Abstract
Alginate microcapsules coated with a permselective poly-L-ornithine (PLO) membrane have been investigated for the encapsulation and transplantation of islets as a treatment for type 1 diabetes. The therapeutic potential of this approach could be improved through local stimulation of microvascular networks in order to meet mass transport demands of the encapsulated cells. Fibroblast growth factor-1 (FGF-1) is a potent angiogenic factor with optimal effect occurring when it is delivered in a sustained manner. In this paper, a technique is described for the generation of multilayered alginate microcapsules with an outer alginate layer that can be used for the delivery of FGF-1. The influence of alginate concentration and composition (high mannuronic acid (M) or guluronic acid (G) content) on outer layer size and stability, protein encapsulation efficiency, and release kinetics was investigated. The technique results in a stable outer layer of alginate with a mean thickness between 113–164 µm, increasing with alginate concentration and G-content. The outer layer was able to encapsulate and release FGF-1 for up to thirty days, with 1.25% of high G alginate displaying the most sustained release. The released FGF-1 retained its biologic activity in the presence of heparin, and the addition of the outer layer did not alter the permselectivity of the PLO coat. This technique could be used to generate encapsulation systems that deliver proteins to stimulate local neovascularization around encapsulated islets.
Keywords: alginate, encapsulation, FGF-1, angiogenesis, neovascularization
Introduction
In recent years, advances have been made in the use of islet transplantation as a treatment for type 1 diabetes owing, in part, to improvements in the procedure for harvesting islets and immunosuppressive drug regimens. 1–3 While clinical islet transplantation has shown some promise, routine application is hindered, because patients must be treated with immunosuppressants and large volumes of islets are required for clinical efficacy. 4,5 Islet encapsulation in permselective biomaterials offers a potential mechanism for overcoming the requirement for immunosuppression. The ability to combine these materials with techniques that promote rapid and stable neovascularization may reduce the number of islets required to achieve improved functional outcomes. 6,7
Alginate microcapsules coated with a permselective poly-L-ornithine (PLO) layer have been investigated for islet cell encapsulation. 5,8 Alginate is composed of (1–4)-linked β-D-mannuronic acid (M-units) and α-L-guluronic acid (G-units) monomers, and is a biocompatible material that has been used for a wide range of biomedical applications, including cell encapsulation and protein delivery. 9–12 The alginate core of the PLO-coated microcapsules serves as a three-dimensional environment for the islets, while the positively charged PLO binds to the alginate core, forming a polycation-polyanion complex that influences the permeability of solutes into the microcapsules. Alginate microencapsulation of viable islets is in clinical trials as a potential treatment for type 1 diabetes. 13,14
Studies have shown that transplanting naked (not encapsulated in a biomaterial) islets simultaneously with proteins that stimulate neovascularization improves the function of the cells in vivo. A bolus of fibroblast growth factor-2 (FGF-2 or basic FGF) or vascular endothelial growth factor (VEGF) at the time of implantation has been shown to improve transplanted islet function, likely due to increased nutrient and oxygen transport as a result of enhanced local vascularization. 15,16 The use of growth factors to stimulate therapeutic neovascularization has shown promise for a number of applications, but the biological effects of the proteins are poor when delivered as a bolus injection. The sustained release of therapeutic proteins, however, tends to enhance and prolong their therapeutic effect.
Fibroblast growth factor-1 (FGF-1 or acidic FGF), promotes neovascularization by acting on numerous cell types, including endothelial cells (ECs), fibroblasts, endothelial progenitor cells, and smooth muscle cells. 17,18 Our lab has recently shown that the sustained delivery of FGF-1 results in a more persistent vascular response than a single bolus delivery. 19–21 The goal of our work is to combine a sustained release method with an islet encapsulation system in order to improve the functionality and success of islet transplantation by local stimulation of neovascularization. In our present work, a procedure to synthesize multilayered alginate microcapsules is described in which an outer alginate layer is generated that can be used as a region for protein encapsulation and sustained release.
Materials and Methods
Chemicals
Low viscosity (20–200 mPa·s) ultra-pure sodium alginate with high mannuronic acid (LVM) and high guluronic acid (LVG) content were purchased from Nova-Matrix (Oslo, Norway). LVM and LVG alginates were reported by the manufacturer to have molecular weights 75–200kDa and G/M ratios of ≤1 and ≥1.5, respectively. PLO hydrochloride, with molecular weight 15,000–30,000, was purchased from Sigma-Aldrich (St.Louis, Mo). Fluorescein conjugated albumin from bovine (FITC-BSA), was obtained from Invitrogen (Eugene, Or). FITC goat anti mouse IgG (FITC-IgG) was purchased from AbD Serotec (Martinsried, Germany). Solutions for alginate microbead synthesis were made using the following chemicals: HEPES, NaCl, and MgCl2 (Fisher Scientific); CaCl2 (Acros).
Multilayer microbead synthesis
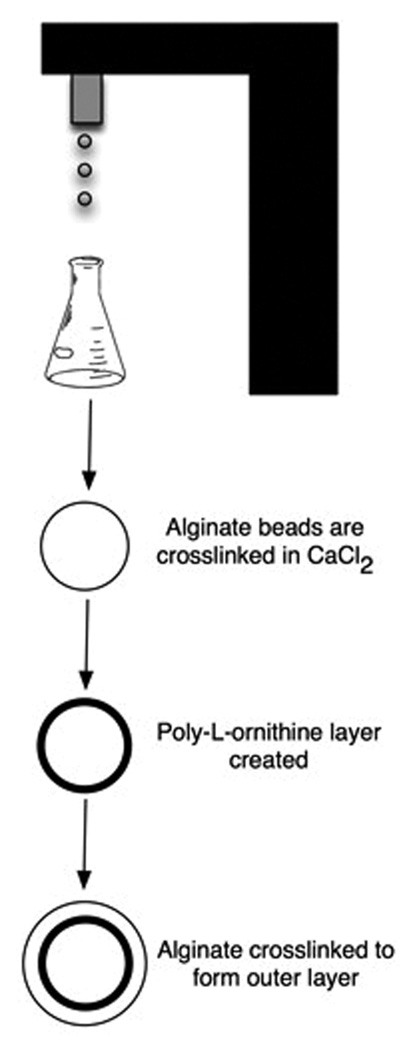
The procedure for generating multilayer microbeads involved first synthesizing PLO coated alginate microbeads followed by the generation of an outer alginate layer (Figure 1). The inner alginate core was synthesized using conditions previously shown to support functional islet encapsulation. 22 Droplets of 1.5% (w/v) LVM alginate were formed using a two-channel air jacket microencapsulator (at air jacket pressure of 15 psi and alginate jacket pressure of 12.5 psi) using a 25-gauge needle. The droplets were expelled into a 100mM CaCl2 crosslinking solution and allowed to incubate for fifteen minutes. The microbeads were washed thrice with a solution of 22mM CaCl2 and 0.9% NaCl for two minutes each. The PLO layer was generated using conditions shown to have appropriate permselectivity for islet encapsulation. 23 This was performed by transferring the microbeads to a 0.1% (w/v) solution of PLO in normal saline and rocked for thirty minutes, resulting in the formation of a PLO coating on the microbeads. Three washes were performed with 22mM CaCl2 and 0.9% NaCl solution to remove residual PLO.
Figure 1.
Schematic of the procedure for synthesizing multilayered alginate microcapsules. The inner alginate core is formed by crosslinking 1.5% LVM alginate in 2mM CaCl2. A PLO coating is then generated on the bead surface before the addition of alginate and crosslinking with CaCl2 to form the outer layer.
The PLO coated microbeads were blotted to remove excess water, and transferred into alginate solutions of varying concentrations and composition. The solutions were rocked for forty-five minutes, allowing alginate time to interact with the PLO layer. Excess alginate was removed, and the outer alginate layer was crosslinked by the addition of 22mM CaCl2. Two additional washes were performed with 2mM CaCl2 and 0.9% NaCl solution to remove unbound alginate. The composition of the outer layer was varied from 1%, 1.25% to 1.5% for both LVG and LVM alginate. In some cases, 5 U/mL heparin was incorporated into the outer layer by directly adding it to the outer alginate solution prior to addition of the microbeads. Immediately after synthesis, the microbeads were imaged using an Axiovert 200 inverted microscope (Carl Zeiss MicroImaging, Inc.) with a 10× objective (1.08 µm/pixel), and the size of the outer alginate layer quantified with AxioVision AC Rel. 4.6. Ten microbeads were measured per condition, and the experiments were repeated three times.
Stability of the outer alginate layer
The microbeads were incubated in saline solution containing a concentration of calcium (2mM) consistent with interstitial fluid and placed in an incubator maintained at 37°C to monitor the stability of the outer layer. The microbeads were imaged daily and the thickness of the outer layer quantified as described in the previous section. The surrounding solution was replenished with fresh solution after imaging.
Radiolabeling of FGF-1
FGF-1 was purchased from Peprotech (Rockyhill, NJ) and radiolabeled with 125I isotope using Iodo-beads (Pierce Chemical Co.). The Iodo-beads were washed with reaction buffer, then placed in a buffer solution containing 125I (1mCi, Perkin Elmer), and allowed to incubate for five minutes. A solution of 50 µg/ml FGF-1 was added to the vial and allowed to react for fifteen minutes, with intermittent mixing. The Iodo-beads were removed from the vial, and the solution was diluted to a final volume of 3 mL before being dialyzed against de-ionized water for 48 hours to remove any unincorporated 125I.
Protein encapsulation and release
Radiolabeled FGF-1 was added to the outer layer alginate solution prior to addition of the PLO-coated alginate microbeads. Encapsulation efficiency was determined by quantifying the fraction of FGF-1 encapsulated in the microbeads measured immediately after constructing the outer layer, relative to the total amount of FGF-1 added to the alginate solution used to create the outer layer. After formation of the outer layer, the microbeads were transferred into a 2mM CaCl2 solution and incubated at 37°C. The entire incubation medium was removed at pre-determined time points to determine release and replaced with fresh solution. Initially, samples were taken every twenty minutes, and then hourly, and daily, as the rate of protein release decreased. Protein content was determined using a gamma-counter (Perkin-Elmer Packard Cobra II Auto Gamma). The percent of FGF-1 released was calculated as the sum of FGF-1 released at a particular time point relative to the total amount encapsulated.
The influence of heparin on the rate of release of FGF-1 was also investigated. FGF-1 with 5 U/mL of heparin was incorporated into the outer alginate layer comprised of 1.25% LVG alginate, and release was quantified in the same manner as described above.
Permeability
The permeability of the microbeads was examined by incubation of the beads in 2mM CaCl2 solutions containing either FITC-IgG (Stokes radius (rs) ~ 5.5 nm) or FITC-BSA (rs ~ 3.6 nm) at a concentration of 2.5 µg/mL. Confocal images of the beads were taken at time zero, 5 hours, and 3 days. All images were obtained using a confocal laser-scanning microscope with a low pass filter of 505 nm and an excitation at 488 nm.
Protein activity
Fifty microbeads with an outer layer formed using 1.25% LVG were synthesized under two conditions: one in which 15 µg/mL of FGF-1 alone was incorporated into the outer alginate solution prior to addition to the microbeads, and the other in which 15 µg/mL of FGF-1 and 5 U/mL heparin were incorporated into the outer layer. The beads were placed in 1mL of Hank’s Buffered Saline Solution (HBSS) containing 5 U/mL heparin. Samples were taken at 12 hours, and the biological activity of released FGF-1 determined using an enzyme immunoassay for quantification of proliferating human umbilical vein endothelial cells (HUVEC). For this assay, HUVECs were seeded in a 96-well plate and grown in endothelial basal media containing 2% fetal bovine serum (FBS), bovine brain extract, gentamicin, hydrocortisone, and EGF, for three days. Cells were then cultured in 0.5% serum in basal media with gentamicin for 24 hours, prior to exposure to release samples for another 24 hours. Bromodeoxyuridine (BrdU), a synthetic nucleoside incorporated into newly synthesized DNA strands, was then added to the wells. Proliferation was determined 24 hours after addition of BrdU by measuring BrdU incorporation using a cell proliferation assay kit purchased from Calbiochem.
Statistical Analyses
The data are expressed as mean ± standard deviation. Significant differences were determined by performing an analysis of variance (ANOVA) with a Student-Newman-Keuls post-test. In all cases, a p < 0.05 was considered to be statistically significant.
Results
Outer layer thickness
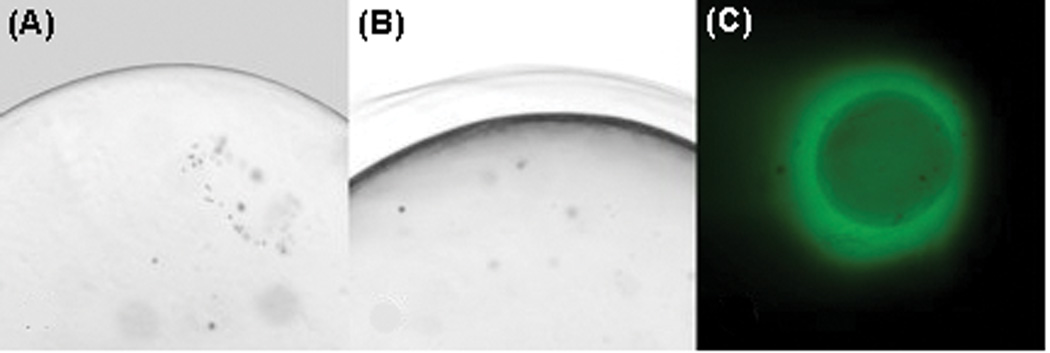
Microbeads were synthesized using 1.5% LVM alginate for the inner core (Figure 2A) and various alginate concentrations for the outer layer: 1%, 1.25% and 1.5% LVM or LVG. A distinct outer layer could be seen on the outside of the bead after crosslinking (Figure 2B). Protein could be encapsulated in this layer formed around the inner alginate core (Figure 2C). The size of the outer alginate layer varied based on the concentration and composition of alginate used (Figure 3). The smallest outer layer was 113±6 µm formed using 1% LVM alginate, while the thickest outer layer, 164.3±3 µm, was made using 1.5% LVG alginate. The results show that, for both LVM and LVG alginate, the size of the outer layer increased with increasing alginate concentration. Moreover, at equal alginate concentrations, outer layers formed using LVG alginate were larger than those made with LVM alginate. At the highest alginate concentrations (1.5% LVM and LVG alginate), the microbeads tended to aggregate, forming large clumps, and it was difficult to consistently isolate individual microbeads. Thus, 1.25% served as the upper limit of the concentration of alginate used to create the outer layer for all subsequent experiments.
Figure 2.
(A) PLO coated microbeads prior to formation of the outer layer. (B) A multilayer alginate microbead with an outer alginate layer that is distinctly visible. (C) FITC-BSA (green) can be seen in the outer layer immediately after synthesis, indicating that this region can be used for protein encapsulation.
Figure 3.
The size of the outer layer of multilayered alginate microbeads varied with the composition and concentration of alginate used. At equal concentrations, it was found that LVG alginate formed larger outer layers than LVM. For both LVM and LVG alginate, the size of the outer layer increased with increasing alginate concentration. All groups were statistically different (p < 0.05) from each other.
Stability of the outer alginate layer
The stability of the outer layer was investigated by incubating microbeads in saline supplemented with calcium levels similar to those found in interstitial fluid. Neither swelling nor degradation of the outer layer was observed, and the size of the outer layer remained constant for all conditions examined over a period of thirty days (Figure 4).
Figure 4.
The size of the outer alginate layer remained stable over a period of thirty days when incubated in a 2mM solution of CaCl2 at 37°C. * indicates that all groups are statistically different from one another, and # denotes that all groups are statistically different from each other except 1% LVM from 1.25% LVG.
FGF-1 encapsulation and release from the outer alginate layer
FGF-1 was encapsulated in the outer layer by suspending it in the alginate solution prior to crosslinking. The encapsulation efficiency for FGF-1 did not vary with alginate concentration or type. The encapsulation efficiencies calculated were as follows: 1% alginate (LVM vs. LVG = 17.8±2.1% and 18.2±1.8%, respectively), and 1.25% alginate (LVM vs. LVG = 20.1±1.7% and 20.3±3.0%, respectively).
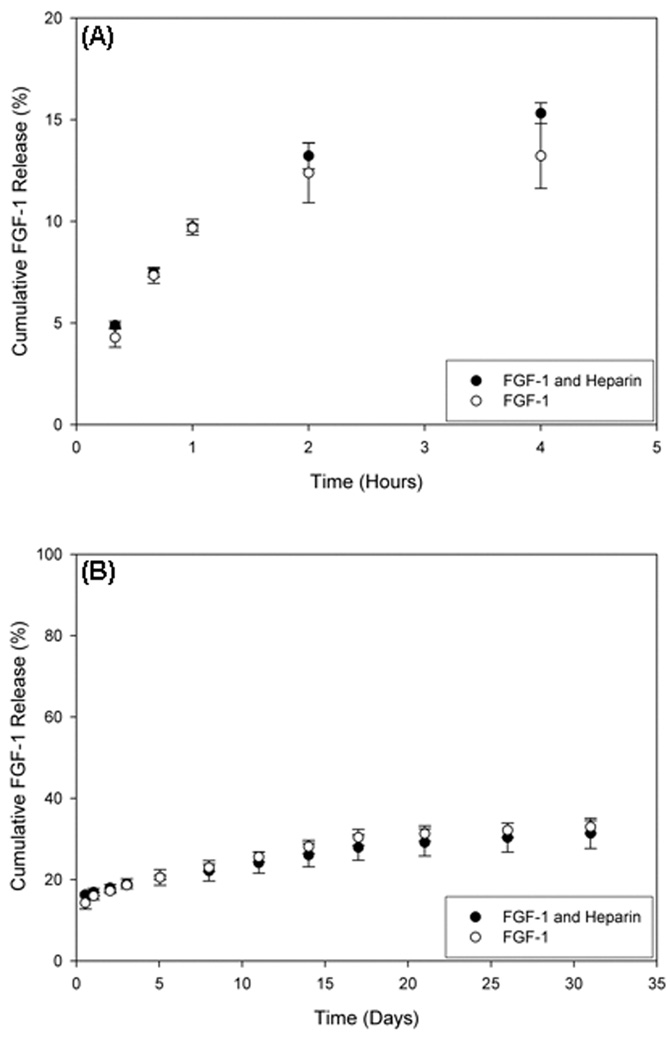
The encapsulation of FGF-1 in the outer layer resulted in a long-term release of the protein into the surrounding fluid. There was an initial burst release rate of protein. The rate of this release varied with alginate composition and concentration used to generate the outer layer. LVG at 1.25% had the lowest initial release with only 18% released after 4 hours compared to greater than 24% in all other conditions (Figure 5A and 5C, showing % and mass of protein released, respectively). Release continued at low levels for over three weeks for all conditions (Figure 5B and 5D). Multilayer microbeads made with 1.25% LVG for the outer alginate layer synthesis had the lowest initial release, and subsequently provided a steady release of FGF-1 for over thirty days.
Figure 5.
Release of FGF-1 from the outer alginate layer varied based on the concentration of LVM and LVG alginate used. (A) and (B) show percent release, and (C) and (D) denote the corresponding mass release of FGF-1 versus time for different outer layer formulations. There is a burst release exhibited for all conditions within the initial 5 hours (A and C), and low-dose continuous release for up to thirty days (B and D).
At all time points and conditions studied, the levels of 125I labeled FGF-1 released were significantly above background. We have previously shown that greater than 0.5 ng/day of FGF-1 is able to stimulate EC proliferation and neovascularization.19 At the given levels of encapsulation, 1% LVM microbeads provided greater than 0.5 ng/day for the first sixteen days, while 1.25% LVM and 1% LVG released more than 0.5 ng/day up to days 18 and 19, respectively. LVG at 1.25% released 61 ng of FGF-1 within the first two hours, with continued release of more than 0.5 ng/day up to day 26. Microbeads were analyzed for remaining protein content after 32 days, and that 38.0±4.6 %, 37.8±5.7 %, 46.5±7.0 % and 64.8±0.9 % of encapsulated FGF-1 still remained in the 1% LVM, 1% LVG, 1.25% LVM and 1.25% LVG microbeads, respectively.
FGF-1 release from the outer layer was also examined with the addition of heparin to outer layers formed with 1.25% LVG. The encapsulation efficiency of FGF-1 in the outer layer containing heparin was 20.6±2.7% (compared to 20.3±3.0% without heparin). Heparin had no significant effect on release kinetics relative to FGF-1 in the absence of heparin (Figure 6).
Figure 6.
Heparin (5 U/mL) was added to the outer layer composed of 1.25% LVG, and FGF-1 release is compared with results in the absence of heparin.
Permeability
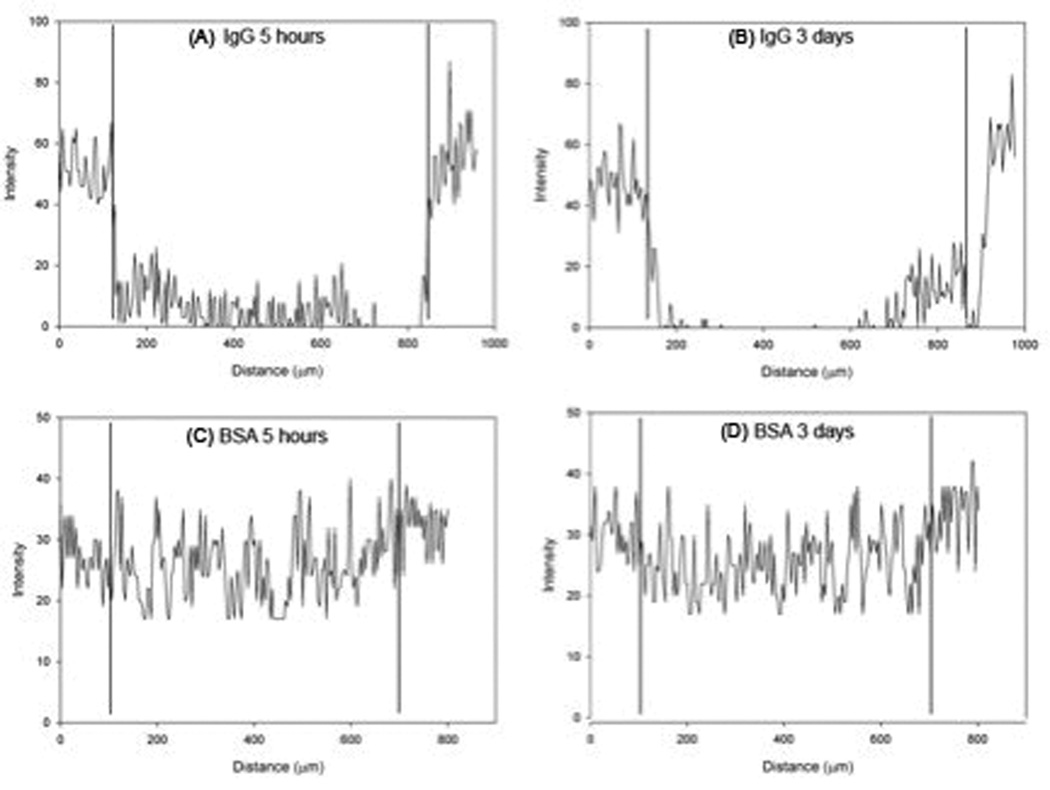
Critical to the success of the system for islet cell encapsulation is that the permeability of the PLO coating is retained after formation of the outer layer. Microbeads were incubated with FITC-labeled IgG (MW 150 kDa, rs ~ 5.5 nm) and BSA (MW 66 kDa, rs ~ 3.6 nm) in order to assess the permselectivity of the PLO coating. Images taken at 5 hours and 3 days illustrate that IgG permeated the outer alginate layer, but PLO inhibited IgG transport into the inner alginate core, where encapsulated cells would reside (Figure 7). The BSA, however, rapidly diffused into the outer layer and could be seen distributed within the alginate at 5 hours. These results are consistent with our previous findings for the permeability of the PLO layer, 23 suggesting that the outer alginate layer created in the present study does not alter PLO permeability.
Figure 7.
Intensity profile of a cross-section of multilayered alginate microbeads incubated with solutions containing fluorescently labeled IgG (A, B) or BSA (C, D). The IgG molecule was not present within the inner alginate core at either (A) 5 hours or (B) 3 days. BSA rapidly diffused into the bead and was present throughout the core at (A) 5 hours and (B) 3 days. Vertical lines designate the position of the PLO coating of the multilayered microbeads.
Protein activity
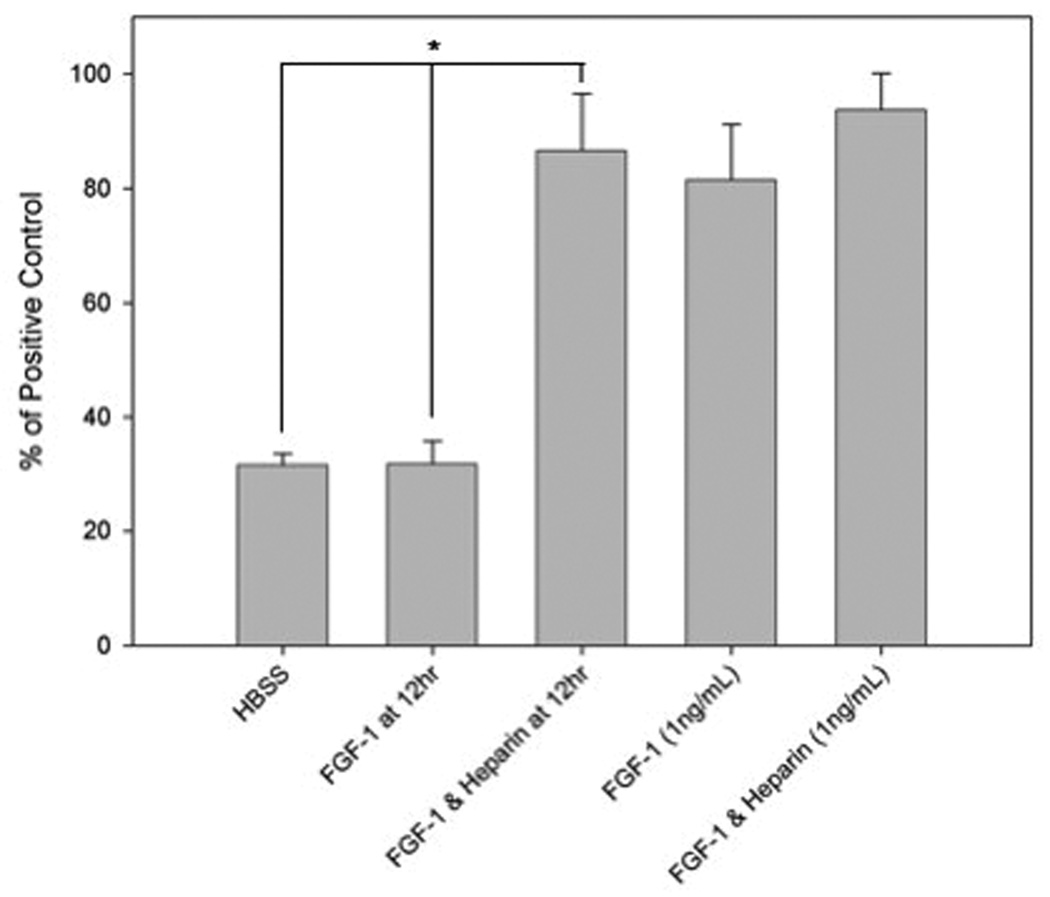
The biological activity of FGF-1 released from the outer layer was studied using a HUVEC proliferation assay. After 12 hours, FGF-1 released from the outer alginate layer containing heparin retained its ability to stimulate HUVEC proliferation (Figure 8). However, FGF-1 released from microbeads in the absence of heparin in the outer layer did not induce greater HUVEC proliferation than the negative control.
Figure 8.
FGF-1 encapsulated with heparin (5 U/mL) in the outer layer released after 12 hours stimulated the proliferation of HUVECs, but FGF-1 without heparin did not. Proliferation data are presented normalized to the positive control (stimulation with 20% FBS). * indicates statistical significance (p < 0.05) in comparison to the negative control (HBSS).
Discussion
The long-term viability of transplanted encapsulated islets is dependent, in part, on its ability to acquire oxygen and nutrients from a vascular blood supply. A biomaterial system that can serve simultaneously as an encapsulation system for islets as well as a sustained delivery system for angiogenic proteins may stimulate vascular growth around the transplanted cells, resulting in improved graft viability. We have shown that in order to achieve persistent neovascularization, a sustained release of FGF-1 is required, in contrast to a single bolus delivery. 20 In this paper, we present an approach for generating multilayered alginate microbeads. The outer layer can be used for the encapsulation and sustained release of FGF-1 while the inner layer can be used for islet encapsulation.
The size of the outer layer ranged from 113 µm to 164 µm. In our studies, the outer layer thickness increased with increasing alginate concentration. In addition, LVG alginate formed thicker outer layers than LVM alginate at equal concentrations. Other researchers have reported a similar relationship between alginate microbead size and alginate composition. 24,25 The di-axial configuration of G units allow for a more favorable interaction with the cation, which results in a greater degree of gelation. 10,26 As described by the ‘egg-box’ model, 27 when sufficient Ca2+ ions are present, it can be expected that the outer layer created using LVG alginate will be thicker than LVM owing to the presence of more G-unit cavities. Properties such as porosity and gel strength differ for LVM and LVG alginates based on the nature and the degree of crosslinking that occurs. A larger fraction of G-units decreases the space available (porosity) for molecules to diffuse, resulting in greater diffusive resistance in alginate with high G content. These differences may help explain the slow rate of release for LVG than LVM alginate at equal concentrations. 10 In addition, high M content results in more rigid gels that may influence the inflammatory response to the implanted material.
Any degradation or swelling of the layer may alter the release kinetics of the entrapped protein. Previous studies have shown that alginate beads without a permselective coating or an additional outer alginate layer undergo significant swelling due to ion exchange. 28 Alginate hydrogels are not degraded by bond cleavage, but instead erode due to ion exchange resulting from calcium and sodium ions in the local environment. Depending on the ionic strength of solutions used to study alginate-based materials, degradation may occur in days or months. 29 In our study, microbeads were incubated in a solution that reflected physiological calcium concentrations in order to approximate in vivo conditions. For both LVG and LVM alginate, the outer layer did not shrink or swell over the course of thirty days, which suggests that the outer layer will be maintained under in vivo conditions. Animal studies must be conducted to gain further insight into the stability of the outer layer in vivo.
Our previous studies have shown that the PLO coating serves as a permselective membrane, which prevents the passage of molecules greater than 120 kDa in size into the inner alginate bead. 23 It is essential that the microcapsules retain this selectivity in the presence of the outer layer. The PLO polymer coating was effective in preventing antibody, but not BSA, diffusion into the inner alginate bead. These results are consistent with prior studies using PLO-coated alginate microbeads for the encapsulation of cells. It is also possible that FGF-1 will diffuse through the PLO membrane and into the inner alginate matrix. The effect of FGF-1 on encapsulated islets is unclear, but FGF-1 is known to activate fibroblast growth factor receptor-1 (FGFR1) on β-cells, which regulates function, survival, and proliferation. 30 The permselectivity of PLO seems to suggest that cells would be protected from molecules that contribute to rejection upon transplantation. However, our results provide only a simple evaluation of PLO as a permselective membrane. Additional studies are required to determine the potential immunological response to cells encapsulated in PLO-coated multilayered alginate microbeads.
We have shown in this study that proteins can be encapsulated and released from the outer alginate layer. FGF-1 in the outer layer of the microbeads was released for over 30 days under experimental infinite sink conditions. The long-term delivery of FGF-1 may stimulate the formation of new vasculature directly towards the transplant site, and in turn, potentially improve the viability of transplanted islets. Previous studies have shown that the concentration of FGF-1 plays an important role in determining the morphology of microvascular networks. We have found that 0.5 ng/day of FGF-1 results in persistent neovascularization.19 In our study, all four batches of microbeads provided a burst release that could stimulate the formation of new vasculature, while the lower doses delivered at later times may lead to persistent neovascularization. Outer layers made with 1.25% LVG but provided a daily release > 0.5 ng/day out to 26 days, which suggests that this may be the optimal condition for use in vivo.
Previous studies have shown that protein diffusion is more restricted by alginate with high G content. 31,32 In addition, the 1.25% G conditions resulted in the thickest outer layer, which would contribute to longer transport times. Diffusion, however, is not likely to be the primary mechanism of release. Release would be complete in a few hours if the release were strictly via ordinary diffusion. Diffusion may contribute to the initial burst release, but other interactions need to occur in order to delay release to the time scale on the order of 30 days. Electrostatic interactions between FGF-1 and negatively charged alginate are unlikely because of FGF-1’s negative charge at neutral pH. 29 Instead, it is possible that FGF-1 interacts with the positively charged PLO layer, which could delay release. The extent of this interaction may be determined in part by the composition of the alginate in the outer layer and how its interactions with the PLO alters availability for interactions with FGF-1. Further studies are needed to better understand the mechanism determining release from the outer layer.
FGF-1 released from the outer layer was active only when it was co-encapsulated with heparin in the outer layer. Immobilization of heparin, or heparin analogs, to biomaterials has been used as a method for prolonging the release of a number of heparin-binding growth factors. 33–35 However, the presence of heparin in the outer alginate layer did not greatly affect the rate of release of FGF-1 in our system. In our experimental approach, heparin was not covalently attached to the alginate, but was incorporated in a form that it could freely diffuse. Heparin facilitates FGF-1 dimerization and activation of its cell surface receptors, and also protects the protein from denaturation by proteolysis or heat inactivation. 36,37 Heparin binding to FGF-1 can increase its half-life 100-fold. 38 The release solution contained heparin in both cases (with or without heparin in the outer layer), so the benefit of facilitating dimerization and receptor interaction upon addition to ECs was always present. In this case, it is likely that the improved activity was due to the improved stability and increased half-life of heparin-bound FGF-1. Biologically enhanced chimeras of FGF-1 could allow for the release of FGF-1 that is active in the absence of exogenous heparin. Heparin-binding growth-associated molecule fibroblast growth factor-1 (HB-GAM/FGF-1) chimeric protein has previously been shown to retain FGF-1’s normal mitogenic properties while exhibiting heparin-independent behavior. 39,40
In conclusion, we have developed a technique for the synthesis of multilayered alginate microcapsules with an outer alginate layer that can be used as a region for the encapsulation and release of FGF-1. Our studies have shown that the outer layer provides a long-term sustained release of FGF-1. The release of FGF-1 can be influenced by the composition and concentration of alginate used to make the outer layer. Indeed, multilayered alginate microbeads have promise for the simultaneous encapsulation of islets in the inner core and FGF-1 in the outer alginate layer, in order to enhance the viability of transplanted islets due to local stimulation of neovascularization.
Acknowledgements
The authors would like to acknowledge support from the National Science Foundation (Grant Nos. 0852048, 0731201, and 0854430), the Veterans Administration (EMB) and the National Institutes of Health (RO1 DK080897). We would also like to thank Jeffery Larson for his contribution to Figure 1 and assistance with confocal microscopy. Mr. Khanna received support from a generous donation from Mr. Edward Ross, and Dr. Moya received support from the Bill & Melinda Gates Foundation.
Contributor Information
Omaditya Khanna, Email: okhanna@iit.edu.
Monica L Moya, Email: moyamon@iit.edu.
Emmanuel C Opara, Email: eopara@wfubmc.edu.
Eric M Brey, Email: brey@iit.edu.
Bibliography
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Menger MD, Messmer K. Pancreatic islet transplantation: isolation, separation, and microvascularization. Wien Klin Wochenschr. 1992;104(15):429–433. [PubMed] [Google Scholar]
- 3.Avila JG, Wang Y, Barbaro B, Gangemi A, Qi M, Kuechle J, Doubleday N, Doubleday M, Churchill T, Salehi P, et al. Improved outcomes in islet isolation and transplantation by the use of a novel hemoglobin-based O2 carrier. Am J Transplant. 2006;6(12):2861–2870. doi: 10.1111/j.1600-6143.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- 4.Opara EC, Kendall WF., Jr Immunoisolation techniques for islet cell transplantation. Expert Opin Biol Ther. 2002;2(5):503–511. doi: 10.1517/14712598.2.5.503. [DOI] [PubMed] [Google Scholar]
- 5.Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. Islet encapsulation: strategies to enhance islet cell functions. Tissue Eng. 2007;13(3):589–599. doi: 10.1089/ten.2006.0183. [DOI] [PubMed] [Google Scholar]
- 6.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45(9):1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 7.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki R, Okada N, Miyamoto H, Yoshioka T, Sakamoto K, Oka H, Tsutsumi Y, Nakagawa S, Miyazaki J, Mayumi T. Cyotomedical therapy for insulinopenic diabetes using microencapsulated pancreatic beta cell lines. Life Sci. 2002;71(15):1717–1729. doi: 10.1016/s0024-3205(02)01724-1. [DOI] [PubMed] [Google Scholar]
- 9.Stabler C, Wilks K, Sambanis A, Constantinidis I. The effects of alginate composition on encapsulated betaTC3 cells. Biomaterials. 2001;22(11):1301–1310. doi: 10.1016/s0142-9612(00)00282-9. [DOI] [PubMed] [Google Scholar]
- 10.Gombotz WR, Wee SF. Protein release from alginate matrices. Advanced Drug Delivery Reviews. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 11.Takka S, Acarturk F. Calcium alginate microparticles for oral administration: I: Effect of sodium alginate type on drug release and drug entrapment efficiency. Journal of microencapsulation. 1999;16(3):275–290. doi: 10.1080/026520499289013. [DOI] [PubMed] [Google Scholar]
- 12.Moya ML, Garfinkel MR, Liu X, Lucas S, Opara EC, Greisler HP, Brey EM. Fibroblast Growth Factor-1 (FGF-1) Loaded Microbeads Enhance Local Capillary Neovascularization. J Surg Res. 2009 doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kizilel S, Garfinkel M, Opara E. The bioartificial pancreas: progress and challenges. Diabetes Technol Ther. 2005;7(6):968–985. doi: 10.1089/dia.2005.7.968. [DOI] [PubMed] [Google Scholar]
- 14.De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia. 1997;40(3):262–270. doi: 10.1007/s001250050673. [DOI] [PubMed] [Google Scholar]
- 15.Rivas-Carrillo JD, Navarro-Alvarez N, Soto-Gutierrez A, Okitsu T, Chen Y, Tabata Y, Misawa H, Noguchi H, Matsumoto S, Tanaka N, et al. Amelioration of diabetes in mice after single-donor islet transplantation using the controlled release of gelatinized FGF-2. Cell Transplant. 2006;15(10):939–944. doi: 10.3727/000000006783981323. [DOI] [PubMed] [Google Scholar]
- 16.Lai Y, Schneider D, Kidszun A, Hauck-Schmalenberger I, Breier G, Brandhorst D, Brandhorst H, Iken M, Brendel MD, Bretzel RG, et al. Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation. 2005;79(11):1530–1536. doi: 10.1097/01.tp.0000163506.40189.65. [DOI] [PubMed] [Google Scholar]
- 17.Becerril C, Pardo A, Montano M, Ramos C, Ramirez R, Selman M. Acidic fibroblast growth factor induces an antifibrogenic phenotype in human lung fibroblasts. Am J Respir Cell Mol Biol. 1999;20(5):1020–1027. doi: 10.1165/ajrcmb.20.5.3288. [DOI] [PubMed] [Google Scholar]
- 18.Ding I, Wu T, Matsubara H, Magae J, Shou M, Cook J, Okunieff P. Acidic fibroblast growth factor (FGF1) increases survival and haematopoietic recovery in total body irradiated C3H/HeNCr mice. Cytokine. 1997;9(1):59–65. doi: 10.1006/cyto.1996.0136. [DOI] [PubMed] [Google Scholar]
- 19.Uriel S, Brey EM, Greisler HP. Sustained low levels of fibroblast growth factor-1 promote persistent microvascular network formation. Am J Surg. 2006;192(5):604–609. doi: 10.1016/j.amjsurg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Moya ML, Lucas S, Francis-Sedlak M, Liu X, Garfinkel MR, Huang JJ, Cheng MH, Opara EC, Brey EM. Sustained delivery of FGF-1 increases vascular density in comparison to bolus administration. Microvasc Res. 2009;78(2):142–147. doi: 10.1016/j.mvr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Moya ML, Cheng MH, Huang JJ, Francis-Sedlak ME, Kao SW, Opara EC, Brey EM. The effect of FGF-1 loaded alginate microbeads on neovascularization and adipogenesis in a vascular pedicle model of adipose tissue engineering. Biomaterials. 31(10):2816–2826. doi: 10.1016/j.biomaterials.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garfinkel MR, Harland RC, Opara EC. Optimization of the microencapsulated islet for transplantation. Journal of Surgical Research. 1998;76:7–10. doi: 10.1006/jsre.1997.5258. [DOI] [PubMed] [Google Scholar]
- 23.Darrabie MD, Kendall WF, Jr, Opara EC. Characteristics of Poly-L-Ornithine-coated alginate microcapsules. Biomaterials. 2005;26(34):6846–6852. doi: 10.1016/j.biomaterials.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Yamagiwa K, Kozawa T, Ohkawa A. Effects of alginate composition and gelling conditions on diffusional and mechanical properties of calcium-alginate gel beads. Journal of Chemical Engineering of Japan. 1995;28:462–467. [Google Scholar]
- 25.Klokk TI, Melvik JE. Controlling the size of alginate gel beads by use of a high electrostatic potential. J Microencapsul. 2002;19(4):415–424. doi: 10.1080/02652040210144234. [DOI] [PubMed] [Google Scholar]
- 26.Becker TA, Kipke DR, Brandon T. Calcium alginate gel: A biocompatible and mechanical stable polymer for endovascular embolization. Journal of biomedical materials research. 2000;54:76–86. doi: 10.1002/1097-4636(200101)54:1<76::aid-jbm9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Draget KI, Stokke BT, Yuguchi Y, Urakawa H, Kajiwara K. Small-angle X-ray scattering and rheological characterization of alginate gels. 3. Alginic acid gels. Biomacromolecules. 2003;4(6):1661–1668. doi: 10.1021/bm034105g. [DOI] [PubMed] [Google Scholar]
- 28.Bajpai S, Sharma S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. Reactive and Functional Polymers. 2004:59. [Google Scholar]
- 29.Moya ML. Ph.D. Thesis. Chicago: Illinois Institute of Technology; 2009. Optimizing alginate microbead delivery system for release of angiogenic protein for neovascularization; p. 119. [Google Scholar]
- 30.Kilkenny DM, Rocheleau JV. Fibroblast growth factor receptor-1 signaling in pancreatic islet beta-cells is modulated by the extracellular matrix. Mol Endocrinol. 2008;22(1):196–205. doi: 10.1210/me.2007-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnology and Bioengineering. 1999;65(5):605–610. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 32.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol Bioeng. 1999;65(5):605–610. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 33.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69(1):149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 34.Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. FASEB J. 1999;13(15):2214–2224. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- 35.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 36.Kang SS, Gosselin C, Ren D, Greisler HP. Selective stimulation of endothelial cell proliferation with inhibition of smooth muscle cell proliferation by fibroblast growth factor-1 plus heparin delivered from fibrin glue suspensions. Surgery. 1995;118(2):280–286. doi: 10.1016/s0039-6060(05)80335-6. discussion 286-7. [DOI] [PubMed] [Google Scholar]
- 37.Pineda-Lucena A, Jimenez MA, Lozano RM, Nieto JL, Santoro J, Rico M, Gimenez-Gallego G. Three-dimensional structure of acidic fibroblast growth factor in solution: effects of binding to a heparin functional analog. J Mol Biol. 1996;264(1):162–178. doi: 10.1006/jmbi.1996.0631. [DOI] [PubMed] [Google Scholar]
- 38.Lopez JJ, Edelman ER, Stamler A, Hibberd MG, Prasad P, Thomas KA, DiSalvo J, Caputo RP, Carrozza JP, Douglas PS, et al. Angiogenic potential of perivascularly delivered aFGF in a porcine model of chronic myocardial ischemia. Am J Physiol. 1998;274(3 Pt 2):H930–H936. doi: 10.1152/ajpheart.1998.274.3.H930. [DOI] [PubMed] [Google Scholar]
- 39.Xue L, Tassiopoulos AK, Woloson SK, Stanton DL, Jr, Ms CS, Hampton B, Burgess WH, Greisler HP. Construction and biological characterization of an HB-GAM/FGF-1 chimera for vascular tissue engineering. J Vasc Surg. 2001;33(3):554–560. doi: 10.1067/mva.2001.112229. [DOI] [PubMed] [Google Scholar]
- 40.Brewster LP, Brey EM, Tassiopoulos AK, Xue L, Maddox E, Armistead D, Burgess WH, Greisler HP. Heparin-independent mitogenicity in an endothelial and smooth muscle cell chimeric growth factor (S130K-HBGAM) Am J Surg. 2004;188(5):575–579. doi: 10.1016/j.amjsurg.2004.07.012. [DOI] [PubMed] [Google Scholar]