Phosphorylation of CLIP-170 by Plk1 and CK2 promotes timely formation of kinetochore–microtubule attachments
CLIP-170 is important for kinetochore–microtubule attachment. The authors show here that CLIP-170 is phosphorylated by Plk1 and CK2 and that this promotes its localization to kinetochores and interaction with dynactin.
Keywords: kinetochore, microtubule attachment, phosphorylation
Abstract
CLIP-170 is implicated in the formation of kinetochore–microtubule attachments through direct interaction with the dynein/dynactin complex. However, whether this important function of CLIP-170 is regulated by phosphorylation is unknown. Herein, we have identified polo-like kinase 1 (Plk1) and casein kinase 2 (CK2) as two kinases of CLIP-170 and mapped S195 and S1318 as their respective phosphorylation sites. We showed that a CK2 unphosphorylatable mutant lost its ability to bind to dynactin and to localize to kinetochores during prometaphase, indicating that the CK2 phosphorylation of CLIP-170 is involved in its dynactin-mediated kinetochore localization. Furthermore, we provide evidence that Plk1 phosphorylation of CLIP-170 at S195 enhances its association with CK2. Finally, we detected defects in the formation of kinetochore fibres in cells expressing the CLIP-S195A and -S1318A, but not the CLIP-S195E and -S1318D, confirming that Plk1- and CK2-associated phosphorylations of CLIP-170 are involved in the timely formation of kinetochore–microtubule attachments in mitosis.
Introduction
Microtubules are active cytoskeletal elements, the dynamics of which are regulated by several classes of microtubule-binding proteins (Akhmanova and Steinmetz, 2008). An interesting group of microtubule-binding proteins is the +TIPs, which associate with the plus ends of microtubules and bridge to microtubule targets, such as kinetochores (Schuyler and Pellman, 2001). CLIP-170, the founding member of the +TIPs (Pierre et al, 1992), is composed of three separate regions: N-terminus, central coiled-coil region, and C-terminus (Figure 1A). In addition to two cytoskeleton-associated protein glycine-rich domains, the N-terminus has three serine-rich regions. The N-terminal domain has an essential function in microtubule targeting, the central domain is responsible for dimerization of the protein, and the C-terminus, containing two zinc-finger domains, interferes with microtubule binding by interacting with the N-terminus (Lansbergen et al, 2004).
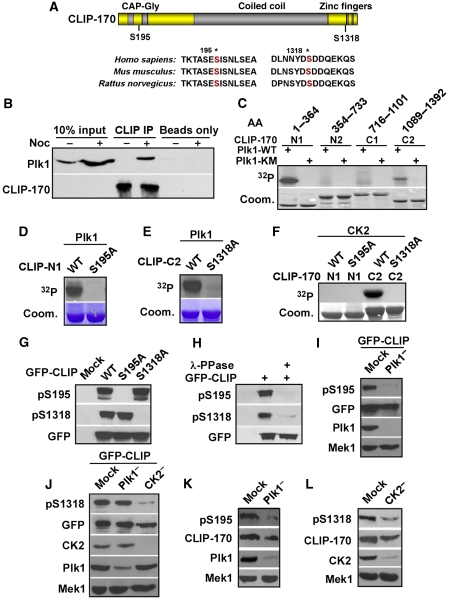
Figure 1.
CLIP-170 is a Plk1/CK2 substrate in vitro and in vivo. (A) Schematic of CLIP-170 with two-identified Plk1/CK2 phosphorylation sites. * marks the two phosphorylation sites. (B) Lysates from HeLa cells that were treated with or without 100 ng/ml nocodazole (Noc) for 12 h were immunoprecipitated with anti-CLIP-170 antibodies, followed by western blotting as indicated. Protein A/G beads only lanes serve as washing controls. (C) GST-Plk1-WT (wild type) or -K82M mutant (kinase defective) was incubated with four purified GST-CLIP-170 fragments (N1, 1–364; N2, 354–733; C1, 716–1101; C2, 1089–1392) in the presence of [γ-32P]ATP. The reaction mixtures were resolved by SDS–PAGE, stained with Coomassie Brilliant Blue (Coom.), and detected by autoradiography. (D, E) Plk1 was incubated with different forms of CLIP-170-N1 (D) or -C2 (E) as in (C). (F) CK2 was incubated with indicated forms of GST-CLIP-170 fragments (N1 or C2). (G) HEK 293T cells were transfected with GFP-CLIP-170 constructs (WT, S195A, or S1318A), treated with nocodazole, and immunoblotted with antibodies indicated. (H) Lysates from 293T cells transfected with GFP-CLIP-170 were treated with λ-phosphatase, followed by western blotting. (I) HeLa cells were depleted of Plk1 with dsRNA, re-transfected with GFP-CLIP-170, treated with nocodazole, and harvested for western blotting. (J) HeLa cells were depleted of Plk1 or CK2 with dsRNA, re-transfected with GFP-CLIP-170, treated with nocodazole, and subjected for western blotting analysis. (K) HeLa cells were Plk1 depleted with dsRNA, treated with nocodazole, and immunoblotted. (L) HeLa cells were CK2 depleted with dsRNA, treated with nocodazole, and immunoblotted.
CLIP-170 has documented functions in microtubule-dependent processes during interphase, such as recruiting dynactin to the microtubule plus ends (Lansbergen et al, 2004) and promoting microtubule rescue (Komarova et al, 2002). CLIP-170 also has an essential function during mitosis (Dujardin et al, 1998; Wieland et al, 2004; Tanenbaum et al, 2006). Deletion of the C-terminal domain of Bik1, the budding yeast homologue of CLIP-170, caused defects in chromosome segregation (Moore et al, 2006). Moreover, knockdown of CLIP-170 in human cells led to mitotic block (Wieland et al, 2004), because of failure of the formation of kinetochore–microtubule attachments (Tanenbaum et al, 2006). However, CLIP-170 knockout mice appear normal and fibroblasts derived from the knockout mice have only minor defects in chromosome alignment (Akhmanova et al, 2005), suggesting that redundant pathways in CLIP-170 function might exist for kinetochore–microtubule interaction (Tanenbaum et al, 2006). In addition, the kinetochore localization of CLIP-170 is dependent on the dynein/dynactin complex (Coquelle et al, 2002; Tai et al, 2002; Tanenbaum et al, 2006). The C-terminal zinc-finger domain of CLIP-170 is responsible for its interaction with dynactin (Lansbergen et al, 2004); however, whether this interaction is regulated by phosphorylation is currently unknown.
Several reports have shown that CLIP-170 is a phosphoprotein and that phosphorylation regulates its microtubule-binding ability (Rickard and Kreis, 1991; Choi et al, 2002). FKBP12-rapamycin-associated protein (FRAP) and Cdc2 are two CLIP-170 kinases identified (Choi et al, 2002; Yang et al, 2009). Biochemical and genetic data from various organisms have documented that Polo-like kinase 1 (Plk1) is involved in many cell-cycle-related events, such as bipolar spindle formation and sister chromatid segregation (Glover et al, 1998; Nigg, 1998). Casein kinase 2 (CK2), the most pleiotropic Ser/Thr-specific protein kinase known to date, is active in both mitosis and interphase. Among the >300 CK2 substrates discovered, are proteins that are implicated in cell-cycle control, DNA replication, and proliferation (Meggio and Pinna, 2003). Herein, we use multiple approaches to show that Plk1 and CK2 phosphorylate CLIP-170. Plk1 and CK2 target S195 and S1318, respectively. Significantly, we show that CK2 phosphorylation of CLIP-170-S1318 is required for its dynein/dynactin-dependent kinetochore localization and that the Plk1-associated phosphorylation of CLIP-170-S195 facilitates its interaction with CK2. Finally, we provide evidence that phosphorylation of CLIP-170 by Plk1 and CK2 is essential for the timely formation of microtubule–kinetochore attachments.
Results
Plk1 phosphorylates CLIP-170 in vitro
In a search for Plk1-interacting proteins, we have identified CLIP-170 as a potential Plk1 target. To determine whether CLIP-170 is a bona fide Plk1-interaction partner, we analysed the association between Plk1 and CLIP-170 in cells. HEK293T cells were co-transfected with GFP-CLIP-170 and Flag-Plk1-wild type (WT) or a kinase-defective mutant Flag-Plk1-K82M (Lee et al, 1995). To block the cells at mitosis, cells were treated with nocodazole. Lysates were subjected to anti-Plk1 immunoprecipitation (IP), and the presence of associated CLIP-170 was determined by immunoblotting (IB). As indicated, over-expressed GFP-CLIP-170 was co-IPed with Flag-Plk1. Interestingly, WT Plk1 formed a less stable complex with CLIP-170 than the Plk1 K82M mutant (Supplementary Figure S1A). This binding profile is suggestive of an enzyme–substrate relationship between Plk1 and CLIP-170.
As CLIP-170 and Plk1 co-immunoprecipitated in transfected cells, we sought to determine whether such an interaction could be detected between the two endogenous proteins. Extracts from asynchronous (−Nocodazole) or mitotic (+Nocodazole) cells were subjected to IP with CLIP-170 antibodies, followed by IB. A complex was detected between endogenous Plk1 and CLIP-170 in mitotic cells, but not in interphase cells (Figure 1B). To map the CLIP-170 domain responsible for Plk1-binding, cells were transfected with GFP-fusion CLIP-170 full length (aa 4–1392), CLIP-170-N (aa 4–309), or CLIP-170-C (aa 348–1392), arrested in mitosis, and harvested for anti-Plk1 IP. Both full-length and the N-terminal domains of CLIP-170 were co-IPed with Plk1 antibodies, suggesting that CLIP-170 interacts with Plk1 through its N-terminus (Supplementary Figure S1B, lanes 4–6).
To determine whether CLIP-170 is a substrate of Plk1, four CLIP-170 fragments were incubated with Plk1 (WT or KM) in the presence of [γ-32P]ATP. Both CLIP-170-N1 (aa 1–364) and CLIP-170-C2 (aa 1089–1392) were phosphorylated by Plk1-WT, but not Plk1-KM. The N-terminus of CLIP-170 served as a more robust substrate for Plk1 than the C-terminus (Figure 1C). To map the phosphorylation sites, we generated a series of serine/threonine to alanine mutations within aa 1–364 and aa 1089–1392 of CLIP-170. A single mutation of S195 or S1318 to alanine was sufficient to completely abolish the phosphorylation signal within aa 1–364 or 1089–1392 of CLIP-170, respectively (Figure 1D and E).
CK2 phosphorylates CLIP-170 in vitro
It has been reported that the Plk1 consensus sequence can be targeted by CK2 as well (Wells et al, 1994; Li et al, 2008). Co-IP experiments confirmed that over-expressed GFP-CLIP-170 associated with endogenous CK2 (Supplementary Figure S1C) and indicated that CLIP-170 associated with CK2 through its N-terminus (Supplementary Figure S1B, lanes 7–9). We next examined whether CLIP-170 is also a substrate of CK2. In vitro kinase assays indicated that CK2 phosphorylated CLIP-170-aa 1089–1392, but not CLIP-170-aa 1–364, and a single mutation of S1318 to alanine was sufficient to completely abolish the phosphorylation signal within aa 1089–1392 (Figure 1F). Moreover, addition of a CK2-specific inhibitor, TBB (Ruzzene et al, 2002), prevented the phosphorylation of CLIP-170 by CK2, confirming the specificity of kinase reactions (Supplementary Figure S1D).
In vivo, Plk1 phosphorylates CLIP-170 at Ser195, whereas CK2 phosphorylates CLIP-170 at Ser1318
To investigate CLIP-170 phosphorylation in vivo, a series of phospho-peptide mapping experiments was performed. HEK293T cells were transfected with GFP-CLIP-170 (WT or mutants), re-transfected with siRNA to deplete Plk1, treated with nocodazole, and incubated with [32P]orthophosphate in the presence or absence of TBB. After lysates were subjected to anti-GFP IP, phosphoproteins were digested with trypsin or endoproteinase Glu-C, and resolved by alkaline PAGE. Introduction of the S195A mutation led to complete disappearance of one phospho-peptide, and Plk1 depletion also reduced the intensity of the same peptide, suggesting that Plk1 kinase activity is required for S195 phosphorylation (Supplementary Figure S1E). Similarly, both the S1318A mutation and TBB treatment resulted in the disappearance of another phospho-peptide, indicating that CK2 is the kinase for S1318 phosphorylation (Supplementary Figure S1F).
To confirm the kinase/substrate relationship, two phospho-specific antibodies (pS195 and pS1318) were generated. A series of control experiments was performed to characterize specificity of the antibodies. First, purified GST-CLIP-170-aa 1–364 (Supplementary Figure S1G) or CLIP-170-aa 1089–1392 (Supplementary Figure S1H) were incubated with Plk1 or CK2 in the presence of unlabelled ATP, followed by anti-pS195 or anti-pS1318 IB. As indicated, an anti-pS195 signal was detected only in the sample that was pre-incubated with Plk1, and an anti-pS1318 signal was detected only in the sample that was pre-incubated with CK2, suggesting that neither of the two antibodies recognizes the unphosphorylatable form of CLIP-170. Furthermore, S195A and S1318A mutations completely abolished the respective anti-pS195 and anti-pS1318 western signal, indicating that the antibodies recognize only phosphorylated forms of CLIP-170 at those two sites. Next, HEK293T cells expressing GFP-CLIP-170 (WT, S195A, or S1318A) were analysed by IB with the two antibodies. Both pS195 and pS1318 signals were detected in cells expressing CLIP-170-WT, but not -S195A or -S1318A, respectively (Figure 1G), indicating that both sites are phosphorylated in vivo. Both pS195 and pS1318 antibodies are specific, as only one major band in each case was observed when they were used for IB with cell lysates (Supplementary Figure S1I and J). Treatment of lysates with λ-phosphatase led to loss of both epitopes, confirming that both sites are phosphorylated in vivo (Figure 1H). To assign the kinases responsible for the two phosphorylation sites identified, RNAi was used to deplete the two possible kinases, Plk1 and CK2. Plk1 depletion inhibited the generation of the pS195 epitope in both over-expressed GFP-CLIP-170 (Figure 1I) and the endogenous protein (Figure 1K), supporting the notion that Plk1 is the major kinase responsible for CLIP-170-S195 phosphorylation. Considering that Plk1 also phosphorylates S1318 in vitro (Figure 1C), we carefully assessed the level of the pS1318 epitope after Plk1 depletion. Plk1 depletion partially decreased the pS1318 epitope (Figure 1J), suggesting that Plk1 is unlikely the major kinase responsible for CLIP-170-S1318 phosphorylation in cells, but Plk1-associated kinase activity might also enhance phosphorylation of S1318. In contrast, CK2 depletion reduced about 90% of the pS1318 epitope in both over-expressed GFP-CLIP-170 (Figure 1J) and the endogenous protein (Figure 1L), indicating that CK2 is the major kinase responsible for CLIP-170-S1318 phosphorylation. We acknowledge that CK2 depletion did not completely abolish the pS1318 epitope, suggesting that additional unidentified kinases might also contribute to phosphorylation of S1318. It has been established that both Plk1 and CK2 prefer a target sequence containing negatively charged residues nearby and that two kinases might share the same sites in some substrates (Wells et al, 1994; Nakajima et al, 2003; Wu and Liu, 2008; Wu et al, 2008). The two phosphorylation sites identified in CLIP-170 (ES195IS and DS1318DD) fit the Plk1 and CK2 target consensus sequence (Figure 1A).
Temporal and spatial regulation of CLIP-170 phosphorylation
As the level of Plk1 is cell-cycle regulated and Plk1 phosphorylates CLIP-170, we asked whether CLIP-170 phosphorylation is regulated throughout the cell cycle. HeLa cells were synchronized with the double thymidine block at the G1/S boundary and released for different times. As shown in Figure 2A, phosphorylation of CLIP-170-S195 starts in late S phase, accumulates in G2, and reaches a peak in mitosis, matching the Plk1-expression pattern. Although phosphorylation of CLIP-170-S1318 is detected in early S phase, the pS1318 epitope does increase as cells go through mitosis (Figure 2A). We also noticed that phosphorylation of S195 peaks at late G2/early mitosis (8–12 h), whereas phosphorylation of S1318 reaches a maximum in late mitosis (14 h). Therefore, we conclude that both CLIP-170-S195 and -S1318 phosphorylation are cell-cycle regulated. One possible reason for the presence of the pS1318 epitope in S phase is the slower dephosphorylation at this site after mitosis, compared with that of the pS195 epitope. The overall patterns of the pS195 and pS1318 epitopes across the cell cycle again suggest that Plk1 activity enhances phosphorylation of S1318, but not absolutely essential, as the level of CK2 is not cell-cycle regulated. In addition, we always observed doublets in pS195, pS1318, and CLIP-170 blots. As antibodies used for these IB were developed in our laboratory and used for IB to detect endogenous proteins for the first time, we asked whether both bands within the doublets were from CLIP-170 or one of the bands detected was due to a non-specific protein. After HeLa cells were depleted of CLIP-170, both upper and lower bands within the doublet disappeared (Supplementary Figure S2A), suggesting that both bands within the doublet detect CLIP-170. Considering that CLIP-170 is a protein with multiple phosphorylation sites, we propose that the doublets in CLIP-170 IB might represent two different modified forms of CLIP-170. To further examine the two phospho-epitopes in late mitosis, HeLa cells were treated with nocodazole to block at prometaphase and released for different times after mitotic shake-off (Figure 2B). As indicated, the pS195 signal was detected in nocodazole-blocked cells and quickly decreased as cells entered into late mitosis (1 h post-release), whereas the pS1318 signal was low in early mitosis, peaked at anaphase (1 h post-release), and remained to be high as cells exited mitosis (100 min post-release). These observations indicated that Plk1 phosphorylates CLIP-170 at early mitosis and that maximum CLIP-170 phosphorylation by CK2 likely occurs at middle/late mitosis.
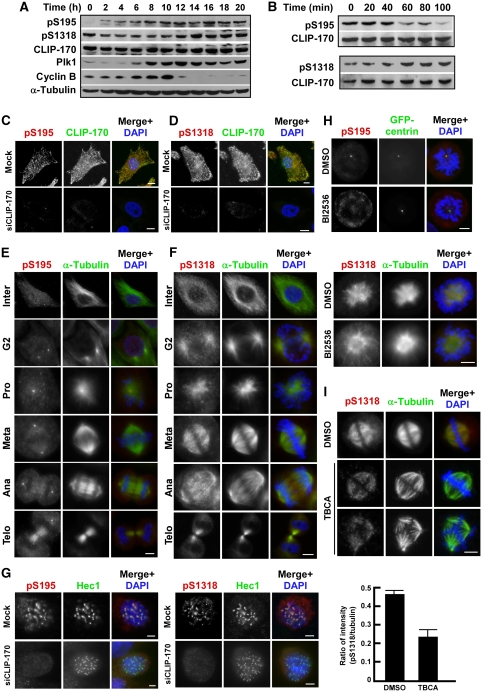
Figure 2.
Temporal and spatial regulation of CLIP-170 phosphorylation in HeLa cells. (A) Cells were synchronized with a double thymidine block (16 h thymidine block, 8 h of release, and a second thymidine treatment for 16 h), released for different times, and harvested for western blotting with antibodies as indicated. (B) After treatment with nocodazole, HeLa cells were collected with mitotic shake-off, released for different times, and analysed by western blotting. (C, D) Cells were depleted of CLIP-170 and co-stained with antibodies against CLIP-170 and pS195 or pS1318. (E, F) Cells were synchronized as in (A), released for 10 h to enrich at mitosis, and co-stained with antibodies against α-tubulin and pS195 or pS1318. (G) Cells were depleted of CLIP-170, treated with nocodazole, and co-stained with antibodies against Hec1 and pS195 or pS1318. (H) HeLa cells stably expressing GFP-centrin (top) or α-tubulin (bottom) were treated with BI2536 and immunostained with pS195 or pS1318. (I) HeLa cells stably expressing GFP-tubulin were synchronized with a triple thymidine block protocol coupled with TBCA treatment (16 h first thymidine block, 6 h release, first TBCA treatment for 6 h, second thymidine block for 12, 6 h release, second TBCA treatment for 6 h, third thymidine block for 12 h, release for 10 h in the presence of TBCA) and immunostained. Ratios between the intensities of pS1318 and α-tubulin staining were indicated in the lower panel. Bars, 5 μm.
We wanted to determine the subcellular localization of phospho-CLIP-170 during cell-cycle progression by immunofluorescence (IF) staining. First, we tested the antibodies on CLIP-170-depleted cells. CLIP-170 was depleted using two different RNAi approaches (Supplementary Figure S2B and C). Moreover, depletion of CLIP-170 by at least 95% led to the disappearance of both pS195 and pS1318 epitopes upon immunostaining, validating the use of these two antibodies in IF (Figure 2C and D; Supplementary Figure S2D). Consistent with the IB results, the pS195 epitope is very low during interphase, and starts to be detected in late G2 phase in centrosomes. Major phospho-CLIP-170-S195 was detected at spindle poles throughout mitosis and some weak phospho-CLIP-170-S195 signal was observed in spindle microtubules in metaphase and anaphase. Finally, phospho-CLIP-170-S195 localizes at mid-bodies in telophase (Figure 2E). In contrast, the pS1318 epitope matches the α-tubulin-staining pattern during mitosis. It localizes to microtubule asters in late G2/prophase, to spindles in metaphase and anaphase, and to mid-bodies in telophase. Furthermore, the overall intensity of the pS1318 epitope increases significantly from G2 to late mitosis (Figure 2F). CLIP-170 localization at various mitotic structures (centrosomes in G2 and prophase, spindles in metaphase and anaphase, and mid-bodies in telophase) was also detected by co-staining with an α-tubulin antibody (Supplementary Figure S2E). It was shown that CLIP-170 localizes to unattached kinetochores at the onset of prometaphase (Tanenbaum et al, 2006) (Supplementary Figure S2F). We were able to detect kinetochore localization of both pS195 and pS1318 epitopes in the absence of microtubule–kinetochore attachments, and both epitopes in kinetochores disappeared upon CLIP-170 depletion (Figure 2G). To confirm the specificity of the subcellular localization of the pS195 and pS1318 epitopes, IF staining was performed in the presence of Plk1 and CK2 inhibitors. Whereas the pS195 epitope was consistently reduced in centrosomes in the presence of BI2536, a Plk1 inhibitor (Brennan et al, 2007), the pattern of the pS1318 epitope was slightly changed after BI2536 treatment (Figure 2H), confirming that Plk1 is mainly responsible for the generation of the pS195, but might affect the pS1318 epitope as well. Incubation of cells with a CK2 inhibitor, TBCA (Pagano et al, 2007), reduced, but did not completely inhibit, pS1318 distribution in spindles (Figure 2I), consistent with the slow dephosphorylation process of this epitope after mitosis (Figure 2A and B). An alternative explanation of these data is that S1318 might be targeted by additional unidentified kinases as well.
As a control, treatment of cells with BI2536 and TBCA did not affect spindle pole localization of total CLIP-170 (Supplementary Figure S2G). Although cells treated with BI2536 resulted in a moderate but consistent reduction of CLIP-170 on kinetochores, TBCA treatment led to a more significant effect in terms of kinetochore localization (Supplementary Figure S2H). Finally, we showed that neither BI2536 nor TBCA treatment affects kinetochore localization of p150glued, the major subunit of the dynactin complex (Supplementary Figure S2I).
Phosphorylation of CLIP-170 at Ser1318 is required for its dynactin-dependent kinetochore localization
CLIP-170 interacts with dynactin through its C-terminus, and this interaction is critical for the essential function of CLIP-170 in the formation of kinetochore–microtubule attachments (Lansbergen et al, 2004; Tanenbaum et al, 2006; Yang et al, 2007). To determine the functional significance of S1318 phosphorylation, we expressed RNAi-resistant rat GFP-CLIP-170 with different substitutions at S1318 in the absence of endogenous protein and analysed the effect of these mutations on its association with dynactin. As indicated, introduction of alanine at S1318 of CLIP-170 reduced its association with p150glued, the major subunit of the dynactin complex (Figure 3A). Furthermore, dynactin associated tightly with CLIP-170-S1318D, emphasizing the significance of CLIP-170-S1318 phosphorylation in its interaction with dynactin. As we have shown that CK2 is the major kinase responsible for CLIP-170-S1318 phosphorylation, we tested whether CK2 inhibition would affect the association of CLIP-170 with dynactin. As indicated, the addition of TBCA reduced the dynactin association with CLIP-170 (Figure 3B). Alternatively, CK2 depletion reduced dynactin association with CLIP-170-WT (Figure 3C). However, we acknowledge that CK2 depletion also inhibits binding of CLIP-170-S1318D to p150Glued, suggesting that S1318 might not be the only site targeted by CK2. Alternatively, CK2 might also target p150Glued, whose phosphorylation is involved in its interaction with CLIP-170.
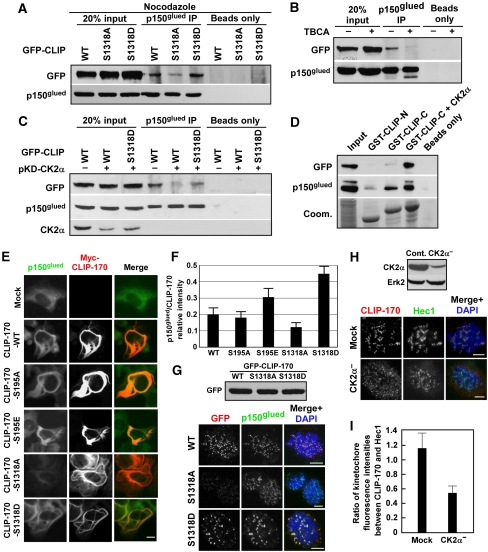
Figure 3.
Phosphorylation of S1318 of CLIP-170 is essential for dynactin-mediated kinetochore localization. (A) HeLa cells were co-transfected with pBS/U6-CLIP-170 and rat GFP-CLIP-170 (WT, S1318A, S1318D) at a ratio of 2:1. After 1 day of transfection, cells were treated with nocodazole for 14 h to block the cells at mitosis. Lysates were subjected to anti-p150glued IP, followed by anti-GFP immunoblot analysis. Protein A/G beads only lanes serve as washing controls. (B) Transfected cells as in (A) were treated with TBCA and subjected to anti-p150glued IP, followed by western blotting. (C) HeLa cells were depleted of CK2α with vector-based RNAi, re-transfected with GFP-CLIP-170, treated with nocodazole, and immunoprecipitated. (D) Lysates from U2OS cells stably expressing GFP-p150glued were incubated with purified GST-CLIP-170-N (aa 4–309), -C (aa 348–1392), or -C (aa 348–1392) pre-incubated with CK2α, subjected to GST pull-down assays, and analysed by western blotting. (E, F) U2OS cells were transfected with myc-CLIP-170 constructs, co-stained with antibodies against p150glued and myc (E), and relative intensity between p150glued versus myc-CLIP-170 was quantified (F). To quantify, the region with p150glued bundles was encircled, and a ratio of fluorescence intensity (p150glued versus myc) within the encircled region was calculated. All the imaging parameters were kept constant during data acquisition. At least 100 cells were quantified for each construct. (G) CLIP-170-depleted HeLa cells were transfected with rat GFP-CLIP-170, treated with nocodazole, and co-stained with antibodies against GFP and p150glued. (H) CK2-depleted HeLa cells were synchronized in mitosis and co-stained with antibodies against CLIP-170 and Hec1. (I) Ratios of kinetochore fluorescence intensities between CLIP-170 and Hec1 staining were quantified (n>50). Bars, 5 μm.
To confirm the phenotype aforementioned, a U2OS cell line stably expressing GFP-p150glued was generated. A GST pull-down assay with lysates from this cell line indicated that the CLIP-170-C-terminus binds to p150glued and that preincubation with CK2 enhanced the binding (Figure 3D). Furthermore, the GFP-p150glued-expressing cell line was used to express myc-tagged CLIP-170 constructs. It has been established that over-expression of CLIP-170 causes the formation of microtubule bundling and that the microtubule localization of dynactin is CLIP-170 dependent (Coquelle et al, 2002). Therefore, over-expression of myc-CLIP-170 in cells stably expressing GFP-p150glued led to the formation of p150glued bundles (Supplementary Figure S3A). Quantification indicated that the relative intensity of GFP-p150glued versus myc-CLIP-170 is dependent on phosphorylation state of CLIP-170-S1318 (Supplementary Figure S3B), but not that of CLIP-170-S195, consistent with the co-IP and GST pull-down data. We also expressed the same myc-CLIP-170 constructs in regular U2OS cells and analysed the endogenous p150glued pattern (Figure 3E). Over-expression of CLIP-170-S1318D had the most dramatic effect in CLIP-170 expression-induced bundle formation of endogenous p150glued (Figure 3F). Taken together, we concluded that CK2 phosphorylation of CLIP-170-S1318 is involved in its association with dynactin during mitosis.
CLIP-170 is targeted to unattached kinetochores in a dynein/dynactin-dependent manner (Coquelle et al, 2002). We have provided evidence that phosphorylation of CLIP-170 by CK2 is required for its association with dynactin. Therefore, we asked whether the phosphorylation of CLIP-170 by CK2 regulates its kinetochore localization during prometaphase. As shown in Supplementary Figure S2F, co-localization of CLIP-170 with Hec1 (a kinetochore marker) or p150glued at kinetochores was detected in nocodazole-treated cells. Next, HeLa cells were transfected with GFP-CLIP-170 (WT, S1318A, S1318D) (Figure 3G, top panel), treated with nocodazole, and stained with anti-GFP antibodies. Both CLIP-170-WT and -S1318D co-localized with dynactin to kinetochores, whereas CLIP-170-S1318A failed to do so (Figure 3G, bottom panel). Moreover, we monitored the kinetochore localization of CLIP-170 in CK2α-depleted cells. HeLa cells were depleted of CK2α (Figure 3H, top panel), and stained for CLIP-170 (Figure 3H, bottom panel). In CK2-depleted cells, only very weak CLIP-170 signal was detected on kinetochores (Figure 3I). In comparison with control cells, CK2α depletion led to nearly 50% reduction of kinetochore-localized CLIP-170, confirming that CK2 phosphorylation has a critical function for recruitment of CLIP-170 onto kinetochores. These observations were consistent with that the TBCA treatment resulted in a reduction of CLIP-170 at kinetochores (Supplementary Figure S2H, low panel).
Plk1-dependent phosphorylation of CLIP-170 does not affect tubulin binding
CLIP-170 binds to microtubules in an N-terminal domain-dependent manner and this interaction is regulated by both conformational changes (Lansbergen et al, 2004) and phosphorylation (Rickard and Kreis, 1991). Considering that Plk1 phosphorylates CLIP-170 at S195, which is at its N-terminus, we examined whether the Plk1 phosphorylation of CLIP-170 regulates its microtubule-binding behaviour. U2OS cells stably expressing various GFP-CLIP-170 constructs were generated. IF staining with antibodies against α-tubulin (Supplementary Figure S4A) and EB1, a +TIP marker (Supplementary Figure S4B), indicated that these GFP-CLIP-170 constructs have the same subcellular localization as the endogenous protein, the plus ends of microtubules, suggesting that phosphorylation at CLIP-170-S195 does not affect its microtubule binding in interphase. In addition, the tubulin-binding patterns of CLIP-170 were not affected by Plk1 or CK2-mediated phosphorylation in mitosis, either (Supplementary Figure S4C). In vitro pull-down analysis was used as an alternate approach. Purified GST-CLIP-170-aa 1–364 with different mutations (WT, S195A, or S195E) all showed similar tubulin-pull-down ability (Figure 4A), indicating that Plk1 phosphorylation of CLIP-170-S195 does not affect its tubulin-binding ability. This observation was supported by a microtubule-pelleting assay in which both S195A and S195E mutants of CLIP-170-aa 1–364 showed the same binding capacity as that of WT CLIP-170 (Figure 4B).
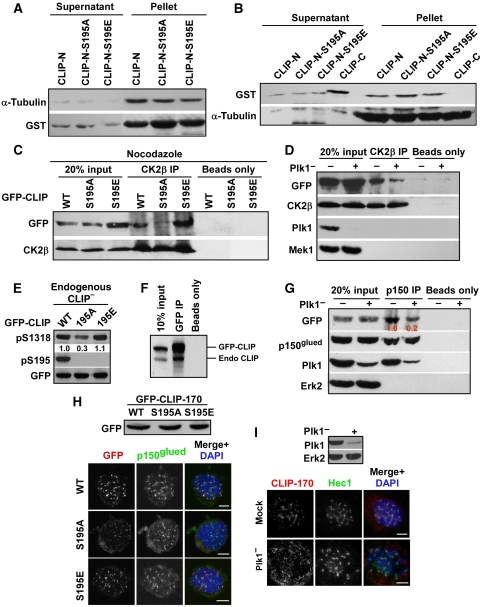
Figure 4.
Plk1 phosphorylation of CLIP-170-S195 is essential for its association with CK2. (A) After incubation with GST-CLIP-170-N-aa 1–364 (WT, S195A or S195E) proteins bound on glutathione-agarose beads, microtubules associated with the GST-fusion proteins were separated by low-speed centrifugation, followed by western blotting. (B) After purified GST-CLIP-170 proteins were incubated with microtubules, the supernatants and pellets were separated by ultracentrifugation. Microtubules and associated materials were analysed by western blot. CLIP-170-N: aa 1–364, CLIP-170-C: aa 1089–1392. (C) CLIP-170-depleted HeLa cells were transfected with rat GFP-CLIP-170, treated with nocodazole, and immunoprecipitated with CK2β antibodies, followed by western blotting. Protein A/G beads only serve as washing controls. (D) HeLa cells were depleted of Plk1, re-transfected with GFP-CLIP-170, treated with nocodazole, and immunoprecipitated with CK2β antibodies, followed by western blotting. Protein A/G beads only serve as washing controls. (E) CLIP-170-S195 phosphorylation affects S1318 phosphorylation. Cells as in (C) were immunoblotted. Numbers indicate the relative pS1318 intensity. (F) 293T cells were transfected with GFP-CLIP-170-S195A and harvested. Lysates were subjected to anti-GFP IP, followed by anti-CLIP-170 western blotting. Positions of GFP-CLIP-170 and endogenous CLIP-170 are indicated on the right. (G) Transfected cells as in (D) were immunoprecipitated with p150glued antibodies, followed by western blotting. Numbers indicate the relative GFP-CLIP-170 co-immunoprecipitated. (H) Kinetochore localization of CLIP-170 is S195-phosphorylation dependent. HeLa cells were co-transfected with pBS/U6-CLIP-170 and rat GFP-CLIP-170 (WT or S195 mutants) at a ratio of 2:1 and immunostained. Efficient protein expression of CLIP-170 constructs is indicated in the upper panel. (I) Kinetochore localization of CLIP-170 is Plk1 dependent. HeLa cells were depleted of Plk1, treated with nocodazole for 2 h, and co-stained with antibodies against CLIP-170 and Hec1. Bars, 5 μm.
Plk1-dependent phosphorylation of CLIP-170 enhances its association with CK2
As we have shown that CLIP-170 associates with both Plk1 and CK2 through its N-terminus, we examined whether Plk1 phosphorylation of CLIP-170-S195 regulates its binding to CK2. As indicated, CLIP-170-S195A showed decreased association with CK2 (Figure 4C). Moreover, CK2 associates tightly with CLIP-170-S195E, suggesting that CLIP-170-S195 phosphorylation enhances its interaction with CK2. To confirm the significance of Plk1 in CK2/CLIP-170 association, Plk1 was depleted in cells expressing GFP-CLIP-170. Plk1 depletion reduced the association between CK2 and CLIP-170 (Figure 4D).
As Plk1 phosphorylation of CLIP-170-S195 is involved in CK2 binding and CK2 is the kinase that targets CLIP-170-S1318, we speculated that Plk1 phosphorylation of S195 might affect the subsequent CK2 phosphorylation of S1318. Indeed, the intensity of the pS1318 epitope in cells expressing CLIP-170-S195A was about 30% of that in cells expressing CLIP-170-WT and -S195E (Figure 4E), supporting the notion that Plk1 likely serves as a priming kinase to create a docking site for CK2, thus enhancing its phosphorylation of CLIP-170-S1318. We might point out that we detected about 70% reduction of the pS1318 epitope only in the absence of endogenous CLIP-170 (compare Figure 4E versus 1G) (Supplementary Figure S4D), suggesting that the endogenous protein might dimerize with GFP-CLIP-170-S195A, thus facilitating the generation of the S1318 epitope (Figure 4F). Overall, our data support the conclusion that Plk1 phosphorylation of CLIP-170-S195 enhances CK2 phosphorylation of CLIP-170-S1318, but is not absolutely required.
As we have shown that the kinetochore localization of CLIP-170 is mediated by CK2 phosphorylation-dependent dynactin association, and that Plk1 phosphorylation of CLIP-170-S195 is involved in its association with CK2, we reasoned that both the dynactin association and kinetochore localization of CLIP-170 might be affected by Plk1-associated kinase activity. As expected, the binding between CLIP-170 and dynactin was reduced by 80% in Plk1-depleted cells (Figure 4G). Moreover, both CLIP-170-WT and -S195E co-localized with dynactin to kinetochores, whereas CLIP-170-S195A failed to do so (Figure 4H). Finally, we consistently observed reduced kinetochore localization of CLIP-170 in Plk1-depleted cells. Compared with control cells, nearly 79% of Plk1-depleted cells showed no detectable kinetochore localization of CLIP-170 (Figure 4I). These observations suggest that Plk1-dependent phosphorylation is involved in the proper kinetochore localization of CLIP-170 during mitosis.
Phosphorylation of CLIP-170 by both Plk1 and CK2 is involved in timely microtubule–kinetochore attachments during mitosis
It has been documented that CLIP-170 has an essential function in microtubule attachments at kinetochores during mitosis (Tanenbaum et al, 2006). We, therefore, examined whether the phosphorylation of CLIP-170 by Plk1 and CK2 is involved in its functions during these processes. To detect defects of microtubule attachments at kinetochores, HeLa cells were synchronized at G1/S border by 20 h thymidine block, released for 8 h, incubated for additional 3 h in nocodazole-containing media, washed two times, released in fresh media for 30 min, and stained for Mad2, which specifically binds to unattached kinetochores during mitosis. A representative Mad2-staining image is shown in Figure 5A. As indicated, about 16% of control cells at prometaphase were Mad2 positive at 30 min post-release, indicating that most of the control cells had achieved microtubule–kinetochore attachments. However, almost 78% of CLIP-170-depleted cells at prometaphase were Mad2 positive (Figure 5B). These observations confirm that CLIP-170 is required for proper microtubule–kinetochore attachments during mitosis. Next, we tested whether CK2 and Plk1 are also involved in these processes. Both CK2 and Plk1 depletions led to much higher percentages of Mad2-positive cells (Figure 5C), indicating that two kinases are involved in the timely formation of kinetochore–microtubule attachments as well.
Figure 5.
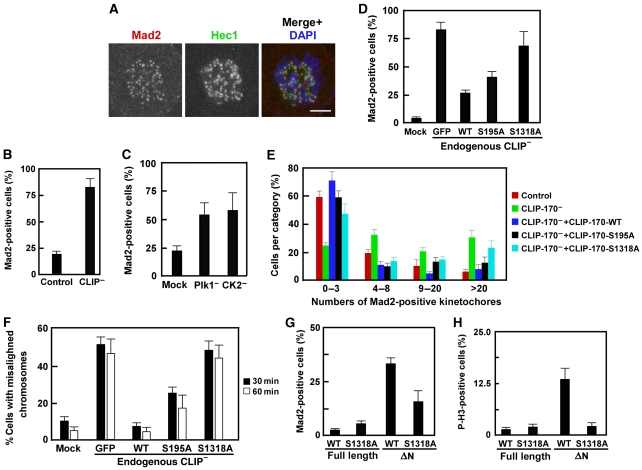
Phosphorylation of CLIP-170 by both Plk1 and CK2 is required for timely microtubule–kinetochore attachments. (A, B) CLIP-170-depleted HeLa cells were synchronized by the double thymidine block, released for 8 h, and treated with nocodazole for 3 h. Cells were released from the nocodazole block for 30 min, co-stained with antibodies against Mad2 and Hec1 (A), and quantified (B). Bars, 5 μm. (C) HeLa cells were depleted of Plk1 or CK2 and processed as in (A). (D–F) HeLa cells were co-transfected with pBS/U6-CLIP-170 and rat GFP-CLIP-170 (WT, S195A, S1318A) at a ratio of 4:1, processed, and stained for Mad2 as in (A). The number of Mad2-positive kinetochores (D, E) and misaligned chromosomes (F) per cell were quantified. (G, H) The CK2 unphosphorylatable mutant CLIP-170-ΔN-S1318A (aa 348–1320) loses the ability to serve as a CLIP-170 dominant-negative form. (G) Transfected cells were treated with nocodazole for 3 h, released for 30 min, and stained with Mad2 antibodies. (H) Transfected cells were synchronized by the double thymidine block, released for 20 h, and stained with phospho-H3 antibodies.
To test whether phosphorylation at S195 and S1318 regulates the essential function of CLIP-170 in the timely formation of microtubule–kinetochore attachments, a series of rescue experiments were performed. After 30 min post-release from nocodazole block, about 83% CLIP-170-depleted cells were detected as Mad2 positive (Figure 5D). Among them, nearly 76% cells showed >3 Mad2-positive kinetochores and about 33% cells had >20 kinetochores with detectable Mad2 signals (Figure 5E). Expression of rat GFP-CLIP-170-WT reduced the Mad2-positive cells to 25%, whereas 35% of GFP-CLIP-170-S195A-transfected cells and, significantly, 64% of GFP-CLIP-170-S1318A-expressing cells remained Mad2 positive (Figure 5D). We also quantified Mad2-positive cells in terms of kinetochore localization in the absence of endogenous CLIP-170. In CLIP-170-WT-transfected cells, only 28% of the population showed >3 Mad2-positive kinetochores, whereas 42 or 54% of the populations exhibited >3 Mad2-positive kinetochores in CLIP-170-S195A- or -S1318A-expressing cells, respectively (Figure 5E). These observations indicate that both WT CLIP-170 and S195A, but not S1318A, can partially reverse the defect in CLIP-170-depletion-induced microtubule–kinetochore attachments. As the kinetochore localization of CLIP-170 is mediated by its interaction with the dynactin complex, we propose that the weak ability of S1318A to reverse the defect is likely due to its low-binding affinity to the dynactin complex. Compared with the very weak ability of S1318A, S195A exhibited partial reverse ability, likely because of over-expression of the protein. In contrast to S1318A, although S195A cannot be phosphorylated by Plk1, it probably still could be partially phosphorylated by CK2 at S1318, especially when over-expressed (Figure 4E). Therefore, S195A, phosphorylated at S1318, would also have the ability to interact with the dynactin complex. Accordingly, S195A showed higher reverse ability than S1318A, which almost completely lost its binding ability with the dynactin complex.
As described above, both CLIP-170-S195A and CLIP-170-S1318A showed reduced rescue ability to CLIP-170 depletion-induced Mad2 accumulation at kinetochores. We asked whether CLIP-170 phosphorylation would directly regulate Mad2 stripping from kinetochores, failure of which would also lead to Mad2 accumulation at kinetochores without causing a defect in kinetochore–microtubule attachments. As shown in Supplementary Figure S5, nocodazole treatment resulted in Mad2 accumulation at kinetochores in cells expressing different forms of GFP-CLIP-170 (WT, S195A, or S1318A). Once the cells were released and preceded to anaphase, Mad2 disappeared from kinetochores in all cells, regardless of CLIP-170 being expressed, indicating that Mad2 stripping from kinetochores was not directly regulated by CLIP-170 phosphorylation.
We also explored possible chromosome alignment defects induced by CLIP-170 depletion and compared the rescue ability of CLIP-170 with different phosphorylation states. Although about 93% control U2OS cells had completely aligned their chromosomes after 1 h release from the nocodazole block, almost 50% of CLIP-170-depleted cells were observed with misaligned chromosomes at 1 h post-release. As expected, expression of CLIP-170-WT reduced the cells with misaligned chromosomes to 6% at 1 h post-release, in comparison with 17% of CLIP-170-S195A-expressing cells and 43% of CLIP-170-S1318A-expressing cells, respectively (Figure 5F). As we propose that CLIP-170 phosphorylation regulates the timely kinetochore–microtubule attachments, it is reasonable that expression of unphosphorylatable CLIP-170 mutants in the absence of endogenous CLIP-170 would induce severe chromosome alignment defects, as kinetochore dynein is required for formation and stabilization of kinetochore fibres and in powering chromosome motion and congression (Yang et al, 2007).
It was previously shown that CLIP-170-ΔN (aa 348–1320) accumulates at kinetochores, consequently, this truncated form of CLIP-170 might compete with and displace endogenous CLIP-170 from kinetochore-binding sites, leading to a dominant-negative inhibition of processes in which CLIP-170 is involved (Dujardin et al, 1998). Therefore, we also observed an increased both Mad2-positive staining at kinetochores (32%) and phospho-histone H3 staining (a mitosis marker) in cells with over-expression of CLIP-170-ΔN. However, only 14% of Mad2-positive cells were detected in CLIP-170-ΔN-S1318A-expressing cells and no mitotic block was observed (Figure 5G and H). These studies also suggest that phosphorylation of CLIP-170 at S1318 is required for its kinetochore localization, which is essential for the timely formation of kinetochore–microtubule attachments.
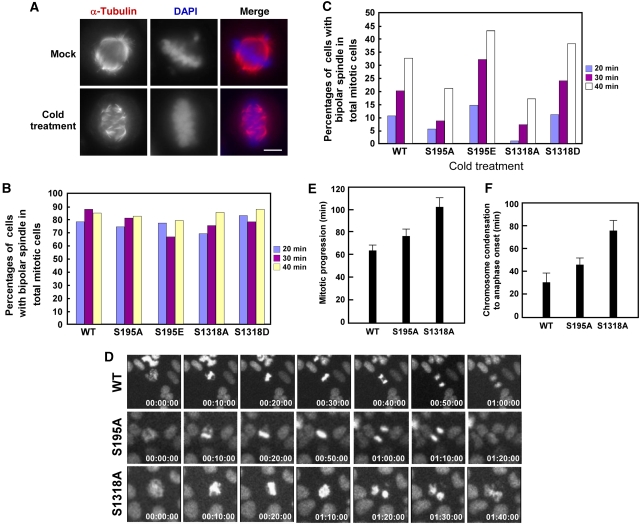
To directly measure microtubule–kinetochore attachments by following kinetochore fibre formation, U2OS cells stably expressing GFP-CLIP-170 constructs were synchronized at prometaphase by nocodazole, released for different times, treated with or without cold, and stained with α-tubulin antibodies (Figure 6A–C). Without cold treatment, kinetics of spindle formation is almost identical for all cell lines expressing different forms of CLIP-170, with normal bipolar spindle formation in about 75% of mitotic cells after release from nocodazole block for 20 min (Figure 6B). In contrast, cold-stable kinetochore–microtubules were detected in 10, 20, and 32% of mitotic cells expressing GFP-CLIP-170-WT after 20, 30, and 40 min release from the block, respectively. Of note, for cells expressing CLIP-170-S195A, a significant reduction of cold-stable kinetochore–microtubules was detected in all three-time points we analysed. For cells expressing CLIP-170-S1318A, only 2, 7, and 17% of mitotic cells had kinetochore fibres after release for 20, 30, and 40 min, respectively (Figure 6C). As expected, kinetochore fibre formation of cells expressing both CLIP-170-S195E and -S1318D was not affected.
Figure 6.
Expression of GFP-CLIP-170-S195A or -S1318A-induced mitotic progression defects. (A–C) Timely kinetochore fibre formation is CLIP-170 phosphorylation dependent. U2OS cells stably expressing GFP-CLIP-170 constructs were treated with thymidine for 24 h, released for 6 h, and blocked with nocodazole for additional 5 h. After nocodazole was washed away, cells were released for different times (20, 30, and 40 min), incubated at 4°C for 20 min, and stained with α-tubulin antibodies. (A) Representative images for spindles we analysed. Bar, 5 μm. (B) Time course of normal bipolar spindle formation in cells stably expressing different forms of CLIP-170. (C) Time course of kinetochore fibre formation in cells stably expressing different forms of CLIP-170. (D) Time-lapse videomicroscopy to illustrate mitotic progression of U2OS cells stably expressing different forms of GFP-CLIP-170 constructs. Images were acquired at the indicated time points after chromosome condensation. (E, F) Histograms indicating the time periods needed to go through entire mitosis (E) or from the start of chromosome condensation to anaphase onset (F) in cells expressing different forms of CLIP-170 (n>5).
As our above data indicate, CLIP-170 phosphorylation is involved in timely creation of microtubule–kinetochore attachments and then loss of phosphorylation should result in defects in mitotic progression. Therefore, time-lapse experiments were performed to examine mitotic progression in live cells expressing different forms of CLIP-170 (Supplementary Movies 1, 2, 3). For cells expressing CLIP-170-WT, <40 min is long enough from start of chromosome condensation to anaphase onset. In striking contrast, CLIP-170-S195A-expressing cells spent at least 50 min and CLIP-170-S1318A-expressing cells need >70 min to proceed to anaphase (Figure 6D–F). Moreover, broad metaphase plates were observed in cells expressing CLIP-170-S1318A, indicating that chromosome congression was aberrant (Figure 6D). We propose that these mitotic defects were most likely induced by lack of timely kinetochore fibre formation in cells expressing unphosphorylatable CLIP-170 mutants.
Discussion
Upstream kinases of CLIP-170
It has been established that CLIP-170 is a phosphoprotein and phosphorylation has been proposed to regulate its microtubule binding (Rickard and Kreis, 1991; Hoogenraad et al, 2000; Choi et al, 2002; Vaughan et al, 2002; Vaughan, 2004). CLIP-170 is phosphorylated at multiple sites and the major phosphorylated residues are serines (Rickard and Kreis, 1991; Choi et al, 2002). The FRAP phosphorylation of CLIP-170 positively regulates its microtubule-binding behaviour (Choi et al, 2002). We showed that Cdc2 phosphorylation of CLIP-170 is involved in inhibition of centrosome reduplication after S phase (Yang et al, 2009). Here, we report that CLIP-170 is phosphorylated at nearly 10 sites (Supplementary Figure S1E and F). Moreover, we identify Plk1 and CK2 as the major kinases responsible for phosphorylation of CLIP-170 at S195 and S1318, respectively. Considering that FRAP phosphorylation of CLIP-170 regulates its MT-binding ability, the phosphorylation likely occurs at interphase. In contrast, Cdc2, Plk1, and CK2-dependent phosphorylation of CLIP-170 is likely to be a G2/M phase event. As CLIP-170 has well-established functions in both interphase and mitosis, it is not surprising that kinases from both interphase and mitosis regulate CLIP-170. Identification of additional kinases for CLIP-170 will help us to further understand the rather complicated regulatory mechanisms of this important molecule.
For some Plk1 substrates, Cdc2 phosphorylation has been shown to generate a docking site to recruit Plk1 towards these substrates (Elia et al, 2003a, 2003b). Here, we noticed that pS195-CLIP-170 starts at G2, peaks at early mitosis, and quickly decreases at late mitosis. The cell-cycle regulation of the pS195 epitope fits well of the Cdc2 activity profile. We thus examined whether the Plk1 phosphorylation of CLIP-170 is regulated in a Cdc2-dependent manner. Towards that end, typical sequential kinase assays were performed (Wu and Liu, 2008). Sequential exposure of GST-CLIP-170 to Cdc2 and Plk1 did not result in a significant increase of phosphorylation of GST-CLIP-170, indicating that the association between CLIP-170 and Plk1 is not Cdc2-phosphorylation dependent (data not shown). However, the data presented here suggest that Plk1 phosphorylation of CLIP-170-S195 enhances CK2-mediated phosphorylation of CLIP-170 at S1318, but is not absolutely essential. Therefore, regulation of CLIP-170 functions by multiple kinases is complex.
CLIP-170 in the timely formation of kinetochore–microtubule attachments
It has been established that CLIP-170 is recruited to kinetochores in a dynactin-dependent manner (Coquelle et al, 2002), but how CLIP-170 recognizes and binds to dynactin at unattached kinetochores is unknown. Moreover, CLIP-170 is quickly displaced from kinetochores once the kinetochore–microtubule attachments are established. How this dynamic behaviour of CLIP-170 is regulated in mitosis is elusive. Here, we provide evidence to support that CK2-dependent phosphorylation of CLIP-170 at S1318 promotes its kinetochore localization through enhancing its interaction with the dynein/dynactin complex. This finding provides one mechanism to explain how CLIP-170 regulates the timely formation of kinetochore–microtubule attachments. As CK2 activity is not cell-cycle regulated, how it specifically phosphorylates CLIP-170 at mitosis is difficult to understand. In this report, we present additional evidence to show that Plk1 phosphorylation of CLIP-170 at S195 could enhance CK2-mediated phosphorylation of CLIP-170, thus increasing CK2 specificity at mitosis.
The function of CLIP-170 during mitosis has been examined by various loss-of-function approaches (Wieland et al, 2004; Tanenbaum et al, 2006). An early report showed that depletion of CLIP-170 caused mitotic block because of chromosome misalignment during metaphase (Wieland et al, 2004). More recently, it was shown that CLIP-170 localizes to unattached kinetochores at prometaphase, and such localization is essential for the formation of kinetochore–microtubule attachments (Tanenbaum et al, 2006). Further detailed kinetochore–microtubule attachment defects were confirmed, as a high level of Mad1, which localizes to unattached kinetochores, was observed in CLIP-170-depleted cells (Tanenbaum et al, 2006). As CLIP-170 directly interacts with kinetochore-localized dynactin, the possible explanation for these observations is that CLIP-170 acts as a hook to guide growing microtubules to kinetochore, thus facilitating timely kinetochore fibre formation (Yang et al, 2007). As both Plk1 and CK2 phosphorylation of CLIP-170 enhances its localization at unattached kinetochores, it is reasonable that we observed both phosphorylation events facilitate the generation of timely microtubule–kinetochore attachments. We propose that CLIP-170 phosphorylation mainly functions in initial formation of microtubule–kinetochore attachments at kinetochores. We first provide evidence that phosphorylation at CLIP-170-S1318 facilitates interaction of CLIP-170 on unattached kinetochores through promoting its binding to dynactin at kinetochores. Second, cold treatment experiments showed that CLIP-170-S195 and -S1318 phosphorylation is involved in timely formation of kinetochore–microtubule attachments, as both GFP-CLIP-170-S195A- and -S1318A-expressing cells exhibit slower kinetochore fibre formation. Third, phosphorylated CLIP-170, especially at S1318, was mainly observed on spindle microtubules. As CLIP-170 is supposed to serve as a bridge between spindle microtubules and unattached kinetochores in generation of attachments, we propose that CLIP-170 phosphorylation would improve the formation of bridge, therefore, facilitating the initial lateral attachments upon nuclear envelope breakdown, which is known to be a dynein-mediated process (Yang et al, 2007). Thus, we want to point out that phosphorylation of CLIP-170 at the two sites we described here is unlikely required to form mature attachments as we show that cells expressing CLIP-170 phosphomutants eventually enter anaphase, a clear indication of functional kinetochore–microtubule attachments. Whether phosphorylation at other sites affects mature attachments waits further investigation.
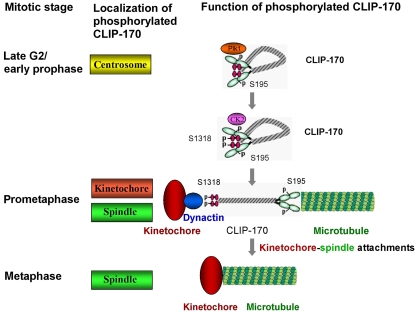
In conclusion, we have shown that CLIP-170 is a substrate for both Plk1 and CK2 and that CK2-dependent phosphorylation of CLIP-170 is involved in its dynactin-dependent kinetochore localization during mitosis. We also conclude that Plk1 phosphorylation of CLIP-170 enhances CK2-mediated phosphorylation of CLIP-170 and that both phosphorylation events are involved in timely microtubule–kinetochore attachments (Figure 7).
Figure 7.
A model to illustrate how Plk1/CK2 phosphorylation of CLIP-170 regulates the timely microtubule–kinetochore attachment. At late G2/early prophase, Plk1 interacts with CLIP-170 at centrosomes and phosphorylates CLIP-170 at S195. Plk1-mediated phosphorylation of CLIP-170 promotes its association with CK2 at its N-terminus, subsequently facilitating CK2 phosphorylation of CLIP-170 at S1318. Upon S1318 phosphorylation, CLIP-170 is recruited to kinetochores through its interaction with the dynein/dynactin complex, which localizes on unattached kinetochores at prometaphase. Finally, CLIP-170 recruits spindle microtubules to unattached kinetochores thus to create timely microtubule–kinetochore attachments.
Materials and methods
Vector construction, RNAi, cell culture, and transfection
Detailed information on the construction of various expression or RNAi vectors, cell culture, and DNA transfection are described in Supplementary data.
Antibodies
Two phospho-specific antibodies against S195 and S1318 of CLIP-170 were generated by Proteintech, Chicago. Briefly, two peptides containing phospho-S195 (NLTKTASESpISNLSEAG) and phospho-S1318 (GDDLNNYDSpDDQEKQSK) were synthesized and used to immunize rabbits. After the antibodies were affinity purified, a series of control experiments was performed to confirm the specificity of the antibodies. Sources of other antibodies are described in Supplementary data.
IP, IB, IF staining, time-lapse videomicroscopy, and kinase assays
Detailed information of IP, IB, and IF staining, time-lapse videomicroscopy, and in vitro kinase assays with purified Plk1 and CK2 are described in Supplementary data.
Supplementary Material
Acknowledgments
We are grateful to Dr Raymond Erikson, in whose laboratory the preliminary experiments were performed, and for generously providing many cell lines and plasmids. We appreciate Dr Gary G Borisy and Dr Holly Goodson for various CLIP-170 expression constructs and CLIP-170 antibody. We appreciate Dr Ji-Xing Cheng and Shirley Bond for the technical assistance for time-lapse experiments. We also thank Eleanor Erikson for critical reading of the paper. XL is a recipient of the Howard Temin Award from the National Cancer Institute (K01 CA114401).
Footnotes
The authors declare that they have no conflict of interest.
References
- Akhmanova A, Mausset-Bonnefont AL, van Cappellen W, Keijzer N, Hoogenraad CC, Stepanova T, Drabek K, van der Wees J, Mommaas M, Onderwater J, van der Meulen H, Tanenbaum ME, Medema RH, Hoogerbrugge J, Vreeburg J, Uringa EJ, Grootegoed JA, Grosveld F, Galjart N (2005) The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis. Genes Dev 19: 2501–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A, Steinmetz MO (2008) Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 9: 309–322 [DOI] [PubMed] [Google Scholar]
- Brennan IM, Peters U, Kapoor TM, Straight AF (2007) Polo-like kinase controls vertebrate spindle elongation and cytokinesis. PLoS One 2: e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Bertram PG, Drenan R, Carvalho J, Zhou HH, Zheng XF (2002) The FKBP12-rapamycin-associated protein (FRAP) is a CLIP-170 kinase. EMBO Rep 3: 988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquelle FM, Caspi M, Cordelieres FP, Dompierre JP, Dujardin DL, Koifman C, Martin P, Hoogenraad CC, Akhmanova A, Galjart N, De Mey JR, Reiner O (2002) LIS1, CLIP-170's key to the dynein/dynactin pathway. Mol Cell Biol 22: 3089–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin D, Wacker UI, Moreau A, Schroer TA, Rickard JE, De Mey JR (1998) Evidence for a role of CLIP-170 in the establishment of metaphase chromosome alignment. J Cell Biol 141: 849–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia AE, Cantley LC, Yaffe MB (2003a) Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 299: 1228–1231 [DOI] [PubMed] [Google Scholar]
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB (2003b) The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115: 83–95 [DOI] [PubMed] [Google Scholar]
- Glover DM, Hagan IM, Tavares AA (1998) Polo-like kinases: a team that plays throughout mitosis. Genes Dev 12: 3777–3787 [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Akhmanova A, Grosveld F, De Zeeuw CI, Galjart N (2000) Functional analysis of CLIP-115 and its binding to microtubules. J Cell Sci 113(Part 12): 2285–2297 [DOI] [PubMed] [Google Scholar]
- Komarova YA, Akhmanova AS, Kojima S, Galjart N, Borisy GG (2002) Cytoplasmic linker proteins promote microtubule rescue in vivo. J Cell Biol 159: 589–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen G, Komarova Y, Modesti M, Wyman C, Hoogenraad CC, Goodson HV, Lemaitre RP, Drechsel DN, van Munster E, Gadella TW Jr, Grosveld F, Galjart N, Borisy GG, Akhmanova A (2004) Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J Cell Biol 166: 1003–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Yuan YL, Kuriyama R, Erikson RL (1995) Plk is an M-phase-specific protein kinase and interacts with a kinesin-like protein, CHO1/MKLP-1. Mol Cell Biol 15: 7143–7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang Y, Liu X (2008) Plk1-dependent phosphorylation regulates functions of DNA topoisomerase II{alpha} in cell cycle progression. J Biol Chem 283: 6209–6221 [DOI] [PubMed] [Google Scholar]
- Meggio F, Pinna LA (2003) One-thousand-and-one substrates of protein kinase CK2? FASEB J 17: 349–368 [DOI] [PubMed] [Google Scholar]
- Moore JK, D'Silva S, Miller RK (2006) The CLIP-170 homologue Bik1p promotes the phosphorylation and asymmetric localization of Kar9p. Mol Biol Cell 17: 178–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E (2003) Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem 278: 25277–25280 [DOI] [PubMed] [Google Scholar]
- Nigg EA (1998) Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol 10: 776–783 [DOI] [PubMed] [Google Scholar]
- Pagano MA, Poletto G, Di Maira G, Cozza G, Ruzzene M, Sarno S, Bain J, Elliott M, Moro S, Zagotto G, Meggio F, Pinna LA (2007) Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem 8: 129–139 [DOI] [PubMed] [Google Scholar]
- Pierre P, Scheel J, Rickard JE, Kreis TE (1992) CLIP-170 links endocytic vesicles to microtubules. Cell 70: 887–900 [DOI] [PubMed] [Google Scholar]
- Rickard JE, Kreis TE (1991) Binding of pp170 to microtubules is regulated by phosphorylation. J Biol Chem 266: 17597–17605 [PubMed] [Google Scholar]
- Ruzzene M, Penzo D, Pinna LA (2002) Protein kinase CK2 inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) induces apoptosis and caspase-dependent degradation of haematopoietic lineage cell-specific protein 1 (HS1) in Jurkat cells. Biochem J 364(Part 1): 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler SC, Pellman D (2001) Microtubule ‘plus-end-tracking proteins': the end is just the beginning. Cell 105: 421–424 [DOI] [PubMed] [Google Scholar]
- Tai CY, Dujardin DL, Faulkner NE, Vallee RB (2002) Role of dynein, dynactin, and CLIP-170 interactions in LIS1 kinetochore function. J Cell Biol 156: 959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Galjart N, van Vugt MA, Medema RH (2006) CLIP-170 facilitates the formation of kinetochore-microtubule attachments. EMBO J 25: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan KT (2004) Surfing, regulating and capturing: are all microtubule-tip-tracking proteins created equal? Trends Cell Biol 14: 491–496 [DOI] [PubMed] [Google Scholar]
- Vaughan PS, Miura P, Henderson M, Byrne B, Vaughan KT (2002) A role for regulated binding of p150(Glued) to microtubule plus ends in organelle transport. J Cell Biol 158: 305–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells NJ, Addison CM, Fry AM, Ganapathi R, Hickson ID (1994) Serine 1524 is a major site of phosphorylation on human topoisomerase II alpha protein in vivo and is a substrate for casein kinase II in vitro. J Biol Chem 269: 29746–29751 [PubMed] [Google Scholar]
- Wieland G, Orthaus S, Ohndorf S, Diekmann S, Hemmerich P (2004) Functional complementation of human centromere protein A (CENP-A) by Cse4p from Saccharomyces cerevisiae. Mol Cell Biol 24: 6620–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZQ, Liu X (2008) Role for Plk1 phosphorylation of Hbo1 in regulation of replication licensing. Proc Natl Acad Sci USA 105: 1919–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZQ, Yang X, Weber G, Liu X (2008) Plk1 phosphorylation of TRF1 is essential for its binding to telomeres. J Biol Chem 283: 25503–25513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li H, Liu XS, Deng A, Liu X (2009) Cdc2-mediated phosphorylation of CLIP-170 is essential for its inhibition of centrosome reduplication. J Biol Chem 284: 28775–28782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Tulu US, Wadsworth P, Rieder CL (2007) Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr Biol 17: 973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.