Abstract
Objective
The objective was to determine if intermittent, low-dose, short-acting GnRH agonist (GnRHag) administration up-regulates pituitary-gonadal function in gonadotropin deficiency (GnD) sufficiently to be of diagnostic or therapeutic value.
Design/Intervention
Low-dose leuprolide acetate was administered SC at 4–5 d intervals up to one year.
Patients
Adult volunteers and GnD patients were studied.
Setting
The studies were performed in a General Clinical Research Center.
Main Outcome Measures
LH, FSH, and sex steroid responses were determined.
Results
In normal men and women, low-dose GnRHag repetitively transiently stimulated gonadotropins in a gender-dimorphic manner. In congenitally GnD deficient men (n=6) and women (n=1), none of whom had a normal LH response to an initial GnRHag test dose, this regimen consistently stimulated LH to the normal baseline range within two weeks. Long-term GnRHag administration to a partially GnD man did not alleviate hypogonadism, however. Women with hypothalamic amenorrhea (n=2) responded normally to a single GnRHag injection; however, repeated dosing did not seem to induce the normal priming effect.
Conclusions
The subnormal LH response to GnRHag of congenital GnD normalized in response to repetitive intermittent GnRHag, but not sufficiently to improve hypogonadism. Hypothalamic amenorrhea patients lacked the priming response to repeated GnRHag, but otherwise had normal hormonal responses to GnRHag. We conclude that intermittent administration of a short-acting GnRHag is of potential diagnostic value in distinguishing hypothalamic from pituitary causes of GnD.
Keywords: gonadotropin deficiency, gonadotropin-releasing hormone agonist, repetitive administration, gonadotropin sexual dimorphism
INTRODUCTION
Patients with gonadotropin deficiency (GnD) typically have subnormal gonadotropin responses to acute GnRH (1, 2) or GnRH agonist (GnRHag) administration (3, 4). Pulsatile GnRH administration usually corrects the gonadotropin deficiency within weeks, which indicates that they are GnRH-deficient (5). These results have not been replicated by administering GnRHag at two-day intervals (6, 7), because of the GnRHag-desensitizing (down-regulating) effect on gonadotropin secretion (8), or at one-week intervals, because this is too infrequent to up-regulate gonadotropin release (9). We reasoned that a stimulatory outcome might be accomplished by developing a regimen of intermediate frequency, low-dose GnRHag administration that would avoid desensitization, yet be sufficiently frequent to prime increasing gonadotropin responses.
We here demonstrate that low-dose leuprolide (10) given at 4–5-day intervals for two weeks repetitively stimulates the pituitary-gonadal axis of normal men and women. We also show that repeated intermittent low-dose GnRHag administration results in increasing LH responsiveness that may be helpful in diagnosing hypothalamic GnRH deficiency, but lacks therapeutic potential in GnD.
METHODS
Subjects
Healthy adult male (n=39) and eumenorrheic female (n=10) volunteers, age 18 to 35 years old, were recruited by advertisement as controls. GnD males (n=6), 15 to 27 years old, were recruited from the endocrinology clinics of the University of Chicago Medical Center. Males had diagnoses of Kallmann’s syndrome (n=2; relatives with KAL-1 mutation (11)), idiopathic congenital panhypopituitarism (n=2), familial hypogonadotropic hypogonadism (n=1) and idiopathic partial gonadotropin deficiency (n=1). The latter was a 24-year old male whose testes were low-normal adult size (10 cc); otherwise, all had prepubertal size testes, and all were sexually infantile with plasma testosterone <50 ng/dl, prepubertal LH and FSH levels, and azoospermia. One female GnD patient had familial hypogonadotropic hypogonadism (n=1, prepubertal 13.25-year old sibling of the above male). The others were sexually mature women with hypothalamic amenorrhea that was idiopathic (n=1; low-normal gonadotropin and estradiol levels) or secondary to chronic disease (n=1, Gitelman’s syndrome; low gonadotropins and estradiol). With the exception of abnormalities mentioned above, all study subjects had unremarkable histories, examinations, and all patients had unremarkable screening tests for chronic endocrine, metabolic, or systemic disorders. Ethnicity was non-Hispanic Black 17%, non-Hispanic White 60%, Hispanic 5% and other 17%. These studies were approved by the University of Chicago Institutional Review Board and were performed after obtaining informed consent.
Study Design
Study 1 (response to single dose GnRHag in healthy volunteers)
At 0700 hr on day 1, after collecting baseline blood every 15 minutes for one hr, leuprolide acetate (GnRHag) injection was given as single dose of 1.0 or 10 μg/kg SC in random order to men in the University of Chicago General Clinical Research Center. Blood samples were then obtained at intervals up to 96 hrs after the injection for LH, FSH, and sex steroids. Women were studied similarly on 1.0 μg/kg GnRHag commencing on day 2–6 of their menstrual cycle.
Study 2 (response to repeated low-dose GnRHag in healthy volunteers)
A subset of healthy males received 1.0 μg/kg GnRHag again on days 5 and day 9. Females (n=5) received two more doses of GnRHag at 5-day intervals. After each injection, blood was sampled at 0–4 hrs and then daily until the next injection. Pelvic ultrasound examination was performed every 2–4 days, and progesterone was evaluated at baseline and 4 days after the last injection.
Study 3 (response of GnD patients to low-dose GnRHag at 5-day intervals)
GnD males received GnRHag 1.0 μg/kg SC every 5 days for 30 days, and GnD females did so for up to 11 days. After an initial 24-hr study following the day 1 GnRHag test dose, baseline and acute (0–4 hr) hormone responses were monitored similarly to Study 2. Pelvic ultrasound was performed every 2–4 days in females.
Study 4 (therapeutic trial of prolonged repetitive GnRHag administration)
Following study 3, the one partially GnD male underwent one year of intermittent GnRHag treatment with variation of interval (4 or 5 days) and/or dose (1.0 or 10 μg/kg). Testosterone cypionate therapy, 200 mg IM every two weeks, was added on day 287 of intermittent GnRHag administration for symptomatic relief and to determine if it would enhance spermatogenesis. Hormonal response testing and testicular ultrasound examinations were performed regularly.
Laboratory and Procedural Methods
Plasma total testosterone and estradiol were measured by commercially available immunoassay kits (testosterone, Coat-A-Count, Diagnostic Products Corporation, Los Angeles, CA; estradiol, Pantex, Santa Monica, CA) (10). Progesterone was measured by radioimmunoassay after preliminary chromatography (12). LH and FSH were measured by immunochemiluminometric assay (Delphia, Wallac, Finland) (13). Real-time testicular and vaginal pelvic ultrasound imaging were performed using an Acuson Sequoia with a 4 Mhz transducer (Acuson, Mountainview, CA) and a GE Voluson 730 5–9 MHz transducer (General Electric Fairfield,CT), respectively
Data Analysis
Baseline values were computed by averaging values from the hour preceding GnRHag injection. The significance of differences among responses to GnRHag was tested by repeated measures ANOVA, after logarithmic transformation as necessary for normalization of data distribution, and between group evaluation was tested by one-way ANOVA. Post hoc evaluation was by Fisher’s protected least significant differences test. Results are reported as mean±SEM, unless otherwise noted. P values < 0.05 were considered statistically significant.
RESULTS
Study 1. Response to Single Dose GnRHag in Healthy Volunteers (Table 1)
Table 1.
LH, FSH and sex steroid response to GnRH agonist administration in healthy volunteers. Mean ± SD (normal range)a.
| Male Controls | Female Controls | |||
|---|---|---|---|---|
| 1.0 μg/kg (n=19) | 10 μg/kg (n=20) | 1.0 μg/kg (n=10) | ||
| LH (U/L) | Baseline | 3.5 ± 1.2 (1.4–6.2)b | 3.6 ±1.8 (1.4–6.2)b | 4.1 ± 2.0 (2.0–7.3) |
| 0.5 HR | 21.8 ± 8.4 (13.0–29.0) | 26.3 ± 17.6 (3.8–55.5) | 13.1 ± 4.6c (6.9–21.5) | |
| 1 HR | 24.6 ± 10.5 (16.3–35.0) | 29.7 ± 19.7 (4.7–62.5) | 14.0 ± 4.6c (7.1–20.0) | |
| 3 HR | 24.7 ± 8.7 (14.4–34.5) | 22.6 ± 11.9 (5.7–40.5) | 54.3 ± 29.8c (19.0–131) | |
| 4 HR | 26.8 ± 9.3 (16.5–39.5) | 23.5 ± 13.2 (4.8–43.5) | 62.7 ± 46.1c (19–187) | |
| 24 HR | 13.0 ± 7.3 (5.5–25.5) | 21.7± 17.0 (3.0–46.8) | 11.6 ± 3.9 (5.1–17.9) | |
| 48 HR | 2.5 ± 0.9d (1.3–3.9) | 4.3 ± 2.7 (1.3–9.2) | 4.3 ± 0.9 (2.7–5.5) | |
| FSH (U/L) | Baseline | 2.9 ± 1.4 (0.9–7.5)b | 3.6 ± 1.7 (0.9–7.5)b | 5.5 ± 1.9c (3.6–9.5) |
| 0.5 HR | 4.7 ± 2.2d (1.6–8.7) | 12.6 ± 11.6 (3.3–31.5) | 7.8 ± 2.4 (5.3–12.8) | |
| 1 HR | 5.7 ± 2.8d (1.8–10.6) | 16.3 ± 15.6 (4.8–48.0) | 8.5 ± 3.8 (2.3–15.9) | |
| 3 HR | 6.7 ± 2.9d (2.1–12.6) | 15.2 ± 12.1 (4.4–33.0) | 20.4 ± 10.6c (6.8–38.0) | |
| 4 HR | 7.5 ± 3.1d (4.4–13.0) | 14.8 ± 10.5 (4.5–37.0) | 24.3 ± 13.7c (8.3–47) | |
| 24 HR | 6.2 ± 3.7d (1.5–12.0) | 17.9 ± 16.5 (5.4–40.0) | 9.9 ± 5.5 (5.2–22) | |
| 48 HR | 3.4 ± 1.7d (1.9–6.0) | 5.6 ± 2.9 (2.8–11.2) | 7.3 ± 4.6 (3.1–17) | |
| T (male, ng/dl)e E2 (female, pg/ml)e |
Baseline | 630 ± 170 (368–879)b | 548 ± 91 (368–879)b | 35 ± 15 (23–73) |
| 24 HR | 884 ± 249 (466–1260) | 816 ± 160 (582–1045) | 172 ± 85 (93–320) | |
| 48 HR | 640 ± 242 (349–1000) | 762 ± 190 (366–994) | 40 ± 20 (16–72) | |
5th to 95th percentile for males, observed range for females
pooled baseline 2.5–97.5th percentile (n=39).
p <0.05 between sexes for same dose
p <0.05 between doses
Abbreviations and conversions to SI units: testosterone (T) ng/dl x 0.0347= nmol/L; estradiol (E2) pg/ml x 3.61= pmol/L
Men
LH peaked at 1–4 hr in 95% of subjects on the low dose, but later (24 hr) in 33% on the larger dose, at which time a statistically significant difference emerged between doses. FSH peaked at 4 hr in 60% on the lower dose and at 24 hr in 56% on the larger dose; values were significantly higher on the larger dose from 0.5–48 hr (p<0.01). The LH/FSH ratio at baseline was 1.3±0.13 (5th–95th percentile 0.35–4.3); that 4 hr post-GnRHag was 4.3±0.5 (2.0–8.3) for the 1.0 μg/kg dose and 2.5±0.4 (0.13–4.8) for the 10 μg/kg dose. Testosterone rose significantly (p<0.0001) at 24 hr by an average of 41±0.05% (111–190) after 1.0 μg/kg and by 53±8% (108–206%) after 10 μg/kg GnRHag; it remained significantly, but variably, above baseline at 48 hr only on the higher dose (p<0.001).
Women
In response to 1.0 μg/kg GnRHag LH rose significantly and steadily from 0.5 through 4 hr and then fell to a level above baseline by 24 hrs. FSH rose steadily from 0.5 hr until 4 hrs, and then fell to baseline by 24 hr. LH and FSH peaks occurred at 3–4 hr in all. LH/FSH ratio was 0.8±0.2 (0.3–1.6) at baseline and 2.8±0.4 (1.1–5.6) at 4 hr. Estradiol rose significantly at 24 hrs in response to GnRHag and returned to baseline by 48 hrs.
Comparison between sexes on 1.0 μg/kg GnRHag
LH levels were similar in both sexes at baseline, but rose faster in males so that they had significantly higher levels than females at 0.5–1 hr (p≤0.002). However, unlike men’s gonadotropin levels, which plateaued, women’s LH levels continued to rise, becoming significantly higher than men’s at 2 hr (p=0.02) through 4 hr (p=0.003). FSH levels were significantly higher in women at baseline (p<0.001), and, like LH, they continued to rise so that the differences increased from 2 hr through 4 hr (p <0.001). By 24–48 hr, both LH and FSH fell to near baseline levels, with women’s levels slightly higher at 48 hr (p<0.005). There was a significant difference between sexes in the LH/FSH ratio at baseline (p=0.01), but not at the 4-hr peak.
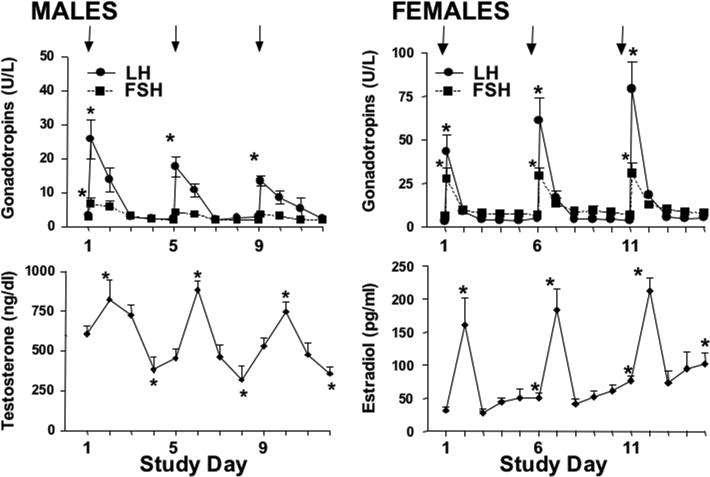
Study 2. Response to Repeated Low-Dose GnRHag in Healthy Volunteers (Figure 1)
Figure 1.
Effect of repeatedly administering GnRHag (1.0 μg/kg) in healthy volunteers. GnRHag (arrows) was given at 4 (males, n=5) or 5 (female, n=5) day intervals. Peak gonadotropin responses (shown) occurred on the day of GnRHag administration. Significant differences from day 1 baseline within a group are shown (* p<0.05). Notice scale differences between males and females.
Men
GnRH agonist 1.0 μg/kg dosing at 4-day intervals stimulated significant LH responses to each injection, but a significant FSH response to only the first. The peak LH decreased (p<0.05) with repeated administration, but the average LH over the 4-hr test interval did not. The LH/FSH ratios at baseline and peak were not changed by repeated GnRHag administration. While testosterone levels rose 24 hrs after each GnRHag injection, they dipped significantly below baseline at 3 days and then began to rebound at 4 days. This suggested that a 4-day interval might not be sufficient time for recovery of normal pituitary-gonadal responsiveness to GnRHag stimulation, so subsequent studies were carried out at 5-day intervals.
Women
GnRHag 1.0 μg/kg administered at 5-day intervals repeatedly stimulated significant, brisk, transient LH and FSH release, with unchanged LH/FSH baseline and peak ratios. Estradiol repeatedly rose 24 hr later, returned to baseline at 48 hrs, and then increased spontaneously over the subsequent 2 days after each injection. Thus, the baseline estradiol on the day of each subsequent GnRHag dose was significantly above the previous baseline. Progesterone levels at study commencement were 31±7 ng/d. In two of the five volunteers, 72–96 hr after the final dose of GnRHag, progesterone rose to 253–439 ng/dl, estradiol secondarily rose to 181–731 pg/ml, and ovarian size increased 3–8 fold in association with ovarian “cyst” development, indicating that ovulation had occurred. Dosing GnRHag at 1.0 μg/kg at 5-day intervals thus seemed optimal for further study.
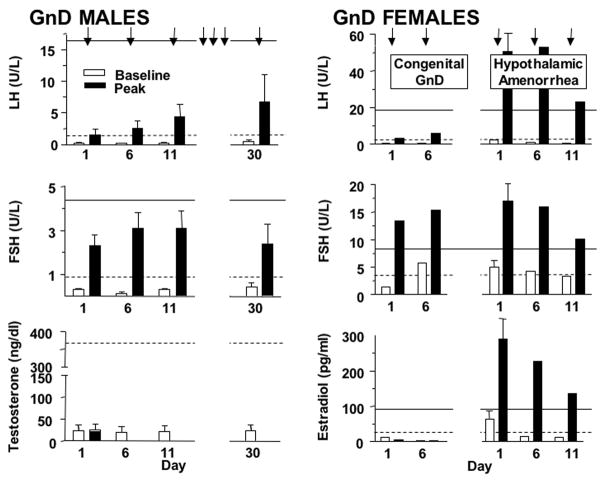
Study 3. Response of GnD Patients to Low-Dose GnRHag at 5-Day Intervals (Figure 2)
Figure 2.
Effect of repeatedly administering GnRHag (1.0 μg/kg) to GnD patients. GnRHag administration was repeated every 5 days (arrows) in both male GnD (n=6) and female GnD (n=3) patients. Testosterone was only sampled at baseline in men, while estradiol was sampled daily, with black bars representing 24 hrs after injection. Baseline and peak responses are indicated by open and closed bars, respectively. The lower limits of baseline (dotted line) and peak levels (solid line) for normal volunteers are shown for comparison. LH and estradiol responses of the congenitally GnD female patient (n=1) were low, but those of the hypothalamic amenorrhea patients (n=2) were normal. Notice scale differences between males and females.
Men
Congenitally GnD males had lower baseline LH (0.1–0.7 U/L) and FSH (0.15–0.6 U/L) than controls. LH rose significantly, but subnormally, after the initial dose of GnRHag, whereas FSH responses were normal in half. The LH peak successively increased with repeated dosing, quickly returning to baseline after each injection. FSH rose into the normal baseline range following the first dose of GnRHag; however, repeated administration did not significantly potentiate the FSH response. The LH/FSH ratio was 0.90±0.38 (range 0.25–2.7) at baseline and 0.65± 0.33 (0.2–2.3) 4 hrs after the first GnRHag dose; those post-GnRHag were all below normal. The peak LH/FSH ratio rose significantly by day 30 (1.3±0.4, p < 0.05), becoming normal in two subjects. Testosterone did not change after repeated GnRHag dosing.
Women
The congenital GnD female had low baseline (0.14 U/L) and peak (3.4 U/L) LH and low baseline (1.4 U/L) but normal peak FSH (13.5 U/L) responses. LH and FSH peaks were slightly higher in response to the second GnRHag injection, but remained subnormal, and estradiol remained <10 pg/ml.
In contrast, the two hypothalamic amenorrhea patients responded normally to the initial GnRHag dose. The study in the subject with secondary hypothalamic amenorrhea was aborted because she developed a 3.4 cm2 cyst in response to the first GnRHag injection. The subject with idiopathic hypothalamic amenorrhea underwent a 2-week study and repeatedly experienced brisk LH, FSH, and estradiol responses that were into the normal range. However, in contrast to normals, these responses waned with each successive GnRHag injection, and estradiol fell back to the control level each time. Her treatment was not followed by hormonal evidence of ovulation.
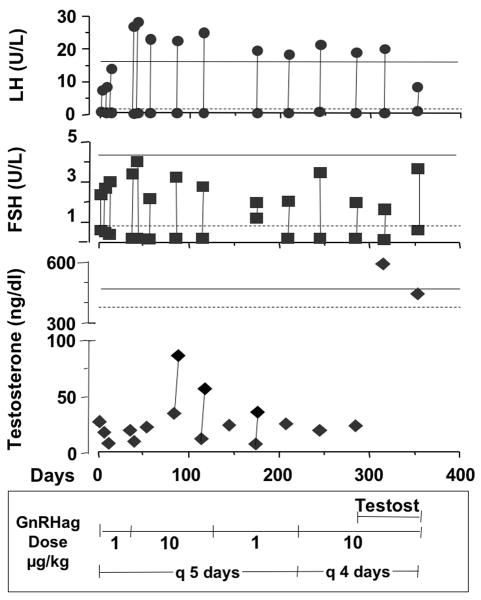
Study 4. Therapeutic Trial of Prolonged Repetitive GnRHag Administration (Figure 3)
Figure 3.
Long term, intermittent GnRH agonist treatment of a partially GnD male. The response to GnRHag dose and interval variation was tested. On days 1–21 the patient was given 1.0 μg/kg GnRHag every 5 days. Between days 22–135 GnRHag dose was increased to 10 μg/kg every 5 days. For days 136–210 the patient was again given 1.0 μg GnRHag every 5 days. On day 211 through the remainder of the evaluation, the GnRHag dose was changed to 10 μg/kg every 4 days. On day 287, depot testosterone (50 mg every 2 weeks, then increased to 100 mg every 2 weeks) was added. The acute hormonal responses to GnRHag administration were tested intermittently as shown; vertical lines connect baseline and peak at each visit (the testosterone response was only evaluated at 24–48 hr on three occasions). There was no obvious effect of the testosterone supplementation on LH and FSH responses. The lower limit of baseline (dotted line) and peak levels (solid line) for normal volunteers at 1.0 μg/kg are shown for comparison.
The one male patient with partial GnD underwent prolonged treatment with intermittent GnRHag. By one month, LH and FSH consistently responded normally but evanescently to GnRHag. Modest testosterone responsiveness developed: levels rose from baselines of 23–28 ng/dl to 36–87 ng/dl 24-hr post-GnRHag. Repeated GnRHag administration at a variety of combinations of dose and interval resulted in only modest testosterone responses, but no semen production. The administration of testosterone did not change the gonadotropin responses to GnRHag administration, the testes did not enlarge, and the semen lacked sperm.
DISCUSSION
This study was designed to determine if GnD patients can achieve normal gonadotropin levels in response to repetitive GnRHag administration at an optimal dose and interval. First, we demonstrated in normal men and women that low-dose GnRHag administration at 4 to 5-day intervals consistently transiently stimulated gonadotropin and sex steroid levels, in a gender-specific manner. Then, in congenitally GnD men and women, none of whom had a normal LH response to an initial GnRHag test dose, we showed that this regimen consistently stimulated LH to approximate the normal baseline range within two weeks. However, long-term GnRHag administration to a partially GnD man did not further potentiate gonadotropin release sufficiently to alleviate hypogonadism. Additionally, women with hypothalamic amenorrhea, unlike congenital GnD, responded normally to a single GnRHag injection, but repeated dosing did not seem to induce a normal priming effect on follicular function.
We here confirm our earlier finding (10) that normal men and women have sexually dimorphic patterns of gonadotropin response to GnRHag administration. Most strikingly, women had a smaller early LH response, but a greater LH peak, as well as higher baseline and peak FSH than men. These data are interpretable in terms of the two-pool model of gonadotropin release (14, 15), which would indicate that normal males have a larger readily releasable pool of gonadotropins than females, but females have a greater capacity to synthesize gonadotropins in response to intense GnRH stimulation, which may be what enables them to mount the mid-cycle gonadotropin surge.
The sex steroid pattern in response to repeated GnRHag administration also differed between normal men and women. After responding to each GnRHag dose, men’s testosterone levels transiently fell below baseline, suggesting Leydig cell desensitization (16, 17). In contrast, women’s baseline estradiol never fell below the control level and indeed continued to climb in tandem with increasing LH responses to GnRHag; furthermore, follicular development culminated in ovulation in two women. It seems as if thecal desensitization to LH was counteracted by FSH stimulation of follicular development and gonadotrope desensitization to GnRHag was counteracted by the positive feedback effect of the resultant estradiol secretion (18).
GnD males responded to GnRHag administration at 5-day intervals with significant potentiation of LH release, but the LH response was subnormal and transient, testosterone did not increase, and the FSH-predominance of severe GnD (9, 19) persisted. One-year’s treatment of a patient with partial GnD, altering the interval of GnRHag administration and varying the dosage by ten-fold, did not lead to sufficiently sustained repetitive LH and FSH responses to normalize testosterone levels or to correct azospermia. Since high intratesticular testosterone levels are important for the initiation of spermatogenesis (20, 21), we attempted supplementing intermittent GnRHag therapy with replacement testosterone; however, this treatment did not improve azoospermia, as has been reported for the combination of FSH and testosterone treatment of GnD men (22). Notably, exogenous testosterone did not alter the LH or FSH response to GnRHag, which is consistent with similar studies with natural GnRH (23–25).
In contrast to the female with congenital GnD, whose responses to GnRHag resembled those of congenitally GnD males, women with hypothalamic amenorrhea had normal LH, FSH and estradiol responses to the first GnRHag test dose, as expected from such studies of GnRH itself (26, 27). Although normal responses recurred with each subsequent GnRHag injection in the one hypothalamic amenorrhea patient tested repetitively, unlike normal women, she did not exhibit a self-priming effect at either the gonadotrope or the ovarian level, and her estradiol responses tended to wane with successive doses.
We conclude that intermittent GnRHag administration may be useful to distinguish hypothalamic from pituitary causes of GnD. However, it does not seem to be a promising form of treatment for hypothalamic GnRH deficiency.
Acknowledgments
This research was supported in part by FD-R-001012, FD-R-001473, RR-00055, UL1RR02499, and T32-DK064582. Thanks to Nancy Perovic, RN for coordinating these studies and to Kristen Wroblewski for biostatistical consultation.
Abbreviations
- GnD
gonadotropin deficiency
- GnRHag
gonadotropin releasing hormone agonist
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reitano JF, Caminos-Torres R, Snyder PJ. Serum LH and FSH responses to the repetitive administration of gonadotropin-releasing hormone in patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1975;41:1035–42. doi: 10.1210/jcem-41-6-1035. [DOI] [PubMed] [Google Scholar]
- 2.Yoshimoto Y, Moridera K, Imura H. Restoration of normal pituitary gonadotropin reserve by administration of luteinizing-hormone-releasing hormone in patients with hypogonadotropic hypogonadism. N Engl J Med. 1975;292:242–5. doi: 10.1056/NEJM197501302920505. [DOI] [PubMed] [Google Scholar]
- 3.Ghai K, Cara JF, Rosenfield RL. Gonadotropin releasing hormone agonist (nafarelin) test to differentiate gonadotropin deficiency from constitutionally delayed puberty in teen-age boys--a clinical research center study. J Clin Endocrinol Metab. 1995;80:2980–6. doi: 10.1210/jcem.80.10.7559884. [DOI] [PubMed] [Google Scholar]
- 4.Ehrmann DA, Rosenfield RL, Cuttler L, Burstein S, Cara JF, Levitsky LL. A new test of combined pituitary-testicular function using the gonadotropin-releasing hormone agonist nafarelin in the differentiation of gonadotropin deficiency from delayed puberty: pilot studies. J Clin Endocrinol Metab. 1989;69:963–7. doi: 10.1210/jcem-69-5-963. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman AR, Crowley WF., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307:1237–41. doi: 10.1056/NEJM198211113072003. [DOI] [PubMed] [Google Scholar]
- 6.Laron Z, Dickerman Z, Ben Zeev Z, Prager-Lewin R, Comaru-Schally AM, Schally AV. Long-term effect of D-Trp6-luteinizing hormone-releasing hormone on testicular size and luteinizing hormone, follicle-stimulating hormone, and testosterone levels in hypothalamic hypogonadotropic males. Fertil Steril. 1981;35:328–31. doi: 10.1016/s0015-0282(16)45381-1. [DOI] [PubMed] [Google Scholar]
- 7.Moore MP, Smith R, Donald RA, Espiner EA, Stronach S. The effects of different dose regimes of D-SER(TBU)6-LHRH-EA10 (HOE 766) in subjects with hypogonadotrophic hypogonadism. Clin Endocrinol (Oxf) 1981;14:93–7. doi: 10.1111/j.1365-2265.1981.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 8.Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 9.Crowley WF, Jr, Beitins IZ, Vale W, Kliman B, Rivier J, Rivier C, et al. The biologic activity of a potent analogue of gonadotropin-releasing hormone in normal and hypogonadotropic men. N Engl J Med. 1980;302:1052–7. doi: 10.1056/NEJM198005083021903. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfield RL, Perovic N, Ehrmann DA, Barnes RB. Acute hormonal responses to the gonadotropin releasing hormone agonist leuprolide: dose-response studies and comparison to nafarelin--a clinical research center study. J Clin Endocrinol Metab. 1996;81:3408–11. doi: 10.1210/jcem.81.9.8784105. [DOI] [PubMed] [Google Scholar]
- 11.Bhagavath B, Xu N, Ozata M, Rosenfield RL, Bick DP, Sherins RJ, et al. KAL1 mutations are not a common cause of idiopathic hypogonadotrophic hypogonadism in humans. Mol Hum Reprod. 2007;13:25–30. doi: 10.1093/molehr/gal108. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfield RL, Barnes RB, Ehrmann DA. Studies of the nature of 17-hydroxyprogesterone hyperresonsiveness to gonadotropin-releasing hormone agonist challenge in functional ovarian hyperandrogenism. J Clin Endocrinol Metab. 1994;79:1686–92. doi: 10.1210/jcem.79.6.7989476. [DOI] [PubMed] [Google Scholar]
- 13.Mortensen M, Rosenfield RL, Littlejohn E. Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab. 2006;91:3786–90. doi: 10.1210/jc.2006-0835. [DOI] [PubMed] [Google Scholar]
- 14.Bremner WJ, Paulsen CA. Two pools of luteinizing hormone in the human pituitary: evidence from constant administration of luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. 1974;39:811–5. doi: 10.1210/jcem-39-5-811. [DOI] [PubMed] [Google Scholar]
- 15.Redding TW, Schally AV, Arimura A, Matsuo H. Stimulation of release and synthesis of luteinizing hormone(LH) and follicle stimulating hormone(FSH) in tissue culture of rat pituitaries in response to natural and synthetic LH and FSH releasing hormone. Endocrinology. 1972;90:764–70. doi: 10.1210/endo-90-3-764. [DOI] [PubMed] [Google Scholar]
- 16.Forest MG, Lecoq A, Saez JM. Kinetics of human chorionic gonadotropin-induced steroidogenic response of the human testis. II. Plasma 17 alpha-hydroxyprogesterone, delta4-androstenedione, estrone, and 17 beta-estradiol: evidence for the action of human chorionic gonadotropin on intermediate enzymes implicated in steroid biosynthesis. J Clin Endocrinol Metab. 1979;49:284–91. doi: 10.1210/jcem-49-2-284. [DOI] [PubMed] [Google Scholar]
- 17.Cigorraga SB, Dufau ML, Catt KJ. Regulation of luteinizing hormone receptors and steroidogenesis in gonadotropin-desensitized leydig cells. J Biol Chem. 1978;253:4297–304. [PubMed] [Google Scholar]
- 18.Young JR, Jaffe RB. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. II. Effects of varying concentrations of estradiol. J Clin Endocrinol Metab. 1976;42:432–42. doi: 10.1210/jcem-42-3-432. [DOI] [PubMed] [Google Scholar]
- 19.Wilson DA, Hofman PL, Miles HL, Unwin KE, McGrail CE, Cutfield WS. Evaluation of the buserelin stimulation test in diagnosing gonadotropin deficiency in males with delayed puberty. J Pediatr. 2006;148:89–94. doi: 10.1016/j.jpeds.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Root A, Steinberger E, Smith K, Steinberger A, Russ D, Somers L, et al. Isosexual pseudoprecocity in a 6-year-old boy with a testicular interstitial cell adenoma. J Pediatr. 1972;80:264–8. doi: 10.1016/s0022-3476(72)80588-2. [DOI] [PubMed] [Google Scholar]
- 21.Steinberger E, Root A, Ficher M, Smith KD. The role of androgens in the initiation of spermatogenesis in man. J Clin Endocrinol Metab. 1973;37:746–51. doi: 10.1210/jcem-37-5-746. [DOI] [PubMed] [Google Scholar]
- 22.Schaison G, Young J, Pholsena M, Nahoul K, Couzinet B. Failure of combined follicle-stimulating hormone-testosterone administration to initiate and/or maintain spermatogenesis in men with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1993;77:1545–9. doi: 10.1210/jcem.77.6.8263139. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein JS, Whitcomb RW, O'Dea LS, Longcope C, Schoenfeld DA, Crowley WF., Jr Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 1991;73:609–20. doi: 10.1210/jcem-73-3-609. [DOI] [PubMed] [Google Scholar]
- 24.Pitteloud N, Dwyer AA, DeCruz S, Lee H, Boepple PA, Crowley WF, Jr, et al. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 2008;93:784–91. doi: 10.1210/jc.2007-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitteloud N, Dwyer AA, Decruz S, Lee H, Boepple PA, Crowley WF, Jr, et al. The relative role of gonadal sex steroids and gonadotropin-releasing hormone pulse frequency in the regulation of follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab. 2008;93:2686–92. doi: 10.1210/jc.2007-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lachelin GC, Yen SS. Hypothalamic chronic anovulation. Am J Obstet Gynecol. 1978;130:825–31. doi: 10.1016/0002-9378(78)90017-0. [DOI] [PubMed] [Google Scholar]
- 27.Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61:851–8. doi: 10.1210/jcem-61-5-851. [DOI] [PubMed] [Google Scholar]