Abstract
“Oh, Jerusalem of gold, and of light, and of bronze…” goes the popular song. But it was another metal that towered above the Jerusalem landscape during the meeting of the International Society for Zinc Biology (ISZB; http://www.iszb.org/), held at Mishkenot Sha’ananim, a whisper away from the Old City walls. More than 100 scientists gathered on 1 to 5 December 2009 to discuss their research on the biology of this metal. Zinc is a double-edged sword. Zinc supplementation accelerates wound healing and growth and promotes an effective immune response. On the other hand, zinc deficiency leads to growth retardation and impaired learning and memory function, and has been linked to mood disorders. At the cellular level, however, uncontrolled increases in zinc concentrations can lead to neuronal cell death and may be involved in neurodegenerative disorders. Through regulation of various intracellular signaling pathways, zinc can accelerate cell growth and possibly contribute to cancer. However, despite the physiological and clinical importance of this metal, research on the molecular basis of these effects is still in its infancy. The 2009 ISZB meeting provided a venue for investigators working on various zinc-related issues to share their thoughts and ideas and to promote the growth of this field.
Introduction
After welcoming remarks by ISZB president Glen Andrews (University of Kansas Medical Center, USA), the meeting began with a short overview of the zinc field by Israel Sekler (Ben Gurion University, Israel) highlighting the major recent discoveries and unsolved questions and challenges that lie ahead. In the first plenary lecture, Ilana Gozes (Tel Aviv University, Israel) described the properties of the zinc-binding peptide NAP, an 8-amino-acid fragment of the activity-dependent neuroprotective protein (ADNP), which is currently undergoing clinical trials for treating Alzheimer’s disease (1). In the second plenary lecture, Bruce Pitt (University of Pittsburgh, USA) focused on the role of zinc and nitric oxide signaling in endothelial cells. He showed that release of zinc from metallothioneins during hypoxia leads to protein kinase C (PKC)–dependent formation of stress fibers that are associated with vascular pulmonary constriction (2).
Zinc Effects on Mood Disorders and Disease States
The meeting continued with talks on the behavioral effects of zinc deficiency, with a focus on depression and depression-related disorders such as anorexia, anxiety, and anhedonia, and the use of zinc as an adjunct to antidepressant therapy. Behavioral effects of zinc have been characterized in humans and experimental animal models (3). Although the potential effects of zinc supplementation in antidepressant treatment has been tested in humans (4), the cellular and molecular mechanisms responsible for the metal’s therapeutic effects are not well understood. Gabriel Nowak (Polish Academy of Sciences, Poland) described the interaction of zinc with serotonin and glutamate receptors, which may cause antidepressant effects (5). John Beattie (Rowett Research Institute, Scotland) described a connection between zinc status, metallothioneins, and secretion of leptin, a hormone that is linked to appetite and metabolism (6). Finally, Cathy Levenson (Florida State University College of Medicine, USA) described how dietary zinc deficiency leads to a p53-dependent decrease in neuronal stem cells proliferation that is associated with depression (7). The role of zinc in cognitive impairment was addressed by Allan Rofe (Hanson Institute, Australia), who showed that the administration of the bacterial endotoxin lipopolysaccharide to pregnant rats caused fetal zinc deficiency, resulting in neuronal cell death and long-term behavioral changes that could be reversed by zinc supplementation (8).
Ananda Prasad (Wayne State University, USA) emphasized in his presentation that subacute zinc deficiencies lead to decreased binding of the transcription factor nuclear factor κB (NF-κB) to DNA as well as decreased interleukin 2 (IL-2) concentrations and IL-2 receptor α abundance in T helper cells (9, 10), thereby accounting for decreased Th1 cytokine function. Besides its effect on cell-mediated immunity, zinc also functions as an antioxidant and anti-inflammatory agent. Fred Askari (University of Michigan, USA) compared studies using zinc homeostasis as maintenance therapy in Wilson’s disease patients. These studies provide insight into the molecular basis of Wilson’s disease, which is caused by a mutation in the gene encoding the copper transporting ATPase, ATP7B (11). Zinc induces production of metallothioneins in the intestine, which bind copper and prevent absorption. Robert Black (Johns Hopkins University, USA) described the success of zinc supplementation in the treatment of childhood diarrheas, a leading cause of death in Third World countries (12). At the cellular level, David Soybel (Harvard Medical School, USA) presented data demonstrating that zinc transport in the stomach is regulated by acid secretion (13), suggesting that inhibition of acid secretion with conventional proton-pump inhibitors may affect zinc homeostasis in the stomach and downstream digestive activities of the gastrointestinal tract. Impaired zinc homeostasis also plays a role in retinal pathologies. Imre Lengyel (University College London, UK) explained that accumulation of zinc in age-related macular degeneration results in aggregation and inhibition of complement factor H, leading to enhanced complement activation and inflammation and ultimately to retinal damage (14).
Zinc Transporters and Their Role in Normal and Disease States
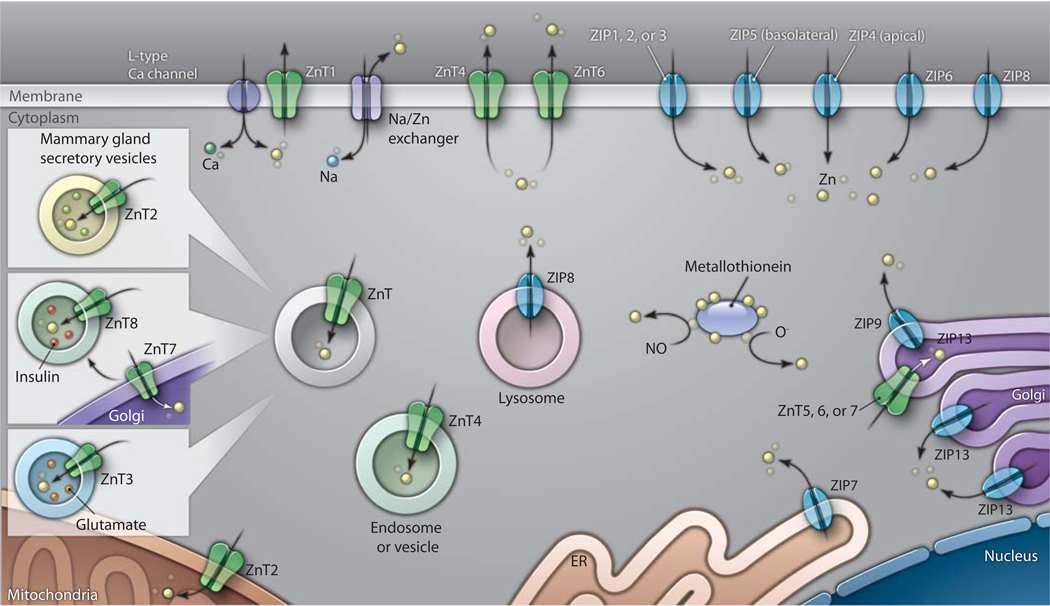
Identification of zinc transporters and investigation into their function and regulation provides insight into the molecular link between these transporters and diseases. Two major zinc transporter families have been described, ZIP (Zrt/Irt-like protein, also known as the SLC39 protein family) and ZnT (zinc transporter, also known as SLC30) (Fig. 1). The ZIP family mediates zinc influx into cells and out of intracellular organelles. Of the 10 ZnT family members that have been identified, all except ZnT1 are found primarily on intracellular membranes, where they control cytoplasmic and organellar zinc concentrations. These transporters promote cell survival as well as regulate zinc availability to zinc-binding proteins (15–17). The distribution and function of the zinc transporter proteins is regulated, as was described in several presentations during this meeting, and changes in their distribution pattern or function have been linked to multiple diseases. ZnT8 is localized to insulin-containing vesicles, and a polymorphism in ZnT8 (Arg325→Trp; R325W) has been linked to susceptibility to type 2 diabetes (18). Guy Rutter (Imperial College, UK) described packaging of pancreatic insulin and glucose and insulin homeostasis in ZnT8 knockout (KO) mice. Intriguingly, glucose resistance was found in the ZnT8 KO mice. He also provided data showing that the R325W polymorphism increases zinc transport activity (19). Giuditta Perozzi (National Research Institute for Food and Nutrition, Italy) discussed homodimerization of ZnT8 and demonstrated that murine ZnT8 is found not only in pancreatic β cells but also in other secretory cell types (20). Christian Sheline (Louisiana State University Health Sciences Center, USA) discussed mechanisms by which impaired zinc homeostasis is associated with both type 1 and type 2 diabetes. He further presented data from in vivo studies indicating that high ZnT8 abundance enhances susceptibility of mice developing streptozotocin- and high-fat diet–induced diabetes. Finally, Irina Korichneva (New Jersey–Robert Wood Johnson Medical School, USA) provided data indicating that zinc excess or deficiency promotes proteolysis of ZnT5 in cardiomyocytes from diabetic rats, which induces alterations in intracellular zinc concentrations and leads to increased PKCε abundance and decreased PKCδ abundance (21). Liping Huang (Western Human Nutrition Research Center, USA) showed that ablation of ZnT7 in mice caused reduced food intake and poor growth, leading to a prediabetic state (22). Furthermore, ZnT7 is also present in β cells in the islets of Langerhans, where it may regulate zinc accumulation and insulin biosynthesis. Insulin secretion may also be indirectly affected by a feedback mechanism in which zinc ions, which are cosecreted with insulin, enter β cells and inhibit cyclic adenosine monophosphate (cAMP) synthesis in β cells (23).
Fig. 1.
Zinc transporters and buffers. Shown is a generic cell with the major intracellular and plasma membrane zinc transporters and buffers, which are found on multiple intracellular organelles and regulate cellular zinc concentrations.
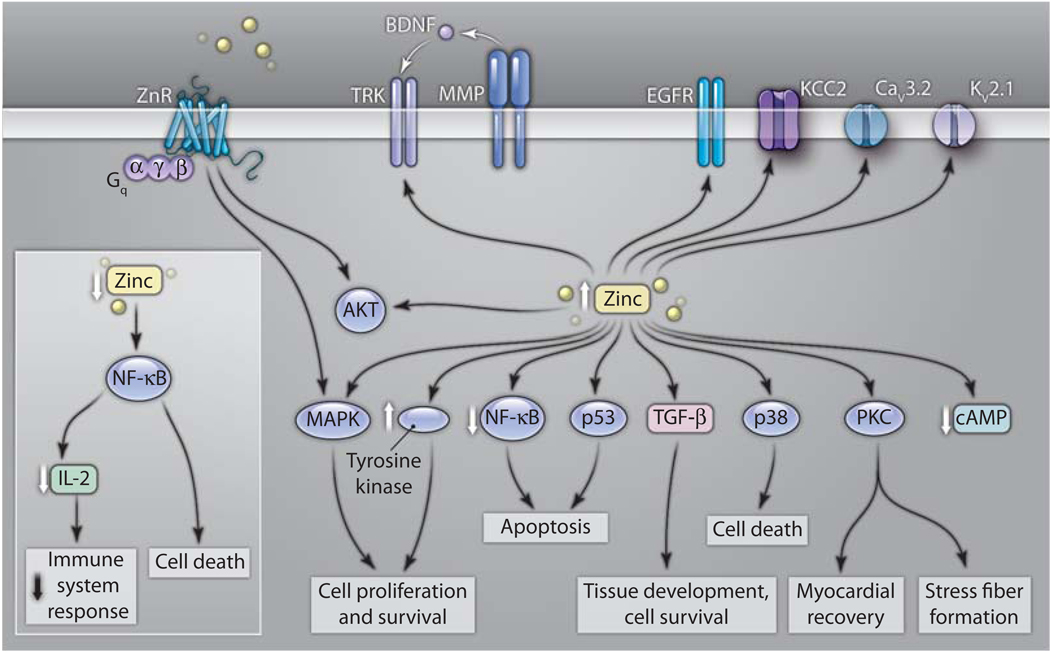
Changes in cytoplasmic or organellar zinc concentrations mediated by zinc transporters affect major cellular signaling pathways and diverse physiological activities (Fig. 2). A presentation by Hajo Haase (Aachen University Hospital, Germany) addressed the diverse effects of zinc signaling on the immune system. Distinct types of zinc signals can regulate the activity of different subsets of signaling proteins (24), which in turn control processes such as cell maturation and development, or the response to pathogens (25, 26). Lothar Rink (Aachen University Hospital, Germany) discussed measurements of labile zinc pools in immune cells and the effects of zinc on signaling pathways such as regulation of cAMP generation, which is involved in immune cell differentiation and function (24). Prathiba Joshi (Emory University School of Medicine, USA) showed that chronic alcohol ingestion or HIV-1 infection reduces zinc concentrations as well as the abundance of ZIP1 and ZnT4 in alveolar macrophages, leading to impaired phagocytosis and increased lung infections (27). Another intriguing aspect of zinc signaling is the link to inflammatory responses. Using in vitro and in vivo sepsis models, Daren Knoell (Davis Heart and Lung Research Institute, USA) showed that Zip8 expression is regulated by the transcription factor NF-κB and is vital in cell survival and innate immune defense (28).
Fig. 2.
Major signaling pathways regulated by intracellular and extracellular zinc. Arrows indicate the up-regulation or down-regulation of these pathways after changes in intracellular or extracellular zinc. The physiological roles of these pathways are also presented. BDNF, brain-derived neurotrophic factor; ZnR is also known as GPR39.
A link between intracellular zinc, cancer, and cell growth was presented by Kathryn Taylor (Tenovus Centre, Wales), who showed that phosphorylation of ZIP family members increases zinc release from stores, which in turn enhances the proliferation and survival of estrogen-resistant breast cancer cells (29). Furthermore, zinc homeostasis in these breast cancer cells is determined by the ability of ZIP7 to shift zinc from the endoplasmic reticulum (ER) to other cell compartments (16, 30). Christer Hogstrand (King’s College, UK) demonstrated that ZIP7-mediated zinc release from the ER is essential for activation of tyrosine kinases that promote proliferation, such as Src, epidermal growth factor receptor (EGFR), and insulin-like growth factor 1 (IGF1) receptor (30). Silencing of ZIP7 results in blocked growth factor responses and decreased cell growth, suggesting that ZIP7 may control intracellular zinc-dependent activation of cellular biochemical pathways related to cancer. Furthermore, the plasma membrane zinc importers, ZIP6 and ZIP10, whose abundance is increased in breast cancers, mediate cell detachment and migration through activation of AKT and glycogen synthase-kinase-3β (GSK-3β). This in turn leads to decreased E-cadherin abundance and enhanced metastasis (31). Jung Hwang (University of Ulsan College of Medicine, South Korea) demonstrated that tamoxifen-induced zinc accumulation in lysosomes can cause cell death in breast cancer cells by initiating autophagy. Similarly, oxidative stress triggers lysosomal zinc accumulation in neurons and leads to cell death (32). Veronica Lopez (Pennsylvania State University, USA) described zinc-dependent survival of breast tumor cells, which show increased abundance of ZIP6 and ZnT2 in these cells, although only attenuation of ZnT2 reduced tumor colony formation (31). Rosa Puca (National Cancer Institute of Rome, Italy) demonstrated a previously unknown role of zinc in regulating the activity of p53 and the homeodomain-interacting protein kinase 2 (HIPK2), which in turn altered cell sensitivity to chemotherapy and tumor growth (33). Zinc signaling was also directly linked to the clinical setting by the use of zinc ionophores. Daren Magda (Pharmacyclics, USA) showed that several versions of the water-soluble zinc ionophore 1-hydroxypyridine-2-thione (ZnHPT) inhibited growth of lung and prostate cancer cells in xenografted mice (34). The role of ZIP1 and zinc permeation in prostate cancer cells was also discussed by Vladimir Kolenko (Fox Chase Cancer Center, USA), who showed that concentrations of zinc and abundance of ZIP1, ZIP2, and ZIP3 decline during progression of prostate cancer, leading to increased activity of NF-κB. Furthermore, ZIP1 overexpression suppressed NF-κB activity and sensitized cultured prostate cancer cells to apoptosis (35, 36).
A series of presentations described pathophysiologies associated with improper distribution or function of these transporters. ZnT2 is found in secretory vesicles in the mammary gland and is responsible for zinc content in milk (37). Studies of ZnT2 function in the mammary gland were presented by Shannon Kelleher (Pennsylvania State University, USA), who identified a mutation in the maternal ZnT2 gene that causes transient neonatal zinc deficiency in humans. She also showed that two isoforms of this protein were localized either to the endosomal and secretory compartment or to the plasma membrane (38). Studies on Zip13 were presented by Toshyuki Fukada (RIKEN Research Center for Allergy and Immunology, Japan), who showed that Zip13 ablation in mice causes reduced maturation of connective tissue cells, leading to malformation of cartilage, bone, and teeth (39). He further showed the involvement of this zinc transporter in bone morphogenetic protein (BMP) and transforming growth factor β (TGF-β) signaling. In addition, mutations in this gene were found in patients with a unique variant of Ehlers-Danlos syndrome, who exhibit clinical symptoms similar to those of the KO mice. Among the zinc transporters with a clear clinical link is Zip4. Humans with acrodermatitis enteropathica have multiple mutations in Zip4 (40, 41). Glen Andrews (University of Kansas Medical Center, USA) showed that mice heterozygous for Zip4 were more sensitive to dietary zinc deficiency. Andrews previously discovered increased abundance of Zip4 in various cancers, and in this context, ZIP4 may suppress apoptosis and enhance cell cycle and invasive behavior. Taiho Kambe (Kyoto University) showed that cell surface mouse ZIP4 was proteolytically cleaved during conditions of prolonged zinc deficiency (42), a process that required an intact endocytotic pathway and left the remaining C-terminal half of ZIP4 still functional as a zinc transporter. Two ZipP4 mutants that block the processing of this transporter are found in Acrodermatitis enteropathica patients. Zinc deficiency increases Zip4 expression in the gut and decreases Zip5 expression in the yolk sac (43). Ben Weaver (University of Kansas Medical Center, USA) identified two candidate zinc-responsive miRNAs that increase Zip5 expression during zinc repletion. Finally, Bing Zhou (Tsinghua University of Beijing, China) presented the use of Drosophila as a model system to study the physiological function of zinc transport proteins. His studies of ZnT1 KO flies revealed a role for this protein in dietary absorption of zinc (44).
Zinc in the Nervous System
In the nervous system, zinc is packaged by a specific transporter (ZnT3) into synaptic vesicles (45). Intracellular zinc that is bound to proteins, such as metallothioneins, is liberated during injurious conditions (46). John Weiss (University of California Davis, USA) discussed how impaired zinc homeostasis and mitochondrial dysfunction lead to calcium deregulation in an in vitro model of brain ischemia (47). A new angle on zinc-triggered apoptosis and the intersection between zinc homeostasis, potassium efflux, and oxidative stress (48) was presented by Patrick Redman (University of Pittsburgh School of Medicine, USA). Redman showed that zinc modulates the insertion of the voltage-dependent K+ channel Kv2.1 into the plasma membrane by coordinating the inactivation of protein tyrosine phosphatase ε with the activation of the kinase p38. Both of these events are necessary for the increased K+ current detected in neurons dying by apoptosis. During ischemia, increased intracellular zinc concentrations also induce calcineurin-dependent dephosphorylation of Kv2.1, which causes dispersal of channel clusters on the plasma membrane. This phenomenon is accompanied by a hyperpolarizing shift in the voltage-dependent activation of delayed-rectifier potassium currents, which may be neuroprotective (49). The K+/Cl− cotransporter KCC2 is inhibited by the increased intracellular zinc concentrations that occur after oxygen-glucose deprivation. This leads to a depolarizing shift in the GABA reversal potential, which may contribute to neuronal injury (50). A role for intracellular zinc as a trigger of neuronal and glial autophagy (32) was described by Jae Koh (University of Ulsan College of Medicine, South Korea). Increased neuronal zinc concentrations are also associated with cell death during epileptogenesis and may enhance the severity of seizures (51). This may be caused, as demonstrated by Yoel Yaari (Hebrew University, Israel), by a zinc-dependent increase in mRNA abundance of and current mediated by the Cav3.2 T-type calcium channel, which is implicated in epileptogenesis (52). Albert Becker (University of Bonn Medical Center, Germany) further showed that in this process zinc activates metal-responsive transcription factor-1 (MTF-1), which then interacts with metal-regulatory elements in the promoter region of Cav3.2. Increased zinc concentrations have also been noted during brain ischemia. William Shuttleworth (University of New Mexico School of Medicine, USA) showed that substantial zinc release from neurons and astrocytes occurs after episodes of spreading depression and may contribute to neurodegeneration in the postischemic period (53). Sangwon Suh (University of California San Francisco, USA) showed that zinc translocation across the synapse contributes to neuronal cell death after ischemia (54). Jonathan Friedman (D-Pharm, Israel) presented studies describing how the lipophilic divalent transition metal chelator DP-b99 decreases zinc concentrations and thus zinc-dependent events, such as the activity of matrix metalloprotease 9 (MMP9) and TNF-α–converting enzyme TACE (55, 56).
The possible roles of zinc in Alzheimer’s disease (AD) and drugs that can alleviate the associated cognitive impairment by modulating zinc concentrations in distinct neuronal and brain regions were discussed. Excessive increases in zinc concentrations have been associated with the onset of AD and neurodegeneration. Jorge Busciglio (University of California Irvine, USA) demonstrated that the formation of β-amyloid (Aβ) oligomers can be disrupted by the metal chelator clioquinol (CQ), which decreases the amount of zinc that is synaptically released (57). Irit Sagi (The Weizmann Institute, Israel) described the structural basis for the toxic, aggregate-promoting effect of zinc on Aβ peptides (58). David Finkelstein (The Mental Health Research Institute, Australia) further showed that CQ also reverses the hyperphosphorylation of tau and increases the abundance of the synaptic protein synaptophysin (59). These changes were associated with improvements in spatial learning and memory retention in mouse AD models. Kevin Barnham (University of Melbourne, Australia) provided surprising evidence that AD-associated brain damage is not simply caused by excessive concentrations of zinc and copper in the plaques, but is also associated with zinc deficiency within neurons (59). He further showed that a CQ-based compound, PTB2, acts as a metal carrier and supplements neurons with zinc and copper, which inhibits GSK-3β, thereby attenuating hyperphosphorylation of tau and activating metal-dependent proteases to enhance Aβ degradation. Because zinc deficiency is associated with impaired learning and memory, the previous finding that ZnT3 KO mice, which are deficient in synaptic zinc, have no learning or memory deficits, was surprising. However, Paul Adlard (The Mental Health Research Institute, Australia) showed that older ZnT3 KO mice do manifest cognitive impairments associated with synaptic dysfunction (60), indicating that synaptic zinc may be required to prevent aging-related learning deficits.
Synaptic zinc is released from glutamatergic nerve terminals in an activity- and Ca-dependent manner (61). There is an ongoing debate on how synaptically released zinc affects synaptic transmission and plasticity (62, 63). This ISZB meeting provided an opportunity for investigators to describe advances in our understanding of the role of synaptic zinc in the normal brain. Richard Dyck (University of Calgary, Canada) provided evidence of dynamic, experience-dependent modulation of zinc concentrations within synaptic terminals and vesicles of cerebral cortical neurons (64). Using mice with a mutant NR2A subunit that renders NMDA receptors insensitive to zinc modulation, Angela Vergnano (Ecole Normale Supérieure, France) provided evidence that zinc blocks postsynaptic NMDA receptors in the CA1 region of the hippocampus (65, 66). Arnaud Ruiz (University of London, UK) discussed a different pathway in which zinc released from the dentate gyrus mossy fibers can indirectly modulate GABAergic neuronal function through a pathway involving interneurons in the hippocampus (67) and thereby modulate feedback inhibition to the dentate granule cells. A metabotropic pathway activated by synaptic zinc through a zinc-sensing G protein–coupled receptor activates postsynaptic neuronal signaling pathways, in particular those mediated by mitogen-activated protein kinases (MAPK) and Ca2+/calmodulin-dependent protein kinase (CAMKII) (68). Atsushi Takeda (University of Shizuoka, Japan) provided additional evidence of a modulatory role for zinc in the hippocampus with his data indicating a role for zinc in the development of long-term potentiation in the hippocampus (69).
Tools for the Detection of Zinc in Biological Systems and Modeling of Zinc Homeostasis
Several methods and applications for detecting “mobile” or “free” zinc (zinc not bound to proteins) in cells and organisms were introduced. Steve Lippard (Massachusetts Institute of Technology, USA) described the application of fluorescent sensors that can quantify the release of mobile zinc (70, 71) to study the release of pancreatic or synaptic zinc. Zijan Guo (Nanjing University, China) then described the use of a ratiometric zinc indicator in developing zebrafish embryos (72). Elisa Tomat (Massachusetts Institute of Technology, USA) described the coupling of zinc dyes to proteins targeted to specific organelles (73). Zhang and Lippard presented the first intracellular MRI sensor for zinc, which can be imaged in the brains of live rodents. Using two-photon and fluorescence microscopy as well as x-ray imaging to dynamically monitor the subcellular localization of zinc, Christoph Fahrni (Georgia Institute of Technology, USA) demonstrated that changes in zinc concentrations accompany various phases of the cell cycle (74). Finally, Carol Fierke (University of Maryland School of Medicine, USA) described a new x-ray and fluorescence microprobe for imaging zinc that revealed the subcellular distribution of zinc in yeast cells (75).
The large number of zinc proteins and their complex interactions suggests that an understanding of protein networks will be required to fully understand their physiological roles. The importance of considering quantitative approaches to understanding cellular zinc homeostasis was explored in a talk by Robert Colvin (Ohio University, USA), who reviewed computational models of zinc homeostasis. He incorporated modeling of metallothionein as a cellular buffer for zinc (76); similar dynamics of zinc coordination and cellular distribution (77) are also suggested by work from Wolfgang Maret (King’s College of London, UK). Wojciech Bal (Polish Academy of Sciences, Poland) described quantitative estimates with intracellular fluorophores of the impact of small molecules on “free” zinc concentrations, emphasizing the relevance of zinc-binding proteins to the actual concentrations of intracellular zinc (78). Hans-Werner Adolph (University of Copenhagen, Denmark) produced several simulations using available data to illustrate errors that can occur when using equilibrium-binding constants to describe “shuttling” of zinc between binding proteins due to conformational changes (79). Finally, Antonio Rosato (University of Florence, Italy) gave an update of current progress on annotating the cellular zinc proteome and toward using that information to describe cellular zinc metallomics and evolutionary relationships in metallomes (80).
Conclusion
The ISZB 2009 meeting described the growing catalog of roles that zinc plays in cell signaling. Previously unknown regulatory and catalytic mechanisms for regulation of zinc homeostasis and detailed characterization of proteins that mediate zinc transport were reported. New tools for monitoring changes in zinc cellular concentration and its localization at the subcellular or whole animal level were presented. Empirical descriptions of zinc deficiency and its symptoms are now being replaced by mechanistic and molecular insights. Transgenic and knockout models of the zinc homeostatic proteins elucidate the specific roles of these pathways. Promising drugs have been developed for rectifying impaired zinc homeostasis and are currently being tested for the treatment of Alzheimer’s disease, diabetes, diarrhea, and stroke. We are confident that the fruits of the collaborations and projects emanating from this meeting will be presented at the next meeting of the ISZB in January 2012 in Melbourne, Australia.
Footnotes
A report on the Meeting of the International Society for Zinc Biology, Jerusalem, Israel, 1 to 5 December 2009.
References and Notes
- 1.Gozes I, Stewart A, Morimoto B, Fox A, Sutherland K, Schmeche D. Addressing Alzheimer’s disease tangles: From NAP to AL-108. Curr. Alzheimer Res. 2009;6:455–460. doi: 10.2174/156720509789207895. [DOI] [PubMed] [Google Scholar]
- 2.Bernal PJ, Leelavanichkul K, Bauer E, Cao R, Wilson A, Wasserloos KJ, Watkins SC, Pitt BR, St Croix CM. Nitric-oxide-mediated zinc release contributes to hypoxic regulation of pulmonary vascular tone. Circ. Res. 2008;102:1575–1583. doi: 10.1161/CIRCRESAHA.108.171264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tassabehji NM, Corniola RS, Alshingiti A, Levenson CW. Zinc deficiency induces depression-like symptoms in adult rats. Physiol. Behav. 2008;95:365–369. doi: 10.1016/j.physbeh.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Siwek M, Dudek D, Paul IA, Sowa-Kucma M, Zieba A, Popik P, Pilc A, Nowak G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: A double blind, placebo-controlled study. J. Affect. Disord. 2009;118:187–195. doi: 10.1016/j.jad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Cichy A, Sowa-Kucma M, Legutko B, Pomierny-Chamiolo L, Siwek A, Piotrowska A, Szewczyk B, Poleszak E, Pilc A, Nowak G. Zinc-induced adaptive changes in NMDA/glutamatergic and serotonergic receptors. Pharmacol. Rep. 2009;61:1184–1191. doi: 10.1016/s1734-1140(09)70182-3. [DOI] [PubMed] [Google Scholar]
- 6.Kwun IS, Cho YE, Lomeda RA, Kwon ST, Kim Y, Beattie JH. Marginal zinc deficiency in rats decreases leptin expression independently of food intake and corticotrophin-releasing hormone in relation to food intake. Br. J. Nutr. 2007;98:485–489. doi: 10.1017/S0007114507730763. [DOI] [PubMed] [Google Scholar]
- 7.Corniola RS, Tassabehji NM, Hare J, Sharma G, Levenson CW. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res. 2008;1237:52–61. doi: 10.1016/j.brainres.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 8.Coyle P, Tran N, Fung JN, Summers BL, Rofe AM. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav. Brain Res. 2009;197:210–218. doi: 10.1016/j.bbr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Prasad AS. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao B, Prasad A, Beck FW, Suneja A, Sarkar F. Toxic effect of zinc on NF-kappaB, IL-2, IL-2 receptor alpha, and TNF-alpha in HUT-78 (Th(0)) cells. Toxicol. Lett. 2006;166:222–228. doi: 10.1016/j.toxlet.2006.07.306. [DOI] [PubMed] [Google Scholar]
- 11.Brewer GJ, Askari F, Lorincz MT, Carlson M, Schilsky M, Kluin KJ, Hedera P, Moretti P, Fink JK, Tankanow R, Dick RB, Sitterly J. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch. Neurol. 2006;63:521–527. doi: 10.1001/archneur.63.4.521. [DOI] [PubMed] [Google Scholar]
- 12.Chang S, El Arifeen S, Bari S, Wahed MA, Rahman KM, Rahman MT, Mahmud AB, Begum N, Zaman K, Baqui AH, Black RE. Supplementing iron and zinc: Double blind, randomized evaluation of separate or combined delivery. Eur. J. Clin. Nutr. 2010;64:153–160. doi: 10.1038/ejcn.2009.127. [DOI] [PubMed] [Google Scholar]
- 13.Kohler JE, Mathew J, Tai K, Blass AL, Kelly E, Soybel DI. Monochloramine impairs caspase-3 through thiol oxidation and Zn2+ release. J. Surg. Res. 2009;153:121–127. doi: 10.1016/j.jss.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nan R, Gor J, Lengyel I, Perkins SJ. Uncontrolled zinc- and copper-induced oligomerisation of the human complement regulator factor H and its possible implications for function and disease. J. Mol. Biol. 2008;384:1341–1352. doi: 10.1016/j.jmb.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Matsuura W, Yamazaki T, Yamaguchi-Iwai Y, Masuda S, Nagao M, Andrews GK, Kambe T. SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: Characterization of the ZIP subfamily I protein in vertebrate cells. Biosci. Biotechnol. Biochem. 2009;73:1142–1148. doi: 10.1271/bbb.80910. [DOI] [PubMed] [Google Scholar]
- 16.Taylor KM. A distinct role in breast cancer for two LIV-1 family zinc transporters. Biochem. Soc. Trans. 2008;36:1247–1251. doi: 10.1042/BST0361247. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T, Ishihara K, Migaki H, Ishihara K, Nagao M, Yamaguchi-Iwai Y, Kambe T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J. Biol. Chem. 2005;280:30956–30962. doi: 10.1074/jbc.M506902200. [DOI] [PubMed] [Google Scholar]
- 18.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 19.Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, da Silva Xavier G, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murgia C, Devirgiliis C, Mancini E, Donadel G, Zalewski P, Perozzi G. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr. Metab. Cardiovasc. Dis. 2009;19:431–439. doi: 10.1016/j.numecd.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Karagulova G, Yue Y, Moreyra A, Boutjdir M, Korichneva I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J. Pharmacol. Exp. Ther. 2007;321:517–525. doi: 10.1124/jpet.107.119644. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Yu YY, Kirschke CP, Gertz ER, Lloyd KK. Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J. Biol. Chem. 2007;282:37053–37063. doi: 10.1074/jbc.M706631200. [DOI] [PubMed] [Google Scholar]
- 23.Dyachok O, Idevall-Hagren O, Sågetorp J, Tian G, Wuttke A, Arrieumerlou C, Akusjärvi G, Gylfe E, Tengholm A. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab. 2008;8:26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- 25.Dubben S, Hönscheid A, Winkler K, Rink L, Haase H. Cellular zinc homeostasis is a regulator in monocyte differentiation of HL-60 cells by 1 alpha, 25-dihydroxyvitamin D3. J. Leukoc. Biol. 2010;87:833–844. doi: 10.1189/jlb.0409241. [DOI] [PubMed] [Google Scholar]
- 26.Haase H, Ober-Blöbaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J. Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- 27.Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am. J. Respir. Cell Mol. Biol. 2009;41:207–216. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cyto-protection in lung epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L1127–L1136. doi: 10.1152/ajplung.00057.2008. [DOI] [PubMed] [Google Scholar]
- 29.Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology. 2008;149:4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 30.Hogstrand C, Kille P, Nicholson RI, Taylor KM. Zinc transporters and cancer: A potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 2009;15:101–111. doi: 10.1016/j.molmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Lopez V, Kelleher SL. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp. Cell Res. 2010;316:366–375. doi: 10.1016/j.yexcr.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Hwang JJ, Lee SJ, Kim TY, Cho JH, Koh JY. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J. Neurosci. 2008;28:3114–3122. doi: 10.1523/JNEUROSCI.0199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puca R, Nardinocchi L, Sacchi A, Rechavi G, Givol D, D’Orazi G. HIPK2 modulates p53 activity towards pro-apoptotic transcription. Mol. Cancer. 2009;8:85. doi: 10.1186/1476-4598-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magda D, Lecane P, Wang Z, Hu W, Thiemann P, Ma X, Dranchak PK, Wang X, Lynch V, Wei W, Csokai V, Hacia JG, Sessler JL. Synthesis and anticancer properties of water-soluble zinc ionophores. Cancer Res. 2008;68:5318–5325. doi: 10.1158/0008-5472.CAN-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golovine K, Makhov P, Uzzo RG, Shaw T, Kunkle D, Kolenko VM. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-kappaB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin. Cancer Res. 2008;14:5376–5384. doi: 10.1158/1078-0432.CCR-08-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makhov P, Golovine K, Uzzo RG, Wuestefeld T, Scoll BJ, Kolenko VM. Transcriptional regulation of the major zinc uptake protein hZip1 in prostate cancer cells. Gene. 2009;431:39–46. doi: 10.1016/j.gene.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowanadisai W, Lönnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 38.Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem. J. 2009;422:43–52. doi: 10.1042/BJ20081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, Higashiyama H, Idaira Y, Asada Y, Kitamura H, Yamasaki S, Hojyo S, Nakayama M, Ohara O, Koseki H, Dos Santos HG, Bonafe L, Ha-Vinh R, Zankl A, Unger S, Kraenzlin ME, Beckmann JS, Saito I, Rivolta C, Ikegawa S, Superti-Furga A, Hirano T, Isalan M. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development: Its involvement in BMP/TGF-beta signaling pathways. PLoS ONE. 2008;3:e3642. doi: 10.1371/journal.pone.0003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews GK. Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochem. Soc. Trans. 2008;36:1242–1246. doi: 10.1042/BST0361242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dufner-Beattie J, Weaver BP, Geiser J, Bilgen M, Larson M, Xu W, Andrews GK. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum. Mol. Genet. 2007;16:1391–1399. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- 42.Kambe T, Andrews GK. Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol. Cell. Biol. 2009;29:129–139. doi: 10.1128/MCB.00963-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biol. Chem. 2007;388:1301–1312. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Wu Y, Zhou B. Dietary zinc absorption is mediated by ZnT1 in Drosophila melanogaster. FASEB J. 2009;23:2650–2661. doi: 10.1096/fj.08-126649. [DOI] [PubMed] [Google Scholar]
- 45.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aizenman E, Stout AK, Hartnett KA, Dineley KE, McLaughlin B, Reynolds IJ. Induction of neuronal apoptosis by thiol oxidation: Putative role of intracellular zinc release. J. Neurochem. 2000;75:1878–1888. doi: 10.1046/j.1471-4159.2000.0751878.x. [DOI] [PubMed] [Google Scholar]
- 47.Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J. Neurosci. 2009;29:1105–1114. doi: 10.1523/JNEUROSCI.4604-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redman PT, Hartnett KA, Aras MA, Levitan ES, Aizenman E. Regulation of apoptotic potassium currents by coordinated zinc-dependent signalling. J. Physiol. 2009;587:4393–4404. doi: 10.1113/jphysiol.2009.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aras MA, Saadi RA, Aizenman E. Zn2+ regulates Kv2.1 voltage-dependent gating and localization following ischemia. Eur. J. Neurosci. 2009;30:2250–2257. doi: 10.1111/j.1460-9568.2009.07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hershfinkel M, Kandler K, Knoch ME, Dagan-Rabin M, Aras MA, Abramovitch-Dahan C, Sekler I, Aizenman E. Intracellular zinc inhibits KCC2 transporter activity. Nat. Neurosci. 2009;12:725–727. doi: 10.1038/nn.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riba-Bosch A, Pérez-Clausell J. Response to kainic acid injections: Changes in staining for zinc, FOS, cell death and glial response in the rat forebrain. Neuroscience. 2004;125:803–818. doi: 10.1016/j.neuroscience.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y, Beck H. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J. Neurosci. 2008;28:13341–13353. doi: 10.1523/JNEUROSCI.1421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dietz RM, Weiss JH, Shuttleworth CW. Contributions of Ca2+ and Zn2+ to spreading depression-like events and neuronal injury. J. Neurochem. 2009;109 suppl. 1:145–152. doi: 10.1111/j.1471-4159.2009.05853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, Chan PH, Swanson RA. Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J. Cereb. Blood Flow Metab. 2008;28:1697–1706. doi: 10.1038/jcbfm.2008.61. [DOI] [PubMed] [Google Scholar]
- 55.Barkalifa R, Hershfinkel M, Friedman JE, Kozak A, Sekler I. The lipophilic zinc chelator DP-b99 prevents zinc induced neuronal death. Eur. J. Pharmacol. 2009;618:15–21. doi: 10.1016/j.ejphar.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Diener HC, Schneider D, Lampl Y, Bornstein NM, Kozak A, Rosenberg G. DP-b99, a membrane-activated metal ion chelator, as neuroprotective therapy in ischemic stroke. Stroke. 2008;39:1774–1778. doi: 10.1161/STROKEAHA.107.506378. [DOI] [PubMed] [Google Scholar]
- 57.Deshpande A, Kawai H, Metherate R, Glabe CG, Busciglio J. A role for synaptic zinc in activity-dependent Abeta oligomer formation and accumulation at excitatory synapses. J. Neurosci. 2009;29:4004–4015. doi: 10.1523/JNEUROSCI.5980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noy D, Solomonov I, Sinkevich O, Arad T, Kjaer K, Sagi I. Zinc-amyloid beta interactions on a millisecond time-scale stabilize non-fibrillar Alzheimer-related species. J. Am. Chem. Soc. 2008;130:1376–1383. doi: 10.1021/ja076282l. [DOI] [PubMed] [Google Scholar]
- 59.Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knockout mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 2010;30:1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J. Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 63.Nakashima AS, Dyck RH. Zinc and cortical plasticity. Brain Res. Brain Res. Rev. 2009;59:347–373. doi: 10.1016/j.brainresrev.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Nakashima AS, Dyck RH. Enhanced plasticity in zincergic, cortical circuits after exposure to enriched environments. J. Neurosci. 2008;28:13995–13999. doi: 10.1523/JNEUROSCI.4645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gielen M, Le Goff A, Stroebel D, Johnson JW, Neyton J, Paoletti P. Structural rearrangements of NR1/NR2A NMDA receptors during allosteric inhibition. Neuron. 2008;57:80–93. doi: 10.1016/j.neuron.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158:126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 67.Ruiz A, Walker MC, Fabian-Fine R, Kullmann DM. Endogenous zinc inhibits GABA(A) receptors in a hippocampal pathway. J. Neurophysiol. 2004;91:1091–1096. doi: 10.1152/jn.00755.2003. [DOI] [PubMed] [Google Scholar]
- 68.Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, Hershfinkel M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 2009;29:2890–2901. doi: 10.1523/JNEUROSCI.5093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeda A, Sakurada N, Ando M, Kanno S, Oku N. Facilitation of zinc influx via AMPA/kainate receptor activation in the hippocampus. Neurochem. Int. 2009;55:376–382. doi: 10.1016/j.neuint.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Nolan EM, Lippard SJ. Small-molecule fluorescent sensors for investigating zinc metalloneurochemistry. Acc. Chem. Res. 2009;42:193–203. doi: 10.1021/ar8001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang XA, Hayes D, Smith SJ, Friedle S, Lippard SJ. New strategy for quantifying biological zinc by a modified zinpyr fluorescence sensor. J. Am. Chem. Soc. 2008;130:15788–15789. doi: 10.1021/ja807156b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qian F, Zhang C, Zhang Y, He W, Gao X, Hu P, Guo Z. Visible light excitable Zn2+ fluorescent sensor derived from an intramolecular charge transfer fluorophore and its in vitro and in vivo application. J. Am. Chem. Soc. 2009;131:1460–1468. doi: 10.1021/ja806489y. [DOI] [PubMed] [Google Scholar]
- 73.Tomat E, Lippard SJ. Imaging mobile zinc in biology. Curr. Opin. Chem. Biol. 2010;2010:225–230. doi: 10.1016/j.cbpa.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sumalekshmy S, Henary MM, Siegel N, Lawson PV, Wu Y, Schmidt K, Brédas JL, Perry JW, Fahrni CJ. Design of emission ratiometric metal-ion sensors with enhanced two-photon cross section and brightness. J. Am. Chem. Soc. 2007;129:11888–11889. doi: 10.1021/ja073240o. [DOI] [PubMed] [Google Scholar]
- 75.Koutmou KS, Casiano-Negroni A, Getz MM, Pazicni S, Andrews AJ, Penner-Hahn JE, Al-Hashimi HM, Fierke CA. NMR and XAS reveal an inner-sphere metal binding site in the P4 helix of the metallo-ribozyme ribonuclease P. Proc. Natl. Acad. Sci. U.S.A. 2010;107:2479–2484. doi: 10.1073/pnas.0906319107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colvin RA, Bush AI, Volitakis I, Fontaine CP, Thomas D, Kikuchi K, Holmes WR. Insights into Zn2+ homeostasis in neurons from experimental and modeling studies. Am. J. Physiol. Cell Physiol. 2008;294:C726–C742. doi: 10.1152/ajpcell.00541.2007. [DOI] [PubMed] [Google Scholar]
- 77.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 78.Piatek K, Hartwig A, Bal W. Physiological levels of glutathione enhance Zn(II) binding by a Cys4 zinc finger. Biochem. Biophys. Res. Commun. 2009;389:265–268. doi: 10.1016/j.bbrc.2009.08.128. [DOI] [PubMed] [Google Scholar]
- 79.Heinz U, Hemmingsen L, Kiefer M, Adolph HW. Structural adaptability of zinc binding sites: Different structures in partially, fully, and heavy-metal loaded states. Chemistry. 2009;15:7350–7358. doi: 10.1002/chem.200900870. [DOI] [PubMed] [Google Scholar]
- 80.Andreini C, Bertini I, Rosato A. Metalloproteomes: A bioinformatic approach. Acc. Chem. Res. 2009;42:1471–1479. doi: 10.1021/ar900015x. [DOI] [PubMed] [Google Scholar]
- 81.Acknowledgments: We thank all the session chairs of the ISZB 2009 Meeting for their helpful comments, suggestions, and insight.