCTTCCTTGTAAATCTCCACCT |
| SCR 16–20Δ20 R NotI | GCGGCCGCTAAGCATTTTGGTGGTTCTGACC |
| SCR 6,7,18–20 SCR 6 F AscI | GGCGCGCCTACCTTGAAACCTTGTGATTATCCAGA |
| SCR 6,7,18–20 SCR 20 R XhoI | CTCGAGCTATCTTTTTGCACAAGTTGGATACTCCA |
| SCR 6,7,18–20 SCR 7,18 F | CCCAGATGCATCGACACCTCCTGTGTGAATCCG |
| SCR 6,7,18–20 SCR 18,7 R | ACAGGAGGTGTCGATGCATCTGGGAGTAGGAGA |
*F: Forward primer from 5’ to 3’ direction. Incorporated AscI sites are underlined.
*R: Reverse primer from 5’ to 3’ direction. Incorporated NotI or XhoI sites are underlined.
FH-related protein 1 (CFHR1) is composed of 5 SCR domains that bear 36%, 45%, 100%, 100% and 97% amino acid identity to SCRs 6, 7, 18, 19 and 20 of human fH, respectively (17, 39). CFHR1 was expressed and purified as described previously (17). FHL-1, an alternatively spliced variant of fH that contains the seven N-terminal fH SCRs with four unique amino acids at the C-terminal end (39) was expressed and purified as described previously (40).
We also constructed a recombinant fH fragment containing (from the N- to the C-terminus) SCRs 6, 7, 18, 19 and 20, that we called SCR 6,7,18–20 (SCR 6,7,18–20 lacked fH SCRs 1–5 and 8–17). In addition, we constructed an fH fragment spanning SCR 6–20. Briefly, overlap extension PCR with pBluescript vector containing either human fH SCR 6–10 or SCR 16–20 as templates for SCR 6,7,18–20 (32) and whole fragment fH (SCR 1–20; a gift from Dr. Michael K. Pangburn, University of Texas Health Science Center, Tyler, TX) was used (primers listed in Table I). The final PCR product encoding SCRs 6–7 fused to SCRs 18–20 and separately, SCR 6–20, were digested with AscI and XhoI and cloned into pCDNA3 (38). The resulting PCR products and clones were verified by DNA sequencing. These plasmids were used to transiently transfect CHO cells. SCR 6,7,18–20 secreted in cell culture supernatants was purified by affinity chromatography using EZview™ Red ANTI-FLAG® M2 affinity gel (Sigma). Protein purity was confirmed by Coomassie-stained SDS-PAGE gels and protein concentration was determined by BCA assay (Pierce Chemicals, [Rockford, IL]). Cell culture supernatant containing SCR 6–20 was spin-concentrated using Amicon Ultra-15 Centrifugal Filter Units (Millipore). Protein concentration was estimated by ELISA using Quidel anti-fH mAb (Cat. No. A229) (32) as the capture Ab and polyclonal goat anti-human fH followed by anti-goat IgG-alkaline phosphatase as the detecting Ab. Purified fH (Complement Technologies) was used to generate a standard curve.
Flow cytometry
Binding of complement proteins and recombinant proteins to N. gonorrhoeae was performed as described above and previously (32). Briefly, 108 bacteria suspended in HBSS++ were incubated with 67 nM fH or other complement related proteins and detected with anti-human fH (Bethyl) at a dilution of 1:400, followed by anti-goat IgG conjugated to Alexafluor 647. Bacteria were similarly incubated using 80 nM fH/Fc fusion proteins in final reaction volumes of 100 µl for 30 min at 37°C and fH/Fc constructs detected using anti-mouse IgG-biotin followed by streptavidin-Alexafluor 647.
Binding of complement proteins and recombinant proteins to CHO/CR3 or CHO/NEO cells was also performed as described above. Briefly, 106 CHO cells were suspended in antibiotic-free Ham’s/F12 media and incubated with fH or fH-related proteins or constructs; fH, CFHR1, FHL-1, and SCR 6, 7, 18–20 (each protein/construct was used at 10 µg/ml, which is equivalent to 67, 268, 231 and 268 nM respectively). CHO cells were similarly incubated for 30 min at 37°C using 80 nM fH/Fc fusion proteins in final reaction volumes of 100 µl. Detection of fH, fH-related proteins/constructs and fH/Fcs to CHO cells was identical to that used for detection to gonococci (see above). In experiments with CHO/CR3 or CHO/NEO cells 50,000 events were recorded.
Association of gonococci with CHO cells
CHO cell lines were seeded in 12-well tissue culture plates (Becton Dickinson and Company [Franklin Lakes, NJ]) at a concentration of 1.25 × 105 epithelial cells/well and grown to confluency. Twelve hours prior to the addition of bacteria to cells, the media was changed to antibiotic-free Ham’s Nutrient mixture F12 medium (Ham’s/F12 media). Cells were then released using enzyme-free PBS based cell dissociation buffer (Invitrogen [Carlsbad, CA]). GFP-expressing gonococci grown to mid-log phase were suspended in antibiotic-free Ham’s/F12 media and pre-incubated for 30 min with purified human fH, fH-related proteins (fH, CFHR1, FHL-1 or fH-related constructs (SCR 6, 7, 18–20 and SCR 6–20) and added to cells at a multiplicity of infection (MOI) of 100 (100 gonococci per CHO cell). Gonococci were pre-incubated initially with 67 nM amounts of each of the proteins or constructs. If enhanced gonococcal association was not observed at these equimolar concentrations using any individual protein or construct, we increased the concentration of the protein under examination to 10 µg/ml, which is equivalent to 67, 268, 231, 268 and 89 nM respectively, and reported that experiment. We also tested: i) non-pre-incubated GFP-expressing gonococci added to CHO-CR3 cells, which instead had been pre-incubated with fH (67 nM), ii) fH (67 nM) and gonococci added to CHO/CR3 cells simultaneously (no pre-incubation involved) and iii) fH (67 nM) pre-incubated gonococci added to CHO/CR3 cells that had also been pre-incubated with fH (67 nM). Infected cell lines were incubated at 37°C, 5% CO2 for 2 hours. Following incubation the reaction mixture were washed twice with Dulbecco’s PBS containing Ca++ and Mg++ (D-PBS++) (Gibco-BRL Life Technologies [Gaithersburg, MD]), resuspended in 600µl of D-PBS++ containing 0.5% bovine serum albumin (BSA), and flow cytometry performed immediately to enumerate bacteria associated with cells. Binding of gonococci to CHO/CR3 and CHO/NEO was detected by monitoring GFP expression of the gonococci by flow cytometry. Gating by cell size (forward scatter) excluded bacteria that were not associated with cells. Data were collected on a LSR II flow cytometer (Becton Dickinson [Franklin Lakes, NJ]) and analyzed using the FlowJo analysis software program (Version 7.2.2, TreeStar Inc. [Ashland, OR]).
Results
fH binds to CR3
We incubated CHO/CR3 cells with purified fH (67 nM) and detected cell-bound fH by flow cytometry. CHO cells transfected with the empty vector that contained the neomycin selection marker (CHO/NEO cells) were used as the negative control. We measured a 4-fold increase in fH binding to CHO/CR3 cells compared to CHO/NEO cells (Figure 1). We also used 10% heat-inactivated NHS as a source of fH and observed similar results (data not shown).
Figure 1. Binding of human fH to CHO/CR3 cells.
CHO cells transfected with human CR3 (CHO/CR3 cells) and CHO cells transfected with a vector containing only the neomycin marker (CHO/NEO control cells). Cells were incubated with purified human fH at a concentration of 67 nM. fH binding to cells was detected by flow cytometry using goat polyclonal anti-human fH as the primary antibody and AlexaFluor A647 conjugated (rabbit) anti-goat IgG as the disclosing antibody. The thick black line represents fH binding to CHO/CR3 cells; the grey shaded graph, fH binding to CHO/NEO cells. The broken black and grey lines represent the controls for CHO/CR3 and CHO/NEO, respectively (no fH added). One representative experiment is shown of three independently performed experiments.
Binding specificity of purified human fH to CR3 was examined using as inhibitors: (a) purified human iC3b, a known ligand for CR3 (33, 41, 42) and (b) mAbs specific for: i) CD11b, CR3’s unique α subunit and ii) CD18, the noncovalently associated β subunit (43). Purified C3b, known to possess diminished binding to CR3 (41, 42), was used as a ‘negative’ control. iC3b completely blocked fH binding to CHO/CR3 cells (Figure 2A; fluorescence decreased to control levels); C3b produced less inhibition (Figure 2B). mAbs specific for CD11b and CD18 each inhibited fH binding to CHO/CR3 cells almost to isotype control levels (Figure 2C). Collectively, these data suggest a specific interaction between fH and CR3 on CHO/CR3 cells.
Figure 2. Specificity of fH binding to CHO/CR3 cells.
Specificity of fH binding to CR3 was determined by incubating CHO/CR3 cells with purified iC3b (220 nM), a ligand for CR3, followed by addition of purified human fH at a concentration of 67 nM. Purified C3b (220 nM) known to bind minimally to CR3 served as a negative control. The thick black line represents fH binding to CHO/CR3 cells incubated with fH alone, the grey shaded graph, binding of fH to CHO/CR3 cells after pre-incubation with: iC3b (A.) or C3b (B.). The broken lines represent the controls (no fH added). (C.) fH binding to CHO/CR3 was blocked by using monoclonal antibodies against either CD11b or CD18 (each used at a concentration of 294 and 526 nM). Numbers in the histogram represent the median fluorescence of the CHO/CR3 cell population. The x-axis represents fluorescence on a log10 scale, the y-axis the number of events. In all experiments, human fH binding was detected using goat polyclonal anti-human fH followed by AlexaFluor A647 conjugated anti-goat IgG. In the control histograms, fH was omitted from the reaction mixture. One representative experiment is shown of three independently performed experiments.
Defining regions in fH that bind to CR3
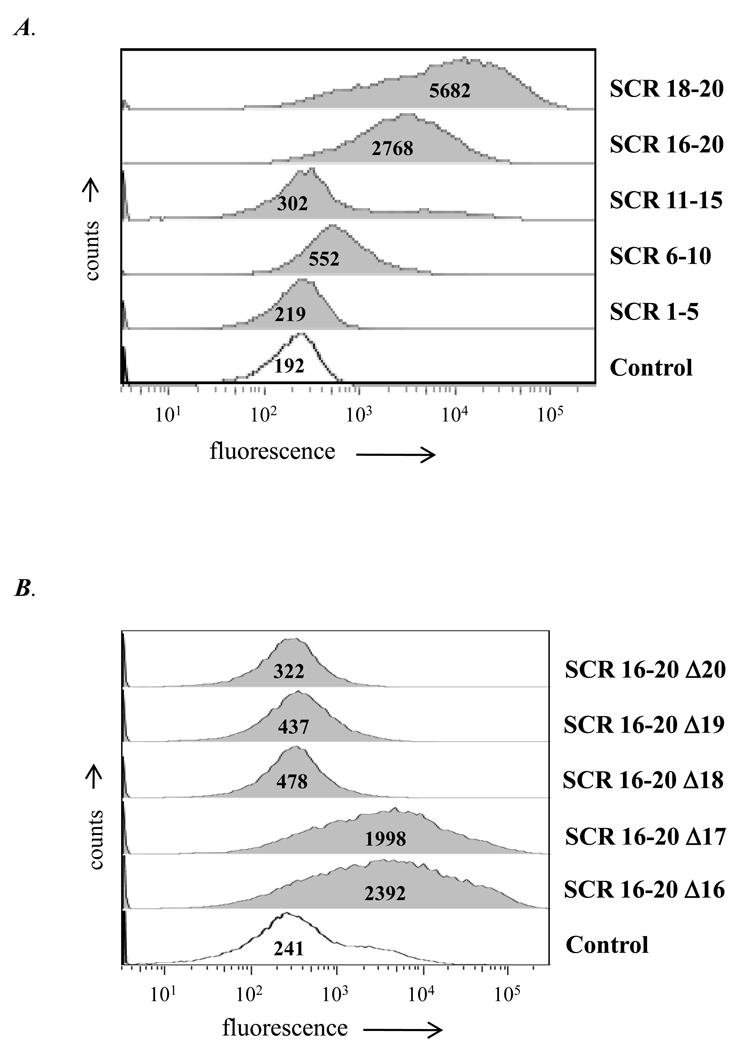
We used contiguous domains of human fH fused to the Fc fragment of mouse IgG2a (fH/ Fc fusion proteins; (32)) to determine SCR region(s) in fH that bound to CR3. SCRs1–5/Fc, SCRs 6–10/Fc, SCRs 11–15/Fc, SCRs 16–20/Fc and SCRs 18–20/Fc that collectively spanned all 20 fH SCRs were examined for binding to CHO/CR3 cells. SCR 16–20/Fc and SCR 18–20/Fc showed the greatest binding to CHO/CR3 (~15- and ~30-fold increase in fluorescence over control levels; Figure 3A). SCR 6–10/Fc also showed increased binding, however the fluorescence intensity was only ~3-fold greater than control levels.
Figure 3. Defining the SCR regions in human fH that bind to CHO/CR3 cells.
A. Binding of fH derived SCR regions/mouse Fc fusion proteins to CHO/CR3 cells. The SCR fragments used spanned the entire length of the fH molecule. B. Binding to CHO/CR3 cells of SCRs 16–20 lacking individual SCRs (16, 17, 18, 18, 19 or 20), each fused to mouse Fc. Biotin labeled goat anti-mouse IgG antibody was followed by streptavidin-labeled AlexaFluor 647 to detect binding of the each SCR to CHO/CR3 cells. SCRs 18, 19 and 20 together were required for binding of the C-terminal region of fH to CR3. Each Fc fusion protein was added at a concentration of 80 nM. The histogram marked “Control” lacked any fH derived SCR region/Fc fusion protein. Numbers in the histogram represent the median fluorescence of the CHO/CR3 cell population. The x-axis represents fluorescence on a log10 scale, the y-axis the number of events. One representative experiment is shown of three independently performed experiments.
In an attempt to further narrow the region in SCR 18–20 that bound to CR3, we deleted individual SCR domains in the background of SCRs 16–20/Fc. As seen in Figure 3A, deleting either SCR 16 or 17 from SCRs 16–20 in the SCRs 16–20/Fc construct had little effect on binding to CHO/CR3 cells. However, deleting either SCRs 18, 19 or 20 singly abrogated binding and suggested that all three SCR domains were necessary for interaction of the C-terminal region of fH with CR3 (Figure 3B).
Defining regions in fH that bind to N. gonorrhoeae strain 252
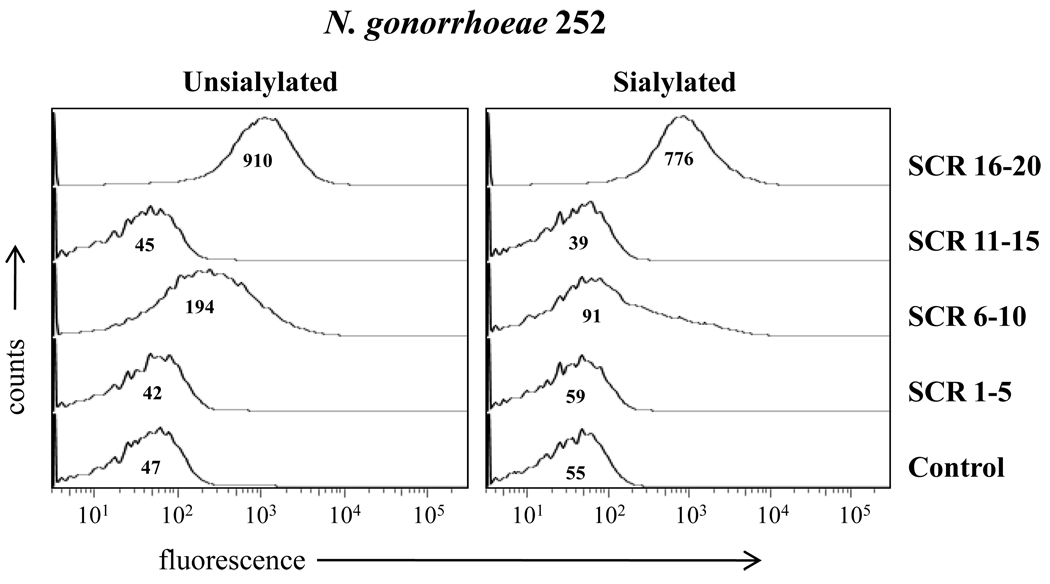
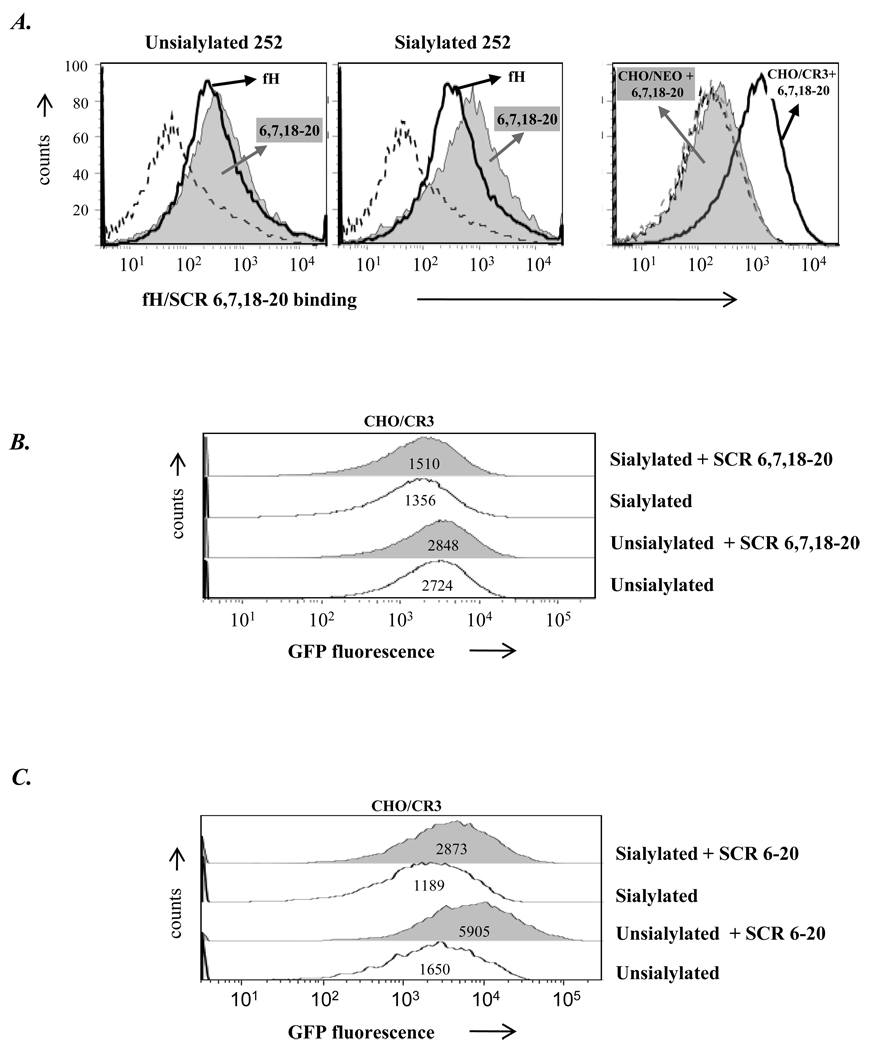
We used N. gonorrhoeae strain 252 (expressing the porin B.1A [PorB.1A] molecule and manifesting stable serum-resistance) that binds to fH (32) to examine whether this strain would bind to CHO/CR3 cells through fH. Before ascertaining gonococcal binding to CHO/CR3 cells through fH, we used the fH fragment/Fc fusion proteins to identify potential differences fH sites that bound to unsialylated versus sialylated gonococci. We observed that while SCR 16–20 bound equally well to strain 252 in both the unsialylated and sialylated states (Figure 4), SCR 6–10 bound unsialylated gonococci (Figure 4, left panel) but minimally to sialylated strain 252 (Figure 4, right panel).
Figure 4. Binding of human fH/murine Fc fusion proteins to N. gonorrhoeae.
Binding of fH derived SCR regions/mouse Fc fusion proteins to unsialylated and sialylated N. gonorrhoeae strain 252. SCR regions spanned the entire length of the fH molecule. Unsialylated strain 252 bound SCR 6–10 and SCR 16–20, sialyated strain 252 bound (only) SCR16–20. Each Fc fusion protein was added at a concentration of 80 nM. The “Control” lacks fusion protein(s). Detection was performed as described in Figure 3. The x-axis represents fluorescence on a log10 scale, the y-axis the number of events. Numbers in the histogram represent the median fluorescence of the bound Fc fusion proteins to N. gonorrhoeae strain 252. One representative experiment is shown of three independently performed experiments.
Binding of CFHR1 and FHL-1 to CR3
The binding of SCRs 18–20/Fc and to a lesser extent SCRs 6–10/Fc to CR3 raised the possibility that two other members of the fH family of proteins, fH-related protein 1 (CFHR1) (44) and fH-like protein 1 (FHL-1) (19) may also bind CR3. The relative homologies of CFHR1 and FHL-1 with equivalent regions in fH are shown schematically in Figures 5A and 5C. We measured strong binding of CFHR1 to CHO/CR3 cells and weaker binding to CHO/NEO cells (Figure 5B). In contrast, FHL-1 bound minimally to CHO/CR3 cells even when the concentration of FHL-1 was increased from 67 nM to 231 nM; no binding was seen to CHO/NEO cells (Figure 5D). These data are consistent with greater binding of SCR 18–20/Fc compared to SCR 6–10/Fc (Figure 3A).
Figure 5. Binding of CFHR1 and FHL-1 to CHO/CR3 and CHO/NEO cells.
A and C are schematic representation of CFHR1 and FHL-1. CFHR1 is the product of a unique gene, and the two N-terminal and the three C-terminal SCRs bear amino acid homology to fH SCRs 6–7 and 18–20 as indicated (39). FHL-1 is an alternatively spliced variant of fH that contains the seven N-terminal fH SCRs and is spliced to a separate exon that encodes for four C-terminal amino acids that are unique to FHL-1; S, F, T and L (as indicated). CHO/CR3 cells and non-transfected (CHO/NEO) cells were incubated either with purified human CFHR1 or FHL-1, each at a concentration of 268 and 231 nM (B and D respectively). The thick black lines represent CFHR1 and FHL-1 binding to CHO/CR3 cells in each of the histograms and the grey shaded graphs, binding to CHO/NEO cells. The broken black line represents controls for the CHO/CR3 cells (no CFHR1 or FHL-1 added). CFHR1 and FHL-1 binding was detected by flow cytometry using polyclonal goat anti-human fH followed by AlexaFluor A647 conjugated anti-goat IgG. The x-axis represents fluorescence on a log10 scale, the y-axis the number of events. One representative experiment is shown of three independently performed experiments.
Binding of CFHR1 and FHL-1 to N. gonorrhoeae strain 252
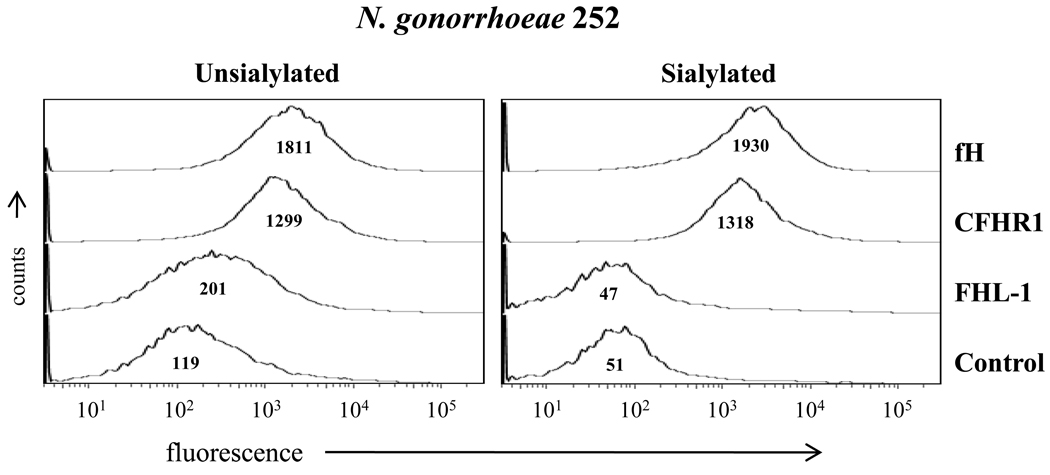
We also examined binding of fH, CFHR1 and FHL-1 to strain N. gonorrhoeae strain 252 in both its unsialylated and sialylated forms. As shown previously (32), unsialylated strain 252 bound fH and CFHR1 similarly but FHL-1 to a lesser extent (Figure 6, left panel); sialylated 252 also bound fH and CFHR1 (Figure 6, right panel). As guided earlier by the modest binding of SCR 6–10 to sialylated N. gonorrhoeae 252, (Figure 4, right panel), FHL-1 did not bind to sialylated strain 252 (Figure 6, right panel). Collectively, these data suggested that the two regions in fH that bind to unsialylated gonococci and CR3 bore similarities (SCR 6–7 and SCR 18–20).
Figure 6. Binding of fH and related derivatives to N. gonorrhoeae.
Binding of fH, CFHR1 and FHL-1 to unsialylated and sialylated N. gonorrhoeae strain 252 each at a concentration of 67 nM. “Control” had fH, CFHR1 or FHL-1 omitted from the reaction mixture. Binding of fH or its derivatives was detected as described in Figure 4. The x-axis represents fluorescence on a log10 scale, the y-axis the number of events. Numbers in the histogram represent the median fluorescence of the bound fH, CFHR1 and FHL-1 to N. gonorrhoeae strain 252. One representative experiment is shown of three independently performed experiments.
FH enhances the association of gonococci with CR3-expressing CHO cells
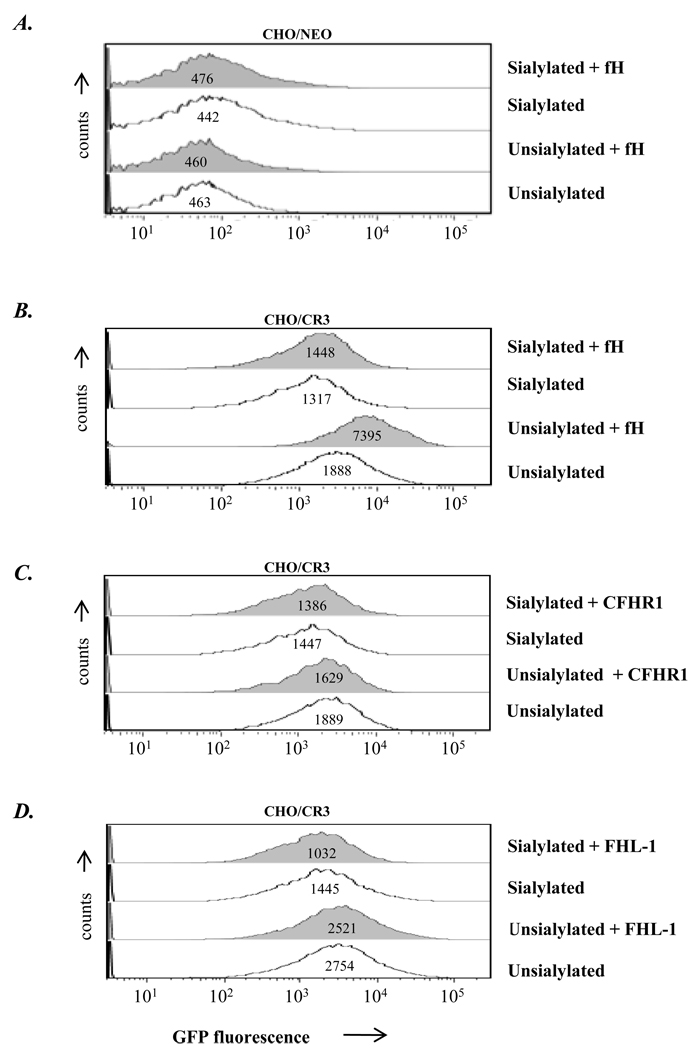
Next, we examined whether fH and its derivatives, CFHR1 and FHL-1 (the latter used as a putative negative control) that had bound to strain 252 enhanced binding of gonococci to CHO/CR3 cells. GFP-expressing N. gonorrhoeae 252 (either unsialylated or sialylated) were used and FACS analysis was performed by gating on CHO cells. Intrinsic binding of 252, unsialylated or sialylated, to CR3 expressing CHO (CHO/CR3) cells, occurred even in the absence of fH or its derivatives affixed to the strain, compared to binding to CHO/NEO cells (Figures 7B, 7C or 7D compared to Figure 7A), consistent with previous observations that gonococci can bind directly to CR3 (2). Full-length fH used at 67 nM concentration further enhanced binding of unsialylated bacteria selectively to CHO/CR3 (Figure 7B, row 3); in contrast, pre-incubation of sialylated strain 252 with 67 nM did not enhance binding to CHO/CR3 (Figure 7B, rows 1 and 2). No differences were founds when: i) non-pre-incubated GFP-expressing gonococci were added to CHO-CR3 cells, which instead had been pre-incubated with fH (67 nM), ii) fH (67 nM) and gonococci were added to CHO/CR3 cells simultaneously (no preincubation involved) and iii) fH (67 nM) pre-incubated gonococci were added to CHO/CR3 cells that had also been pre-incubated with fH (67 nM) (data not shown). We also examined another strain of N. gonorrhoeae Por1B.1A, strain UU1 (35) that binds fH in the unsialylated state under these conditions, but with weaker fluorescence intensity than 252 and a third strain, F62, that does not bind detectable amounts of fH in the unsialylated state (32). fH (67 nM) increased binding of unsialylated strain UU1 to CHO/CR3 cells but did not increase binding of unsialylated strain F62 (data not shown).
Figure 7. Incubation of N. gonorrhoeae strain 252 with fH enhances bacterial binding to CHO/CR3 cells.
Binding of GFP-expressing unsialylated or sialylated gonococci (strain 252) to CHO/NEO (control) cells (A) or CHO/CR3 cells (B) in the presence (grey shaded histograms) or absence (histograms depicted by solid lines) of fH (concentration, 67 nM); determined by flow cytometry. Bacteria were added to CHO cells at an MOI of 100:1. (C) Binding experiments performed using CHO/CR3 cells and organisms coated with CFHR1 and (D) using FHL-1. Events were gated to include CHO cells only (with or without bound bacteria and excluding free bacteria). GFP fluorescence (on the x-axis) reflects the bacteria associated with CHO cells, the y-axis the number of events. Numbers in the histogram represent the GFP median fluorescence of the CHO/NEO and CHO/CR3 cell population. One representative experiment is shown of three independently performed experiments.
CFHR1 and FHL-1 did not increase binding of unsialylated strain 252 to CHO/CR3 cells (Figures 7C and D) even when concentrations were each increased from 67 nM to 268 and 231 nM, respectively. Although CFHR1 contains both potential binding sites (within SCRs 6–7 and 18–20) to CR3 and gonococcal strain 252, the sequence homologies of the N-terminal SCRs 6 and 7 segments in CFHR1 are only 36 and 45% compared to SCRs 6 and 7 in fH and may therefore not have been effective in binding to CR3 via one site and strain 252 via the other.
We speculated that unsialylated gonococci may engage CR3 in a cooperative fashion via fH using one of the individual binding regions, SCRs 6–10 or SCRs 16–20 that also bound gonococci (shown in Figure 4, left panel), or alternatively that other sites on CR3 may be used for direct binding to N. gonorrhoeae. To more faithfully isolate the SCRs possibly involved in bridging CR3 and gonococci to enhance binding to CHO/CR3 cells, we constructed a mutant fH molecule that comprised (from the N- to C-terminus) fH SCRs 6, 7, 18, 19 and 20 (SCR 6,7,18–20). SCR 6,7,18–20 did not enhance the association of strain 252 with CHO/CR3 (Figure 8B) (concentration increased to 268 nM) although it maintained its ability to bind to unsialylated (and sialylated) gonococci (Figure 8A, left and middle panel) and to CHO/CR3 (Figure 8A, right panel). These data suggested that in addition to the mere presence of both the binding sites for bacteria and CR3, spatial orientation of these domains provided by the intervening SCRs (SCR 8–17) present in the full length fH molecule was required for enhanced binding of fH-coated unsialylated gonococci to CR3-expressing cells (Figure 7B). To examine this possibility, we constructed and used an fH recombinant protein, SCR 6–20, which in addition to containing CR3 and bacterial binding SCRs 6–7 and 18–20, also contained the intervening SCRs 8–17. Incubation of N. gonorrhoeae strain 252 SCR 6–20 (67 nM) enhanced the association of bacteria with CHO/CR3 cells by both unsialylated and sialylated gonococci (Figure 8C) suggesting a key role for the SCRs 8–17 in providing a ‘spacer’ for bridging gonococci and CR3-expressing cells. This result precluded the need to test SCR6–20 for separate binding to gonococci and CR3. In addition, SCR 6–20 bridging of sialylated gonococci to CHO/CR3 was modestly enhanced compared to full length fH (Figure 8C compared to 7B) suggesting improved binding of SCR 6 when it is located in a terminal position.
Figure 8. Binding of N. gonorrhoeae to CHO/CR3 cells via fH SCRs 6, 7, 18–20 (SCR 6,7,18–20) and SCR 6–20.
A. SCR 6,7,18–20 binding to strain 252, unsialylated (left panel) and sialylated (middle panel). Strain 252 was incubated with 67 nM of SCR 6,7,18–20 (grey shaded graph) or with 67 nM of fH (thick black line). The broken black line (control) represents samples where neither fH nor SCR 6,7,18–20 was added. The third panel shows binding of SCR 6,7,18–20 to CHO cells. CHO/CR3 or CHO/NEO cells were incubated with SCR 6,7,18–20 (268 nM); binding of SCR 6,7,18–20 to cells was assessed by flow cytometry. The thick black line represents binding of SCR 6,7,18–20 to CHO/CR3 cells and grey shaded graph to CHO/NEO cells. Control (no added SCR 6,7,18–20) for CHO/CR3 are shown by the broken black line. Binding of fH and SCR 6,7,18–20 were detected as described in Figure 4. B. Incubation of GFP-expressing strain 252 (unsialylated or sialylated) with SCR 6,7,18–20 does not increase binding of bacteria to CHO/CR3 cells, determined by flow cytometry as described in Figure 7. Numbers in the histogram represent the GFP median fluorescence of the CHO/CR3 cell population. C. Binding of recombinant protein SCR 6–20 to GFP-expressing strain 252 increases association of bacteria with CHO/CR3 cells in both the unsialylated and sialylated forms at a concentration of 67 nM as determined by flow cytometry (described in Figure 7). The x-axis represents fluorescence on a log10 scale, the y-axis the number of events. Numbers in the histogram represent the GFP median fluorescence of the CHO/CR3 cell population. Axes are as described in Figure 2. One representative experiment is shown of two independently performed experiments.
Discussion
In this study we have characterized the interactions between fH, FHL-1 and CFHR1 with human CR3 and have shown that fH, but not FHL-1 or CFHR1, can serve to bridge gonococci with CR3-expressing cells. Thus, in addition to playing an important role in gonococcal pathogenesis by enhancing the ability of bacteria to resist killing by human complement (10), fH may also enhance the association of gonococci with CR3 expressing cells. The redundancy of cell adhesion mechanisms employed by gonococci would enable it to bind to a variety of cell types that it may encounter in vivo. Using primary cervical epithelial cells, Edwards et al (6, 45) have shown that gonococcal binding to CR3 is mediated through a cooperative interaction that involves gonococcal porin and pilus and iC3b deposited on the bacteria by alternative pathway activation (6, 45, 46). Our data indicate that gonococci also use fH to associate with CR3.
Using a model system (CHO cells) we demonstrated that expression of CR3 augmented binding of fH. The specificity of the interaction was confirmed by inhibition of binding using mAbs against CR3 and soluble iC3b, the primary ligand for CR3. It is noteworthy that while iC3b at a ~3-fold molar excess (Fig. 2A) blocked fH binding to CHO/CR3, fH at a ~3-fold molar excess over iC3b resulted in only a ~20% decrease in the fluorescence of iC3b binding to CHO/CR3 (data not shown). Thus, fH is not likely to interfere with iC3b/CR3 interactions in vivo. Although our studies have been performed in a model cell line, the ability of fH to bind to CR3 expressed on human cells such as PMNs (25) and facilitate adhesion to microbes (47) indicates that our findings are likely to be pathophysiologically relevant.
Based on results of binding experiments with recombinant mutant fH fusion proteins, FHL-1 and CFHR1, two regions in fH (SCR 6–7 and SCR 18–20) were found to bind to CR3. Interestingly, the same two regions bound to unsialylated PorB.1A-expressing gonococci. SCR 6–7 and SCR 18–20 appear to be critical for binding to several other microbes and cell surfaces (reviewed in (48, 49). The importance of appropriate spatial orientation of the two binding regions in fH to enable it to bridge gonococci to CR3 was illustrated by observing that loss of the intervening SCRs (8 through 17) prevented adhesion of bacteria to CHO/CR3 (Figure 8B). A newly proposed functional structure of fH suggests that the SCR 12–14 region is the site of a bend or a hinge (50) and substantiates the proposed folded-back conformation of monomeric fH (51). Such a bend or hinge may permit SCR 6 and 7 tethered organisms to establish binding to CR3 via nearby SCRs 18–20 that are folded back. This resultant increase in proximity of SCRs 6 and 7 to SCRs 18–20 may enhance the potential for cooperation between SCR 6–7 and SCR 18–20 in fH mediated binding of N. gonorrhoeae to CR3 expressing cells (modeled in Figure 9).
Figure 9. Proposed model of fH-mediated enhancement of the N. gonorrhoeae-CR3 interactions.
CR3 and PorB.1A-expressing gonococci (labeled as N.g) both interact with human fH either through SCRs 6–7 or 18–20. Binding of the full-length fH to gonococci through SCR 6–7 or SCR 18–20 permits SCRs 18–20 or SCRs 6–7 respectively, to engage either N. gonorrhoeae or CR3. Bridging of gonococci and CR3 by fH may also require the intervening domains (SCRs 8–17) to provide appropriate spatial orientation for bridging to occur possibly accompanied by a bend or a hinge at SCR 12–14 (50) to permit “fold-back” (51) to bring the two binding sites into closer apposition. No binding to both N. gonorrhoeae and CR3 by FHL-1 (through SCRs 6–7 alone) or CFHR1 (through the fully homologous fH SCRs 18–20 alone) occurs because there is only a single binding site. Binding to both structures by SCR 6,7,18–20 also would not occur because both binding sites are juxtaposed and steric hindrance by either N. gonorrhoeae or CR3, when bound, would prevent binding to the other. Binding to both structures by SCR 6–20 occurs because SCRs 1–5 do not participate in binding.
Another noteworthy finding was that fH enhanced the association of unsialylated bacteria to CHO/CR3 cells; binding of sialylated bacteria to CHO/CR3 was only marginally increased. Two possibilities, not mutually exclusive, could be invoked. First, that sialylation of LOS is associated with enhanced negative surface charge of the gonococcal surface and could contribute to increased forces of repulsion between the bacteria and the negatively charged cell surface. A second consideration is that only those fH molecules that bind to bacteria through SCR 6–7 contribute to adhesion to CR3 – that is, a stable bridge is formed only when SCR 18–20 engages CR3. Binding of fH SCR 6–7 containing molecules to strain 252 is greatly decreased when LOS was sialylated (Figure 4 and 6). Therefore, in this instance binding of most fH molecules to bacteria would occur through SCR 18–20 leaving only SCR 6–7 (binds weakly to CR3) available. We observed that the SCR 6–20 construct facilitated bridging of sialylated gonococci over that produced by full-length fH, prompting the suggestion that terminally located SCR 6 may be more effective in binding to gonococci and allowing SCR 18–20 to bridge to CR3. Male urethral cells that posses the asialoglycoprotein receptor bind to unsialylated gonococci directly via the lacto-N-neotetraose (LNT) structure of gonococcal LOS (52); however, LOS sialylation does not affect gonococcal invasion into primary endo- or ectocervical cells (45). Primary cervical cells secrete all components of the alternative pathway and deposition of iC3b on bacteria may overcome the potential inhibitory effects of LOS sialic acid on bacteria-cell adhesion to facilitate invasion (6). The inhibitory effects of LOS sialylation is also cell specific; sialylation inhibits gonococcal opacity protein (Opa) mediated binding of gonococci to Chang epithelial cells and ME-180 endocervical cells (53), but not to HEC-1B and PC-3 cells (53). Because adherence and invasion of gonococci to host cells is a complex process (54, 55) we employed a reductionist approach here to investigate one of these variables.
Strains that cause DGI and other strains that may cause asymptomatic gonococcal infection often express the PorB.1A molecule (56–58); these strains bind fH directly (29) and do not require sialylation. Sialylation and desialylation of other gonococci in the genital tract are dynamic processes; neuraminidases (sialidases) elaborated by vaginal microflora (54) and by cervical epithelia (59, 60) may result in desialylation of gonococci. Here we have shown that PorB.1A strains that are not sialylated and bind fH adhere more efficiently to CR3-expressing cells. The present study may contribute an explanation of why PorB.1A-exressing gonococci invade epithelial cells in the genital tract, often without producing clinical signs or symptoms, and from there sometimes disseminate.
In conclusion, this study has shown that fH plays an important role in enhancing binding of unsialylated gonococci to eukaryotic cells that express CR3. Unsialylated gonococci with fH attached in addition to other complement components may be ubiquitous in the lumen of the genital tract and be adept in binding female epithelial cells, in particular via the CR3 receptor. Further, the regions in fH that interact with CR3 have been characterized and are in accordance with recent observations using PMNs. These studies could pave the way for future studies that examine the functional consequences of fH bound to gonococci when they interact with CR3-expressing non-phagocytic as well as phagocytic cells. These data also provide another ligand (CR3) for select members of the fH family of proteins to target at sites of inflammation.
Acknowledgements
We gratefully acknowledge the gift of plasmid pEG2 from Dr. Myron Christodoulides (University of Southampton Medical School, Southampton, UK), plasmid with the whole fH fragment from Dr. Michael K. Pangburn (University of Texas Health Science Center, Tyler, TX), and CR3 transfected Chinese Hamster Ovary (CHO) cells from Dr. Douglas T. Golenbock (University of Massachusetts Medical School, Worcester, MA).
Abbreviations used in this paper
- fH
factor H
- CCP
complement control protein domain
- SCR
short consensus repeat
- FHL-1
factor H-like molecule 1
- CFHR1
factor H-related protein 1
- DGI
disseminated gonococcal infection
- CMP-NANA
5’-cytidinemonophospho-N-acetylneuraminic acid
- LOS
lipooligosaccharide
- HBSS++
Hanks’ balanced salt solution with calcium and magnesium
- CR3
complement receptor 3
- CHO/CR3
Chinese hamster ovary cells transfected with CR3
- CHO/NEO
Chinese hamster ovary cells transfected with vector alone
Footnotes
“This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, hold the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version-derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
This work was supported by grants from the National Institute of Health AI32725, AI 084048 (P.A.R.) and AI054544 (S.R.), and from the Deutsche Forschungs Gemeinschaft (P.F.Z).
References
- 1.Harvey HA, Post DM, Apicella MA. Immortalization of human urethral epithelial cells: a model for the study of the pathogenesis of and the inflammatory cytokine response to Neisseria gonorrhoeae infection. Infect Immun. 2002;70:5808–5815. doi: 10.1128/IAI.70.10.5808-5815.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JL, Brown EJ, Ault KA, Apicella MA. The role of complement receptor 3 (CR3) in Neisseria gonorrhoeae infection of human cervical epithelia. Cell Microbiol. 2001;3:611–622. doi: 10.1046/j.1462-5822.2001.00140.x. [DOI] [PubMed] [Google Scholar]
- 3.Edwards JL, Shao JQ, Ault KA, Apicella MA. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect Immun. 2000;68:5354–5363. doi: 10.1128/iai.68.9.5354-5363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehlers MR. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect. 2000;2:289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 5.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JL, Brown EJ, Uk-Nham S, Cannon JG, Blake MS, Apicella MA. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol. 2002;4:571–584. doi: 10.1046/j.1462-5822.2002.t01-1-00215.x. [DOI] [PubMed] [Google Scholar]
- 7.Esparza-Gordillo J, Soria JM, Buil A, Almasy L, Blangero J, Fontcuberta J, Rodriguez de Cordoba S. Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics. 2004;56:77–82. doi: 10.1007/s00251-004-0660-7. [DOI] [PubMed] [Google Scholar]
- 8.Jozsi M, Zipfel PF. Factor H family proteins and human diseases. Trends Immunol. 2008;29:380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Ripoche J, Day AJ, Harris TJ, Sim RB. The complete amino acid sequence of human complement factor H. Biochem J. 1988;249:593–602. doi: 10.1042/bj2490593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris KM, Aden DP, Knowles BB, Colten HR. Complement biosynthesis by the human hepatoma-derived cell line HepG2. J Clin Invest. 1982;70:906–913. doi: 10.1172/JCI110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwaeble W, Zwirner J, Schulz TF, Linke RP, Dierich MP, Weiss EH. Human complement factor H: expression of an additional truncated gene product of 43 kDa in human liver. Eur J Immunol. 1987;17:1485–1489. doi: 10.1002/eji.1830171015. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Forrester JV, Xu H. Synthesis of complement factor H by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments. Exp Eye Res. 2007;84:635–645. doi: 10.1016/j.exer.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Friese MA, Hellwage J, Jokiranta TS, Meri S, Peter HH, Eibel H, Zipfel PF. FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol Immunol. 1999;36:809–818. doi: 10.1016/s0161-5890(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 15.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skerka C, Zipfel PF. Complement factor H related proteins in immune diseases. Vaccine. 2008;26 Suppl 8:I9–I14. doi: 10.1016/j.vaccine.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Haupt K, Kraiczy P, Wallich R, Brade V, Skerka C, Zipfel PF. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J Infect Dis. 2007;196:124–133. doi: 10.1086/518509. [DOI] [PubMed] [Google Scholar]
- 18.Heinen S, Hartmann A, Lauer N, Wiehl U, Dahse HM, Schirmer S, Gropp K, Enghardt T, Wallich R, Halbich S, Mihlan M, Schlotzer-Schrehardt U, Zipfel PF, Skerka C. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114:2439–2447. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn S, Skerka C, Zipfel PF. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H1. J Immunol. 1995;155:5663–5670. [PubMed] [Google Scholar]
- 20.Zipfel PF, Skerka C. FHL-1/reconectin: a human complement and immune regulator with cell-adhesive function. Immunol Today. 1999;20:135–140. doi: 10.1016/s0167-5699(98)01432-7. [DOI] [PubMed] [Google Scholar]
- 21.Hellwage J, Kuhn S, Zipfel PF. The human complement regulatory factor-H-like protein 1, which represents a truncated form of factor H, displays cell-attachment activity. Biochem J. 1997;326(Pt 2):321–327. doi: 10.1042/bj3260321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQuillen DP, Gulati S, Ram S, Turner AK, Jani DB, Heeren TC, Rice PA. Complement processing and immunoglobulin binding to Neisseria gonorrhoeae determined in vitro simulates in vivo effects. J Infect Dis. 1999;179:124–135. doi: 10.1086/314545. [DOI] [PubMed] [Google Scholar]
- 23.Oglesby TJ. The Complement System in Reproduction. In: Volanakis JE, Frank MM, editors. Human Complement System Health Disease. New York: Marcel Dekker; 1998. pp. 355–373. [Google Scholar]
- 24.Alexander JJ, Hack BK, Cunningham PN, Quigg RJ. A protein with characteristics of factor H is present on rodent platelets and functions as the immune adherence receptor. J Biol Chem. 2001;276:32129–32135. doi: 10.1074/jbc.M101299200. [DOI] [PubMed] [Google Scholar]
- 25.DiScipio RG, Daffern PJ, Schraufstatter IU, Sriramarao P. Human polymorphonuclear leukocytes adhere to complement factor H through an interaction that involves alphaMbeta2 (CD11b/CD18) J Immunol. 1998;160:4057–4066. [PubMed] [Google Scholar]
- 26.Karpman D, Manea M, Vaziri-Sani F, Stahl AL, Kristoffersson AC. Platelet activation in hemolytic uremic syndrome. Semin Thromb Hemost. 2006;32:128–145. doi: 10.1055/s-2006-939769. [DOI] [PubMed] [Google Scholar]
- 27.Gulati S, Cox A, Lewis LA, Michael FS, Li J, Boden R, Ram S, Rice PA. Enhanced factor H binding to sialylated Gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in Gonococci. Infect Immun. 2005;73:7390–7397. doi: 10.1128/IAI.73.11.7390-7397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madico G, Ngampasutadol J, Gulati S, Vogel U, Rice PA, Ram S. Factor H binding and function in sialylated pathogenic neisseriae is influenced by gonococcal, but not meningococcal, porin. J Immunol. 2007;178:4489–4497. doi: 10.4049/jimmunol.178.7.4489. [DOI] [PubMed] [Google Scholar]
- 29.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, Rice PA. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cannon JG, Buchanan TM, Sparling PF. Confirmation of association of protein I serotype of Neisseria gonorrhoeae with ability to cause disseminated infection. Infect Immun. 1983;40:816–819. doi: 10.1128/iai.40.2.816-819.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunham RC, Plummer F, Slaney L, Rand F, DeWitt W. Correlation of auxotype and protein I type with expression of disease due to Neisseria gonorrhoeae. J Infect Dis. 1985;152:339–343. doi: 10.1093/infdis/152.2.339. [DOI] [PubMed] [Google Scholar]
- 32.Ngampasutadol J, Ram S, Gulati S, Agarwal S, Li C, Visintin A, Monks B, Madico G, Rice PA. Human Factor H Interacts Selectively with Neisseria gonorrhoeae and Results in Species-Specific Complement Evasion. J Immunol. 2008;180:3426–3435. doi: 10.4049/jimmunol.180.5.3426. [DOI] [PubMed] [Google Scholar]
- 33.Levitz SM, Tabuni A, Kozel TR, MacGill RS, Ingalls RR, Golenbock DT. Binding of Cryptococcus neoformans to heterologously expressed human complement receptors. Infect Immun. 1997;65:931–935. doi: 10.1128/iai.65.3.931-935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golenbock DT, Liu Y, Millham FH, Freeman MW, Zoeller RA. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J Biol Chem. 1993;268:22055–22059. [PubMed] [Google Scholar]
- 35.Wetzler LM, Blake MS, Gotschlich EC. Characterization and specificity of antibodies to protein 1 of Neisseria gonorrhoeae produced by injection with various protein 1-adjuvant preparations. J Exp Med. 1988;168:1883–1897. doi: 10.1084/jem.168.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christodoulides M, Everson JS, Liu BL, Lambden PR, Watt PJ, Thomas EJ, Heckels JE. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol Microbiol. 2000;35:32–43. doi: 10.1046/j.1365-2958.2000.01694.x. [DOI] [PubMed] [Google Scholar]
- 37.McQuillen DP, Gulati S, Rice PA. Complement-mediated bacterial killing assays. Methods Enzymol. 1994;236:137–147. doi: 10.1016/0076-6879(94)36013-8. [DOI] [PubMed] [Google Scholar]
- 38.Visintin A, Halmen KA, Latz E, Monks BG, Golenbock DT. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J Immunol. 2005;175:6465–6472. doi: 10.4049/jimmunol.175.10.6465. [DOI] [PubMed] [Google Scholar]
- 39.Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, Kraiczy P, Noris M, Remuzzi G. Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans. 2002;30:971–978. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]
- 40.Shaughnessy J, Lewis LA, Jarva H, Ram S. Functional comparison of the binding of factor H short consensus repeat 6 (SCR 6) to factor H binding protein from Neisseria meningitidis and the binding of factor H SCR 18 to 20 to Neisseria gonorrhoeae porin. Infect Immun. 2009;77:2094–2103. doi: 10.1128/IAI.01561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross GD, Reed W, Dalzell JG, Becker SE, Hogg N. Macrophage cytoskeleton association with CR3 and CR4 regulates receptor mobility and phagocytosis of iC3b-opsonized erythrocytes. J Leukoc Biol. 1992;51:109–117. doi: 10.1002/jlb.51.2.109. [DOI] [PubMed] [Google Scholar]
- 42.Diamond MS, Garcia-Aguilar J, Bickford JK, Corbi AL, Springer TA. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zipfel PF, Jokiranta TS, Hellwage J, Koistinen V, Meri S. The factor H protein family. Immunopharmacology. 1999;42:53–60. doi: 10.1016/s0162-3109(99)00015-6. [DOI] [PubMed] [Google Scholar]
- 45.Edwards JL, Apicella MA. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell Microbiol. 2002;4:585–598. doi: 10.1046/j.1462-5822.2002.00212.x. [DOI] [PubMed] [Google Scholar]
- 46.Edwards JL, Apicella MA. I-domain-containing integrins serve as pilus receptors for Neisseria gonorrhoeae adherence to human epithelial cells. Cell Microbiol. 2005;7:1197–1211. doi: 10.1111/j.1462-5822.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 47.Losse J, Zipfel PF, Jozsi M. Factor H and factor H-related protein 1 bind to human neutrophils via complement receptor 3, mediate attachment to Candida albicans, and enhance neutrophil antimicrobial activity. J Immunol. 2010;184:912–921. doi: 10.4049/jimmunol.0901702. [DOI] [PubMed] [Google Scholar]
- 48.Blom AM, Hallstrom T, Riesbeck K. Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol Immunol. 2009;46:2808–2817. doi: 10.1016/j.molimm.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Kraiczy P, Wurzner R. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol Immunol. 2006;43:31–44. doi: 10.1016/j.molimm.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt CQ, Herbert AP, Kavanagh D, Gandy C, Fenton CJ, Blaum BS, Lyon M, Uhrin D, Barlow PN. A new map of glycosaminoglycan and C3b binding sites on factor H. J Immunol. 2008;181:2610–2619. doi: 10.4049/jimmunol.181.4.2610. [DOI] [PubMed] [Google Scholar]
- 51.Aslam M, Perkins SJ. Folded-back solution structure of monomeric factor H of human complement by synchrotron X-ray and neutron scattering, analytical ultracentrifugation and constrained molecular modelling. J Mol Biol. 2001;309:1117–1138. doi: 10.1006/jmbi.2001.4720. [DOI] [PubMed] [Google Scholar]
- 52.Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol Microbiol. 2001;42:659–672. doi: 10.1046/j.1365-2958.2001.02666.x. [DOI] [PubMed] [Google Scholar]
- 53.van Putten JP, Grassme HU, Robertson BD, Schwan ET. Function of lipopolysaccharide in the invasion of Neisseria gonorrhoeae into human mucosal cells. Prog Clin Biol Res. 1995;392:49–58. [PubMed] [Google Scholar]
- 54.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merz AJ, So M. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu Rev Cell Dev Biol. 2000;16:423–457. doi: 10.1146/annurev.cellbio.16.1.423. [DOI] [PubMed] [Google Scholar]
- 56.Hildebrandt JF, Mayer LW, Wang SP, Buchanan TM. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978;20:267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buchanan TM, Hildebrandt JF. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981;32:985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandstrom EG, Knapp JS, Reller LB, Thompson SE, Hook EW, 3rd, Holmes KK. Serogrouping of Neisseria gonorrhoeae: correlation of serogroup with disseminated gonococcal infection. Sex Transm Dis. 1984;11:77–80. doi: 10.1097/00007435-198404000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Paulesu L, Pessina GP. Cyclic changes of sialidase in human cervical mucus. Int J Biochem. 1982;14:561–563. doi: 10.1016/0020-711x(82)90035-0. [DOI] [PubMed] [Google Scholar]
- 60.Flori F, Secciani F, Capone A, Paccagnini E, Caruso S, Ricci MG, Focarelli R. Menstrual cycle-related sialidase activity of the female cervical mucus is associated with exosome-like vesicles. Fertil Steril. 2007;88:1212–1219. doi: 10.1016/j.fertnstert.2007.01.209. [DOI] [PubMed] [Google Scholar]