Abstract
From routine in vitro drug-transporter inhibition assays, observed inhibition is typically assumed from direct interaction with the transporter. Other mechanisms that possibly reduce substrate uptake are not frequently fully examined. The objective of this study was to investigate the association of transporter inhibition with drug cytotoxicity. From a pool of drugs that were identified as known ASBT or OCTN2 inhibitors, twenty one drugs were selected to screen inhibitory potency of their prototypical substrate and cytotoxicity against three human sodium-dependent solute carrier (SLC) transporters: apical sodium-dependent bile acid transporter (ASBT), organic cation/carnitine transporter (OCTN2), and the excitatory amino acid transporter 4 (EAAT4) in stable cell lines. Twenty drugs showed apparent inhibition in OCTN2-MDCK and ASBT-MDCK. Four dihydropyridine calcium channel blockers were cytotoxic to MDCK cells, and the observed cytotoxicity of three of them accounted for their apparent OCTN2 inhibition, and consequently were classified as non-OCTN2 inhibitors. Meanwhile, since their cytotoxicity only moderately contributed to ASBT inhibition, these three were still considered ASBT inhibitors. Four other drugs showed apparent inhibition in EAAT4-HEK cells, and cytotoxicity of three drugs corresponded with their inhibition of this transporter. Therefore, cytotoxicity significantly affected EAAT4 observations. Results showed the potential of cytotoxicity as a mechanism that can account for apparent in vitro transporter inhibition. Drug cytotoxicity varied in different cell lines, which could increase false positives for pharmacophore development.
Keywords: Cytotoxicity, HEK cells, inhibition, MDCK cells, transporter
1. Introduction
Computational screening is a popular approach to rapidly identify potential inhibitors of transporters [1], where “hits” are then experimentally validated via cell-based inhibition assays. In addition to specific drug binding to the protein, other possible modes of drug inhibition of a transporter can be regarded as false positives but are not frequently considered. Such modes of inhibition include cell toxicity, modulation of the energy source for active transport, and non-specific interaction of a drug with the cell membrane. Chemical modification of protein thiol groups [2,3] and interference in binding interactions by compound aggregates have recently been identified as non-specific mechanisms for compound candidates to inhibit enzymes [4,5].
The objective of this study was to evaluate cytotoxicity as a mechanism for apparent transporter inhibition. Twenty one drugs, along with the Na+/K+ pump inhibitor ouabain, were evaluated against three solute carrier proteins: human apical sodium-dependent bile acid transporter (ASBT; SLC10A2), human organic cation/carnitine transporter (OCTN2; SLC22A5), and the excitatory amino acid transporter 4 (EAAT4; SLC1A6). Previously, using a combined in vitro and pharmacophore-based approach, several drugs were found to be inhibitors of ASBT or the OCTN2 [6,7]. Eleven ASBT inhibitors and ten OCTN2 inhibitors were selected to screen against these three transporters. Ouabain was used to evaluate the effect of sodium gradient modulation [8]. Drug cytotoxicity was also evaluated.
ASBT, OCTN2, and EAAT4 were selected since they are all sodium-dependent, active influx transporters, and recognize dissimilar substrates with little known overlap for inhibitors. The transporters are energized by co-transporting sodium ion down the membrane sodium gradient. ASBT is responsible for the intestinal recovery of bile acids recovery [9]. Substrate translocation is coupled with sodium in a 2:1 sodium: bile acid stoichiometry [10]. OCTN2 mediates the reabsorption of organic cations in the kidney, particularly carnitine [11]. OCTN2-mediated translocation can be either sodium-dependent or sodium-independent, although translocation of L-carnitine is sodium-dependent [12]. EAAT4 is enriched in the Purkinje cells of the cerebellum and is a subtype of the five known human glutamate transporters in neurons [13]. It precisely regulates extracellular glutamate concentrations to maintain critical signaling yet avoiding excitotoxicity by uptake glutamate from the synaptic cleft [14]. Both OCTN2 and ASBT have hydrophobes as features in common, while ASBT prefers a negative charge and OCTN2 requires a positive ionisable feature [6,7,15]. A general EAAT pharmacophore includes two acidic functional groups and a protonatable nitrogen [16].
Results from drug screening of three sodium-depended transporters in this study indicate that for some drugs, their cytotoxicity contributed to apparent transporter inhibition, and we identified cytotoxicity differences between MDCK and HEK cells.
2. Materials and Methods
2.1. Materials
[3H]-Taurocholic acid and [3H]-glutamic acid were purchased from Perkin Elmer (Waltham, MA). [3H]-L-carnitine was purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Taurocholate and glutamic acid were obtained from Sigma Aldrich (St. Louis, MO). L-carnitine was purchased from Spectrum Pharmacy Products (Tucson, AZ). Fetal bovine serum (FBS), trypsin, and Dulbecco’s modified Eagle’s medium (DMEM) were procured from Invitrogen Corporation (Carlsbad, CA). WST reagent was purchased from Roche Applied Science (Indianapolis, IN). All drugs and ouabain were obtained from Sigma Chemical (St. Louis, MO), Alexis Biochemicals (San Diego, CA), AK Scientific (Mountain View, CA), LKT Labs (St. Paul, MN), Spectrum Chemicals & Laboratory Products (Gardena, CA), Spectrum Pharmacy Products (Tucson, AZ), or TCI America (Portland, OR).
2.2. Cell Culture
ASBT-MDCK [17], OCTN2-MDCK [7], and EAAT1-HEK [18] cells have been characterized and were cultured as previously described.
Briefly, stably transfected ASBT-MDCK cells and OCTN2-MDCK were grown at 37 °C, 90% relative humidity, and 5% CO2 atmosphere and fed every two days. Media comprised DMEM supplemented with 10% fetal bovine serum, 50 units/mL penicillin, and 50 μg/mL streptomycin. Geneticin was used at 1 mg/mL to maintain selection pressure. Cells were passaged every 4 days or after reaching 90% confluence. EAAT4 stably transfected EAAT4-HEK cells were grown at 37 °C, 90% relative humidity, and 5% CO2 atmosphere and fed every two days. Growth media comprised DMEM (include 110 mg/L sodium pyruvate), supplemented with 50 units/mL penicillin and 50 μg/mL streptomycin. Hygromycin was used for selection at 50 μg/mL. Cells were passaged after reaching 70% confluence.
2.3 Inhibition Study
Inhibition studies were performed using 21 drugs: 11 potent ASBT inhibitors (i.e. lansoprazole, amlodipine, fluvastatin, indomethacin, latanoprost, lovastatin, nicardipine, nifedipine, nisoldipine, propafenone, simvastatin, tioconazole) [6]; and 10 potent OCTN2 inhibitors (i.e. desloratadine, carvedilol, chlorpheniramine, clozapine, diltiazem, imipramine, imatinib, thioridazine, verapamil, vinblastine) [7]. Amlodipine, diltiazem, thioridazine, and verapamil were known to inhibit both transporters.
Inhibition studies were conducted as previously described [7]. Briefly, stably transfected ASBT-MDCK and OCTN2-MDCK cells were seeded in 12 well cluster plates (Corning; NY) at a density of 1.5 million cells/well. Uptake studies were performed in triplicate on the fifth day. EAAT4-HEK cells were seeded in 12 well poly-D lysine coated plates (BD BioCoat; Bedford, MA) at a density of 80,000 cells/well and uptake studies were performed after 48 hours. ASBT-MDCK, OCTN2-MDCK, and EAAT4-HEK cells were exposed to donor solution containing 2.5 μM taurocholate (spiked with 0.5 μCi/mL [3H]-taurocholate), 2.5 μM L-carnitine (0.5 μCi/mL [3H]-L-carnitine), and 1 μM glutamate (0.5 μCi/mL [3H]-glutamic acid), respectively, in the presence of a drug. Substrate concentrations used were equivalent to half Kt. For low water soluble compounds, 1–2.5% DMSO was included in transport buffer, which has been shown to not affect transporter kinetics.[19] A drug was denoted as an apparent inhibitor if substrate uptake was reduced to 80% or less.
2.4 Cytotoxicity Studies
ASBT-MDCK and OCTN2-MDCK cells were seeded at a density of 50,000 cells/well, while EAAT4-HEK were seeded at 30,000 cells/well, in 96 well plates and grown for 48 hr. Cells were washed thrice with HBSS and incubated with donor solution containing drug for 10 min to simulate the uptake studies. After 10 min of exposure, cells were washed thrice with HBSS, and 10 μl of cell proliferation reagent WST-1 in 100 μL of HBSS was added to each well, followed by an incubation period of 4 hr. Absorbance was measured at 440 nm using a SpectraMax 384 Plus plate reader (Molecular Devices; Sunnyvale, CA). All studies were performed in triplicate. A drug was denoted as cytotoxic if cell viability was less than or equal to 80%.
3. Results
3.1 Transporters Inhibition and Cytotoxicity
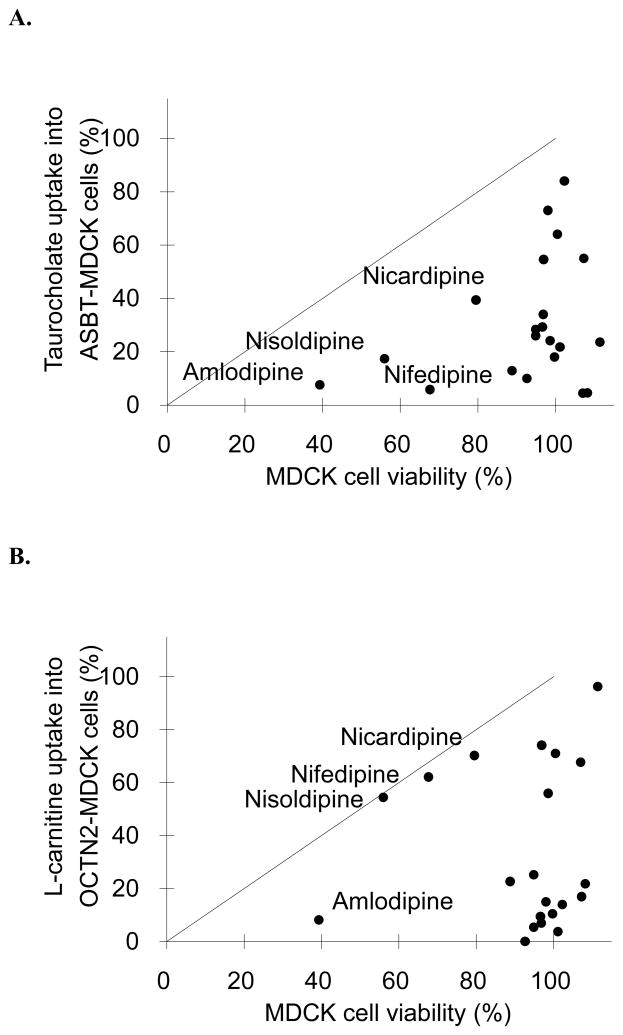
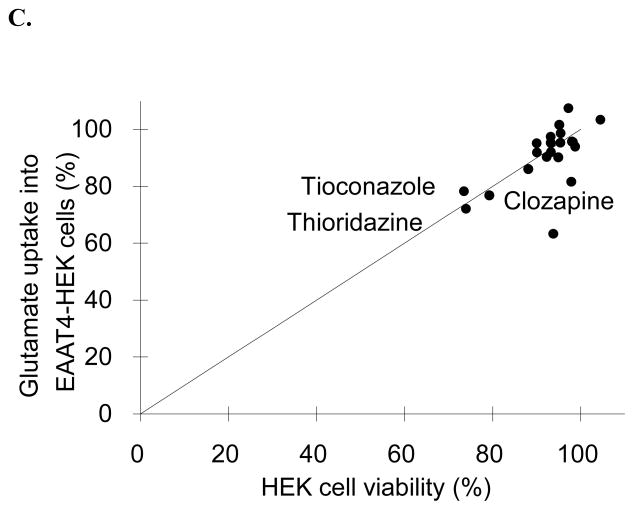
Twenty one FDA approved drugs were subjected to ASBT, OCTN2, and EAAT4 inhibition in vitro assays, as well as cytotoxicity assay. Fig. 1 plots inhibition vs. cytotoxicity results for each transporter. Inhibition results are presented in terms of percent of substrate (i.e. taurocholate, L-carnitine, or glutamate, respectively). Drugs were considered cytotoxic if they reduced the cell viability more than 20%, and their names are labeled in Fig. 1.
Fig. 1.
Relationship between transporter inhibition and cytotoxicity for 21 drugs. Cell lines were (A) ASBT-MDCK, (B) OCTN2-MDCK, and (C) EAAT4-HEK, which employed taurocholate, L-carnitine, and glutamate as substrates, respectively. The line of unity is drawn on each figure. Linear regression showed slope=0.409, r2=0.100, and p=0.163 for panel A; slope=0.045, r2=0.001, and p=0.908 for panel B; and slope=0.862, r2=0.392, and p=0.002 for panel C. In all three transporter assays, some compounds that reduced substrate uptake also showed cytotoxicity. However, most drugs that inhibited transport were not cytotoxic. Drugs that showed marked cytotoxicity are labeled in each figure.
In ASBT-MDCK assay (Fig. 1A), 16 out of 21 drugs were not cytotoxic. Linear regression of data in Fig. 1A indicate no association between ASBT inhibition and cytotoxicity in MDCK cell lines (r2=0.100, p=0.163). For example, the most potent ASBT inhibitor, fluvastatin, reduced taurocholate uptake to 4.51%, yet its cell viability was 107%. The dihydropyridine subclass of calcium channel blockers (i.e. amlodipine, nisoldipine, nifedipine, and nicardipine) showed apparent inhibition and also cytotoxicity, suggesting that their cytotoxicity contributed to the apparent ASBT inhibition. For example, only 39.3% cells were viable after incubating with 500 μM amlodipine for 10 min; 100 μM nisoldipine or nifedipine reduced the mean cell viability to 56.0% and 67.7% respectively. However, because taurocholate uptake was reduced to a greater extent than cell viability, their inhibition was not solely attributed to cytotoxicity. Even after considering their cytotoxicity, these four drugs still caused approximately 30–60% inhibition, and were concluded to be inhibitors of ASBT. However, cytotoxicity was a confounding variable.
Fig. 1B shows there is no relationship between cytotoxicity in MDCK cells and OCTN2 inhibition (r2=0.001 and p=0.908). As above, amlodipine, nisoldipine, nifedipine, and nicardipine were cytotoxic to MDCK cells. In contrast to ASBT, there was a strong correlation between OCTN2 inhibition and cytotoxicity for nisoldipine, nifedipine, and nicardipine, such that these compounds’ OCTN2 inhibition was entirely due to their cytotoxicity. Therefore, these three drugs were not classed as OCTN2 inhibitors. Amlodipine however is still considered an inhibitor, in spite of 60% of cells being non-viable, as the drug reduced L-carnitine uptake over 90%.
The linear regression indicates a correlation between cytotoxicity in HEK cells and EAAT4 inhibition (r2=0.392 and p=0.002) (Fig. 1C). Thioridazine (73.9% of viable cells), tioconazole (73.5%), and clozapine (79.2%) caused modest cytotoxicity, which corresponded with percent inhibition, such that these three compounds were also non-inhibitors of EAAT4. No dihydropyridine was cytotoxic to HEK cells. Interestingly, fluvastatin reduced glutamate uptake to about 60%, without cytotoxicity.
3.2 Comparison of OCTN2 and ASBT Inhibition
The results described above were further examined in terms of drug inhibition between OCTN2 and ASBT since both transporters were stably transfected in MDCK cells. Fig. S1 (supplementary data) illustrates the relationship of inhibition between OCTN2 and ASBT. Overall, for data in Fig. S1, drug inhibition of ASBT and OCTN2 was not correlated (linear slope=0.015, r2=0.0004, and p=0.933).
3.3 Comparison of Inhibition Across Three Transporters
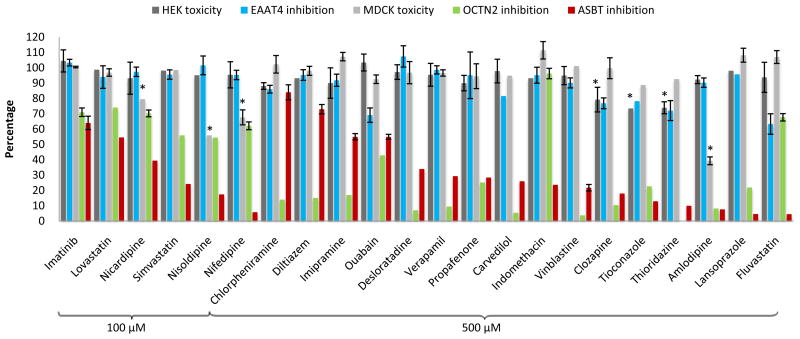
Fig. 2 re-plots cytotoxicity and inhibition results. The corresponding data are listed in Table S1 (supplementary data). For each 100 μM and 500 μM concentration, drugs are listed in order of increasing ASBT inhibition. Table 1 summarizes the conclusions of inhibitors, with consideration of the cytotoxicity effect on apparent inhibition. Drugs that only inhibited ASBT were indomethacin, nisoldipine, nicardipine, and nifedipine. The drug that only inhibited OCTN2 was chlorpheniramine. Most drugs, surprisingly, inhibited ASBT and OCTN2. Fluvastatin inhibited all three transporters.
Fig. 2.
Drug inhibition results from the three different transporters, along with cytotoxicity results. Cytotoxicity is in terms of the percentage of EAAT1-HEK cells (black bars) and ASBT-MDCK cells (grey bars) that were viable. Inhibition is in terms of the percent uptake of glutamate into EAAT4-HEK cells (blue bars), percent uptake of carnitine into OCTN2-MDCK cells (green patterned bars), and percent uptake of taurocholate into ASBT-MDCK cells (red bar), compared to no-drug control. For each inhibitor concentration (i.e. 100 μM and 500 μM), compounds are listed in order of ASBT inhibition potency from lowest to highest (left to right). * Indicates cell viability decreased 20% or more. Data are summarized as mean (SEM) of three measurements.
Table 1.
Summary of inhibition and cytotoxicity results of 21 drugs and ouabain in ASBT-MDCK, OCTN2-MDCK, and EAAT4-HEK cell lines.
| Drugs | ASBT | OCTN2 | EAAT4 | |||
|---|---|---|---|---|---|---|
| Apparent inhibition | inhibitor | Apparent inhibition | inhibitor | Apparent inhibition | inhibitor | |
| Amlodipinea | + | + | + | + | ||
| Chlorpheniramine | + | + | ||||
| Clozapineb | + | + | + | + | + | |
| Desloratadine | + | + | + | + | ||
| Diltiazem | + | + | + | + | ||
| Fluvastatin | + | + | + | + | + | + |
| Indomethacin | + | + | ||||
| Imatinib | + | + | + | + | ||
| Imipramine | + | + | + | + | ||
| Lansoprazole | + | + | + | + | ||
| Latanoprost | + | + | + | + | ||
| Nicardipinea | + | + | + | |||
| Nifedipinea | + | + | + | |||
| Nisoldipinea | + | + | + | |||
| Lovastatin | + | + | + | + | ||
| Propafenone | + | + | + | + | ||
| Simvastatin | + | + | + | + | ||
| Tioconazoleb | + | + | + | + | + | |
| Thioridazineb | + | + | + | + | + | |
| Verapamil | + | + | + | + | ||
| Vinblastine | + | + | + | + | ||
Fifteen drugs were assessed to be inhibitors of both ASBT and OCTN2 without being cytotoxic. Fluvastatin inhibited all three transporters. + denotes active. No symbol denotes not active (i.e. < 20% inhibition).
Drug caused at least 20% cytotoxicity to MDCK cells.
Drug caused at least 20% cytotoxicity to HEK cells.
Ouabain is a metabolic inhibitor that inhibits the Na+/K+ pump [8]. The Na+/K+ pump is a primary transporter and is expressed in the basolateral membrane. Inhibition of the Na+/K+ pump eliminates the sodium gradient across the cell membrane, leading to compromised functioning of sodium-dependent transporters. Taurocholate uptake was reduced about 2-fold in the presence of 500 μM ouabain. Similarly, 500 μM ouabain also reduced L-carnitine uptake into OCTN2-MDCK to 42.9% and glutamate uptake into EAAT4-HEK cells to 69.1%, without reducing cell viability in the 10 min cytotoxicity assay (Fig. 2).
4. Discussion
We have evaluated drug inhibition of three SLC sodium-dependent transporters, which possess different substrate requirements. From a pool of drugs that were known to inhibit either ASBT or OCTN2, the majority inhibited both ASBT and OCTN2. Only one drug, fluvastatin, inhibited EAAT4, suggesting the cellular membrane sodium gradient was not altered by the remaining drugs. Both ASBT and OCTN2 were stably transfected in MDCK cells, while EAAT4 was not transfected in MDCK cells but in HEK cells. For the majority of compounds, which inhibited ASBT and OCTN2 but not EAAT4, the inhibition could be caused by either specific binding to the transporters, or an interaction in MDCK cells that is not present in HEK cells.
4.1 Phenothiazines
Chlorpromazine, thioridazine, and clozapine are phenothiazines and are amphiphilic. Phenothiazines have been extensively studied in in vitro models. They express selective cytotoxicity and antiproliferative activity, and induced apoptosis in various cell lines [20,21]. Though the underlying mechanisms of these effects remain unclear, phenothiazines interact with DNA [22,23], modulate signaling pathway [24,25], and induce oxidative stress [26]. In this study, chlorpromazine, thioridazine, and clozapine reduced cell viability to 88.1%, 73.5%, and 79.2% in HEK cells, respectively, but were not cytotoxic in MDCK cells.
4.2 Dihydropyridines
Cytoxicity of dihydropyridines has not been documented in previous publications, although nicardipine can form aggregates in solution, which could cause unspecific binding to the protein [4,5]. Besides ASBT and OCTN2, dihydropyridines were also found to be potent inhibitors of P-glycoprotein (P-gp) [27], breast cancer resistance protein (BCRP) [28], Multiple drug resistance protein 1 (MRP1) [29], ATP-binding cassette transporter ABCG2 [30], equilibrative nucleoside transporters (ENT-1, ENT-2) [31], and the adenosine transporter [32]. Since nicardipine, nifedipine, nisoldipine, and amlodipine were cytotoxic in MDCK cells in the current study, studies that employ this model system for evaluation of dihydropyridines should interpret their apparent inhibition with caution.
4.3 Fluvastatin
A previously described ASBT quantitative pharmacophore was composed of one hydrogen bond acceptor, three hydrophobic features, and an additional five excluded volume features [6]. In contrast, a qualitative OCTN2 pharmacophore model consisted of three hydrophobic features and a positive ionizable feature [7]. A pharmacophore for EAAT4 is not available. However, an EAAT pharmacophore model indicates inhibitors share the same orientation of the two acids and the protonatable nitrogen, and the distance between the two carboxylic carbons may vary from 3.7 to 4.9 Å [16]. The ASBT and OCTN2 pharmacophores have been shown to reliably predict new inhibitors in our hands, but have little overlap with only multiple hydrophobic features common to both.
While EAAT4 prefers different ligands than ASBT and OCTN2, fluvastatin inhibited all three transporters without being cytotoxic. The inhibition mechanism could conceivably be modulation of the intracellular sodium gradient. Fluvastatin down-regulates the Na+/Ca2+ exchanger in cardiomyoblast H9c2 cells [33]. Fluvastatin also reduced substrate uptake in an equally modest fashion in OCTN2 and EAAT4 (approximate 35% inhibition), but was a potent inhibitor for ASBT (over 90% inhibition). While a possible fluvastatin affect on the sodium gradient cannot be excluded, previous analyses indicate fluvastatin competitively inhibits ASBT [6]. Moreover, fluvastatin may act as a promiscuous inhibitor of transporters since it has been reported to inhibit human proton-coupled small peptide carrier (hPepT1) [34], human organic anion transporters (OATs) [35], P-gp [36], and human monocarboxylate transporter 4 (MCT4) [37]. It is therefore not inconceivable that the 3,5-dihydroxyhept-6-enoic acid moiety of fluvastatin (right side) shares the features required for a EAAT4 pharmacophore, as exemplified in the glutamate structure (supplementary data Fig. S2). Meanwhile, the remaining portion of fluvastatin (left side) has features required for OCTN2 and ASBT inhibitor pharmacophores in addition to overlap with other transporter pharmacophores.
4.4 Correlation of Cytotoxicity and Inhibition
Overall, cytotoxicity did not extensively affect ASBT. Twenty drugs showed apparent inhibition and all were concluded to be inhibitors. OCTN2 inhibition results were moderately impacted by cytotoxicity. Twenty drugs showed apparent OCTN2 inhibition, but only 17 were OCTN2 inhibitors. Cytotoxicity significantly influenced EAAT4 observations. Out of four drugs that showed apparent inhibition, only one was an EAAT4 inhibitor. Of course, drug cytotoxicity can depend on drug concentrations. For example, amlodipine at 500 μM was remarkably cytotoxic in MDCK cells (39.3±2.6%), although it did not reduce cell viability at 100 μM (101±6%).
This study shows a potential association between transporter inhibitions with drug cytotoxicity. Cytotoxicity is often neglected in in vitro drug screening studies. Disregarding cytotoxicity may over-estimate the number of inhibitors obtained. Cytotoxic molecules are also false positives, in terms of pharmacophore development.
In vitro drug cytotoxicity may be variable among different cell lines. Dihydropyridines were clearly cytotoxic to MDCK cells but not to HEK cells. Meanwhile, thioridazine, tioconazole, and clozapine reduced cell viability in HEK cells but not in MDCK cells. A potential reason is differential drug metabolism, where a toxic metabolite is generated in one cell line but not another [38]. Intracellular ATP content, as the cellular energy source for apoptosis, can also be different among different cell lines and result different cytotoxicity [39]. Intracellular drug concentration is determined by passive permeability and active transporter uptake, which can vary across cell lines. Furthermore, intracellular transporters can modulate intracellular drug disposition and hence cytotoxicity [40].
Both MDCK and HEK cell lines are extensively used as an expression tool for recombinant proteins, including transporter [41,42]. Results here show cytotoxicity in one cell line cannot always predict toxicity in another cell line. Therefore, a parallel cytotoxicity assay is suggested for future transporter studies, particularly for those compounds exhibiting apparent inhibition. Recognition of the cytotoxicity mechanisms for specific cell lines may improve screening results in many areas of pharmaceutical interest.
In summary, in this study twenty drugs caused apparent inhibition in OCTN2-MDCK and ASBT-MDCK. All 20 were considered ASBT inhibitors However, the cytotoxicity of nicardipine, nifedipine, and nisoldipine was correlated with their OCTN2 inhibition, such that they were not considered inhibitors of OCTN2. Four drugs caused apparent inhibition in EAAT4-HEK. Among these four, thioridazine, tioconazole, and clozapine were cytotoxic to HEK cells and they were not EAAT4 inhibitors since their cytotoxicity corresponds to their EAAT4 inhibition. Fluvastatin was the only drug that inhibited all three transporters without being cytotoxic. While a possible affect on the sodium gradient cannot be excluded, fluvastatin inhibited ASBT in a specific manner due to its strong inhibition potency of ASBT, compared to OCTN2 and EAAT4. This is the first time it has been shown as an EAAT4 inhibitor and this may be useful as a starting point for designing additional inhibitors. Other statins such as lovastatin and simvastatin used in this study did not appreciably inhibit EAAT4. Overall, although most compounds did not cause cytotoxicity in these transporter assays, cytotoxicity did impact inhibitor determinations for OCTN2 and especially for EAAT4. To avoid false positives in transporter inhibition studies, we should therefore be vigilant and a cytotoxicity assay is suggested as an important parallel test when performing transporter inhibition, especially for potent inhibitors.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grant DK67530. The authors kindly acknowledge Drs Xin Ming and Dhiren R. Thakker (University of North Carolina-Chapel Hill) for providing the hOCTN2-MDCK cell line. The authors kindly acknowledge Svetlana Vidensky and Dr. Jeffrey D. Rothstein (Johns Hopkins University) for providing the stably transfected EAAT4-HEK cell line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang C, Ekins S, Bahadduri P, Swaan PW. Pharmacophore-based discovery of ligands for drug transporters. Adv Drug Deliv Rev. 2006;58:1431–50. doi: 10.1016/j.addr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metz JT, Huth JR, Hajduk PJ. Enhancement of chemical rules for predicting compound reactivity towards protein thiol groups. J Comput Aided Mol Des. 2007;21:139–44. doi: 10.1007/s10822-007-9109-z. [DOI] [PubMed] [Google Scholar]
- 3.Huth JR, Song D, Mendoza RR, Black-Schaefer CL, Mack JC, Dorwin SA, Ladror US, Severin JM, Walter KA, Bartley DM, Hajduk PJ. Toxicological evaluation of thiol-reactive compounds identified using a la assay to detect reactive molecules by nuclear magnetic resonance. Chem Res Toxicol. 2007;20:1752–9. doi: 10.1021/tx700319t. [DOI] [PubMed] [Google Scholar]
- 4.Coan KE, Shoichet BK. Stoichiometry and physical chemistry of promiscuous aggregate-based inhibitors. J Am Chem Soc. 2008;130:9606–12. doi: 10.1021/ja802977h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidler J, McGovern SL, Doman TN, Shoichet BK. Identification and prediction of promiscuous aggregating inhibitors among known drugs. J Med Chem. 2003;46:4477–86. doi: 10.1021/jm030191r. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, Ekins S, Raufman JP, Polli JE. Computational models for drug inhibition of the human apical sodium-dependent bile acid transporter. Mol Pharmaceutics. 2009;6:1591–603. doi: 10.1021/mp900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diao L, Ekins S, Polli JE. Novel inhibitors of human organic cation/carnitine transporter (hOCTN2) via computational modeling and in vitro testing. Pharm Res. 2009;26:1890–900. doi: 10.1007/s11095-009-9905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chipperfield AR, Whittam R. Ouabain binding to the sodium pump. Nature. 1973;242:62–3. doi: 10.1038/242062a0. [DOI] [PubMed] [Google Scholar]
- 9.Wong MH, Oelkers P, Dawson PA. Identification of a mutation in the ileal sodium-dependent bile acid transporter gene that abolishes transport activity. J Biol Chem. 1995;270:27228–34. doi: 10.1074/jbc.270.45.27228. [DOI] [PubMed] [Google Scholar]
- 10.Weinman SA, Carruth MW, Dawson PA. Bile acid uptake via the human apical sodium-bile acid cotransporter is electrogenic. J Biol Chem. 1998;273:34691–5. doi: 10.1074/jbc.273.52.34691. [DOI] [PubMed] [Google Scholar]
- 11.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem. 1998;273:20378–82. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- 12.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–51. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 13.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 14.Bohmer C, Philippin M, Rajamanickam J, Mack A, Broer S, Palmada M, Lang F. Stimulation of the EAAT4 glutamate transporter by SGK protein kinase isoforms and PKB. Biochem Biophys Res Commun. 2004;324:1242–8. doi: 10.1016/j.bbrc.2004.09.193. [DOI] [PubMed] [Google Scholar]
- 15.Baringhaus KH, Matter H, Stengelin S, Kramer W. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. II. A reliable 3D QSAR pharmacophore model for the ileal Na(+)/bile acid cotransporter. J Lipid Res. 1999;40:2158–68. [PubMed] [Google Scholar]
- 16.Mennini T, Fumagalli E, Gobbi M, Fattorusso C, Catalanotti B, Campiani G. Substrate inhibitors and blockers of excitatory amino acid transporters in the treatment of neurodegeneration: critical considerations. Eur J Pharmacol. 2003;479:291–6. doi: 10.1016/j.ejphar.2003.08.078. [DOI] [PubMed] [Google Scholar]
- 17.Balakrishnan A, Sussman DJ, Polli JE. Development of stably transfected monolayer overexpressing the human apical sodium-dependent bile acid transporter (hASBT) Pharm Res. 2005;22:1269–80. doi: 10.1007/s11095-005-5274-8. [DOI] [PubMed] [Google Scholar]
- 18.Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, Bowers WJ, Federoff HJ, Sternweis PC, Rothstein JD. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- 19.Rais R, Gonzalez PM, Zheng X, Wring SA, Polli JE. Method to screen substrates of apical sodium-dependent bile acid transporter. AAPS J. 2008;10:596–605. doi: 10.1208/s12248-008-9069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motohashi N, Kawase M, Satoh K, Sakagami H. Cytotoxic potential of phenothiazines. Curr Drug Targets. 2006;7:1055–66. doi: 10.2174/138945006778226624. [DOI] [PubMed] [Google Scholar]
- 21.Nordenberg J, Fenig E, Landau M, Weizman R, Weizman A. Effects of psychotropic drugs on cell proliferation and differentiation. Biochem Pharmacol. 1999;58:1229–36. doi: 10.1016/s0006-2952(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 22.Sakagami H, Takahashi H, Yoshida H, Yamamura M, Fukuchi K, Gomi K, Motohashi N, Takeda M. Induction of DNA fragmentation in human myelogenous leukaemic cell lines by phenothiazine-related compounds. Anticancer Res. 1995;15:2533–40. [PubMed] [Google Scholar]
- 23.Zhelev Z, Ohba H, Bakalova R, Hadjimitova V, Ishikawa M, Shinohara Y, Baba Y. Phenothiazines suppress proliferation and induce apoptosis in cultured leukemic cells without any influence on the viability of normal lymphocytes. Phenothiazines and leukemia. Cancer Chemother Pharmacol. 2004;53:267–75. doi: 10.1007/s00280-003-0738-1. [DOI] [PubMed] [Google Scholar]
- 24.Shin SY, Kim SY, Kim JH, Min DS, Ko J, Kang UG, Kim YS, Kwon TK, Han MY, Kim YH, Lee YH. Induction of early growth response-1 gene expression by calmodulin antagonist trifluoperazine through the activation of Elk-1 in human fibrosarcoma HT1080 cells. J Biol Chem. 2001;276:7797–805. doi: 10.1074/jbc.M009465200. [DOI] [PubMed] [Google Scholar]
- 25.Hadjimitova V, Bakalova R, Traykov T, Ohba H, Ribarov S. Effect of phenothiazines on protein kinase C- and calcium-dependent activation of peritoneal macrophages. Cell Biol Toxicol. 2003;19:3–12. doi: 10.1023/a:1022061513581. [DOI] [PubMed] [Google Scholar]
- 26.Eghbal MA, Tafazoli S, Pennefather P, O’Brien PJ. Peroxidase catalysed formation of cytotoxic prooxidant phenothiazine free radicals at physiological pH. Chem Biol Interact. 2004;151:43–51. doi: 10.1016/j.cbi.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Katoh M, Nakajima M, Yamazaki H, Yokoi T. Inhibitory potencies of 1,4-dihydropyridine calcium antagonists to P-glycoprotein-mediated transport: comparison with the effects on CYP3A4. Pharm Res. 2000;17:1189–97. doi: 10.1023/a:1007568811691. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Gupta A, Wang H, Zhou L, Vethanayagam RR, Unadkat JD, Mao Q. BCRP transports dipyridamole and is inhibited by calcium channel blockers. Pharm Res. 2005;22:2023–34. doi: 10.1007/s11095-005-8384-4. [DOI] [PubMed] [Google Scholar]
- 29.Zhou XF, Coburn RA, Morris ME. Effects of new 4-aryl-1,4-dihydropyridines and 4-arylpyridines on drug efflux mediated by multidrug resistance-associated protein 1. J Pharm Sci. 2005;94:2256–65. doi: 10.1002/jps.20406. [DOI] [PubMed] [Google Scholar]
- 30.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–51. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 31.Li RW, Tse CM, Man RY, Vanhoutte PM, Leung GP. Inhibition of human equilibrative nucleoside transporters by dihydropyridine-type calcium channel antagonists. Eur J Pharmacol. 2007;568:75–82. doi: 10.1016/j.ejphar.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 32.van Rhee AM, Jiang JL, Melman N, Olah ME, Stiles GL, Jacobson KA. Interaction of 1,4-dihydropyridine and pyridine derivatives with adenosine receptors: selectivity for A3 receptors. J Med Chem. 1996;39:2980–9. doi: 10.1021/jm9600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda S, Matsuoka I, Iwamoto T, Kurose H, Kimura J. Down-regulation of Na+/Ca2+ exchanger by fluvastatin in rat cardiomyoblast H9c2 cells: involvement of RhoB in Na+/Ca2+ exchanger mRNA stability. Mol Pharmacol. 2005;68:414–20. doi: 10.1124/mol.104.000786. [DOI] [PubMed] [Google Scholar]
- 34.Ekins S, Johnston JS, Bahadduri P, D’Souza VM, Ray A, Chang C, Swaan PW. In vitro and pharmacophore-based discovery of novel hPEPT1 inhibitors. Pharm Res. 2005;22:512–7. doi: 10.1007/s11095-005-2505-y. [DOI] [PubMed] [Google Scholar]
- 35.Takeda M, Noshiro R, Onozato ML, Tojo A, Hasannejad H, Huang XL, Narikawa S, Endou H. Evidence for a role of human organic anion transporters in the muscular side effects of HMG-CoA reductase inhibitors. Eur J Pharmacol. 2004;483:133–8. doi: 10.1016/j.ejphar.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Goard CA, Mather RG, Vinepal B, Clendening JW, Martirosyan A, Boutros PC, Sharom FJ, Penn LZ. Differential interactions between statins and P-glycoprotein: Implications for exploiting statins as anticancer agents. Int J Cancer. 2010 doi: 10.1002/ijc.25295. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi M, Otsuka Y, Itagaki S, Hirano T, Iseki K. Inhibitory effects of statins on human monocarboxylate transporter 4. Int J Pharm. 2006;317:19–25. doi: 10.1016/j.ijpharm.2006.02.043. [DOI] [PubMed] [Google Scholar]
- 38.Lash LH, Putt DA, Cai H. Drug metabolism enzyme expression and activity in primary cultures of human proximal tubular cells. Toxicology. 2008;244:56–65. doi: 10.1016/j.tox.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–40. [PubMed] [Google Scholar]
- 40.Govindarajan R, Leung GP, Zhou M, Tse CM, Wang J, Unadkat JD. Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol. 2009;296:G910–G922. doi: 10.1152/ajpgi.90672.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Braun A, Hammerle S, Suda K, Rothen-Rutishauser B, Gunthert M, Kramer SD, Wunderli-Allenspach H. Cell cultures as tools in biopharmacy. Eur J Pharm Sci. 2000;11 (Suppl 2):S51–S60. doi: 10.1016/s0928-0987(00)00164-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.