Abstract
The current study addresses whether alterations in osteoclasts (OCs) derived from oim/oim mice, an established model of moderate-to-severe OI, are present. Bone marrow cells from oim/oim and wildtype (+/+) mice were cultured on bone slices in the presence of MCSF and RANKL and evaluated at days 0, 1, 2, 4, and 7. OCs were identified by tartrate-resistant acid phosphatase (TRAP) staining, and bone slice resorption pits were analyzed by reflection microscopy. Flow cytometry was used to examine CD51 (integrin αV) and CD61 (integrin β3) markers. Confocal microscopy was used to assess changes in OC morphology and resorption. There was no difference between the OC precursors of the two genotypes in expression of CD51 and CD61 markers. At day 2, the bone slices seeded with oim/oim cells had a greater percentage of mononuclear cells associated with resorption pits compared to +/+ bone slices. At day 4, the diameter and area of oim/oim OCs were larger compared to the +/+ OCs, and the number of nuclei per OC was also greater for the oim/oim group. At day 7, the oim/oim OCs contained more F-actin rings compared to the +/+ OCs, and the number of OCs in the oim/oim group was greater compared to the +/+ group. The resorbed area of bone slices for the oim/oim group was also greater compared to the +/+ group at day 7. In conclusion, oim/oim mononuclear resorbing cells and OCs showed cellular changes and greater resorptive activity compared to +/+ cells, features that likely contribute to dysregulated bone remodeling in OI.
Keywords: osteogenesis imperfecta, osteoclasts, resorptive activity, bone, mice
Osteogenesis imperfecta (OI) is a heritable disease of the connective tissues caused primarily by heterogeneous mutations in the genes encoding for type I collagen. Phenotypically, it is characterized by abnormal bone mineralization, tissue fragility, and skeletal deformities. When assessed by biochemical markers, conflicting results have been reported on bone turnover in children with OI. Urinary hydroxyproline values were elevated in some cases [Baron et al., 1983], whereas other studies reported normal or even decreased values [Antoniazzi et al., 1996; Cepollaro et al., 1999]. Histomorphometric analyses performed on bone samples obtained from standard biopsy sites have also yielded conflicting bone turnover information. Zeitlin et al. [2003] found increased resorption and number of osteoid surfaces in children with OI compared to age-matched controls, whereas McCarthy et al. [1997] found that resorption surfaces as well as osteoid surfaces were below normal in adults with OI. Insight into bone turnover abnormalities in OI could be gained by studies on isolated osteoclasts (OCs), but to date, there have been no such studies in either OI patients or in animal models of OI.
OCs are specialized cells capable of breaking down both inorganic (hydroxyapatite) and organic (collagen and non-collagenous proteins) components of bone, dentin, and mineralized cartilage [Hayashi et al., 1998]. Osteoclastic bone resorption is associated with multiple steps, including OC precursor differentiation into mature OCs, attachment of OCs to calcified tissues, development of a ruffled border and clear zone, and the secretion of acids and lysosomal enzymes into the space beneath the ruffled border. A defect in any of these steps could result in the alteration of morphology, fusion, or resorptive function and contribute to dysregulated bone turnover.
The current study seeks to characterize OCs derived from oim/oim mice, an established animal model of moderate-to-severe OI, in an in vitro osteoclastogenesis culture system. These mice exhibit cortical thinning, bowing of long bones, fractures, and bone deformities characteristic of human OI type III [Chipman et al., 1998]. Several studies have also demonstrated reduced bone mechanical properties in these mice [Saban et al., 1995; McBride et al., 1998; Camacho et al., 1999], and a recent study [Kalajzic et al., 2002] found that compared to wildtype (+/+) mice, bone remodeling was increased in oim/oim mice. To further assess how OC abnormalities may contribute to the bone fragility phenotype, we compared the morphology and function of mononuclear resorbing cells and mature OCs from oim/oim and wildtype mice. The results from these studies provide evidence that abnormal OC function may play an important role in the increased bone turnover phenomena in this animal model of OI.
MATERIALS AND METHODS
Animals
All animal work was done under an IACUC-approved protocol. Bone marrow from wildtype (+/+) and oim/oim mice between 5 and 8 weeks of age was utilized in this study. Mice were weaned at 3 weeks of age and housed in a light-controlled environment (12-h light–dark cycles) with up to 4 mice/500 cm2 cage according to genotype and sex. One +/+ or oim/oim mouse was used to harvest bone marrow cells in each experiment. Mice were killed by CO2 inhalation, and total body weight obtained prior to dissection.
Osteoclast Cultures
Bone marrow was harvested as described previously with slight modifications [Murrills et al., 1990]. Briefly, the femurs and tibias of mice were aseptically removed and dissected free of adhering tissue. The bone ends were cut off and bone marrow was flushed from the diaphysis with a syringe and 25-gauge needle, and collected in primary culture medium (alpha modified essential medium [α-MEM] containing l-glutamine, nucleoside (GIBCO, Invitrogen Corp.), supplemented with 10% fetal bovine serum (GIBCO), and 1% antibiotics [penicillin and streptomycin; GIBCO]). Red blood cells were removed by treatment with H2O-phosphate-buffered saline (PBS) solution. Bone marrow cells were cultured overnight in a 25 ml cell culture flask in the presence of 5 ng/ml recombinant murine M-CSF (PeproTech, Rocky Hill, NJ). Non-adherent cells were collected and plated at a density of 1 × 105/well in 96-well plates with devitalized bovine bone slices (4 × 4 mm2) on the bottom of each well of the plate. After removal of non-adherent cells by rinsing in PBS, the bone slices were transferred to another 96-well plate and cultured in primary culture medium in the presence of 20 ng/ml M-CSF and 60 ng/ml recombinant murine RANKL (PeproTech). The slices were then incubated at 37°C for 7 days in a 5% CO2 atmosphere. Media containing M-CSF and RANKL were changed every 2 days.
Flow Cytometry
Two characteristic OC markers, CD51 (integrin αV) and CD61 (integrin β3 chain) [Miyamoto et al., 2000], were assessed to study the OC phenotype expression in the mixed cell population from bone marrow (day 0) and non-adherent cells after overnight culture in the presence of M-CSF (day 1). Samples were analyzed from five separate experiments (N = 5). Cells were incubated with R-phycoerythrin (R-PE)-conjugated rat anti-mouse CD51 (integrin αV chain; Pharmingen San Diego, CA) and monoclonal antibody fluorescein isothiocyanate (FITC) conjugated hamster anti-mouse CD61 (Integrin β3 chain monoclonal antibody; Pharmingen) and analyzed using a flow cytometer (FACSCalibur; Becton Dickinson, San Jose, CA).
Confocal Microscopy Studies
A modified version of the method of Lakkakorpi and Vaananen [1991] was used for the immunofluorescence microscopy. Bone slices harvested with intact cells at days 2, 4, and 7 of culture were rinsed with PBS, fixed with 3.7% paraformaldehyde and 3% sucrose in PBS for 5 min at room temperature, rinsed in PBS, permeabilized in HEPES-Triton X-100 buffer for 5 min at 0°C and rinsed in PBS. Fluorescein-labeled phalloidin (Molecular Probes, Inc.) at 5 units/ml was utilized to stain F-actin. The bone slices were washed in PBS and then the nuclei were counterstained with TO-PRO-3 (Molecular Probes) before mounting onto slides. Bone slices were mounted in prolonged anti-fade mounting medium (Molecular Probes) and viewed with a confocal laser scanning microscope (Carl Zeiss, Jena, Germany), equipped with Leica software. Bone slices were viewed with 63× immersion objectives with appropriately chosen pinholes using 256 × 256 image format. For the analysis of OC precursors at day 2 and the multinuclear cell (MNC) imaging study at days 4 and 7, ten areas or ten MNCs from five areas (290 nm × 290 nm) were acquired on each slice (as described below for the assessment of percent resorption of bone slices). For the study of resorptive activity of mononuclear cells at day 2, a mononuclear cell with a resorption pit under its cell body was counted as a resorbing mononuclear cell. For the morphology study of mature MNCs, the total area occupied by MNCs, the longest diameter of MNCs, the number of nuclei inside the MNCs and the number of F-actin rings per MNC were assessed. Samples were analyzed from five separate experiments with two replicate slices at each time point (N = 5).
Tartrate-Resistant Acid Phosphatase (TRAP) Staining and Assay
Bone slices were harvested at days 4 and 7 for TRAP staining (N = 5 per timepoint). Bone slices with adherent cells were fixed with 3.7% formaldehyde solution in PBS for 15 min at room temperature and stained for TRAP using a commercial kit (Sigma Diagnostics, acid phosphatase, leukocyte kit) for 60 min. MNCs were defined as TRAP-positive cells containing three or more nuclei. The bone slices were scanned using bright field optics on a Nikon Eclipse 800 upright microscope and 10× objective with Metamorph image analyzer (Universal Imaging Corp., Downington, PA). For each bone slice, five images (0.182 mm2) were obtained, one at each corner and one in the center of the slice. The number of TRAP-positive MNCs was counted and data were expressed as number of cells divided by the area of the image (number/mm2).
Assessment of Bone Resorption
Bone resorption was assayed using the disaggregated OC resorption assay originated by Boyde et al. [1984] and Chambers et al. [1984]. After TRAP staining analysis, the bone slices were soaked in 1M NH4OH for 3 min to remove adherent cells. Resorption pits were examined by a reflectance microscope attached to a Spectrum Spotlight FT-IR imaging system (PerkinElmer Instruments, Boston, MA). For each bone slice, five images (1.33 mm2) were obtained, one at each corner and one in the center of the slice. Resorption pit area measurements were performed using Image J v1.33 software (National Institutes of Health). There were two replicate slices for each time point in each experiment (N = 13).
Statistical Analysis
Data from the mature MNC confocal study, TRAP staining, and resorption pit studies were examined by two-way ANOVA complemented with Bonferroni’s test when the difference between mean values was significant at P < 0.05. Culture timepoint and genotype were the two factors in the two-way ANOVA analysis. A student’s t-test was used to compare differences between +/+ and oim/oim mice for body weight, cell number and day 2 resorption variables. Data are expressed as mean ± standard deviation.
RESULTS
Animal Weight and Harvested Cell Numbers
The average body weight of the +/+ mice was greater than that of oim/oim mice (28.0 ± 2.34 g vs. 21.6 ± 1.98 g, P < 0.05), and the number of harvested bone marrow cells from the +/+ mice was greater than that from the oim/oim mice (9.78 ± 1.91 × 106/ml vs. 6.78 ± 1.40 × 106/ml, P < 0.01). After normalizing for body weight, the difference was no longer significant (0.35 ± 0.06 × 106/ml/g vs. 0.31 ± 0.06 × 106/ml/g, P > 0.05). After overnight culture, there was also no significant difference in non-adherent cell number between +/+ and oim/oim mice (7.07 ± 2.52 × 106/ml vs. 5.41 ± 2.22 × 106/ml, P > 0.05). Similarly, when normalized to body weight, the number of non-adherent cells after overnight culture was equivalent between the two genotypes (0.25 ± 0.10 × 106/ml/g vs. 0.25 ± 0.09 × 106/ml/g). For this reason, all other variables measured were not corrected for body weight.
Surface Marker Expression of OC Precursors
In freshly harvested bone marrow cells and after overnight culture, both +/+ and oim/oim mice strongly expressed CD51 and CD61 (data not shown). Thus, there was no phenotypic difference between the two genotypes based on expression of these markers.
Confocal Microscopy Results
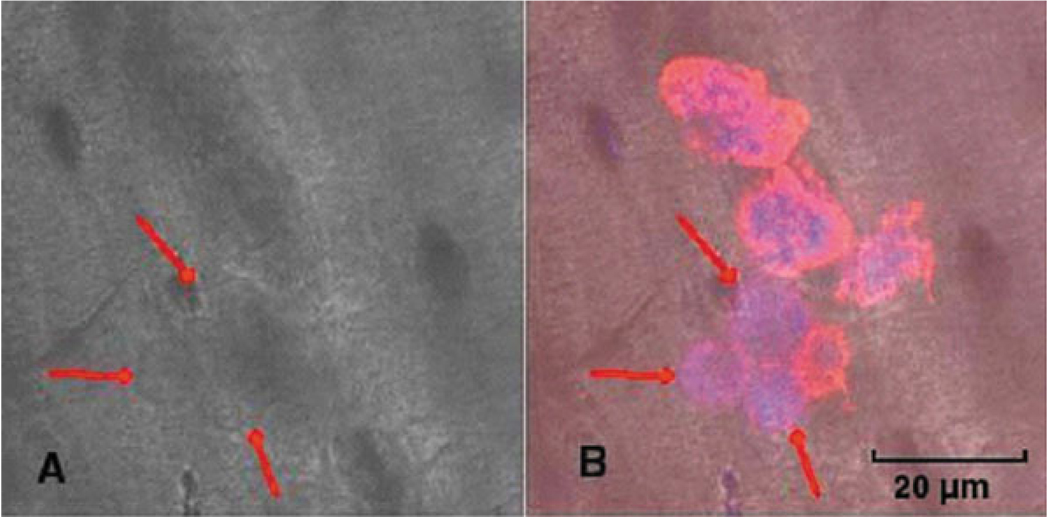
Mononuclear cells started to resorb bone at day 2 of culture (Fig. 1). There was a significantly greater percentage of resorbing mononuclear cells in the oim/oim group compared to the +/+ group (43.4 ± 6.00% vs. 21.9 × 11.0%, P < 0.05).
Fig. 1.
Resorbing mononuclear cells on bone slices present at day 2 of culture. A: Transmission image of the resorption pit formed by monocytes obtained from oim/oim mice. B: The same region as in (A) with monocytes stained for F-actin at the periphery of cells and nuclei inside the cells.
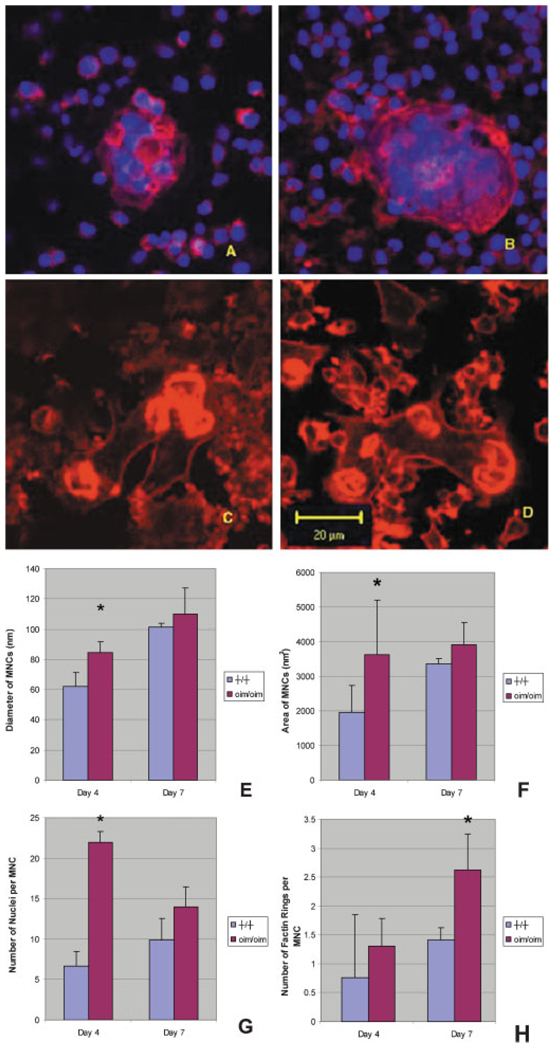
At day 4, the morphology and number of nuclei of the oim/oim MNCs were very different compared to the +/+ (Fig. 2). The MNCs from the oim/oim mice had a larger cell diameter and area compared to +/+ mice, and the number of nuclei per MNC in the oim/oim mice was nearly three times that of the +/+ group. These differences were not present at day 7. However, there were cytoskeletal differences at day 7 as manifested by a greater number of F-actin rings in the oim/oim MNCs compared to +/+ mice.
Fig. 2.
Confocal images of multinuclear cells (MNCs) from +/+ (A) and oim/oim (B) mice at day 4 showed increased nuclear number in oim/oim mice MNCs compared to +/+ mice. Confocal images of MNCs from +/+ (C) and oim/oim (D) mice at day 7 showed increased number of F-actin rings on the MNCs in oim/oim mice compared to +/+ mice. At day 4 the diameter (E) and area (F) of the oim/oim mice MNCs were larger compared to the +/+ mice (*P < 0.05). The number of nuclei per MNC in oim/oim mice was nearly three times that of the +/+ at day 4 (*P < 0.01) (G). At day 7, MNCs in oim/oim mice contained a larger the number of F-actin rings compared to those of the +/+ mice (*P < 0.05) (H).
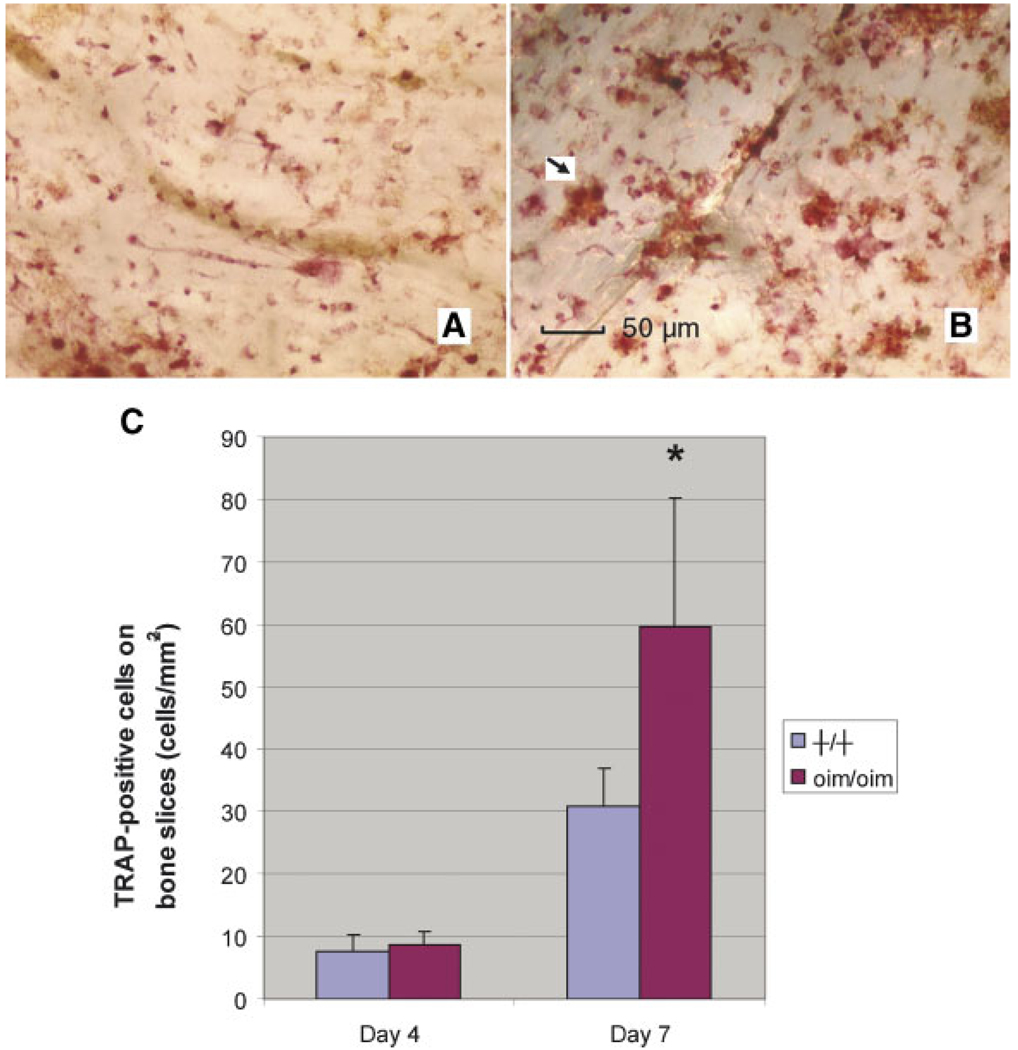
TRAP Staining
At day 2 of culture, the morphology of the +/+ and oim/oim cells changed from spindle-shaped to round. At day 3, MNCs began to appear in both the +/+ and oim/oim culture. At day 4, numerous MNCs were present in both the +/+ and oim/oim culture, and all the MNCs and mononucleated cells were TRAP positive (Fig. 3). There was no significant difference in TRAP-positive cell number between +/+ and oim/oim groups at day 4. At day 7 however, there were more TRAP-positive oim/oim MNCs compared to +/+ MNCs on bone slices.
Fig. 3.
TRAP-positive cells at day 7 on bone slices seeded with cells from +/+ (A) and oim/oim (B) mice. (C) At day 7, the number of MNCs from the oim/oim mice was greater compared to the +/+ mice (*P < 0.05). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
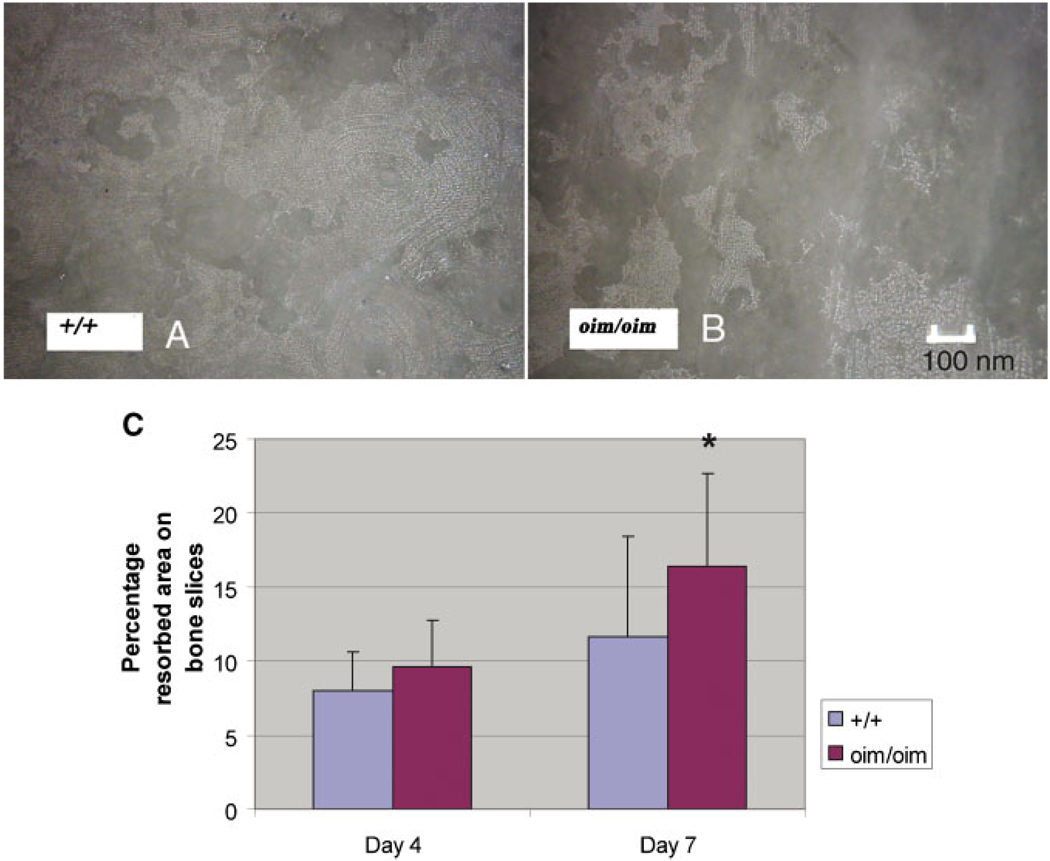
Reflectance Light Microscopic Evaluation of Resorption Pit Area
There were no significant differences in the percentage of resorbed area of the bone slices between the two genotypes at day 4, but at day 7, the oim/oim bone slices had a greater percentage of resorbed area compared to the +/+ bone slices (Fig. 4).
Fig. 4.
Images of resorption pits on bone slices at day 7 seeded with cells from +/+ (A) and oim/oim (B) mice. No difference in resorbed area was found at day 4, but increased resorption was found in the oim/oim group compared to the +/+ group at day 7 (*P < 0.01) (C). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The current study is the first to investigate isolated OCs derived from an animal model of OI, the oim/oim mouse. We found that the oim/oim mice generated an increased number of OCs that were larger, had a greater number of nuclei and F-actin rings per OC, and displayed increased resorptive activity compared to +/+ mice. All these parameters point to an increased potential for bone resorption. These results are consistent with a previous histological and cellular study of bone turnover in the oim/oim mice that found increased bone resorption parameters compared to control mice [Kalajzic et al., 2002]. Those results pointed to defective osteoblast function coupled with increased resorption, the severity of which is linked to phenotype and ultimately leads to quantitatively decreased bone formation and variable or heterogeneous mineralization, as has been found in other studies [Camacho et al., 1999; Phillips et al., 2000]. The current data also have clinical implications, especially given the success of anti-resorptive therapy in OI patients [Glorieux, 2001]. The fact that bisphosphonates can act directly on OCs is strong evidence that in addition to the established osteoblast abnormality, it is likely that abnormal OC activity is present in OI patients.
Bone marrow stromal stem cells are the most convenient source of OC precursors for studies such as the current one. OC precursors from these stem cells generally express all OC markers [Takeshita et al., 2000], and therefore can provide insight into the early stages of OC differentiation. As demonstrated in our data, the +/+ mice had a greater body weight compared to the oim/oim mice of a similar age with a corresponding increase in the number of bone marrow cells. Thus, the number of OC precursor cells normalized to body weight was not significantly different between the +/+ and oim/oim groups. In our OC phenotype study, we found both +/+ and oim/oim stromal cells expressed integrin αVβ3 and integrin α3, two markers of mature OCs. Taken together, we can conclude that it is unlikely that the number of OC precursors, or that an integrin-associated defect in the pre-OC phenotype in bone marrow cells, contributes to abnormalities in bone turnover in the oim/oim mouse.
We did, however, find specific changes in the oim/oim mice evident in the early stages of osteoclastogenesis with mononuclear cells. The majority of previous in vitro culture studies have not included evaluation of the resorptive capability of mono- and binuclear cells, as they are not classically defined as OCs [Burgess et al., 1999]. Most of the studies evaluated the mature OC, which is a fully functional OC with a large cell size, multi-nuclei, and associations with obvious resorptive pits. Thus, it was not possible to unambiguously distinguish mononuclear OC precursors from other mononuclear cells in the culture. However, three-dimensional data acquisition by confocal microscopy enables high enough resolution and sensitivity to permit such assessments [Boyde, 2005]. The current study evaluated the interface between the monocytic OC precursor and the bone substrate surface, and identified shallow resorption pits underneath the cell body of mononuclear cells. We defined these monocytes as mononuclear resorbing cells. Similar findings of mononuclear cells with the capability to resorb bone in vitro have been described in a recent study by Dempster et al. [2005], and in earlier studies by Prallet et al. [1992]; Sarma and Flanagan [1996]; Nicholson et al. [2000]; and Mundy et al. [1978]. Although the OC precursors in the current study did not show mature OC characteristics, they did start to resorb bone around their attachment site, with the resorption pit present relative to cell body size. It is possible that similar functionally active monocytes with OC markers in bone marrow contribute to the increased bone turnover phenomena in the oim/oim mouse model and in OI patients. However, the underlying molecular basis of this change in the OC cell line function remains to be determined.
OCs in the oim/oim mice also showed marked morphological changes compared to +/+ mice in the later developmental stage of osteoclastogenesis. As demonstrated, the number of nuclei contained in oim/oim OCs was three-fold greater compared to those of +/+ mice. Increased fusion function was also reflected by the altered morphology of MNCs in the oim/oim mice at day 4, where we found significantly larger MNCs compared to +/+ mice. Typically, human OCs contains 1–10 nuclei [Teitelbaum et al., 1997]. In some bone diseases marked by increased resorption, such as Paget’s disease, the number of OC nuclei can reach as high as 100 [Reddy et al., 1999]. In vitro, increased numbers of OCs are formed from Pagetic precursors, and the cells are larger and contain more nuclei than controls [Noor and Shoback, 2000]. Similar morphologic and fusion function changes may be reflective of the increased resorptive activities of OC precursors in oim/oim mice, although the specific mechanism underlying these changes is also not known.
Further alterations in the osteoclastogenesis pathway were reflected in the greatly increased number of MNCs in the oim/oim culture, where there were twice as many MNCs compared to the +/+ group at day 7. This finding supports the concept that increased OC differentiation is also involved in the dysregulated bone remodeling in the oim/oim mouse model, and the greater number of F-actin rings reflect cytoskeletal changes that accompany the increased differentiation. To resorb bone, the OC must become polarized and change its cytoskeletal arrangement, in particular the F-actin ring structure which overlies the sealing zone in the surface region between the OC and bone [Vaananen and Horton, 1995; Nakamura et al., 1996]. Typically, there is a significant correlation between F-actin ring formation and bone resorption [Holtrop et al., 1981]. Thus, the change in the cytoskeleton in MNCs in the oim/oim mice was expected to be linked to increased resorptive activity, as indeed it was at day 7 of culture.
Notwithstanding these novel findings of OC abnormalities in this mouse model of OI, defective osteoblast function plays an exceedingly important role in the clinical findings of higher bone turnover in OI. Histomorphometric studies have shown that the bone formation rate per osteoblast surface was decreased in types I, III, and IV OI [Rauch et al., 2000] and that a reduced amount of trabecular bone and thinner cortices were present in OI bone [Baron et al., 1983]. Thus, the combined data from cellular and histomorphometric studies point to defective osteoblast function linked to increased resorption in OI patients. Therefore, it is likely that abnormal signaling from the oim/oim osteoblast to the oim/oim OC could be an initial requirement for the increased OC activity. Recent studies have yielded significant insight into the molecular mechanisms that govern communication between osteoblasts and OC precursor cells leading to osteoclastogenesis [Jimi et al., 1996; Takeda et al., 1999; Katagiri and Takahashi, 2002; Wittrant et al., 2004; Roodman, 2006; Wada et al., 2006]. In OI, studies have shown that extracellular matrix has a significant influence in the development and differentiation of many cell types in the oim/oim mouse [Kalajzic et al., 2002], and in OI patients [Fedarko et al. 1995]. Taken together, a defect in bone marrow stromal cells, osteoblast-OC signaling, or bone matrix could all conceivably contribute to activation of OC precursors and the functional changes in mature OCs derived from oim/oim mice. Clearly, further studies of these interactions are required to ascertain what role these mechanisms may play in bone turnover in OI.
In summary, the current study demonstrates for the first time that alterations in OC precursors and in mature OCs are associated with increased resorptive activity in an animal model of OI. Further studies that utilize in vitro osteoclastogenesis assays coupled with osteoblasts and bone matrix substrates may yield additional insight into the basis of the increased bone turnover in this animal model of OI, and, by analogy, in patients with OI.
ACKNOWLEDGMENTS
This study was supported in part by NIH AR48337 (NPC) and NIH AR39191 (DD), and utilized the facilities of the Musculoskeletal Repair and Regeneration Core Center (NIH AR46121).
Grant sponsor: NIH; Grant numbers: AR48337, AR39191; Grant sponsor: Musculoskeletal Repair and Regeneration Core Center (NIH); Grant number: AR46121.
REFERENCES
- Antoniazzi F, Bertoldo F, Mottes M, Valli M, Sirpresi S, Zamboni G, Valentini R, Tato L. Growth hormone treatment in osteogenesis imperfecta with quantitative defect of type I collagen synthesis. J Pediatr. 1996;129:432–439. doi: 10.1016/s0022-3476(96)70077-x. [DOI] [PubMed] [Google Scholar]
- Baron R, Gertner JM, Lang R, Vignery A. Increased bone turnover with decreased bone formation by osteoblasts in children with osteogenesis imperfecta tarda. Pediatr Res. 1983;17:204–207. doi: 10.1203/00006450-198303000-00007. [DOI] [PubMed] [Google Scholar]
- Boyde A. Confocal optical microscopy. In: Duke PJ, Michette AD, editors. Modern microscopies: Techniques and applications. New York: Plenum; 2005. pp. 185–204. [Google Scholar]
- Boyde A, Ali NN, Jones SJ. Resorption of dentine by isolated osteoclast in vitro. Br Dent J. 1984;156:216–220. doi: 10.1038/sj.bdj.4805313. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Qian YX, Kaufman S, Ring B, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL. The ligand for osteoprotegein (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145:527–538. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho NP, Hou L, Toledano TR, Ilg WA, Brayton CF, Raggio CL, Root L, Boskey AL. The material basis for reduced mechanical properties oim mice bones. J Bone Miner Res. 1999;14:264–272. doi: 10.1359/jbmr.1999.14.2.264. [DOI] [PubMed] [Google Scholar]
- Cepollaro C, Gonnelli S, Pondrelli C, Montagnani A, Martini S, Bruni D, Gennari C. Osteogenesis imperfecta: Bone turnover, bone density, and ultrasound parameters. Calcif Tissue Int. 1999;65:129–132. doi: 10.1007/s002239900670. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Revell PA, Fuller K, Athanasou NA. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984;66:383–399. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
- Chipman S, Sweet HO, McBride DJ, Jr, Davison MT, Marks CS, Jr, Shudiner AR, Wenstrup R, Rowe DW, Shapiro JR. Defective proá 2(I) collagen synthesis in a recessive mutation in mice: A model of human osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;90:1701–1705. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster DW, Hughes-Begos CE, Plavetic-Chee K, Brandao-Burch A, Cosman F, Nieves J, Neubort S, Lu SS, Iida-Klein A, Arnett T, Lindsay R. Normal human osteoclasts formed from peripheral blood monocytes express PTH type 1 receptors and are stimulated by PTH in the absence of osteoblasts. J Cell Biochem. 2005;95:139–148. doi: 10.1002/jcb.20388. [DOI] [PubMed] [Google Scholar]
- Fedarko NS, Robey PG, Vetter UK. Extracellular matrix stoichiometry in osteoblasts from patients with osteogenesis imperfecta. J Bone Miner Res. 1995;10:1122–1129. doi: 10.1002/jbmr.5650100718. [DOI] [PubMed] [Google Scholar]
- Glorieux FH. The use of bisphosphonates in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2001;14:1491–1495. [PubMed] [Google Scholar]
- Hayashi SI, Yamane T, Miyamoto A, Hemmi H, Tagaya H, Tanio Y, Kanda H, Yamazaki H, Kunisada T. Commitment of differentiation of stem cells to the osteoclast lineage. Biochem Cell Biol. 1998;76:911–922. [PubMed] [Google Scholar]
- Holtrop ME, Cox KA, Clark MB, Holick MF, Anast CS. 1,25-Dihydroxycholecalciferol stimulates osteoclast in rate bones in the absence of parathyroid hormone. Endocrinology. 1981;108:2293–2301. doi: 10.1210/endo-108-6-2293. [DOI] [PubMed] [Google Scholar]
- Jimi E, Akiyama S, Tsurukai Osteoclast function is activated by osteoblastic cells through a mechanism involving cell-to-cell. Endocrinology. 1996;137:2187–2190. doi: 10.1210/endo.137.5.8612568. [DOI] [PubMed] [Google Scholar]
- Kalajzic I, Terzic J, Rumboldt Z, Mack K, Naprta A, Ledgard F, Gronowicz G, Clark SH, Rowe DW. Osteoblastic response to the defective matrix in the osteogenesis imperfecta murine (oim) mouse. Endocrinology. 2002;143:1594–1601. doi: 10.1210/endo.143.5.8807. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–159. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi PT, Vaananen HK. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J Bone Miner Res. 1991;6:817–826. doi: 10.1002/jbmr.5650060806. [DOI] [PubMed] [Google Scholar]
- McBride DJ, Jr, Shapiro JR, Dunn MG. Bone geometry and strength measurements in aging mice with the oim mutation. Calcif Tissue Int. 1998;62:172–176. doi: 10.1007/s002239900412. [DOI] [PubMed] [Google Scholar]
- McCarthy EF, Earnest K, Rossiter K, Shapiro J. Bone histomorphometry in adults with type IA osteogenesis imperfecta. Clin Orthop Relat Res. 1997;336:254–262. doi: 10.1097/00003086-199703000-00034. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Arai F, Ohneda O, Takagi K, Anderson DM, Suda T. An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligand. Blood. 2000;96:4335–4343. [PubMed] [Google Scholar]
- Mundy GR, Varani J, Orr W, Gondek MD, Ward PA. Resorbing bone is chemotactic for monocytes. Nature. 1978;275:132–135. doi: 10.1038/275132a0. [DOI] [PubMed] [Google Scholar]
- Murrills RJ, Stein LS, Fey CP, Dempster DW. The effects of parathyroid hormone (PTH) and PTH-related peptide on osteoclast resorption of bone slices in vitro: An analysis of pit size and resorption focus. Endocrinology. 1990;127:2648–2653. doi: 10.1210/endo-127-6-2648. [DOI] [PubMed] [Google Scholar]
- Nakamura I, Takahashi N, Sasaki T, Jimi E, Kurokawa T, Suda T. Chemical and physical properties of the extracellular matrix are required for the actin ring formation in osteoclasts. J Bone Miner Res. 1996;11:1873–1879. doi: 10.1002/jbmr.5650111207. [DOI] [PubMed] [Google Scholar]
- Nicholson GC, Malakellis M, Collier FM, Cameron PU, Holloway WR, Gough TJ, Gregorio-King C, Kirkland MA, Myers DE. Induction of osteoclasts from CD14-positive human peripheral blood mononuclear cells by receptor activator of nuclear factor kappaB ligand (RANKL) Clin Sci (Lond) 2000;99:133–140. [PubMed] [Google Scholar]
- Noor M, Shoback D. Paget’s disease of bone: Diagnosis and treatment update. Curr Rheumatol Rep. 2000;2:63–67. doi: 10.1007/s11926-996-0071-x. [DOI] [PubMed] [Google Scholar]
- Phillips CL, Bradley DA, Schlotzhauer CL, Bergfeld M, Libreros-Minotta C, Gawenis LR, Morris JS, Clarke LL, Hillman LS. Oim mice exhibit altered femur and incisor mineral composition and decreased bone mineral density. Bone. 2000;27:219–226. doi: 10.1016/s8756-3282(00)00311-2. [DOI] [PubMed] [Google Scholar]
- Prallet B, Male P, Neff L, Baron R. Identification of a functional mononuclear precursor of the osteoclast in chicken medullary bone marrow cultures. J Bone Miner Res. 1992;7:405–414. doi: 10.1002/jbmr.5650070408. [DOI] [PubMed] [Google Scholar]
- Rauch F, Travers R, Parfitt AM, Glorieux FH. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone. 2000;26:581–589. doi: 10.1016/s8756-3282(00)00269-6. [DOI] [PubMed] [Google Scholar]
- Reddy SV, Menaa C, Singer FR, Demulder A, Roodman GD. Cell biology of Paget’s disease. J Bone Miner Res. 1999;14:3–8. doi: 10.1002/jbmr.5650140203. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Regulation of osteoclast differentiation. Ann N Y Acad Sci. 2006;1068:100–109. doi: 10.1196/annals.1346.013. [DOI] [PubMed] [Google Scholar]
- Saban J, Zussman MA, Havey R, Patwardhan AG, Schneider GB, King D. Heterogyzous oim mice exhibit a mild form of osteogenesis imperfecta. Bone. 1995;19:575–579. doi: 10.1016/s8756-3282(96)00305-5. [DOI] [PubMed] [Google Scholar]
- Sarma U, Flanagan AM. Macrophage colony-stimulating factor induces substantial osteoclast generation and bone resorption in human bone marrow cultures. Blood. 1996;88:2531–2540. [PubMed] [Google Scholar]
- Takeda S, Yoshizawa T, Nagai Y. Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: Studies using VDR knockout mice. Endocrinology. 1999;140:1005–1008. doi: 10.1210/endo.140.2.6673. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Kaji K, Kudo A. Identification and characterization of new osteoclast progenitor with Marcophage phenotypes being able to differentiate into mature osteoclast. J Bone Miner Res. 2000;15:1477–1488. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Tondravi MM, Ross FP. Osteoclasts, macrophages, and the molecular mechanisms of bone resorption. J Leukoc Biol. 1997;61:381–388. doi: 10.1002/jlb.61.4.381. [DOI] [PubMed] [Google Scholar]
- Vaananen H, Horton M. The osteoclast clear zone is specialized cell-extracellular matrix adhersion structure. J Cell Sci. 1995;108:2732. doi: 10.1242/jcs.108.8.2729. [DOI] [PubMed] [Google Scholar]
- Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wittrant Y, Theoleyre S, Chipoy C, Padrines M, Blanchard F, Heymann D, Redini F. RANKL/RANK/OPG: New therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta. 2004;1704:49–57. doi: 10.1016/j.bbcan.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Zeitlin L, Fassier F, Glorieux FH. Modern approach to children with osteogenesis imperfecta. J Pediatr Orthop B. 2003;12:77–87. doi: 10.1097/01.bpb.0000049567.52224.fa. [DOI] [PubMed] [Google Scholar]