Hyphal growth in Candida albicans requires the phosphorylation of Sec2 by the Cdc28-Ccn1/Hgc1 kinase
This study establishes that Sec2, the guanine exchange factor (GEF) of the Rab GTPase Sec4, which has a central function in polarized exocytosis, undergoes CDK-dependent phosphorylation/relocalization in response to hypha-inducing signals.
Keywords: cyclin-dependent kinase, polarized exocytosis, polarized growth, Spitzenkörper
Abstract
Polarized growth is a fundamental property of cell growth and development. It requires the delivery of post-Golgi secretory vesicles to the site of polarized growth. This process is mediated by Rab GTPases activated by their guanine exchange factors (GEFs). The human fungal pathogen, Candida albicans, can grow in a budded yeast form or in a highly polarized hyphal form, and thus provides a model to study this phenomenon. During hyphal, but not yeast growth, secretory vesicles accumulate in an apical body called a Spitzenkörper, which acts to focus delivery of the vesicles to the tip. Post-Golgi transport of secretory vesicles is mediated by the Rab GTPase Sec4, activated by its GEF Sec2. Using a combination of deletion mapping, in vitro mutagenesis, an analogue-sensitive allele of Cdc28 and an in vitro kinase assay, we show that localization of Sec2 to the Spitzenkörper and normal hyphal development requires phosphorylation of Serine 584 by the cyclin-dependent kinase Cdc28. Thus, as well as controlling passage through the cell cycle, Cdc28 has an important function in controlling polarized secretion.
Introduction
Polarized growth is a fundamental property of cell growth and development. In the development of specialized cells such as the outgrowth of axons and dendrites from the cell body of neurons, or the extension of plant pollen tubes, new membrane material is inserted at the growing tip by the process of polarized exocytosis, in which post-Golgi vesicles travel along cytoskeletal tracks to fuse with the plasma membrane. Fungal hyphae also show highly polarized tip growth. This polarized growth is driven by a subapical structure called a Spitzenkörper, which is rich in secretory vesicles that contain enzymes for the biosynthesis of cell walls, as well as providing the material for the expansion of the plasma membrane necessary for hyphal elongation. (Girbardt, 1957, 1969; Grove and Bracker, 1970; Grove et al, 1970; Bracker et al, 1997; Harris et al, 2005; Steinberg, 2007). According to the Vesicle Supply Centre model, secretory vesicles accumulate in the Spitzenkörper before onward transport to the hyphal surface, possibly transported along actin cables (Bartnicki-Garcia et al, 1989, 1995). The proximity of the Spitzenkörper to the tip ensures that secretory vesicles arrive in greater densities at the hyphal tip than subapical regions, resulting in polarized tip growth.
The human fungal pathogen Candida albicans can grow in three developmentally distinct states: hyphae, pseudohyphae and yeast (Sudbery et al, 2004). The yeast form closely resembles the budding yeast Saccharomyces cerevisiae; the pseudohyphal form consists of chains of elongated cells with constrictions at the septal junctions; the hyphal form consists of chains of tube-like cells that have no constrictions at the septal junctions. In pseudohyphae, cell elongation can be so extreme that they superficially resemble hyphae; however, multiple lines of evidence suggest that there are fundamental differences between hyphae and pseudohyphae, including the organization of the cell cycle and modes of polarized growth (reviewed in Sudbery et al, 2004). Polarized growth at the tip of the pseudohyphal bud is restricted to the first part of the cell cycle (Crampin et al, 2005); whereas in hyphae, growth from the tip is continuous (Soll et al, 1985). We have shown that the myosin light chain, Mlc1 fused to YFP (Mlc1-YFP), is localized to a subapical spot at hyphal tips that resembles a Spitzenkörper and is continuously present throughout the cell cycle (Crampin et al, 2005). In contrast, Mlc1-YFP is localized to a surface crescent in pseudohyphae that is only present during the first part of the cell cycle. On the basis of this observation, we proposed that polarized growth in C. albicans hyphae, but not pseudohyphae, is driven by a vesicle-rich Spitzenkörper, which we have subsequently confirmed by observing that the vesicle-associated proteins Sec2 and Sec4 co-localize with Mlc1 in this apical spot, whereas they localize to a surface crescent in pseudohyphae (present work and L Jones, unpublished observations). Understanding the mechanism of the developmental switch from yeast to hyphae thus requires an understanding of why secretory vesicles accumulate in the Spitzenkörper in hyphae, but not in yeast or pseudohyphae.
Polarized secretion has been extensively studied in the related budding yeast S. cerevisiae (for a review, see Park and Bi, 2007). Post-Golgi secretory vesicles travel along actin cables to sites of polarized growth in which they dock with a multiprotein complex called the exocyst before fusion with the plasma membrane, mediated by the interaction of v-Snares on the vesicles and t-SNAREs on the plasma membrane. The actin cables are generated at sites of polarized growth by a second multiprotein complex called the polarisome and the motive force for vesicle transport is provided by the class V myosin, Myo2, complexed to its regulatory light chain Mlc1. These late stages of the secretory pathway require the Rab GTPase, Sec4, in its active GTP-bound form, which mediates the docking of secretory vesicles with the exocyst through its interaction with the exocyst subunit Sec15 (Walworth et al, 1989; Guo et al, 1999). Sec4 is attached to the secretory vesicles and thus localizes to sites of polarized growth at the tip of the young buds and at the site of septum formation during cytokinesis (Goud et al, 1988; Novick et al, 1988; Novick and Brennwald, 1993; Walch-Solimena et al, 1997). Localization of Sec4 and its conversion to its active GTP-bound state is mediated by its GEF, Sec2, which is also found on secretory vesicles at sites of polarized growth (Walch-Solimena et al, 1997). In temperature-sensitive mutants of Sec2, Sec4 is only partially localized at the permissive temperature; at the non-permissive temperature, Sec4 is completely delocalized and electron microscopy shows that secretory vesicles are randomly distributed throughout the bud and mother cell (Walch-Solimena et al, 1997). Thus, activation of Sec4 by Sec2 is required for the transport of secretory vesicles and/or their retention at sites of polarized growth.
S. cerevisiae Sec2 is composed of 759 amino acids (Nair et al, 1990). Its GEF activity is located in an N-terminal domain (1–161), which also contains a coil-coil region, which promotes its dimerization (Walch-Solimena et al, 1997; Dong et al, 2007). Sec2 is recruited to secretory vesicles by an upstream GTPase, encoded by a pair of redundant genes YPT31/32, which weakly binds Sec2 between residues 161 and 374 (Ortiz et al, 2002). Sec2 also physically interacts with Sec15, the effector of Sec4 (Medkova et al, 2006). The region in which Sec15 interacts with Sec2 overlaps the region in which Ypt31/32 interacts and the two proteins compete for Sec2 binding. Deletion mapping located a critical 58-residue window, 450–508, which is required both for localization of Sec2 to sites of polarized growth and for its attachment to secretory vesicles (Elkind et al, 2000). The temperature-sensitive sec2-59 allele is a nonsense mutation that encodes a truncated protein that lacks this domain (1–374) (Nair et al, 1990), whereas another temperature-sensitive mutation, sec2-78, results in a cysteine to tyrosine substitution at residue 478 within this localization domain (Elkind et al, 2000). Importantly, Sec2 is phosphorylated within the 450–508 region (Elkind et al, 2000). Although the phosphorylated residue or residues and the kinase responsible have not so far been identified, Sec2 localization has been shown to be directly or indirectly dependent on the cyclin-dependent kinase Cdc28 (McCusker et al, 2007). Mutant Sec2 proteins lacking the localization domain show increased affinity to Sec15 and are found complexed to Sec15 in the cytosol rather than on secretory vesicles (Medkova et al, 2006). The current model is that after docking with exocyst, the Sec2/Sec15 complex is released into the cytosol. To participate in a new cycle of secretory vesicle transport and docking, Sec15 must be exchanged for Ypt31/32. The localization domain acts to lower the affinity of Sec2 for Sec15 and thus facilitate the interaction with Ypt31/32 (Novick et al, 2007).
Owing to the central function played by Sec2 in the localization of secretory vesicles to polarized growth, we have studied the function of Sec2 in the accumulation of secretory vesicles in the C. albicans Spitzenkörper. We show that Sec2 localizes to the Spitzenkörper in hyphae, to a surface crescent in pseudohyphae and to sites of septum formation in yeast. We reasoned that the differences of Sec2 localization in the different growth forms may reflect its differential phosphorylation. We, therefore, sort to identify the site or sites of phosphorylation, the kinase(s) responsible, and to determine the physiological effect of phosphorylation. We show that phosphorylation of S584 is required both for normal hyphal morphology and localization of Sec2 to the Spitzenkörper. We show that soon after induction, S584 is phosphorylated by Cdc28 partnered by the cyclins Hgc1 or Ccn1 and that this phosphorylation is required to localize Sec2 to the Spitzenkörper during hyphal growth. Moreover, the early phosphorylation of Sec2 is not dependent on the hyphal-specific-transcription programme initiated by the signal transduction pathways mediated by Cph1 and Efg1.
Results
Sec2 localizes to a Spitzenkörper in hyphae
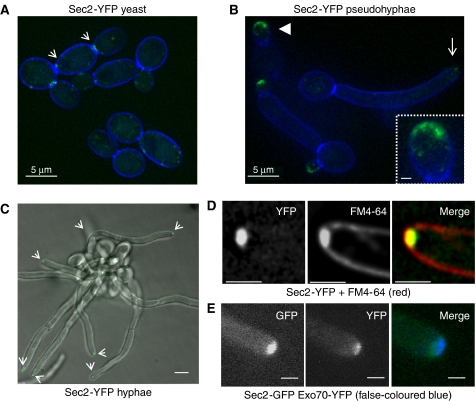
To localize Sec2, we generated a C-terminal fusion to YFP in a sec2Δ/SEC2 heterozygous parent, so that Sec2-YFP was the sole form of Sec2. This strain grew and formed hyphae normally, showing the Sec2-YFP fusion protein to be functional. The intracellular location of Sec2-YFP in cells growing in the different morphological forms was examined (Figure 1). In yeast, Sec2-YFP was observed to localize to punctate spots and to the cytokinetic ring at the mother-bud neck (Figure 1A). In pseudohyphae, Sec2 was located to a surface crescent at the tip of short elongated buds, it was absent at the tip of long buds and reappeared at the cytokinetic ring (Figure 1B). In hyphae, Sec2 localized to an apical spot in the majority of hyphae (87% n=65), which was continuously present (Figure 1C). A faint ring was also transiently visible at the site of septum formation (not shown). Thus, the pattern of localization is different in hyphae compared with yeast and pseudohyphae. The apical spot co-localized with FM4-64, a Spitzenkörper marker (Crampin et al, 2005) (Figure 1D). Exocyst and polarisome components localize to a surface crescent, which is spatially and dynamically distinct from the Spitzenkörper (Crampin et al, 2005, Jones and Sudbery, unpublished observations). Sec2-YFP also localized to a distinct location compared with Exo70, an exocyst component (Figure 1E). We have also found in FRAP experiments that Sec2-YFP has the dynamic properties of a Spitzenkörper component, clearly distinct from those of exocyst and polarisome components (Jones and Sudbery, in revision). Thus, in hyphae, the secretory vesicle component, Sec2, localizes to the Spitzenkörper, supporting the conclusion that a vesicle-rich Spitzenkörper forms at the tip of hyphae in this fungus.
Figure 1.
Sec2 localizes to the Spitzenkörper in hyphae. A sec2Δ/SEC2-YFP strain was grown as yeast, hyphae and pseudohyphae and the localization of Sec2-YFP visualized by fluorescence microscopy. (A) In yeast cells, Sec2-YFP forms punctuate patches and a ring at the mother-bud neck during cytokinesis (arrows). (B) In pseudohyphae, Sec2-YFP localizes to an apical crescent in short buds (arrow head, enlarged in inset); however, it becomes depolarized in long buds (barbed arrow). (C) In hyphae, Sec2-YFP localizes to a bright apical spot in hyphae corresponding to a Spitzenkörper (arrows). (D) Sec2-YFP co-localizes with the Spitzenkörper marker FM4-64. E: Sec2-GFP does not co-localize with the exocyst component Exo70-YFP. Cell outlines were visualized by counterstaining with Calcofluor white (A, B, D) or DIC microscopy (C). Note the blue fluorescence produced by Calcofluor white in this image may not be reproduced well by some printers. Scale bars: A, B, main panel; C, 5 μm; D, E, 1 μm; inset panel B, 0.5 μm.
Sec2 requires phosphorylation on residue S584 to support hyphal growth
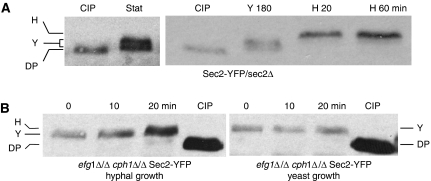
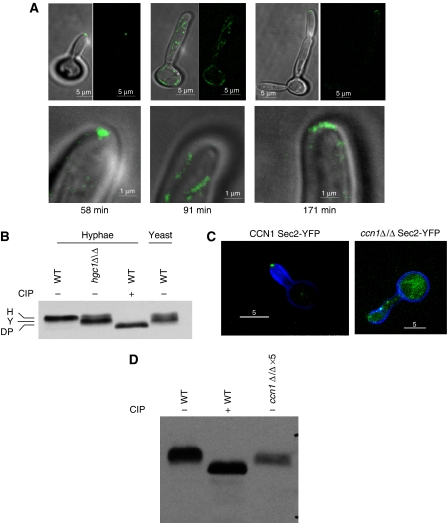
We investigated whether different patterns of phosphorylation during yeast and hyphal growth could be detected in C. albicans Sec2-YFP by an electrophoretic band shift (Figure 2). Sec2-YFP extracted from stationary phase and growing yeast cells showed a reduced electrophoretic mobility compared with the sample treated with calf intestinal phosphatase (CIP) (annotated DP in Figure 2A). In most, but not all experiments, the band-shifted protein resolved into two separate bands (annotated Y in Figure 2A). When stationary phase yeast cells were induced to form hyphae, the pattern changed to a single, hyper-retarded band, suggesting it was hyper-phosphorylated (annotated H in Figure 2A). This change was evident within 20 min, well before hyphal germ tubes evaginated from the unbudded mother cell (30–35 min). Thus, in stationary phase and growing yeast cells, Sec2 is constitutively phosphorylated, probably on more than one residue, but the pattern of phosphorylation rapidly changes upon hyphal induction and precedes the appearance of hyphal germ tubes.
Figure 2.
Sec2 exists in multiple phosphorylated forms. (A) Sec2-YFP isolated from stationary phase cells (Stat, left panel) or cells growing in yeast-promoting conditions for 180 min (Y180, right panel) migrates as a doublet, which is retarded compared with Sec2-YFP dephosphorylated by CIP treatment. In this, and subsequent figures, annotation of the bands are as follows: H, hyphal-specific band; Y, double band visible in yeast and stationary phase cells (this band does not always resolve into a double band and so appears as a single band migrating between the dephosphorylated and hyphal forms); DP, Sec2 dephosphorylated by CIP treatment. (B) In hyphal-inducing conditions, Sec2-YFP still shows a hyper-phosphorylated band in cells where the hyphal-transcription programme is blocked by the efg1Δ/Δ cph1Δ/Δ mutations.
Environmental cues that induce hyphal formation are sensed by a network of signal transduction pathways and result in a hyphal-specific programme of transcription (Biswas et al, 2007; Brown et al, 2007). The most important of these is the cAMP-dependent pathway, which targets the Efg1-transcription factor. A second pathway operates through Cph1, a MAP kinase that is homologous to the Fus3/Kis1 MAP kinase that mediates the pheromone response and pseudohyphal formation in S. cerevisiae. A C. albicans mutant lacking both Cph1 and Efg1 is unable to form hyphae and fails to initiate the hyphal-specific programme of transcription (Lo et al, 1997). We made use of this mutant to ask whether the hyphal-specific programme of transcription was required for the rapid appearance of the hyphal-specific pattern of Sec2 phosphorylation. In a cph1Δ/Δ efg1Δ/Δ strain growing as yeast, Sec2-YFP migrated with the reduced electrophoretic mobility characteristic wild-type yeast form compared with the CIP-treated protein from wild-type cells (Figure 2B). Interestingly, when this strain was induced to form hyphae, Sec2-YFP underwent a time-dependent band shift to a hyper-phosphorylated species, characteristic of the hyphal form, despite the complete inability of this strain to form hyphae. Thus, the rapid phosphorylation of Sec2 upon hyphal induction does not depend upon hyphal-specific gene transcription.
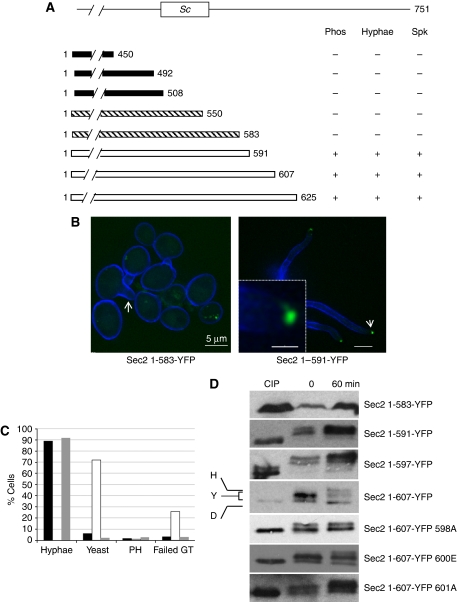
To map the site or sites phosphorylated in hyphae, we constructed a series of C-terminal deletions in such a way that the resulting truncated protein was fused to YFP. C. albicans is an obligate diploid. We constructed the Sec2-truncated alleles using both a parent strain, which was a wild-type diploid, so that a normal copy of Sec2 was also expressed, and also in sec2Δ/SEC2 heterozygous parent strain, so that the truncated Sec2 protein was the sole form of Sec2 expressed. We determined whether the truncated protein was phosphorylated using band shifts in western blots and whether the Sec2-YFP was localized to the Spitzenkörper upon hyphal induction. In addition, this approach also allowed us to define the extent of the N-terminal region of Sec2 protein required for viability and the extent of the region required for normal hyphal growth. The results are summarized in Figure 3. In Figure 3A, black bars indicate deletions where we conclude the truncated Sec2-YFP strain is unable to support viability because the deletion could not be constructed in a heterozygous sec2Δ/SEC2 parent, but could be constructed in a wild-type parent; hatched bars indicate deletions where the truncated Sec2 could support viability, but which could not programme normal hyphal growth; open bars indicate deletions where the truncated Sec2 could support viability and normal hyphal growth.
Figure 3.
Deletion mapping to dissect Sec2 function. (A) C-terminal truncations fused to YFP were constructed in sec2Δ/SEC2 or SEC2/SEC2 parents and challenged to form hyphae. Phosphorylation, identified through a band shift, formation of morphologically normal hyphae and localization of the truncated Sec2-YFP to a Spitzenkörper were recorded. Black bars: the indicated truncations are presumed to remove Sec2 sequences required to support viability because they could not be constructed in a sec2Δ/SEC2 parent, but could be readily constructed in an SEC2/SEC2 parent. Hatched bars: the truncated Sec2 protein could support viability as they could be constructed in a sec2Δ/SEC2 parent, but could not form normal hyphae. White bars: the truncated Sec2 protein constructed in a sec2Δ/SEC2 parent could support viability and formed normal hyphae. The box marked Sc denotes the region equivalent to the 58 residue required for Sec2 localization and phosphorylation in S. cerevisiae. (B) Cells expressing only Sec21−583-YFP cannot form normal hyphae and the truncated Sec2-YFP is not polarized (left panel). Some cells appeared to form a germ tube, but failed to elongate (arrow). Cells expressing only Sec1−591-YFP formed normal hyphae and Sec2 is localized to a Spitzenkörper (right panel). Inset shows enlargement of tip indicated by arrow. Scale bars: main panels 5 μm, inset 1 μm. (C) Quantitation of Sec2-YFP (black bars), Sec21−583-YFP (white bars) and Sec1−591-YFP (grey bars) cells cultured in hyphal-inducing conditions for 90 min (n⩾70). PH=pseudohyphae. Failed GT=failed germ tube illustrated by arrow in the Sec21−583-YFP image in panel B. (D) Stationary phase cells (time 0) were induced to form hyphae for 60 min (60). Dephosphorylated CIP-treated extracts were used as a control. Autoradiographs of the truncations with the indicated end points and Sec21−607-YFP truncations with the indicated substitutions.
Residues 492–550 in CaSec2 correspond to 450–508 in ScSec2, the 58-residue window required for localization and which contains a phospho-acceptor site or sites. In C. albicans, this region is apparently required for viability (Figure 3A). Three rounds of deletion mapping defined an 8-residue window (amino acids 583–591) that was required for the formation of normal hyphae, localization of Sec2-YFP to the Spitzenkörper and for its phosphorylation. Cells expressing only Sec21−550-YFP or Sec21−583-YFP were viable, but when induced to form hyphae, hyphal development was rudimentary or absent (Figure 3B and C) and truncated Sec2-YFP protein showed no polarization (Figure 3B). In contrast, cells expressing only Sec21−591-YFP formed morphologically normal hyphae in which Sec21−591-YFP localized to the Spitzenkörper (Figure 3B). In western blots (Figure 3D), we consistently observed that Sec21−550-YFP and Sec21−583-YFP did not show any difference in electrophoretic mobility compared with CIP-treated controls and so were probably not phosphorylated, whereas Sec21−591-YFP was band shifted, and so phosphorylated. We conclude that residues between 583 and 591 contain a residue(s) that is/are phosphorylated and that this phosphorylation event is required for normal hyphal growth.
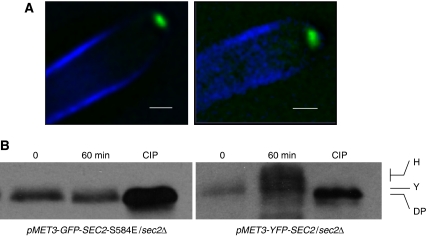
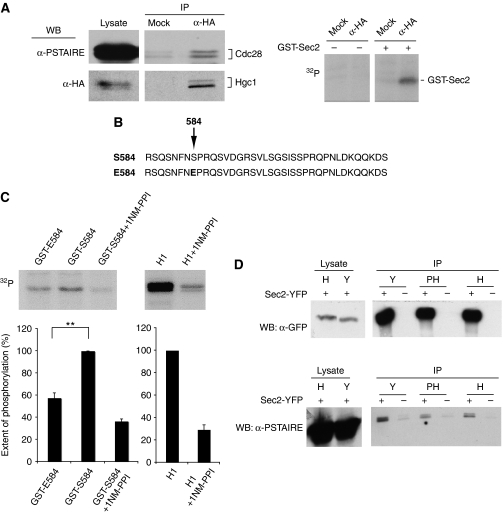
The sequence 583 NSPRQSVDG 591 contains two possible phosphorylation sites: S584 and S588. To investigate which of these is phosphorylated and to determine the physiological significance of the phosphorylation, we attempted to mutate these sites to the non-phosphorylatable alanine residue, in a sec2Δ/SEC2 heterozygote parent, so that only the mutated allele was expressed. We could readily make the S588A allele, which resulted in a strain, which produced morphologically normal hyphae, but were unable to create a strain in which the only copy of SEC2 carried the S584A allele. However, we could readily generate the phosphomimetic glutamate substitution at this site to form the S584E allele, which formed normal hyphae. Thus, it seems likely that S584 is the phospho-acceptor residue. If this is the case, then the Sec2 S584E protein should not show the hyphal pattern of phosphorylation, even though it can support normal hyphal growth. For unknown reasons, we were unable to C-terminally tag this allele, despite persistent attempts. However, we could construct an N-terminally tagged pMET3-green fluorescent protein (GFP)-SEC2 S584E allele where GFP-Sec2 S584E was expressed from the regulatable MET3 promoter (Care et al, 1999). We adjusted the culture conditions so that the level of Sec2 S584E expressed from the MET3 promoter was comparable with Sec2-YFP expressed from its native promoter, as judged by western blots. In these conditions, cells expressing only GFP-Sec2 S584E formed morphologically normal hyphae and GFP-Sec2 584E localized to the Spitzenkörper (Figure 4A). However, GFP-Sec2 S584E failed to show the large band shift upon phosphatase treatment that is characteristic of the wild-type protein, although a small band shift was evident (Figure 4B). Thus, we conclude that phosphorylation of S584 is necessary for hyphal growth.
Figure 4.
SEC2 S584E does not show the hyphal-specific pattern of phosphorylation, but forms hyphae normally. (A) Both pMET-YFP-SEC2-S584E/sec2Δ and pMET-YFP-SEC2/sec2Δ strains form hyphae normally and both YFP-Sec2 and YFP-Sec2 S584E are localized to a Spitzenkörper. (B) As with the C-terminal fusion, YFP-Sec2 shows a band shift in yeast, which shows a further shift during hyphal growth. YFP-Sec2 S584E shows a small band shift compared with the CIP-treated sample, but does not show a further shift in the hyphal sample. Scale bars: 5 μm.
Although Sec21−591-YFP supported hyphal growth, the hyper-retarded band, characteristic of hyphal growth was not evident, suggesting that the hyper-phosphorylation of Sec2 observed in hyphae is not actually required for hyphal growth. We, therefore, sought to identify the additional phosphorylation event evident in hyphae and to investigate whether or not it is actually required for hyphal growth. To this end, we constructed a further series of C-terminal truncations to map the additional site. A Sec21−607 truncation showed the hyphal pattern of phosphorylation, thus locating the phosphorylated residue to the region between residues 597 and 607 (Figure 3D). This region contains three potential serine phospho-acceptor sites located at 598, 600 and 601. We introduced substitutions at each of these sites into the Sec21−607-YFP truncation. An alanine substitution at S598 abolished the hyper-phosphorylation; whereas substitutions at each of the other sites had no effect on the pattern of phosphorylation (Figure 3D). We tested the ability of Sec21−607 S598A-YFP to support hyphal growth. The ability to form hyphae in liquid culture was normal as was the ability to maintain hyphal growth in colonies on agar plates induced by Spider medium and serum (data not shown). Thus, as suggested by the ability of the Sec21−591-YFP to support hyphal growth, the additional phosphorylation event observed in hyphae is not actually necessary for Sec2 to support hyphal growth. The ability of the all the other serine substitutions in this window to form hyphae was also normal. It is interesting to note that residue S601 occurs in the same SPRQ context as S584. Nevertheless, substituting this residue had no effect on the pattern of phosphorylation or on its ability to form hyphae.
We sought to identify the kinase responsible for the phosphorylation of S584. Sec2 has recently been reported to be targeted by Cbk1, a kinase that is essential for hyphal growth (McNemar and Fonzi, 2002; Kurischko et al, 2008). To investigate the function of Cbk1, we constructed strains containing Sec2-YFP, which contained a kinase-dead mutation in the Cbk1 active site or in the S570 site required for its activation by phosphorylation. Although neither of these strains could form hyphae, Sec2 was still phosphorylated normally (data not shown). Thus, Cbk1 is not responsible for targeting Sec2 S584. Tpk1 and Tpk2 are a pair of cAMP-dependent kinases activated in hyphal-inducing conditions and are required for hyphal development (Sonneborn et al, 2000; Bockmuhl et al, 2001). A cell lacking both kinases is not viable. We, therefore, used a tpk1Δ/Δ tpk2Δ/tpk2-1as strain in which the sole cAMP-dependent kinase catalytic unit could be inhibited by the ATP analogue 1NM-PP1, to investigate whether this kinase targets Sec2 (Bishop et al, 2000). Again, as in the case of Cbk1, Sec2 was phosphorylated normally after the addition of 1NM-PP1, despite the inability of this strain to form hyphae (data not shown).
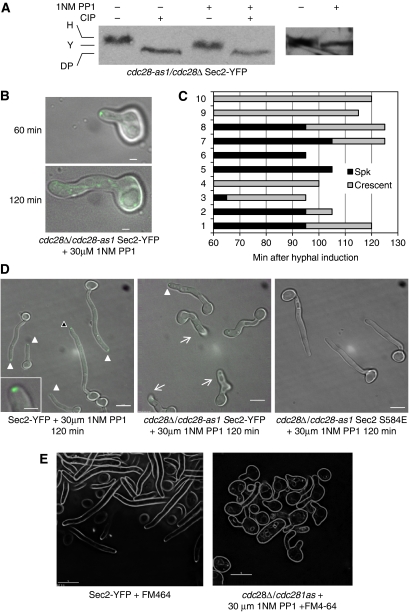
The context of S584 is an SP site which forms the core motif recognized by cyclin-dependent kinases (Cdks) (Endicott et al, 1999). In C. albicans, the cyclin-dependent kinase Cdc28 is known to be required for normal hyphal growth (Sinha et al, 2007). We tested the hypothesis that S584 phosphorylation is dependent on Cdc28 by determining whether Sec2 shows the hyphal pattern of phosphorylation in cells lacking Cdc28 activity. As Cdc28 is essential, we constructed a strain SEC2-YFP cdc28-1as strain in which Cdc28 activity can be inhibited by the ATP analogue 1NM-PP1 (Bishop et al, 2000). Western blots showed that Sec2-YFP phosphorylation was reduced (Figure 5A).
Figure 5.
Sec2 phosphorylation and localization is Cdc28 dependent. (A) When Cdc28-1as is inhibited, Sec2-YFP shows the yeast, but not the hyphal pattern of phosphorylation upon hyphal induction. Stationary phase cells were size fractionated by elutriation and induced to form hyphae. Samples were collected after 180 min. (B–D) Sec2 localization and hyphal formation is inhibited by 1NM-PP1 in cells expressing only Cdc28-1as. Stationary phase cdc28-1as/cdc28Δ SEC2-YFP; CDC28/CDC28 SEC2-YFP or cdc28Δ/cdc28-as1 SEC2 S584E cells were induced to form hyphae in the presence of 30 μM 1NM-PP1 on the surface of agarose in concavity microscope slides and incubated in parallel at 38°C on a microscope stage heated by a temperature hood. (B) The same representative cdc28-1as/cdc28Δ SEC2-YFP cell imaged after 60 and 120 min as indicated. (C) 60 min after induction, 10 cdc28-1as/cdc28Δ SEC2-YFP cells were selected, their morphology and pattern of Sec2-YFP localization recorded at intervals. The duration of the Spitzenkörper or crescent pattern localization pattern is graphically represented by black bars or grey bars, respectively. Cessation of any apical localization is shown by the termination of the grey bars. (D) After 120 min, representative fields of cells of each genotype were recorded. Solid arrows show the presence of a Spitzenkörper, an example, highlighted by the black arrowhead, is enlarged in inset of the left panel. Barbed arrows in the middle panel show cells, which cannot be recorded in a single focal plane because the hypha has grown into the agarose substratum. In these cells, care was taken to examine them by using an extended focal range to verify the absence of any apical Sec2-YFP localization. (E) Inhibition of Cdc28 1as by 1NM PP1 prevents Spitzenkörper formation. Spitzenkörper was visualized by uptake of FM4-64, 90 min after hyphal induction. Scale bars: panel B, 1 μm; panel D, E, 5 μm; inset 1 μm.
We noticed some impairment of hyphal growth in the SEC2-YFP cdc28-1as mutant, even when the inhibitor was not present. Thus, in the following experiments to examine the morphological effects of Cdc28 inhibition, we used a strain with a non-mutated CDC28 gene as a control. In the presence of the inhibitor, the SEC2-YFP cdc28-1as strain formed swollen, slow growing hyphae (Figure 5D and E). These hyphae were conspicuously curved showing that they were defective in hyphal guidance. They also prematurely invaded the agar substratum and so could not be imaged in a single focal plane, in contrast to wild-type control hyphae (Figure 5C, barbed arrows, middle panel). So the lack of guidance control extended to the Z dimension as well as the X–Y dimension. We examined the localization of Sec2-YFP when Cdc28 was inhibited. Just after evagination (60 min after hyphal induction), a Spitzenkörper-like structure was present in the majority of cells (Figure 5B). However, localization of the Spitzenkörper could not be maintained and Sec2-YFP first reverted to a crescent-like organization and then disappeared from the tip altogether (Figure 5B). To quantify this effect, we followed cells growing on an agarose substratum on concavity slides in hyphal-inducing conditions (Figure 5C). At 60 min, when the observations were commenced, Sec2-YFP was localized to a spot in 7 out of 10 cells. By 120 min, none of the cells showed Sec2-YFP localizing to a spot. At the end of this time, other cells growing on the slides were examined. Only one out of a further 22 cells showed Sec2-YFP localizing to a spot and the majority showed no apical localization of Sec2-YFP (Figure 5D middle panel). (These cells were examined in an extended focal range to ensure that fluorescence from Sec2-YFP was genuinely absent). In the same experiment, control cells carrying the wild-type CDC28 gene were incubated on concavity slides in the same temperature hood. After 120 min, all 50 cells examined showed Sec2-YFP localizing to an apical spot. These cells also grew in a more-or-less linear manner and did not invade the agarose substratum (Figure 5D, left panel).
To test the hypothesis that S584 is the critical residue, we introduced the phosphomimetic SEC2 584E allele into the cdc28-1as strain (sec2 S584E cdc28Δ/cdc28-1as). This allele rescued the growth and morphological defects caused by Cdc28 inhibition (Figure 5D, right panel). After 180 min growth, the mean length of the of the cdc28 1asSEC2 584E hyphae was nearly twice that of the cdc28-1as strain (27.48±1.89 versus 14.98±1.34 μm, n=70) and the mean width of the cdc28-1as hyphae was 35% greater than the cdc28-1asSEC2 584E hyphae (1.35±0.035 versus 1.02±0.032 μm, n=70). Taken together, these observations show that phosphorylation of Sec2 S584 is necessary for normal hyphal growth, and that this phosphorylation is directly or indirectly dependent on the activity of Cdc28. To investigate whether the Spitzenkörper formed in the absence of Sec2, we used the Spitzenkörper marker FM4-64. When Cdc28 was inhibited in a cdc28-1as strain, FM4-64 no longer localized to an apical spot, as it did in a wild-type strain (Figure 5E). Quantitation of this experiment showed that in the wild-type strain 30/42 hyphae displayed an apical spot, whereas only 4/66 hyphae examined formed a spot when Cdc28 was inhibited. Thus, when phosphorylation of Sec2 to the Spitzenkörper is prevented by inhibition of Cdc28, the Spitzenkörper does not form.
Hyphal growth requires the cyclins Hgc1 and Ccn1. Hgc1 is homologous to S. cerevisiae Cln1/2. It is specifically expressed during hyphal growth and cells lacking Hgc1 develop abnormal, swollen hyphae (Zheng et al, 2004). As we have shown that Sec2 phosphorylation is dependent on Cdc28, we investigated whether Hgc1 was required for Sec2 phosphorylation. An hgc1Δ/Δ SEC2-YFP strain was constructed and these cells were grown in hyphal-inducing conditions on concavity slides in a temperature hood. At intervals, the morphology and Sec2-YFP was examined in 10 cells growing on the same slide, Figure 6A shows the images recorded from one of the cells, which is representative of all the cells in this and other similar experiments. Initially, germ tubes evaginated normally and Sec2-YFP localized to a Spitzenkörper, as was the case when Cdc28 was directly inhibited. However, after 90 min, polarized growth ceased and Sec2 was no longer polarized to the Spitzenkörper (Figure 6A). Growth subsequently resumed, but the resulting filament had a pseudohyphal morphology and Sec2-YFP localized to a surface crescent typical of pseudohyphae. Western blots of cell lysates prepared after cessation of hyphal-form growth showed that the phosphorylation of Sec2 was reduced (Figure 6B).
Figure 6.
Cells lacking Hgc1 are unable to maintain hyphal growth and show the yeast pattern of phosphorylation. (A) Frames from a timelapse video of an hgcΔ/Δ Sec2-YFP cell induced to form hyphae showing morphology and localization of Sec2-YFP. Times refer to minutes after stationary phase cells were re-inoculated into hyphal-inducing conditions. Top row merged DIC and fluorescent images (left) and fluorescent images alone (right). Bottom row shows detail of the tip of the same cells shown in the top row. Ten other timelapse videos gave similar results. Scale bars: top row, 5 μm; bottom row, 1 μm. (B) Sec2-YFP shows the yeast pattern of phosphorylation in cells lacking Hgc1. Hyphal cells were grown for 90 min after induction of hyphal growth, yeast cells were grown for 120 min. (C) Sec2-YFP is delocalized and hyphal tips swollen in a cell lacking Ccn1. Images taken 60 min after induction of hyphae. (D) Sec2-YFP shows the yeast pattern of phosphorylation and is destabilized in cells lacking Ccn1. Protein content of cell lysates was determined with a Bradford assay. To visualize the Sec2 YFP signal from a ccn1Δ/Δ strain, it was necessary to increase the amount of cell lysate five-fold compared with the wild-type control and the signal was still weaker. Scale bars: 5 μm.
We also investigated the function of Ccn1 in Sec2-YFP localization and phosphorylation. It has also been reported that this cyclin is required for hyphal morphogenesis. We also found that ccn1Δ/Δ mutants formed abnormal hyphae, but only in nutritionally poor synthetic medium and even then the phenotype was inconsistent. Interestingly, we found that in those cultures in which hyphae development was abnormal, Sec2 appeared destabilized in ccn1Δ/Δ mutants and in which hyphae development was abnormal, Sec2 localization to the Spitzenkörper was aberrant and Sec2 did not show the hyphal pattern of phosphorylation (Figure 6C and D).
We wished to investigate whether Cdc28-Hgc1 kinase targets Sec2 directly. In a first approach, we set out to test whether it could phosphorylate the wild-type S584 protein, but not the S584E mutant protein, in vitro. C. albicans Sec2 is 751 amino acids in length and contains 94 serines and 52 threonines including a TP dipeptide and two further SP dipeptides, in addition to the SP site at S584–5. With this large number of potential phosphorylation sites, it is likely that in non-physiological in vitro conditions, a purified Cdc28 kinase preparation would phosphorylate Sec2 at other sites apart from S584 and generate a positive signal even where the S584E mutant protein was used as a substrate. Thus, the specificity of Hgc1-Cdc28 for the S584 site would be difficult to establish by this means. To overcome this problem, we sought to generate a Sec2-derived peptide encompassing the sequence surrounding S584. In addition to the cdk core motif, stable binding and efficient phosphorylation of cdk target substrates can be dependent on additional C-terminal sequences that bind to a conserved hydrophobic patch in the relevant cyclin (Schulman et al, 1998). As this hydrophobic patch is conserved in Hgc1 (residues 100–156), we constructed a Sec2-derived 38-amino-acid peptide corresponding to amino acids 577–605, which was expressed as a GST fusion in Escherichia coli.
Anti-HA immunoprecipitates of lysates derived from a cdc28-1as strain containing HA-tagged Hgc1 were highly enriched for Hgc1 and Cdc28 (Figure 7A, left-hand panels). Immunoprecipitated HGC1-Cdc28, but not mock immunoprecipitates, were capable of phosphorylating the GST-Sec2 polypeptide in the presence of [32P]ATP (Figure 7A, right-hand panel). To establish whether Hgc1-Cdc28 phophorylates S584, we created a GST-Sec2 fusion peptide containing the S584E substitution (Figure 7B), and compared the extent of phosphorylation between wild-type and mutant GST-Sec2 peptide substrates (Figure 7C). When the S584E fusion peptide was used as substrate, the signal was reduced by about 50%, consistent with the notion that S584 is at least one of the sites phosphorylated by Hgc1-Cdc28 (Figure 7C). Kinase activity against the GST-Sec2 peptide, as well as the canonical cdk substrate histone H1, was significantly inhibited in the presence of 25 μm 1NM-PP1 (Figure 7C) confirming the specificity of the reaction.
Figure 7.
Cdc28-Hgc1 directly targets Sec2. (A) (Left-hand panels): cell lysates were immunoprecipitated with anti-HA antibodies, and after electrophoresis, immunoprecipitates and lysates samples were subjected to western blotting with anti-HA (lower panels) and anti-PSTAIRE (upper panels). Right-hand panels: Hgc1-Cdc28 1as or mock immunoprecipitates were incubated in absence and presence of purified GST-Sec2 peptide in the presence of [32P] ATP and, after electrophoresis, subjected to autoradiography. (B) GST fusion peptide substrates containing either the wild-type S584 residue or the E584 substitution. (C) Hgc1-Cdc28 1as immunoprecipitates were incubated with purified GST-Sec2 S584 peptide, GST-Sec2 S584E peptide or histone H1 in the presence of [32P] ATP and 25 μM 1NM-PPI where indicated and, after electrophoresis, subjected to autoradiography. Quantitation shows the results of three independent experiments. Error bars are standard errors of the mean. Difference in extent of phosphorylation between GST-S584 and GST-S584E (significance P<0.01). (D) Sec2 co-immunoprecipitates with Cdc28. Sec2-YFP was immunoprecipitated from yeast (Y), pseudohyphae (PH) and hyphae (H) expressing Sec2 or Sec2-YFP as indicated. Parallel western blots loaded with equal amounts of the immunoprecipitate or cell lysate were probed with a monoclonal anti-GFP antibody or anti-PSTAIRE antibody, which recognizes Cdc28.
In a second approach, we investigated whether we could detect a stable physical association between Cdc28 and Sec2. To do this, lysates from Sec2-YFP or control untagged Sec2 containing strains were subjected to immunoprecipitation using an anti-GFP monoclonal antibody and, after electrophoresis and western blotting, probed either with an anti-GFP or anti-PSTAIRE antibody. Figure 7D shows that Cdc28 was indeed present in the Sec2 immunoprecipitate.
Discussion
Previously, we have shown that, Mlc1 localizes to a subapical spot in C. albicans hyphae, whereas in yeast and pseudohyphae, it localizes to a surface crescent. We suggested that the spot in hyphae was analogous to a Spitzenkörper, a subapical body rich in secretory vesicles that is thought to drive polarized growth in filamentous fungi. In this work, we have shown that Sec2 also localizes to an apical spot in hyphae, which is spatially distinct from the localization of Exo70, an exocyst component that localizes to a surface crescent. Other work in our laboratory has shown that Sec4 similarly localizes to an apical spot (Jones and Sudbery, in revision). This localization is spatially distinct from the localization of polarisome and exocyst components. Moreover, we have also shown that Spitzenkörper components are more dynamic in FRAP experiments than exocyst or polarisome components. Both Sec2 and Sec4 are known to associate with secretory vesicles (Goud et al, 1988; Walch-Solimena et al, 1997); thus the localization of these proteins to an apical spot adds further weight to the hypothesis that the polarized growth of C. albicans is driven by an accumulation of secretory vesicles.
Phosphorylation of Serine 584 is required for hyphal growth
In this work, we have addressed the mechanism, which leads to the appearance of the Spitzenkörper in C. albicans hyphae, but not pseudohyphae or yeast forms. We focused on Sec2 because previous research has shown that it has a central function in mediating the flow of secretory vesicles from the Golgi to sites of polarized growth. We reasoned that the function of Sec2 may be modulated by differential phosphorylation. Using a C-terminal YFP fusion as a tag, we showed that Sec2 is phosphorylated and that the pattern changes upon induction of hyphal growth. We constructed a series of C-terminal truncations to map the phosphorylation events required for viability and for hyphal growth. Truncations that extended beyond residue 550, which deleted the region corresponding to the 58-amino-acid localization domain in ScSec2, were not capable of supporting viability. Truncation alleles Sec21−550-YFP and Sec21−583-YFP were still capable of supporting growth of yeast cells, but not hyphal development and were not phosphorylated. Sec21−591-YFP supported hyphal growth and localized to a Spitzenkörper and was phosphorylated, but only in the basal yeast pattern. Thus, deletion mapping identified an 8-amino-acid window between residues 583 and 591 that was required for hyphal growth, localization of secretory vesicles to the Spitzenkörper and phosphorylation.
There are two serines 584 and 588 that are potential phospho-acceptor sites within the critical 8-amino-acid region. A non-phosphorylatable alanine substitution for serine at 588 (S588A) formed hyphae normally, but the sec2 S584A mutant was apparently non-functional because we were unable to construct a strain in which this allele provided the sole source of Sec2. However, a phosphomimetic substitution of glutamate for serine at 584, S584E, resulted in a functional protein that still supported hyphal growth, but no longer displayed the pronounced band shift characteristic of the wild-type protein. Thus, S584 is likely the important phospho-acceptor site and phosphorylation of this residue is required for hyphal development. Sec21−591-YFP showed the basal, but not the hyphal pattern of phosphorylation. This suggested that the additional pattern of phosphorylation observed in hyphae was not actually necessary to support hyphal growth. We confirmed this conclusion by identifying S598 as the residue specifically phosphorylated in hyphal growth and showing that a Sec2 S598A allele is still able to support hyphal growth. Interestingly, S598 was identified in a proteome-wide survey of phosphorylation sites in C. albicans (Beltrao et al, 2009). Although we were unable to identify a physiological function for this phosphorylation event, it is possible that it has a subtle function in the induction or maintenance of hyphal growth in vivo.
S584 is phosphorylated by Cdc28
To identify the kinase responsible, we first investigated the possible involvement of two candidate kinases. Cbk1 is an evolutionarily conserved kinase, which is required for polarized growth in diverse fungal species. Recently, it has been reported that in S. cerevisiae, it interacts with and phosphorylates Sec2 in vitro and is required for the localization of Sec2 and Sec4 in vivo (Kurischko et al, 2008). Tpk1 and Tpk2 encode catalytic subunits of cAMP-dependent protein kinase, which is part of the signal transduction pathway that induces hyphal development in response to environmental cues (Sonneborn et al, 2000; Bockmuhl et al, 2001). Although the absence of activity of both of these kinases blocked hyphal development, Sec2 was still phosphorylated. Thus, neither of these two candidate kinases is required for phosphorylation of Sec2. However, we obtained evidence that suggested that Cdc28 partnered by the cyclin Hgc1, and possibly Ccn1, was directly or indirectly required for the phosphorylation of S584. First, we constructed a cdc28-1as allele that allows Cdc28 activity to be conditionally repressed by the addition of 1NM-PP1. Biochemical assays reported elsewhere confirm that this allele of Cdc28 is indeed inhibited by 1NM-PP1 (Sinha et al, 2007). When Cdc28 activity was inhibited, Sec2-YFP showed the yeast, but not the hyphal pattern of phosphorylation. As a consequence, hyphal development was abnormal, and although Sec2-YFP was initially localized to a spot, its localization rapidly degenerated first to a crescent, and then disappeared altogether. Importantly, the phosphomimetic S584E allele rescues the effects of Cdc28 inhibition. Second, we showed that in the absence of Hgc1, phosphorylation of Sec2 was reduced and its localization was abnormal in a similar manner to the effects of Cdc28 inhibition. We examined whether Cdc28-Hgc1 directly phosphorylates Sec2 S584 by an in vitro kinase assay using a peptide spanning the site as a substrate. We showed that Cdc28-Hgc1 isolated from hyphae phosphorylated a peptide spanning the S584 region in vitro, but that phosphorylation of a peptide containing the S584E substitution was reduced by about 50%. The residual phosphorylation was presumably due to seven other potential phospho-acceptor sites in the peptide. Moreover, we showed that Sec2 co-immunoprecipitates with Cdc28 demonstrating a physical interaction in vivo between the kinase–cyclin complex and Sec2. Thus, it seems likely that the Cdc28-Hgc1 directly targets S584.
The SEC2 584E allele was unable to rescue the effects of the hgc1Δ/hgc1Δ and ccn1Δ/Δ mutations. On its own, this observation is not surprising as Hgc1 is known to have other functions in hyphal development, for example Hgc1 mediates the phosphorylation of Rga2, a negative regulator of polarized growth, acting as a GAP for Cdc42 (Zheng et al, 2007). Moreover, it is known that Cdc28 partnered by Ccn1 mediates the phosphorylation of the septin Cdc11 by Cdc28 at early times after hyphal induction (Sinha et al, 2007). Thus, the more surprising observation is that the S584E allele rescues the effect of inhibiting Cdc28-1as with 1NM-PP1. We speculate that this may be due to residual activity of Cdc28 activity in the presence of the inhibitor that facilitates the rescue. Support for this conjecture comes from the in vitro kinase assay where we observed that the activity of Cdc28 1as was reduced, but not abolished by the presence of the inhibitor.
What is the physiological function of S584 phosphorylation? In S. cerevisiae, post-Golgi transport of secretory vesicles is mediated by the Mlc1/Myo1 motor complex and interactions of this complex with Sec2 has been reported. We have preliminary evidence that confirms an interaction between Sec2 and Mlc1 and suggests that the interaction is different in hyphae compared with yeast. We speculate that the hyphal-specific phosphorylation pattern of Sec2 facilitates its efficient exit from the Golgi and transport to the Spitzenkörper by promoting its interaction with the Mlc1/Myo2 motor complex.
Materials and methods
Media and growth conditions
Unless stated otherwise, cultures were grown on YEPD (2% glucose, 2% Difco Bacto peptone and 1% Difco Bacto yeast extract) plus 80 mg/l uridine. SD medium consists of 0.67% w/v yeast nitrogen base (Difco), 2% w/v glucose, 80 mg/l each of histidine, uridine and arginine. Yeast form growth was promoted by adjusting the pH to 6.0 and incubating at 25°C. Pseudohyphal growth was promoted by adjusting the pH to 5.5 and incubating at 36°C. Hyphal growth was promoted by adding 20% calf serum (Sigma Aldrich, St Louis), adjusting the pH to 7.0 and incubating at 37°C (Sudbery, 2001).
Strain construction
Strains constructed are listed in Supplementary Table 1, and the oligonucleotides used are listed in Supplementary Table 2. All strains were derived from BWP17 unless stated otherwise. Gene deletions and C- and N-terminal YFP fusions were performed as previously described (Wilson et al, 1999; Gerami-Nejad et al, 2001, 2004; Gola et al, 2003; Schaub et al, 2006; Court and Sudbery, 2007).
Site-directed mutagenesis was carried out as previously described (Court and Sudbery, 2007). Oligonucleotides used are listed in Supplementary Table 2. The cdc28-1as mutant allele was F85G equivalent to the F88G allele shown to inactivate S. cerevisiae Cdc28 in the presence of the 1NM-PP1 inhibitor (Bishop et al, 2000). In C. albicans, the Cdc28 in this F85G allele has been shown to be inhibited by 5 μM 1NM-PP1 using an in vitro kinase assay (Sinha et al, 2007). In the case of the sec2Δ/sec2 S583E cdc28Δ/cdc28-1as strain, the continued presence of the cdc28-1as and sec2 S583E alleles were verified a second time after the experiments were completed by re-amplification by PCR and re-sequencing. The re-sequencing also confirmed the absence of a wild-type Cdc28 allele, which might have arisen by chromosome duplication (Supplementary Figure S1).
Extraction of total cell protein
Stationary phase cells were re-inoculated into fresh media and grown under the conditions as specified for different experiments. Cells were harvested and lysed with glass beads at 4°C using a FastPrep beadbeater fitted with cryohead (MP Bio, Cambridge, UK). Beads and cellular debris were removed by centrifugation at 11 000 r.p.m. for 5 min at 4°C. To study protein phosphorylation, cells were lysed in 50 mM Tris pH8, 50 mM NaF, 5 mM EDTA, 25 mM NaCl, 100 μM Na3VO4 to inhibit phosphatase action, protease inhibitors (Complete protease inhibitor cocktail, Roche, Welwyn Garden City, UK) and 1.6 mM PMSF. Samples that were to be treated with phosphatase were extracted in a simple buffer of 50 mM Tris pH8 and protease inhibitors (Complete protease inhibitor cocktail, Roche). Phosphatase treatment was carried out by incubating protein extracts prepared without phosphatase inhibitors with CIP (New England Biolabs, Ispwich, MA) for 90 min at 37°C.
Western blots
Western blots were carried out as previously described (Wightman et al, 2004; Crampin et al, 2005; Court and Sudbery, 2007). The anti-GFP monoclonal antibody (Roche Biosciences, Lewes, UK) was diluted 3.3 × 10−4. Anti-PSTAIRE (Sigma Aldrich) was diluted 10−3. Secondary antibodies conjugated with horseradish peroxidase were diluted 3.3 × 10−4. All dilutions were made with 5% w/v non-fat dried milk powder in TBS-T buffer.
Co-immunoprecipitation
Clarified protein extracts were prepared as described above and GFP-tagged proteins were purified using μMACS Epitope Tag Protein Isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The YFP fusion protein and any interacting proteins were eluted in SDS-loading buffer.
Kinase assay
See Supplementary data for full details.
Cdc28-1as inhibition
Stationary phase cells were size fractionated using a Beckman JE 5.0 elutriating rotor (Beckman Coulter, High Wycombe, UK). Large unbudded cells were re-inoculated into hyphal-inducing conditions in the presence of 5 μm 1NM-PP1 (Calbiochem, San Diego, CA) or 3.2 × 10−2 % DMSO (w/v)-solvent control.
Microscopy and live cell imaging
Localization of Sec2-YFP to the Spitzenkörper is dependent on vigorous hyphal growth, which requires imaging of live cells suspended in SD medium plus 20% serum on a microscope slide and maintained at 38°C during image acquisition using a microscope incubation chamber (Solent Scientific, Segensworth, UK). Wide field epifluorescence microscopy and differential interference contrast (DIC) microscopy was carried out using a Delta Vision RT microscope (Applied Precision Instruments, Seattle) using an Olympus 100 × UPlanAPo NA 1.35 lens (Olympus Tokyo, Japan). Images were acquired and deconvolved with Softworx™ software. Images are projections of the deconvolved Z-stack unless otherwise stated. In the timelapse experiment shown in Figure 6, the exposure times were kept to a minimum to result to avoid phototoxicity. This resulted in a grey background in the area outside that occupied by the cells in the image. This background was removed by adjusting the threshold across the whole image at the black side of the spectrum using the channels spectrum facility in Softworx. To visualize the cell outline in fluorescent images, cells were counterstained with 1 μg/ml Calcofluor white (Fluorescent Brightener 28, Sigma Aldrich). Where appropriate, the blue channel has been differentially enhanced to ensure that the outline of the cells was clearly visible. Co-localization of Exo70-YFP and Sec2-GFP-Sec4 was carried out using a Zeiss Laser Scanning META confocal microscope running Release 4.0. SP2 software. The 514 nm HFT laser in conjunction with a Long pass 530 filter was used to image YFP and GFP was imaged with a 488 HFT laser and Long pass 505 filter. To visualized the Spitzenkörper, cells were incubated with 40μM FM4-64 (Invitrogen, Eugene, Oregon) for 5 min at 37°C.
Supplementary Material
Acknowledgments
Amy Bishop and Rachel Lane made an equal contribution to the generation of the experimental work described in this paper. Bernardo Chapa-y-Lazo constructed the cdc28-1as allele. Peter Sudbery conceived the strategic approach, carried out some of the microscopy and wrote the paper. Richard Beniston and Carl Smythe carried out the kinase assay in collaboration with Amy Bishop. We thank Christophe d'Enfert for the tpk1Δ/Δ tpk2Δ/tpk2 1as strain and Haoping Liu and Yue Wang for both supplying ccn1Δ/Δ and hgc1Δ/Δ strains and Yue Wang for the 6HA-HGC1 CDC28-1as/Δ strain. Rachel Lane was in receipt of a BBSRC research studentship. Richard Benson was supported by a Yorkshire Cancer Research grant number S304. This work was funded by BBSRC grant number BB/H003126/1. Delta Vision Microscope Images were obtained using the facilities of the Sheffield University Light Microscope Facility (LMF) supported by Wellcome Trust grant Number GR077544AIA.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bartnicki-Garcia S, Bartnicki DD, Gierz G, Lopezfranco R, Bracker CE (1995) Evidence that Spitzenkorper behavior determines the shape of a fungal hypha—a test of the hyphoid model. Exp Mycol 19: 153–159 [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S, Hergert F, Gierz G (1989) Computer-simulation of fungal morphogenesis and the mathematical basis for hyphal (tip) growth. Protoplasma 153: 46–57 [Google Scholar]
- Beltrao P, Trinidad JC, Fiedler D, Roguev A, Lim WA, Shokat KM, Burlingame AL, Krogan NJ (2009) Evolution of phosphoregulation: comparison of phosphorylation patterns across yeast species. Plos Biol 7; doi:10.1371/journal.pbio.1000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM (2000) A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407: 395–401 [DOI] [PubMed] [Google Scholar]
- Biswas S, Van Dijck P, Datta A (2007) Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev 71: 348–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmuhl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF (2001) Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol 42: 1243–1257 [DOI] [PubMed] [Google Scholar]
- Bracker CE, Murphey DJ, Lopez-Franco R (1997) Laser microbeam manipulation of cell morphogenesis in growing hyphae. In Functional Imaging of Optical Manipulation of Living Cells, Farkas, DL, Tromberg BJ (eds) Washington: International Society for Optical Engineering, Bellingham [Google Scholar]
- Brown AJP, Argimon S, Gow NAR (2007) Signal transduction and morphogenesis in Candida albicans. In Biology of the Fungal Cell, Howard RJ, Gow NAR (eds) Berlin: Springer. pp 167–194 [Google Scholar]
- Care RA, Trevethick J, Binley KM, Sudbery PE (1999) The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol Microbiol 34: 792–798 [DOI] [PubMed] [Google Scholar]
- Court H, Sudbery P (2007) Regulation of Cdc42 GTPase activity in the formation of hyphae in Candida albicans. Mol Biol Cell 18: 265–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampin H, Finley K, Gerami-Nejad M, Court H, Gale C, Berman J, Sudbery PE (2005) Candida albicans hyphae have a Spitzenkorper that is distinct from the polarisome found in yeast and pseudohyphae. J Cell Sci 118: 2935–2947 [DOI] [PubMed] [Google Scholar]
- Dong G, Medkova M, Novick P, Reinisch KM (2007) A catalytic coiled coil: structural insights into the activation of the Rab GTPase Sec4p by Sec2p. Mol Cell 25: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind NB, Walch-Solimena C, Novick PJ (2000) The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles. J Cell Biol 149: 95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott JA, Noble MEM, Tucker JA (1999) Cyclin-dependent kinases: inhibition and substrate recognition. Curr Opin Struct Biol 9: 738–744 [DOI] [PubMed] [Google Scholar]
- Gerami-Nejad M, Berman J, Gale C (2001) Cassettes for PCR-mediated construction of green, yellow and cyan fluorescent protein fusions in Candida albicans. Yeast 18: 859–880 [DOI] [PubMed] [Google Scholar]
- Gerami-Nejad M, Hausauer D, McClellan M, Berman J, Gale C (2004) Cassettes for the PCR-mediated construction of regulatable alleles in Candida albicans. Yeast 21: 429–436 [DOI] [PubMed] [Google Scholar]
- Girbardt M (1957) Der Spitzenkörper von polystictus versicolor. Planta 50: 47–50 [Google Scholar]
- Girbardt M (1969) Ultrastructure of apical region of fungal hyphae. Protoplasma 67: 413 [Google Scholar]
- Gola S, Martin R, Walther A, Dunkler A, Wendland J (2003) New modules for PCR-based gene targeting in Candida albicans: rapid and efficient gene targeting using 100 bp of flanking homology region. Yeast 20: 1339–1347 [DOI] [PubMed] [Google Scholar]
- Goud B, Salminen A, Walworth NC, Novick PJ (1988) A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma-membrane in yeast. Cell 53: 753–768 [DOI] [PubMed] [Google Scholar]
- Grove SN, Bracker CE (1970) Protoplasmic organization of hyphal tips amoung fungi: vesicles and Spitzenkörper. J Bacteriol 104: 989–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove SN, Bracker CE, Morre DJ (1970) An ultrastructural basis for hyphal tip growth in Pythium ultimum. Am J Botany 57: 245–266 [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P (1999) The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J 18: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SD, Read ND, Roberson RW, Shaw B, Seiler S, Plamann M, Momany M (2005) Polarisome meets Spitzenkorper: microscopy, genetics, and genomics converge. Euk Cell 4: 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurischko C, Kuravi VK, Wannissorn N, Nazarov P, Husain M, hang C, hokat KM, Caffery JM, Luca FC (2008) The yeast LATS/Ndr kinase Cbk1 regulates growth via Golgi-dependent glycosylation and secretion. Mol Biol Cell 19: 5559–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939–949 [DOI] [PubMed] [Google Scholar]
- McCusker D, Denison C, Anderson S, Egelhofer TA, Yates JR, Gygi SP, Kellogg DR (2007) Cdk1 coordinates cell-surface growth with the cell cycle. Nat Cell Biol 9: 506–515 [DOI] [PubMed] [Google Scholar]
- McNemar MD, Fonzi WA (2002) Conserved serine/threonine kinase encoded by CBK1 regulates expression of several hypha-associated transcripts and genes encoding cell wall proteins in Candida albicans. J Bacteriol 184: 2058–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medkova M, France YE, Coleman J, Novick P (2006) The rab exchange factor Sec2p reversibly associates with the exocyst. Mol Biol Cell 17: 2757–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J, Muller H, Peterson M, Novick P (1990) Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. J Cell Biol 110: 1897–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Brennwald P (1993) Friends and family—the role of the Rab GTPases in vesicular traffic. Cell 75: 597–601 [DOI] [PubMed] [Google Scholar]
- Novick PJ, Goud B, Salminen A, Walworth NC, Nair J, Potenza M (1988) Regulation of vesicular traffic by a GTP-binding protein on the cytoplasmic surface of secretory vesicles in yeast. Cold Spring Harb Symp Quant Biol 53: 637–647 [DOI] [PubMed] [Google Scholar]
- Novick P, Medkova M, Dong G, Hutagalung A, Reinisch K, Grosshans B (2007) Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem Soc Trans 34: 683–686 [DOI] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P (2002) Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol 157: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HO, Bi EF (2007) Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev 71: 48–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub Y, Dunkler A, Walther A, Wendland J (2006) New pFA-cassettes for PCR-based gene manipulation in Candida albicans. J Basic Microbiol 46: 416–429 [DOI] [PubMed] [Google Scholar]
- Schulman BA, Lindstrom DL, Harlow E (1998) Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc Natl Acad Sci USA 95: 10453–10458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha I, Wang YM, Philp R, Li CR, Yap WH, Wang Y (2007) Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev Cell 13: 421–432 [DOI] [PubMed] [Google Scholar]
- Soll DR, Herman MA, Staebell MA (1985) The involvement of cell wall expansion in the two modes of mycelium formation of Candida albicans. J Gen Microbiol 131: 2367–2375 [DOI] [PubMed] [Google Scholar]
- Sonneborn A, Bockmuhl DP, Gerads M, Kurpanek K, Sanglard D, Ernst JF (2000) Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol Microbiol 35: 386–396 [DOI] [PubMed] [Google Scholar]
- Steinberg G (2007) Hyphal growth: a tale of motors, lipids, and the Spitzenkorper. Euk Cell 6: 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery PE (2001) The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localisation. Mol Microbiol 41: 19–31 [DOI] [PubMed] [Google Scholar]
- Sudbery PE, Gow NAR, Berman J (2004) The distinct morphogenic states of Candida albicans. Trends Microbiol 12: 317–324 [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ (1997) Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol 137: 1495–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Goud B, Kabcenell AK, Novick PJ (1989) Mutational analysis of Sec4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J 8: 1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Davis D, Mitchell AP (1999) Rapid hypothesis testing in Candida albicans through gene disruption with short homology regions. J Bacteriol 181: 1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang Y, Wang Y (2004) Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J 23: 1845–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XD, Lee RTH, Wang YM, Lin QS, Wang Y (2007) Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J 26: 3760–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.