Abstract
Reproductive abnormalities in alligators exposed to contaminants in Lake Apopka, Florida, USA represent a clear example of endocrine disruption in wildlife. Several of these contaminants that are not able to bind to mammalian estrogen receptors (such as atrazine and cyanazine) have previously been reported to bind to the alligator estrogen receptor from oviductal tissue. Binding of known Lake Apopka contaminants to full length estrogen receptors alpha from human (hERα) and alligator (aERα) was assessed in a side-by-side comparison within the same assay system. Baculovirus-expressed recombinant hERα and aERα were used in a competitive binding assay. Atrazine and cyanazine were not able to bind to either receptor. p,p′-Dicofol was able to bind to aERα with a concentration inhibiting 50% of binding (IC50) of 4 μM, while only partially displacing 17β-estradiol (E2) from hERα and yielding a projected IC50 of 45 μM. Chemicals that only partially displaced E2 from either receptor, including some dichlorodiphenyltrichloroethane (DDT) metabolites and trans-nonachlor, appeared to have higher affinity for aERα than hERα. p,p′-Dicofol-mediated transcriptional activation through aERα and hERα was assessed to further explore the preferential binding of p,p′-dicofol to aERα over hERα. p,p′-Dicofol was able to stimulate transcriptional activation in a similar manner with both receptors. However, the in vitro results obtained with p,p′-dicofol were not reflected in an in vivo mammalian model, where Kelthane™ (mixed o,p′-and p,p′-dicofol isomers) did not elicit estrogenic effects. In conclusion, although there was no evidence of exclusively species-specific estrogen receptor binders, some xenoestrogens, especially p,p′-dicofol, had a higher affinity for aERα than for hERα.
Keywords: Binding assay, Endocrine disruption, Steroid receptors, Transcriptional activation, Rat uterotrophic assay
INTRODUCTION
The demasculinization of male alligators in a highly contaminated site in Florida, USA (Lake Apopka) represents one of the most well-known cases of endocrine disruption in wildlife. Sources of contamination to Lake Apopka have included agricultural run-off, sewage treatment facility effluent, as well as a serious pesticide spill in 1980 composed of dicofol, dichlorodiphenyltrichloroethane (DDT), and DDT metabolites [1]. In addition to decreased population size, juvenile alligators from Lake Apopka were found to have an abundance of reproductive system issues including small penis size, testicular abnormalities and decreased testosterone in males; and ovarian abnormalities and elevated 17β-estradiol (E2) in females [2,3]. A proposed mechanism for these negative reproductive effects in juvenile alligators is early exposure to estrogenic contaminants [2].
Vonier et al. [4] confirmed that many of the contaminants identified in Lake Apopka were able to compete with E2 for binding to alligator estrogen receptor (aER) derived from adult female alligator oviductal tissue. Interestingly, they found that the herbicides atrazine, cyanazine, and alachlor were able to bind to aER with concentrations inhibiting 50% of binding (IC50s) of 20.7, 19, and 27.5 μM, respectively [4], whereas others have reported that these compounds do not bind to human estrogen receptor (hER) [5]. These findings allude to the potential for species-specific endocrine disruptors. However, there has not been a direct side-by-side comparison of chemical binding to hER and aER within the same system to confirm these observations.
Recently, we developed an estrogen receptor competitive binding assay for use in multi-species comparisons [6]. This system uses baculovirus expressed full-length recombinant ERs in a 96-well plate competitive binding assay. Following the isolation and cloning of the full length American alligator (Alligator mississippiensis) ERa by Katsu et al. [7] (GenBank accession no. AB115909), we synthesized the receptor and inserted the full-length sequence into a baculovirus expression vector. Proper functionality of aERα was previously confirmed in our system through saturation and competitive binding assays with well-characterized, model steroids [6]. The dissociation constant (Kd) of aERα for E2 was found to be 0.44±0.039 nM [6], which is in good agreement with the 0.5 nM Kd determined by Vonier et al. [8] with aER from alligator oviductal tissue.
In the present study, our competitive binding system was used to assess the affinity of Lake Apopka contaminants for aERα and hERα in a side-by-side comparison. The present study addresses whether the preferential binding of atrazine and cyanazine observed using aER derived from alligator tissue is maintained when full-length recombinant receptors are used. Additionally, other Lake Apopka contaminants were assessed for their ability to bind to aERα and hERα. The direct comparison of chemical binding to aERα versus hERα in a uniform platform allows for more definitive determination of potential species-specific effects, with the receptor being the only variable.
Following the parallel competitive binding analysis, the preferential binding of p,p′-dicofol to aERα over hERα was explored further. A CV1 transcriptional activation assay was used to determine whether p,p′-dicofol resulted in greater transcriptional activation of ER-responsive genes through aERα versus hERα. This assay is well-suited for cross-species comparisons because the receptor of choice is transiently transfected into the CV1 cells. A second, more sensitive transcriptional activation assay employing endogenous hERα and hERβ was used to confirm results obtained with hERα in the CV1 transcriptional activation assay.
Due to differences among mammals and alligators in terms of validated protocols for determining estrogenic effects in vivo and the particular difficulties of working with live alligators, we could not compare the biological significance of the observed in vitro results with a parallel in vivo assessment of dicofol effects in mammals and alligators. Therefore, we performed an in vivo mammalian assay of estrogencity to explore whether the weak estrogenic activity of dicofol observed with hERα in vitro was biologically significant and subsequently compared our results to available in vivo alligator results in the discussion section. In the rat uterotrophic assay, the in vivo estrogenic activity of mixed o,p′- and p,p′-dicofol was compared to that of the potent endogenous estrogen, E2, and a weaker estrogen of similar in vitro potency as p,p′-dicofol, bisphenol A (BPA).
MATERIALS AND METHODS
Chemicals
Atrazine (Chemical Abstracts Service [CAS] no. 1912-24-9, purity 99.1%), BPA (CAS no. 80-05-7, purity >99%), cyanazine (CAS no. 21725-46-2, purity 99.8%), p,p′-dicofol (CAS no. 115-32-2, purity 97.6%), 1-Chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]benzene (o,p′-DDD; CAS no. 53-19-0; purity >99%), 1-Chloro-4-[2,2-dichloro-1-(4-chlorophenyl)ethyl]benzene (p,p′-DDD; CAS no. 72-54-8; purity 97%), 1-Chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethenyl]benzene (o,p′-DDE; CAS no. 3424-82-6; purity 99.5%), 1-Chloro-4-[2,2-dichloro-1-(4-chlorophenyl)ethenyl]benzene (p,p′-DDE; CAS no. 72-55-9; purity 99%), 1-Chloro-2-[2,2,2-trichloro-1-(4-chlorophenyl)ethyl]benzene (o,p′-DDT; CAS no. 789-02-6; purity 98.9%), 1-Chloro-4-[2,2,2-trichloro-1-(4-chlorophenyl)-ethyl]benzene (p,p′-DDT; CAS no. 50-29-3; purity 99.1%), 17β-Estradiol (CAS no. 50-28-2, purity 98%), cis-Nonachlor (CAS no. 5103-73-1, purity 99.9%), and trans-Nonachlor (CAS no. 39765-80-5, purity 99.1%) were purchased from Sigma (USA). The Fluvestrant or ICI 182,780 (CAS no. 129453-61-8, purity > 99%) was purchased from Tocris Bioscience (USA). Radiolabeled [2,4,6,7,16,17-3H(N)] 17β-estradiol was purchased from PerkinElmer (USA). Kelthane™ (CAS no. 115-32-2; 95% pure dicofol composed of 80% p,p′- and 20% o,p′-dicofol, lot no. 687) was purchased from Rohm and Haas Company (USA) for use in the rat uterotrophic assay. 17β-Estradiol benzoate purchased from Sigma and BPA (CAS no. 80-05-7, purity > 99%, lot 49f-0368) acquired from Aldrich (USA) were used in the rat uterotrophic assay. All stock solutions for the binding and transactivation assays were made up using 100% ethanol as a solvent, except in the T47D-KBluc assay where dimethylsulfoxide ([DMSO]; Baxter Healthcare, USA) was used as the solvent for ICI and estradiol stocks. Stock solutions for the uterotrophic assays were made up in corn oil (Sigma). The purities of all chemicals used in the present study were determined by their manufacturers.
Receptors
Baculovirus constructs
Human ERα in a baculovirus expression vector (AcVHER) was a generous gift from C. Klinge [9]. The ERα sequence from the American alligator (Alligator mississippiensis; GenBank accession no. AB115909 [7]) was publicly available. The synthesis of aERα and sub-cloning into the expression plasmid, pVL1393, and construction of baculovirus expressing aERα (AcVAER) was described previously [6]. GenERαl techniques used in insect cell culture and baculovirus manipulation can be found elsewhere [10,11]. A high volume of receptors were produced by infecting insect Sf21 cells with baculovirus constructs in 50 ml suspension cultures. Briefly, Sf21 cells were infected at a multiplicity of infection of one, incubated for 72 h at 1 × 106 cells/ml, and centrifuged at 700 × g for 10 min. The pellet was suspended in 50 ml high salt TEDG buffer (10 mM Tris, 1.5 mM ethylenediaminetetraacetic acid, 1 mM dithiothreitol, 10% glycerol with 400 mM KCl, 1 mM sodium molybdate, and 1 mM phenylmethanesulphonylfluoride), freeze-thawed on ice 3 times, clarified by centrifugation (12,000 × g at 4°C for 30 min) and stored at −80° until use.
Receptor plasmids for CV1 transactivation assay
The hERα contained in the pCMV-XL6 plasmid was purchased from OriGene (USA). The full length aERα synthesized sequence described above was cloned into plasmid pUC57 (pchAR-57) and then subcloned into the EcoR1 site of pIRES2-EGFP (Clontech Laboratories, USA), yielding plasmid pAlligER-g.
Cell-free binding assay
The AcVAER and AcVHER infected cell lysates were used based on the protocol developed by Williams and Gorski [12]. A detailed description of the methods can be found in Rider et al. [6]. Briefly, experiments were performed in 96-well round bottom plates and all components were kept on ice or at 4°C throughout. Each experiment consisted of 3 replicates each of total binding wells, non-specific binding wells, 8 concentrations of the E2 standard, and 10 concentrations of up to three test compounds. Either buffer (total binding), unlabeled E2 (non-specific binding), E2 standards, or test compounds were added to wells along with 1 nM/well [3H] E2 and receptor. Plates were incubated overnight (18-24 h) at 4°C. Free ligand was separated from bound ligand by addition of 5% dextran-coated charcoal solution to each well followed by 10 min of gentle shaking and centrifugation at 1000 × g for 5 min. A 50 μl sample of the supernatant from each well was transferred to a scintillation vial and radioactivity was measured on a Beckman LS 5000TD scintillation counter (USA). Experiments were repeated three times.
Transactivation assays
CV1 transactivation assay with exogenous hERα and aERα
A comparison of hERα- and aERα-mediated transcriptional activation of estrogen responsive genes by E2 and p,p′-dicofol was performed in CV1 cells (monkey kidney line; ATCC) using a protocol modified from Wong et al. [13]. The CV1 cells were plated at a density of 150,000 cells/well in 6 well plates. Following a 24 h incubation, cells were transiently transfected with 0.5 μg/well of either hERα or aERα and 1 μg/well reporter (3×-ERE-TATA-Luc) using 6 μl/well FuGene® HD (Roche, Germany) according to the manufacturer's instructions. The reporter, containing three copies of vitellogenin estrogen response element (ERE), was a generous gift from D. McDonnell [14]. Twenty-four hours after transfection, cells were exposed to the following treatments: solvent, 1 μM ICI (estrogen receptor antagonist), E2 (0.01, 0.1, 1, or 10 nM), 10 nM E2 + 1 μM ICI, or p,p′-dicofol (1, 3, 10, 30, 100, 300, 1000, 3000, 10,000, or 30,000 nM). Following an overnight incubation, cells were rinsed with phosphate buffered saline and harvested with 200 μl/well lysis buffer. A 50 μl aliquot of lysate was transferred to a cuvet and luminescence was measured on a Monolight 2010 luminometer (Analytical Luminescence Laboratory, USA).
T47D-KBluc transactivation assay with endogenous hERα and hERβ
The T47D-KBluc transcriptional activation assay was used to confirm the results acquired with p,p′-dicofol and hERα in the CV1 transcriptional activation assay. T47D-KBluc cells are T47D human breast cancer cells which express hERα and hERβ and have been stably transfected with the estrogen-responsive lucifERαse reporter (3x-ERE-TATA-Luc). Detailed methods for the T47D-KBluc transcriptional activation assay can be found in Wilson et al. [15]. Briefly, cells were plated at a density of 10,000 cells/well in 96-well luminometer plates. Following overnight incubation, media was replaced with 100 μl/well of dosing media containing either solvent, E2 (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100 pM), 30 pM E2 + 1 μM ICI, or p,p′-dicofol (1, 3, 10, 30, 100, 300, 1000, 3000, 10,000, 30,000 nM) for a 24 h incubation period. Cells were then rinsed with phosphate buffered saline and harvested by addition of 25 μl lysis buffer (Promega, USA) to each well. Luciferase activity was measured as relative light units on a LumiStar OPTIMA microtiter plate luminometer (BMG LABTECH, USA) following the addition of reaction buffer and D-luciferin (Promega). Two independent experiments containing four replicate wells per treatment were performed.
Rat uterotrophic assay
Twenty-five, ninety day old Long Evans ovariectomized female rats (Charles Rivers Labs) were housed two per cage in clear polycarbonate cages (20 × 25 × 47 cm) containing laboratory-grade, heat-treated pine shavings after receipt (Northeastern Products, USA). Environmental conditions remained constant throughout the study period at 14:10 h light:-dark reverse photoperiod (lights out at 11:00 a.m., 20-24°C temperature, and 40–50% relative humidity). Rats were provided with Purina Rat Chow 1501 and filtered municipal (Durham, NC, USA) drinking water ad libitum. Six to seven rats per treatment group were dosed via subcutaneous injection for two consecutive days with either vehicle alone, 25 μg 17β-estradiol benzoate dissolved in 100 ml corn oil, or 200 mg/kg/d Kelthane™ (mixed p,p′-and o,p′-dicofol) or BPA in corn oil.
In the morning of the third day, all rats received a subcutaneous injection of 0.5 mg progesterone in 100 ml corn oil. Vaginal smears were performed following progesterone injection to determine whether vaginal cytology was altered in an estrogenic manner by the treatments. Each primed female was then paired with a proven stud male rat in a bedding-free cage to observe mating behavior under dim light during the dark phase of the animals' activity cycle. The first five mounts were observed and instances of lordosis response (ventral arching of the spine) displayed by the female in response to the mount were recorded. The lordosis to mount ratio (lordosis quotient) was then calculated by dividing mounts with lordosis by mounts without lordosis. Following behavioral testing, females were anesthetized with CO2 and euthanized by decapitation and the wet weight of the uterus was recorded. The present study was conducted under protocols approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee (U.S. Environmental Protection Agency, Research Triangle Park).
Statistical analysis
Competitive binding data were graphed in Prism and fit with the one site competition function (GraphPad Prism version 5, USA). In cases where binding curves flattened before reaching 100% displacement of radiolabeled E2, only the sloping portion of the curve was used in the curve analysis. The t tests were performed on the IC50's of each chemical to detect statistical differences in the affinities of chemicals for aERα and hERα using SigmaStat (Systat Software, USA). Relative binding affinities were calculated by dividing the IC50 of E2 by the IC50 of the test compound for each receptor and then multiplying by 100. Chemicals that displaced less than 50% E2 at the highest soluble concentration were considered non-binders, while chemicals that displaced 50% or greater, but less than 80% were considered equivocal binders.
Transcriptional activation assay data were converted from relative light units to log fold induction. The data were then analyzed using PROC GLM one-way analysis of variance with SAS® 9.1 software (SAS Institute, USA).
The behavioral, vaginal cytology and uterine weight data from the rat uterotrophic assay were analyzed with PROC GLM followed by LSMEANS t tests with SAS 9.1 software.
RESULTS
Competitive binding to hERα and aERα
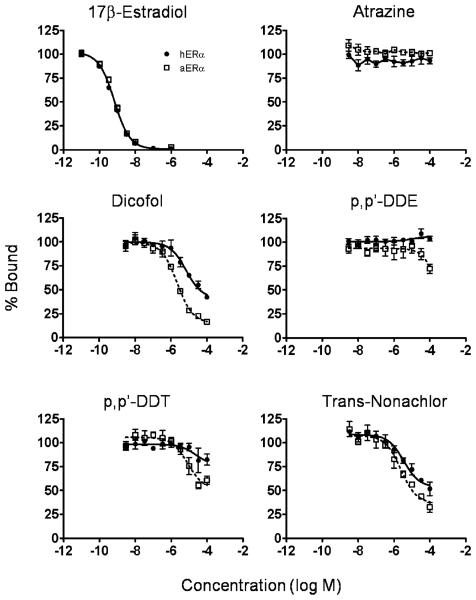
Competitive binding of chemicals to hERα and aERα was compared (Table 1, Fig. 1). In general, although many of the chemicals were classified as non-binders, there was a tendency for some chemicals to bind with slightly greater affinity to aERα than hERα. Atrazine, cyanazine, o,p′-DDD, p,p′-DDD, p,p′-DDE, and p,p′-DDT were classified as non-binders to both receptors because they did not displace at least 50% of the radioligand. However, some of the non-binders partially displaced E2 from one or both receptors: p,p′-DDT, o,p′-DDD, and p,p′-DDE displaced approximately 42, 38, and 28% E2 from aERα, respectively and displaced approximately 31, 18, and 0% from hERα, respectively. Both o,p′-DDE and cis-nonachlor were equivocal binders to hERα and aERα. trans-Nonachlor was classified as an equivocal binder to aERα, displacing a maximum of 68%, and a non-binder to hERα, displacing 48% E2 from hERα. p,p′-Dicofol demonstrated the greatest difference in affinity for the two receptors with an IC50 for aERα of 4 μM: an order of magnitude lower than the 45 μM IC50 for hERα (Table 1, Fig. 1). p,p′-Dicofol displaced up to 83% of E2 from aERα and was an equivocal binder to hERα, displacing a maximum of 58% E2. Although, cis-Nonachlor did not completely displace E2 from either receptor, it was able to bind to both receptors with approximately equal affinity. Bisphenol A and o,p′-DDT demonstrated complete binding curves and bound with similar affinity to both receptors (Table 1).
Table 1.
Relative binding affinities for endocrine disrupting chemicals to human and alligator estrogen receptors alpha. The values represent the mean concentration inhibiting 50% of binding (IC50) ±standard error from three replicate assays.
| Human |
Alligator |
|||
|---|---|---|---|---|
| Chemical | IC50 (log M) | RBA | IC50 (log M) | RBA |
| 17β-Estradiol | −9.22±0.036 | 100 | −9.13±0.043 | 100 |
| Atrazine | NB | NB | ||
| Cyanazine | NB | NB | ||
| Bisphenol A | −5.87±0.12 | 0.05 | −5.97±0.090 | 0.07 |
| p,p′-Dicofol | EB (−4.35±0.078) | 0.001 | −5.40±0.066* | 0.02 |
| o,p′-DDD | NB | NB | ||
| p,p′-DDD | NB | NB | ||
| o,p′-DDE | EB (−3.66±0.10) | 0.003 | EB (−4.07±0.10) | 0.009 |
| p,p′-DDE | NB | NB | ||
| o,p′-DDT | −6.02±0.06 | 0.06 | −6.01±0.088 | 0.08 |
| p,p′-DDT | NB | NB | ||
| cis-Nonachlor | EB (−4.63±0.084) | 0.003 | EB (−4.90±0.10) | 0.006 |
| trans-Nonachlor | NB | EB (−4.79±0.077) | 0.005 | |
EB – Equivocal binder: displaced greater than 50%, but less than 80% at the highest soluble concentration.
IC50 – Concentration that inhibits 50% of binding
NB – Non-binder: displaced less than 50% 17b-estradiol at the highest soluble concentration.
o,p′-DDD – 1-Chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]benzene
o,p′-DDE – 1-Chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethenyl]benzene
o,p′-DDT – 1-Chloro-2-[2,2,2-trichloro-1-(4-chlorophenyl)ethyl]benzene
p,p′-DDD – 1-Chloro-4-[2,2-dichloro-1-(4-chlorophenyl)ethyl]benzene
p,p′-DDE – 1-Chloro-4-[2,2-dichloro-1-(4-chlorophenyl)ethenyl]benzene
p,p′-DDT – 1-Chloro-4-[2,2,2-trichloro-1-(4-chlorophenyl)ethyl]benzene
Indicates aERα value differs from hERα value by p<0.001. Values are IC50±standard error (log M) from three independent experiments.
Relative binding affinties (RBAs) were calculated by dividing the IC50 of 17β-estradiol for each of the receptors by the IC50 of the target compound for that receptor and multiplying by 100.
Fig. 1.
Competitive binding curves for select chemicals with human estrogen receptor alpha (hERα) and alligator estrogen receptor alpha (aERα) from a cell-free assay. Data were fit with a one site competition model (except atrazine which did not compete for binding): solid line for hERα and dotted line for aERα. For the 17β-estradiol data, a single line fit both hERα and aERα data sets. Each data point represents the average value from at least three replicate experiments standard error.
Transcriptional activation with hERα and aERα
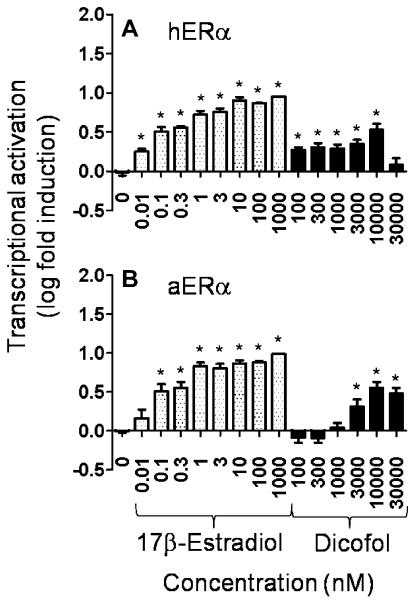
The CV1 transcriptional assay was used to further explore the observed difference in binding affinity of p,p′-dicofol for aERα over hERα. Both receptors performed well in the CV1 transcriptional activation assay. The E2 positive control elicited transcriptional activation that was ablated at the 10 nM E2 level with concurrent treatment with the ER antagonist ICI (data not shown) indicating that the effect was ER mediated. Both the E2 positive control and p,p′-dicofol induced transcription of an estrogen responsive gene with aERα and hERα (Fig. 2). However, the dose-response to p,p′-dicofol with hERα was not as clear due to an induction of transcriptional activation at the lowest concentrations of E2 and p,p′-dicofol tested. The high concentration of p,p′-dicofol (30 μM) resulted in cell distress as indicated by an observed increase in detached cells, which likely explains the drop off in transcriptional activation at that concentration (Fig. 2).
Fig. 2.
Transcriptional activation of an estrogen-responsive reporter gene by 17β-estradiol or p,p′-dicofol in CV1 cells. CV1 cells were transfected with either (A) human estrogen receptor (hERα)or(B) alligator estrogen receptor alpha (aERα) and 3x-ERE-TATA-Luc. Relative light units were converted to log fold induction. Each point represents the mean from four to five replicate assays containing two duplicates per treatment ± standard error. Asterisks indicate that values were significantly greater than solvent control (p < 0.05).
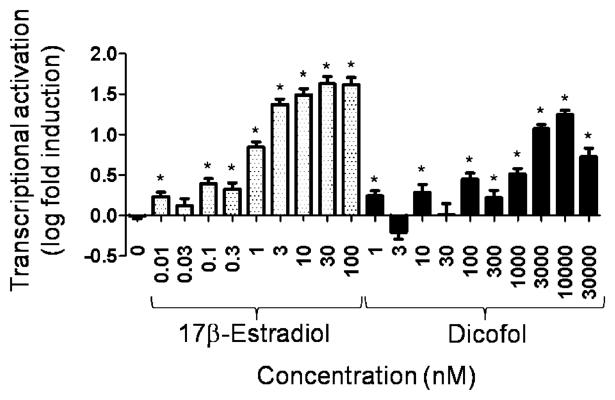
The T47D-KBluc transcriptional activation assay was used to confirm the positive estrogenic effect of p,p′-dicofol observed in the CV1 assay with hERα (Fig. 3). The E2 positive control resulted in dose dependent transactivation which was reversed at the 30 pM concentration of E2 with ICI (data not shown). p,p′-Dicofol clearly elicited a dose-dependent increase in the transcriptional activation of the estrogen-dependent reporter (Fig. 3). Again, the 30 μM concentration of p,p′-dicofol was toxic to the cells, as evidenced by increased cell detachment and cytopathology, and, thus, the decreased level of transcriptional activation seen at that concentration was attributed to cytotoxicity.
Fig. 3.
Transcriptional activation of an estrogen-responsive reporter gene by 17β-estradiol or p,p′-dicofol in the T47D-KBluc assay. T47D breast cancer cells containing endogenous human estrogen receptor alpha and beta were stably transfected with 3x-ERE-TATA-Luc. Cells were exposed to either 17b-estradiol or p,p′-dicofol. Relative light units were converted to log fold induction. Each point represents the mean from two replicate assays containing four duplicates per treatment ± standard error. Asterisks indicate that values were significantly greater than solvent control (p < 0.05).
In vivo estrogenicity assay with xenoestrogens
17β-Estradiol and BPA both induced significant increases in the percentages of nucleated epithelial cells in the vaginal lavage, uterine weight, and lordosis quotient as compared to the vehicle control (Table 2). In contrast, mixed o,p′- and p,p′- dicofol treatment did not induce any estrogenic effect, indicating that it was not estrogenic in vivo even at the high dose level tested (Table 2).
Table 2.
Comparison of in vivo estrogenic potency of mixed p,p′and o,p′ dicofol, bisphenol A (a weak estrogen), and 17β-estradiol positive control using the rat uterotrophic assay. Some of the corn oil control, 17b-estradiol, and bisphenol A data were previously published (Gray and Ostby [31]).
| Corn oil (0.1 ml/day) Control |
17β-Estradiol (25 μg/rat/day) Strong estrogen |
Bisphenol A (200 mg/kg) Weak estrogen |
Dicofol (200 mg/kg) Unknown |
|
|---|---|---|---|---|
| Number of Females | 7 | 6 | 6 | 6 |
| % Nucleated epithelial cells in vaginal lavage at necropsy | 8.6±5.5 | 83.3±2.1** | 71.1±7.9** | 10.8±6.1 |
| Uterine wet weight | 92.6±10.0 | 383.9±13.8** | 283.6±24.1** | 81.3±5.9 |
| Lordosis behavior quotient (%) | 0±0 | 96.7±3.3** | 100±0** | 0±0 |
Indicates a significant difference from the corn oil control at p≤0.0001.
DISCUSSION
Pollutants found in Lake Apopka, Florida, which are associated with reproductive problems in alligators, were assessed for their ability to bind to aERα and hERα using a competitive binding assay in which the only variable was the receptor. Some of the test chemicals had a slightly higher affinity for aERα than hERα. The most striking difference was seen with p,p′-dicofol, which had an affinity an order of magnitude higher for aERα than hERα. This difference was further assessed by evaluating p,p′-dicofol using in vitro ER-mediated transcriptional activation assays and in a short-term mammalian uterotrophic assay for estrogenic activity. The limited data available on in vivo effects of dicofol on alligators are discussed below.
Previous competitive binding assays using alligator oviductal tissue as the receptor source found that multiple environmentally relevant chemicals exhibited binding to aER [4,16]. These studies utilized receptor preparations that likely contained aERα and aERβ, as well as other steroid receptors, and did not include a parallel comparison to chemical binding with a representative mammalian ER [4,16]. Several of the chemicals that were able to bind to oviduct-derived aER (atrazine, cyanazine, alachlor, heptachlor, chlordane, and lindane) were inactive in the E-screen assay in which estrogenic compounds stimulate proliferation of human MCF-7 cells [5]. The triazine herbicide, atrazine, in particular, has been found to be a non-binder to ERs from a diverse array of species in multiple in vitro assays [17-19].
In the present study, a recently developed competitive binding assay system [6] was used to assess the binding of contaminants previously found in Lake Apopka alligator eggs [20] to full-length recombinant aERα and hERα where the only variable was the receptor. Contrary to the findings of Vonier et al. [4], atrazine and cyanazine were not able to bind to either aERα or hERα at concentrations up to 100 μM. At this time, the possibility cannot be ruled out that ERb, which was likely present in the oviductal aER preparation, could bind preferentially to the triazine herbicides, thereby accounting for the discrepancy between studies. Interestingly, Harris et al. found that the mouse showed a greater ER subtype selectivity than either the rat or human indicating that subtype selectivity could be species-dependent [21]. Sequencing of the full-length aERβ is necessary to test this hypothesis.
The results of the present study indicate that aERα appears to be slightly more sensitive to xenoestrogen binding than hERα. For example, even though the DDT metabolites, p,p′-DDE and p,p′-DDT, are classified as non-binders, they displaced more E2 from aERα than from hERα (Figure 1). p,p′-Dicofol had the most notable preference for aERα over hERα (Fig. 1, Table 1). Transcriptional activation assays were used to determine whether this order of magnitude difference in IC50s observed in a competitive binding assay would remain intact at the next level of biological complexity.
The CV1 transcriptional activation assay allows for a cross-species comparison because it does not rely on an endogenous ER, but depends on the transient transfection of the receptor of choice. Although it appears clear that p,p′-dicofol was able to induce transcription with aERα and hERα, the dose-response achieved with hERα is less defined due to the high background level of transcriptional activation seen with all treatments except the vehicle control. Due to the difficulty in interpreting these results, the only conclusion that can be drawn is that p,p′-dicofol is able to stimulate transcriptional activation of estrogen responsive genes through both aERα and hERα with median effective concentration (EC50) values of 2.8 × 10−6 M and 1.7 × 10−6 M, respectively. It is further confirmed that p,p′-dicofol was able to activate transcription with hER by using the T47D-KBluc transcriptional activation assay, which employs the endogenous hERα and hERβ and is more sensitive. The results of this assay demonstrate that p,p′-dicofol has the ability to activate transcription through hER with an EC50 of 1.5 × 10−6 M. The CV1 and T47D-KBluc assays displayed similar results, with p,p′-dicofol eliciting maximal transactivation of the estrogen responsive gene at the 10 μM concentration in both assays. Similarly, Hoekstra et al. found that p,p′-dicofol (EC50 1.6 × 10−6 M) and racemic (±) o,p′-dicofol (EC50 4.2 × 10−6 M) were weak hER agonists whereas, the chiral enantiomer (−) o,p′-dicofol was a more potent hER agonist (EC50 5.1 × 10−7 M) [22]. These transcriptional activation assays did not reflect the greater binding affinity of p,p′-dicofol for aERα over hERα observed in the competitive binding assay. One potential explanation for this discrepancy of in vitro results could be the fact that mammalian cells were used in the transcriptional activation assays. Perhaps subtle species differences in cellular components complicate the parallel comparison of transcriptional activation in cell-based assays.
The next question was whether the observed in vitro responses were indicative of organismal level responses; in effect, is the estrogenic response maintained in vivo? Previous studies have tested whether various environmental chemicals are estrogenic using the sex reversal response in reptiles with environmental sex determination [23-26]. Many reptilian species use temperature as a major factor in sex determination [27], but this factor can be overcome by exposure of the developing embryo to an estrogenic chemical during a narrow embryonic window of sensitivity. Overriding a male-producing temperature with an estrogen can redirect sex from male to female. This response has been demonstrated with a number of contaminants in freshwater turtles and alligators [23-26]. Specifically, atrazine and dicofol exposure of alligator embryos has been examined.
In ovo exposure of alligator embryos, incubated at 33°C, at stage 19.5 (just prior to the period of sex determination) to an 83.4% p,p′-and 15.7% o,p′-dicofol mixture (single topical egg exposure to 0.14, 1.40, or 14.0 ppm) tended to induce sex reversal (male to female) in a non-dose dependent fashion [28], suggestive of estrogenic activity in this species, which supports the in vitro receptor activity data obtained in the present study. However, it is possible that the weak estrogenicity observed in vivo could be due to the o,p′-dicofol present in the mixture. More work is needed to confirm and characterize the potential estrogenic effect of dicofol in the alligator and other non-mammalian species.
Previous studies examining atrazine exposure of alligators in ovo (33°C; stage 21; single topical egg exposure to 0.14, 1.40, or 14.0 ppm) produced no sex reversal at any concentration [29], but did alter gonadal aromatase activity and not plasma E2 concentrations [24]. Importantly, the sex reversal response is complex and involves an interaction of temperature and chemical exposure, as it was reported that in ovo exposure of alligator embryos to 100 ppb p,p′-DDE caused no male to female sex reversal at 33.5°C (a temperature usually producing 100% males) whereas at 32°C (mixed sex ratio) a slight female sex bias was observed [25]. There was very slight displacement of E2 by p,p′-DDE from the aERα in our studies reported here, suggesting that p,p′-DDE is not an estrogen agonist and the sex reversal reported by Milnes et al. [25] might be due to other factors following in ovo exposure: such as changes in the hepatic biotransformation of steroids thus altering the hormonal milieu, or a combination of variables not completely understood at this time (e.g., functioning and expression of receptor cofactors such as heat shock proteins that are very sensitive to temperature).
As with the reptilian sex reversal assay, the rat uterotrophic assay is a gold standard in vivo test for estrogenicity in mammals [30]. In this assay, we were able to measure estrogen-mediated endpoints in the brain, vagina, and uterus. In contrast to the reported trend in sex reversal in alligators [28], we found that mixed p,p′-and o,p′-dicofol isomers did not elicit any estrogenic effect in the rat. In other words, the partial displacement of E2 from hERα and the modest activation of an estrogen-responsive gene did not translate into a biological response at the high dose (200 mg/kg/d) of mixed p,p′- and o,p′-dicofol tested.
Taken together, the evidence suggests that there could be a species difference in sensitivity to the estrogenic effects of p,p′-dicofol and that this difference in sensitivity was reflected in the in vitro competitive binding test system. However, the potential in vivo effects of pure p,p′-dicofol require confirmation of the trend reported by Rooney et al. using the mixed isomers [28].
Although some quantitative differences were observed in affinity of the test chemicals for aERα versus hERα, the fact that there are no examples, to date, of a chemical binding with moderate or high affinity to the ER of one species and not binding at all to the ER of another species indicates that mammalian ERs are appropriate surrogates for other species in in vitro tests used for the screening of chemicals for estrogenicity. Additional testing with an expanded chemical set is needed to support this observation. It is clear that many complex factors contribute to species sensitivity to endocrine disrupting compounds. Therefore, while the use of mammalian receptors for initial screening of chemicals appears to be adequate for prioritizing chemicals with ER binding activity, further testing in multiple species is necessary to actually characterize the biological effects associated with xenoestrogens.
Acknowledgement
Funding was provided by the North Carolina State University and U.S. Environmental Protection Agency (U.S. EPA) Cooperative Training Program CT833235-01-0 and the National Institute of Health Pathway to Independence Award 1K99ES016806. The receptor constructs discussed herein are available upon written request. The research described in this study has been reviewed by the National Health Environmental Effects Research Laboratory, U.S. EPA, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does the mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- 1.Woodward AR, Percival HF, Jennings ML, Moore CT. Low clutch viability of american alligators on Lake Apopka. Fla Sci. 1993;56:52–63. [Google Scholar]
- 2.Guillette LJ, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex-hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994;102:680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillette LJ, Pickford DB, Crain DA, Rooney AA, Percival HF. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol. 1996;101:32–42. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- 4.Vonier PM, Crain DA, McLachlan JA, Guillette LJ, Arnold SF. Interaction of environmental chemicals with the estrogen and progesterone receptors from the oviduct of the American alligator. Environ Health Perspect. 1996;104:1318–1322. doi: 10.1289/ehp.961041318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO. The E-screen assay as a tool to identify estrogens - an Update on estrogenic environmental-pollutants. Environ Health Perspect. 1995;103:113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rider CV, Hartig PC, Cardon MC, Wilson VS. Development of a competitive binding assay with recombinant estrogen receptors from multiple species. Toxicol Lett. 2009;184:85–89. doi: 10.1016/j.toxlet.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Katsu Y, Bermudez DS, Braun EL, Helbing C, Miyagawa S, Gunderson MP, Kohno S, Bryan TA, Guillette LJ, Iguchi T. Molecular cloning of the estrogen and progesterone receptors of the American alligator. Gen Comp Endocrinol. 2004;136:122–133. doi: 10.1016/j.ygcen.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Vonier PM, Guillette LJ, McLachlan JA, Arnold SF. Identification and characterization of estrogen and progesterone receptors from the oviduct of the American alligator (Alligator mississippiensis) Biochem Biophys Res Commun. 1997;232:308–312. doi: 10.1006/bbrc.1997.6274. [DOI] [PubMed] [Google Scholar]
- 9.Klinge CM. Role of estrogen receptor ligand and estrogen response element sequence on interaction with chicken ovalbumin upstream promoter transcription factor (COUP-TF) J Steroid Biochem Mol Biol. 1999;71:1–19. doi: 10.1016/s0960-0760(99)00124-7. [DOI] [PubMed] [Google Scholar]
- 10.O'Reilly DR, Miller LK, Luckow VA. Baculovirus Expression Vectors: A Laboratory Manual. W.H. Freeman and Company; New York, NY, USA: 1992. p. 347. [Google Scholar]
- 11.Summers MD, Smith GE. A Manual of Methods for Baculovirus Vectors and Insect Cell Culture Procedures Texas Agriculture Experiment Station Bulletin No 1555. Texas A&M University; College Station, TX, USA: 1987. pp. 1–55. [Google Scholar]
- 12.Williams D, Gorski J. Equilibrium binding of estradiol by uterine cell suspensions and whole uteri in vitro. Biochemistry. 1974;13:5537–5542. doi: 10.1021/bi00724a013. [DOI] [PubMed] [Google Scholar]
- 13.Wong CI, Kelce WR, Sar M, Wilson EM. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. Jo Biol Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- 14.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ER beta) of the human estrogen receptor modulates ER alpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 15.Wilson VS, Bobseine K, Gray LE. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci. 2004;81:69–77. doi: 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- 16.Guillette LJ, Vonier PM, McLachlan JA. Affinity of the alligator estrogen receptor for serum pesticide contaminants. Toxicology. 2002;181:151–154. doi: 10.1016/s0300-483x(02)00272-x. [DOI] [PubMed] [Google Scholar]
- 17.Matthews J, Celius T, Halgren R, Zacharewski T. Differential estrogen receptor binding of estrogenic substances: a species comparison. J Steroid Biochem Mol Biol. 2000;74:223–234. doi: 10.1016/s0960-0760(00)00126-6. [DOI] [PubMed] [Google Scholar]
- 18.Rider CV, Hartig PC, Cardon M, Wilson VS. Comparison of chemical binding to recombinant fathead minnow and human estrogen receptors alpha (ERa) in whole cell and cell-free assay systems. Environ Toxicol Chem. 2009;28:2175–2181. doi: 10.1897/09-018.1. [DOI] [PubMed] [Google Scholar]
- 19.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Heinz GH, Percival HF, Jennings ML. Contaminants in American alligator eggs fromm lakes Apopka, Griffin and Okeechobee, Florida. Environ Monit Assess. 1991;16:277–285. doi: 10.1007/BF00397615. [DOI] [PubMed] [Google Scholar]
- 21.Harris HA, Bapat AR, Gonder DS, Frail DE. The ligand binding profiles of estrogen receptors alpha and beta are species dependent. Steroids. 2002;67:379–384. doi: 10.1016/s0039-128x(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 22.Hoekstra PF, Burnison BK, Garrison AW, Neheli T, Muir DCG. Estrogenic activity of dicofol with the human estrogen receptor: Isomerand enantiomer-specific implications. Chemosphere. 2006;64:174–177. doi: 10.1016/j.chemosphere.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 23.Bergeron JM, Crews D, McLachlan JA. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994;102:780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crain DA, Guillette LJ, Rooney AA, Pickford DB. Alteration in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milnes MR, Bryan TA, Medina JG, Gunderson MP, Guillette L. Developmental alterations as a result of in ovo exposure to the pesticide metabolite p,p′-DDE in Alligator mississippiensis. Gen Comp Endocrinol. 2005;144:257–263. doi: 10.1016/j.ygcen.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Willingham E, Crews D. Sex reversal effects of environmentally relevant xenobiotic concentrations on the red-eared slider turtle, a species with temperature-dependent sex determination. Gen Comp Endocrinol. 1999;113:429–435. doi: 10.1006/gcen.1998.7221. [DOI] [PubMed] [Google Scholar]
- 27.Crews D. Sex determination: where environment and genetics meet. Evolution and Development. 2003;5:1–6. doi: 10.1046/j.1525-142x.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- 28.Rooney AA. Variation in the endocrine and immune system of juvenile alligators: Environmental influence on physiology. University of Florida; Gainesville, FL, USA: 1998. PhD thesis. [Google Scholar]
- 29.Crain DA, Spiteri ID, Guillette LJ. The functional and structural observations of the neonatal reproductive system of alligators exposed in ovo to atrazine, 2,4-D, or estradiol. Toxciol Ind Health. 1999;15:180–185. doi: 10.1191/074823399678846565. [DOI] [PubMed] [Google Scholar]
- 30.Kanno J, Onyon L, Peddada S, Ashby J, Jacob E, Owens W. The OECD program to validate the rat uterotrophic bioassay. Phase 2: Dose-response studies. Environ Health Perspect. 2003;111:1530–1549. doi: 10.1289/ehp.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray LE, Ostby J. Effects of pesticides and toxic substances on behavioral and morphological reproductive development: Endocrine versus nonendocrine mechanisms. Toxicol Ind Health. 1998;14:159–184. doi: 10.1177/074823379801400111. [DOI] [PubMed] [Google Scholar]